Abstract
Background
Tenofovir disoproxil fumarate (TDF) can cause proximal tubular damage and chronic kidney disease in HIV-infected individuals. Urine α1-microglobulin (α1m), a low molecular weight protein indicative of proximal tubular dysfunction, may enable earlier detection of TDF-associated tubular toxicity.
Study Design
Cross-sectional
Setting & Participants
883 HIV-infected and 350 uninfected men enrolled in the Multicenter AIDS Cohort Study
Predictors
HIV infection and TDF exposure
Outcome
Urine α1m levels
Results
Urine α1m was detectable in 737 (83%) HIV-infected and 202 (58%) uninfected men, respectively (p<0.001). Among the HIV-infected participants, 573 (65%) were current TDF users and 112 (13%) were past TDF users. After multivariable adjustment including demographics, traditional kidney disease risk factors and eGFR, HIV infection was associated with 136% higher urine α1m levels (95%CI: 104,173) and 1.5-fold prevalence of detectable α1m (95%CI: 1.3,1.6). When participants were stratified by TDF exposure, HIV infection was associated with higher adjusted α1m levels by 164% among current users (95%CI: 127,208), 124% among past users (95%CI: 78,183), and 76% among never users (95%CI: 45,115). Among HIV-infected participants, each year of cumulative TDF exposure was associated with 7.6% higher α1m levels (95%CI: 5.4,9.9) in fully adjusted models, a 4-fold effect size relative to advancing age (1.8% per year; 95%CI: 0.9, 2.7). Each year since TDF discontinuation was associated with 4.9% lower α1m levels (95%CI: −9.4,−0.2) among past users.
Limitations
Results may not be generalizable to women.
Conclusions
Compared with uninfected men, HIV-infected men had higher urine α1m levels. Among HIV-infected men, cumulative TDF exposure was associated with incrementally higher α1m levels, whereas time since TDF discontinuation was associated with progressively lower α1m levels. Urine α1m appears to be a promising biomarker for the detection and monitoring of TDF-associated tubular toxicity.
Tenofovir disoproxil fumarate (TDF) is a nucleotide analog reverse transcriptase inhibitor that is prescribed worldwide for the treatment of HIV infection. Due to its efficacy, tolerability and availability as a once daily medication, TDF is recommended by the United States (US) Department of Health and Human Services (http://aidsinfo.nih/gov) and the HIV Medicine Association of the Infectious Diseases Society of America1 as a component of several first-line combination antiretroviral regimens. In addition to its global use for the treatment of HIV, TDF was recently approved by the FDA for pre-exposure prophylaxis of individuals at high risk for HIV acquisition,2–4 and it remains an effective therapy for hepatitis B virus infection.5,6 Although pre-marketing studies suggested a favorable safety profile,7 TDF use has been associated with the development of acute kidney injury, proteinuria, chronic kidney disease (CKD), and the Fanconi syndrome of proximal tubular dysfunction.8–12 Kidney biopsy series of patients with TDF-associated kidney injury have demonstrated flattening of proximal tubular epithelial cells and widespread mitochondrial abnormalities.13,14 Notably, the active drug, tenofovir, is eliminated in urine through active secretion by proximal tubular epithelial cells,15 with tenofovir influx and efflux mediated by organic anion transporters and multidrug resistance proteins, respectively.16,17
The serum creatinine concentration is an insensitive marker of early kidney damage, as more than 50% of glomerular filtration function may be lost before serum creatinine is above the normal laboratory range.18,19 Because the proximal tubule is the primary site of TDF-associated nephrotoxicity, biomarkers that are sensitive markers of proximal tubular dysfunction may enable the detection of tubular toxicity at earlier stages. α1-microglobulin (α1m) is a 26-kDa lipocalin that is freely filtered at the glomerulus but reabsorbed by proximal tubular epithelial cells under healthy conditions;20 elevated urine α1m levels therefore indicate proximal tubular dysfunction.21 In the Women's Interagency HIV Study, we recently found that HIV-infected women had higher urine α1m levels compared with HIV-uninfected women.22 Furthermore, urine α1m levels were associated with subsequent kidney function decline and mortality independent of traditional and HIV-related risk factors, eGFR, and albuminuria, suggesting that proximal tubular dysfunction may lead to irreversible kidney damage. However, because our prior study utilized urine specimens that were collected prior to the widespread use of TDF, we were unable to evaluate the associations of TDF exposure with urine α1m levels.
In this contemporary study of men enrolled in the Multicenter AIDS Cohort Study, we evaluated the associations of HIV infection and TDF exposure with proximal tubular dysfunction, measured by urine α1m levels. Then, we examined the associations of other antiretroviral medications and clinical factors with urine α1m levels.
Methods
Study Population and Design
The Multicenter AIDS Cohort Study (MACS) is an ongoing, prospective cohort study designed to describe the epidemiology and natural history of HIV infection among men who have sex with men. A total of 6,972 HIV-infected and uninfected men were enrolled between 1984 and 2003 from four sites in the US: Baltimore, Chicago, Los Angeles and Pittsburgh.23 Participants attend semiannual visits that include standardized questionnaires, a physical examination, and collection of biological specimens.
The MACS Kidney Study was designed as a nested cohort study to investigate the onset and progression of kidney disease among HIV-infected men, using stored urine and serum samples. Urine specimens were refrigerated immediately after collection, and centrifuged at 5000×g to remove cellular debris. The supernatant was aliquoted into 1cc vials and then stored at −80°C until biomarker measurement was undertaken. This cross-sectional study of kidney damage included all 883 HIV-infected men with urine samples collected between October 1, 2009 and September 30, 2011, and a random sample of 350 uninfected men with available urine specimens from this time period.
The institutional review boards of participating institutions approved the study protocol (IRB #10-00827), and informed consent was obtained from all study participants. This study was also approved by the University of California, San Francisco, and San Francisco VA Medical Center committees on human research.
Antiretroviral Medication Exposure
Antiretroviral (ARV) medication exposure was ascertained using self-reported data from each MACS participant collected at semi-annual visits. ARVs with less than 5% prevalence of use at the time of biomarker measurement were not included as candidate covariates in our analyses. Cumulative exposure was defined as the sum of current and historical exposure durations for each participant. Current duration was defined as duration on therapy at the time of biomarker measurement.
Urine Biomarker Measurements
Urine α1m was measured at the Cincinnati Children's Hospital Medical Center Biomarker Laboratory using a commercially available assay (Siemens BNII nephelometer, Munich, Germany). The detectable limit of the α1m assay was 0.53 mg/dl. Urine specimens were in continuous storage at −80°C until biomarker measurement without prior freeze-thaw. Laboratory personnel performing the biomarker assays were blinded to participants' clinical information.
Covariates
The following demographic and clinical characteristics were tested as candidate covariates in multivariable models: age, race/ethnicity, diabetes mellitus (fasting glucose ≥126mg/dL; hemoglobin A1c ≥6.5%; or self-reported history of diabetes and diabetes medication use), systolic and diastolic blood pressure, hypertension (systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg; or self-reported history of hypertension and antihypertensive medication use), cigarette smoking status (current, past, or never), LDL and HDL cholesterol, triglycerides, body mass index (BMI), waist circumference, and hepatitis C virus (HCV) infection (confirmed by detectable HCV RNA following a positive HCV antibody result). Candidate HIV-related characteristics included: current CD4 lymphocyte count, nadir CD4 lymphocyte count, history of clinical AIDS diagnosis,24 current and peak plasma HIV RNA level, and time-averaged historical HIV RNA level. Urine albumin, total protein, and creatinine were measured using a Siemens Dimension Xpand plus HM clinical analyzer (Siemens, Munich, Germany). Glomerular filtration rate was estimated using the CKD-EPI equation for creatinine (eGFR).25 CKD was defined by the presence of an eGFR<60 ml/min/1.73m2. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 5 imputations to yield ~95% relative efficiency.26 The percentage of missing observations for each covariate ranged from 0% to 26% (Table S1).
Statistical Analysis
Approximately one-fourth of participants had undetectable urine α1m, and the distribution among those with detectable α1m was right-skewed (Figure 1). Due to the left censored nature of the data, we analyzed α1m by two approaches: 1) as a log-transformed continuous variable using models that accommodate left censored data, and 2) as a dichotomous variable (detectable vs. undetectable).
Figure 1. Distribution of urine α1m levels in MACS participants (N=883) by HIV status and TDF use.
Empirical distributions of urine α1m levels with model-based density from Tobit regression. Test for difference in location: p<0.001. Test for homogeneity of variance: p=0.013. Proportions with undetectable values are represented as vertical bars.
We first stratified men into four categories based on HIV status and TDF use (never, past, or current) and compared demographic and clinical characteristics using the chi-square and Kruskal-Wallis tests for categorical and continuous variables, respectively. We then used multivariable generalized gamma regression models to evaluate the associations of HIV infection with urine α1m, and to identify clinical factors associated with α1m. Similar to the Tobit regression method, generalized gamma regression models accommodate left censored data by including undetectable values, and also allow log-transformation of urine α1m to normalize its right-skewed distribution. Results were back-transformed to produce estimated percentage differences in urine α1m attributable to each predictor. As a secondary approach, we used Poisson relative risk regression with a robust variance estimator27 to assess the association of HIV infection with detectable urine α1m. Models were adjusted sequentially for demographics, traditional kidney risk factors, and eGFR. We then adjusted for albuminuria, a clinical marker of glomerular injury, and urine creatinine, to account for urine tonicity. To assess for effect modification by race, we also performed race-stratified analyses and evaluated interactions of HIV infection and race for the α1m outcomes.
Next, we constructed smoothing splines using generalized additive models in order to examine the relationship of TDF exposure with urine α1m levels. We then used multivariable generalized gamma regression models to examine associations of TDF with urine α1m while controlling for traditional kidney disease risk factors and HIV-related factors, using stepwise backward selection (α=0.05) to remove candidate variables that were not associated with the outcome. We used Bayesian model averaging as an alternative model building approach.28 Models constructed using the two approaches were very similar. TDF exposure was analyzed continuously (per year of total duration and per year of current duration) and categorically (current, past, or never exposure). We additionally evaluated duration off TDF as a continuous predictor of α1m. We then stratified participants by race to determine whether TDF exposure had similar associations with urine α1m in African Americans and Caucasians.
To evaluate associations of other ARVs with urine α1m levels, we first examined ARVs individually, in models controlling for traditional and HIV-related risk factors. Because individual ARVs are used in combination and therefore inter-correlated, we used the least absolute shrinkage and selection operator (LASSO) method to determine which of multiple ARVs were associated with α1m.29
As a sensitivity analysis, we also evaluated the associations of HIV and TDF exposure with urine protein/creatinine ratio, to compare the performance of urine α1m with this clinically available measure. We implemented Bayesian model averaging using the BMA package for R and LASSO using the glmnet package for R (Foundation for Statistical Computing, Vienna). All other analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study population by HIV status and TDF use
Among the 883 HIV-infected and 350 uninfected men included in this study, the median age was 52 years, and approximately one-third of participants were African American (Table 1). Among the HIV-infected participants, 65% (n=573) were receiving TDF at the time of urine collection, while 13% (n=112) were past TDF users. Median TDF exposure duration was 4.4 years among current users (IQR: 2.8, 6.4). Among past TDF users, the median TDF exposure duration was 2.4 years (IQR: 1.0, 4.6) and the median time since TDF discontinuation was 2.3 years (IQR: 1.2, 4.6). Diabetes mellitus was more common among past TDF users (27%) compared with the other study participants (14%), while hypertension and HCV infection were present in approximately 50% and 10% of participants, respectively. Compared with uninfected participants, current TDF users and never TDF users, past TDF users had the lowest eGFR and highest prevalence of albuminuria. HIV-infected men who never received TDF had the highest CD4 lymphocyte counts and lowest historical prevalence of AIDS, while current TDF users had the highest prevalence of HIV viral suppression. Only 5% (n=46) of HIV-infected participants had never received ARV therapy.
Table 1.
Characteristics of MACS participants, stratified by HIV status and tenofovir use
| HIV-negative | HIV-positive | |||
|---|---|---|---|---|
| Never TDF | Past TDF | Current TDF | ||
| N | 350 | 187 | 112 | 573 |
| Age (y) | 54 (49–62) | 53 (49–58) | 53 (48–59) | 51 (45–57) |
| Race | ||||
| Black | 102 (29%) | 65 (35%) | 36 (32%) | 169 (29%) |
| White | 229 (65%) | 116 (62%) | 72 (64%) | 344 (60%) |
| Other | 19 (5%) | 6 (3%) | 4 (4%) | 60 (10%) |
| Diabetes mellitus | 44 (15%) | 20 (14%) | 25 (27%) | 65 (14%) |
| Systolic BP (mm Hg) | 128 (116–136) | 130 (120–139) | 126 (114–137) | 125 (115–134) |
| Diastolic BP (mm Hg) | 78 (71–84) | 81 (75–86) | 75 (68–83) | 77 (71–84) |
| Hypertension | 155 (47%) | 95 (53%) | 57 (56%) | 222 (43%) |
| Antihypertensive use | 117 (34%) | 80 (43%) | 49 (44%) | 175 (31%) |
| Hepatitis C | 33 (9%) | 17 (9%) | 13 (12%) | 57 (10%) |
| Cigarette smoking | ||||
| Current | 82 (24%) | 52 (28%) | 33 (31%) | 174 (31%) |
| Past | 174 (51%) | 86 (47%) | 48 (45%) | 244 (43%) |
| Never | 85 (25%) | 45 (25%) | 26 (24%) | 145 (26%) |
| LDL (mg/dL) | 115 (92–137) | 105 (88–132) | 104 (79–130) | 108 (88–132) |
| HDL (mg/dL) | 50 (41–60) | 46 (38–56) | 48 (39–54) | 45 (38–54) |
| TG (mg/dL) | 108 (76–157) | 133 (94–199) | 166 (113–257) | 134 (94–202) |
| Body Mass Index (kg/m2) | 27 (24–32) | 26 (23–29) | 26 (23–33) | 26 (24–30) |
| Waist Circumference (cm) | 97 (89–107) | 92 (84–101) | 93 (87–103) | 94 (87–102) |
| eGFR (ml/min/1.73m2) | 89 (78–100) | 92 (81–104) | 77 (58–97) | 92 (77–104) |
| eGFR<60ml/min/1.73m2 | 13 (4%) | 14 (8%) | 31 (28%) | 31 (5%) |
| Albuminuria * | 29 (8%) | 34 (18%) | 25 (23%) | 88 (16%) |
| Current CD4 (cells/mm3) | 607 (450–808) | 515 (347–641) | 572 (405–741) | |
| Nadir CD4 (cells/mm3) | 317 (207–432) | 260 (149–369) | 287 (177–415) | |
| History of AIDS | 20 (11%) | 29 (26%) | 74 (13%) | |
| HIV Viral Load (copies/mL) | ||||
| <80 | 114 (62%) | 85 (77%) | 496 (87%) | |
| 80–2,000 | 24 (13%) | 9 (8%) | 44 (8%) | |
| 2,000–9,999 | 17 (9%) | 10 (9%) | 3 (1%) | |
| >10,000 | 30 (16%) | 6 (5%) | 27 (5%) | |
Data are presented as median (interquartile range) or numbers (percent). Interquartile ranges and percentages were calculated among participants with non-missing data.
Abbreviations: eGFR, estimated glomerular filtration rate; TDF, tenofovir disoproxil fumarate.
defined as a positive urine dipstick result (≥1+) or urine albumin-creatinine ratio >30 mg/g
Association of HIV infection with urine α1m
Compared with uninfected men, HIV-infected men had higher median α1m levels and prevalence of detectable α1m (Table 2). After multivariate adjustment including demographics, traditional kidney disease risk factors and eGFR, HIV infection was associated with 136% higher urine α1m levels, and a 45% higher prevalence of detectable α1m. When we stratified HIV-infected participants by TDF exposure history, HIV infection remained associated with higher adjusted α1m levels by 76% among never users, 124% among past users, and 164% among current users. Additional adjustment for ACR and urine creatinine only mildly attenuated the effect sizes.
Table 2.
Association of HIV infection with urine α1m levels, overall and stratified by tenofovir use
| HIV-negative | HIV-positive | ||||
|---|---|---|---|---|---|
| Overall vs HIV-negative | Never TDF vs HIV-negative | Past TDF vs HIV-negative | Current TDF vs HIV-negative | ||
| N | 346 | 878 | 184 | 111 | 572 |
| Urine α1m (mg/dL), Median (IQR) 1 | 0.61 (<0.53–1.25) | 1.51 (0.72–3.19) | 1.02 (0.55–2.35) | 2.13 (0.71–4.27) | 1.62 (0.79–3.32) |
| % Difference 2 , HIV+ vs HIV− (95% CI) | |||||
| Models | |||||
| Demographic-adjusted3 | Ref | 146 (111, 186) | 76 (44, 116) | 174 (115, 247) | 174 (115, 247) |
| Multivariable-adjusted4 | Ref | 136 (104, 173) | 76 (45, 115) | 124 (78, 183) | 164 (127, 208) |
| Multivariable-adjusted+ACR+UCr5 | Ref | 94 (71, 119) | 39 (18, 64) | 80.9 (48, 121) | 119 (93, 149) |
| Detectable urine α1m, n(%) | 202 (58%) | 737 (83%) | 144 (77%) | 92 (82%) | 490 (86%) |
| Prevalence Ratio,6 HIV+ vs HIV− (95% CI) | |||||
| Models | |||||
| Demographic-adjusted3 | Ref | 1.46 (1.33, 1.60) | 1.33 (1.18, 1.50) | 1.42 (1.26, 1.60) | 1.51 (1.37, 1.66) |
| Multivariable-adjusted4 | Ref | 1.45 (1.32, 1.59) | 1.34 (1.19, 1.51) | 1.35 (1.19, 1.54) | 1.50 (1.37, 1.65) |
| Multivariable-adjusted+ACR+UCr5 | Ref | 1.33 (1.23, 1.45) | 1.22 (1.09, 1.35) | 1.24 (1.10, 1.39) | 1.38 (1.27, 1.50) |
Median (IQR) estimates include those with undetectable α1m values. The detectable limit for the α1m assay was 0.53 mg/dL.
Estimated percentage difference in α1m attributable to HIV infection, with HIV-negative participants as reference group.
Adjusted for age and race
Adjusted for age, race, diabetes mellitus, hypertension, antihypertensive use, history of cardiovascular disease, illicit drug use, hepatitis C infection, and eGFR
Adjusted for albumin-creatinine ratio and urine creatinine in addition to factors listed above
Prevalence ratio for detectable α1m in HIV-positive vs HIV-negative participants, with HIV-negative participants as reference group.
Abbreviations: α1m, α1-microglobulin; ACR, albumin-creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; IQR, interquartile range; TDF, tenofovir disoproxil fumarate; UCr, urine creatinine.
Median urine protein/creatinine ratios were 100 mg/g (IQR: 70, 162) among HIV-infected participants and 68 mg/g (IQR: 51, 92) among uninfected participants (Table S2). In multivariable-adjusted analyses, HIV infection was associated with higher urine protein/creatinine ratio by 54% overall (95% CI: 43, 65), 35% among never users (95% CI: 21, 49), 63% among past users (44, 85), and 59% among current users (95% CI: 47, 72). Urine protein/creatinine ratio was positively correlated with urine α1m in both HIV-infected (r=0.58, p<0.001) and uninfected (r=0.25, p<0.001) participants.
Among both HIV-infected and uninfected participants, African Americans had higher median urine α1m levels and higher prevalence of detectable α1m, as compared with Caucasians (Table S3). Median α1m levels were 2-fold higher in HIV-infected men relative to uninfected men among African Americans (1.9 vs. 0.9 mg/dL) and nearly 3-fold higher among Caucasians (1.4 vs. 0.5). In multivariable models adjusting for traditional kidney risk factors, eGFR, ACR and urine creatinine, HIV infection was associated with 48% higher α1m levels among African Americans, and with 120% higher α1m levels among Caucasians (p-value for HIV/race interaction = 0.003).
Association of TDF exposure with urine α1m
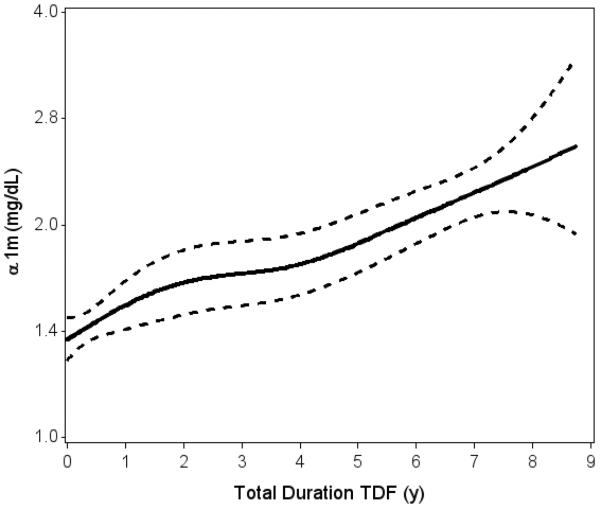
Current and past users of TDF had higher urine α1m levels relative to HIV-infected men who never used TDF and to uninfected men (Figure 1). Duration of TDF exposure was linearly associated with urine α1m (p<0.001, Figure 2) and proteinuria (p<0.001, Figure S1), although the slope appeared steeper for α1m as compared with proteinuria (8.3% per year of exposure vs. 3.3% in unadjusted analysis). In analyses that adjusted for demographics, traditional kidney risk factors and HIV-related factors (Table 3), each year of cumulative TDF exposure was associated with approximately 8% higher urine α1m levels (p<0.001), an approximately 4-fold effect size relative to advancing age (2% per year). There was minimal attenuation of effect size after additional adjustment for eGFR, ACR and urine creatinine. Among current TDF users, each year of TDF exposure was associated with approximately 7% higher urine α1m (p<0.001) in fully adjusted models. When TDF exposure was modeled as a categorical variable, current and past TDF users had higher adjusted urine α1m levels by 56% (p<0.001) and 29% (p=0.02), respectively, compared with HIV-infected men who never received TDF. Among participants previously exposed to TDF, each year since TDF discontinuation was associated with 5% lower urine α1m levels (p=0.04).
Figure 2. Association of cumulative TDF exposure with urine α1m levels in HIV-infected MACS participants (N=883).
Spline plot displaying unadjusted association of TDF exposure duration with urine α1m levels, calculated from generalized additive models. Solid line denotes predicted urine α1m level; dotted lines represent 95% confidence bounds. Highest 2.5% of values were truncated. p<0.001 for association of TDF duration with urine α1m; p=0.41 for tests of non-linearity.
Table 3.
Association of TDF exposure with levels of urine α1m among HIV-infected MACS participants (N = 883)
| Model 1: Adjusted for traditional kidney and HIV-related risk factors1 | Model 2: Adjusted for Model 1 + eGFR, Urine Creatinine, and ACR | |||
|---|---|---|---|---|
| TDF Exposure2 | % Estimate (95% CI) | P Value | % Estimate (95% CI) | P Value |
| Cumulative TDF exposure (y) | 8.4 (5.6, 11.2) | <0.001 | 7.6 (5.4, 9.9) | <0.001 |
| Current TDF duration (y) | 6.8 (4.1, 9.5) | <0.001 | 6.9 (4.7, 9.1) | <0.001 |
| Current vs never TDF use | 50.2 (25.3, 79.9) | <0.001 | 55.6 (33.9, 80.8) | <0.001 |
| Past vs never TDF use | 42.9 (10.8, 84.2) | 0.006 | 28.5 (3.9, 58.9) | 0.02 |
| Duration off TDF3 (y) | −2.5 (−8.2, 3.6) | 0.41 | −4.9 (−9.4, −0.2) | 0.04 |
Estimated percentage difference in biomarker attributable to TDF exposure. Models adjust for age, race, diabetes mellitus, CD4 lymphocyte count, body mass index (BMI) and TDF exposure
TDF exposure variables enter the model individually, not simultaneously
Analyses restricted to current and past TDF users (n=685)
Abbreviations: α1m, α1-microglobulin; ACR, albumin-creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; TDF, tenofovir disoproxil fumarate; y, years.
In multivariable adjusted analyses, each year of cumulative TDF exposure was associated with 3.2% higher urine protein/creatinine ratio (95% CI: 1.7, 4.7). Compared with never users, current and past TDF users had levels of urine protein/creatinine ratio that were 22% (95% CI: 11, 35) and 26% (95% CI: 9, 45) higher, respectively (Table S4).
In race-stratified analyses, each year of cumulative TDF exposure was associated with higher urine α1m by 6.5% (95% CI: 1.6, 11.6; p=0.009) among African Americans and 9.3% (95% CI: 5.8, 12.9; p<0.001) among Caucasians (p-value for TDF*race interaction = 0.36).
Associations of cumulative ARV exposure and other clinical factors with urine α1m
Next, we evaluated the associations of ARVs other than TDF and clinical factors with urine α1m levels. Compared with the other ARVs, TDF had the highest prevalence of use and largest effect size on α1m levels (Table S5). In multivariable models adjusting for demographics and clinical characteristics, each year of exposure to emtricitabine was associated with 7.7% higher urine α1m (p<0.001), while ritonavir and lopinavir were each associated with 2.8% (p=0.03) and 6.8% (p<0.001) higher urine α1m per year of exposure. Of note, among emtricitabine, ritonavir, and lopinavir users, the proportions of individuals who simultaneously received TDF were 98%, 72%, and 67%, respectively. Zidovudine was the only ARV showing a statistically significant association with lower urine levels of α1m (−2.0% per year exposure, p=0.02).
To account for simultaneous use of multiple ARVs, we used the LASSO method to select and adjust for the subset of ARVs most strongly associated with urine α1m levels (Table 4). Using this approach, only TDF and lopinavir use remained significantly associated with higher urine α1m levels, by 7.5% and 4.9% per year of exposure, respectively. Compared to individuals who never received tenofovir or lopinavir, adjusted urine α1m levels were 97% higher in participants who received tenofovir and lopinavir simultaneously (95% CI: 58, 145), 42% higher in participants who received tenofovir without lopinavir (95% CI: 17, 72), and 53% higher in participants who received lopinavir without tenofovir (95% CI: −6, 150).
Table 4.
Demographic and clinical factors associated with urine α1m in HIV-infected MACS participants (N=883)
| Univariate | Multivariate1 | |||
|---|---|---|---|---|
| Parameter | % Estimate2 (95% CI) | P Value | % Estimate (95% CI) | P Value |
| Age (per year) | 1.9 (0.9, 2.8) | <0.001 | 1.8 (0.9, 2.7) | <0.001 |
| African-American vs Caucasian | 38.0 (17.1, 62.5) | <0.001 | 39.3 (18.4, 63.9) | <0.001 |
| Other/Latino vs Caucasian | 3.2 (−22.4, 37.1) | 0.83 | 3.3 (−2.2, 37.2) | 0.82 |
| Body mass index (kg/m2) | −3.1 (−4.9, −1.2) | 0.001 | −2.9 (−4.7, −1.1) | 0.002 |
| Diabetes mellitus | 26.0 (1.7, 56.0) | 0.03 | 24.7 (0.9, 54.1) | 0.04 |
| CD4 lymphocyte count (per doubling) | −17.6 (−24.6, −9.9) | <0.001 | −18.3 (−25.2, −10.8) | <0.001 |
| Tenofovir (per year exposure) | 5.2 (1.5, 8.9) | 0.005 | 7.5 (4.6, 10.3) | <0.001 |
| Zidovudine (per year exposure) | −1.5 (−3.1, 0.2) | 0.09 | ||
| Abacavir (per year exposure) | 1.6 (−0.9, 4.3) | 0.21 | ||
| Emtricitabine (per year exposure) | 3.2 (−1.3, 7.8) | 0.17 | ||
| Ritonavir (per year exposure) | 1.2 (−1.4, 3.8) | 0.38 | ||
| Lopinavir (per year exposure) | 5.5 (2.1, 8.9) | 0.001 | 4.9 (1.7, 8.2) | 0.003 |
Multivariate models include demographic and clinical factors listed, and antiretroviral medications selected by LASSO method.
Estimated percentage difference in α1m attributable to each factor
Other clinical factors associated with higher urine α1m included older age, African American race, lower body mass index, diabetes mellitus, and lower CD4 lymphocyte count.
Discussion
With an expanding population of TDF users worldwide, nephrotoxicity has become a common clinical problem in persons with HIV. Early detection of tubular toxicity could enable clinicians to quantify risks of therapy and ensure patient safety. In this large cross-sectional study, we found that HIV-infected men had higher urine levels of α1m compared with uninfected men. Among the HIV-infected participants, current and past TDF users had substantially higher urine α1m levels compared with men who never received TDF, and each year of TDF exposure was incrementally associated with higher urine α1m levels. Notably, among past TDF users, each year since TDF discontinuation was associated with progressively lower α1m levels. In conjunction with our prior work demonstrating the prognostic significance of urine α1m for CKD progression and mortality, these findings highlight α1m as a promising biomarker of TDF-associated tubular toxicity.
Consistent with prior literature, we observed positive associations between tenofovir exposure and proteinuria, but the relative effect sizes were substantially stronger for urine α1m as compared with proteinuria. For example, relative to never users of tenofovir, current users had 50% higher urine α1m levels, compared with 22% higher protein/creatinine ratios. Notably, the majority of tenofovir users in this cohort had urine protein/creatinine ratios in the normal range (below 200 mg/g), despite their having substantially higher urine α1m levels compared with nonusers. Our observations suggest that subclinical tubular dysfunction is common among tenofovir users, and that proteinuria is not an optimally sensitive marker of TDF-associated tubular damage. Future studies should rigorously compare urine α1m with other markers of tubular dysfunction (such as glucosuria, phosphaturia, uricosuria, and metabolic acidosis) to determine whether urine α1m is the earliest indicator of tubular dysfunction.
α1-microglobulin was first isolated in urine samples of patients with tubular damage from chronic cadmium poisoning.30 Subsequent studies characterized α1m as a size- and charge- heterogeneous lipocalin that circulates in plasma in free and protein-bound forms.20 The 26-kDa unbound α1m is freely filtered at the glomerulus and reabsorbed by proximal tubular epithelial cells, via the endocytic receptor megalin.31,32 In the presence of proximal tubular damage, the filtered loads of α1m and other low molecular weight proteins, including β2-microglobulin and retinol binding protein, are incompletely reabsorbed, resulting in higher levels in urine.21,33 Wu et al. observed higher urine α1m levels among individuals with drug-induced interstitial nephritis, as compared with age- and sex-matched controls.34 Among participants with drug-induced interstitial nephritis, urine α1m levels correlated positively with the severity of inflammatory infiltration, interstitial edema, and tubular atrophy on kidney biopsy samples. Furthermore, in a study of children with acute tubular necrosis following cardiopulmonary bypass surgery, α1m was one of the earliest biomarkers detectable by proteomic analysis of urine samples, with levels rising by 3-fold within 2 hours of surgery, compared with controls who did not develop acute kidney injury (p<0.01).35
TDF-associated kidney injury localizes to the proximal tubule, due to active secretion of tenofovir by proximal tubular epithelial cells.13,15 Hence, markers of proximal tubular dysfunction, may be particularly useful in detecting toxicity from TDF. Nishijima et al. reported a 10% prevalence of kidney tubular dysfunction (defined as three or more abnormalities in: urine α1m, β2-microglobulin, N-acetyl-beta-D-glucosaminidase, fractional excretion of phosphorus, or fractional excretion of uric acid) among 190 HIV-infected individuals receiving tenofovir.36 Labarga et al. previously found that tenofovir users had a higher prevalence of tubular dysfunction (defined by at least two of the following: glucosuria, hyperaminoaciduria, hyperphosphaturia, or β2-microglobulinuria), compared with antiretroviral-naïve individuals (22% vs 12%, p<0.001).37 Hall et al. also reported higher urine levels of retinol-binding protein and N-acetyl-beta-D-glucosaminidase among HIV-infected tenofovir users, compared with non-users or antiretroviral-naïve patients.38 In contrast to the markers of tubular dysfunction examined by these studies, urine α1m levels have been associated with elevated risks for subsequent kidney function decline and mortality. In a large cohort of HIV-infected women who were not exposed to tenofovir at the time of biomarker measurement, we previously reported a 2.1-fold risk of incident CKD and 1.6-fold mortality risk over 8 years, for HIV-infected women in the highest vs lowest tertiles of urine α1m.22 The current study builds upon prior literature by demonstrating a dose response between tenofovir exposure and urine α1m levels, and it supports a potential mechanistic link between TDF use, tubular dysfunction, and the subsequent development of CKD.
Our finding that lopinavir exposure was associated with higher urine α1m levels is consistent with prior literature reporting synergistic nephrotoxicity when TDF is co-administered with protease inhibitors. In a trial of 741 HIV-infected women randomized to receive TDF/emtricitabine with either lopinavir/ritonavir or nevirapine, lopinavir/ritonavir users had an adjusted odds ratio of 3.1 (95%CI: 1.2, 8.1) for renal events (defined as: creatinine rise to ≥2 mg/dL or creatinine clearance <50 ml/min, causing interruption or discontinuation of TDF) compared with nevirapine users, over 2 years of follow-up.39 Protease inhibitors may interfere with tenofovir efflux from proximal tubular cells by inhibiting the multidrug resistance proteins responsible for tenofovir transport into the tubular lumen.17,40,41 Other studies have suggested that enhanced intestinal absorption of tenofovir accounts for this drug interaction.42–44 We also observed elevated urine α1m levels among individuals who received lopinavir without concomitant TDF, although the association did not reach statistical significance. Further studies are needed to verify this finding and to elucidate potential mechanisms by which lopinavir might exert direct kidney toxicity.
We found that African-Americans have higher urine α1m levels than Caucasians, an observation that is supported by our previous findings in the Women's Interagency HIV Study.22 Although the relative associations of HIV infection with urine α1m levels appeared stronger in Caucasians than in African Americans, these results may have been driven by elevated urine α1m levels among the uninfected African American participants. Large cohort studies have demonstrated that HIV-infected African Americans have a higher incidence of ESRD relative to Caucasians, and experience faster progression from CKD to ESRD.45–47 Although specific polymorphisms on the APOL1 gene appear to account for a portion of this racial disparity,48–50 we recently reported that the high-risk APOL1 genotype was not associated with urine α1m levels in a cross-sectional evaluation of HIV-infected African-American women with well-preserved kidney function.51 In the present study, we performed race-stratified analyses of TDF with urine α1m levels to examine potential differences in susceptibility to TDF-associated proximal tubular dysfunction, and we observed no statistically significant interactions by race. Further studies are needed to validate these findings and to evaluate alternate mechanisms leading to tubular dysfunction among HIV-infected African Americans.
Our study has several implications for clinical care. The measurement of urine α1m, in combination with other biomarkers of tubular damage, could constitute a novel method for the detection and monitoring of tubular toxicity while on therapy with TDF. Future studies should evaluate longitudinal changes in α1m levels among TDF users, and whether these changes are associated with the development of CKD. Second, little is known regarding the potential reversibility of tubular dysfunction following cessation of TDF. Although we found that time since TDF exposure was associated with lower α1m levels, the persistence of high α1m levels among past TDF users suggests incomplete recovery. Finally, recognition of nephrotoxicity at its earliest stages may be particularly important for the growing population of HIV-uninfected individuals receiving TDF as pre-exposure prophylaxis.
There are important limitations to this study. First, because this was a study of men, the results may not be generalizable to women. However, our earlier work in the WIHS cohort revealed that urine α1m levels in HIV-infected women were strongly predictive of incident CKD and all-cause mortality.22 Additionally, there is no known pathophysiologic basis for a gender-based interaction between TDF exposure and kidney injury. Second, we did not have access to the clinical reasons for TDF discontinuation. However, the presence of lower eGFR and higher prevalence of CKD in past TDF users, as compared with current or never users, suggests that nephrotoxicity may have led to the discontinuation of TDF. Finally, although we adjusted for multiple potential confounders, we cannot exclude the possibility of residual confounding.
In conclusion, in this large cohort of men with predominantly normal kidney function, HIV infection and TDF exposure were associated with higher urine levels of α1m, a marker of proximal tubular dysfunction. If these findings are validated in future studies, urine α1m may be a useful indicator of TDF-associated tubular dysfunction.
Supplementary Material
Association of cumulative TDF exposure with urine protein/creatinine ratio in HIV-infected MACS participants (N=867)
Proportion of missing data for variables included in multiple imputation model
Association of HIV infection with urine protein/creatinine ratio, overall and stratified by tenofovir use
Race-stratified associations of HIV infection with urine α1m levels
Association of TDF exposure with urine protein/creatinine ratio among HIV-infected MACS participants (N=867)
Associations of cumulative antiretroviral medication exposure1 (per year) with urine α1m levels, without simultaneous adjustment for other ARVs
Acknowledgements
Funding Sources MACS Kidney Study is funded by grant 1 R01 AG034853-01A2 (PI, Shlipak), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Richard Elion, Richard Elion, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D'Souza (Co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Alvaro Muoz, Derek Ng, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The funders of this study had no role in study design; collection, analysis or interpretation of data; manuscript preparation; or the decision to submit the report for publication. The MACS website is located at http://www.statepi.jhsph.edu/macs/macs.html.
Footnotes
The results of this study were presented as a poster at the American Society of Nephrology annual conference held in San Diego, November 2015.
Disclosure Statement The authors have no conflicts of interest to disclose.
Contributions: research idea and study design: VJ, MGS; data acquisition: LPJ, MDW, FJP, BM, MB; data analysis/interpretation: VJ, RS, MME, MGS; statistical analysis: RS; supervision or mentorship: CRP, JHI, MGS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. VJ takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140(1):132–143. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 7.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrobial agents and chemotherapy. 2001;45(10):2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonjoch A, Juega J, Puig J, et al. High Prevalence of Signs of Renal Damage Despite Normal Renal Function in a Cohort of HIV-Infected Patients: Evaluation of Associated Factors. AIDS patient care and STDs. 2014;28(10):524–529. doi: 10.1089/apc.2014.0172. [DOI] [PubMed] [Google Scholar]
- 9.Flandre P, Pugliese P, Cuzin L, et al. Risk factors of chronic kidney disease in HIV-infected patients. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(7):1700–1707. doi: 10.2215/CJN.09191010. [DOI] [PubMed] [Google Scholar]
- 10.Rifkin BS, Perazella MA. Tenofovir-associated nephrotoxicity: Fanconi syndrome and renal failure. Am J Med. 2004;117(4):282–284. doi: 10.1016/j.amjmed.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(5):773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. Aids. 2012;26(7):867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Laboratory investigation; a journal of technical methods and pathology. 2009;89(5):513–519. doi: 10.1038/labinvest.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney international. 2010;78(11):1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 15.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrobial agents and chemotherapy. 2006;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides, nucleotides & nucleic acids. 2001;20(4–7):641–648. doi: 10.1081/NCN-100002341. [DOI] [PubMed] [Google Scholar]
- 17.Cihlar T, Ray AS, Laflamme G, et al. Molecular assessment of the potential for renal drug interactions between tenofovir and HIV protease inhibitors. Antiviral therapy. 2007;12(2):267–272. [PubMed] [Google Scholar]
- 18.Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3(2):481–490. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassirer JP. Clinical evaluation of kidney function--glomerular function. The New England journal of medicine. 1971;285(7):385–389. doi: 10.1056/NEJM197108122850706. [DOI] [PubMed] [Google Scholar]
- 20.Akerstrom B, Logdberg L, Berggard T, Osmark P, Lindqvist A. alpha(1)-Microglobulin: a yellow-brown lipocalin. Biochimica et biophysica acta. 2000;1482(1–2):172–184. doi: 10.1016/s0167-4838(00)00157-6. [DOI] [PubMed] [Google Scholar]
- 21.Weber MH, Verwiebe R. Alpha 1-microglobulin (protein HC): features of a promising indicator of proximal tubular dysfunction. Eur J Clin Chem Clin Biochem. 1992;30(10):683–691. [PubMed] [Google Scholar]
- 22.Jotwani V, Scherzer R, Abraham A, et al. Association of Urine alpha1-Microglobulin with Kidney Function Decline and Mortality in HIV-Infected Women. Clinical journal of the American Society of Nephrology : CJASN. 2015;10(1):63–73. doi: 10.2215/CJN.03220314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 24.Castro KGWJ, Slutsker L, et al. Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults: Center for Disease Control and Prevention 1992. 1993. 1992. [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilks WR, Richardson S, Spiegehalter DJ. Markov chain Monte Carlo in practice. Chapman & Hall; London: 1996. [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 28.Hoeting JMD, Raftery A, Volinsky C. Bayesian Model Averaging: A Tutorial. Statistical Science. 1999;14:382–401. [Google Scholar]
- 29.Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Statist Soc B. 1996;58:267–288. [Google Scholar]
- 30.Ekstrom B, Peterson PA, Berggard I. A urinary and plasma alpha1-glycoprotein of low molecular weight: isolation and some properties. Biochemical and biophysical research communications. 1975;65(4):1427–1433. doi: 10.1016/s0006-291x(75)80388-3. [DOI] [PubMed] [Google Scholar]
- 31.Leheste JR, Rolinski B, Vorum H, et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. The American journal of pathology. 1999;155(4):1361–1370. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strober W, Waldmann TA. The role of the kidney in the metabolism of plasma proteins. Nephron. 1974;13(1):35–66. doi: 10.1159/000180368. [DOI] [PubMed] [Google Scholar]
- 33.Christensen EI, Nielsen S. Structural and functional features of protein handling in the kidney proximal tubule. Semin Nephrol. 1991;11(4):414–439. [PubMed] [Google Scholar]
- 34.Wu Y, Yang L, Su T, Wang C, Liu G, Li XM. Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(11):1954–1959. doi: 10.2215/CJN.02370310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR. Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56(4):632–642. doi: 10.1053/j.ajkd.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishijima T, Shimbo T, Komatsu H, et al. Urinary beta-2 microglobulin and alpha-1 microglobulin are useful screening markers for tenofovir-induced kidney tubulopathy in patients with HIV-1 infection: a diagnostic accuracy study. J Infect Chemother. 2013;19(5):850–857. doi: 10.1007/s10156-013-0576-y. [DOI] [PubMed] [Google Scholar]
- 37.Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. Aids. 2009;23(6):689–696. doi: 10.1097/QAD.0b013e3283262a64. [DOI] [PubMed] [Google Scholar]
- 38.Hall AM, Edwards SG, Lapsley M, et al. Subclinical tubular injury in HIV-infected individuals on antiretroviral therapy: a cross-sectional analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;54(6):1034–1042. doi: 10.1053/j.ajkd.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Mwafongo A, Nkanaunena K, Zheng Y, et al. Renal events among women treated with tenofovir/emtricitabine in combination with either lopinavir/ritonavir or nevirapine. Aids. 2014;28(8):1135–1142. doi: 10.1097/QAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiser JJ, Carten ML, Aquilante CL, et al. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clinical pharmacology and therapeutics. 2008;83(2):265–272. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 41.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. Journal of acquired immune deficiency syndromes. 2006;43(3):278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 42.Tong L, Phan TK, Robinson KL, et al. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrobial agents and chemotherapy. 2007;51(10):3498–3504. doi: 10.1128/AAC.00671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vishnuvardhan D, Moltke LL, Richert C, Greenblatt DJ. Lopinavir: acute exposure inhibits P-glycoprotein; extended exposure induces P-glycoprotein. Aids. 2003;17(7):1092–1094. doi: 10.1097/01.aids.0000060380.78202.b5. [DOI] [PubMed] [Google Scholar]
- 44.Washington CB, Duran GE, Man MC, Sikic BI, Blaschke TF. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1998;19(3):203–209. doi: 10.1097/00042560-199811010-00001. [DOI] [PubMed] [Google Scholar]
- 45.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O'Hare AM. The impact of HIV on chronic kidney disease outcomes. Kidney international. 2007;72(11):1380–1387. doi: 10.1038/sj.ki.5002541. [DOI] [PubMed] [Google Scholar]
- 46.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. The Journal of infectious diseases. 2008;197(11):1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak M. Risk factors for end-stage renal disease in HIV-infected individuals: traditional and HIV-related factors. AJKD. 2012 doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Human genetics. 2010;128(3):345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peralta CA, Bibbins-Domingo K, Vittinghoff E, et al. APOL1 Genotype and Race Differences in Incident Albuminuria and Renal Function Decline. Journal of the American Society of Nephrology : JASN. 2015 doi: 10.1681/ASN.2015020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jotwani V, Shlipak MG, Scherzer R, et al. APOL1 Genotype and Glomerular and Tubular Kidney Injury in Women With HIV. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;65(6):889–898. doi: 10.1053/j.ajkd.2015.02.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of cumulative TDF exposure with urine protein/creatinine ratio in HIV-infected MACS participants (N=867)
Proportion of missing data for variables included in multiple imputation model
Association of HIV infection with urine protein/creatinine ratio, overall and stratified by tenofovir use
Race-stratified associations of HIV infection with urine α1m levels
Association of TDF exposure with urine protein/creatinine ratio among HIV-infected MACS participants (N=867)
Associations of cumulative antiretroviral medication exposure1 (per year) with urine α1m levels, without simultaneous adjustment for other ARVs