Summary
Background and Objectives
Plasminogen appears to affect brain inflammation, cell movement, fibrinolysis, neuronal excitotoxicity and cell death. However, brain tissue and circulating blood plasminogen may have different roles and, there is large individual variation in blood plasminogen levels. The aim of this study was to determine the integrated effect of blood plasminogen levels on ischemic brain injury.
Methods
We examined thromboembolic stroke in mice with varying, experimentally-determined, blood plasminogen levels. Ischemic brain injury, blood-brain barrier breakdown, matrix metalloproteinase-9 expression and microvascular thrombosis were determined.
Results
Within the range of normal variation, plasminogen levels were strongly associated with ischemic brain injury (p<0.0001); higher blood plasminogen levels had dose-related, protective effects (p<0.0001). Higher plasminogen levels were associated with increased dissolution of the middle cerebral artery thrombus (p<0.0001). Higher plasminogen levels decreased blood-brain barrier breakdown (p<0.05), matrix metalloproteinase-9 expression (p<0.01) and reduced microvascular thrombosis (p<0.0001) in the ischemic brain. In plasminogen-deficient mice, selective restoration of blood plasminogen levels reversed the harmful effects of plasminogen deficiency on ischemic brain injury. Specific inhibition of thrombin also reversed the effect of plasminogen deficiency on ischemic injury by diminishing microvascular thrombosis, blood-brain barrier breakdown and matrix metalloproteinase-9 expression.
Conclusions
Variation in blood plasminogen levels, within the range seen in normal individuals, had marked effects on experimental ischemic brain injury. Higher plasminogen levels protected against ischemic brain injury, decreased blood-brain barrier breakdown, matrix metalloproteinase-9 expression and microvascular thrombosis. The protective effects of blood plasminogen appear to be mediated largely through reduction of microvascular thrombosis in the ischemic territory.
Keywords: Blood-brain Barrier, Cerebral Infarction, Matrix Metalloproteinase-9, Plasminogen, Thrombosis, Thromboembolism
Introduction
Ischemic stroke affects nearly 17 million people a year, leaving nearly half of patients disabled or dead. Thrombosis is the most common acute cause of ischemic stroke, but the mechanisms that modify the severity of subsequent brain injury are still poorly understood. Plasminogen and its derivative enzyme plasmin (P(g)) participate in processes that may affect outcomes in ischemic stroke such as fibrinolysis, inflammation, cell movement, neuronal excitotoxicity and neuronal death etc. [1]. Plasminogen (Pg) is a liver-synthesized, blood protein that circulates in the vasculature and it is also expressed in the brain in the cortex, hippocampus and the cerebellum of adult animals [2]. Since P(g) has several pathophysiologic roles, which may be different in the brain or vascular compartments, it has proven challenging to define the integrated effect of P(g) on acute ischemic stroke.
Several studies have examined the effects of P(g) within the brain tissue. Direct injections of P(g) into the brain parenchyma enhance neuronal apoptosis, brain tissue damage and inflammatory cell infiltration [3]. Within the brain parenchyma, P(g) has been shown to contribute to excitotoxic neuronal cell death, in a non-fibrin-dependent manner [4], in part by acting as a chemokine activator of MCP-1 [5]. Plasmin has been linked to microglial activation [6] and laminin degradation [7] that alter the survival of neurons and astrocytes. Pg potentiates the toxic effects of thrombin and may contribute to intracerebral brain bleeding [8].
The overall effects of vascular or blood P(g) on ischemic brain injury are also poorly understood. Nagai et al. found that P(g) deficiency increased ischemic neurodegeneration after surgical arterial ligation [9]. P(g) appears to enhance inflammatory responses such as neutrophil accumulation and activation of matrix metalloproteinase-9 (MMP-9) which may worsen acute brain and vascular reperfusion injury [10, 11]. In addition, cell-based models suggest that Pg may contribute to blood-brain barrier (BBB) breakdown [12, 13]. Although P(g) plays a well-established role in fibrinolysis, normal endogenous fibrinolysis typically has minimal effects on establishing arterial reperfusion in clinical or experimental models of ischemic stroke [14, 15]. It is also perplexing that nearly eight-fold variations in blood Pg levels have been described in normal individuals, yet Pg levels have not been correlated with stroke risk and severe human Pg deficiency is not associated with an increased risk of vascular thrombosis [16-18].
The aim of these experiments was to determine the integrated effects of blood Pg levels on ischemic brain injury in a well-established model of cerebral thromboembolism that has pathophysiologic relevance to human ischemic stroke [19]. Our data show that ~two-fold variations in blood P(g) levels, which are within the range seen in normal individuals, significantly alter ischemic brain injury following cerebral thromboembolism. Microvascular thrombosis appears to play a causative role as selective inhibition of that process compensates for the deleterious effects of P(g) deficiency on brain infarction. These data suggest that circulating Pg levels may contribute to the variation in outcomes in patients with thromboembolic ischemic stroke.
Materials and methods
Proteins and reagents
Reagents were purchased from the following sources: Pg-depleted human fibrinogen (Enzyme research laboratories, IN); human Glu-Pg (Athens Res. and Tech. GA): bovine thrombin (Sigma, St. Louis, MO); citrated frozen human plasma (Lampire Biological Laboratories, PA); lysine-sepharose (GE healthcare); tPA (Genetech Inc.);125I-fibrinogen (Perkin-Elmer, MA); argatroban (Enzo life sciences, NY); all the other reagents if not specified (Sigma, St. Louis, MO).
Measurement of Pg levels and in vitro plasma clot lysis
Plasma Pg concentrations (n=10-13 per group) were measured by a chromogenic assay as we have described previously [20, 21]. Mouse plasma clots were formed by mixing CaCl2 (20 mM), thrombin (2U/ml) and trace 125I-fibrinogen in Tris buffer (50 mM Tris, 100 mM NaCl, pH 7.4) in a test tube at 37 °C for 40 min. Clot lysis was determined in duplicate by measuring the release of radioactive fibrin degradation products into the supernatant by gamma scintillation counting (Cobra II Packard) at different time points after the addition of tPA (10 nM) [22]. To study human plasma clot lysis, plasma was depleted of Pg by passing through lysine-sepharose beads [23, 24] and was later supplemented with different concentrations of purified Pg. Clot lysis was measured by clot turbidity assay as we have described previously [21, 25]. Data was analyzed by Graph Pad Prism (La Jolla, CA) and experiments were repeated 3-5 times for quantitative analysis (n=3-5 ± SEM).
Thromboembolic Ischemic stroke
Animal studies were approved by the Institutional Animal Care and Use Committee. Adult male and female Pg+/+ and Pg−/− mice on a C57Black/6J background (Jackson Labs, Bar Harbor, ME) were interbred to obtain Pg+/+, Pg+/− and Pg−/− mice. Male and female mice of 6-12 weeks of age were used for thromboembolic stroke study essentially as we have described [19]. Plasma clots made with pooled fresh frozen normal mouse plasma and 125I-fibrinogen (~5000 cpm) were embolized to the origin of middle cerebral artery (MCA). A laser Doppler flow (LDF) probe mounted on the skull verified appropriate embolization by ≥ 80% drop in hemispheric blood flow. In some experimental groups, mice were treated by contralateral jugular vein infusion with the thrombin inhibitor, argatroban (10 mg/kg dose dissolved in 0.9% saline at 0.56 mg/ml concentration) beginning 5 min prior and continuing after induction of stroke. In Pg supplementation experiments, human Glu-Pg (10 mg/kg) was administered through the jugular vein 45 min before injecting clots. Mice groups were euthanized 6 h after thromboembolism, blood was collected by cardiac puncture and whole body saline perfusion was extensively performed before harvesting brain tissue.
Analysis of thrombolysis, brain infarction, swelling and hemorrhage
Dissolution of the MCA thrombus was calculated by comparing the ratio of the initial 125I-fibrin radioactivity counts in the injected clot to the residual counts in the brain ex vivo after stroke by gamma scintillation counting (Cobra II Packard) as we have described [22]. Brains were sectioned (2 mm) coronally and the percent brain swelling, hemorrhage (before 2,3,5-triphenyl tetrazolium chloride (TTC)) staining and infarction volume (after TTC staining) were calculated using Image Pro Plus 6.2 (Media Cybernetics, Bethesda, MD) essentially as we have described [19, 22].
Immunofluorescence staining and analysis
Formalin fixed paraffin embedded brain sections (5 μm) were used for immunofluorescence staining after deparaffinization (Safeclear II, Fisher Diagnostics, MI) as described previously [19, 22]. Antigen retrieval was performed by heat-induced epitope retrieval 98 °C in 10 mM sodium citrate buffer, pH 6.2 for 20 min. Sections were blocked with 10% normal donkey serum for 1 h at room temperature, incubated with primary antibody in 2% normal donkey serum overnight at 4 °C and probed with fluorophore-conjugated donkey secondary antibodies The primary antibodies include rabbit anti-mouse collagen IV (Karlan Research Products Corporation, # ECM451), goat anti-collagen type IV (Southern Biotech, AL, #1340), goat anti-mouse albumin (Abcam, #ab19194), rabbit anti-mouse fibrinogen (MyBioSource, CA, #MBS315814), goat anti-mouse MMP-9 (R&D systems, #AF 909) and MMP-3 (R&D Systems AF548). The secondary antibodies were: DyLight® 488 donkey anti-rabbit & DyLight® 549 donkey anti-goat (Jackson ImmunoResearch Laboratories, PA), Alexa Fluor® 488 donkey anti-rat & Alexa Fluor® 555 donkey anti-rabbit (Life technologies, CA). The cell nuclei were counterstained with DAPI using Vectashield hardset mounting media (Vector Laboratories, CA). Slides were scanned for fluorescence imaging with Aperio image fluorescence scanner (AperioScanScope, Vista, CA). Total fluorescence area or density was quantitated in at least 15 different fields at 20x magnification (100 μm) of both hemispheres of each brain using Image Pro Plus 6.2 and expressed as the ratio or percentage area or intensity of fluorescence in the infarct to the non-infarct hemisphere. Martius Scarlet Blue (MSB) staining was done with MSB kit according to the manufacturer's instructions (Atom Scientific) as we have described [22]. BBB breakdown or leakage was also determined as the percent area of the ischemic hemisphere (vs. the non-ischemic area as a control) with albumin immunostaining (=100% x (albumin stained area of the ischemic hemisphere – albumin stained area of the non-ischemic hemisphere)/total ischemic hemisphere area)).
SDS-PAGE and Immunoblotting of brain lysates
After 6 h stroke in mice, ischemic and non-ischemic hemispheres of brains were snap frozen in liquid nitrogen and stored at −80 °C until further use for immunoblotting of fibrin(ogen) and MMP-9 [26-28]. Brain tissue (50 mg) was homogenized in lysis buffer (0.5 M Tris-HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA) containing 1.5 % SDS and 25 mM EACA (ε-aminocaproic acid) (for plasmin inhibition). Protein was estimated by bicinchoninic acid assay using homogenized tissue supernatant after centrifugation. Proteins (50 μg) were electrophoresed on 10% reducing SDS-PAGE gels and transferred to polyvinylidenedifluoride membrane. The membranes were blocked with 1% BSA in PBS containing 0.1% Tween 20. Fibrin(ogen) and MMP-9 were probed with rabbit anti-mouse fibrinogen antibody (MyBiosource) at 1:3000 dilution and goat anti-mouse MMP-9 (1:1000) antibodies respectively, overnight at 4 °C and, detected with anti-rabbit and anti-goat IRDye Li-COR secondary antibodies (LI-COR biosciences, NE). The membranes were scanned on LI-COR Odyssey scanner and pixel density was measured by NIH Image J software. Gelatinase zymography was performed as described [26].; additional details are available in the Supplemental Data.
Statistical analysis
Statistical analysis was done in Graph Pad Prism 5.0 software (San Diego, CA). Data are represented as the mean ± standard error. Normally distributed data were analyzed by an unpaired Student's t-test or a one way ANOVA using the Neuman–Keuls correction. Non-parametric data were analyzed by a Mann–Whitney test or a one way Kruskal Wallis analysis using Dunn's correction. A two-tailed p<0.05 was considered statistically significant. Linear regression was done using Graph Pad Prism.
Results
Effects of varying Pg levels on fibrinolysis and ischemic brain injury following cerebral thromboembolism
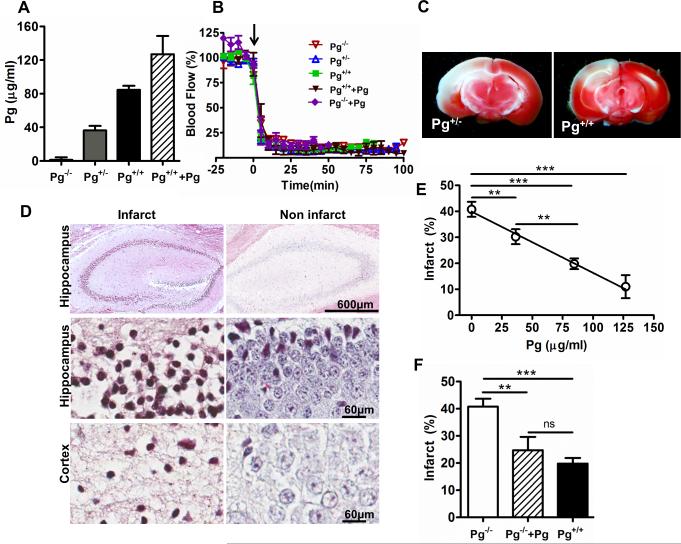
To determine the effect of Pg blood levels on ischemic stroke, we examined mice with different blood Pg levels, ranging from Pg deficiency to supra-physiologic levels due to Pg supplementation (Fig. 1A). Wild-type Pg+/+ mice had slightly more than two-fold higher mean Pg levels (84.5± 4.8 μg/ml) than heterozygous Pg+/− mice (36.3± 5.2 μg/ml, Fig. 1A). Since the Pg system is activated early after ischemic stroke, we assessed the acute effects of these differences 6 h after MCA thromboembolism. Hemispheric blood flow fell by at least 80% after MCA thromboembolism in all mice and remained suppressed (Fig. 1B). Brain infarction was 50% larger in Pg+/− (Fig. 1C, TTC-stained representative images) by comparison to Pg+/+ mice with normal Pg levels (Fig. 1E, p<0.01). The cerebral infarction was further confirmed by MSB staining showing that infarct regions of brain (hippocampus and cortex) were associated with cell death characterized by constricted or lost nuclei as compared to non-infarct regions showing intact cell morphology (Fig. 1D). These studies showed a significant, inverse dose-dependent relationship between blood Pg levels and brain infarction (r=−0.72, p<0.0001; Fig. 1E). To determine whether the enhanced brain injury was due to deficiency of blood Pg vs. brain Pg, we supplemented blood Pg levels in Pg−/− mice by intravenous Pg administration prior to thromboembolism. By comparison to Pg-deficient Pg−/− mice, supplementation of blood Pg levels decreased ischemic brain injury significantly, to levels that were not significantly different from that seen in normal Pg+/+ mice (Fig. 1F).
Figure 1. Pg levels show an inverse dose-response relationship with the extent of brain infarction after MCA thromboembolism.
(A) Plasma Pg levels in mice with different Pg genotypes (Pg−/−, Pg+/−, Pg+/+ and Pg+/+ mice with increased Pg levels) as measured by Pg activation assay. (B) Representative tracings of laser Doppler blood flow in the ischemic hemispheric in mice after thromboembolism. Arrow indicates the time when clot was injected (C) Representative TTC-stained brain sections from Pg+/− and Pg+/+ mice after stroke as labeled. (D) Representative MSB stained images of hippocampus (4x and 40x) and cortex (40x) regions of infarct and non-infarct brain hemispheres after 6 h ischemic stroke showing cell death. (E) Regression analysis (line graph) shows a significant inverse dose-relationship between Pg levels and the extent of brain infarction in Pg−/−, Pg+/− and Pg+/+ mice (n=43 mice, 10-14 per group) and Pg+/++Pg mice (Pg+/+ mice with increased Pg level) n=6.**p<0.01, ***p<0.001 (F) Intravascular Pg (10 mg/Kg) supplementation to Pg−/− mice (n=5) reduced the cerebral infarct size to levels comparable to Pg+/+ mice (bar graph) as measured through TTC staining after 6 h stroke. **p<0.01, ***p<0.001, ns (non- significant).
A two-way analysis of variance showed that endogenous Pg levels were strongly associated with the severity of brain infarction (p<0.0001), but the sex of the mouse was not (p=0.94). There was no significant relationship between brain hemorrhage and Pg levels or genotype (p=0.39); sex also did not significantly affect the risk of hemorrhage (p=0.16).
Pg levels affect fibrinolysis of middle cerebral artery thrombus
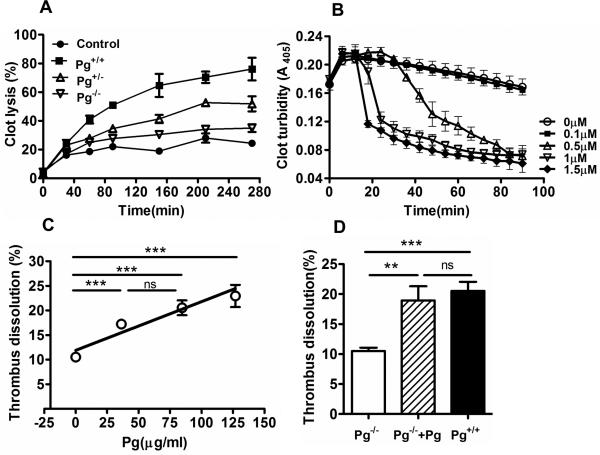
In vitro, plasma clots formed from Pg+/+ mouse showed the highest amount of fibrinolysis, followed by clots from Pg+/− mice, followed by Pg−/− mice (Fig. 2A). In a similar fashion, higher Pg levels also accelerated the dissolution of human plasma clots (Fig. 2B). Dissolution of the MCA thrombus appeared slightly greater in Pg+/+ mice than in Pg+/− mice; however this did not achieve statistical significance (Fig. 2C). Nevertheless, in studies that included Pg+/+ mice with increased Pg levels, there was a linear dose relationship between the Pg levels and MCA thrombus dissolution (Fig. 2C, r=0.68, p<0.0001). A two-way analysis of variance confirmed that blood Pg levels affected thrombus dissolution (p< 0.01) but sex did not (p=0.44, data not shown). Restoration of blood Pg levels in Pg−/− mice significantly increased MCA thrombus dissolution by comparison to Pg−/− mice, to levels that were not significantly different from that seen in normal Pg+/+ mice (Fig. 2D).
Figure 2. Relationship between plasma Pg levels and in vitro and in vivo thrombus dissolution.
(A) tPA (10 nM) mediated dissolution of clots (% clot lysis) made from fresh frozen plasma from Pg−/−, Pg+/− and Pg+/+ mice. Control represents Pg−/− plasma clot without tPA. (n=3-5 ± SEM) (B) Pg-depleted human plasma was supplemented with purified human Pg and tPA (14 nM). Clot dissolution was measured by the decrease in clot turbidity at 405 nm at 37 °C. (n=3-5 ± SEM). (C) The line graph shows that plasma Pg levels were directly proportional to MCA thrombus dissolution (%) in Pg−/−, Pg+/− and Pg+/+ mice (n= 43 mice, 10-14 per group) and Pg+/++Pg mice (n=6) during stroke. ***p<0.001. (D) Intravascular Pg (10 mg/Kg) supplementation to Pg−/− mice significantly enhanced the dissolution of the MCA thrombus after 6 h stroke. Mean ± SE, n= 6 per group. **p<0.01, ***p<0.001, ns (not significant).
Higher Pg levels are associated with decreased BBB breakdown and reduced MMP-9 expression
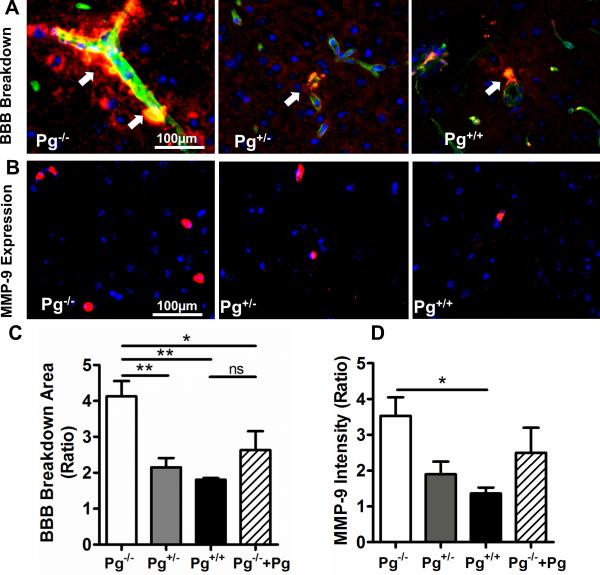
In vitro models of the BBB suggest that Pg may enhance barrier breakdown due to plasmin's proteolytic effects during stroke [12]. Breakdown of the BBB leads to the extravasation of blood proteins such as albumin into the brain and is a major determinant of brain injury after stroke [27, 28]. The leakage or extravasation of albumin outside collagen IV-stained brain blood vessels was examined by immunofluorescence microscopy (Fig. 3A) as we have described [19]. BBB breakdown was more than 2-fold greater in Pg−/− mice than in Pg+/+ (p<0.01); it was also significantly greater in comparison to Pg+/− mice (p<0.01, Fig. 3C bar graph). The area of BBB breakdown, assessed as a percentage of the ischemic hemisphere, was also greater in Pg−/− mice (28.5±5.6%) and in Pg+/− mice (24.7±5.2%) than in Pg+/+ mice (8.3±3.9%, p<0.05). Pg supplementation of Pg−/− mice reduced BBB breakdown (Fig. 3C, p<0.05). BBB breakdown was detected in the infarct as well as in peri-infarct cortical and hippocampal regions of the ischemic hemisphere. In addition to the injury to the BBB, we also analyzed the expression of MMP-9, an inflammatory marker associated with cerebral infarction. Endogenous Pg deficiency in Pg−/− mice was associated with a 2-3 fold increase in MMP-9 expression in the brain (Fig. 3B and 3E) vs. Pg+/− and Pg+/+ mice (p<0.05). Pg supplementation of Pg−/− mice was associated with a non-significant reduction in MMP-9 expression. Pg−/− mice also showed ~2-fold increase in MMP-9 levels by immunoblotting vs. Pg+/+ mice (Fig. S1A). SDS-PAGE gelatin zymography showed that MMP-9 was mainly present as pro-MMP-9 six hours post-stroke (Fig. S1B) as described by previous studies [29]. MMP-9 expression was predominantly localized to microvessels (Fig. S2) with smaller amounts seen in the brain parenchyma. MMP-3 expression was also significantly increased in Pg−/− mice in comparison to Pg+/− or Pg+/+ mice as determined by immunofluorescence staining (Fig. S3)[30, 31].
Figure 3. Higher Pg levels are associated with decreased BBB breakdown and reduced vascular MMP-9 expression after stroke.
(A) Leakage (arrows) of albumin (red) into the brain parenchyma outside collagen IV-stained (green) blood vessels was assessed in the ischemic hemispheres by comparison to the non-ischemic hemispheres of Pg−/−, Pg+/− and Pg+/+ mice. Merged images (20x) show areas of overlapping staining for collagen IV and albumin (yellow) and DAPI-stained nuclei in blue. (B) Representative immunofluorescence images (20x) showing MMP-9 expression (red) in the ischemic hemispheres of Pg−/−, Pg+/− and Pg−/− mice as detected by goat anti-mouse MMP-9 primary antibody followed by donkey anti-goat DyLight® 549. (C) The bar graphs show the ratio of the total area of albumin staining (in 20x fields) and (D) MMP-9 fluorescence intensity in the ischemic vs. the non-ischemic hemisphere of each mice group (mean ± SE). n= 4-5 per group, *p<0.05, **p<0.01, ns (not significant).
Pg levels affect development of microvascular thrombosis
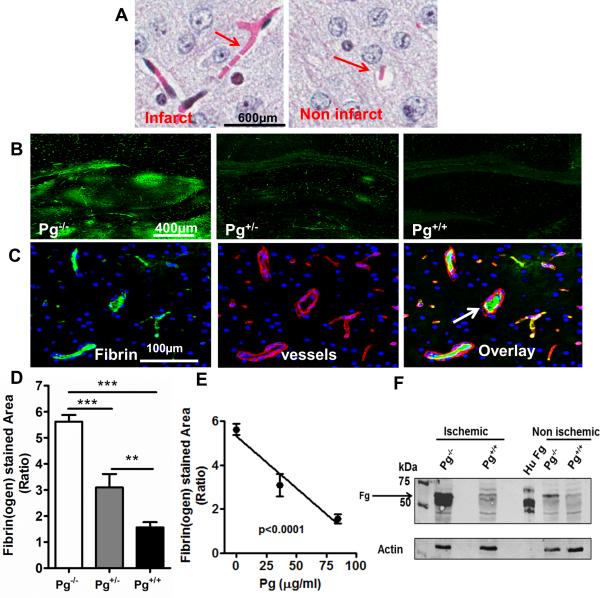
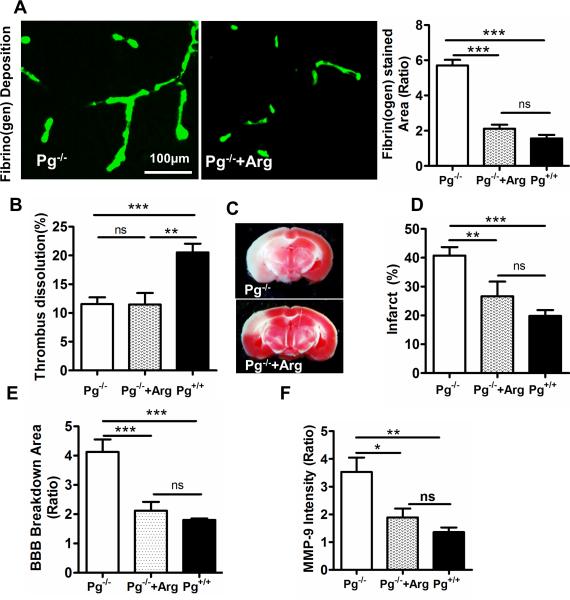
Brain ischemia promotes microvascular thrombosis [32-34] which causes vascular obstruction and may further enhance ischemic injury. There is indirect evidence that P(g) may affect microvascular thrombosis in ischemic stroke [22]. Microvascular thrombosis in the brain was assessed by MSB staining (Fig. 4A) that showed increased fibrin deposition in infarct vs non-infarct brain hemisphere. Immunofluorescence staining showed that fibrin(ogen) deposition was prominent in the hippocampus and cortex of Pg−/− mice (Fig. 4B). Microvascular thrombosis was examined by fibrin(ogen) deposition inside collagen IV-stained blood vessels (Fig. 4C). Microvascular thrombosis was significantly reduced in Pg+/− vs. Pg−/− mice (Fig. 4D) and Pg+/+ mice showed a further decrease in fibrin(ogen) deposition by comparison to Pg+/− (Fig. 4D p<0.01 and Fig. S4A). There was a significant inverse dose-response relationship between Pg levels and fibrinogen deposition (Fig. 4E). These findings were confirmed by immunoblotting studies, which showed that total fibrin(ogen) content in the brains of Pg−/− mice was nearly 3-fold higher (p<0.05) than that was found in Pg+/+ mice (Fig. 4F and Fig. S4B).
Figure 4. Higher plasma Pg levels are associated with reduced microvascular thrombosis in the ischemic brain.
(A) MSB stained brain images showing microvascular thrombosis (pink fibrin) in the ischemic and non-ischemic hemisphere (B) Representative immunofluorescence images (6x) of the hippocampal region of ischemic hemispheres of Pg−/−, Pg+/−, and Pg+/+ mice showing areas of fibrin(ogen) deposition (green). Rabbit anti-mouse fibrin(ogen) and Dylight® 488 donkey anti-rabbit were used as primary and secondary antibodies respectively. (C) Representative brain images from Pg−/− mice (20x) showing fibrino(gen) (green), collagen IV-stained blood vessels (red) and a merged image showing intravascular fibrin(ogen) (white arrow) in blood vessels. For fibrin(ogen) and blood vessels, rabbit anti-mouse fibrin(ogen) and goat anti-human collagen IV were used as primary antibodies respectively. Dylight® 488 donkey anti rabbit and Alexa Fluor® 555 donkey anti-goat respectively were used as secondary antibodies. (D) Quantitation by digital imaging of the fibrino(gen) stained area in 15 different fields at 20x magnification and expressed in the bar graph as ratio of ischemic vs non-ischemic hemispheres of Pg−/−, Pg+/−, and Pg+/+ mice. Mean ± SE, n= 5 per group. (E) Regression analysis (line graph) shows that fibrin(ogen) deposition in brain was inversely correlated to Pg levels. (F) Immunoblotting of brain samples with rabbit anti-mouse fibrino(gen) antibody showed fibrin(ogen (α,β chains; Fg) in the ischemic and non-ischemic hemisphere. Purified human fibrinogen (Hu Fg) was used as a positive control.
Microvascular thrombosis enhances ischemic brain injury
These studies indicate that Pg levels affected the dissolution of the MCA thrombus and the development of microvascular thrombosis. Although the role of the MCA thrombus in stroke is established, there is debate whether microvascular thrombosis is a cause or a consequence of increased ischemic injury. To examine whether the enhanced microvascular thrombosis associated with Pg deficiency plays a causal role in ischemic brain injury, we used argatroban, a selective thrombin inhibitor to suppress the development of microvascular thrombosis without affecting dissolution of the MCA thrombus [35]. In thromboembolic stroke, argatroban significantly reduced microvascular thrombosis (Fig. 5A, p<0.01 bar graph) in Pg−/− mice to levels comparable to that observed in Pg+/+ mice, as assessed by immunofluorescence staining for fibrin(ogen) deposition. Interestingly, argatroban treatment of Pg−/− mice did not affect dissolution of the MCA thrombus (Fig. 5B, p= 0.84). The selective reduction in microvascular thrombosis in Pg−/− mice was associated with a significant decrease in cerebral infarction (TTC staining Fig. 5C) to the level observed in Pg+/+ mice which was significantly different than the injury observed in control Pg−/− mice not receiving argatroban (Fig. 5D, p<0.01). This decrease in microvascular thrombosis in argatroban-treated Pg−/− mice was further associated with reduced BBB breakdown and diminished MMP-9 expression, and was comparable to that in Pg+/+ mice (Figs. 5E, F). These data suggest that, independent of its effects on the culprit MCA thrombus, Pg had protective effects on the ischemic brain that are due to its suppression of microvascular thrombosis.
Figure 5. Reducing microvascular thrombosis diminishes brain injury in Pg−/− mice even in the absence of changes in MCA thrombus dissolution.
(A) Microvascular thrombosis was significantly diminished in Pg−/− mice by argatroban (arg) treatment as quantitatively determined by immunofluorescence staining. The bar graph represents the ratio of fibrin(ogen) stained area in ischemic/non-ischemic hemisphere. (B) MCA thrombus dissolution was unaffected in Pg−/− mice with and without argatroban treatment as determined ex vivo by 125I-fibrin radioactivity in brain. (C) Reductions in microvascular thrombosis by argatroban are associated with significant decreases in brain infarction in Pg−/− mice as measured through TTC staining after 6 h stroke. (D) Bar graph shows the percent brain infarct in Pg−/− mice after argatroban treatment in comparison to untreated Pg−/− mice and Pg+/+ mice. Mean ± SE, n= 5 per group, **p<0.01, ***p<0.001, ns (not significant). (E,F) Argatroban treatment significantly decreased BBB breakdown (E) and MMP-9 expression (F) in Pg−/− mice as quantitated in ischemic vs non-ischemic hemisphere in Pg−/− mice after argatroban treatment vs Pg−/− or Pg+/+ mice.
Discussion
Pg is present in both vascular and neuronal compartments of the brain. It has diverse roles in fibrinolysis, inflammation, maintenance of endothelial barriers and other processes that may have opposing effects during the pathogenesis of complex processes such as ischemic stroke. Despite the marked variations in blood Pg levels in normal individuals, there is no firm clinical data to link Pg levels to stroke risk or outcomes. These studies provide the first evidence that variations in endogenous blood Pg levels, within the range of that seen in normal humans, have marked dose-related effects on ischemic stroke outcomes. Pg levels modulated brain infarction, endogenous fibrinolysis, microvascular thrombosis, inflammation and breakdown of the BBB. Restoration of blood Pg levels, in Pg−/− mice lacking both brain and blood Pg, was sufficient to reduce acute ischemic injury to the levels seen in mice with normal blood and brain Pg levels. Studies with direct thrombin inhibition in Pg−/− mice suggest that a significant component of the protective effects of Pg levels in stroke may be due to suppression of microvascular thrombosis in the ischemic hemisphere.
Variations in endogenous Pg levels affected ischemic brain injury, dissolution of the MCA thrombus and microvascular thrombosis. These data are consistent with recent findings that promoting endogenous plasmin activity or fibrinolysis, by inactivating its principal inhibitor (α2-antiplasmin), reduces microvascular thrombosis, ischemic brain injury and mortality after thromboembolic stroke [19, 22]. It is notable that even in the absence of effects on the MCA thrombus, suppression of microvascular thrombosis markedly reduced brain infarction. Although not specifically examined in that study, the marked suppression of microvascular thrombosis by Pg may explain the enhanced ischemic neurodegeneration observed in Pg-deficient mice by Nagai et al. after surgical MCA ligation [9].
It is important to note that our study focused on the integrated acute effects of Pg during the first six hours after thromboembolism, when the fibrinolytic system is dynamic and it is known to have significant effects on stroke outcomes. As such the observations on MMP-9 expression, BBB breakdown and microvascular thrombosis are limited to the early phase of ischemic injury after stroke. The strong dose-response relationships observed in this study provide compelling evidence for a direct effect of Pg on ischemic stroke; still, this study did not examine other molecules in the fibrinolytic pathway that also may affect outcomes. A recent stroke study suggests that Pg also has long term protective effects on neurological functional recovery after stroke by affecting axonal remodeling [36]. While MMP-9, MMP-3 and BBB breakdown are enhanced in Pg−/− mice, additional experiments will be necessary to determine whether there is a mechanistic link between BBB breakdown and MMP-3, 9, or the other MMPs and tissue inhibitors of metalloproteinase proteins implicated in this process [37].
To date there is surprisingly little evidence that Pg deficiency enhances intravascular thrombosis, though Pg deficiency is associated with enhanced extravascular fibrin deposition [38, 39]. However, the extravascular deposition of fibrin observed in Pg-deficient mice specifically in the ischemic area may reflect the tendency for intravascular fibrin to move or extravasate outside of blood vessels [40]. In Pg deficiency, vascular patency also may be maintained by non-plasmin fibrinolytic mechanisms mediated by macrophages or MMP-9 etc.[41, 42]. While Pg deficiency does not trigger acute intravascular thrombosis in mice or humans, it has been associated with decreased clearance of experimental thrombi in mice [39, 43]. The present studies demonstrate that physiologic variation in Pg levels affects the clearance of macrovascular thrombi and the development of microvascular thrombi. Longer duration studies are warranted to assess the role of P(g) in thrombo-inflammatory mechanisms in brain parenchyma involving microglial cells, neurons, macrophages and processes during the subacute and chronic recovery phase after ischemic stroke.
To conclude, in experimental, thromboembolic stroke endogenous Pg levels were strongly associated with ischemic brain injury, dissolution of cerebral thromboemboli, MMP-9 expression, microvascular thrombosis and breakdown of BBB. This suggests that physiologic variations in Pg levels may affect outcomes after thromboembolic stroke in humans.
Supplementary Material
Essentials.
Physiologic variations in blood plasminogen (Pg) levels may affect ischemic stroke outcomes.
We tested Pg effects in a model with translational relevance to human thromboembolic stroke.
A dose-response exists between Pg levels and brain injury, fibrinolysis, barrier breakdown.
Higher Pg levels reduce microvascular thrombosis and improve outcomes in ischemic stroke.
Acknowledgments
We thankfully acknowledge Dr. Inna P. Gladysheva for reviewing the manuscript and her valuable suggestions.
Sources of Funding: This research was supported in part by National Institute of Health grants to G.L. Reed (HL58496, HL92750, and NS73147).
Footnotes
Author's contribution: G. L. Reed, S. Singh conceived, designed the work. A. K. Houng, S. Singh and D. Wang performed the work. G. L. Reed, A. K. Houng, S. Singh and D. Wang analyzed and interpreted the data and S. Singh and G. L. Reed wrote the manuscript.
Disclosures:
G. L. Reed is a founder of Translational Sciences and reports grants from National Institutes of Health. The other authors have no disclosures.
References
- 1.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–54. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- 2.Basham ME, Seeds NW. Plasminogen expression in the neonatal and adult mouse brain. J Neurochem. 2001;77:318–25. doi: 10.1046/j.1471-4159.2001.t01-1-00239.x. [DOI] [PubMed] [Google Scholar]
- 3.Xue M, Del Bigio MR. Acute tissue damage after injections of thrombin and plasmin into rat striatum. Stroke. 2001;32:2164–9. doi: 10.1161/hs0901.095408. [DOI] [PubMed] [Google Scholar]
- 4.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–52. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheehan JJ, Zhou C, Gravanis I, Rogove AD, Wu YP, Bogenhagen DF, Tsirka SE. Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J Neurosci. 2007;27:1738–45. doi: 10.1523/JNEUROSCI.4987-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheehan JJ, Tsirka SE. Fibrin-modifying serine proteases thrombin, tPA, and plasmin in ischemic stroke: a review. Glia. 2005;50:340–50. doi: 10.1002/glia.20150. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin catalyzed degradation of laminin. Cell. 1997;91:917–25. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Plasminogen potentiates thrombin cytotoxicity and contributes to pathology of intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2008;28:506–15. doi: 10.1038/sj.jcbfm.9600547. [DOI] [PubMed] [Google Scholar]
- 9.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–4. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 10.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–68. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 11.Harris AK, Ergul A, Kozak A, Machado LS, Johnson MH, Fagan SC. Effect of neutrophil depletion on gelatinase expression, edema formation and hemorrhagic transformation after focal ischemic stroke. BMC Neurosci. 2005;6:49. doi: 10.1186/1471-2202-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niego B, Freeman R, Puschmann TB, Turnley AM, Medcalf RL. t-PA-specific modulation of a human blood-brain barrier model involves plasmin-mediated activation of the Rho kinase pathway in astrocytes. Blood. 2012;119:4752–61. doi: 10.1182/blood-2011-07-369512. [DOI] [PubMed] [Google Scholar]
- 13.Niego B, Medcalf RL. Plasmin-dependent modulation of the blood-brain barrier: a major consideration during tPA-induced thrombolysis? J Cereb Blood Flow Metab. 2014;34:1283–96. doi: 10.1038/jcbfm.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grotta JC, Alexandrov AV. tPA-associated reperfusion after acute stroke demonstrated by SPECT. Stroke. 1998;29:429–32. doi: 10.1161/01.str.29.2.429. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Zhou W, Heiland S, Marti HH, Veltkamp R. A translationally relevant thromboembolic stroke model for the study of secondary hemorrhage after thrombolysis in rats. Brain research. 2011;1368:346–54. doi: 10.1016/j.brainres.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 16.Tait RC, Walker ID, Conkie JA, Islam SI, McCall F, Mitchell R, Davidson JF. Plasminogen levels in healthy volunteers--influence of age, sex, smoking and oral contraceptives. Thromb Haemost. 1992;68:506–10. [PubMed] [Google Scholar]
- 17.Schuster V, Hugle B, Tefs K. Plasminogen deficiency. J Thromb Haemost. 2007;5:2315–22. doi: 10.1111/j.1538-7836.2007.02776.x. [DOI] [PubMed] [Google Scholar]
- 18.Suri MF, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2010;29:497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houng AK, Wang D, Reed GL. Reversing the deleterious effects of alpha2-antiplasmin on tissue plasminogen activator therapy improves outcomes in experimental ischemic stroke. Experimental neurology. 2014;255C:56–62. doi: 10.1016/j.expneurol.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed GL, 3rd, Matsueda GR, Haber E. Synergistic fibrinolysis: combined effects of plasminogen activators and an antibody that inhibits alpha 2-antiplasmin. Proc Natl Acad Sci U S A. 1990;87:1114–8. doi: 10.1073/pnas.87.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sazonova IY, Thomas BM, Gladysheva IP, Houng AK, Reed GL. Fibrinolysis is amplified by converting alpha-antiplasmin from a plasmin inhibitor to a substrate. J Thromb Haemost. 2007;5:2087–94. doi: 10.1111/j.1538-7836.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 22.Reed GL, Houng AK, Wang D. Microvascular Thrombosis, Fibrinolysis, Ischemic Injury, and Death After Cerebral Thromboembolism Are Affected by Levels of Circulating alpha2- Antiplasmin. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.304530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deutsch DG, Mertz ET. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970;170:1095–6. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 24.Onundarson PT, Francis CW, Marder VJ. Depletion of plasminogen in vitro or during thrombolytic therapy limits fibrinolytic potential. J Lab Clin Med. 1992;120:120–8. [PubMed] [Google Scholar]
- 25.Singh S, Bhando T, Dikshit KL. Fibrin-targeted plasminogen activation by plasminogen activator, PadA, from Streptococcus dysgalactiae. Protein Sci. 2014;23:714–22. doi: 10.1002/pro.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park KP, Rosell A, Foerch C, Xing C, Kim WJ, Lee S, Opdenakker G, Furie KL, Lo EH. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009;40:2836–42. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–8. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krueger M, Hartig W, Reichenbach A, Bechmann I, Michalski D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One. 2013;8:e56419. doi: 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–9. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Lijnen HR, Silence J, Van Hoef B, Collen D. Stromelysin-1 (MMP-3)-independent gelatinase expression and activation in mice. Blood. 1998;91:2045–53. [PubMed] [Google Scholar]
- 31.Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost. 2007;5:1732–9. doi: 10.1111/j.1538-7836.2007.02628.x. [DOI] [PubMed] [Google Scholar]
- 32.Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly ES, Jr., Kottirsch G, Pinsky DJ. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest. 1998;102:1301–10. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petty MA, Wettstein JG. Elements of cerebral microvascular ischaemia. Brain Res Brain Res Rev. 2001;36:23–34. doi: 10.1016/s0165-0173(01)00062-5. [DOI] [PubMed] [Google Scholar]
- 34.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–94. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 35.Kikumoto R, Tamao Y, Tezuka T, Tonomura S, Hara H, Ninomiya K, Hijikata A, Okamoto S. Selective inhibition of thrombin by (2R,4R)-4-methyl-1-[N2-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl++ +) sulfonyl]-l-arginyl)]-2-piperidinecarboxylic acid. Biochemistry. 1984;23:85–90. doi: 10.1021/bi00296a014. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Li Y, Qian J, Cui Y, Chopp M. Plasminogen deficiency causes reduced corticospinal axonal plasticity and functional recovery after stroke in mice. PLoS One. 2014;9:e94505. doi: 10.1371/journal.pone.0094505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–9. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 39.Ploplis VA, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow EF, Collen D. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995;92:2585–93. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 40.Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25:1847–53. doi: 10.1161/01.str.25.9.1847. discussion 53-4. [DOI] [PubMed] [Google Scholar]
- 41.Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco PM. Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med. 2001;193:793–802. doi: 10.1084/jem.193.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witting PK, Zeng B, Wong M, McMahon AC, Rayner BS, Sapir AJ, Lowe HC, Freedman SB, Brieger DB. Polymorphonuclear leukocyte phagocytic function increases in plasminogen knockout mice. Thromb Res. 2008;122:674–82. doi: 10.1016/j.thromres.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Lijnen HR, Carmeliet P, Bouche A, Moons L, Ploplis VA, Plow EF, Collen D. Restoration of thrombolytic potential in plasminogen-deficient mice by bolus administration of plasminogen. Blood. 1996;88:870–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.