Abstract
Background and aims
Atherosclerosis is a major cause of heart attack and stroke. Inflammation plays a critical role in the development of atherosclerosis. Since the cytoplasmic adaptor molecule TRAF3IP2 (TRAF3-Interacting Protein 2) plays a causal role in various autoimmune and inflammatory diseases, we hypothesized that TRAF3IP2 mediates atherosclerotic plaque development.
Methods
TRAF3IP2/ApoE double knockout (DKO) mice were generated by crossing TRAF3IP2−/− and ApoE−/− mice. ApoE−/− mice served as controls. Both DKO and control mice were fed a high-fat diet for 12 weeks. Plasma lipids were measured by ELISA, atherosclerosis by en face analysis of aorta and plaque cross-section measurements at the aortic valve region, plaque necrotic core area, collagen and smooth muscle cell content by histomorphometry, and aortic gene expression by RT-qPCR.
Results
The plasma lipoprotein profile was not altered by TRAF3IP2 gene deletion in ApoE−/− mice. While total aortic plaque area was decreased in DKO female, but not male mice, the plaque necrotic area was significantly decreased in DKO mice of both genders. Plaque collagen and smooth muscle cell contents were increased significantly in both female and male DKO mice compared to respective controls. Aortic expression of proinflammatory cytokine (Tumor necrosis factor α, TNFα), chemokine (Chemokine (C-X-C motif) Ligand 1, CXCL1) and adhesion molecule (Vascular cell adhesion molecule 1, VCAM1; and Intercellular adhesion molecule 1, ICAM1) gene expression were decreased in both male and female DKO mice. In addition, the male DKO mice showed a markedly reduced expression of extracellular matrix (ECM)-related genes, including TIMP1 (Tissue inhibitor of metalloproteinase 1), RECK (Reversion-Inducing- Cysteine-Rich Protein with Kazal Motifs) and ADAM17 (A Disintegrin And Metalloproteinase 17).
Conclusions
TRAF3IP2 plays a causal role in atherosclerotic plaque development and vulnerability, possibly by inducing the expression of multiple proinflammatory mediators. TRAF3IP2 could be a potential therapeutic target in atherosclerotic vascular diseases.
Introduction
Atherosclerosis is an important contributing factor for cardiovascular diseases such as heart attack and stroke (1). It is one of the major leading causes of death in Western countries and its incidence is increasing alarmingly in developing countries (1). Atherosclerosis results in gradual narrowing of arterial lumen due to lipid accumulation and infiltration of immune cells into the subintimal space, and involves complex cellular and molecular interactions, leading to plaque development, rupture, and thrombosis (3). Although the major stages involved in atherosclerotic plaque development and stability have been well described, the molecular mechanisms underlying its pathogenesis are not fully understood. Identifying newer molecules and determining their role in atherosclerosis development and progression will help us better understand the disease process and the development of novel therapeutic strategies.
TRAF3-Interacting Protein 2 (TRAF3IP2) is a cytoplasmic adaptor molecule and activator of the transcription factors nuclear factor κB (NF-κB) and activator protein-1 (AP-1) in I kappa B kinase (IKK)- and c-Jun N-terminal kinase (JNK)-dependent manner (20, 21). NF-κB and AP-1 regulate the expression of various pro-atherogenic mediators, including proinflammatory cytokines, chemokines, adhesion molecules and ECM degrading MMPs, that play critical roles in atherosclerotic plaque formation and progression (30).
The major cell types involved in atherosclerotic plaque formation (e.g., endothelial cells, smooth muscle cells, and macrophages) express TRAF3IP2 (39–44). High glucose and ox-LDL induce TRAF3IP2 expression and endothelial dysfunction in vitro (40, 44). Angiotensin II (AngII), aldosterone, interleukin-18 (IL-18), and advanced oxidation protein products (AOPPs) that play a role in atherogensis induce TRAF3IP2 expression in vitro (34, 39, 41–43), suggesting that TRAF3IP2 could play a role in atherosclerosis. Therefore, we hypothesized that TRAF3IP2 mediates atherosclerotic plaque development and progression.
Materials and methods
Generation of TRAF3IP2/ApoE double knockout (DKO) mice
All animal studies were approved by the Institutional Animal Care and Use Committee at Tulane University in New Orleans, LA, and conformed to the Guide for the Care and Use of Laboratory Animals published by the NIH. TRAF3IP2−/− mice (C57Bl/6 background) were previously described (8). ApoE−/− mice (C57Bl/6 background) were purchased from The Jackson Laboratory (Ann Harbor, ME). TRAF3IP2 and ApoE heterozygous mice (TRAF3IP2+/−/ApoE+/−) were generated by crossing TRAF3IP2−/− and ApoE−/− mice, and the double knockout mice (TRAF3IP2−/−/ApoE−/−; DKO) by intercrossing TRAF3IP2+/−/ApoE+/− mice. Lack of TRAF3IP2 expression in aortas of DKO mice was confirmed by RT-qPCR (Table 2).
Table 2.
Gene expression in aortas from ApoE−/− and DKO mice fed a Western diet
| Target gene | ApoE−/− female | DKO female | ApoE−/− male | DKO male |
|---|---|---|---|---|
| IL-6 | 1.00±0.45 | 1.37±0.80 | 1.00±0.44 | 0.08±0.04 |
| IL-18 | 1.00±0.60 | 0.87±0.44 | 1.00±0.34 | 1.269±0.23 |
| TNFα | 1.00±0.64 | 0.18±0.13 | 1.00±0.60 | 0.30±0.06 |
| ICAM1 | 1.00±0.25 | 0.28±0.18 | 1.00±0.23 | 0.15±0.04* |
| VCAM1 | 1.00±0.26 | 0.29±0.15 | 1.00±0.23 | 0.57±0.27 |
| MCP1 | 1.00±0.25 | 0.99±0.72 | 1.00±0.05 | 0.73±0.33 |
| CXCL1 | 1.00±0.09 | 0.32±0.17* | 1.00±0.33 | 0.20±0.07* |
| AT1A | 1.00±0.32 | 0.85±0.47 | 1.00±0.69 | 0.23±0.07 |
| AT2 | 1.00±0.13 | 0.33±0.24 | 1.00±0.88 | 0.42±0.15 |
| MMP2 | 1.00±0.33 | 0.64±0.40 | 1.00±0.42 | 0.31±0.12 |
| MMP9 | 1.00±0.14 | 5.84±2.90 | 1.00±0.33 | 2.00±1.47 |
| MMP14 | 1.00±0.53 | 0.52±0.38 | 1.00±0.33 | 0.66±0.16 |
| TIMP1 | 1.00±0.11 | 0.53±0.57 | 1.00±0.10 | 0.40±0.07* |
| TRAF3IP2 | 1.00±0.34 | - | 1.00±0.23 | - |
| RECK | 1.00±0.40 | 0.73±0.31 | 1.00±0.13 | 0.25±0.05* |
| ADAM10 | 1.00±0.82 | 7.40±5.20 | 1.00±0.053 | 1.00±0.25 |
| ADAM17 | 1.00±0.25 | 0.57±0.37 | 1.00±0.06 | 0.23±0.04* |
IL-6, interleukin-6; IL-18, interleukin-18; TNFα, tumor necrosis factor α; ICAM1, intercellular adhesion molecule 1; VCAM1, vascular cell adhesion molecule; AT1A, angiotensin II receptor, type 1a; AT2, angiotensin II receptor, type 2; MMP, matrix metalloproteinase; TIMP1, tissue inhibitor of matrix metalloproteinase 1; TRAF3IP2, TRAF3-Interacting Protein 2; RECK, reversion-inducing cysteine-rich protein with kazal motifs; ADAM, a disintegrin and metalloproteinase. 18S rRNA served as the endogenous invariant control, and relative gene expression in ApoE−/− female mice were taken as ‘1’. DKO; double knockout mice (TRAF3IP2−/−/ApoE−/−). Values are mean ± SEM (n = 3–5).
indicates significance at p<0.05 when compared between ApoE−/− and DKO mice belonging to the same gender. Statistical significance was calculated by Student’s t-test.
Genotyping
A genomic polymerase chain reaction (PCR) was used to identify the wild-type and mutant alleles of TRAF3IP2 (WT product: 250bp; mutant product: 453bp) and ApoE (WT product: 145bp; mutant product: 250bp) (Supplemental Figure 1). TRAF3IP2: primer 1- 5’-CTGGCATGTTTTCTCTTGTTTC-3’; primer 2: 5’-GCCTTCTAAAGAAACTGGCTTC-3’; primer 3: 5’-CAATCATGTGTTCAGTCAGC-3’; primer 4: 5’-GCTCTATGGCTTCTGAGG-3’. The PCR conditions were as follows: denaturation at 95°C for 30 s, annealing at 55°C for 45 s, and extension at 72°C for 30 s. ApoE: primer 1: 5’-GCCTAGCCGAGGGAGAGCCG-3’; primer 2: 5’-TGTGACTTGGGAGCTCTGCAGC-3’; primer 3: 5’-GCCGCCCCGACTGCATCT-3’. PCR conditions were as follows: denaturation at 94°C for 30 s, annealing at 68°C for 40 s, and extension at 72°C for 60 s.
Diet
Eight week-old male and female DKO and ApoE−/− mice (n=10–15/groups) were fed a Western diet (42% of total calories from fat; 0.15% cholesterol; #TD88137, Harlan-Teklad diets) for twelve weeks. Body weights were recorded weekly and blood was collected by cardiac puncture prior to euthanasia.
Atherosclerosis quantification
Mice were anesthetized and perfused initially with saline and then with 4% paraformaldehyde plus 5% sucrose. Heart and entire aorta were dissected and fixed overnight in 4% paraformaldehyde plus 5% sucrose. Atherosclerosis was quantified by en face analysis of aorta and determination of plaque cross-sectional area at the aortic root. For en face analysis, adventitial fat was removed from the aorta and stained with Oil Red O and opened longitudinally, pinned en face and photographed. The total arterial surface area and total plaque were determined by Image-Pro PLUS v 6.0 software (Media Cybernetics Inc.). The extent of lesion development was determined as a percentage of the total area of the aorta that was occupied by Oil Red O-positive atherosclerotic lesions. For determination of plaque cross-sectional area, serial 6μm-thick sections from the entire aortic root area were stained with hematoxylin and eosin (H&E). Plaque cross-sectional area was determined by quantifying the plaque area in images (DP70 digital camera) using Image-Pro PLUS software. The mean value of plaque cross-sectional areas from 3 sections was used to estimate the extent of atherosclerosis in each animal.
Features of plaque stability
The necrotic core area was determined in aortic valve plaques by quantifying acellular area (H&E negative), which also contains cholesterol crystals. Aortic valve sections were stained with Masson’s trichrome, photographed and collagen positive area was quantified. Smooth muscle cell content in plaques was determined by immunohistochemistry of aortic valve sections. Sections were incubated with mouse alpha-smooth muscle actin (αSMA)-antibody (Millipore) or isotype-matched mouse IgG (Abcam), followed by secondary antibody and Avidin, Alexa Flour® 488 conjugate (ThermoFisher). Nuclei were stained with DAPI. Images were taken using a fluorescent microscope and αSMA-positive area in the plaques was quantified.
Quantitative real-time RT-qPCR
Mice were anesthetized and perfused with saline, the whole aorta was dissected and cleaned in RNAlater® (ThermoFisher) and stored at −80°C until further use. Total RNA was isolated using Trizol reagent (Sigma). Total RNA (0.5 μg) was reverse transcribed into cDNA using a reverse transcription kit. Collagen, type I, α1 (ColIα1; Assay ID: Mm00801666), Collagen, type III, α1 (ColIIIα1; Assay ID: Mm1254476), Matrix metalloproteinase 2 (MMP2; Assay ID: Mm01253621), Matrix metalloproteinase 9 (MMP9; Assay ID: mm00600163), Matrix metalloproteinase (MMP14; Assay ID: Mm01318969), Tissue inhibitor of metalloproteinase 1 (TIMP1, Assay ID: Mm00441818), Reversion-Inducing-Cysteine-Rich Protein with Kazal Motifs (RECK, Assay ID: Mm01342144), A disintegrin and metalloproteinase domain 10 (ADAM10, Assay ID: Mm00545742), ADAM17 (Assay ID: Mm00456428), Tumor necrosis factor α (TNFα, Assay ID: Mm00443258), Interleukin-6 (IL-6, Assay ID: Mm00446191), IL-18 (Assay ID: Mm00434226), Intercellular adhesion molecule (ICAM1, Assay ID: Mm01175876), Vascular cell adhesion molecule 1 (VCAM1, Assay ID: Mm00449197), Monocyte chemoattractant protein 1 (MCP 1, Assay ID: Mm00441242), Chemokine (C-X-C) ligand 1 ( CXCL1, Assay ID: Mm00433859), Angiotensin II receptor, type 1 (AGTR1A, Assay ID: Mm01957722) and Angiotensin II receptor, Type 2 (AGTR2; Assay ID: Mm01341373) mRNA levels were determined by RT-qPCR using TaqMan® probes (Applied Biosystems). Data were analyzed using the 2−ΔΔ Ct method. 18S (Assay ID. Hs99999901) served as the endogenous invariant control, and all data were normalized to corresponding 18S levels.
Statistical analysis
All data are expressed as mean ± SEM. Statistical significance was determined by student’s t-test. Differences are considered significant if the p value is less than or equal to 0.05.
Results
TRAF3IP2 gene deletion does not alter body weight gain and plasma lipid profile in ApoE−/− mice
Initial body weights were similar between ApoE−/− and DKO male mice, whereas DKO female mice had higher body weights compared to ApoE−/− female mice (20.67±0.49g vs. 18.2±0.28; p <0.01) (Supplemental Figure 2). Increases in body weight were similar between gender-matched ApoE−/− and DKO mice during the twelve-week Western diet feeding (5.82±0.39g vs. 5.46±0.65g with p >0.05 for females; 10.20±1.15g vs. 10.70±0.76g with p >0.05 for males) (Supplemental Figure 2). Plasma triglycerides, total cholesterol, VLDL/LDL cholesterol, and HDL cholesterol levels were similar in the gender-matched ApoE−/− and DKO mice (Table 1), though DKO male mice showed a strong trend towards an increase in plasma HDL cholesterol levels (56.7±16.7 vs. 26.7±9.8; p <0.10) (Table 1).
Table 1.
Plasma lipid profile of ApoE−/− and DKO mice fed a Western diet
| ApoE− female | DKO female | ApoE−/− male | DKO male | |
|---|---|---|---|---|
| Triglycerides (mg/dL) | 97.0±19.2 | 114.6±40.4 | 96.69±39.23 | 205.2±40.1 |
| Total cholesterol (mg/dL) | 1502±142 | 1748±127 | 2048±207 | 2070±183 |
| VLDL/LDL cholesterol (mg/dL) | 1189±152 | 1161±79.4 | 1292±99.0 | 1030±140 |
| HDL cholesterol (mg/dL) | 23.5±6.50 | 21.5±6.00 | 26.7±9.8 | 56.7±16.7 |
Values are represented in mean ± SEM; n=4–7 mice/group. DKO, double knockout mice (TRAF3IP2−/−/ApoE−/−). Comparisons were made between ApoE−/− and DKO mice belonging to the same gender. Statistical significance was calculated by Student’s t-test.
TRAF3IP2 gene deletion reduced atherosclerosis in ApoE−/− female mice, but not in male mice
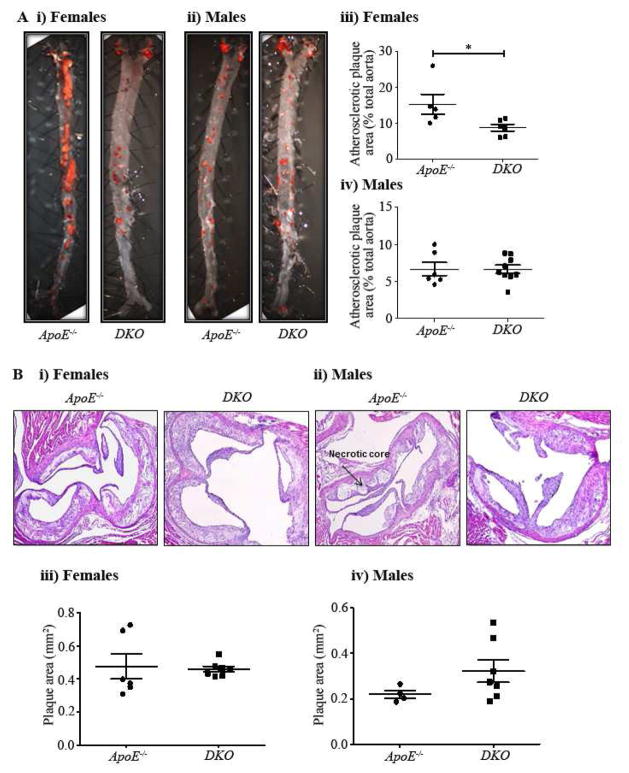
En face analysis of aorta revealed that DKO females had 42.8% reduced total plaque area when compared to ApoE−/− females after twelve weeks on a Western diet (5.2±2.8% vs 8.72±0.93%; p <0.05; Figure 1Ai & iii), whereas no such changes were observed in the DKO male mice (Figure 1Aii & iv). Morphometric analysis of aortic root plaque revealed no significant change in plaque cross-sectional area between the DKO mice of both genders compared to their controls (Figure 1Bi–iv).
Fig. 1. Total aortic plaque burden and aortic root plaque cross-sectional area in ApoE−/− and DKO mice fed a Western diet.
A(i), Representative Oil Red O-stained en face aortas from ApoE−/− and DKO female mice. A(ii), Representative Oil Red O-stained en face aortas from ApoE−/− and DKO male mice. A(iii), Quantitative assessment of plaque area in ApoE−/− and DKO female mice. A(iv), Quantitative assessment of plaque area in ApoE−/− and DKO male mice. B(i), Representative aortic root cross sections from ApoE−/− and DKO female mice (magnification X100). B(ii). Representative aortic root cross sections from ApoE−/− and DKO male mice (magnification X100). B(iii). Quantitative assessment of plaque area in ApoE−/− and DKO female mice. B(iv). Quantitative assessment of plaque area in ApoE−/− and DKO male mice. Values are mean ± SEM. Statistical significance was calculated by Student’s t-test (*p <0.05).
TRAF3IP2 gene deletion decreased plaque vulnerability in both ApoE−/− female and male mice, as evidenced by increased plaque stability features
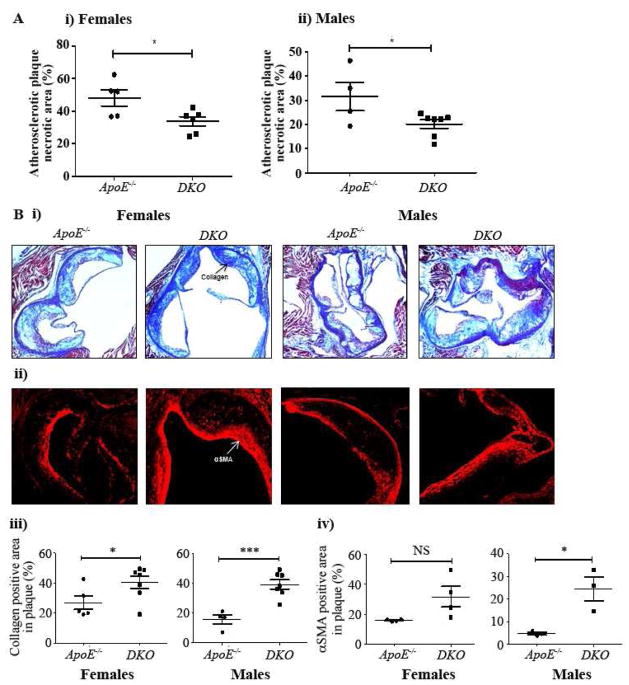
H&E staining of aortic root plaques sections revealed 30% decrease in necrotic area in the Western diet-fed DKO female mice when compared with ApoE−/− female mice (33.66±2.86% vs. 48.08±4.96%; p <0.05; Figure 2Ai). Similarly, DKO male mice showed 36% decrease in the necrotic core area in aortic root plaques when compared to that of ApoE−/− male mice (20.19±1.8% vs. 31.57±5.89%; p <0.05; Figure 2Aii).
Fig. 2. Necrotic core, collagen-positive and smooth muscle cell-positive areas in aortic root valve plaques in ApoE−/− and DKO mice fed a Western diet.
A(i), Quantitative assessment of the aortic root plaque necrotic area in ApoE−/− and DKO female mice. A (ii). Quantitative assessment of the aortic root plaque necrotic area in ApoE−/− and DKO male mice. B (i). Representative aortic root cross sections stained with Masson’s trichrome from ApoE−/− and DKO female and male mice. B(ii), Representative aortic root cross sections for αSMA of ApoE−/− and DKO female and male mice (magnification X200). B(iii), Quantitative assessment of collagen- positive area in ApoE−/− and DKO female and male mice. B(iv), Quantitative assessment of α SMA-positive area in ApoE−/− and DKO female and male mice. Values are mean ± SEM. Statistical significance was calculated by Student’s t-test (*p <0.05, *** p <0.001). NS, not significant.
Masson’s trichrome staining of aortic root plaques revealed a significant 49.9% increase in collagen content in DKO female mice when compared to that of ApoE−/− female mice (40.35±4.1% vs. 26.91±4.5%; p <0.05; Figure 2Bi & iii), whereas a 148.6% increase was observed in DKO male mice when compared to that of ApoE−/− males (39.26±3.1% vs. 15.79±3.0%; p <0.01; Figure 2B i & iii).
Immunohistochemistry for smooth muscle cell content in aortic root sections revealed a strong trend towards an increase in αSMA-positive SMC area in atherosclerotic lesions in DKO females compared to ApoE−/− female mice (28.14±6.4% vs. 13.89±2.12%; p <0.10; Figure 2B ii & iv). The αSMA-positive area in atherosclerotic lesions was increased significantly by 419.5% in DKO males compared to that of ApoE−/− males (24.73±5.3% vs 4.76±0.65%; p <0.05; Figure 2B ii & iv).
TRAF3IP2 gene deletion decreased aortic expression of proinflammatory cytokines, chemokines and adhesion molecules in ApoE−/− mice
CXCL1 mRNA levels were significantly decreased by 62% in aortas of DKO female mice compared to that of ApoE−/− female mice (Table 2), whereas a trend towards decrease was observed in TNFα, ICAM1, VCAM1 and AT2 gene expression (Table 2). ICAM1 and CXCL1 mRNA levels were decreased significantly by 85% and 80% respectively in aortas of Western diet-fed DKO male mice compared to that of ApoE−/− male mice, whereas a trend towards decrease was observed in IL-6, TNFα, VCAM1 and AT1A gene expression (Table 2).
TRAF3IP2 gene deletion altered aortic mRNA levels of ECM-related proteins in ApoE−/− mice
Gene expressions of TIMP1, RECK and ADAM17 were significantly decreased by 60%, 75%, and 76%, respectively, in aortas of DKO male mice compared to that of ApoE−/− male mice (Table 2). No significant changes were observed in gene expression of ECM-related proteins in aortas of DKO females, except for TIMP1 which showed a strong trend towards decrease, and MMP9 and ADAM10 showed a trend towards increase when compared to ApoE−/− female mice (Table 2).
Discussion
TRAF3IP2 was initially discovered as an upstream activator of NF-κB and AP-1 transcription factors (20, 21). It plays a critical role in IL-17 signaling, as it directly interacts with the IL-17 receptor to activate various downstream inflammatory pathways (7). TRAF3IP2 ubiquitinates the bound TNF receptor-associated factor 6 (TRAF6) and activates IKK, resulting in IκB phosphorylation, degradation and nuclear translocation of NF-κB (22). TRAF3IP2 also activates AP-1 via IKKγ/JNK interaction (39). Its activation increases the stability of mRNAs encoding chemokines like CXCL1 through TRAF2/TRAF5 complex (35). Our previous studies showed that inflammatory mediators such as IL-18, AngII, aldosterone, AOPPs, ox-LDL and ROS induce TRAF3IP2 expression, which in turn activates NF-κB and AP-1, and induction of proinflammatory cytokines in various cell types of the cardiovascular system (34, 39, 41–43). Previous studies from our laboratory have shown that silencing TRAF3IP2 by siRNA and shRNA decreased cardiac fibroblast migration and cardiomyocyte death in vitro, respectively (39, 41). Results from both animal and human studies showed elevated IL-18, IL-17, and AngII levels and activation of JNK and IKK signaling in atherosclerotic plaques (10–12, 23, 26, 28, 33, 37). Recent studies also showed that aldosterone promotes atherosclerosis and plaque inflammation (25). Elevated TRAF2, 5 and 6 protein levels were also reported in human atherosclerotic plaques (46). Together, these observations support a possible causal role for TRAFF3IP2 in atherogenesis.
Plaque stability is a critical determinant of its vulnerability to rupture. Stable plaques have lesser necrotic core with thicker fibrous caps, higher number of SMCs, and increased deposition of ECM proteins like collagens, whereas unstable plaques have larger necrotic area with thinner fibrous caps, and lower SMC and collagen content. In this study TRAF3IP2 gene deletion increased features of plaque stability in both male and female ApoE−/− mice as evidenced by decreased necrotic core and increased SMC content and collagen levels. Size of necrotic core is influenced by the rate of monocyte recruitment, differentiation, foam cell formation and death (29). In a previous study, we showed that ox-LDL activates endothelial cell and monocyte attachment in vitro via TRAF3IP2-dependent increases in ICAM1 and VCAM1 expression (40). In the present study, aortic expression of ICAM1 and VCAM1 was reduced in the DKO mice, possibly suggesting that reduced endothelial cell activation in these mice might have resulted in reduced monocyte recruitment. These mice also showed significantly reduced CXCL1 expression, a chemokine known to mediate ox-LDL-mediated monocyte recruitment (45), further supporting our hypothesis. Matsuyama et al. showed that TRAF6 activation promotes monocyte differentiation by regulating cell-ECM interactions suggesting that TRAF3IP2-null mice might have also had reduced monocyte differentiation due to reduced TRAF6 signaling (24).
The role of TRAF3IP2 in foam cell formation and apoptosis is not studied yet, but signaling cascades involving TRAF3IP2 were shown to regulate these critical steps. Activated NF-κB was reported in atherosclerotic plaques from human and animal models (6). Hematopoietic cell- and endothelial cell-specific inhibition of NF-κB resulted in decreased atherosclerosis, whereas macrophage- and myeloid-cell-specific inhibition of NF-κB resulted in increased atherosclerosis with elevated macrophage apoptosis (15). Studies showed that JNK pathway is critical for foam cell formation (32). The role of IL-17, an upstream activator of TRAF3IP2, in atherosclerosis is inconclusive, as the studies demonstrated contrasting effects (16). Though, cell-specific inhibition of signaling molecules regulated by TRAF3IP2 have shown divergent outcomes on plaque stability, alteration of these pathways by global knockdown of TRAF3IP2 might have altered signaling cascades towards increased plaque stability. Interestingly, the DKO mice showed reduced aortic expression of TNFα in both male and female mice, which could be one of the possible mechanisms involved in decreased necrotic core, as TNFα is an important mediator of macrophage apoptosis (38). In male mice, reduced aortic expression of IL-6, AT1A and ADAM17 along with TNFα might have further improved plaque stability features, as they were shown to play a casual role in atherosclerotic plaque development and vulnerability (14, 17, 36).
The progression of stable to unstable plaques is characterized by an increase in SMC apoptosis, resulting in reduced number of SMCs, which in turn reduces ECM deposition (2). Activated NF-κB was detected in SMCs from human atherosclerotic plaques (4), and both IKK/NF-κB and JNK/AP-1 play a role in VSMC apoptosis (13, 19, 27). JNK has also been shown to mediate TNFα-induced autophagy in human VSMCs (18). In this study, TRAF3IP2 gene deletion increased plaque SMC and collagen content in both the genders but to a greater extent in males, thus providing the first evidence that TRAF3IP2 mediates vulnerable plaque formation. Perhaps, reduced JNK signaling due to TRAF3IP2 gene deletion might have resulted in decreased SMC apoptosis and increased plaque stability features, nullifying the effects of reduced NF-κB signaling. TNFα is also an important mediator of SMC apoptosis (5) and decreased TNFα expression might have contributed to increased fibrous cap formation and a stable plaque in the DKO mice.
MMPs play a critical role in plaque stability, as they regulate ECM degradation and VSMC proliferation and migration. In the DKO mice of either gender, a trend towards increased MMP9 expression was observed along with reduced expression of its endogenous inhibitors, TIMP1 in female mice and RECK in male mice, suggesting a possible local increase in MMP activity in atherosclerotic plaques of the DKO mice. Increased MMP activity can lead to either increased plaque stability by facilitating SMC proliferation and migration or decreased plaque stability by increasing collagen degradation. It is thus plausible that increased local MMP activity might have promoted VSMC proliferation and migration resulting in a stable plaque with thicker fibrous cap in the DKO mice.
Although features of plaque stability are enhanced in both the genders, total aortic plaque burden was reduced in females, but not males, suggesting that plaque stability is gender-independent, whereas plaque development is gender dependent in the DKO mice. However, it is not known whether female sex hormones regulate TRAF3IP2 expression. In a recent study, TRAF3IP2 has been shown to play a role in estrogen-deficient osteoporosis (9), suggesting that estrogen might regulate TRAF3IP2 expression by mechanisms not yet described. Further, deletion of TRAF6, a downstream target of TRAF3IP2, in endothelial cells resulted in reduced atherosclerosis in females but not males, further supporting our observations (31). A limitation of our study is that, though we have determined the effects of global deletion of TRAF3IP2 on atherosclerotic plaque formation and composition, the contribution of TRAF3IP2 from individual cell types on the observed phenotype has not been determined.
In conclusion, we have demonstrated for the first time a causal role for TRAF3IP2 in atherosclerosis. TRAF3IP2 gene deletion not only decreased plaque development, but also increased features of plaque stability in ApoE−/− mice. Interestingly, TRAF3IP2 gene deletion failed to modulate systemic cholesterol levels. However, its lack of expression blunted proinflammatory cytokine, chemokine, adhesion molecule, and MMP expression. Thus, TRAF3IP2 could be a potential therapeutic target in atherosclerotic vascular diseases. Our future studies will determine whether gelatin nanoparticle or adeno-associated virus-mediated delivery of TRAF3IP2 siRNA or shRNA will blunt development of atherosclerosis and promote plaque stability in ApoE−/− mice fed a Western diet.
Supplementary Material
Highlights.
TRAF3IP2 gene deletion reduces atherosclerosis in female ApoE−/− mice.
TRAF3IP2 gene deletion decreases plaque necrotic core size in both genders.
TRAF3IP2 gene deletion increases features of plaque stability.
TRAF3IP2 gene deletion inhibits aortic proinflammatory and extracellular matrix genes.
TRAF3IP2 could be a potential therapeutic target in atherosclerotic vascular diseases.
Acknowledgments
Financial support
BC is a recipient of the Department of Veterans Affairs Research Career Scientist award and is supported by VA Office of Research and Development Biomedical Laboratory Research and Development Service Award I01-BX002255 and the NIH/NHLBI grant HL-86787. PD is supported by NHLBI grants HL-70241 and HL-80682. US is supported by the Intramural Research Program of the NIH/NIAID. The contents of this report do not represent the views of the Department of Veterans Affairs or the United States government.
Abbreviations
- Act1
activator of NF-κB
- AP-1
activator protein-1
- ADAM
A Disintegrin and metalloproteinase domain-containing protein
- AGTR
angiotensin II receptor
- AngII
angiotensin II
- AOPPs
advanced oxidation protein products
- ApoE
Apolipoprotein E
- IKK
IκB kinase
- CXCL1
chemokine (C-X-C motif), ligand 1
- ECM
Extracellular matrix
- HDL
High-density lipoprotein
- ICAM1
intercellular adhesion molecule 1
- IκB
inhibitory kappa B
- IL
interleukin
- JNK
c-Jun amino-terminal kinase
- LDL
Low-density lipoprotein
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor κB
- Ox-LDL
Oxidized low-density lipoprotein
- ROS
reactive oxygen species
- RECK
Reversion-Inducing-Cysteine-Rich Protein with Kazal Motifs
- ROS
reactive oxygen species
- αSMA
alpha smooth muscle actin
- TIMP
Tissue inhibitor of metalloproteinase
- TNFα
Tumor necrosis factor α
- VCAM1
Vascular cell adhesion molecule
- TRAF
TNF Receptor Associated Factor
- TRAF3IP2
TRAF3-Interacting Protein 2
- VLDL
Very low-density lipoprotein
- VSMC
vascular smooth muscle cell
Footnotes
Conflict of interest
The authors declared that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Archives of medical research. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circulation research. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circulation research. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 4.Bourcier T, Sukhova G, Libby P. The nuclear factor kappa-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. The Journal of biological chemistry. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- 5.Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1553–1558. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- 6.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. Journal of Clinical Investigation. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. The Journal of biological chemistry. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 8.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H, Siebenlist U. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. Journal of immunology (Baltimore, Md : 1950) 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeSelm CJ, Takahata Y, Warren J, Chappel JC, Khan T, Li X, Liu C, Choi Y, Kim YF, Zou W, Teitelbaum SL. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. Journal of cellular biochemistry. 2012;113:2895–2902. doi: 10.1002/jcb.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovascular research. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 11.Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, Stellos K, Little KM, Lasitschka F, Doesch A, Hakimi M, Dengler TJ, Giese T, Blessing E, Katus HA, Gleissner CA. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. Journal of immunology (Baltimore, Md : 1950) 2014;193:4344–4355. doi: 10.4049/jimmunol.1400181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, Hakimi M, Bockler D, Katus HA, Gleissner CA. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic research in cardiology. 2011;106:125–134. doi: 10.1007/s00395-010-0135-y. [DOI] [PubMed] [Google Scholar]
- 13.Erl W, Hansson GK, de Martin R, Draude G, Weber KS, Weber C. Nuclear factor-kappa B regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells. Circulation research. 1999;84:668–677. doi: 10.1161/01.res.84.6.668. [DOI] [PubMed] [Google Scholar]
- 14.Eto H, Miyata M, Shirasawa T, Akasaki Y, Hamada N, Nagaki A, Orihara K, Biro S, Tei C. The long-term effect of angiotensin II type 1a receptor deficiency on hypercholesterolemia-induced atherosclerosis. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:1631–1642. doi: 10.1291/hypres.31.1631. [DOI] [PubMed] [Google Scholar]
- 15.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell metabolism. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Gong F, Liu Z, Liu J, Zhou P, Liu Y, Lu X. The paradoxical role of IL-17 in atherosclerosis. Cellular immunology. 2015;297:33–39. doi: 10.1016/j.cellimm.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Holdt LM, Thiery J, Breslow JL, Teupser D. Increased ADAM17 mRNA expression and activity is associated with atherosclerosis resistance in LDL-receptor deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1097–1103. doi: 10.1161/ATVBAHA.108.165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunology and cell biology. 2006;84:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 19.Larroque-Cardoso P, Swiader A, Ingueneau C, Negre-Salvayre A, Elbaz M, Reyland ME, Salvayre R, Vindis C. Role of protein kinase C delta in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells. Cell death & disease. 2013;4:e520. doi: 10.1038/cddis.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonardi A, Chariot A, Claudio E, Cunningham K, Siebenlist U. CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10494–10499. doi: 10.1073/pnas.190245697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, Haag M, Stark GR. Act1, an NF-kappa B-activating protein. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10489–10493. doi: 10.1073/pnas.160265197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Science signaling. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 24.Matsuyama W, Faure M, Yoshimura T. Activation of discoidin domain receptor 1 facilitates the maturation of human monocyte-derived dendritic cells through the TNF receptor associated factor 6/TGF-beta-activated protein kinase 1 binding protein 1 beta/p38 alpha mitogen-activated protein kinase signaling cascade. Journal of immunology (Baltimore, Md : 1950) 2003;171:3520–3532. doi: 10.4049/jimmunol.171.7.3520. [DOI] [PubMed] [Google Scholar]
- 25.McGraw AP, Bagley J, Chen WS, Galayda C, Nickerson H, Armani A, Caprio M, Carmeliet P, Jaffe IZ. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. Journal of the American Heart Association. 2013;2:e000018. doi: 10.1161/JAHA.112.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijer CA, Le Haen PAA, van Dijk RA, Hira M, Hamming JF, van Bockel JH, Lindeman JH. Activator protein-1 (AP-1) signalling in human atherosclerosis: results of a systematic evaluation and intervention study. Clinical Science (London, England : 1979) 2012;122:421–428. doi: 10.1042/CS20110234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzler B, Hu Y, Dietrich H, Xu Q. Increased expression and activation of stress-activated protein kinases/c-Jun NH(2)-terminal protein kinases in atherosclerotic lesions coincide with p53. The American journal of pathology. 2000;156:1875–1886. doi: 10.1016/S0002-9440(10)65061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair J, Ghatge M, Kakkar VV, Shanker J. Network Analysis of Inflammatory Genes and Their Transcriptional Regulators in Coronary Artery Disease. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polykratis A, van Loo G, Xanthoulea S, Hellmich M, Pasparakis M. Conditional targeting of tumor necrosis factor receptor-associated factor 6 reveals opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis. Circulation. 2012;126:1739–1751. doi: 10.1161/CIRCULATIONAHA.112.100339. [DOI] [PubMed] [Google Scholar]
- 32.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell metabolism. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 34.Somanna NK, Yariswamy M, Garagliano JM, Siebenlist U, Mummidi S, Valente AJ, Chandrasekar B. Aldosterone-induced cardiomyocyte growth, and fibroblast migration and proliferation are mediated by TRAF3IP2. Cellular signalling. 2015;27:1928–1938. doi: 10.1016/j.cellsig.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nature immunology. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka H, Matsumura I, Nakajima K, Daino H, Sonoyama J, Yoshida H, Oritani K, Machii T, Yamamoto M, Hirano T, Kanakura Y. GATA-1 blocks IL-6-induced macrophage differentiation and apoptosis through the sustained expression of cyclin D1 and bcl-2 in a murine myeloid cell line M1. Blood. 2000;95:1264–1273. [PubMed] [Google Scholar]
- 37.Tenger C, Sundborger A, Jawien J, Zhou X. IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:791–796. doi: 10.1161/01.ATV.0000153516.02782.65. [DOI] [PubMed] [Google Scholar]
- 38.Tran TM, Temkin V, Shi B, Pagliari L, Daniel S, Ferran C, Pope RM. TNFalpha-induced macrophage death via caspase-dependent and independent pathways. Apoptosis : an international journal on programmed cell death. 2009;14:320–332. doi: 10.1007/s10495-009-0311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valente AJ, Clark RA, Siddesha JM, Siebenlist U, Chandrasekar B. CIKS (Act1 or TRAF3IP2) mediates Angiotensin-II-induced Interleukin-18 expression, and Nox2-dependent cardiomyocyte hypertrophy. Journal of molecular and cellular cardiology. 2012;53:113–124. doi: 10.1016/j.yjmcc.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valente AJ, Irimpen AM, Siebenlist U, Chandrasekar B. OxLDL induces endothelial dysfunction and death via TRAF3IP2. Inhibition by HDL3 and AMPK activators. Free radical biology & medicine. 2014;70:117–128. doi: 10.1016/j.freeradbiomed.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valente AJ, Sakamuri SS, Siddesha JM, Yoshida T, Gardner JD, Prabhu R, Siebenlist U, Chandrasekar B. TRAF3IP2 mediates interleukin-18-induced cardiac fibroblast migration and differentiation. Cellular signalling. 2013;25:2176–2184. doi: 10.1016/j.cellsig.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valente AJ, Yoshida T, Clark RA, Delafontaine P, Siebenlist U, Chandrasekar B. Advanced oxidation protein products induce cardiomyocyte death via Nox2/Rac1/superoxide-dependent TRAF3IP2/JNK signaling. Free radical biology & medicine. 2013;60:125–135. doi: 10.1016/j.freeradbiomed.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valente AJ, Yoshida T, Izadpanah R, Delafontaine P, Siebenlist U, Chandrasekar B. Interleukin-18 enhances IL-18R/Nox1 binding, and mediates TRAF3IP2-dependent smooth muscle cell migration. Inhibition by simvastatin. Cellular signalling. 2013;25:1447–1456. doi: 10.1016/j.cellsig.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatesan B, Valente AJ, Das NA, Carpenter AJ, Yoshida T, Delafontaine JL, Siebenlist U, Chandrasekar B. CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cellular signalling. 2013;25:359–371. doi: 10.1016/j.cellsig.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Z, Subramanian P, Sevilmis G, Globke B, Soehnlein O, Karshovska E, Megens R, Heyll K, Chun J, Saulnier-Blache JS, Reinholz M, van Zandvoort M, Weber C, Schober A. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell metabolism. 2011;13:592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Zirlik A, Bavendiek U, Libby P, MacFarlane L, Gerdes N, Jagielska J, Ernst S, Aikawa M, Nakano H, Tsitsikov E, Schonbeck U. TRAF-1, -2, -3, -5, and -6 are induced in atherosclerotic plaques and differentially mediate proinflammatory functions of CD40L in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1101–1107. doi: 10.1161/ATVBAHA.107.140566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.