SUMMARY
The cerebellum has long been recognized for its role in motor co-ordination, but it is also increasingly appreciated for its role in complex cognitive behavior. Historically, the cerebellum has been overwhelmingly understudied compared to the neocortex in both humans and model organisms. However, this tide is changing as advances in neuroimaging, neuropathology, and neurogenetics have led to clinical classification and gene identification for numerous developmental disorders that impact cerebellar structure and function associated with significant overall neurodevelopmental dysfunction. Given the broad range in prognosis and associated medical and neurodevelopmental concerns accompanying cerebellar malformations, a working knowledge of these disorders and their causes is critical for obstetricians, perinatologists, and neonatologists. Here we present an update on the genetic causes for cerebellar malformations that can be recognized by neuroimaging and clinical characteristics during the prenatal and postnatal periods.
Keywords: Cerebellum, Neuroimaging, Magnetic resonance imaging, Genetics, Brain malformation, Neurodevelopment
1. Introduction
Cerebellar malformations are now widely diagnosed during pregnancy and associated with significant morbidity and mortality in the newborn period and throughout life. Given the broad range in prognosis and associated medical concerns, a working knowledge of these disorders and their causes is essential for obstetricians, perinatologists and neonatologists. For clinicians, it is most relevant to organize cerebellar malformations by their clinical and imaging features, which then directs additional diagnostic testing, medical monitoring for associated complications, and counseling about prognosis, treatment and recurrence risk. Distinguishing genetic disorders from similar conditions caused by extrinsic factors, such as infection, stroke, or prematurity, is particularly important to provide quality patient care. Cerebellar malformations may be classified as predominantly involving the cerebellum or involving both the cerebellum and brainstem. They may occur in isolation or as part of broader syndromes involving multiple systems. Though the cerebellum has long been recognized for its role in motor co-ordination, it also shapes the functions of other brain regions, especially cognition and affect, by processing external sensory and internally generated information to influence neocortical circuit refinement. Thus, not surprisingly, most cerebellar malformations are associated with neurodevelopmental issues affecting multiple domains: motor, communication, cognition, emotional regulation, and executive function. Human cerebellar development begins around the ninth gestational week and continues beyond birth. This protracted developmental timeline makes the human cerebellum particularly vulnerable to insult, especially during 24–40 weeks of gestation, when considerable neurogenesis in the external granule cell layer results in a five-fold increase in cerebellar size. Malformations that arise early in development typically affect both cerebellum and brainstem, whereas, later in development, cerebellar malformations have less effect on the pons. Here we present some of the most frequently occurring and best understood human cerebellar malformations and their genetic causes.
2. Epidemiology of cerebellar malformations
There are few population-based prevalence data for cerebellar malformations, due to several factors. Neuroimaging studies are required for diagnosis, but are variably performed depending on the clinical circumstances and resources available. When neuroimaging studies are performed, cerebellar malformations are often under-recognized. Within the few population-based cohorts, patients diagnosed with cerebellar malformations have the most severe clinical features [1]. Finally, cerebellar malformations are found in cohorts of individuals with autism, but rarely listed as a diagnosis. Dandy–Walker malformation (DWM) is frequently reported as the most prevalent cerebellar malformation, but estimated prevalences vary considerably among available studies (1/3000 to 1/30,000) [2]. Thus, the true prevalence for cerebellar malformations collectively, or for specific disorders, is mostly unknown.
3. Clinical features of cerebellar malformations
The clinical classification of cerebellar disorders provides critical information for accurate prognostic and recurrence risk counseling. The most useful diagnostic categories also guide subsequent evaluation and medical management. Furthermore, a clinical diagnosis may facilitate identification of the underlying genetic cause that can then be used for prenatal diagnosis and carrier testing. Since clinical and neuroimaging features of specific cerebellar malformations overlap considerably, correctly classifying the cerebellar malformation in any particular patient requires a comprehensive approach that integrates pre- and postnatal medical history, physical examination, neuroimaging, and laboratory testing.
Prenatal ultrasound and magnetic resonance imaging (MRI) are now widely used to evaluate the fetal brain, and many cerebellar malformations are recognizable before birth (Fig. 1); however, the sensitivity and specificity of prenatal neuroimaging is not known. Prenatal ultrasound can identify cerebellar hypoplasia, abnormal fluid collections in the posterior fossa, or poor delineation of posterior fossa landmarks. Additional evaluation during pregnancy can involve fetal MRI, genetic amniocentesis, cell-free fetal DNA testing, and evaluation for in-utero infection. Despite thorough evaluation during pregnancy, a specific etiology is often not identified until after birth. For patients who elect to terminate a pregnancy, fetal autopsy after termination without a prolonged interval before delivery provides the best diagnostic information and should be offered.
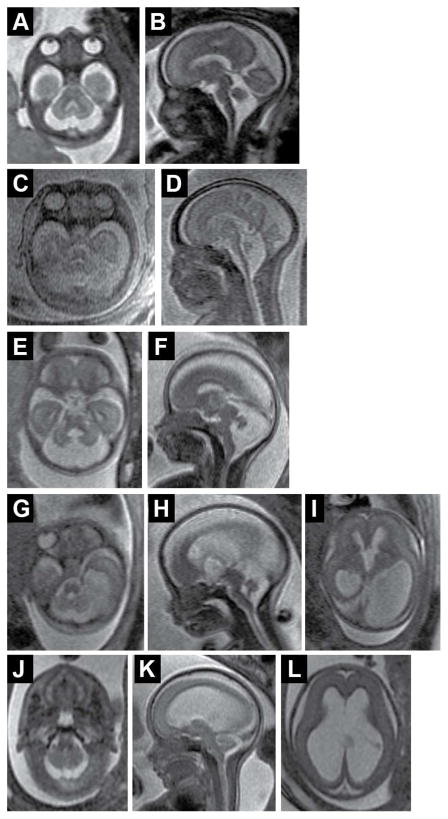
Fig. 1.
Fetal neuroimaging of cerebellar malformations. (A, B) Axial and sagittal views of a 22-week gestation fetus with confirmed VLDLR-related cerebellar hypoplasia. Note that cerebellar hypoplasia more severely affects the vermis; there are no primary fissure and other vermis landmarks on the sagittal view. (C, D) Axial and sagittal views of a 25-week gestation fetus with TSEN54-related pontocerebellarhyplasia. Note marked hypoplasia of the vermis, hemispheres and pons. (E, F) Axial and sagittal views of a 20-week gestation fetus with Joubert syndrome. Note the more severely affected vermis with dysplastic brainstem. (G–I) Axial and sagittal views of a 20-week gestation fetus with POMGNT1-related muscle–eye–brain disease demonstrating cerebellar hypoplasia of vermis and hemispheres, “kinked brainstem” and severe, asymmetric lateral ventriculomegaly. (J–L) Axial and sagittal views of a 20-week gestation fetus with rhombencephalosynapsis demonstrating small cerebellum without an obvious vermis and severe ventriculomegaly with partially absent septum (diagnosis was confirmed by autopsy).
The postnatal clinical presentation of patients with cerebellar malformations is typically non-specific; features include hypotonia, motor delay, nystagmus, and decreased visual attention. Severely affected patients can present with apnea, feeding difficulties, aspiration, spasticity, lack of developmental progress, and seizures. Signs of cranial nerve dysfunction, including abnormal eye movements, ptosis, facial palsy, hearing impairment, and facial/corneal anesthesia, may be observed. Ophthalmologic evaluation may reveal chorioretinal coloboma or retinal dystrophy in patients with Joubert syndrome (JS), various structural eye abnormalities in patients with cobblestone malformations, or other eye movement abnormalities. Mildly affected patients may have relatively isolated cranial nerve dysfunction, as in Duane retraction syndrome and horizontal gaze palsy with progressive scoliosis. Cognitive impairment is frequent, but not universal, among patients with cerebellar malformations, and autistic features are also observed. Not surprisingly, cerebellar malformations are associated with a wide range of neurodevelopmental outcomes.
Recognizing the clinical features associated with cerebellar dysfunction can aid in identifying patients with cerebellar malformations and trigger the need for neuroimaging; recognizing the idiosyncratic features associated with specific diagnoses may help to differentiate specific cerebellar disorders (Tables 1 and 2 and sections below). Extreme prematurity or intrauterine infection may indicate a non-genetic etiology; however, these are usually diagnoses of exclusion. Laboratory testing can also differentiate patients. For example, creatine kinase is elevated in patients with cobblestone malformations, protein glycosylation profiles are abnormal in congenital disorders of glycosylation (CDGS), and hyperglycemia with markedly decreased or absent insulin is seen in patients with PTF1A-related cerebellar and pancreatic agenesis.
Table 1.
Typical clinical, imaging, and genetic characteristics of specific cerebellar malformations with known genetic cause.a
| Disorder | Clinical features | Imaging features | Causes/testing | Inheritance |
|---|---|---|---|---|
| BRF1-related PCH | Dysmorphic facial features, short stature, microcephaly, dental anomalies. NDV impairment. |
Cerebellar vermis hypoplasia, thin corpus callosum, flat midbrain and pons | BRF1 | AR |
| CASK-related PCH | Typically females (rarely males), progressive microcephaly, variable hearing loss. Severe NDV impairment. |
Small pons and cerebellum (may be mild), proportionate cerebellar vermis and hemisphere hypoplasia, microcephaly | CASK | X-linked |
| CDGS Type 1a | Abnormal fat distribution, retinitis pigmentosa, other diverse features: failure to thrive, elevated transaminases, coagulopathy, hypothyroidism, hypogonadism, seizures, stroke-like episodes, substantial ataxia. Mild to severe NDV impairment. |
Pontocerebellar hypoplasia with progressive atrophy, cortical atrophy in some patients | PMM2, transferrin glycosylation analysis | AR |
| Cerebellar hyperplasia | Highly variable features of Alexander disease, fucosidosis, Sotos, Williams, Costello, MCAP/MPPH syndromes | Large cerebellum, cerebellar ectopia, cerebellum wrapped around brainstem | Genomic imbalances, genes for associated disorders | Various depending on specific disorder |
| CHARGE Syndrome | Coloboma, heart defects, choanal atresia, retarded growth and development, genital and inner ear abnormalities | Small cerebellar vermis often with some upward rotation, mildly enlarged posterior fossa, abnormal hemisphere foliation | CHD7 | De-novo AD |
| Chudley–McCullough syndrome | Hearing loss, mild or no ataxia. Mild NDV impairment. |
Cerebellar hemisphere dysplasia, inferior cerebellar vermis hypoplasia, partial ACC, frontal subcortical heterotopia, frontal PMG, arachnoid cysts | GPSM2 | AR |
| Cockayne syndrome | Growth retardation, dysmorphic facial features, sensitivity to UV radiation. Mild to severe NDV impairment. |
Small pons and cerebellum, calcifications mostly in putamen, progressive, generalized cerebral and cerebellar atrophy | ERCC6 | AR |
| Dandy–Walker malformation | Macrocephaly frequent. Variable cognitive, NDV impairment. Axenfield–Rieger syndrome (FOXC1). |
Vermis hypoplasia, variable hemisphere hypoplasia, large posterior fossa, upwardly rotated vermis | FOXC1, ZIC1/ZIC4 deletions (rare) | Sporadic (recurrence risk <5%) |
| Dystroglycanopathies (Walker–Warburg syndrome, muscle– eye–brain disease, Fukuyama muscular dystrophy) | Eye involvement (cataracts, coloboma, high myopia), muscle involvement (variably increased CK). Severe NDV impairment. |
Kinked brainstem, large tectum, cerebellar hypoplasia, subcortical cerebellar cysts, cobblestone cerebral cortex, abnormal myelination | FKRP, FKTN, GPR56, GTDC2, ISPD, LARGE, POMGNT1, POMT, POMT1, POMT2 | AR |
| Joubert syndrome | Hypotonia, alternating apnea and tachypnea (improves with age); other diverse features: retinal dystrophy, coloboma, liver fibrosis, nephronophthisis, polydactyly, substantial ataxia. Variable NDV impairment, usually moderate. |
Vermis hypoplasia, long/thick, horizontal SCPs, encephalocele (rare), foramen magnum cephalocele (occasional), anterior midbrain and dorsal medulla heterotopia (occasional), PMG and CC agenesis (rare) | AHI1, ARL13B, B9D1, B9D2, C2CD3, C5ORF42, CC2D2A, CEP41, CEP104, CEP290, CSPP1, IFT172, INPP5E, KIAA0586, KIF7, MKS1, NPHP1, OFD1, RPGRIP1L, TCTN1, TCTN2, TCTN3, TMEM67, TMEM107, TMEM138, TMEM216, TMEM231, TMEM237 | AR, X-linked (OFD1 only) |
| Lissencephaly-related | Spasticity, seizures. Severe NDV impairment in patients with RELN mutations. Broad range of NDV outcome in patients with tubulinopathies and ARX mutations. |
Heterogeneous. RELN: pachygyria with mildly thickened cortex. ARX: variable spectrum, lissencephaly with moderately thick cortex (more severe posteriorly), small to absent basal ganglia, CC agenesis, small pons with normal cerebellum. Tubulinopathies: highly variable spectrum of PMG to lissencephaly, enlarged tectum, dysmorphic basal ganglia, thin/absent CC. |
RELN, ARX, TUBA1A | AR (RELN), X-linked (ARX), de novo AD (TUBA1A) |
| PCH Type 1 | Spinal muscular atrophy | Hypoplastic pons (often mild), proportional vermis and hemispheres hypoplasia | EXOSC3 (majority), RARS2, TSEN54, VRK1 | AR |
| PCH Type 2 | Neonatal encephalopathy, severe progressive microcephaly, increased tone, dyskinesia, seizures, cortical visual impairment. NDV impairment usually profound. |
Postmigrational microcephaly, small pons and cerebellum, atrophic appearing cortex, thin CC, vermis less hypoplastic than hemispheres | TSEN54 (majority), TSEN2, TSEN34 | AR |
| PCH Type 3 | Hypotonia, hyperreflexia, seizure onset in infancy. Severe NDV impairment. |
Small pons and cerebellum, reduced cerebral white matter | PCLO | AR |
| PCH Type 4/5 | Severe PCH2 | TSEN54, TSEN2, TSEN34 | AR | |
| PCH Type 6 | Elevated cerebrospinal fluid lactate | Small pons and cerebellum, vermis more severely hypoplastic than hemispheres | RARS2 | AR |
| PCH Type 8 | Acquired microcephaly, increased extremity tone and contractures. Moderate to severe NDV impairment. |
Small pons and cerebellum, proportionate vermis and hemisphere hypoplasia | CHMP1A | AR |
| PCH Type 9 | Progressive microcephaly, seizure onset in infancy, hypertonia, visual impairment. Severe NDV impairment. |
Small cerebellum (with atrophy) and pons with ventral flattening, mega cisterna magna, brainstem “figure of 8” appearance. Generalized atrophy of cerebral cortex and CC hypoplasia |
AMPD2 | AR |
| PCH Type 10 | Progressive microcephaly and neurodegeneration features by 6 months, absent or delayed speech, progressive spasticity, spontaneous seizures. Severe NDV impairment. |
Small cerebellum, pons and CC with atrophy | CLP1 | AR |
| Poretti–Boltshauser syndrome | Ataxia, myopia, intellectual disability, language impairment | Cerebellar dysplasia with cysts | LAMA1 | AR |
| PTF1A-related PCH | Congenital diabetes, intrauterine growth retardation, microcephaly. Severe NDV impairment. |
Small to absent cerebellum, small pons | PTF1A | AR |
| Ritscher–Schinzel/3C syndrome | Craniofacial and limb abnormalities, cardiac defects. NDV impairment. |
Dandy–Walker malformation, cerebellar vermis hypoplasia, posterior fossa cysts, ventricular dilatation | CCDC22 | X-linked |
| VLDLR-related cerebellar hypoplasia | Dysequilibrium, quadripedal locomotion reported in some, substantial ataxia. Moderate to severe NDV impairment. |
Cerebellar hypoplasia with decreased foliation (vermis worse than hemispheres), simplified cortical gyral pattern | VLDLR | AR |
| WNT1-related PCH | Osteogenesis imperfecta, unilateral ptosis, autism and seizures reported in some. Mild to severe NDV impairment. |
Small brainstem, midbrain and cerebellum (normal to unilateral hypoplasia to complete absence); Chiari reported in one patient with normal intelligence | WNT1 | AR |
| X-linked intellectual disability with cerebellar hypoplasia | Moderate NDV impairment | Mild vermis hypoplasia, mild cerebellar hemisphere hypoplasia, variable ventriculomegaly | OPHN1 | X-linked |
PCH, pontocerebellarhyplasia; NDV, neurodevelopmental; AR, autosomal recessive; CDGS, congenital disorders of glycosylation; MCAP, megalencephaly–capillary malformation–polymicrogyria; MPPH, megalencephaly–polydactyly–polymicrogyria–hydrocephalus; AD, autosomal dominant; ACC, agenesis of the corpus callosum; PMG, polymicrogyria; UV, ultraviolet; CK, creatine kinase; SCP, superior cerebellar peduncles; CC, corpus callosum.
Note that information published for many of the disorders is quite limited, so the full spectrum of characteristics is likely broader than shown in this table.
Table 2.
Typical clinical, imaging, and genetic characteristics of cerebellar malformations without known genetic cause.
| Disorder | Clinical features | Imaging features | Causes/testing | Inheritance |
|---|---|---|---|---|
| OCCS | Orbital cysts, micro/anophthalmia, focal skin defects and appendages | Severely hypoplastic vermis, normal or hypoplastic cerebellar hemispheres, enlarged dysplastic tectum, thick, vertical SCPs, CC agenesis, frontal PMG | Unknown | Sporadic |
| PCH type 7 | Ambiguous or female external genitalia with 46,XY karyotype, hypergonadotrophic hypogonadism, hypotonia, seizures. Severe NDV impairment |
Small pons and cerebellum, enlarged ventricles, thin corpus callosum | Unknown | AR |
| PHACE syndrome | Segmental hemangioma (usually head and neck), intracranial and great vessel abnormalities. Variable NDV impairment in <50% (usually mild). |
Unilateral cerebellar hypoplasia with or without vermis involvement, DWM. Dysmorphic or absent major vessels. Occasional heterotopia, PMG. |
Unknown | Sporadic |
| PTCD | Hearing loss, trigeminal anesthesia/corneal scarring, dysphagia, variable cardiac and vertebral/rib defects, substantial ataxia. Moderate to severe NDV impairment. |
Hypoplastic pons, mildly hypoplastic cerebellum, “cap” of white matter on dorsum of pons, markedly hypoplastic middle and inferior cerebellar peduncles | No specific testing | Sporadic |
| RES all types | Alopecia, trigeminal anesthesia (GLH syndrome), head shaking (usually “figure 8” pattern), hyperactivity/impulsivity, VACTERL features (<50%), variable ataxia. Full range of NDV outcome. |
Absent septum pellucidum, aqueductal stenosis, fused colliculi, posterior holoprosencephaly (rare), absent olfactory bulbs (<50%) | Unknown | Sporadic (one recurrence reported) |
OCCS, oculocerebrocutaneous syndrome; SCP, superior cerebellar peduncles; CC, corpus callosum; PMG, polymicrogyria; PCH, pontocerebellarhyplasia; NDV, neurodevelopmental; AR, autosomal recessive; PHACE, Posterior fossa malformations, Hemangioma, Arterial anomalies, Cardiac defects, and Eye anomalies; DWM, Dandy–Walker malformation; PTCD, pontine tegmental cap dysplasia; RES, rhombencephalosynapsis; GLH, Gómez–López–Hernández; VACTERL, Vertebral defects, Anal atresia, Cardiac defects, Tracheo-Esophageal fistula, Renal defects, and Limb defects.
4. Specific malformations with known genetic causes
4.1. Predominantly cerebellar malformations
A malformed cerebellum may be abnormally small, dysplastic, or unusually large. The vermis and both hemispheres may be equally or disproportionately affected. Primary malformations of the pons, midbrain, and supratentorial structures are also seen in a substantial subset of patients. The wide range in morphological presentations results from the diversity of causes, including chromosomal abnormalities, specific genetic syndromes, and extrinsic factors.
4.1.1. Dandy–Walker malformation
Dandy–Walker malformation (MIM 220200) is a heterogeneous disorder defined by a hypoplastic, upwardly rotated vermis, an enlarged fourth ventricle, and an enlarged posterior fossa with an elevated confluence of sinuses (Fig. 2B). Typically, the cerebellar hemispheres are less affected than the vermis, and the brainstem is normal to moderately hypoplastic. DWM can occur with additional brain abnormalities including agenesis of the corpus callosum (ACC) and hydrocephalus, but more often it occurs as an isolated brain-imaging finding. The clinical features and developmental outcomes vary widely. Patients may exhibit symptoms ranging from intellectual disability to autism or they may be completely unaware of any deficits until diagnosed as adults for unrelated reasons [3]. The recurrence risk in isolated DWM is low at an estimated 1–5% [4], suggesting de-novo, somatic mosaic, or complex genetic causes.
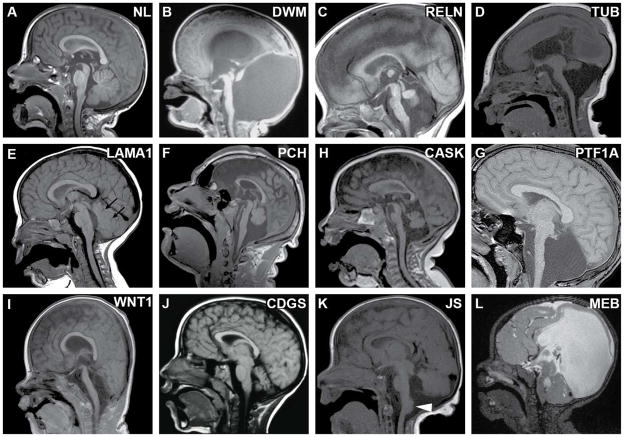
Fig. 2.
Sagittal views of cerebellar malformations with known genetic causes. (A) Unaffected individual for comparison. (B) Dandy–Walker malformation (unknown cause) with hypoplastic, rotated vermis and marked enlargement of 4th ventricle and posterior fossa. (C) Cerebellar hypoplasia in a patient with biallelic RELN mutations, demonstrating hypoplastic brainstem and characteristic absent folia of the vermis; note the normal tectum. (D) Tubulinopathy (TUBA1A mutation) with brainstem hypoplasia, vermis hypoplasia, lissencephaly and microcephaly; note the large, dysplastic tectum. (E) Mild cerebellar vermis hypoplasia, abnormal 4th ventricle shape, and small cysts (arrows) in a patient with biallelic LAMA1 mutations. (F) Pontocerebellarhyplasia (PCH) (homozygous TSEN54 mutation) with hypoplastic brainstem and vermis (which is less affected than hemispheres); note the normal tectum. (G) PCH in a patient with congenital diabetes; note the extremely small vermis and flat pons with preserved tectum. (H) CASK-related PCH; note that the pons is not severely affected in this patient. (I) Cerebellar agenesis, with severe pontine and midbrain hypoplasia in a severely affected patient with biallelic WNT1 mutations. (J) Congenital disorder of glycosylation Type 1a due to biallelic PMM2 mutations. (K) TCTN2-related Joubert syndrome with vermis hypoplasia (obscured by hemispheres in this image), horizontal superior cerebellar peduncles, large dysplastic tectum and heterotopia at the dorsal cervicomedullary junction (arrowhead). (L) Muscle–eye–brain disease due to POMGNT1 mutations; note the markedly hypoplastic and dysplastic brainstem, cerebellar cysts, abnormal tectum, and hydrocephalus. Adapted with permission from Doherty et al. [12], except for panel (I) which is from Aldinger et al. [40].
Few genes have been implicated in rare cases of DWM, including genomic imbalances that are part of a congenital syndrome and rare single gene disorders [5–8]. FOXC1-related DWM is associated with multiple congenital anomalies, especially eye malformations consistent with Axenfeld–Rieger syndrome [6]. Congenital anomalies associated with FOXC1-related DWM in severely affected patients overlap with Ritscher–Schinzel, or 3C (cranio-cerebello-cardiac) syndrome. Recently, mutations in CCDC22 were found in X-linked cases of 3C syndrome, suggesting that CCDC22 mutations may be a new cause of DWM [8]. Though these patients were noted to have DWM, limited neuroimaging data were reported to substantiate this diagnosis [8]. ZIC1/4-related DWM is also associated with multiple congenital anomalies, including dysmorphic facial features and abnormal development of the eyelids. Recently, exome sequencing identified autosomal dominant mutations in LAMC1 and NID1 as the cause of DWM with encephalocele in two families [7]. Despite these genetic advances, the genetic cause remains unknown in the majority of DWM patients.
Case reports and small case-series also suggest that extrinsic factors may contribute to DWM. For example, serial fetal prenatal neuroimaging identified evidence of prenatal hemorrhage above the cerebellum in a patient postnatally diagnosed with DWM [9]. Two comprehensive population-based studies suggest that clomiphene citrate exposure and twinning may be additional non-genetic risk factors for DWM. Additional rigorous studies are needed to assess prenatal risk factors for DWM.
4.1.2. Cerebellar hypoplasia
Cerebellar hypoplasia (CH) refers to an underdevelopment of the cerebellum. This category of cerebellar malformation is distinct from DWM in that it does not involve a concurrent enlargement of the posterior fossa, and almost all individuals exhibit cognitive and motor impairments. CH is a feature of many different disorders and it is often a non-specific feature associated with genomic imbalances. CH is frequently associated with additional brain abnormalities, including lissencephaly, cortical dysplasia, microcephaly and heterotopia, pointing to specific genetic causes.
The lissencephaly spectrum of brain malformations is caused by defects in either the reelin pathway or microtubule formation and function. Patients with autosomal recessive mutations in RELN have pachygyria and an extremely hypoplastic cerebellum with very little foliation and disproportionate effects on the vermis (Fig. 2C) [10]. Additional clinical features include profound developmental disability, microcephaly, sloping forehead, seizures, and congenital lymphedema. In contrast, patients with autosomal recessive mutations in VLDLR, a reelin receptor, exhibit mild pachygyria and a mildly small cerebellum that retains some foliation [11]. Reelin is transiently expressed by neurons within superficial layers in both the cerebral cortex and cerebellum and regulates radial neuronal migration.
Mutations in the alpha- and beta-tubulins are a major cause of brain malformations, especially lissencephaly, pachygyria, and polymicrogyria, collectively referred to as tubulinopathies. Mutations in TUBA1A, TUBA8, TUBB2A, TUBB2B, TUBB3, TUBB4A, and TUBB are associated with a range of clinical features from isolated congenital fibrosis of the extraocular muscles to severe intellectual disability, quadriplegic cerebral palsy, seizures, cranial neuropathies and hydrocephalus [12,13]. Neuroimaging features include cortical dysgenesis (lissencephaly or polymicrogyria), malformation of cranial nerves, and basal ganglia dysplasia, often with cerebellar and pontine hypoplasia, and defects in the corpus callosum, anterior commissure and internal capsule (Fig. 2D). Dysmorphic features are infrequently reported, and, surprisingly, other organ systems are not affected. Most occurrences are sporadic and due to de-novo mutations, but rare recurrences have been reported due to germline mosaicism and autosomal recessive inheritance [14].
Additional genes and extrinsic exposures have been associated with CH. Mutations in CHD7 account for the majority of patients with CHARGE syndrome (MIM-214800), characterized by Coloboma, Heart defects, choanal Atresia, Retardation of growth and development, Genital and Ear abnormalities [15]. Neuroimaging reveals that more than a third of these patients have a slightly rotated, mildly to moderately hypoplastic cerebellar vermis, a mildly enlarged posterior fossa, and abnormal cerebellar hemisphere foliation [16]. CHD7 loss-of-function disrupts critical gene expression in the isthmus organizer, a transient embryonic structure that directs early cerebellar development [16]. Mutations in OPHN1 are associated with CH, ventriculomegaly, intellectual disability, seizures and mildly dysmorphic facial features that are characteristic in males and occasionally in females [17]. Retinoic acid exposure during early pregnancy has been associated with severe cerebellar vermis hypoplasia with variable effects on the hemispheres. Additional clinical features include microtia, micrognathia, cleft palate, conotruncal heart defects, thymic defects, and retinal abnormalities [18].
4.1.3. Cerebellar hyperplasia
Cerebellar hyperplasia, or macrocerebellum, is a rare neuroimaging finding that occurs in isolation, or is coincident with a variety of neurodevelopmental disorders, including genomic imbalances and specific overgrowth syndromes, including megalencephaly–capillary malformation (MCAP) and megalencephaly–polydactyly–polymicrogyria–hydrocephalus (MPPH) (reviewed by Poretti et al. [19]). Recently, significant progress has been made in understanding the pathophysiology that leads to brain overgrowth in MCAP/MPPH. Clinical features of MCAP/MPPH include seizures, capillary malformations, macrocephaly, and polydactyly, and diagnosis is often based on cerebral cortical overgrowth and polymicrogyria [20,21]. Though cerebellar size is normal at birth, patients often develop a macrocerebellum with normal posterior fossa size. This may progress into cerebellar ectopia/Chiari I malformation, causing clinically associated symptoms (posterior headache, dysphagia, stridor) and hydrocephalus. It is unknown whether the cerebellar overgrowth is a feature of generalized brain overgrowth, or whether there are distinct mechanisms that specifically influence cerebellar overgrowth. Most MCAP and MPPH patients have activating de-novo mutations in PIK3R2 and PIK3CA, respectively, that result in increased cell growth [20,21]. Mutations affecting the PI3K–AKT–mTOR pathway are found in a variety of cancers, opening the possibility of using drugs in development for cancer treatment to reduce brain overgrowth and neurological issues in patients with MCAP/MPPH.
4.1.4. Cerebellar dysplasia
Any part of the cerebellum can be dysplastic, from small focal regions within one hemisphere to abnormal foliation throughout the cerebellum [22]. Hypoplastic cerebella are frequently also dysmorphic, as observed in tubulinopathies and cobblestone malformations. Chudley–McCullough syndrome (CMS; MIM 604213) is an autosomal recessive disorder in which patients have striking disorganization of the inferior cerebellar hemisphere folia and additional brain abnormalities, including frontal polymicrogyria with subcortical heterotopia, corpus callosum hypogenesis, and arachnoid cysts. Clinical presentation includes severe neonatal sensorineural hearing loss and hydrocephalus that may require shunting in some patients. Surprisingly, these patients typically are not dysmorphic, lack additional congenital anomalies, and have relatively mild developmental problems [23]. CMS is caused by biallelic truncating mutations in GPSM2 that encodes a GTPase regulator required for correct orientation of stem cell divisions in multiple tissues [24]. The cerebellar dysplasia present in CMS is likely due to abnormal cell division, but the precise mechanism remains unknown. Poretti–Boltshauser syndrome (PBS; MIM 150320) is characterized by cerebellar dysplasia, cysts, and vermis hypoplasia (Fig. 2E) with and without retinal dystrophy and is caused by mutations in LAMA1 [25,26]. The superior cerebellar peduncles are long in some patients, though their appearance differs from the classic molar-tooth appearance that defines JS. The clinical features of PBS include motor and speech delay with variable cognitive impact [25,26]. Finally, cerebellar dysplasia with cysts is a prominent feature in cobblestone malformations and GPR56-related brain malformations (described below).
4.2. Cerebellar and brainstem malformations
4.2.1. Pontocerebellar hypoplasia
Pontocerebellar hypoplasia (PCH) occurs in several disorders that are distinguishable by neuroimaging findings and associated clinical features (Fig. 2F). Historically, PCH has been divided into 10 numbered types. However, genetic and phenotypic overlap is the rule rather than the exception in PCH, so diagnostic terms that include the genetic cause and the clinical/neuroimaging features are more informative, e.g. “TSEN54-related PCH.” PCH type 1 (MIM 607596 and 614678), associated with mutations in EXOSC3 [27] and VRK1 [28], is characterized by moderate PCH on neuroimaging in combination with spinal muscular atrophy, resulting in substantial global weakness and decreased or absent reflexes. PCH types 2 (MIM 277470, 612389, and 612390), 4 (MIM 225753) and 5 (MIM 610204) have more severe hypoplasia and are caused predominantly by mutations in genes that encode tRNA splicing endonucleases (TSEN54, TSEN34 and TSEN2) [12]. Patients with PCH type 2 represent the less severe end of the spectrum with early hyperreflexia, developmental delay, and feeding problems, eventually developing spasticity and involuntary movements in childhood, whereas patients with PCH type 4 represent the severe end of the spectrum characterized by polyhydramnios, severe hyperreflexia, contractures, and early death due to central respiratory failure. Microcephaly is present at birth in patients with PCH4, whereas microcephaly develops over time in PCH2. Seizures are frequent in both groups. A typical feature of TSEN-related PCH is more severe involvement of the cerebellar hemispheres versus the vermis (Fig. 2D) [29]. PCH type 6 (MIM 611523) is associated with elevated CSF lactate and caused by mutations in RARS2 [30].
PCH types 3 (MIM 608027), 9 (MIM 615809), and 10 (MIM 615803) are distinguishable from other PCH types because they show evidence of progressive atrophy in addition to the characteristic neuroimaging features of PCH. PCH type 3 is associated with optic atrophy and caused by mutations in PCLO, but has only been described in one family [31]. PCH type 9 is characterized by severely delayed psychomotor development, progressive microcephaly, spasticity and seizures and caused by mutations in AMPD2 [32]. PCH type 10 is characterized by slow, progressive, neurodegenerative features or static encephalopathy by six months of age and is caused by mutations in CLP1 [33,34]. Neuroimaging for these patients revealed mild atrophy of the cerebellum, pons, and corpus callosum and progressive microcephaly. Additional clinical features include spasticity, seizures and delayed myelination with occasional dysmorphic features and axonal sensorimotor neuropathy.
Mutations in BRF1 (MIM 616202) were recently identified among patients diagnosed with a cerebellar–facial–dental syndrome and neuroimaging anomalies that resemble TSEN-related PCH [35]. Reported neuroimaging findings include a thin corpus callosum, a flat brainstem, and cerebellar vermis hypoplasia. Clinical features include intellectual disability, short stature, and prenatal microcephaly. BRF1 is a subunit of the RNA polymerase III transcription initiation factor that synthesizes tRNA, providing a molecular link to TSEN-related PCH.
PCH type 8 (MIM 614961) is characterized by severe PCH with proportionate involvement of cerebellar vermis and hemispheres and relative preservation of cerebellar folia and caused by recessive loss-of-function mutations in CHMP1A [36]. Patients have moderate to severe developmental delay, acquired microcephaly, increased extremity tone and contractures. CHMP1A appears to facilitate the repression of CDKN2A expression by the transcriptional repressor BMI1, resulting in decreased proliferation and stem cell renewal. PCH type 7 (MIM 614969) is the only PCH type for which the genetic cause is not yet known.
Mutations in CASK on the X chromosome are associated with PCH. CASK loss-of-function mutations are associated with prenatal or postnatal microcephaly, PCH (Fig. 2H), and severe intellectual disability in females; a more severe presentation occurs in affected males [37]. In contrast, hypomorphic or mosaic mutations in CASK cause a milder phenotype associated with variable intellectual disability with or without nystagmus [38].
Patients with PTF1A-related cerebellar hypoplasia present with neonatal diabetes due to pancreatic agenesis (Fig. 2G) [39]. Published neuroimaging revealed a severely hypoplastic pons, and asymmetric to complete absence of the cerebellum [39]. In mice, loss of Ptf1a causes cell fate misspecification in the cerebellar ventricular zone, which causes some neuroprogenitors to migrate into the brainstem and others to die, resulting in cerebellar agenesis later in development. Asymmetric or complete cerebellar agenesis (Fig. 2I) is also seen in patients with WNT1-related osteogenesis imperfecta [40]. Among patients with WNT1 mutations and severe intellectual and motor deficits, neuroimaging revealed prominent brainstem and cerebellar hypoplasia that ranged from mild hypoplasia to complete cerebellar agenesis, often with left–right asymmetry. This striking asymmetry is most often reported as an isolated anomaly, presumably due to prenatal hemorrhage within the posterior fossa or as part of PHACE syndrome (see below).
Pons and cerebellar hypoplasia with progressive volume loss is characteristic of CDGS (MIM 212065, Fig. 2J). Patients present with hypotonia and developmental delay, but can also display a striking variety of other features including abnormal fat distribution, coagulopathy, retinal degeneration, peripheral neuropathy, stroke-like episodes, and seizures. The range of developmental outcomes is broad. CDG Type1a is autosomal recessive, is caused by biallelic mutations in PMM2, and is required for N-glycosylation of proteins [41]. The identity and role of N-glycosylated proteins during hindbrain development remain under investigation.
Many causes of PCH remain undefined, although atypical presentations of disorders such as VLDLR-related disequilibrium syndrome or pre- and postnatal insults – often due to complications of extreme prematurity – may explain additional subsets of patients [42].
4.2.2. Joubert syndrome/molar tooth malformation
Joubert syndrome is defined by a characteristic brain malformation including: cerebellar vermis hypoplasia or dysplasia; long, thick, elevated superior cerebellar peduncles; a thin midbrain–hindbrain junction; and a deep interpeduncular fossa producing the “molar tooth sign” on axial MRI (Fig. 2K). Patients present with neonatal hypotonia, abnormal eye movements, and alternating apnea and tachypnea. Polydactyly is present in a few patients, and subsets of patients develop retinal dystrophy, nephronophthisis, and liver fibrosis. Additional brain malformations may be present, including polymicrogyria, brainstem and cortical heterotopia, agenesis of the corpus callosum, and/or cephalocele [43]. Diffusion tensor imaging can further show laterally displaced and dysmorphic deep cerebellar nuclei, hypoplastic medial lemnisci, and absent transverse fibers in the central vermis and deficient superior cerebellar peduncle decussation. JS is thought to be a frrequent cause of congenital ataxia, with a prevalence of ~1/80,000 in Northern Europeans [44]. The >30 genes implicated in JS encode proteins that function in and around the primary cilium, the cellular antenna that mediates a variety of signaling processes. JS is one of a new class of disorders called ciliopathies, named for their overlapping clinical features and shared pathophysiology involving cilium dysfunction [45,46]. Brain malformations seen in JS may result from defects in midline fusion of the developing vermis [47], defects in sonic hedgehog-mediated neural tube patterning and cerebellar granule cell proliferation, and abnormal cilium-dependent neuronal migration and axon guidance. Diagnosing JS is important because it is recessive and carries a 25% recurrence risk. Additionally, patients are at risk for progressive retinal, kidney and liver disease, which requires surveillance and treatment to prevent additional complications.
4.2.3. Cobblestone malformations
Cobblestone malformations comprise a broad spectrum of clinically and genetically overlapping disorders resulting from defects in the pial limiting membrane and the attachment of radial glial fibers thereto [48]. The Walker–Warburg phenotype represents the severe end of the spectrum with profoundly small and dysmorphic cerebellar hemispheres, often with cysts, absent vermis and very small brainstem, and other major brain malformations: cobblestone cerebral cortex, abnormal white matter, anomalous corpus callosum, enlarged dysplastic tectum, and hypoplastic/dysplastic brainstem (Fig. 2I). The cerebellar cortical and subcortical cysts represent small areas of pia/subarachnoid space herniating inward through gaps in the pial limiting membrane. Patients frequently have hydrocephalus, various eye abnormalities, seizures, hypotonia, and/or muscular dystrophy (creatine kinase levels 2-fold to 15-fold normal). The muscle–eye–brain phenotype represents more moderately affected patients with milder cerebellar and brainstem hypoplasia (Fig. 2L), and Fukuyama muscular dystrophy represents the mild end of the spectrum that still includes brain malformation. Recessive mutations in multiple genes (POMT1 MIM 607423, POMT2 MIM 607439, POMGNT1 MIM 606822, FKTN MIM 607440, FKRP MIM 606596, LARGE MIM 603590, ISPD MIM 614631, and GTDC2 MIM 614830) may cause overlapping phenotypes across the entire spectrum of disease. Autosomal recessive mutations in GPR56 (MIM 606854) are distinguishable from other cobblestone malformations due to the presence of bilateral frontoparietal polymicrogyria [49]. A substantial proportion of patients with cobblestone malformations remains unexplained.
5. Specific malformations with unknown genetic cause
5.1. Rhombencephalosynapsis
Rhombencephalosynapsis (RES) is a unique cerebellar malformation in which the vermis is deficient or absent and the hemispheres are fused across the midline (Fig. 3A,B). A range of severity is observed, from a small cerebellum with absent vermis and fused deep cerebellar nuclei, to milder forms with partially deficient vermis and cerebellar ectopia (inferior and superior) due to disproportionate size between cerebellum and posterior fossa. RES is often associated with aqueductal stenosis, midline fusion of the colliculi, absent septum pellucidum, and dysplastic corpus callosum. The severity of RES detected on neuroimaging correlates with clinical and developmental outcome [50].
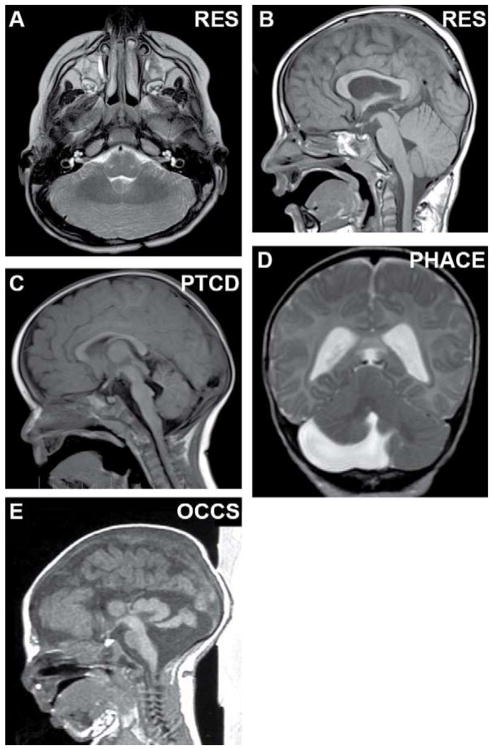
Fig. 3.
Cerebellar malformations without known genetic causes. (A, B) Axial and sagittal views of rhombencephalosynapsis with absent vermis, fusion of the hemispheres, and lack of vermis morphology on sagittal view. (C) Sagittal view of pontine tegmental cap dysplasia with dorsal “cap”, absence of the ventral pons and mild vermis hypoplasia. (D) Coronal view of unilateral cerebellar hypoplasia in PHACE syndrome (Posterior fossa malformations, Hemangioma, Arterial anomalies, Cardiac defects and Eye anomalies). (E) Sagittal view of massively enlarged tectum and vermis hypoplasia with preserved pons in oculocerebrocutaneous syndrome. (B, C) Adapted with permission from Doherty et al. [12].
Patients with RES often display a characteristic figure-of-eight headshaking behavior, hyperkinesis, and impulsivity. Three recognizable categories have been proposed: (i) Gómez–López–Hernández syndrome (MIM 601853) combining RES with scalp alopecia and trigeminal anesthesia; (ii) RES plus features of VACTERL association (Vertebral defects, Anal atresia, Cardiac defects, Tracheo-Esophageal fistula, Renal defects and Limb defects); and (iii) RES with atypical holoprosencephaly, most severely affecting the occipital lobes [50]. Patients have motor delays and a broad range of cognitive function, from profound developmental disability to normal intelligence. Although presumed to be genetic, only one possible recurrence has been reported. Without genetic causes and no animal models to recapitulate RES, the underlying mechanism remains unknown.
5.2. Pontine tegmental cap dysplasia
Pontine tegmental cap dysplasia (PTCD) (MIM 614688) is characterized by cerebellar hypoplasia and absence of the ventral pontine prominence, with a dorsal pons ectopic “cap” of transverse axon pathways (Fig. 3C). Additional neuroimaging findings include hypoplastic or absent inferior cerebellar peduncles, and hypoplastic middle cerebellar peduncles. Patients display multiple cranial nerve deficits including hearing loss, trigeminal anesthesia, facial paralysis, and swallow dysfunction [51]. Some patients also have congenital heart, kidney, vertebral and rib defects. Severely affected patients have a more “beak-like” prominence on the dorsal pons, sometimes with a kinked brainstem. Some patients die early in life, presumably due to apnea, respiratory insufficiency, or aspiration. Neurodevelopmental outcome does not overlap with the typical population, but some patients are ambulatory and can communicate with words, signs, and keyboarding. PTCD is presumed to be genetic. However, no recurrences or genetic causes have been identified.
5.3. PHACE syndrome
PHACE syndrome (Posterior fossa malformations, Hemangioma, Arterial anomalies, Cardiac defects and Eye anomalies) (MIM 606519) is a widespread vascular neurocutaneous disorder. Abnormalities involving many organ systems require multi-disciplinary management. Typically, patients present in the neonatal period with an increasingly apparent segmental head and/or neck hemangioma, and specific criteria have been developed to establish the diagnosis [52]. Neuroimaging has revealed a range of brain malformations in patients with PHACE syndrome, most frequently, unilateral cerebellar hypoplasia (Fig. 3D) on the side of the hemangioma. Other neuroimaging abnormalities include DWM and cerebellar dysplasia [52]. Management includes imaging of the cerebral vasculature to determine the risk for stroke, and propranolol may be used to treat the hemangiomas. PHACE appears to be associated with increased risk of abnormal neurodevelopmental outcome, particularly when neuroimaging abnormalities are present, though limited outcome data have been reported [53]. Nonetheless, many patients do not have severe developmental problems. No familial recurrences of PHACE syndrome have been reported, suggesting de-novo, somatic mosaic, or complex genetic causes.
5.4. Oculocerebrocutaneous syndrome
Oculocerebrocutaneous syndrome (OCCS) (MIM 164180), or Dellman syndrome, is a rare multiple congenital malformation syndrome characterized by eye, brain, and skin anomalies. Clinical features include orbital cysts and anophthalmia or microphthalmia, focal aplastic or hypolpastic skin defects, skin appendages, and brain malformations. Neuroimaging findings include frontal predominant polymicrogyria and periventricular nodular heterotopia, enlarged lateral ventricles or hydrocephalus, agenesis of the corpus callosum, and a characteristic mid-hindbrain malformation [54]. The mid-hindbrain malformation includes an enlarged and dysplastic tectum, cerebellar vermis agenesis, small cerebellar hemispheres, and a large posterior fossa cyst (Fig. 3E). Whereas the eye and skin findings overlap with several other cutaneous syndromes, the pattern of brain malformations is unique. Similar to the other sporadic disorders in this section, OCCS is presumed to be due to de-novo, somatic mosaic, or complex genetic causes.
6. Approach to genetic diagnosis
Accurate diagnosis of patients with cerebellar malformations requires a holistic, team approach. As with all patient evaluations, careful review of history and physical examinations are the first step to generate a differential diagnosis. A high quality brain MRI is essential for diagnosis, which can often be accomplished without sedation in neonates. For straightforward findings, local review of the neuroimaging may be sufficient; however, review by experts is often revealing for difficult-to-diagnose patients. Based on thorough review of the history, examination and neuroimaging by the primary medical team and subspecialist consultants, additional laboratory, genetic and ancillary testing may be indicated to support or exclude specific diagnoses, and to evaluate for associated complications. A detailed review of medical literature, online resources such as GeneReviews, OMIM, and proprietary databases may be helpful.
7. Conclusions
Modern genetic and genomic techniques have revolutionized the diagnosis of cerebellar malformation disorders. Previously, few specific causes were known and genetic testing was not highly informative. Now, the genetic cause can be identified in many patients with distinct cerebellar malformations. Powerful techniques can detect a broad spectrum of variants, from single nucleotide changes to large genomic imbalances. We are now transitioning from targeted single-gene or panel testing to sequencing the majority of coding DNA (the exome) or even the whole genome. Whole genome sequencing can potentially detect most single nucleotide changes and genomic imbalances, resulting in a high yield using a single test. To maximize the utility of this type of testing, progress must be made in our understanding of normal human genetic variation and in our ability to identify DNA variants associated with disease. Extensive functional analysis of sequence changes is further required to understand their biological impact, as well as empiric phenotyping work to correlate genetic variation with clinical features.
Specific genetic diagnosis in a fetus or neonate with a cerebellar malformation is improving care for patients and their families, by providing: (i) diagnostic, carrier and prenatal testing; (ii) more accurate prognostic and recurrence risk information; (iii) avoidance of additional, unnecessary diagnostic testing; (iv) early diagnosis of associated complications through medical monitoring; (v) reduction in stress caused by diagnostic uncertainty; and (vi) relief of parental guilt and anxiety for causing their child’s disability. Defining molecular pathways underlying normal and abnormal human cerebellar development is critical toward developing specific therapies. It is uncertain whether early developmental brain anomalies can be corrected, but many gene products required for brain development are later required for continuing brain function, so interventions targeting the affected pathways may be possible. For example, loss-of-function JS genes disrupt protein localization to the primary cilium where several neurotransmitter receptors are normally present, likely contributing to the intellectual disability, behavior problems, and mental health issues experienced by these patients. In the future, pharmacological treatments may be able to address ongoing brain dysfunction caused by ciliary receptor mislocalization.
Beyond direct benefits to patients with cerebellar malformations, understanding these disorders may inform other areas of human health and disease. Developing treatments for neurological dysfunction caused by trauma, infection, and neurodegenerative conditions depends on a solid understanding of human cerebellar development and function. Similarly, successful treatment of oculomotor, vestibular, and respiratory control dysfunction will benefit from basic knowledge about how these neurological systems develop in humans. In addition to functions typically assigned to the cerebellum, mounting evidence indicates that the cerebellum plays a role in autism, mental health, and cognitive disorders. Though recent progress in understanding normal and abnormal human cerebellar development has been remarkable, further advances in neuroimaging, genetics, and animal modeling foretell continued progress in the future.
Practice points.
Distinguishing different cerebellar malformation conditions is important for prognostic and recurrence risk counseling, medical monitoring for complications, and treatment decisions.
Diagnosis requires a multidisciplinary team approach including perinatologists, neonatologists, neurologists, and geneticists.
Cerebellar malformations are often caused by de-novo dominant mutations as well as autosomal recessive and X-linked mechanisms.
Cerebellar malformation conditions have overlapping neuroimaging and clinical phenotypes.
Research directions.
-
Bench research:
identify the genetic causes of “unsolved” disorders;
dissect underlying mechanisms of genetic and non-genetic malformations;
develop mechanism-specific treatments.
-
Clinical research:
identify environmental causes of “unsolved” disorders;
delineate the phenotypic spectrum (i.e. natural history) associated with specific genetic causes;
test gene-specific treatments of the future.
Acknowledgments
Funding source
Support for brain malformation research from NIH to D.D. (R01NS064077).
Footnotes
Conflict of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Long A, Moran P, Robson S. Outcome of fetal cerebral posterior fossa anomalies. Prenat Diagn. 2006;26:707–10. doi: 10.1002/pd.1485. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch J-F, Pierre-Kahn A, Renier D, Sainte-Rose C, Hoppe-Hirsch E. The Dandy–Walker malformation: a review of 40 cases. J Neurosurg. 1984;61:515–22. doi: 10.3171/jns.1984.61.3.0515. [DOI] [PubMed] [Google Scholar]
- 3.Osenbach RK, Menezes AH. Diagnosis and management of the Dandy–Walker malformation: 30 years of experience. Pediatr Neurosurg. 1992;18:179–89. doi: 10.1159/000120660. [DOI] [PubMed] [Google Scholar]
- 4.Murray JC, Johnson JA, Bird TD. Dandy–Walker malformation: etiologic heterogeneity and empiric recurrence risks. Clin Genet. 1985;28:272–83. doi: 10.1111/j.1399-0004.1985.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 5.Grinberg I, Northrup H, Ardinger H, Prasad C, Dobyns WB, Millen KJ. Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy–Walker malformation. Nat Genet. 2004;36:1053–5. doi: 10.1038/ng1420. [DOI] [PubMed] [Google Scholar]
- 6.Aldinger KA, Lehmann OJ, Hudgins L, et al. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25. 3 Dandy–Walker malformation. Nat Genet. 2009;41:1037–42. doi: 10.1038/ng.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbro BW, Mahajan VB, Gakhar L, et al. Mutations in extracellular matrix genes NID1 and LAMC1 cause autosomal dominant Dandy–Walker malformation and occipital cephaloceles. Hum Mutat. 2013;34:1075–9. doi: 10.1002/humu.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolanczyk M, Krawitz P, Hecht J, et al. Missense variant in CCDC22 causes X-linked recessive intellectual disability with features of Ritscher–Schinzel/3C syndrome. Eur J Hum Genet. 2015;23:633–8. doi: 10.1038/ejhg.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichiecchio A, Decio A, Di Perri C, Parazzini C, Rossi A, Signorini S. “Acquired” Dandy–Walker malformation and cerebellar hemorrhage: usefulness of serial MRI. Eur J Paediatr Neurol. 2016;20:188–91. doi: 10.1016/j.ejpn.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 10*.Hong SE, Shugart YY, Huang DT, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26:93–6. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- 11.Boycott KM, Bonnemann C, Herz J, et al. Mutations in VLDLR as a cause for autosomal recessive cerebellar ataxia with mental retardation (dysequilibrium syndrome) J Child Neurol. 2009;24:1310–15. doi: 10.1177/0883073809332696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Doherty D, Millen KJ, Barkovich AJ. Midbrain and hindbrain malformations: advances in clinical diagnosis, imaging, and genetics. Lancet Neurol. 2013;12:381–93. doi: 10.1016/S1474-4422(13)70024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahi-Buisson N, Poirier K, Fourniol F, et al. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014;137:1676–1700. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- 14.Oegema R, Cushion TD, Phelps IG, et al. Recognizable cerebellar dysplasia associated with mutations in multiple tubulin genes. Hum Mol Genet. 2015;24:5313–25. doi: 10.1093/hmg/ddv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vissers LE, van Ravenswaaij CM, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955–7. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- 16.Yu T, Meiners LC, Danielsen K, et al. Deregulated FGF and homeotic gene expression underlies cerebellar vermis hypoplasia in CHARGE syndrome. Elife. 2013;2:e01305. doi: 10.7554/eLife.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philip N, Chabrol B, Lossi AM, et al. Mutations in the oligophrenin-1 gene (OPHN1) cause X linked congenital cerebellar hypoplasia. J Med Genet. 2003;40:441–6. doi: 10.1136/jmg.40.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammer EJ, Chen DT, Hoar RM, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–41. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- 19.Poretti A, Mall V, Smitka M, et al. Macrocerebellum: significance and pathogenic considerations. Cerebellum. 2012;11:1026–36. doi: 10.1007/s12311-012-0379-1. [DOI] [PubMed] [Google Scholar]
- 20.Riviere JB, Mirzaa GM, O’Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44:934–40. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K–AKT3–mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–5. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demaerel P. Abnormalities of cerebellar foliation and fissuration: classification, neurogenetics and clinicoradiological correlations. Neuroradiology. 2002;44:639–46. doi: 10.1007/s00234-002-0783-1. [DOI] [PubMed] [Google Scholar]
- 23.Chudley AE, McCullough C, McCullough DW. Bilateral sensorineural deafness and hydrocephalus due to foramen of Monro obstruction in sibs: a newly described autosomal recessive disorder. Am J Med Genet. 1997;68:350–6. doi: 10.1002/(sici)1096-8628(19970131)68:3<350::aid-ajmg19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24*.Doherty D, Chudley AE, Coghlan G, et al. GPSM2 mutations cause the brain malformations and hearing loss in Chudley–McCullough syndrome. Am J Hum Genet. 2012;90:1088–93. doi: 10.1016/j.ajhg.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poretti A, Hausler M, von Moers A, et al. Ataxia, intellectual disability, and ocular apraxia with cerebellar cysts: a new disease? Cerebellum. 2014;13:79–88. doi: 10.1007/s12311-013-0521-8. [DOI] [PubMed] [Google Scholar]
- 26.Aldinger KA, Mosca SJ, Tetreault M, et al. Mutations in LAMA1 cause cerebellar dysplasia and cysts with and without retinal dystrophy. Am J Hum Genet. 2014;95:227–34. doi: 10.1016/j.ajhg.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan J, Yourshaw M, Mamsa H, et al. Mutations in the RNA exosome component gene EXOSC3 cause pontocerebellar hypoplasia and spinal motor neuron degeneration. Nat Genet. 2012;44:704–8. doi: 10.1038/ng.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renbaum P, Kellerman E, Jaron R, et al. Spinal muscular atrophy with pontocerebellar hypoplasia is caused by a mutation in the VRK1 gene. Am J Hum Genet. 2009;85:281–9. doi: 10.1016/j.ajhg.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Budde BS, Namavar Y, Barth PG, et al. tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Nat Genet. 2008;40:1113–18. doi: 10.1038/ng.204. [DOI] [PubMed] [Google Scholar]
- 30.Edvardson S, Shaag A, Kolesnikova O, et al. Deleterious mutation in the mitochondrial arginyl-transfer RNA synthetase gene is associated with pontocerebellar hypoplasia. Am J Hum Genet. 2007;81:857–62. doi: 10.1086/521227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed MY, Chioza BA, Rajab A, et al. Loss of PCLO function underlies pontocerebellar hypoplasia type III. Neurology. 2015;84:1745–50. doi: 10.1212/WNL.0000000000001523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akizu N, Cantagrel V, Schroth J, et al. AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell. 2013;154:505–17. doi: 10.1016/j.cell.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaffer AE, Eggens VR, Caglayan AO, et al. CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell. 2014;157:651–63. doi: 10.1016/j.cell.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karaca E, Weitzer S, Pehlivan D, et al. Human CLP1 mutations alter tRNA biogenesis, affecting both peripheral and central nervous system function. Cell. 2014;157:636–50. doi: 10.1016/j.cell.2014.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borck G, Hog F, Dentici ML, et al. BRF1 mutations alter RNA polymerase III-dependent transcription and cause neurodevelopmental anomalies. Genome Res. 2015;25:155–66. doi: 10.1101/gr.176925.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mochida GH, Ganesh VS, de Michelena MI, et al. CHMP1A encodes an essential regulator of BMI1-INK4A in cerebellar development. Nat Genet. 2012;44:1260–4. doi: 10.1038/ng.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Najm J, Horn D, Wimplinger I, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008;40:1065–7. doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- 38.Moog U, Kutsche K, Kortum F, et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet. 2011;48:741–51. doi: 10.1136/jmedgenet-2011-100218. [DOI] [PubMed] [Google Scholar]
- 39.Sellick GS, Barker KT, Stolte-Dijkstra I, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–5. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- 40.Aldinger KA, Mendelsohn NJ, Chung BH, et al. Variable brain phenotype primarily affects the brainstem and cerebellum in patients with osteogenesis imperfecta caused by recessive WNT1 mutations. J Med Genet. 2015 Dec 15; doi: 10.1136/jmedgenet-2015-103476. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Matthijs G, Schollen E, Pardon E, et al. Mutations in PMM2, a phosphomannomutase gene on chromosome 16p13, in carbohydrate-deficient glycoprotein type 1 syndrome (Jaeken syndrome) Nat Genet. 1997;16:88–92. doi: 10.1038/ng0597-88. [DOI] [PubMed] [Google Scholar]
- 42.Messerschmidt A, Brugger PC, Boltshauser E, et al. Disruption of cerebellar development: potential complication of extreme prematurity. Am J Neuroradiol. 2005;26:1659–67. [PMC free article] [PubMed] [Google Scholar]
- 43.Poretti A, Huisman TA, Scheer I, Boltshauser E. Joubert syndrome and related disorders: spectrum of neuroimaging findings in 75 patients. Am J Neuroradiol. 2011;32:1459–63. doi: 10.3174/ajnr.A2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroes HY, Fransen van de Putte DE, Ravesloot CJ, Lindhout D. The birth prevalence of Joubert syndrome: a population based study in the Netherlands. Eur J Hum Genet. 2007;15:68. [Google Scholar]
- 45*.Romani M, Micalizzi A, Valente EM. Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet Neurol. 2013;12:894–905. doi: 10.1016/S1474-4422(13)70136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parisi M, Glass I. GeneReviews. University of Washington; Seattle: 1993. Joubert syndrome and related disorders. [PubMed] [Google Scholar]
- 47.Lancaster MA, Gopal DJ, Kim J, et al. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat Med. 2011;17:726–31. doi: 10.1038/nm.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godfrey C, Clement E, Mein R, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130:2725–35. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- 49.Piao X, Hill RS, Bodell A, et al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–6. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- 50*.Ishak GE, Dempsey JC, Shaw DW, et al. Rhombencephalosynapsis: a hindbrain malformation associated with incomplete separation of midbrain and forebrain, hydrocephalus and a broad spectrum of severity. Brain. 2012;135:1370–86. doi: 10.1093/brain/aws065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Barth PG, Majoie CB, Caan MW, et al. Pontine tegmental cap dysplasia: a novel brain malformation with a defect in axonal guidance. Brain. 2007;130:2258–66. doi: 10.1093/brain/awm188. [DOI] [PubMed] [Google Scholar]
- 52.Metry D, Heyer G, Hess C, et al. Consensus Statement on Diagnostic Criteria for PHACE Syndrome. Pediatrics. 2009;124:1447–56. doi: 10.1542/peds.2009-0082. [DOI] [PubMed] [Google Scholar]
- 53.Tangtiphaiboontana J, Hess CP, Bayer M, et al. Neurodevelopmental abnormalities in children with PHACE syndrome. J Child Neurol. 2013;28:608–14. doi: 10.1177/0883073812450073. [DOI] [PubMed] [Google Scholar]
- 54.Moog U, Jones MC, Bird LM, Dobyns WB. Oculocerebrocutaneous syndrome: the brain malformation defines a core phenotype. J Med Genet. 2005;42:913–21. doi: 10.1136/jmg.2005.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]