Abstract
Identifying environmental exposures that cause adverse mammary gland outcomes in rodents is a first step in disease prevention in humans and domestic pets. ‘Whole mounts’ are an easy and inexpensive tissue preparation method that can elucidate typical or abnormal mammary gland morphology in rodent studies. Here we propose procedures to facilitate the use of whole mounts for histological identification of grossly noted tissue alterations. We noted lesions in mammary whole mounts from 14 month old CD-1 mice that were not found in the contralateral gland hematoxylin and eosin (H&E)-stained section. Whole mounts were removed from the slide and carefully processed to produce high quality histological sections that mirrored the quality of the original H&E-stained section in order to properly diagnose the unidentified gross abnormalities. Incorporation of this method into testing protocols which focus on low-dose human relevant chemicals and endocrine disruptors will increase the chances of identifying lesions in the gland and reduce the risk of false negative findings. This method can be especially invaluable when lesions are not always palpable during the course of the study or visible at necropsy, or when a single cross-section of the mammary gland is otherwise used for detecting lesions.
Keywords: mammary gland, whole mount, contralateral, development, breast, pathology
Introduction
Whole mounts are an invaluable tool used primarily in rodent studies to illustrate the progression of normal or chemically-altered mammary gland epithelial growth, development, function and disease. When collected during various stages of development, the whole mount provides information regarding 1) early development of mammary tissue before endogenous hormone changes, 2) pre- and peripubertal epithelial alterations in offspring as a consequence of chemical transfer through placental/lactational exposure 3) early signs of lactational impairment in dams when evaluated during mid-pregnancy, 4) influences of circulating endogenous hormone or endocrine disrupting chemical (EDC) exposures and 5) gross pre- and neoplastic lesions that can be verified and properly diagnosed using histopathology (Rudel et al. 2011). The latter examples often involve tissues collected in adulthood at final necropsy in studies involving chemicals with known or suspected endocrine disrupting activities, although mammary tissue can and should be collected at other life stages.
Historically, rodent mammary glands were prepared and evaluated as a representative cross-section that included the skin and very little mammary epithelial tissue. Current recommendations (Davis and Fenton, 2013) include using coronal or longitudinal sections to improve the ability to observe any abnormalities; the whole mount also provides an image of the entire gland and therefore is another acceptable technique of choice for histopathological evaluation of the mammary gland. The mammary whole mount is typically prepared from the inguinal 4th and 5th glands on one side of the animal. Glands from this region are easier to access compared to the thoracic glands thus preserving the glands natural architecture when spread onto a charged slide. One set of inguinal glands are mounted onto the slide, fixed, stained and covered with a coverslip, or sealed in a bag of methyl salicylate for temporary or permanent storage, and the contralateral gland may be processed for histology (Davis and Fenton, 2013).
When using this approach, a comparative visualization of a whole mount along with H&E-stained sections prepared for histologic examination can provide an excellent appreciation of the morphological and cellular alterations occurring in the tissue, especially in cases where a palpable tumor is not observed. However, acquiring and evaluating glands from both sides of the animal may not always be feasible (i.e. tissue required for RNA or protein analysis or due to insufficient funding), or gross abnormalities may occur asymmetrically. The latter was the case during the analysis of a current study where the abnormal findings in the whole mounts were disproportionately higher compared to the contralateral histopathology findings. In response, we developed an inexpensive and streamlined protocol to examine H&E-stained paraffin sections of the original mammary gland whole mount using brightfield microscopy. The development of a standardized protocol will facilitate tissue comparison between labs and may limit the need for additional tissues originally processed for H&E staining.
Materials and Methods
Whole Mount and Contralateral Gland Preparation
Whole mounts were prepared by excising the 4th and 5th inguinal mammary glands from adult female Crl: CD1 (ICR) mice (CD-1; Charles River Laboratory, Raleigh, NC) in an on-going study (collected at 14 mo of age) or from animals that failed to deliver healthy litters (for practicing sectioning and technique specifics). The whole mounted gland was sandwiched between parafilm (Bemis, Neenah, WI) and another glass slide with slight pressure applied to flatten the gland and increase the surface area for fixation and staining. Fixation (Carnoy’s), staining (carmine alum) and defatting (xylene) were performed as previously described in detail in Davis and Fenton (2013).
The contralateral gland was removed and fixed in 10% neutral buffered formalin (Fisher Scientific, Fairlawn, NJ) for 48 hours and dehydrated in 70% ethanol to minimize adipose distortion that can occur during sectioning. Within 48 hr following fixation, samples were embedded in paraffin, sectioned at 5μm, and stained with H&E on an automated platform (Leica ST2050 Multistainer Workstation, Buffalo Grove, IL). H&E-stained sections were visualized on an Olympus BX41 (Olympus Scientific Solutions Americas Corp., Waltham, MA), digitally captured on an Olympus DP70 camera and examined by a board certified veterinary pathologist (S.H.B.).
Whole Mount to H&E Preparation
We recommend that before the sectioned tissue stages of this protocol are started, that high quality images of the intact whole mounts be taken for any future analyses. The first step in preparing mammary whole mounts for sectioning was removal of the glass coverslip. Each whole mount was placed in a glass Coplin jar and immersed overnight in xylene, followed by two additional fresh xylene soaks; 6 hr and overnight, respectively. Covers fall off during this process. Slides were held perpendicular to the bottom of a glass petri dish containing xylene and the tissue was scraped from the slide with a sharp disposable razor blade beginning at the top of the slide. To ensure the tissue was removed intact, the blade was slowly and carefully pulled down the slide, parallel to the tissue. This allowed the gland to fall into the xylene at the bottom of the glass petri dish, minimizing air drying of the sample. The tissue was then placed in a histology cassette for processing. When necessary, larger glands were halved at the midline where the pad narrows and the tissues were placed into two separate cassettes, one labeled for the top half and the other for the bottom half. Since the tissue was thin, we do not advise placing both halves in the same cassette. The cassettes were held in xylene for up to 2 hr. before being placed on the tissue processor (Leica Microsystems TP1020, Chicago, IL). Glands were processed in xylene for 30 min. This step was repeated and followed by immersion in a 1:1 xylene: molten paraffin (30 min). The cassettes were then transferred to the next station, which consisted of molten paraffin (1 h), followed by another incubation in molten paraffin (2 h). Processor settings included a temperature of 60°C and the vacuum on for all stations. See Table 1 for a summary of the tissue processing schedule. Finally, the mammary pad was embedded in paraffin with the flat surface down (side adjacent to the glass slide). Prior to sectioning, the paraffin blocks were incubated at −20°C for 1 hr. This step aided in obtaining quality sections for histopathology. Glands from animals not included in our study (small litters or gestation timing off the group average by more than 12 hr) were sectioned at 4 μm (superior quality vs. a thicker section (6 μm); Figure 1) using a low profile blade (Sakura, Finetek, Inc. USA, Torrance, CA). All slides were incubated overnight at 37°C prior to an automated staining with H&E, as described above for the contralateral gland. Tissue de-staining was unnecessary, since very little of the whole mount carmine alum staining remained in the tissue sections.
Table 1.
Mammary gland whole mount tissue processing schedule
| Process | Temperature (°C) | Time (min) |
|---|---|---|
| Xylene | 37° | 30 |
| 1:1 xylene: molten paraffin | 60° | 30 |
| Molten paraffin | 60° | 60 |
| Molten paraffin | 60° | 120 |
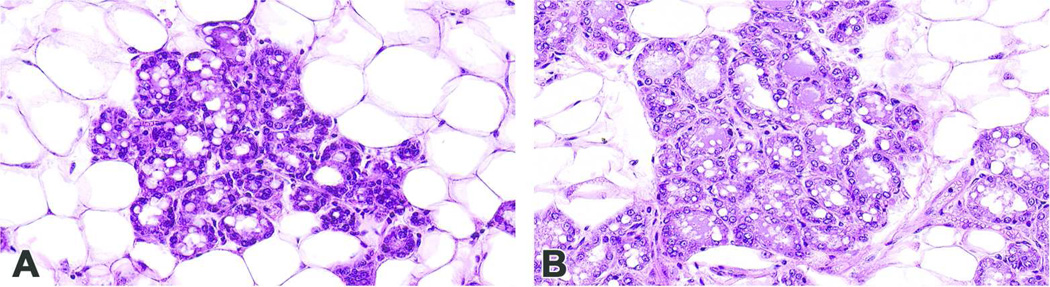
Figure 1. Determining an optimal thickness to prepare mammary gland sections.
Mammary gland sections of (A) 6 µm and (B) 4 µm thickness. Due to the increased clarity and cellular distinction in (B), the 4 μm thickness is preferred. Magnification 40x. Mammary tissues were obtained from animals otherwise destined for euthanization.
Scanning
High quality archival whole mount images were recorded to preserve intact structures for future assessments. Images were prepared by scanning the whole mount mammary gland glass slide on a flatbed scanner (Epson Perfection V750 Pro, Epson America, Long Beach, CA).
Digital images of H&E-stained slides were captured on the Aperio AT2™ slide scanner (Leica) using Imagescope™ software (v.12.1, Leica). White balance correction and image resizing were completed using Adobe Photoshop™ (Adobe Photoshop Creative Cloud 2014.0.0).
Results
It is important to note that the intact mammary gland whole mount, displaced with xylene from the glass slide, is not optimized for sectioning in the traditional histologic sense. In the current situation, the whole mount was permeated with a heavy layer of mounting media, such as Permount (Sigma Aldrich), so that a coverslip may be placed over the thick mammary pad without the introduction of air bubbles. In developing this protocol, the whole mount paraffin blocks from animals not included in our study were originally sectioned at 6 µm. The sections appeared thick, piling up of cells was evident, and specific cellular detail was insufficient (Figure 1A). Tissue blocks sectioned at 4 µm yielded superior results with cellular features and stromal area infiltrates more easily discernable (Figure 1B).
This modification in tissue block sectioning is being used for a large on-going study. The histopathology findings of the H&E-stained longitudinal sections from the contralateral gland of 14 month old female CD-1 mice in our study were inconsistent with whole mounts collected from those same animals, and were found to contain mammary lesions. Two tissue samples with conflicting diagnoses were chosen to illustrate the effectiveness of this method; however, multiple abnormalities were identified and will be described elsewhere in detail. Histopathologic evaluation of the original contralateral H&E-stained tissue section revealed a normal gland with no histologic findings. Normal mammary gland architecture consisted of ducts lined by a single layer of simple cuboidal epithelial cells and supported by a layer of myoepithelial cells. Ductal lumens were evident and the adipocytes were uniform throughout the sections (Two examples; Figure 2A and 3A). In contrast, gross visualization of the contralateral whole mount revealed abnormal gland architecture (Figures 2B and 3B; boxed area) characterized by increased opacity around ducts and stromal structures of the mammary gland. It was not possible to determine whether these areas of opacity represented inflammatory, hyperplastic or neoplastic changes without an examination of a representative H&E-stained section of the gland.
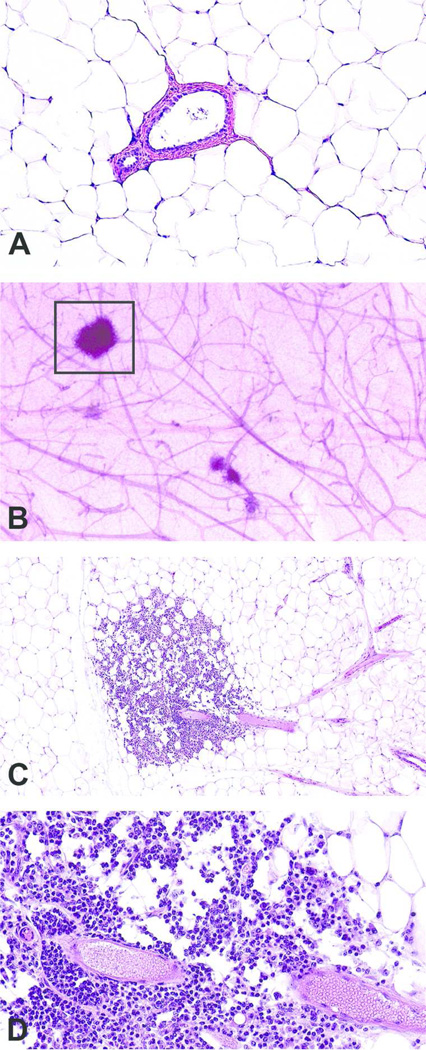
Figure 2. Mammary gland section of perivascular inflammation using the whole mount method.
Normal mammary gland section with no histopathologic findings, formalin-fixed H&E-stained, magnification 10x (A); Increased opacity around ducts and stromal structures (box) in the contralateral carmine-stained mammary whole mount (B); At low magnification, in the contralateral mammary gland H&E prepared from the whole mount (boxed area shown), there are clusters of mononuclear cells around the blood vessel and extending into the adjacent adipose tissue magnification 20x (C); Higher magnification illustrated that the inflammation is composed of perivascular lymphocytes; magnification 40x (D). Tissue samples are from a 14 mo. old female CD-1 mouse.
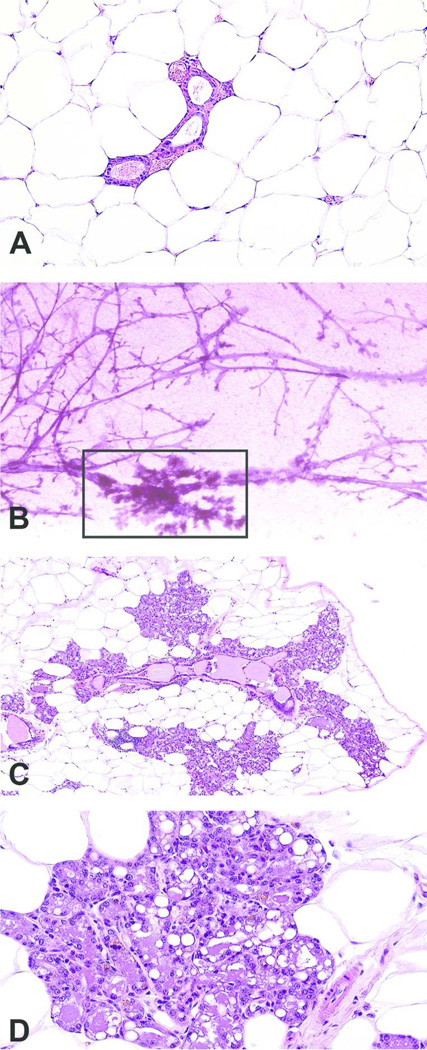
Figure 3. Mammary gland section of lobular hyperplasia using the whole mount method.
Normal mammary gland section with no histopathologic findings, formalin fixed an H&E-stained, magnification 10x (A); Increased opacity around ducts and stromal structures (box) in the contralateral carmine-stained mammary whole mount. (B); At low magnification, in the contralateral mammary gland prepared from the whole mount (boxed area), there is enlargement of the lobule due to an increase in the number and size of cells (lobular alveolar hyperplasia); magnification 10x (C); Higher magnification illustrated enlarged lobules consisting of increased numbers of normal alveolar epithelial cells which were well differentiated and often vacuolated. Alveolar lumens often contained proteinaceous fluid; magnification 40x (D). Tissue samples are from a second individual 14 mo. old female CD-1 mouse.
Results from examination of the sectioned, H&E-stained whole mounts are shown in Figures 2C-D and 3C-D. Samples from one 14 mo old animal (Figure 2C-D) contained lesions diagnosed as perivascular inflammation; characterized by an increased number of perivascular lymphocytes that extended into the adjacent adipose tissue. A portion of the whole mount of a second virgin 14 mo old animal (Figure 3B) was morphologically similar to the appearance of the mammary gland during pregnancy (mid-gestation) or to the mammary gland of male rats (Filgo et al., submitted), characterized by extensive lobular budding. A full evaluation of H&E-stained sections from the whole mount (Figure 3C-D) revealed lobular alveolar hyperplasia characterized by focal to multifocal enlarged lobules comprised of increased numbers of relatively normal alveolar epithelial cells. Alveolar epithelial cells were well differentiated, round, often vacuolated and formed a single concentric layer around ducts that typically contained proteinaceous fluid. Ducts were lined by a single layer of well-differentiated columnar epithelial cells. Alveoli and ducts were normally distributed and there was no evidence of adipocyte compression or cellular atypia.
Discussion
Histopathological evaluation plays an essential role in identifying abnormalities in the mammary gland that are not grossly obvious. This is especially important when evaluating low-dose exposure to EDCs and chemical mixtures that approximate human exposure levels. Whole mammary gland mounts depict the intact morphological landscape and allows an accurate assessment of where and how many abnormalities are occurring. Use of both whole mount and H&E-stained section evaluation is ideal, but not always practical; however, sole reliance on one method can potentially result in missing significant findings. This is especially true when a single H&E-stained mammary gland section is used for histopathological evaluation.
This sectioning method highlights the dual utility and benefits of the mammary gland whole mount. In the two examples discussed, inflammation and lobular hyperplasia were identified in the H&E-stained representative whole gland mount section, but not in the H&E-stained representative from the contralateral mammary gland. This technique is applicable and effective at identifying and quantifying a range of abnormalities, especially in mammary glands of chemically treated or genetically modified rodents. The regular evaluation of mammary tissue from mice and rats during various life stages of development (i.e. wean, puberty and early pregnancy) is highly recommended, due to its small tissue size and relatively low epithelial density. We anticipate that this method will 1) serve as a standardized protocol to reduce inter-lab variability, 2) increase awareness within the scientific community of the ease with which the mammary gland can be incorporated into studies at minimal added cost (Keane et al. 2015), and 3) stimulate collection of the mammary gland into chemical test guideline studies, which may or may not include evaluations (OECD TG443 and NTP), fail to focus on specific mammary gland developmental endpoints (Makris 2011) or are not collected at all (OPPTS 890.1450, US EPA 2009 and OPPTS 890.1500 2009b).
Although it is probable that other histochemical and immunohistochemical staining may be applied to sections derived from whole mounts, it was beyond the scope of this work and further testing would be necessary to make these determinations. Furthermore, it may be possible to prepare smaller sections of abnormal tissue (i.e., punch-biopsy samples) in the same fashion as the whole gland; however, those technical variables were not tested and would require further efforts. It is hoped that evaluation of the entire mammary gland using a combination of whole mounts and their representative H&E-stained sections will minimize the possibility of false negatives in a rodent study, and lead to increased awareness of environmental factors that adversely modify mammary development or function.
Acknowledgments
The authors would like to acknowledge Tina Jones, Pam Ovwigho and Natasha Clayton for their technical expertise and assistance with this project.
References
- 1.Rudel RA, Fenton SE, Ackerman JM, Euling SY, Makris SL. Environmental exposures and mammary gland development: State of the science, public health implications, and research recommendations. Environmental Health Perspectives. 2011;119:1053–1061. doi: 10.1289/ehp.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis B, Fenton S. Haschek and Rousseaux’s Handbook of Toxicologic Pathology. New York: Elsevier Academic Press; 2013. Mammary gland; pp. 2665–2694. [Google Scholar]
- 3.Keane KA, Parker GA, Regan KS, Picut C, Dixon D, Creasy D, Giri D, Hukkanen RR. Scientific and Regulatory Policy Committee (SRPC) Points to Consider: Histopathology Evaluation of the Pubertal Development and Thyroid Function Assay (OPPTS 890.1450, OPPTS 890.1500) in Rats to Screen for Endocrine Disruptors. Toxicol Pathol. 2015 Dec;43(8):1047–1063. doi: 10.1177/0192623315579943. Epub 2015 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OECD. OECD Guidelines for the Testing of Chemicals, Section 4. Paris: OECD Publishing; 2012. Test No. 443: Extended One-Generation Reproductive Toxicity Study. DOI: http://dx.doi.org/10.1787/9789264185371-en. [Google Scholar]
- 5.National Toxicology Program. Specifications for the Conduct of Studies to Evaluate the Reproductive and Developmental Toxicity of Chemical, Biological, and Physical Agents in Laboratory Animals for the National Toxicology Program. Research Triangle Park, NC: National Toxicology Program, National Institute of Environmental Health Sciences; 2011. [Accessed March 18, 2016]. http://ntp.niehs.nih.gov/ntp/test_info/finalntp_reprospecsmay2011_508.pdf. [Google Scholar]
- 6.Makris SL. Current assessment of the effects of environmental chemicals on the mammary gland in guideline rodent studies by the U.S. Environmental Protection Agency (EPA), Organisation for Economic Co-operation and Development (OECD), and National Toxicology Program (NTP) Environmental Health Perspectives. 2011;119:1047–1052. doi: 10.1289/ehp.1002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Environmental Protection Agency. EDSP Test Guidelines OPPTS 890.1450 Pubertal Development and Thyroid Function in the Intact Juvenile/Peripubertal Female Rats. EPA 740-C-09-009. Washington, DC: U.S. Environmental Protection Agency; 2009. [Google Scholar]
- 8.U.S. Environmental Protection Agency. EDSP Test Guidelines OPPTS 890.1500 Pubertal Development and Thyroid Function in Intact Juvenile/Peripubertal Male Rats. EPA 740-C-09-012. Washington, DC: U.S. Environmental Protection Agency; 2009b. [Google Scholar]