Abstract
Calcium (Ca2+) is an essential ligand that binds its primary intracellular receptor Calmodulin (CaM) to trigger a variety of downstream processes and pathways. Central to the actions of Ca2+/CaM is the activation of a highly conserved Ca2+/CaM kinase (CaMK) cascade, which amplifies Ca2+ signals through a series of subsequent phosphorylation events. Proper regulation of Ca2+ flux is necessary for whole-body metabolism and disruption of Ca2+ homeostasis has been linked to a variety of metabolic diseases. Herein, we provide a synthesis of recent advances that highlight the roles of the Ca2+/CaM kinase axis in key metabolic tissues. An appreciation of this information is critical in order to understand the mechanisms by which Ca2+/CaM-dependent signaling contributes to metabolic homeostasis and disease.
Keywords: Calcium, Calmodulin, CaMKK2, CaMKIV, AMPK
Calmodulin is the intracellular receptor for Ca2+
Calcium (Ca2+) serves as one of the most ubiquitous and critical second messengers that regulate a plethora of downstream cellular signaling events. In response to an external stimulus, Ca2+ release from intracellular stores or influx from the extracellular space through ion channels, leads to its binding to target proteins and subsequent regulation of their activity. Ca2+ is a crucial regulatory ligand, and among its many binding partners, calmodulin (CaM) serves as its primary intracellular receptor for controlling essentially every aspect of cellular biology (Box 1). The Ca2+/CaM complex controls the activity of over 120 enzymes and proteins, including but not limited to transcription factors, ion channels and pumps, phosphatases, and kinases [1].
Box 1. The Ca2+/CaM Structure-Function Relationship.
Since Ca2+ is such a pervasive signal, it is essential for its receptor CaM to have structural plasticity to dynamically regulate the panoply of Ca2+-dependent target proteins and downstream pathways. Over the nearly forty years of research on CaM, there have been a number of excellent reviews that summarize details of its structure and physicochemical properties [1–3]. CaM is a small, highly conserved, dumbbell-shaped protein expressed by all eukaryotic cells. In humans, CaM is encoded by three distinct genes (CALM1, CALM2, CALM3), each having unique alternative regulation, tissue-specificity, and alternative splicing, yet surprisingly, producing identical proteins [4]. Structurally, each of its two lobes (N- and C-terminus) consists of a pair of EF hand motifs, each of which can bind two Ca2+ ions. In the absence of Ca2+, the N-terminus of CaM adopts a closed conformation while the C-terminal lobe remains in a semi-open conformation with a slightly exposed hydrophobic patch. A rise in intracellular Ca2+ leads to saturation of Ca2+ binding to CaM, which induces remodeling of both lobes into a more open conformation that exposes hydrophobic patches that promote its stable interaction with target proteins [2,3]. A helix connecting the two lobular domains of CaM also confers incredible flexibility to the molecule, allowing the Ca2+/CaM complex to adopt an infinite variety of conformations, each of which is unique for recognition and regulation of a specific binding partner [1].
A key Ca2+/CaM-dependent kinase cascade emerges
While the majority of early CaM studies were focused on characterizing its sequence, structure and cellular functions (Box 1), subsequent efforts resulted in the discovery and characterization of key Ca2+/CaM downstream targets (Figure I in Box 2). The compendium of Ca2+/CaM target proteins has been stratified into six (6) classes according to the nature of their interaction with the ligand-receptor complex and its effect on their activity [1]. In this review we primarily focus on class E and F targets, which are activated upon binding Ca2+/CaM, and whose regulation and activities collectively define the Ca2+/CaM-dependent kinase cascade (Fig. 1). In response to external stimuli, pulsatile increases in intracellular Ca2+ are sensed by CaM and the Ca2+/CaM complex adopts an increased affinity for class E and F target proteins [1]. However, class E targets, which include Ca2+/CaM-dependent kinases (CaMK) I and IV, require further phosphorylation by class F proteins Ca2+/CaM-dependent kinase kinase 1 and 2 (CaMKK1, CaMKK2). Activated CaMKK2 also phosphorylates the α subunit of AMP-activated protein kinase (AMPK), and forms a multimeric protein complex comprised of Ca2+/CaM, CaMKK2, and the AMPKα and β subunits [5] (Fig. 1). Importantly, the regulatory gamma (γ) subunit of AMPK that senses changes in the ATP:AMP ratio is not required for this interaction, so that activation of AMPK in this scenario is unique to fluctuations in intracellular Ca2+. Moreover, the CaMKK2 and AMPKα interaction occurs via their kinase domains, and CaMKK2 must exist in its activated conformation to interact with AMPKα, but not with its other kinase substrates, CaMKI and CaMKIV [6]. In this manner, full activation of the Ca2+/CaM-dependent kinase cascade effector molecules culminates in appropriate regulation of their cellular functions.
Box 2. CaM Kinase Historical Overview.
Since calcium (Ca2+) is the 5th most abundant element in the earth’s crust and the human body, its emergence as a ubiquitous and pervasive second messenger was unpredicted. While Ca2+ exists at mM concentrations in biological fluids, a similar elevation of free Ca2+ in any living cell would be catastrophic. Therefore, free Ca2+ within cells is tightly regulated in the nM range, and cells have adapted multiple mechanisms to accomplish this, including the evolutionary emergence of numerous Ca2+ pumps and channels associated with plasma, mitochondrial and intracellular membranes as well as a plethora of Ca2+-binding proteins [56]. Many of these proteins function to buffer, sense or monitor the frequency, amplitude or diffusion of Ca2+ within the cell. However, only one Ca2+-binding protein, calmodulin (CaM), is essential, ubiquitously expressed and serves as the primary intracellular receptor for Ca2+ thereby mediating most of the ion’s second messenger functions [1].
CaM was originally identified in snake venom as an activator of cyclic nucleotide phosphodiesterase (PDE) [57] and subsequently shown to require [59] and bind Ca2+ [60]. Shortly thereafter, the protein was independently “discovered” by several investigators working on different biological systems and given different names. The name calmodulin was proposed to investigators in this fledgling field in 1978 as a unifying name and adopted within a year or two. For a review on its early history please see [2,61]. Since its discovery, CaM has been the subject of almost 32,000 papers cited in PubMed (Figure I in Box 2). Over 120 CaM-binding partners have been identified and include members of virtually every major class of cellular proteins.
Early roles for CaM in endocrinology and metabolism were demonstrated by its participation in the action of FSH in testicular Sertoli cells by regulating a cyclic AMP PDE [62] and as an integral, regulatory subunit of the heterotetrameric metabolic enzyme phosphorylase kinase (a complex also regulated by cAMP) [63], respectively. These early findings presaged the subsequent discovery that, in addition to cAMP-mediated pathways, CaM regulates a variety of other intracellular signaling cascades involving nitric oxide, soluble phospholipids, nuclear receptors and membrane receptors/ion channels [56]. Other CaM targets include several Ca2+ pumps/channels, the protein phosphatase, calcineurin, and a number of CaM-dependent protein kinases (CaMK) [64]. While the early CaM field was driven by studies on CaM (Figure I in Box 2) [65] the large upswing in CaM publications mirrors the cloning and subsequent mechanistic studies on the CaMKs [64,66,67]. Biochemical studies of CaMKIV [68] and CaMKI [69], revealed that each required not only CaM binding, but also phosphorylation of a Thr on the activation loop to exhibit maximal activity. These findings led to the discovery of two upstream CaM kinase kinases (CaMKK) CaMKK1 [70] and CaMKK2 [71]. The requirement for one protein kinase to regulate another was reminiscent of the MAP kinase cascade and by analogy named the CaM kinase cascade. CaMKK2 was shown to activate not only CaMKI and CaMKIV but also the master metabolic protein kinase, AMPK [72]. Thus, CaMKK2 has emerged as the primary CaMK participating in regulation of metabolic homeostasis.
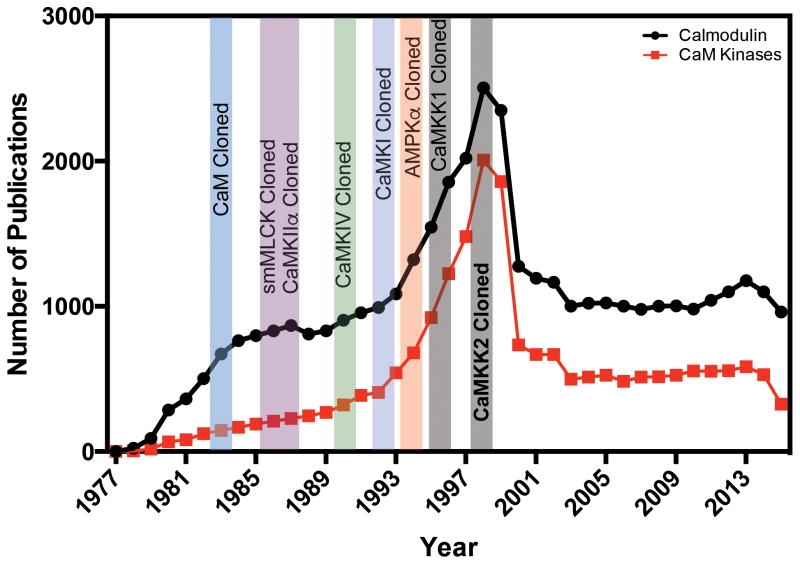
Figure I, Box 2. Timeline of CaM- and CaMK-related publications.
Graphic representation of the historical increase in the number of PubMed® publications on CaM (black line) and the Ca2+/CaM-dependent kinases (CaMK) (red line). Discoveries highlighted include cloning of the genes for the following cascade components: CaM - 1981 [65]; smooth muscle myosin light chain kinase (smMLCK) - 1986 [66]; CaM kinase II α (CaMKIIα) - 1987 [67]; CaMKIV - 1991 [68]; CaMKI - 1993 [69]; AMP-activated protein kinase α (AMPKα) - 1995 [72]; CaMK kinase 1 (CaMKK1) - 1995 [70]; CaMKK2 - 1997 [71].
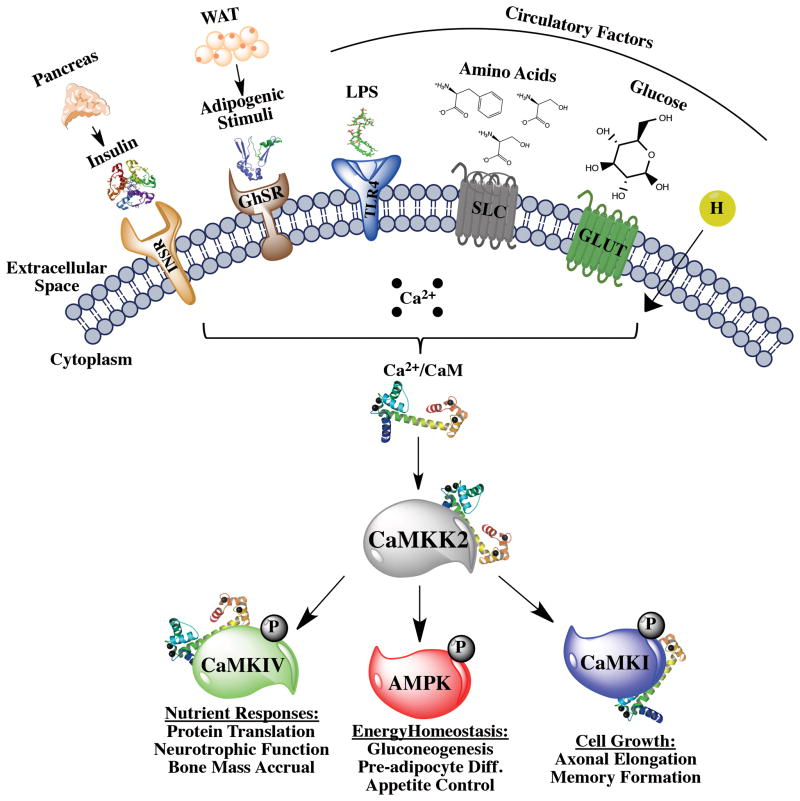
Figure 1. The Ca2+/CaM-dependent kinase cascade mediates pleiotropic metabolic responses to physiologic and pathophysiologic stimuli.
Upstream extracellular signals such as insulin from the pancreas, adipogenic stimuli from white adipose tissue (WAT), and lipopolysaccharide (LPS), amino acids, hormones and glucose from the circulation bind to their respective receptors that trigger a rise in intracellular Ca2+ concentration and accumulation of Ca2+/CaM targets such as CaMKK2. The increased affinity and binding of Ca2+/CaM for CaMKK2 results in an increase in CaMKK2 kinase activity, which phosphorylates and activates CaMKIV, AMPK and CaMKI. Activation of CaMKI is involved in regulation of cell growth, as observed in neurite elongation and branching [73] as well as during cell cycle control [74]. CaMKK2-dependent activation of AMPK leads to regulation of energy balance, particularly in the brain [10], liver [33] and adipose [44]. Regulation of CaMKIV activity results in control of protein synthesis and gene expression programs responsive to nutrients [35] and hormones [14].
Tissue-specific metabolic regulation by the Ca2+/CaM-dependent kinase cascade
Communication among different metabolic tissues and organs ensures proper energy flow and balanced caloric intake and utilization to maintain organismal energy homeostasis. Ca2+/CaM signaling is critical for mediating the downstream effects of hormones, metabolites, inflammatory agents and neuroendocrine signals that coordinate tissue crosstalk to balance the energy equation. Epidemiological studies have demonstrated that several hallmarks of metabolic syndrome, including obesity and diabetes, are associated with abnormal serum Ca2+ levels [7,8]. As discussed previously, the Ca2+/CaM complex mediates the amplification of Ca2+ signals through activation of a tightly regulated set of protein kinases, which themselves have critical functions in key metabolic tissues. An overview of the recent developments in Ca2+/CaM-dependent kinase cascade research as they pertain to metabolic homeostasis is discussed below and summarized in Fig. 2. Since virtually all of the physiologic actions relevant to metabolism illustrated in Fig. 2 are common to cascades using CaMKK2 as the most upstream kinase, this review will focus exclusively on these pathways.
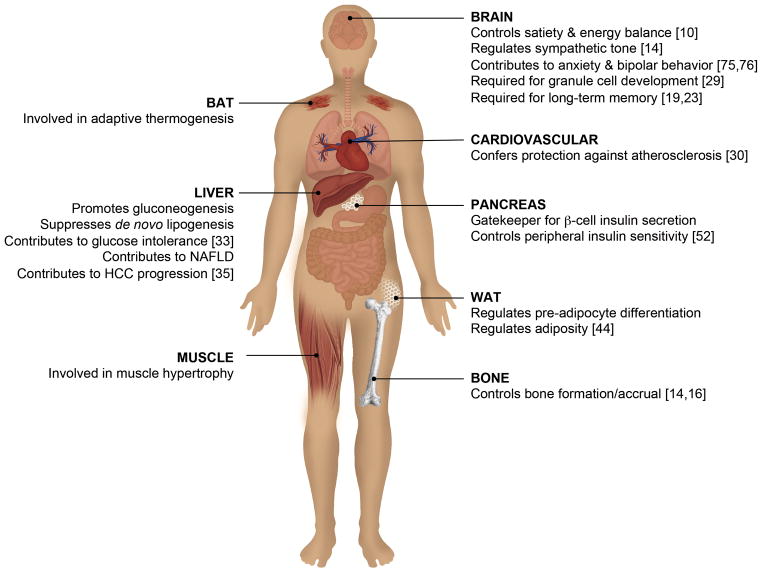
Figure 2. CaMKK2 regulates whole body energy homeostasis through coordinating the actions of key metabolic tissues.
In the brain, CaMKK2 and AMPK function to control appetite and energy homeostasis [10], whereas CaMKK2-mediated activation of CaMKIV is required for cerebellar granule cell development [29]. CaMKK2 is required for regulation of sympathetic tone [14] and long-term memory formation [19,23], and is implicated in anxiety and bipolar disorder [75,76]. Within the liver, CaMKK2 regulates AMPK to promote gluconeogenesis while suppressing de novo lipogenesis [33]. The CaMKK2/CaMKIV axis in the liver also contributes to non-alcoholic fatty liver disease (NAFLD) and is instrumental during progression of hepatocellular carcinoma [35]. In white adipose tissue (WAT), CaMKK2 phosphorylates AMPK to regulate adiposity and pre-adipocyte differentiation [44]. In brown adipose tissue (BAT), CaMKK2 plays a role in adaptive thermogenesis. In the pancreas, CaMKK2 acts as a gatekeeper for β-cell insulin secretion and controls peripheral insulin sensitivity [52]. Within the cardiovascular system, CaMKK2 confers protection against atherosclerosis [30]. In bone, CaMKK2 also functions to regulate bone mass accrual [14,16].
Brain
The brain consumes nearly 70% of total body glucose and its normal functions depend on an optimal range of glucose levels. As part of its role in providing centralized control over the entire organism, the vertebrate brain, particularly the hypothalamus, is responsible for integrating metabolic signals from peripheral organs and tissues [9]. CaMKK2 is highly expressed in the arcuate nucleus (ARC) of the hypothalamus and plays a fundamental role in whole body energy homeostasis [10]. In the fasted state, the orexigenic peptide ghrelin is released from ghrelinergic cells of the intestine and binds its cognate receptor (GHSR, or growth hormone secretagogue receptor) on presynaptic neurons that express the orexigenic hormones Neuropeptide Y (NPY) and Agouti-Related Protein (AgRP), resulting in release of Ca2+ from intracellular stores. Orexin A, an appetite-stimulating neuropeptide, acts via voltage-gated L-type Ca2+ channels to increase intracellular Ca2+ in NPY-producing hypothalamic neurons [11]. Together these Ca2+ signals lead to activation of CaMKK2 and phosphorylation of AMPK, which are necessary for regulating the hypothalamic expression of NPY and AgRP. These findings provide a mechanism for how two orexigenic signals, ghrelin and orexin A, function through CaMKK2 and its target AMPK in specific cell populations of the hypothalamus to control appetite and energy homeostasis [10, 11]. In fact, mice devoid of CaMKK2 fail to respond to ghrelin, have reduced NPY expression, and consequently consume less food when compared to pair-fed wild-type (WT) mice. These findings partially explain why CaMKK2-null mice are protected against high fat diet (HFD)-induced weight gain, insulin resistance, and glucose intolerance [10].
Brain-derived serotonin is a neurotransmitter that modulates appetite and energy expenditure upon binding Htr1a and Htr2b (5-hydroxytryptamine (serotonin) receptor 1A and 2B, respectively) receptors on hypothalamic ARC neurons, triggering cAMP response element-binding protein (CREB)-mediated regulation of genes that favor feeding [12,13]. Conversely, serotonin binding to Htr2c receptors on ventromedial hypothalamic (VMH) neurons induces CaMKIV phosphorylation via CaMKK2 [12]. Active CaMKIV phosphorylates CREB, which in this case promotes expression of genes that decrease sympathetic tone [14]. Since the sympathetic nervous system inhibits osteoblast proliferation and promotes bone resorption by osteoclasts [15], CaMKK2 deletion in VMH neurons decreases peripheral bone mass accrual [14]. A recent study described a dual regulatory role by which CaMKK2 activation of CaMKIV prevents premature differentiation of mesenchymal stem cells into bone matrix-producing osteoblasts, while CaMKK2 activity leads to CREB phosphorylation and subsequent differentiation of osteoclasts. The increase in trabecular bone mass and osteoblast number and the accompanying decrease in osteoclasts were therefore explained by the shift from catabolic to anabolic pathways in CaMKK2-deficient mice during bone remodeling and homeostasis [16]. The phenotypic differences observed in bone mass accrual arising from CaMKK2 loss are best explained by its cell intrinsic functions. The serotonin studies utilized VMH-specific CaMKK2 deletion [14], leaving CaMKK2 function intact in osteoclast and osteoblast progenitors. In whole body CaMKK2 ablation studies [16], the cell autonomous role of CaMKK2 in the osteoblast and osteoclast progenitors has perhaps shifted the balance towards osteoblast differentiation to such an extent that the contribution of sympathetic tone on bone mass accrual becomes secondary or even negligible.
Higher order brain functions are essential for satisfying the desire to feed—the brain facilitates behavioral adaptations required for food accrual while generating a memory of the events for the next round of feeding [17,18]. The Ca2+/CaM kinase cascade up-regulates and activates CREB to induce downstream gene expression programs necessary for long-term memory (LTM) formation and different types of learning [19–22]. CaMKK2 is required during spatial memory formation and for CREB up-regulation and activation but not for contextual fear LTM [19,23]. Downstream of CaMKK2 action lies CaMKIV, which is necessary for contextual LTM [21]. CaMKIV’s role during spatial learning remains largely undefined. Since CaMKIV is activated after LTP at hippocampal CA1 synapses [24], and CaMKK2 is indispensable during hippocampal memory formation [23], it is likely that CaMKIV acts downstream of CaMKK2 during spatial learning to establish late CA1 LTP.
In addition to learning and memory, metabolic syndrome and cerebral ischemia are both associated with impaired energy utilization and dysregulated Ca2+ signaling [25]. It is not surprising that metabolic syndrome is a major risk factor for stroke, which also includes cognitive decline as a persistent pathology [26]. Ca2+/CaM-dependent kinases are associated with endogenous protective functions against cerebrovascular damage. CaMKK2- and CaMKIV-null mice present aggravated brain injuries and behavioral deficits following middle cerebral artery occlusion (MCAO) [27,28]. CaMKIV-knockout mice presented more severe ischemic injuries and displayed higher mortality rates than CaMKK2−/− mice, suggesting that perhaps CaMKIV is downstream of CaMKK2. Phosphorylation of CaMKIV leads to its nuclear translocation and subsequent phosphorylation of histone deacetylase 4 (HDAC4), which is exported from the nucleus, allowing the transcription of CREB, a neuronal survival factor [27,28]. In a similar fashion, both CaMKK2 and CaMKIV are instrumental for the proliferation and survival of cerebellar granule cell neurons, by phosphorylating CREB [29]. CaMKK2/CaMKIV-regulated CREB expression and activation [29] promotes transcription of pro-survival genes, including Bdnf (brain derived neurotrophic factor) during granule cell development [29] and Bcl2 during ischemia [27,28].
Interestingly, both CaMKK2 and CaMKIV preserve the integrity of the blood-brain barrier (BBB). MCAO-treated CaMKK2−/− and CaMKIV−/− mice displayed loss of BBB collagen IV protein and reduced expression of extracellular matrix-degrading, Ca2+-dependent matrix metalloproteinases (MMP) 2/9 [27,28]. It was speculated that CaMKK2 confers protection to endothelial cells that primarily constitute the BBB proper [28]. CaMKK2 phosphorylates both AMPK and SIRT1, a class III histone deacetylase, in mouse aortic endothelium. This dual activation induces anti-inflammatory events in the cytoplasm and nuclear transcription of antioxidants [30]. The mechanism involving the Ca2+/CaM kinase cascade that confers endothelial cell homeostasis in the BBB remains to be fully elucidated, although ischemic models strongly suggest it involves CaMKIV activation by CaMKK2 [27,28] in contrast to that observed in the aortic endothelium that requires AMPK [30].
Distinct from CaMKIV, the role of AMPK is controversial in neuronal ischemic injury. Although stroke induces increased phosphorylation of AMPK, no differences were observed in activated AMPK levels between sham-treated mice and those treated with a selective CaMKK2 inhibitor (STO-609) prior to MCAO [27]. These findings suggest that AMPK activation during stroke is independent of CaMKK2 activity, and that the neuroprotective effects of CaMKK2 are not mediated via AMPK. As reviewed in Ronnett et al. [31], numerous studies provide conflicting claims regarding the benefits and disadvantages of AMPK activation following stroke. However, these reports failed to specifically examine AMPK phosphorylation by CaMKK2. A recent study on neonatal hypoxia-ischemic brain injury demonstrated the pathological activation of the CaMKK2-AMPK axis in neurons [32]. Interestingly, while inactivation of AMPK during the course of ischemic injury promotes neuronal survival, inhibiting AMPK prior to the insult sensitizes the neurons and exacerbates cell death [31]. Collectively, these studies highlight the importance of external inputs, cell types and developmental events that in part reflect the metabolic contributions of the Ca2+/CaM kinase cascade in the brain and the choice of effector molecules that confer cell-specific responses that are impacted by perturbations in metabolic homeostasis.
Liver
While hypothalamic CaMKK2 plays a central role in controlling whole body energy homeostasis [10], little was known about its function in peripheral metabolic tissues. In a study by Anderson et al. [33], WT mice were pair fed to match the consumption level of CaMKK2-null mice maintained on HFD, given that CaMKK2 deletion reduces appetite [10]. Although pair feeding marginally slowed weight gain in WT mice, it failed to improve glucose tolerance [33]. Reduced food intake therefore is insufficient to fully account for the improved metabolic status of CaMKK2−/− mice, suggesting a role for Ca2+/CaM signaling via CaMKK2 in peripheral metabolic tissues.
During prolonged starvation, the liver provides glucose and ketone bodies, both of which are energy sources for the brain [34]. The liver is the central metabolic organ responsible for maintenance of plasma glucose levels and regulating the level of metabolites in blood, including amino acids and fatty acids (FAs) packaged as very low-density lipoproteins (VLDL) for transport [34]. Hepatocytes, which constitute the parenchymal cells of the liver, are essential for its metabolic capacity and express CaMKK2. Acute deletion of hepatic CaMKK2 is sufficient to lower blood glucose and improve glucose tolerance in fed and fasted mice [33]. Primary hepatocytes (PH) isolated from CaMKK2-null mice produced less glucose, which was accompanied by reduced expression of the gluconeogenic genes glucose-6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase (PEPCK), owing to the cell autonomous role of CaMKK2 in phosphorylating hepatic HDAC5 [33]. Similar to HDAC4 function in neurons, HDAC5 phosphorylation relieves transcriptional repression of peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1-α) and activates the expression of gluconeogenesis-related genes [33]. CaMKK2−/− mice also displayed higher acyl-carnitine levels compared to those of WT, as the mice progressed from the fed to fasted states. In vitro studies confirmed that CaMKK2 ablation increased both lipogenesis and lipid oxidation, concomitant with a decrease in glucose utilization in the liver, both of which are consistent with a switch from glucose to fat metabolism [33]. The increased FA oxidation observed in CaMKK2-knockout mice could also be a compensatory response to the overall reduced glucose utilization in these animals, which would account for their steatotic livers observed in response to prolonged fasting that are unaccompanied by other pathophysiological symptoms [33]. Thus, the contribution of Ca2+/CaM signaling through CaMKK2 in regulating energy homeostasis not only involves hypothalamic control of appetite [10], but also a coordinated function in peripheral tissues such as the liver for maintenance of basal glucose levels by controlling hepatic glucose production [33] (Fig. 3).
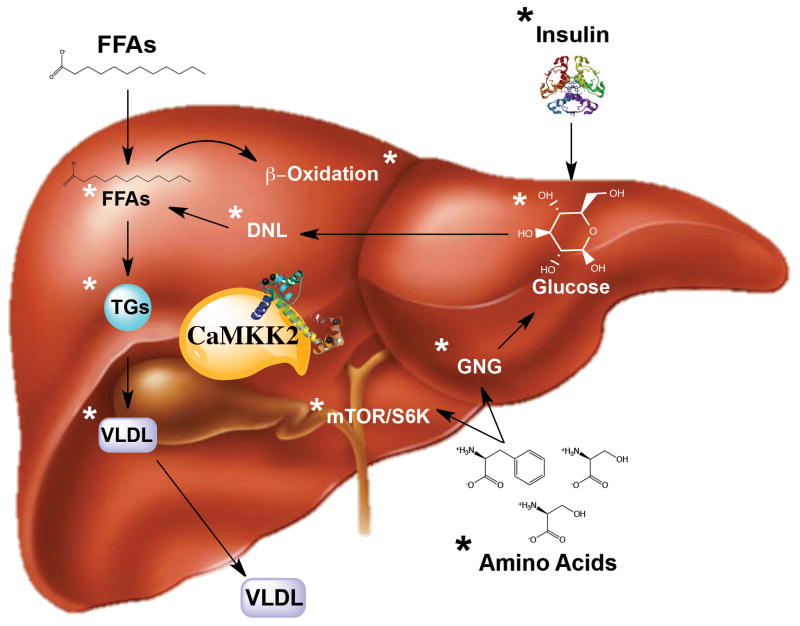
Figure 3. Liver-centric metabolic functions of CaMKK2.
In the liver, ablation of CaMKK2 impacts the metabolic flexibility of numerous pathways as indicated by the asterisks (*). Loss of CaMKK2 in response to high fat diet feeding reduces levels of free fatty acids (FFAs), triglyercides (TGs) and very low-density lipoproteins (VLDL) as well as low densitiy lipoproteins and cholesterol (not depicted) [52]. Consistent with these observations, hepatic-specific ablation of CaMKK2 is sufficient to confer improved insulin sensitivity [33]. Primary hepatocytes devoid of CaMKK2 show increased de novo lipogenesis along with increased β-oxidation [33]. In the context of hepatic cancer, CaMKK2 is essential for driving mTOR/S6K signaling to promote protein synthesis that increases cancer cell proliferation [35].
Hepatocellular carcinoma (HCC) is among the leading causes of cancer-related deaths worldwide and, particularly in the U.S., its rise in incidence has been directly linked to the onset of adult obesity [38]. Ca2+, as previously described, has been epidemiologically linked to obesity, and several studies have also implicated its role in HCC. CaMKK2 expression is markedly up-regulated in HCC compared to matched normal tissue and similarly in several human and murine hepatic cancer cell lines [35]. Survival of HCC patients also inversely correlates with high CaMKK2 expression. Consistent with these findings, knockdown or pharmacological inhibition of CaMKK2 greatly reduced colony formation and proliferation of liver cancer cells, which was rescued by overexpression of exogenous CaMKK2, but not kinase mutant versions of the protein. CaMKK2 activity is therefore instrumental in the cell growth machinery of liver cancer cells [35]. CaMKK2 contributes to hepatic cancer cell growth primarily through CaMKIV, which together with CaMKK2 provides a scaffold for the coordinated regulation of the mammalian target of rapamycin/ribosomal protein S6 kinase, 70 kDa (mTOR/S6K) proteins that respond to nutrient status to drive protein synthesis [35] (Fig. 3). CaMKIV has been previously shown to be up-regulated in human and rat HCC [34]. Ablation of CaMKIV in liver cancer cell lines phenocopies CaMKK2 deletion, and although rescue experiments with CaMKK2 re-expression restored protein synthesis and proliferation defects in both CaMKK2- or CaMKIV-knockdown cells, CaMKIV overexpression was insufficient to completely rescue the loss of CaMKK2 [35].
In vivo xenograft studies revealed that loss of CaMKK2 in PHM1 cells (p53-null/Myc-transformed liver cancer cells) subcutaneously injected into nude mice retarded tumor growth and reduced tumor volume compared to WT PHM1-derived tumors [35]. Furthermore, pharmacological treatment of nude mice harboring WT PHM1-derived tumors with STO-609 was sufficient to suppress tumor outgrowth. In a diethylnitrosamine (DEN) murine model for hepatic cancer, STO-609 treatment resulted in a 20% reduction in tumor volume while vehicle-treated mice showed a 50% increase in tumor volume as measured via PET/CT imaging [35]. CaMKK2 activity is required for aggressive liver cancer growth, and its inhibition is sufficient to block the downstream signaling machinery, leading to attenuation of tumor growth in vivo. Given that CaMKK2−/− mice are resistant to HFD-induced obesity [33], a major risk factor for HCC, and are protected from LPS-induced fulminant hepatitis [37], it is not surprising that CaMKK2 significantly contributes to cancer cell proliferation. Perhaps even more significant is the potential for targeting the CaMKK2/CaMKIV signaling axis by using STO-609 as a therapeutic recourse. Several studies have focused on Ca2+ signaling components, and CaMKK2 in particular, to explore new therapeutic avenues for combatting fatty liver disease and cancer [39,40]. Baicalin, a polyphenolic compound, decreased fat, liver, and whole body weights in HFD-fed mice by inhibiting the CaMKK2-AMPK pathway [39], while bortezomib, a proteasome inhibitor and multiple myeloma therapeutic agent, activated CaMKK2 protein synthesis in human breast cancer cell lines [40]. Only time, further studies, and eventually human clinical trials will reveal the feasibility of such a strategy.
Adipose
Following feeding stimulated by orexigenic signals in the brain, glucose and VLDL produced by the liver are converted into triacylglycerides (TAG) in adipose tissue. During fasting or exercise, TAGs are hydrolyzed into glycerol and free fatty acids (FFAs), which are then transported to the liver for use during gluconeogenesis. Adipocytes therefore largely function to balance lipid storage and release, to coincide with the feeding to fasting transition [41]. Obesity, metabolic syndrome, and their associated complications are often characterized by adipose tissue dysfunction. These dysfunctions include increased macrophage recruitment, abnormal microvasculature, and perturbed adipokine production [42]. Disrupted Ca2+ signaling accompanying obesity is also associated with attenuated differentiation of pre-adipocyte progenitors [43]. CaMKK2-null mice display increased adiposity when fed standard chow due to both increases in size and number of adipocytes [44]. CaMKK2 expression is suppressed once pre-adipocytes differentiate into mature adipocytes, while in vitro ablation or pharmacological inhibition of CaMKK2 increased adipogenesis [44]. Taken together, these findings suggested that CaMKK2 is essential during this differentiation process. Increased intracellular Ca2+ leads to CaMKK2 activation of AMPK in pre-adipocytes, which together maintain expression of preadipocyte factor-1 (Pref-1). Pref-1, in turn, maintains expression of Sox9 through phosphorylation of ERK1/2. Sox9 inhibits transcription of early adipogenic factors such as C/EBPβ and C/EBPδ and eventually blunts other downstream events that maintain a differentiated adipocyte state [44]. CaMKK2 and AMPK therefore play cell autonomous roles in pre-adipocytes to maintain an undifferentiated state [44], similar to their roles in bone remodeling [16], cerebellar granule cell development and granulopoiesis [45]. Thus, Ca2+/CaM signaling consistently functions within several progenitor cell populations to maintain their undifferentiated, proliferative state, and employs a cell-autonomous and unique mechanism involving CaMKK2.
Although CaMKK2 is not expressed in mature adipocytes [44], studies have reported such expression in adipose exposed to exogenous hormonal stimulation [46,47]. In response to thyroid hormone, increased Ca2+ concentration and AMPK activation via CaMKK2 was observed in adipocytes hypomorphic for Lkb1, an alternative upstream kinase for AMPK activation [47]. Meanwhile, CaMKK2 reportedly regulates AMPK action in response to glucagon, which then phosphorylates acetyl-CoA carboxylase 1 (ACC1) in adipocytes. Glucagon-induced phosphorylation of ACC1 attenuates its activity during FA synthesis [46]. Since increased intracellular Ca2+ within adipocytes was not induced by glucagon, the mechanism for AMPK activation remains to be clarified and reconciled with previous findings. One possibility is that glucagon up-regulates CaMKK2 expression, as this gene is a stimulus-regulated event in a variety of cell types [35,48–50].
Pancreas
Two key regulators of liver metabolism are the pancreatic hormones glucagon and insulin. Although the balance between Ca2+ influx and efflux critical to the regulation of insulin production by pancreatic beta (β) cells is well appreciated [51], the molecular underpinnings that contribute to this process are poorly understood. Recent reports demonstrate the key roles of Ca2+ signaling and Ca2+/CaM kinase cascade components in insulin production and secretion. CaMKK2 expression is largely correlated with β cells and only marginally with glucagon-producing α cells. Moreover, CaMKK2 is not required for maintenance of islet structure, β cell polarity, or hormone production [52]. In fact, loss of CaMKK2 led to increased insulin content per islet and increased pancreatic islet insulin secretion. Therefore, it appears that CaMKK2 is an important component of the Ca2+-mediated response to glucose signaling in β cells, acting as a molecular rheostat to regulate insulin secretion and perhaps production. The activity of CaMKK2 facilitates pancreatic overproduction and over-secretion of insulin during chronic metabolic stress such as HFD feeding, which contributes to insulin resistance and glucose intolerance in WT mice. CaMKK2-null mice fed a standard chow have plasma insulin levels that are marginally higher than WT mice, but still within normal physiological levels. Chronic metabolic stress fails to further induce insulin production or secretion because CaMKK2, which is required for these processes, is absent [52]. Combined with its roles in controlling satiety (brain) [10], metabolic conversion (liver) [33] and storage (adipose) [44], these newly appreciated functions of CaMKK2 in the pancreas also fractionally contribute to the protection from diet-induced glucose intolerance and insulin resistance observed in CaMKK2−/− mice.
The exact mechanism by which CaMKK2 regulates insulin secretion and/or production remains to be explored. Perhaps loss of CaMKK2 results in increased secretory granules and/or increased number of granules poised for secretion at the plasma membrane. Other reports suggest that following an increase in intracellular Ca2+, CaMKK2 acts through AMPK to regulate insulin secretion and β cell survival in response to either uridine diphosphate [53] or to adipose tissue derived leptin [54]. Nevertheless, the mechanism by which CaMKK2 regulates insulin secretion is an area ripe for investigation and could even be exploited for treatment of T2DM.
CaMKK2 is also involved in lineage programming of pancreatic α to β cells [55], which is an attractive therapeutic strategy for type 1 diabetes (T1D) that is characterized by loss of β cell mass. Analysis of transcriptomic, proteomic, and phosphoproteomic profiles from α and β cells found that CaMKK2 had decreased expression and phosphorylation in β cells compared to α cells [55]. In vitro knockdown of CaMKK2 in α cells or treatment with STO-609 led to the increased expression of insulin and other key β cell markers, although glucagon expression was also increased. These data present a strong potential for inhibition of this kinase during α-to-β transdifferentiation [55]. Pancreatic α and β cells derive from the same endocrine progenitor during development. Knowing that CaMKK2 functions in cell fate decisions in cerebellar granule cells, adipose [44], bone [16], and granulocyte-monocyte progenitors [45], it is likely that CaMKK2 also plays a role during the differentiation of these pancreatic cell types. CaMKK2-null mice had pancreatic islets that appeared morphologically normal and retained glucagon-expressing cells that were presumably α cells [52]. However, the expression pattern of other definitive α and β cells were not particularly scored [52], and would be an interesting follow up to investigate the role of CaMKK2 in pancreatic islet cell differentiation. It is plausible that deletion of CaMKK2 in endocrine progenitors either endowed α cells the ability to produce insulin and/or skewed the differentiation towards β cell progenitors.
Concluding Remarks and Future Perspectives
In this review, we provide a synthesis of the most recent and critical reports highlighting the roles of the Ca2+/CaM-dependent kinase cascade in organismal metabolic regulation. The kinase cascade triggered by the ligand-receptor binding of Ca2+ to CaM is a tightly regulated mechanism ubiquitous to every key metabolic tissue, which confers pleiotropic actions ranging from regulation of food intake to energy expenditure and fuel storage. Aside from rekindling these previously underappreciated concepts, this review emphasizes the potential of inhibiting members of this kinase cascade for treatment of metabolic diseases such as obesity, diabetes, and even cancer. Of the kinases in this Ca2+/CaM-dependent pathway, CaMKK2 currently appears to be the most attractive therapeutic target. Inhibition of CaMKK2 in mice results in attenuated food intake, promoted weight loss, and generally improved whole body glucose homeostasis [10,33]. CaMKK2 also inhibits progenitor differentiation into mature adipocytes [44]. Investigation into the action of CaMKK2 in pancreatic cell types suggests a prophylactic role against developing insulin resistance [52]. The potential for targeting this kinase to induce α-to-β cell transdifferentiation could impact T1D treatment strategies [55]. Also, the cell autonomous functions of CaMKK2 in macrophages [37], which play key roles in a variety of metabolic tissues, offers an alternative strategy for attenuating the pleiotropic actions of this kinase by pharmacological inhibition. Finally, in vivo administration of STO-609 to mice harboring hepatic tumors significantly regressed tumor burden [35]. STO-609 is currently the most selective and potent pharmacologic inhibitor against CaMKK2. The limited availability of information regarding the pharmacokinetic properties and toxicity of STO-609 as well as the optimal method of packaging and administration of the inhibitor are all issues that must be addressed before one can consider STO-609 as a candidate therapeutic agent for pathologies arising from metabolic dysfunction.
Despite all the information summarized in this review regarding the critical roles of Ca2+/CaM-dependent kinase cascade components in whole body energy balance, a number of key questions remain unanswered (see Outstanding Questions). CaMKK2’s function remains largely undefined in a variety of metabolic tissues, such as brown adipose tissue, cardiac and skeletal muscle, spleen, and kidney. Within skeletal muscle in particular, although CaMKK2 does not appear to be expressed in this tissue, congenic ablation of CaMKK2 results in overt muscle hypertrophy. These data suggest CaMKK2 might regulate myogenic humoral factors, which represents yet another area that is primed for further investigation.
Outstanding Questions Box.
What are the roles of CaMKK2 signaling in unexplored metabolic tissues (i.e. brown adipose tissue, skeletal and cardiac muscle, spleen and kidney)?
What signals (i.e. hormones, metabolites) control the gene expression of members of the Ca2+/CaM/CaMK axis (i.e. Calm, CaMKK2, AMPK, CaMKIV) in response to physiologic or pathophysiological conditions? Is the expression of these pathway components coordinately regulated?
What are the cell-specific functions of the Ca2+/CaM-dependent kinase pathway components? Are the known functions of Ca2+/CaM-dependent kinases attributed to common cell types that are ubiquitously present in all metabolic organs (i.e. macrophages)?
What is the subcellular localization of CaMKK2? Is CaMKK2 bound or tethered to intracellular membranes? Does CaMKK2 shuttle between the cytoplasm and nucleus? What mechanisms control the intracellular trafficking and/or retention of CaMKK2?
Can alternate and/or additional targets/substrates for CaMKK2 be identified? If so, what are their cell- or tissue-specific functions downstream of Ca2+/CaM/CaMKK2 action?
Does CaMKK2 contribute to metabolic reprogramming by controlling fuel substrate utilization?
Aside from consensus binding sequences within the 5′ untranslated region of the human CaMKK2 gene for the transcription factors Ikaros, Runx1, and GATA1 [5], very little is known about regulation of CaMKK2 and CaMKIV gene expression. How are the CaMKK2 and CaMKIV genes controlled? An up-regulation in CaMKK2 expression was observed over the course of hepatic tumor progression in human and mouse samples [35], and CaMKIV expression was likewise increased in human and rat liver tumors [36]. It will be interesting to determine the distinct mechanisms for regulating the expression of these genes during pathologic versus physiologic conditions, or whether these events are merely the result of compensatory processes. Moreover, both CaMKK2 and CaMKIV have pleiotropic and cell-autonomous functions in various metabolic organs. Are there tissue- or cell-specific mechanisms in place to regulate the expression of these kinases?
Analysis of bulk tissue tends to dilute the contribution of individual and underrepresented cell types relative to that of the parenchymal cell type. It was previously demonstrated in the liver that fractionation of the hepatic cell types prior to transcriptomic analyses revealed unique, cell-centric gene expression patterns generated in response to oncogenic or chronic metabolic stress [56]. Performing OMICs-based analyses on individual cell populations will not only uncover signaling nodes specific for that cell type, but are likely to unveil cell-specific functions for proteins and/or protein families. Fractionation of tissues into individual cell types could help determine whether any of the Ca2+/CaM-dependent kinase pathway components, particularly CaMKK2, have cell-specific functions.
The precise distribution of the CaMKK2 protein within the cell is poorly understood. Determining the subcellular localization of the active versus inactive forms of this kinase, in basal versus stimulated states, will shed light on unappreciated functions of CaMKK2. Various studies implicate CaMKK2 activity with phagocytosis [37], secretion [52], and autophagy [57], which involve trafficking of membrane-associated and soluble proteins. Whether CaMKK2 is tethered to any intracellular membranes and what mechanisms are in place to regulate its trafficking within the cell are important questions that warrant further investigation.
Only recently has it been discovered that a kinase other than Lkb1 could phosphorylate AMPK. CaMKK2 activates the AMPKα subunit in response to increases in intracellular Ca2+ concentration, which is then followed by complex formation involving CaMKK2, AMPKα and β subunits and Ca2+/CaM [5]. As CaMKI, CaMKIV and AMPK represent the most well characterized substrates of CaMKK2, it is likely that additional substrates exist that may open doors for unappreciated physiological functions of this kinase. The availability of specific pharmacological inhibitors for any new CaMKK2 substrates will be greatly beneficial, especially for assessing their potential as putative therapeutic agents.
Trends Box.
Calmodulin is the intracellular receptor for Ca2+ that controls the downstream actions of the CaMK cascade that contributes to metabolic homeostasis.
CaMKK2 contributes to the maintenance of organismal energy homeostasis such that CaMKK2−/− mice are protected from diet-induced obesity, glucose intolerance, and insulin resistance.
CaMKK2 activates CaMKIV to regulate sympathetic tone and bone mass accrual, protect neurons from ischemia-related injuries, maintain the integrity of the blood-brain barrier, and regulate protein synthesis to control hepatic tumor cell growth.
The pleiotropic functions of CaMKK2 downstream of Ca2+/CaM for coordinating energy homeostasis underscore the importance of developing suitable inhibitors against CaMKK2 for treatment of metabolic-related diseases.
Acknowledgments
This work was supported by NIH GM033976 to A.R.M. The authors acknowledge the joint participation by Adrienne Helis Malvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine and the “CaMKK2 inhibitors for therapeutic treatment of hepatic cancer” program.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends in cell biology. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 2.Means AR, Dedman JR. Calmodulin--an intracellular calcium receptor. Nature. 1980;285:73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- 3.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Berchtold MW, Egli R, Rhyner JA, Hameister H, Strehler EE. Localization of the human bona fide calmodulin genes CALM1, CALM2, and CALM3 to chromosomes 14q24-q31, 2p21.1-p21.3, and 19q13.2-q13.3. Genomics. 1993;16:461–465. doi: 10.1006/geno.1993.1211. [DOI] [PubMed] [Google Scholar]
- 5.Racioppi L, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J Biol Chem. 2012;287:31658–31665. doi: 10.1074/jbc.R112.356485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MF, Anderson KA, Means AR. Characterization of the CaMKKbeta-AMPK signaling complex. Cell Signal. 2011;23:2005–2012. doi: 10.1016/j.cellsig.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.dos Santos LC, de Padua Cintra I, Fisberg M, Martini LA. Calcium intake and its relationship with adiposity and insulin resistance in post-pubertal adolescents. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association. 2008;21:109–116. doi: 10.1111/j.1365-277X.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- 8.Torres MR, da Ferreira TS, Carvalho DC, Sanjuliani AF. Dietary calcium intake and its relationship with adiposity and metabolic profile in hypertensive patients. Nutrition. 2011;27:666–671. doi: 10.1016/j.nut.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Coll AP, Yeo GSH. The hypothalamus and metabolism: integrating signals to control energy and glucose homeostasis. Current Opinion in Pharmacology. 2013;13:970–976. doi: 10.1016/j.coph.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, et al. Hypothalamic CaMKK2 Contributes to the Regulation of Energy Balance. Cell Metabolism. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Wu WN, Wu PF, Zhou J, Guan XL, Zhang Z, et al. Orexin-A activates hypothalamic AMP-activated protein kinase signaling through a Ca(2)(+)-dependent mechanism involving voltage-gated L-type calcium channel. Mol Pharmacol. 2013;84:876–887. doi: 10.1124/mol.113.086744. [DOI] [PubMed] [Google Scholar]
- 12.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav VK, Oury F, Tanaka KF, Thomas T, Wang Y, et al. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208:41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oury F, Yadav VK, Wang Y, Zhou B, Liu XS, et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 2010;24:2330–2342. doi: 10.1101/gad.1977210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42:837–840. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Cary RL, Waddell S, Racioppi L, Long F, Novack DV, et al. Inhibition of Ca(2)(+)/calmodulin-dependent protein kinase kinase 2 stimulates osteoblast formation and inhibits osteoclast differentiation. J Bone Miner Res. 2013;28:1599–1610. doi: 10.1002/jbmr.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracis C, Gurarie E, Van Moorter B, Goodwin RA. Memory Effects on Movement Behavior in Animal Foraging. PLoS ONE. 2015;10:e0136057. doi: 10.1371/journal.pone.0136057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janmaat KRL, Ban SD, Boesch C. Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Animal Behaviour. 2013;86:1183–1205. [Google Scholar]
- 19.Peters M, Mizuno K, Ris L, Angelo M, Godaux E, et al. Loss of Ca2+/Calmodulin Kinase Kinase β Affects the Formation of Some, But Not All, Types of Hippocampus-Dependent Long-Term Memory. The Journal of Neuroscience. 2003;23:9752–9760. doi: 10.1523/JNEUROSCI.23-30-09752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno K, Ris L, Sánchez-Capelo A, Godaux E, Giese KP. Ca2+/calmodulin kinase kinase alpha is dispensable for brain development but is required for distinct memories in male, though not in female, mice. Mol Cell Biol. 2006:26. doi: 10.1128/MCB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho N, Liauw JA, Blaeser F, Wei F, Hanissian S, et al. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, et al. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno K, Antunes-Martins A, Ris L, Peters M, Godaux E, et al. Calcium/calmodulin kinase kinase beta has a male-specific role in memory formation. Neuroscience. 2007:145. doi: 10.1016/j.neuroscience.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 24.Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, et al. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- 25.Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010;277:3622–3636. doi: 10.1111/j.1742-4658.2010.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, et al. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCullough LD, Tarabishy S, Liu L, Benashski S, Xu Y, et al. Inhibition of calcium/calmodulin-dependent protein kinase kinase α and calcium/calmodulin-dependent protein kinase IV is detrimental in cerebral ischemia. Stroke. 2013:44. doi: 10.1161/STROKEAHA.113.001030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, McCullough L, Li J. Genetic deletion of calcium/calmodulin-dependent protein kinase kinase β (CaMKK β) or CaMK IV exacerbates stroke outcomes in ovariectomized (OVXed) female mice. BMC Neuroscience. 2014;15:1–10. doi: 10.1186/s12868-014-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokubo M, Nishio M, Ribar TJ, Anderson KA, West AE, et al. BDNF-mediated cerebellar granule cell development is impaired in mice null for CaMKK2 or CaMKIV. J Neurosci. 2009;29:8901–8913. doi: 10.1523/JNEUROSCI.0040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen L, Chen Z, Zhang F, Cui X, Sun W, et al. Ca2+/calmodulin-dependent protein kinase kinase β phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proceedings of the National Academy of Sciences. 2013;110:E2420–E2427. doi: 10.1073/pnas.1309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. Journal of Neurochemistry. 2009;109:17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousset CI, Leiper FC, Kichev A, Gressens P, Carling D, et al. A dual role for AMP-activated protein kinase (AMPK) during neonatal hypoxic–ischaemic brain injury in mice. Journal of Neurochemistry. 2015;133:242–252. doi: 10.1111/jnc.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson KA, Lin F, Ribar TJ, Stevens RD, Muehlbauer MJ, et al. Deletion of CaMKK2 from the Liver Lowers Blood Glucose and Improves Whole-Body Glucose Tolerance in the Mouse. Molecular Endocrinology. 2012;26:281–291. doi: 10.1210/me.2011-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 35.Lin F, Marcelo KL, Rajapakshe K, Coarfa C, Dean A, et al. The camKK2/camKIV relay is an essential regulator of hepatic cancer. Hepatology. 2015;62:505–520. doi: 10.1002/hep.27832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura N, Tai Y, Sugimoto K, Kobayashi R, Konishi R, et al. Enhanced expression and activation of Ca2+/calmodulin-dependent protein kinase IV in hepatocellular carcinoma. Cancer. 2000;89:1910–1916. doi: 10.1002/1097-0142(20001101)89:9<1910::aid-cncr6>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Racioppi L, Noeldner PK, Lin F, Arvai S, Means AR. Calcium/Calmodulin-dependent Protein Kinase Kinase 2 Regulates Macrophage-mediated Inflammatory Responses. Journal of Biological Chemistry. 2012;287:11579–11591. doi: 10.1074/jbc.M111.336032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World Journal of Gastroenterology: WJG. 2010;16:3603–3615. doi: 10.3748/wjg.v16.i29.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xi Y, Wu M, Li H, Dong S, Luo E, et al. Baicalin Attenuates High Fat Diet-Induced Obesity and Liver Dysfunction: Dose-Response and Potential Role of CaMKKβ/AMPK/ACC Pathway. Cellular Physiology and Biochemistry. 2015;35:2349–2359. doi: 10.1159/000374037. [DOI] [PubMed] [Google Scholar]
- 40.Deshmukh RR, Dou QP. Proteasome inhibitors induce AMPK activation via CaMKKβ in human breast cancer cells. Breast Cancer Research and Treatment. 2015;153:79–88. doi: 10.1007/s10549-015-3512-2. [DOI] [PubMed] [Google Scholar]
- 41.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 43.Jensen B, Farach-Carson MC, Kenaley E, Akanbi KA. High extracellular calcium attenuates adipogenesis in 3T3-L1 preadipocytes. Exp Cell Res. 2004;301:280–292. doi: 10.1016/j.yexcr.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Lin F, Ribar TJ, Means AR. The Ca2+/Calmodulin-Dependent Protein Kinase Kinase, CaMKK2, Inhibits Preadipocyte Differentiation. Endocrinology. 2011;152:3668–3679. doi: 10.1210/en.2011-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng EC, Racioppi L, Means AR. A cell-intrinsic role for CaMKK2 in granulocyte lineage commitment and differentiation. J Leukoc Biol. 2011;90:897–909. doi: 10.1189/jlb.0311152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng I-C, Chen Z, Sun W, Li Y-S, Marin TL, et al. Glucagon regulates ACC activity in adipocytes through the CAMKKβ/AMPK pathway. American Journal of Physiology -Endocrinology and Metabolism. 2012;302:E1560–E1568. doi: 10.1152/ajpendo.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gormand A, Henriksson E, Ström K, Jensen TE, Sakamoto K, et al. Regulation of AMP-activated protein kinase by LKB1 and CaMKK in adipocytes. Journal of Cellular Biochemistry. 2011;112:1364–1375. doi: 10.1002/jcb.23053. [DOI] [PubMed] [Google Scholar]
- 48.Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. The EMBO Journal. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krebs J, Means RL, Honegger P. Induction of calmodulin kinase IV by the thyroid hormone during the development of rat brain. J Biol Chem. 1996;271:11055–11058. doi: 10.1074/jbc.271.19.11055. [DOI] [PubMed] [Google Scholar]
- 50.Liu YY, Brent GA. A complex deoxyribonucleic acid response element in the rat Ca(2+)/calmodulin-dependent protein kinase IV gene 5′-flanking region mediates thyroid hormone induction and chicken ovalbumin upstream promoter transcription factor 1 repression. Mol Endocrinol. 2002;16:2439–2451. doi: 10.1210/me.2001-0324. [DOI] [PubMed] [Google Scholar]
- 51.Gromada J, Frokjaer-Jensen J, Dissing S. Glucose stimulates voltage- and calcium-dependent inositol trisphosphate production and intracellular calcium mobilization in insulin-secreting beta TC3 cells. Biochem J. 1996;314( Pt 1):339–345. doi: 10.1042/bj3140339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcelo KL, Ribar T, Means CR, Tsimelzon A, Stevens RD, et al. Research Resource: Roles for Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) in Systems Metabolism. Mol Endocrinol. 2016:me20161021. doi: 10.1210/me.2016-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balasubramanian R, Maruoka H, Jayasekara PS, Gao Z-G, Jacobson KA. AMP-activated protein kinase as regulator of P2Y6 receptor-induced insulin secretion in mouse pancreatic β-cells. Biochemical Pharmacology. 2013;85:991–998. doi: 10.1016/j.bcp.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park S-H, Ryu S-Y, Yu W-J, Han YE, Ji Y-S, et al. Leptin promotes KATP channel trafficking by AMPK signaling in pancreatic β-cells. Proceedings of the National Academy of Sciences. 2013;110:12673–12678. doi: 10.1073/pnas.1216351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choudhary A, Hu He K, Mertins P, Udeshi ND, Dančík V, et al. Quantitative-Proteomic Comparison of Alpha and Beta Cells to Uncover Novel Targets for Lineage Reprogramming. PLoS ONE. 2014;9:e95194. doi: 10.1371/journal.pone.0095194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Means AR, Kahl CR, Crenshaw DG, Dayton JS. Traversing the Cell Cycle: The Calcium/Calmodulin Connection. In: Carafoli E, Klee C, editors. Calcium as a Cellular Regulator. New York: Oxford University Press; 1999. [Google Scholar]
- 57.Crawford SE, Estes MK. Viroporin-mediated calcium-activated autophagy. Autophagy. 2013;9:797–798. doi: 10.4161/auto.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheung WY. Cyclic 3′,5′-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970;38:533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- 59.Kakiuchi S, Yamazaki R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3′,5′-nucleotide phosphodiesterase (3) Biochem Biophys Res Commun. 1970;41:1104–1110. doi: 10.1016/0006-291x(70)90199-3. [DOI] [PubMed] [Google Scholar]
- 60.Wang JH, Desai R. A brain protein and its effect on the Ca2+-and protein modulator-activated cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1976;72:926–932. doi: 10.1016/s0006-291x(76)80220-3. [DOI] [PubMed] [Google Scholar]
- 61.Cheung WY. Calmodulin plays a pivotal role in cellular regulation. Science. 1980;207:19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- 62.Fakunding JL, Tindall DJ, Dedman JR, Mena CR, Means AR. Biochemical actions of follice-stimulating hormone in the sertoli cell of the rat testis. Endocrinology. 1976;98:392–402. doi: 10.1210/endo-98-2-392. [DOI] [PubMed] [Google Scholar]
- 63.Shenolikar S, Cohen PT, Cohen P, Nairn AC, Perry SV. The role of calmodulin in the structure and regulation of phosphorylase kinase from rabbit skeletal muscle. Eur J Biochem. 1979;100:329–337. doi: 10.1111/j.1432-1033.1979.tb04175.x. [DOI] [PubMed] [Google Scholar]
- 64.Colomer-Font J, Means AR. Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in Health and Disease. In: Carafoli E, Brini M, editors. Calcium Signalling and Disease. Dordrecht, The Netherlands: Springer Science; 2007. pp. 169–214. [DOI] [PubMed] [Google Scholar]
- 65.Putkey JA, Ts’ui KF, Tanaka T, Lagace L, Stein JP, et al. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983;258:11864–11870. [PubMed] [Google Scholar]
- 66.Guerriero V, Jr, Russo MA, Olson NJ, Putkey JA, Means AR. Domain organization of chicken gizzard myosin light chain kinase deduced from a cloned cDNA. Biochemistry. 1986;25:8372–8381. doi: 10.1021/bi00374a007. [DOI] [PubMed] [Google Scholar]
- 67.Hanley RM, Means AR, Ono T, Kemp BE, Burgin KE, et al. Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science. 1987;237:293–297. doi: 10.1126/science.3037704. [DOI] [PubMed] [Google Scholar]
- 68.Means AR, Cruzalegui F, LeMagueresse B, Needleman DS, Slaughter GR, et al. A novel Ca2+/calmodulin-dependent protein kinase and a male germ cell-specific calmodulin-binding protein are derived from the same gene. Mol Cell Biol. 1991;11:3960–3971. doi: 10.1128/mcb.11.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Picciotto MR, Czernik AJ, Nairn AC. Calcium/calmodulin-dependent protein kinase I. cDNA cloning and identification of autophosphorylation site. J Biol Chem. 1993;268:26512–26521. [PubMed] [Google Scholar]
- 70.Tokumitsu H, Enslen H, Soderling TR. Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. Molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J Biol Chem. 1995;270:19320–19324. doi: 10.1074/jbc.270.33.19320. [DOI] [PubMed] [Google Scholar]
- 71.Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, et al. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- 72.Gao G, Widmer J, Stapleton D, Teh T, Cox T, et al. Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochim Biophys Acta. 1995;1266:73–82. doi: 10.1016/0167-4889(94)00222-z. [DOI] [PubMed] [Google Scholar]
- 73.Wayman GA, Kaech S, Grant WF, Davare M, Impey S, et al. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J Neurosci. 2004;24:3786–3794. doi: 10.1523/JNEUROSCI.3294-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kahl CR, Means AR. Regulation of cyclin D1/Cdk4 complexes by calcium/calmodulin-dependent protein kinase I. J Biol Chem. 2004;279:15411–15419. doi: 10.1074/jbc.M312543200. [DOI] [PubMed] [Google Scholar]
- 75.Scott JW, Park E, Rodriguiz RM, Oakhill JS, Issa SM, et al. Autophosphorylation of CaMKK2 generates autonomous activity that is disrupted by a T85S mutation linked to anxiety and bipolar disorder. Sci Rep. 2015;5:14436. doi: 10.1038/srep14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erhardt A, Lucae S, Unschuld PG, Ising M, Kern N, et al. Association of polymorphisms in P2RX7 and CaMKKb with anxiety disorders. Journal of Affective Disorders. 2007;101:159–168. doi: 10.1016/j.jad.2006.11.016. [DOI] [PubMed] [Google Scholar]