Abstract
Background
Medication non-adherence is a known risk factor for adverse outcomes in the general population. However, little is known about the association of pre-dialysis medication adherence among patients with advanced chronic kidney disease with mortality following their transition to dialysis.
Study Design
Observational study.
Setting & Participants
32,348 US veterans who transitioned to dialysis during 2007–2011.
Predictors
Adherence to treatment with cardiovascular drugs, ascertained from pharmacy database records using proportion of days covered (PDC) and persistence during the pre-dialysis year.
Outcomes
Post-dialysis initiation all-cause and cardiovascular mortality, using Cox models with adjustment for confounders.
Results
The mean age of the cohort was 72±11 (SD) years, among whom 96% were male, 74% were white, 23% were African American, and 69% were diabetic. During median follow-up of 23 (IQR, 9–36) months, 18,608 patients died. Among patients with PDC >80%, there were 14,006 deaths (mortality rate, 283 [95% CI, 278–288]/1000 patient-years [PY]); among patients with PDC >60%–80%, there were 3,882 deaths (mortality rate, 294 [95% CI, 285–304]/1000 PY); among patients with PDC ≤60%, there were 720 deaths (mortality rate, 291 [95% CI, 271–313]/1000 PY). Compared to patients with PDC >80%, adjusted HR for post-dialysis initiation all-cause mortality for patients with PDC >60%–80% was 1.12 (95% CI, 1.08–1.16) and for patients with PDC ≤60% was 1.21 (95% CI, 1.11–1.30). In addition, compared to patients showing medication persistence, adjusted HR risk for post-dialysis initiation all-cause mortality for patients with non-persistence was 1.11 (95% CI, 1.05–1.16). A similar trend was detected for cardiovascular mortality and in subgroup analyses.
Limitations
Large number of missing values; the results may not be generalizable to women or the general US population.
Conclusions
Pre-dialysis cardiovascular medication non-adherence is an independent risk factor for post-dialysis mortality among advanced chronic kidney disease patients transitioning to dialysis. Further studies are needed to assess whether interventions targeting adherence improve survival after dialysis initiation.
Keywords: transition to dialysis, medication adherence, treatment compliance, proportion of days covered (PDC), medication possession ratio (MPR), drug therapy, cardiovascular mortality, mortality, advanced chronic kidney disease, anti-hypertensive medications, statins, aspirin, cardiovascular pharmacotherapy, pharmacy database analysis
Mortality rates in patients with end-stage renal disease (ESRD) continue to be high.(1) Therefore, identification and correction of modifiable risk factors influencing all-cause mortality in ESRD patients is of paramount importance.
Only a limited number of interventions, such as timely arteriovenous fistula creation and adequate access to specialist care during the pre-dialysis period, have been shown to be associated with better outcomes in ESRD patients.(2–6) Cardiovascular disease is the leading cause of mortality in pre-dialysis patients,(7) and anti-hypertensive medications, statins and aspirin are widely used in the cardiovascular risk management of these patients.(8) Adherence to pharmacotherapy in general hypertensive populations has been linked to reduced risk of various outcomes.(9–13) However, little is known about the association of medication adherence during the pre-dialysis period with all-cause and cardiovascular mortality after dialysis initiation.
We investigated the association of adherence to medications targeting cardiovascular risk in the last year prior to initiating dialysis with all-cause and cardiovascular mortality after dialysis initiation in a cohort of US veterans with advanced chronic kidney disease (CKD) transitioning to dialysis. We applied three methods of adherence determination using pharmacy databases: (1) proportion of days covered (PDC) and (2) medication possession ratio (MPR) to evaluate compliance (the extent to which patients follow prescribed dosing regimens), and (3) persistence with drug therapy (duration of time from initial drug dispensation to “unauthorized” discontinuation). We hypothesized that lower medication adherence results in higher all-cause and cardiovascular mortality.
METHODS
Study Population
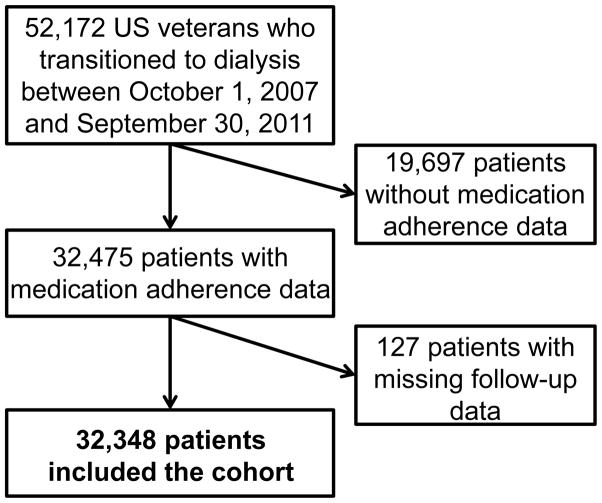
We analyzed data from the Transition of Care in CKD (TC-CKD) study, a retrospective cohort study examining US veterans with CKD transitioning to dialysis from October 1, 2007, through September 30, 2011. A total of 52,172 patients were identified from the US Renal Data System (USRDS). We excluded patients whose medication adherence could not be calculated due to missing pharmacy data (n = 19,697), and those who had lack of follow-up data (n = 127). The final cohort consisted of 32,348 patients (Figure 1).
Figure 1.
Flow chart of patients’ selection
Covariates
Data from the USRDS Patient and Medical Evidence files were used to determine patients’ baseline demographic information and type of vascular access at the time of dialysis initiation. We used the national VA Corporate Data Warehouse LabChem data files to extract data about pre-dialysis serum creatinine.(14) Other laboratory variables were collected from the Decision Support System National Data Extracts Laboratory Results file,(15) and baseline values were defined as the last quarterly average before dialysis initiation or the second-from-last quarterly average if the last data point was missing. Data on medication exposure was obtained from both Centers for Medicare & Medicaid Services (CMS, Medicare Part D) and US Department of Veterans Affairs (VA) pharmacy dispensation records.(16) Patients who received at least one dispensation of outpatient medication within one year of dialysis initiation were recorded as having been treated with these medications. Information about comorbidities at the time of dialysis initiation was extracted from the VA Inpatient and Outpatient Medical SAS Datasets,(17) and from CMS datasets using diagnostic and procedure codes. Cardiovascular/cerebrovascular disease was defined as the presence of diagnostic codes for coronary artery disease, angina, myocardial infarction, or cerebrovascular disease. We calculated the Charlson Comorbidity Index score using the Deyo modification for administrative data sets, without including kidney disease.(18)
Exposure Variables
Figure S1 (provided as online supplementary material) depicts schematics of different methods of adherence calculation. The PDC was defined as the proportion of days with drug available in the measurement period, capped at 100%. The MPR was calculated as the percentage of total days covered by the dispensed drug supply during the measurement period. Numerically, MPR can take values between 0 and >100%.(19, 20) For medication persistence the following algorithm was used: persistence was coded as being 1 (present) if a patient refilled each subsequent prescription with gaps not exceeding 60 days; otherwise, it was coded as 0 (absent, or non-persistent).(20)
Detailed information about each prescription was collected during the last year before dialysis for the following cardiovascular drugs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, calcium channel blockers, ß-blockers, α-blockers, direct vasodilators, diuretics (loop and thiazide), aspirin and statins. The index date was the date of the first available prescription (in the last year before dialysis initiation), regardless of any prescriptions before this date. The last prescription had to be dispensed before dialysis initiation, and the full prescription period was included in the denominator, regardless of whether the supply lasted until after the dialysis initiation date. Only outpatient prescriptions were taken into account. Any inpatient time period was added to the denominator. The averaged values of the PDCs and MPRs of all medication groups were used as exposure variables in analyses. Medication adherence was categorized as follows: (1) for PDC: >80%, >60%–≤80%, and ≤60%; (2) for MPR: ≥100%, >80%–<100%, >60%−≤80%, and ≤60%. We dichotomized medication persistence as average persistence of <50% or >50%, derived from individual drug prescription refills. The PDCs and MPRs were also treated as continuous variables to examine non-linear associations using restricted cubic spline analyses.
Outcome Assessment
The co-primary outcomes of this study were all-cause and cardiovascular mortality after dialysis initiation. Death dates were obtained from USRDS and the VA Vital Status Files (up to December 27, 2012). Cause of death was obtained from USRDS (up to October 6, 2011).
Statistical Analysis
Data are presented as number (percentage) for categorical variables and as mean ± standard deviation or median (interquartile range [IQR]), as appropriate. Categorical variables were compared with χ2 tests. Continuous variables were compared using t tests or Mann–Whitney U tests, or ANOVA, as appropriate. We used Cox proportional hazard regressions to determine the association of medication adherence with all-cause and cardiovascular mortality, considering the time from the date of dialysis initiation until the event of interest. Patients were followed up until death or other censoring events including kidney transplantation, loss to follow-up, or until December 27, 2012, whichever happened first. For cardiovascular mortality, patients were followed up until death or other censoring events, or until October 6, 2011. The influence of potential confounders was analyzed by incremental adjustments based on a priori considerations: unadjusted (model 1); age, sex, race/ethnicity (whites, African-Americans, Hispanics, and others), and marital status (married, divorced, single, and widowed) and ZIP code (model 2); model 2 plus comorbid conditions (diabetes mellitus, congestive heart failure, cardiovascular/cerebrovascular disease, depression, anxiety and Charlson Comorbidity Index) and type of vascular access (model 3); and model 3 plus blood/serum hemoglobin, bicarbonate, albumin, urea nitrogen, and last estimated glomerular filtration rate (eGFR) before ESRD (model 4). Model 4 had high proportions of missing data (40%–50%, see below); therefore, we used model 3 as the main multivariable adjusted model.
Restricted cubic spline models were used to investigate non-linearity in model 3. The associations of PDC with all-cause mortality were examined in subgroups of patients categorized by age, race, Charlson Comorbidity Index level, and the presence of diabetes mellitus, congestive heart failure, cerebro- and cardiovascular disease. Interactions were formally tested for by the inclusion of interaction terms.
For model 4, we had only 13,693 (42%) patients with complete data available. Missing values were not imputed in primary analyses, but were substituted after adding the laboratory data (blood hemoglobin [49% missing], serum bicarbonate [47% missing], serum albumin [49% missing], serum urea nitrogen [45% missing] and last outpatient eGFR [23% missing]) with the use of multiple imputation procedures (creating 5 datasets) using STATA’s “mi” set of commands in sensitivity analyses. We also assessed the association of separate PDCs of each medication category with all-cause mortality as sensitivity analysis.
Compared with patients excluded due to missing medication data, included patients were older, more likely to be male, white (74% versus 70%), had higher arteriovenous fistula rate (23% versus 14%) and had higher prevalence of diabetes (69% versus 55%), cardiovascular/cerebrovascular disease (50% versus 29%), hypertension (91% versus 69%) and congestive heart failure (60% versus 50%) (Table S1).
P values are two-sided and reported as significant at <0.05 for all analyses. All analyses were conducted using STATA MP Version 14 (STATA Corp LP, College Station, TX). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
RESULTS
Baseline Characteristics
The mean age of the cohort at baseline was 72±11 (standard deviation) years; 96% were male, 74% were white, 23% were African American, and 69% were diabetic. The median of the last pre-ESRD outpatient eGFR was 16 (IQR, 10–26) ml/min/1.73m2. Baseline characteristics of patients categorized by PDC categories are shown in Table 1. Patients with a higher PDC (>80%) were older; more likely to be white and married; more likely to initiate dialysis with an arteriovenous fistula; more likely to be on a statin and on angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; had a higher prevalence of hypertension; had higher serum albumin and calcium; lower serum phosphate, PTH, total and low-density lipoprotein cholesterol levels, and urine albumin-creatinine ratio; and more favorable metabolic and anemia markers (Table 1). Table S2 shows the adherence parameters in different medication groups. In individual medication groups, PDC, MPR and non-persistence were very similar (Table S2).
Table 1.
Baseline characteristics of study population overall and by PDC categories
| All (N=32,348) | PDC>80% (n=24,455) | PDC >60%–80% (n=6,633) | PDC≤60% (n=1,260) | P | |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age (y) | 72 ±11 | 73 ±11 | 71 ±12 | 67 ±13 | <0.001 |
| Male sex | 31,045 (96) | 23,627 (97) | 6,264 (94) | 1,154 (92) | <0.001 |
| Race | <0.001 | ||||
| White | 24,064 (74) | 18,943 (78) | 4,410 (67) | 711 (56) | |
| African American | 7,531 (23) | 4,966 (20) | 2,049 (31) | 516 (41) | |
| Marital Status | <0.001 | ||||
| Married | 18,654 (58) | 14,576 (60) | 3,529 (54) | 549 (44) | |
| Non-married | 13,242 (42) | 9,561 (40) | 2,996 (46) | 685 (56) | |
| Comorbidities | |||||
| Charlson Comorbidity Index | 5 [3–7] | 5 [3–7] | 5 [3–7] | 5 [3–7] | 0.003 |
| Diabetes | 22,144 (69) | 16,605 (69) | 4,694 (71) | 845 (68) | <0.001 |
| Cardiovascular/cerebrovascular diseases | 16,117 (50) | 12,278 (50) | 3,263 (49) | 576 (46) | 0.004 |
| Myocardial Infarction | 9,674 (30) | 7,361 (31) | 1,979 (30) | 334 (27) | 0.02 |
| Congestive Heart Failure | 19,270 (60) | 14,348 (59) | 4,147 (63) | 775 (62) | <0.001 |
| Peripheral Vascular Disease | 13,608 (43) | 10,435 (43) | 2,699 (41) | 474 (38) | <0.001 |
| Hypertension | 28,764 (91) | 21,923 (91) | 5,796 (89) | 1,045 (85) | <0.001 |
| Cerebrovascular Diseases | 10,743 (34) | 8,203 (34) | 2,170 (33) | 370 (30) | 0.005 |
| Dementia | 989 (3) | 718 (3) | 219 (3) | 52 (4) | 0.03 |
| Chronic Pulmonary Diseases | 14,924 (47) | 11,207 (46) | 3,147 (48) | 570 (46) | 0.09 |
| Connective Tissue Diseases | 1,486 (5) | 1,157 (5) | 286 (4) | 43 (3) | 0.04 |
| Peptic Ulcer Diseases | 2,757 (9) | 2,036 (8) | 602 (9) | 119 (10) | 0.08 |
| Mild Liver Diseases | 3,695 (12) | 2,601 (11) | 890 (14) | 204 (16) | <0.001 |
| Moderate to Severe Liver | 785 (2) | 532 (2) | 205 (3) | 48 (4) | <0.001 |
| Diseases | |||||
| Paraplegia and Hemiplegia | 1,182 (4) | 881 (4) | 246 (4) | 55 (4) | 0.4 |
| Malignancy | 8,110 (25) | 6,297 (26) | 1,554 (24) | 259 (21) | <0.001 |
| Metastatic Carcinoma | 1,033 (3) | 792 (3) | 202 (3) | 39 (3) | 0.7 |
| Depression | 9,957 (31) | 7,221 (30) | 2,265 (34) | 471 (38) | <0.001 |
| Anxiety | 2,102 (6) | 1,586 (6) | 442 (7) | 74 (6) | 0.6 |
| AIDS/HIV | 281 (1) | 167 (1) | 81 (1) | 33 (3) | <0.001 |
| Vascular Access | <0.001 | ||||
| Arteriovenous fistula | 7,494 (23) | 5,802 (24) | 1,474 (22) | 218 (17) | |
| Arteriovenous graft | 716 (2) | 556 (2) | 142 (2) | 18 (1) | |
| Catheter | 3,140 (10) | 2,328 (10) | 687 (10) | 125 (10) | |
| Unknown | 20,998 (65) | 15,769 (64) | 4,330 (65) | 899 (71) | |
| Laboratory results | |||||
| Serum Sodium (mEq/L) | 139 (3) | 139 (3) | 139 (3) | 139 (3) | <0.001 |
| Blood Hemoglobin (g/dL) | 10.6 (1.6) | 10.7 (1.6) | 10.5 (1.6) | 10.2 (1.7) | <0.001 |
| Serum Albumin (g/dL) | 3.39 (0.6) | 3.42 (0.6) | 3.32 (0.6) | 3.18 (0.7) | <0.001 |
| Serum Potassium (mEq/L) | 4.51 (0.6) | 4.51 (0.6) | 4.50 (0.6) | 4.51 (0.6) | 0.2 |
| Serum Urea Nitrogen (mg/dL) | 64 (48–80) | 64 (49–80) | 63 (48–81) | 61 (48–78) | 0.4 |
| Serum Glucose (mg/dL) | 130 (50) | 130 (50) | 131 (52) | 134 (54) | 0.2 |
| Blood Hemoglobin A1c (%) | 6.8 (1.4) | 6.8 (1.4) | 6.9 (1.6) | 7.0 (1.7) | 0.02 |
| Serum Cholesterol | |||||
| Total (mg/dL) | 145 (119–176) | 143 (118–173) | 150 (123–185) | 159(130–199) | <0.001 |
| LDL (mg/dL) | 78 (59–102) | 76 (58–100) | 82 (61–111) | 91 (66–124) | <0.001 |
| HDL (mg/dL) | 36 (29–45) | 36 (29–44) | 37 (30–46) | 38 (30–48) | <0.001 |
| Serum Triglycerides (mg/dL) | 120 (83–176) | 121 (83–176) | 118 (84–175) | 117 (81–168) | 0.3 |
| Serum Calcium (mg/dL) | 8.71 (0.8) | 8.75 (0.8) | 8.58 (0.8) | 8.38 (0.9) | <0.001 |
| Serum Phosphate (mg/dL) | 5.22 (1.4) | 5.20 (1.4) | 5.28 (1.4) | 5.45 (1.4) | <0.001 |
| Serum Alkaline Phosphate (U/L) | 84 (65–111) | 82 (65–109) | 87 (67–115) | 90 (72–123) | <0.001 |
| Serum intact PTH (pg/mL) | 221 (126–368) | 212 (120–349) | 249 (149–416) | 301(167–496) | <0.001 |
| Serum Bicarbonate (mEq/L) | 22.9 (4.2) | 23.0 (4.2) | 22.6 (4.3) | 22.1 (4.3) | <0.001 |
| White Blood Cells (1000/mm3) | 7.4 (6.0–9.1) | 7.4 (6.1–9.1) | 7.3 (5.9–9.0) | 7.3 (5.9–9.1) | 0.008 |
| Urine ACR, mg/g | 349 (34–1810) | 299 (32–1565) | 477 (51–2181) | 2412 (341–4606) | <0.001 |
| Body Mass Index (kg/m2) | 30.0 (6.6) | 30.1 (6.7) | 29.7 (6.5) | 28.8 (6.3) | <0.001 |
| Last outpatient eGFR (mL/min/1.73m2) | 16 (10–26) | 16 (10–26) | 15 (10–25) | 17 (10–30) | 0.9 |
| Medication use | |||||
| Vitamin D analog | 10,549 (33) | 8,030 (33) | 2,182 (33) | 337 (27) | <0.001 |
| β-blocker | 24,778 (77) | 18,560 (76) | 5,286 (80) | 932 (74) | <0.001 |
| α-blocker | 12,055 (37) | 9,399 (38) | 2,343 (35) | 313 (25) | <0.001 |
| Bicarbonate | 4,629 (14) | 3,494 (14) | 974 (15) | 161 (13) | 0.2 |
| Calcium channel blocker | 22,080 (68) | 18,848 (69) | 4,449 (67) | 783 (62) | <0.001 |
| Diuretic | 26,199 (81) | 19,591 (80) | 5,614 (85) | 994 (79) | <0.001 |
| EPO | 7,655 (24) | 5,730 (23) | 1,659 (25) | 266 (21) | 0.003 |
| NSAID | 34 (0) | 28 (0) | 4 (0) | 2 (0) | 0.4 |
| Phosphate binder | 8,740 (27) | 6,458 (26) | 1,920 (29) | 362 (29) | <0.001 |
| ACEi/ARB | 19,117 (59) | 14,593 (60) | 3,876 (58) | 648 (51) | <0.001 |
| Direct Vasodilator | 1,483 (5) | 1,093 (4) | 347 (5) | 43 (3) | 0.004 |
| Aspirin | 7,865 (24) | 5,782 (24) | 1,796 (27) | 287 (23) | <0.001 |
| Statin | 22,684 (70) | 17,432 (71) | 4,545 (69) | 707 (56) | <0.001 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: bilirubin in mg/dL to μmol/L, ×17.1; calcium in mg/dL to mmol/L, ×0.2495; cholesterol in mg/dL to mmol/L, ×0.02586; glucose in mg/dL to mmol/L, ×0.05551; triglycerides in mg/dL to mmol/L, ×0.01129; serum urea nitrogen in mg/dL to mmol/L, ×0.357;
Abbreviations: AIDS/HIV: Acquired Immune Deficiency Syndrome/Human Immunodeficiency Virus; PDC: Proportion of Days Covered; PTH: Parathyroid Hormone; ACR, albumin-creatinine ratio; ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; LDL, low-density lipoprotein; HDL, high-density lipoprotein; EPO, erythropoietin; NSAID, nonsteroidal anti-inflammatory drug
Predialysis PDC and All-Cause and Cardiovascular Mortality
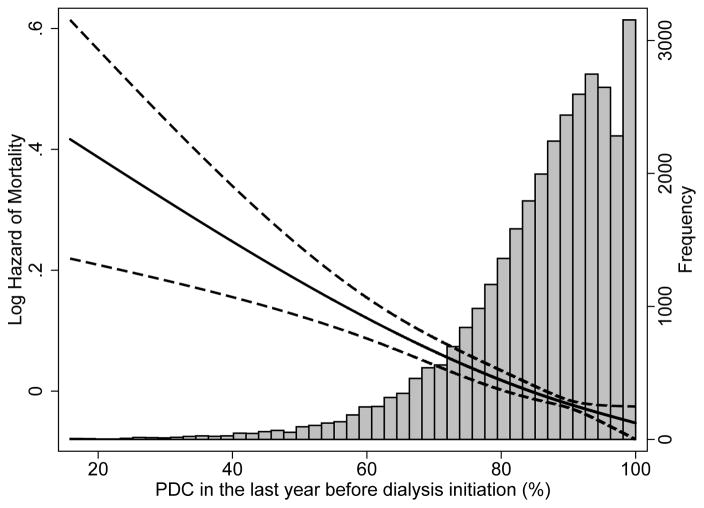
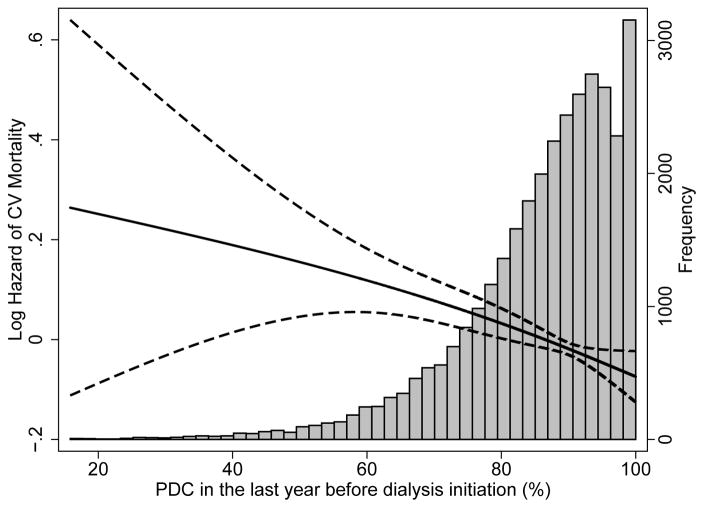
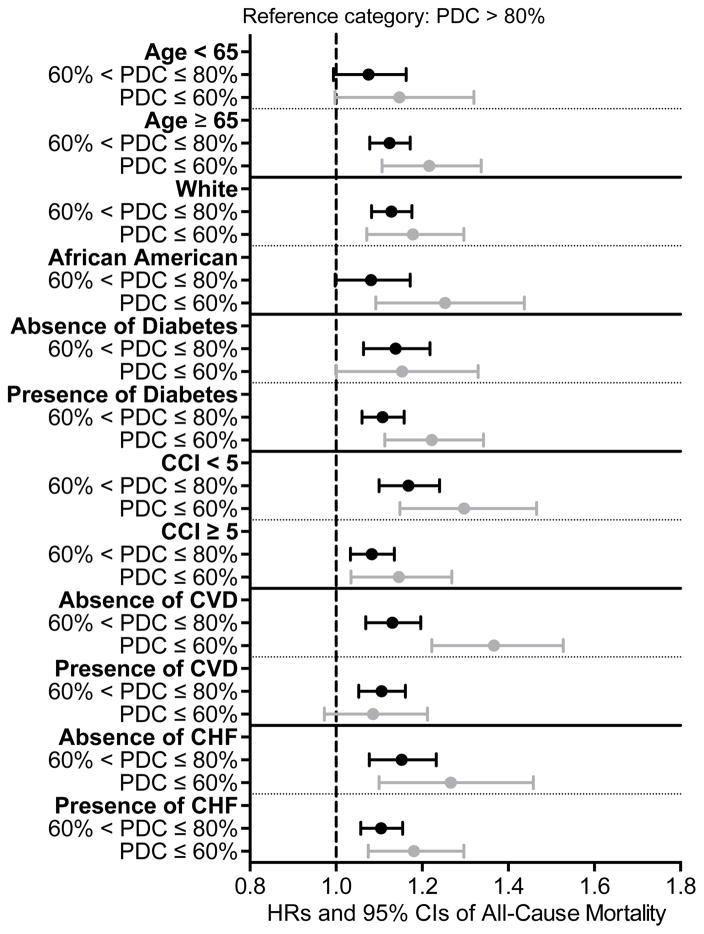
The median follow-up time was 23 (IQR, 9–36) months. There were 18,608 deaths (58%; mortality rate, 286 [95% CI, 281–290]/1000 patient-years [PY]) in the entire cohort, with 14,006 deaths (57%; mortality rate, 283 [95% CI, 278–288]/1000 PY) in patients with PDC >80%, 3,882 deaths (59%; mortality rate, 294 [95% CI, 285–304]/1000 PY) in patients with PDC >60%–80%, and 720 deaths (57%; mortality rate, 291 [95% CI, 271–313]/1000 PY) in patients with PDC ≤60%. Compared to patients with PDC >80%, patients with PDC >60%–80% were at higher risk for all-cause mortality (hazard ratio [HR]: 1.04; 95% CI: 1.00–1.08), while patients with PDC ≤60% had similar risk (HR, 1.02; 95% CI, 0.95–1.10) in the unadjusted model (Table 2). After adjustment for socio-demographic parameters and comorbidities, patients with PDC >60%–80% (HR, 1.12; 95% CI, 1.08–1.16), as well as patients with PDC ≤60% (HR, 1.21; 95% CI, 1.11–1.30) had significantly higher risk of mortality compared to patients with PDC >80% (Table 2). Similar trends were detected after further adjustment for laboratory variables, although the number of observations decreased substantially due to missing values (Table 2). In the multiple imputation model, patients with PDC >60%–80% (HR, 1.11; 95% CI, 1.07–1.15), as well as patients with PDC ≤60% (HR, 1.20; 95% CI, 1.12–1.30) reported significantly higher risk for all-cause mortality compared to patients with PDC >80%. Figure 2 shows an inverse linear association between PDC and all-cause (panel A) and cardiovascular (panel B) mortality in analyses using restricted cubic splines. A higher risk of death was also observed in the various studied subgroups (Figure 3). Table S3 shows the associations between the different PDCs of each medication category and all-cause mortality. The PDCs of loop and thiazide diuretics and of ß-blockers showed significant inverse association with all-cause mortality (Table S3). Similar results were found for cardiovascular mortality (Table 3).
Table 2.
Association of PDC with all-cause mortality after dialysis initiation
| Model 1 (32,348 patients, 18,608 events) | Model 2 (30,943 patients, 17,789 events) | Model 3 (30,592 patients, 17,610 events) | Model 4 (13,693 patients, 6,904 events) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| PDC >80% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| PDC >60%–80% | 1.04 (1.00–1.08) | 0.04 | 1.17 (1.12–1.21) | <0.001 | 1.12 (1.08–1.16) | <0.001 | 1.06 (1.00–1.13) | 0.05 |
| PDC ≤60% | 1.02 (0.95–1.10) | 0.5 | 1.31 (1.21–1.41) | <0.001 | 1.21 (1.11–1.30) | <0.001 | 1.18 (1.03–1.36) | 0.02 |
Abbreviations: CI: confidence interval; HR: hazard ratio; PDC: proportion of days covered
Note: Model1: unadjusted; Model 2: adjusted for age, sex, race/ethnicity, marital status and ZIP code; Model 3: adjusted for model 2 plus Charlson Comorbidity Index and presence of diabetes, congestive heart failure, cardiovascular/cerebrovascular disease, presence of depression, presence of anxiety and type of vascular access; and Model 4: adjusted for model 3 plus blood/serum hemoglobin, bicarbonate, albumin, urea nitrogen, last estimated glomerular filtration rate before end-stage renal disease.
Figure 2.
Association between percentage of days a subject had medication available (proportion of days covered, PDC) in the last year before ESRD and post-dialysis initiation all-cause mortality (panel A) and cardiovascular mortality (panel B) using fractional polynomials and restricted cubic splines (model adjusted for age, sex, race, marital status, ZIP code, CCI, presence of diabetes, congestive heart failure, cardiovascular/cerebrovascular disease, presence of depression, presence of anxiety and type of vascular access)
Figure 3.
Association between percentages of days a subject had medication available (proportion of days covered, PDC) in the last year before ESRD and post-dialysis initiation mortality in different subgroups of patients using adjusted Cox regression analyses (model adjusted for age, sex, race, marital status, ZIP code, CCI, presence of diabetes, congestive heart failure, cardiovascular/cerebrovascular disease, presence of depression, presence of anxiety and type of vascular access)
Table 3.
Association of PDC with cardiovascular mortality after dialysis initiation
| Model 1 (32,065 patients, 5,375 events) | Model 2 (30,692 patients, 5,132 events) | Model 3 (30,366 patients, 5,066 events) | Model 4 (13,606 patients, 1,951 events) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| PDC >80% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| PDC >60%–80% | 1.03 (0.97–1.10) | 0.3 | 1.16 (1.08–1.24) | <0.001 | 1.11 (1.03–1.18) | 0.004 | 1.05 (0.94–1.17) | 0.4 |
| PDC ≤60% | 1.00 (0.87–1.15) | 0.9 | 1.24 (1.07–1.43) | 0.005 | 1.15 (0.99–1.33) | 0.06 | 1.09 (0.83–1.43) | 0.5 |
Abbreviations: CI: confidence interval; HR: hazard ratio; PDC: proportion of days covered
Note: Model1: unadjusted; Model 2: adjusted for age, sex, race/ethnicity, marital status and ZIP code; Model 3: adjusted for model 2 plus Charlson Comorbidity Index and presence of diabetes, congestive heart failure, cardiovascular/cerebrovascular disease, presence of depression, presence of anxiety and type of vascular access; and Model 4: adjusted for model 3 plus blood/serum hemoglobin, bicarbonate, albumin, urea nitrogen, last estimated glomerular filtration rate before end-stage renal disease
Predialysis MPR, Medication Persistence, and All-Cause and Cardiovascular Mortality
Compared to patients with MPR >80%–<100%, patients with MPR >60%–≤80% (HR, 1.11; 95% CI, 1.06–1.17) and patients with MPR ≤60% (HR, 1.19; 95% CI, 1.08–1.30) had significantly higher risk of all-cause mortality; meanwhile, patients with MPR ≥100% (HR, 0.96; 95% CI, 0.93–0.99) had significantly lower risk for all-cause mortality after adjustment for socio-demographic parameters and comorbidities (Table S4). Similar trends were detected after further adjustment for laboratory variables (Table S4). In the multiple imputation model, patients with MPR >60%−≤80% (HR, 1.10; 95% CI, 1.05–1.15), as well as patients with MPR ≤60% (HR, 1.18; 95% CI, 1.08–1.29) had significantly higher risk for all-cause mortality, while patients with MPR ≥100% (HR, 0.96; 95% CI, 0.93–0.99) had significantly lower risk for all-cause mortality compared to patients with MPR >80%-<100%. Figure S2 shows the association between MPR and all-cause (panel A) and cardiovascular (panel B) mortality using restricted cubic splines. Results were similar in all examined subgroups (Figure S3). Similar trends were found with cardiovascular mortality (Table S5).
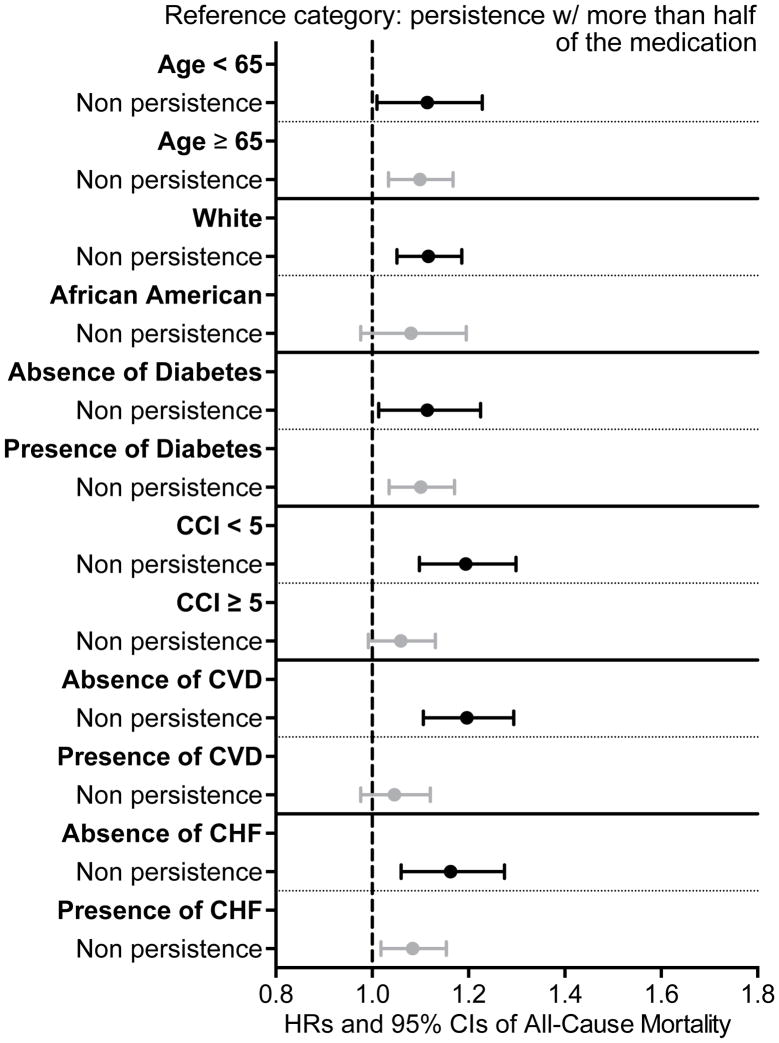
Compared to patients who were persistent with their medication refills, non-persistent patients (HR, 1.11; 95% CI, 1.05–1.16) had significantly higher risk for all-cause mortality (Table 4). Similar results were detected (HR, 1.10; 95% CI, 1.05–1.16) after further adjustment for laboratory variables in our multiple imputation model, in subgroup analyses (Figure 4) and for cardiovascular mortality (Table 5).
Table 4.
Association of medication persistence with all-cause mortality after dialysis initiation
| Model 1 (32,348 patients, 18,608 events) | Model 2 (30,943 patients, 17,789 events) | Model 3 (30,592 patients, 17,610 events) | Model 4 (13,693 patients, 6,904 events) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Persistence with >50% of medication | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Non persistence | 0.99 (0.94–1.04) | 0.8 | 1.16 (1.10–1.22) | <0.001 | 1.11 (1.05–1.16) | <0.001 | 1.08 (0.99–1.17) | 0.06 |
Abbreviations: CI: confidence interval; HR: hazard ratio
Note: Medication persistence with 60-d gap for >50% of medications. Model1: unadjusted; Model 2: adjusted for age, sex, race/ethnicity, marital status and ZIP code; Model 3: adjusted for model 2 plus Charlson Comorbidity Index plus presence of diabetes, congestive heart failure, cardiovascular/cerebrovascular disease, presence of depression, presence of anxiety and type of vascular access; and Model 4: adjusted for model 3 plus blood/serum hemoglobin, bicarbonate, albumin, urea nitrogen, last estimated glomerular filtration rate before end-stage renal disease
Figure 4.
Association between medication persistence (60 days gap for more than 50% of medications) in the last year before ESRD and post-dialysis initiation mortality in different subgroups of patients using adjusted Cox regression analyses (model adjusted for age, sex, race, marital status, ZIP code, CCI, presence of diabetes, congestive heart failure, cardiovascular/cerebrovascular disease, presence of depression, presence of anxiety and type of vascular access)
Table 5.
Association of medication persistence (60 days gap for more than 50% of medications) with cardiovascular mortality after dialysis initiation
| Model 1 (32,065 patients, 5,375 events) | Model 2 (30,692 patients, 5,132 events) | Model 3 (30,366 patients, 5,066 events) | Model 4 (13,606 patients, 1,951 events) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Persistence with >50% of medication | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Non persistence | 0.99 (0.91–1.09) | 0.9 | 1.15 (1.04–1.26) | 0.004 | 1.11 (1.06–1.22) | 0.04 | 1.06 (0.91–1.23) | 0.5 |
Abbreviations: CI: confidence interval; HR: hazard ratio
Note: Persistence with 60-d gap for >50% of medications. Model1: unadjusted; Model 2: adjusted for age, sex, race/ethnicity, marital status and ZIP code; Model 3: adjusted for model 2 plus Charlson Comorbidity Index and presence of diabetes, congestive heart failure, cardiovascular/cerebrovascular disease, presence of depression, presence of anxiety and type of vascular access; and Model 4: adjusted for model 3 plus blood/serum hemoglobin, bicarbonate, albumin, urea nitrogen, last estimated glomerular filtration rate before end-stage renal disease
DISCUSSION
In a large cohort of patients with advanced CKD, we examined the association between one-year pre-dialysis adherence to cardiovascular medications and all-cause and cardiovascular mortality following dialysis initiation. We utilized a pharmacy database analysis to assess two parts of medication adherence: adherence and persistence.(21) Inadequate adherence to cardiovascular pharmacotherapy was associated with reduced survival, independent of demographic, co-morbidity and laboratory characteristics.
Several pre-dialysis demographic and co-morbidity factors such as age, gender, race, socioeconomic status, and co-morbidities were linked to post-dialysis initiation survival.(22–25) These factors are difficult or impossible to modify but bear importance in the risk stratification and estimation of prognosis after initiating renal replacement therapy. For example, a new prediction risk score was recently developed based on demographic and co-morbidity characteristics to help with shared decision-making about dialysis initiation in elderly ESRD patients.(25) On the other hand, it is equally important to understand potentially modifiable pre-dialysis risk factors and behaviors influencing survival after initiating dialysis. Timely arteriovenous fistula placement and pre-dialysis care involving a nephrology specialist were shown to be associated with reduced all-cause mortality in the post-dialysis initiation period.(2–6, 26) The quality of pre-dialysis care as defined by the number of provider visits before ESRD onset was also shown to influence survival. One study found that patients having ≥3 pre-dialysis visits in the six-month period before dialysis initiation had 28% higher survival compared to patients who had <3 visits during the same time period.(27) In addition to the number of visits, nephrology care of <6 months’ duration before ESRD onset was linked to 23%–27% higher one-year all-cause mortality in two recent studies.(28, 29)
To our knowledge, no other studies have attempted to evaluate the influence of pre-dialysis adherence to cardiovascular medications on all-cause and cardiovascular mortality after initiation of dialysis. However, adequate adherence to cardiovascular medications has been shown to be associated with better outcomes in the general population.(9, 13, 30) A large meta-analysis including 1,978,919 individuals concluded that good medication adherence to anti-hypertensive drugs was associated with 45% lower risk of all-cause mortality and good adherence to statins was associated with 29% reduced risk of death.(11) Our study involved a population of patients with advanced CKD transitioning to dialysis, and its results further strengthen the overall importance of adherence to cardiovascular drugs. Because cardiovascular death is the main cause of morbidity and mortality in patients with CKD,(7) it is biologically plausible that patients adherent to cardiovascular drugs have better survival as they may retain better cardiovascular health and live longer once they progress to ESRD. Therefore, adherence to cardiovascular medications should be monitored and reinforced.
Adherence to medications is a complex behavior that is influenced by a broad array of factors, including those related to the patient, the condition, the therapy, the socio-economic background, and the health-care system. Therefore, providers should be familiar with available methods for adherence screening(21) and routinely apply them while treating patients with CKD. The PDC, MPR, and persistence methods that were used in the current study are indirect screening methods for the evaluation of medication-taking behavior based on pharmacy database evaluation.(31, 32) In the absence of a “gold standard” of adherence assessment, pharmacy database analysis is becoming the most practical way to assess “real-world” adherence, especially using large databases. This method is easily quantifiable and objective. In addition, it allows evaluation of two aspects of medication-taking behavior: (1) adherence: the extent to which patients follow prescribed dosing regimens (assessed by PDC and MPR), and (2) persistence: the duration from initiation to “unauthorized” discontinuation of therapy. In the current study, we modified the approach to persistence assessment and used a prescription date closest to the 1-year pre-dialysis mark as the initial date and evaluated subsequent 12-month persistence. The PDC and MPR are both related to the number of available medication doses given out in relationship to the number of days during the period of interest. The key difference is that PDC is capped at 100% as the number of days covered by a drug cannot exceed 100%. Numerically, MPR can exceed 100% and therefore it can account for medication overfills; alas, it has been contended that MPR might overestimate medication adherence.
Our study has a large sample size and event numbers, and is representative of male veterans who received care in the VA system in the entire United States. This study must be interpreted in light of several limitations. Our study was observational, and hence, the results do not allow us to infer causality but merely associations. Most of our patients consisted of male US veterans; therefore, the results may not be generalizable to women or the general US population. Even though we adjusted our analyses for a variety of important covariates as potential confounders, we cannot eliminate the possibility of unmeasured confounders, such as proteinuria and quality of nephrology care. Several limitations of pharmacy database analysis need to be acknowledged. Although we applied three accepted methods of adherence determination using pharmacy databases, we did not have data about discontinuation orders for these drugs, so we were not able to differentiate between the discontinuation by indication versus self-discontinuation (i.e. non-adherence) by the patients. The dispensation of medicine does not guarantee its consumption, nor does it give any information about when medications are taken by patients. Additionally, patients should be enrolled in a closed pharmacy system; in our cohort, it is possible that some veterans received medications outside of the two evaluated pharmacy systems (VA and Medicare Part D). Another limitation of our study is that we only included patients who survived until dialysis initiation; therefore we were not able to examine chronic kidney failure patients receiving conservative management (i.e., no dialysis). Finally, we had large amounts of missing data for some laboratory values; therefore we were not able to include these variables in our main multivariable model. However, models that included these variables as well as the multiple imputation models led to similar conclusions.
Adherence to cardiovascular medications is emerging as a novel risk factor for mortality after initiating dialysis. Poor pre-dialysis medication compliance and persistence in the year preceding ESRD onset are associated with increased all-cause and cardiovascular mortality. Our findings may have important implications for the management of pre-dialysis patients due to the potentially modifiable nature of medication-taking behavior. It would be very practical if pharmacies and/or insurance companies could start the routine provision of pharmacy dispensation records with calculations of compliance and persistence, which would allow providers to have an opportunity to discuss barriers and encourage medication adherence. Future prospective studies are needed to understand adherence barriers and to develop measures enhancing adherence to cardiovascular drugs in patients with advanced CKD.
Supplementary Material
Table S1: Baseline characteristics of included versus excluded patients.
Table S2: Adherence parameters in different medication groups.
Table S3: Association of each PDC drug category with all-cause mortality after dialysis initiation.
Table S4: Association of MPR with all-cause mortality after dialysis initiation.
Table S5: Association of MPR with CV mortality after dialysis initiation.
Figure S1: Different methods of adherence calculation.
Figure S2: Association between MPR in last year pre-ESRD and postdialysis initiation all-cause and CV mortality.
Figure S3: Association between MPR in last year pre-ESRD and postdialysis initiation mortality in different subgroups.
Acknowledgments
Opinions expressed in this study are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs.
Support: This study is supported by grant U01DK102163 from the National Institutes of Health to Drs Kovesdy and Kalantar-Zadeh, and by resources from the VA. Support for VA/CMS data is provided by the VA, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (project numbers SDR 02-237 and 98-004). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: Drs Kovesdy and Kalantar-Zadeh are employees of the VA.
Contributions: Study concept and design: all authors; acquisition of data: CPK, MZM, ES, JLL, JJ, CMR, VAR, MS, PKP; data analysis/interpretation: MZM, CPK, KS, and KK=Z. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. CPK and MZM take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by 2 external peer reviewers, a Statistical Editor, a Co-Editor, and the Editor-in-Chief.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;65(6 Suppl 1):S1–S306. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2(1):89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 3.Winkelmayer WC, Owen WF, Jr, Levin R, Avorn J. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol. 2003;14(2):486–492. doi: 10.1097/01.asn.0000046047.66958.c3. [DOI] [PubMed] [Google Scholar]
- 4.Avorn J, Bohn RL, Levy E, et al. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med. 2002;162(17):2002–2006. doi: 10.1001/archinte.162.17.2002. [DOI] [PubMed] [Google Scholar]
- 5.Ishani A, Gilbertson DT, Kim D, Bradbury BD, Collins AJ. Predialysis care and dialysis outcomes in hemodialysis patients with a functioning fistula. Am J Nephrol. 2014;39(3):238–247. doi: 10.1159/000358843. [DOI] [PubMed] [Google Scholar]
- 6.Jungers P, Massy ZA, Nguyen-Khoa T, et al. Longer duration of predialysis nephrological care is associated with improved long-term survival of dialysis patients. Nephrol Dial Transplant. 2001;16(12):2357–2364. doi: 10.1093/ndt/16.12.2357. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;(3):1–150. [Google Scholar]
- 9.Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 10.Kettani FZ, Dragomir A, Cote R, et al. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke. 2009;40(1):213–220. doi: 10.1161/STROKEAHA.108.522193. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 12.Roy L, White-Guay B, Dorais M, Dragomir A, Lessard M, Perreault S. Adherence to antihypertensive agents improves risk reduction of end-stage renal disease. Kidney Int. 2013;84(3):570–577. doi: 10.1038/ki.2013.103. [DOI] [PubMed] [Google Scholar]
- 13.Degli Esposti L, Saragoni S, Benemei S, et al. Adherence to antihypertensive medications and health outcomes among newly treated hypertensive patients. Clinicoecon Outcomes Res. 2011;3:47–54. doi: 10.2147/CEOR.S15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Deptartment of Veterans Affairs HSRaDS, VA Information Resource Center. VIReC Resource Guide: VA Corporate Data Warehouse. Hines, IL: VA Information Resource Center; 2012. [Google Scholar]
- 15.US Department of Veterans Affairs HSRaDS, VA Information Resource Center. VIReC Research User Guide: Veterans Health Administration Decision Support System Clinical National Data Extracts. 2. Hines, IL: VA Information Resource Center; 2009. [Google Scholar]
- 16.VA Information Resource Center (VIReC) VIReC Research User Guide: VHA Pharmacy Prescription Data nE. Hines, IL: VA Information Resource Center; 2008. [Google Scholar]
- 17.US Department of Veterans Affairs. VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006–2007H. IL: VA Information Resource Center; 2007. [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457–464. [PMC free article] [PubMed] [Google Scholar]
- 20.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 21.Gosmanova EO, Kovesdy CP. Adherence to antihypertensive medications: is prescribing the right pill enough? Nephrol Dial Transplant. 2015;30(10):1649–1656. doi: 10.1093/ndt/gfu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett BJ, Parfrey PS, Morgan J, et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis. 1997;29(2):214–222. doi: 10.1016/s0272-6386(97)90032-9. [DOI] [PubMed] [Google Scholar]
- 23.Miskulin DC, Meyer KB, Martin AA, et al. Comorbidity and its change predict survival in incident dialysis patients. Am J Kidney Dis. 2003;41(1):149–161. doi: 10.1053/ajkd.2003.50034. [DOI] [PubMed] [Google Scholar]
- 24.Soucie JM, McClellan WM. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol. 1996;7(10):2169–2175. doi: 10.1681/ASN.V7102169. [DOI] [PubMed] [Google Scholar]
- 25.Thamer M, Kaufman JS, Zhang Y, Zhang Q, Cotter DJ, Bang H. Predicting Early Death Among Elderly Dialysis Patients: Development and Validation of a Risk Score to Assist Shared Decision Making for Dialysis Initiation. Am J Kidney Dis. 2015;66(6):1024–1032. doi: 10.1053/j.ajkd.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek SH, Ahn S, Lee SW, et al. Outcomes of predialysis nephrology care in elderly patients beginning to undergo dialysis. PLoS One. 2015;10(6):e0128715. doi: 10.1371/journal.pone.0128715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan SS, Xue JL, Kazmi WH, et al. Does predialysis nephrology care influence patient survival after initiation of dialysis? Kidney Int. 2005;67(3):1038–1046. doi: 10.1111/j.1523-1755.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 28.Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86(2):392–398. doi: 10.1038/ki.2014.15. [DOI] [PubMed] [Google Scholar]
- 29.Singhal R, Hux JE, Alibhai SM, Oliver MJ. Inadequate predialysis care and mortality after initiation of renal replacement therapy. Kidney Int. 2014;86(2):399–406. doi: 10.1038/ki.2014.16. [DOI] [PubMed] [Google Scholar]
- 30.Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD. Continuation of statin treatment and all-cause mortality: a population-based cohort study. Arch Intern Med. 2009;169(3):260–268. doi: 10.1001/archinternmed.2008.552. [DOI] [PubMed] [Google Scholar]
- 31.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 32.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Baseline characteristics of included versus excluded patients.
Table S2: Adherence parameters in different medication groups.
Table S3: Association of each PDC drug category with all-cause mortality after dialysis initiation.
Table S4: Association of MPR with all-cause mortality after dialysis initiation.
Table S5: Association of MPR with CV mortality after dialysis initiation.
Figure S1: Different methods of adherence calculation.
Figure S2: Association between MPR in last year pre-ESRD and postdialysis initiation all-cause and CV mortality.
Figure S3: Association between MPR in last year pre-ESRD and postdialysis initiation mortality in different subgroups.