Abstract
Metastasis, drug resistance and recurrence in cancer are regulated by the tumor microenvironment. This review describes recent advances in understanding how cancer cells respond to extracellular environmental cues via integrins, how to build engineered microenvironments to study these interactions in vitro and how nanomaterials can be used to detect and target tumor microenvironments.
Keywords: Integrin, mechanobiology, nanomaterials, drug resistance, metastasis
Introduction
Cells are responsive to the biochemical and biomechanical features of the extracellular matrix (ECM). These responses (e.g., proliferation, apoptosis, migration and differentiation) are crucial for health at all levels. Cancer cells share many of the responsive properties of healthy cells. However, they differ from normal cells because their responses to ECM are often dysregulated and they exhibit drug resistance and the ability to form recurrent tumors. Understanding how cancer cells respond to ECM cues provides access to new avenues for treatment complementary to our current arsenal of cancer therapies.
In this short review, we will discuss recent advances in understanding how cancer cells respond to their environment via integrins with a particular focus on the mechanobiological properties of the ECM (Figure 1). We will also present recent findings on how the size-scale provided by nanomaterials can be used to change these environments for therapeutic purposes.
Figure 1.
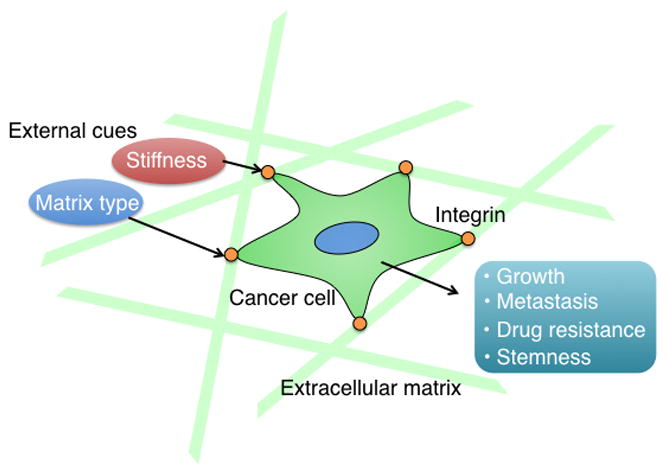
Representative examples of how external cues dictate cell behavior.
Integrins and cancer cells
Integrins are transmembrane glycoproteins that mediate interactions between cells and the ECM and link the ECM to the cytoskeleton [1]. Integrins sense the ECM and relay this information to downstream signaling pathways to control cell movement, growth and gene expression [1]. This function is of considerable importance in cancer because the ability of integrins to interact with the ECM in the tumor microenvironment and sense biochemical and biomechanical perturbations in this ECM is a key determinant of tumor growth and progression to metastatic disease [2]. Therefore the ECM is a key determinant of tumor growth and progression to metastatic disease [2]. Indeed, the literature is full of studies that describe the contribution of integrins to various aspects of cancer cell behavior. Some of the more interesting and provocative work in this area that has emerged recently relates to the importance of ECM–integrin interactions in the function of cancer stem cells (CSCs) and metastasis and the ability of integrins to transduce mechanical forces generated by the ECM (see below). Although these roles are not mutually exclusive, they will be discussed separately here.
CSCs
Most tumors harbor a population of cells with characteristics of stem cells including the ability to self-renew and populate new tumors [3,4]. This population, often referred to as CSCs or tumor-initiating cells, is resistant to most standard chemotherapies and thought to contribute to tumor dormancy and recurrence. CSCs differ from non-CSCs in their expression of specific integrins and these integrins are frequently used as cell surface markers to enrich for CSC populations [5]. Notable examples include the α6 integrins (α6β1 and α6β4) and the αvβ3 integrin [2]. Moreover, it has become increasingly apparent that specific integrins contribute to sustaining stem cell properties by transducing cues from the ECM present in the CSC niche [6–8]. CSC niches are complex microenvironments primarily comprising CSCs, non-CSCs (cancer-associated fibroblasts, inflammatory cells) and ECM, as well as secreted factors (cytokines, growth factors) [8]. The CSC niche can be considered as a specialized form of the tumor microenvironment that functions to maintain stem cell properties, including self-renewal [8].
The biochemical and biomechanical properties of the ECM in the CSC niche exert profound effects on CSC function. Tenascins, periostin and laminins are among the ECM proteins that have been implicated in regulating CSC function [2,8]. The laminins are of particular interest in this regard because they exemplify the specificity of ECM cues in controlling CSCs. Among this large family of ECM proteins, one laminin (LM511) appears to be crucial for maintaining stemness. Breast CSCs, for example, deposit a LM511 matrix that sustains stem cell properties (self-renewal and tumor initiation; Figure 2) [7]. Surface-bound LM511 can be used as a marker to enrich for CSCs and, potentially, to detect CSCs in tumor sections [7]. Interestingly, LM511 is recognized by a specific, cytoplasmic domain splice variant of the α6β1 integrin (α6Bβ1) and this interaction activates a crucial signaling pathway involving the Hippo transducer TAZ which is necessary for the function of breast CSCs [7]. These findings reveal the exquisite specificity of ECM–integrin interactions in regulating stemness because only one of the two splice variants of the α6β1 integrin has the ability to recognize a specific laminin, which is crucial for stemness. This specificity provides opportunities for therapeutic targeting of CSCs, which has become the holy grail of cancer therapy. Future work should focus on understanding the unique biochemical and biomechanical properties of LM511 that confer stemness compared to other laminins and the recognition of this laminin by α6Bβ1.
Figure 2.
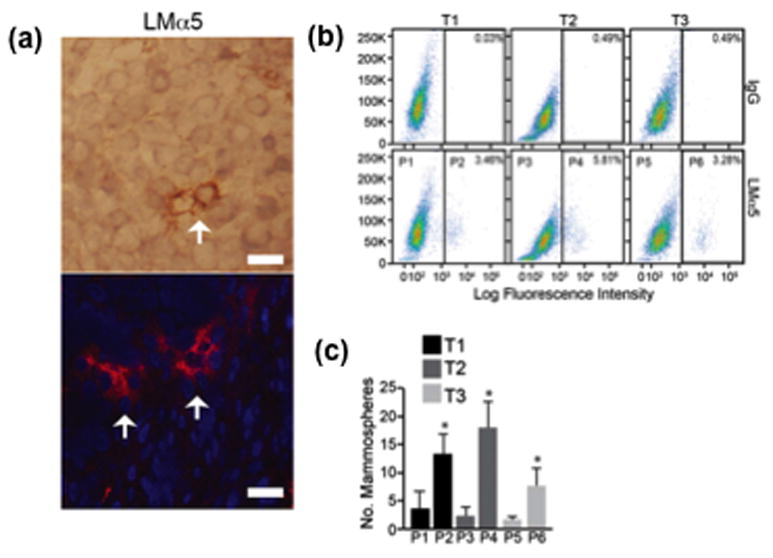
Surface-bound LM511 identifies breast cancer cells with stem cell properties. (a) Sections of human breast tumors were stained with an antibody that recognizes the a5 subunit of LM511 using either immunohistochemistry (top) or immunofluorescence (bottom). Note the small number of cells with intense LMa5 staining on their surfaces (arrows). (b) Three primary human breast tumors (T1, T2 and T3) were dissociated and sorted by FACS using a LMa5 Ab. Cells with low surface-bound LMa5 (P1, P3 and P4) were compared with cells with high surface-bound LMa5 (P2, P4 and P6) for their ability to form mammospheres. (c) Note that cells with high surface-bound LMa5 had the ability to form mammospheres. Adapted, with permission, from [7].
The ability of integrins to promote stemness can occur independently of binding to ECM. This scenario is exemplified best by studies on the αvβ3 integrin, which contributes to the function of mammary and breast CSCs by enhancing the expression of Slug, a transcription factor that is a master regulator of stemness [9]. Antagonists that compete for αvβ3 ligand binding have no effect on mitigating stem cell properties [10]. The ability of αvβ3 to promote stemness in the absence of ECM engagement provides autonomy and self-sufficiency to CSCs. Nevertheless, it is also evident that CSCs are dependent on cues from secreted factors, as well as other ECM–integrin interactions as described above. Clearly, a systems approach is needed to integrate the complex array of signals from the CSC niche and those intrinsic to CSCs that impact stemness.
Metastasis
The concept of the premetastatic niche provides an ideal example of the importance of external cues in cancer progression. This niche is a specialized microenvironment, comprising ECM (especially tenascin-C and periostin) and other molecules, which forms at potential metastatic sites in response to cues from the primary tumor [11,12]. Understanding how the primary tumor communicates the formation of the niche before the arrival of tumor cells is a fascinating problem. Recent studies implicate a key role for exosomes, which are small membrane vesicles that contain DNA, RNA, proteins and lipids [13]. Exosomes formed from primary tumor cells have the potential to home in distant organs and facilitate formation of the premetastatic niche [14].
An exciting advance in this field is the discovery that exosome integrins determine the organ specificity of the premetastatic niche and subsequent metastasis [15]. Exosomes destined for the lung, for example, express the α6β4 integrin, which is a laminin receptor [16], and are taken up by laminin-rich cells in the lung [14]. The fusion of these exosomes and their associated content with resident cells contributes to the formation of the premetastatic niche. These data substantiate previous work implicating the α6β4 integrin in metastasis and they strengthen its potential as a therapeutic target [17]. They also raise several provocative and timely questions. Given that laminin is present in all organs, what is distinct about the laminin in the lung (isoform specificity, location, mechanical properties) that enables it to function as a cue for α6β4-containing exosomes? Another intriguing consideration is how the formation of specific exosomes is regulated in the primary tumor. Given that exosomes are formed by the fusion of multivesicular endosomes with the plasma membrane [7], what are the signals that trigger the formation of α6β4-containing exosomes for example? Is their formation linked to integrin endocytosis and recycling [18]? It is worth noting in this context that hypoxia selects for cells with metastatic potential [19], increases exosome formation and stimulates trafficking of the α6β4 integrin [20].
Mechanobiology and cancer
Studying the ability of a cell to sense and respond to mechanical cues has emerged as a field in itself over the past 20 years, and the importance of the mechanical environment on cell and/or tissue behavior is now appreciated by engineers and biologists alike. Growing evidence is proving that the ECM plays a crucial part in regulating tumor growth and metastasis [21], and stiffness is one of the ECM features responsible for tumor evolution. Tumor ECM is notoriously stiff (up to tens of kPa) relative to healthy tissue (hundreds of Pa) [22]; a difference that is associated with extensive changes in the metastatic potential of cells within the tumor [23]. The cellular response to mechanical influences from the ECM is mediated by integrins that not only provide tissue-specific cell attachment to ECM proteins but also activate biochemical signaling networks in response to static and dynamic mechanical forces [24]. A salient example of this phenomenon that relates to the previous discussion of integrins is that simply increasing ECM stiffness is sufficient to induce the malignant transformation of normal mammary epithelial cells, a process that is mediated by α6β4 integrin signaling [25,26].
Tumor stiffening is derived from an increase in fibrillar type 1 collagen, deposited by cancer-associated fibroblasts (CAFs) [27] and adipogenic stem cells (ASCs) [28]. During tumor growth, collagens become increasingly fibrillar and organize into tight bundles. Some propose that collagen reorganization [29] and tumor (and stroma) stiffness [30] could be used as a prognostic tool for metastatic risk or progression in patients via imaging or minimally invasive biopsy.
Metastasis
After cells have invaded the primary tumor stroma and intravasated local vasculature, those few cells that are able to survive the journey will eventually encounter secondary tissues that are biochemically and mechanically diverse. The mechanosensing of cells and these destination tissues is a newer, but active, area of research. Researchers are using a variety of techniques to quantify the rigidity of these tissue sites. Examples include brain tissue (1–2 kPa in Young’s modulus) [31], lung (2–8 kPa) [32], trabecular and cortical bone being (~10 GPa) and bone marrow (0.3–24.7 kPa) [33]. Engineers are using these analyses as inspiration to create in vitro model systems that capture the mechanical features of these diverse tissues [34], with the goal of elucidating the mechanisms of this mechanosensing, and eventually developing drugs to abrogate it.
Preliminary evidence points to tissue mechanics, alongside ECM binding [35], as one aspect that controls tissue-specific metastatic spread. Kostic et al. showed that cells that typically spread to certain tissues (e.g., brain, bone) also migrated faster and grew faster on substrates that were mechanically close to those destination tissues [36]. In a comparative study, McGrail et al. demonstrated that ovarian cancer cell lines proliferated more and migrated faster on softer gels compared with breast cancer cells [37]. The authors suggest that this difference in migration rates mirrors the observation that ovarian cancer generally metastasizes to softer tissues than breast cancer. More-complex systems have emerged with the ability to combine mechanical cues with other facets of the tumor microenvironment, including vascularization [38], or tissue-specific features, typically from decellularized tissue sources [39]. What is needed to catapult these phenomenological studies into therapeutic targets is to identify the signaling networks downstream of integrin binding responsible for translating the mechanical signaling to metastasis. One recent example is the Rho/ROCK pathway – implicated in stiffness-mediated metastasis [27]. Another is the emerging role of YAP/TAZ as mechanotransducting nodes in the Hippo pathway [40]. Coupling these in vitro systems with phosphoproteomic and genomic sequencing could eventually connect the important studies in carefully controlled microenvironments to interrogation of integrin-mediated signaling for in vivo and clinical utility to stop metastasis from stiff tumors, or halt their ability to reach secondary sites with a certain mechanical profile.
Drug resistance
Drug resistance in cells and patients is a well known problem in cancer. During drug development, potential drug resistance can be missed, because chemotherapeutics and small molecule drugs are screened in ‘plastic’ microenvironments before preclinical and clinical trials, and these plastic environments do not resemble the natural environment of a cell or tumor. There is currently emphasis on the intracellular and genetic mechanisms of drug resistance. However, to make a paradigm shift in this field, many are now beginning to account for the mechanical environment of the cell as it responds to drugs. Given that mechanics regulate metastasis and tumor growth, similar intracellular proteomic networks could be rewired during tumor stiffening that hinder the efficacy of cancer drugs.
The ideal in vitro drug-testing platform to parse the role of mechanics in drug response would present matrix cues (such as stiffness and cell-binding peptides) relevant to what cancer cells experience in vivo in a 3D, high-throughput manner. A recent example is a 4D lung model, in which circulating tumor cells were equally resistant to cisplatin in the model system as they were in vivo, results that were not captured on 2D surfaces [41]. Similarly, combining ovarian cancer cells with fibroblasts in a representative ECM system recapitulated in vivo drug response better than monoculture models [42]. These types of organotypic representations of tissue for drug screening are likely to become increasingly popular with the advent of patient-derived cultures [43]. These patient-derived systems are relatively low-throughput, and a high-throughput aspect is crucial to making it accessible to pharmaceutical companies. Synthetic hydrogels have been adapted into multi-well plates to overcome this throughput issue, the first of which uses polyacrylamide (PAA) gels to show that stiffness regulated cancer cell growth in the presence of a few candidate drugs [44]. Other 2D systems have more recently emerged, including other mechanisms to prepare PAA gels [45], and those based on polyethylene glycol (PEG) [46]. These examples include not only response to common chemotherapeutics (e.g., paclitaxel) [45] but also how signaling networks perturbed during ECM stiffening provide resistance to receptor tyrosine kinase inhibitors (e.g., sorafenib, lapatinib) [46,47]. Ideally, future work will translate these initial results into better-informed drug treatment and monitoring, with delivery systems incorporating tissue-specific targeting facilitated by nanomaterials.
Nanomaterials for regulating cancer cell behaviors
Nanomaterials are materials with sizes between 1 and 100 nm, corresponding to a size-scale ranging from that of small proteins to large cellular organelles. They can be engineered in a particular way to represent some of the key features of ECM, such as hydrophobicity, charge and topology, providing unique tools for fundamental studies of cell–environment interactions and therapeutic applications [48].
Chemical environment and cell behavior
We have discussed above how the specific chemical signals provided by integrins and the mechanical environment of the ECM regulate cancer cell behavior. The physicochemical environment of the ECM likewise plays a significant part, as demonstrated by the role of cell glycosylation in cancer progression. This interplay has been studied using chemically modified surfaces of substrates, however control of chemical functionality and topology remain a challenge. Nanomaterials provide a tool for controlling both aspects by ‘painting’ on pre-functionalized nanomaterials. As an example, Cui et al. have recently used polymer-functionalized nanoparticles to demonstrate that hydrophobic nanomaterials inhibit HeLa cell proliferation [49]. This study presented one axis of the physicochemical environment; however the ECM presents a range of stimuli including charge and topology as well as hydrophobicity. Tang et al. have used small (2 nm core) gold nanoparticles functionalized with a wide range of surface ligands to probe cell proliferation with four different cancer cell lines (Figure 3) [50]. These studies showed that cationic functionality enhanced cell proliferation. More interesting, however, was the fact that specific chemical moieties (e.g., aromatic groups) had differential responses between the cell lines studied. This outcome suggests that modulation of the local chemical environment of cells provides a potential strategy for selectively regulating cancer cell behavior, as discussed below.
Figure 3.
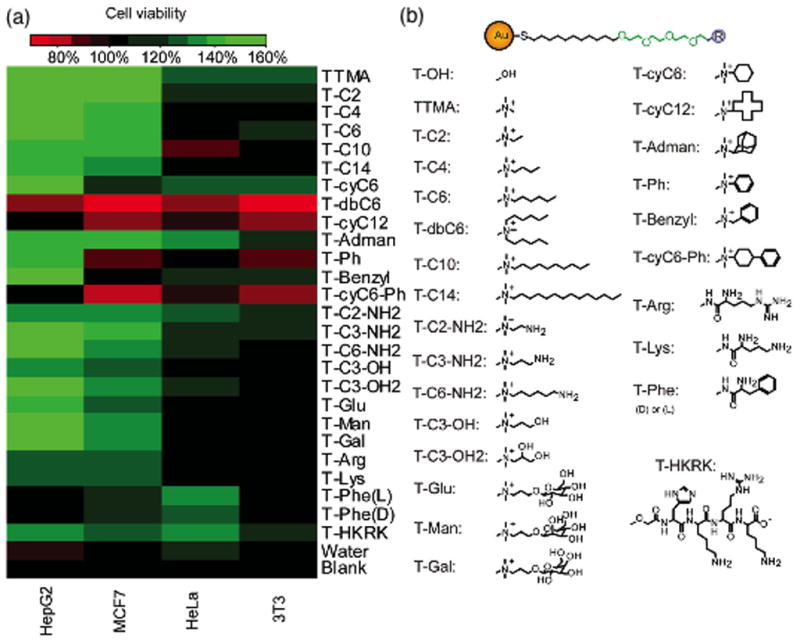
Relationship between cell viabilities of four different cancer cells and different AuNP coatings for 2 nm core AuNPs immobilized on polystyrene surfaces. (a) A heat map of cell viability on different AuNP coatings. (b) Structures of ligands on the AuNPs. Adapted, with permission, from [50].
The above studies used 2D cell culture techniques, which are clearly an abstraction of the 3D nature of tumors. Grzincic and Murphy studied the effects of gold nanorods in 3D collagen matrices, discovering that these nanomaterials accelerated cell migration of breast tumor cells, enabling a rounded ‘amoeboid-like’ phenotype [51] not normally observed in 2D systems.
Nanomaterials as environmental therapeutics
The broadest application of nanomaterials in medicine has been as delivery vehicles for therapeutic small molecules and biomacromolecules [52]. They have also been used as intracellular self-therapeutics for regulating cellular processes. Nanomaterials have recently emerged that mimic and/or alter the cellular environment, providing new strategies for nanotherapeutics. One particularly interesting application was the use of nanoparticles coated with ECM from tumor-associated fibroblasts to capture cancer cells, thereby preventing peritoneal metastasis [53]. Another example of a nanotherapeutic approach lies in altering stemness of CSCs. The ability of CSCs to self-renew and differentiate into multi-lineage provides a challenging yet potentially high-payoff target for therapeutic strategies. Just as environmental effects can be used to regulate normal stem cell differentiation, CSCs can be induced to differentiate through environmental cues. For instance, gadolinium fullerene Gd@C82(OH)22 was reported to block epithelial-to-mesenchymal transition of triple-negative breast cancer cells [54], without any toxicity to normal mammary epithelial cells. Gd@C82(OH)22 successfully suppressed breast tumor growth in vivo. Hydrophobicity appears to be an important consideration in CSC interactions, because graphene-oxide (GO) was also reported to work as a therapeutic strategy against CSCs via de-stemming, as demonstrated through a lack of spheroid formation [55].
Concluding remarks
The biochemical and biomechanical microenvironment of tumors is a key factor in metastasis and drug resistance. Understanding the part these factors play in cancer progression provides new strategies for therapeutics. We present here how biomolecules (e.g., integrins) and nanomaterials alter cancer cell behavior through chemical means, and how mechanobiology is a key factor in understanding and treating cancer progression. Taken together, this work shows that targeted presentation of external cues has the potential to complement current intracellular cancer therapy strategies.
Supplementary Material
Highlights.
Biomolecules including integrins are key factors in cancer progression
Nanomaterials provide tools for regulating the chemical environment of cells
Mechanobiology is an emerging determinant in designing cancer therapies
Acknowledgments
A.M. acknowledges support from the NIH (CA168464 and CA203439), V.R. acknowledges GM 077173 and A.M., S.P. and V.R. received support from the University of Massachusetts System President’s Science and Technology Initiative Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Seguin L, et al. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25:234–240. doi: 10.1016/j.tcb.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Farahani E, et al. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis. 2014;35:747–759. doi: 10.1093/carcin/bgu045. [DOI] [PubMed] [Google Scholar]
- 6.Lathia JD, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells. Genes Dev. 2015;29:1–6. doi: 10.1101/gad.253682.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plaks V, et al. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desgrosellier JS, et al. Integrin αvβ3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Dev Cell. 2014;30:295–308. doi: 10.1016/j.devcel.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seguin L, et al. An integrin β3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oskarsson T, et al. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo M, et al. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 14.Minciacchi VR, et al. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EC, et al. The Integrin α6β4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elizabeth A, et al. Mobilization and activation of a signaling competent α6β4 integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev. 2005;24:413–423. doi: 10.1007/s10555-005-5133-4. [DOI] [PubMed] [Google Scholar]
- 18.Caswell PT, et al. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL. The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863:382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon S, et al. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the α6β4 integrin. Cancer Res. 2005;65:2761–2769. doi: 10.1158/0008-5472.CAN-04-4122. [DOI] [PubMed] [Google Scholar]
- 21.Duda DG, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Provenzano PP, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhuri O, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–988. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 26.Shaw LM, et al. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 27.Samuel MS, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo BR, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:310ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conklin MW, Keely PJ. Why the stroma matters in breast cancer: insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adh Migr. 2012;6:249–260. doi: 10.4161/cam.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fenner J, et al. Macroscopic stiffness of breast tumors predicts metastasis. Sci Rep. 2014;4 doi: 10.1038/srep05512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budday S, et al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater. 2015;46:318–330. doi: 10.1016/j.jmbbm.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luque T, et al. Local micromechanical properties of decellularized lung scaffolds measured with atomic force microscopy. Acta Biomater. 2013;9:6852–6859. doi: 10.1016/j.actbio.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 33.Jansen LE. Mechanics of intact bone marrow. J Mech Behav Biomed Mater. 2015;50:299–307. doi: 10.1016/j.jmbbm.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrick WG, et al. PEG-phosphorylcholine hydrogels as tunable and versatile platforms for mechanobiology. Biomacromolecules. 2013;14:2294–2304. doi: 10.1021/bm400418g. [DOI] [PubMed] [Google Scholar]
- 35.Barney LE, et al. A cell-ECM screening method to predict breast cancer metastasis. Integr Biol. 2015;7:198–212. doi: 10.1039/c4ib00218k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostic A, et al. Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS One. 2009;4:e6361. doi: 10.1371/journal.pone.0006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGrail DJ, et al. Actomyosin tension as a determinant of metastatic cancer mechanical tropism. Phys Biol. 2015;12:026001. doi: 10.1088/1478-3975/12/2/026001. [DOI] [PubMed] [Google Scholar]
- 38.Ehsan SM, et al. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol. 2014;6:603–610. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villasante A, et al. Bioengineered human tumor within a bone niche. Biomaterials. 2014;35:5785–5794. doi: 10.1016/j.biomaterials.2014.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 41.Vishnoi M, et al. Circulating tumor cells from a 4-dimensional lung cancer model are resistant to cisplatin. J Thorac Cardiovasc Surg. 2014;148:1056–1063. doi: 10.1016/j.jtcvs.2014.05.059. discussion 1063–1054. [DOI] [PubMed] [Google Scholar]
- 42.Kenny HA, et al. Quantitative high throughput screening using a primary human three-dimensional organotypic culture predicts in vivo efficacy. Nat Commun. 2015;6:6220. doi: 10.1038/ncomms7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Wetering M, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilghman RW, et al. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One. 2010;5:e12905. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zustiak S, et al. Multiwell stiffness assay for the study of cell responsiveness to cytotoxic drugss. Biotechnol Bioeng. 2014;111:396–403. doi: 10.1002/bit.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen TV, et al. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials. 2014;35:5749–5759. doi: 10.1016/j.biomaterials.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 47.Lin CH, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 49.Cui W, et al. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomedicine. 2012;8:46–53. doi: 10.1016/j.nano.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Tang R, et al. Rapid coating of surfaces with functionalized nanoparticles for regulation of cell behavior. Adv Mater. 2014;26:3310–3314. doi: 10.1002/adma.201306030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grzincic EM, Murphy CJ. Gold nanorods indirectly promote migration of metastatic human breast cancer cells in three-dimensional cultures. ACS Nano. 2015;9:6801–6816. doi: 10.1021/acsnano.5b03362. [DOI] [PubMed] [Google Scholar]
- 52.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotech. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 53.De Vlieghere E, et al. Tumor-environment biomimetics delay peritoneal metastasis formation by deceiving and redirecting disseminated cancer cells. Biomaterials. 2015;54:148–157. doi: 10.1016/j.biomaterials.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, et al. Gd-met allofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat Commun. 2015;6:5988. doi: 10.1038/ncomms6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiorillo M, et al. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget. 2015;6:3553–3562. doi: 10.18632/oncotarget.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


