Abstract
Purpose
The muscle quality index (MQI) was proposed as a measure to quantify age-related alterations in muscle function. It is unknown if the MQI predicts mortality.
Methods
This was a population-based cohort study from the Third National Health and Nutrition Survey (NHANES III; 1988–1994). The MQI was quantified using a timed sit-to-stand test, body mass, and leg length. Vital status was obtained through the National Center for Health Statistics. We fit multivariable-adjusted regression models to estimate the Hazard Ratio (HR) and 95% Confidence Interval (CI) between the MQI and mortality.
Results
During 14.6 years of follow-up, 3,299 (73.1%) of 4,510 study participants died. Lower MQI was associated with a higher risk of mortality (Ptrend<0.001). The multivariable-adjusted HR for mortality was 1.50 (95% CI: 1.15–1.96) for those in the lowest quintile of MQI compared to the highest quintile. The association between MQI and mortality was stronger among males [highest versus lowest quintile of MQI, HR: 1.37 (95% CI: 1.00–1.87); Ptrend=0.001] compared to females [highest versus lowest quintile of MQI, HR: 1.27 (95% CI: 0.89–1.83); Ptrend=0.044; Pinteraction=0.005].
Conclusions
The MQI predicts mortality, and may differ between males and females. Additional research examining the MQI is warranted.
Keywords: physical function, mobility, aging, physical activity, muscle strength
INTRODUCTION
Two of the most important changes related to aging are the deterioration of muscle quantity and muscle quality [1]. Older adults lose approximately 1% of muscle mass, 2% of muscle strength, and 3% of muscle power annually [2]. The importance of muscle mass has been well-described among older adults [3]. However, less is known about the importance of muscle quality [1,4]. The muscle quality index (MQI) was recently proposed as a measure to quantify age-related alterations in muscle function [4]. The MQI quantifies lower-extremity muscle function using anthropometric parameters and the timed sit-to-stand test [5]. The MQI may be more sensitive than other measures of muscle quality, such as relative strength [strength per unit of muscle mass [6]], because it accounts for the velocity of muscle shortening, reflecting the quality of neural innervation of muscle tissue [7]. The MQI is distinct from the sit-to-stand test by incorporating body mass and leg length [8], which modify the relationship between sit-to-stand time and lower-extremity muscle strength [5]. The capacity of the MQI to predict distal health outcomes, such as mortality, has not been described.
The goal of this study was to understand the relationship between the MQI and mortality. We tested the hypothesis that the MQI would be sufficiently sensitive to distinguish risk for mortality among a nationally-representative sample of community-dwelling midlife and older adults. As an exploratory aim, we tested the hypothesis that the prognostic capacity of the MQI differed between males and females.
METHODS
Study Design and Sample
The Third National Health and Nutrition Examination Survey, 1988–1994 (NHANES III) was a stratified multistage study conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention, to provide health information on a nationally-representative sample of U.S. civilians [9]. The NHANES III sample does not include persons residing in nursing homes, members of the armed forces, institutionalized persons, or US nationals living abroad. Participants provided written informed consent prior to completing any study-related activities. Participants in this analysis included adults of age ≥60 years with the requisite study measures necessary to calculate the MQI, as described below.
Muscle Quality Index
The MQI was quantified using a timed sit-to-stand test, body mass, and leg length to calculate a power index that is expressed in watts (W) [5]. The MQI was calculated as follows: , where 0.5 (m), L (m), g (m/s2), and T represent the height of the chair, leg length, acceleration of gravity (9.8 m/s2), and time required to complete the sit-to-stand test, respectively. Using a stopwatch, sit-to-stand time was calculated as the time to stand from a seated position and return to sitting consecutively five times. The use of arms was not allowed during the sit-to-stand test. Body mass was measured using a digital scale with the participant dressed in an exam gown without shoes. Leg length was taken as the sum of upper leg length and knee height. Upper leg length was measured from the inguinal crease to the patella along the midline of the thigh. Knee height was measured with the participant sitting on an examination table with the legs hanging, using a sliding caliper placed under the heel of the leg to the anterior surface of the thigh, above the condyles of the femur. The MQI is correlated with knee-extensor cross-sectional area, measured using magnetic resonance imaging (r=0.801; P<0.001), and knee-extensor strength, measured using isometric dynamometry (r=0.730; P<0.001) [5].
Mortality Outcome
Vital status was identified using the National Death Index (NDI) database through December 31, 2006. Participants were linked to the NDI database using probabilistic matching that included 12 identifiers such as Social Security number, sex, and date of birth [10]. The National Center for Health Statistics found that 96.1% of deceased participants and 99.4% of living participants were correctly classified using the probabilistic matching algorithm [11]. The National Center for Health Statistics removed select subject characteristics in the file to prevent re-identification of study participants. The publically released survival data are nearly identical to the restricted-use NHANES III mortality-linked file [12].
Covariates
Demographic information including age, sex, and race were reported using a standardized questionnaire [13]. Behavioral and clinical information including smoking status, self-rated health, hospitalization, and falls were reported using a standardized questionnaire [13]. Cognitive function was quantified using the short-portable version of the mini mental status exam to form a score that ranges from 0 to 17, with higher scores indicating better cognition [14]. The presence of comorbid health conditions was reported by asking participants if a healthcare provider had ever told them that they had any of the following: hypertension, diabetes, hyperlipidemia, COPD, cancer, arthritis, myocardial infarction, stroke, congestive heart failure, or kidney disease. Hemoglobin, albumin, c-reactive protein, glycated hemoglobin, insulin, glucose, and creatinine were quantified using standardized laboratory assay procedures that have been described in detail [15,16]. Poor balance was defined as the inability to maintain a full tandem stand for 10 seconds [17]. Gait speed was assessed using a four-meter walk with a stopwatch.
Statistical Analysis
Continuous variables are presented as means (standard error: SE), and categorical variables are presented as percentages (%). Multivariable linear regression was used to identify baseline correlates of the MQI. Cox proportional hazards regression models were used to estimate the hazard ratio (HR) and 95% confidence interval (95% CI) to quantify the association of MQI quintiles and mortality. The assumption of proportional hazards was confirmed using log-log plots. Five regression models were specified to systematically understand the relationship between the MQI and mortality after incrementally accounting for important covariates. Covariates were selected and included in regression models on the basis of biologic plausibility, statistical evidence of confounding, and previously established prognostic importance among adults. To determine if the relationship between MQI and mortality differed between subgroups of males and females we included a statistical interaction term between the MQI and sex in the Cox proportional hazards regression models. Male and female subgroup-stratified analyses are presented to facilitate interpretation. The C-statistic using receiver operating characteristic (ROC) analysis was conducted to determine if the MQI provided incremental discriminative capacity beyond that of the sit-to-stand test to predict mortality [18]. In a post hoc power analysis, our study sample had sufficient statistical power to detect a hazard ratio ≥1.1 between extreme quintiles of the MQI. The threshold for statistical significance for all analyses was P<0.05. All statistical analyses incorporated sample weights to account for nonresponse bias, multistage sampling probabilities, and the subpopulation of participants included in this analytic sample [19]. Stata/SE v.13.1 statistical software was used for all analyses.
RESULTS
Baseline Characteristics Associated with Mortality
Participant characteristics stratified by vital status (Table 1) demonstrate that various demographic (e.g., age, sex, race), clinical (e.g., smoking status, cognitive function, comorbid health conditions, self-rated health, hospitalization, falls), biochemical (e.g., albumin, c-reactive protein, glycated hemoglobin, insulin, glucose, creatinine) and physical (e.g., poor balance, gait speed) characteristics associated with mortality.
Table 1.
Participant characteristics, overall and stratified by survival status
| Characteristic | Overall (N=4,510) [mean (SE) or n (%)] | Died During Follow-Up
|
P-Value | |
|---|---|---|---|---|
| Yes (N=3,299) | No (N=1,211) | |||
| Age, years | 69.9 (0.13) | 71.7 (0.16) | 65.7 (0.18) | <0.001 |
| Sex, % | ||||
| Male | 44.0% | 47.0% | 37.4% | <0.001 |
| Female | 56.0% | 53.0% | 62.6% | |
| Race | ||||
| White | 89.9% | 90.4% | 88.8% | <0.001 |
| Black | 7.9% | 8.3% | 7.2% | |
| Other | 2.2% | 1.3% | 4.0% | |
| Body Mass, kg | 73.6 (0.34) | 73.5 (0.41) | 73.9 (0.60) | 0.647 |
| Leg Length, cm | 90.0 (0.01) | 90.1 (0.01) | 90.0 (0.01) | 0.058 |
| Smoking Status, % | ||||
| Never | 43.7% | 39.7% | 52.7% | <0.001 |
| Former | 40.9% | 42.2% | 37.9% | |
| Current | 15.4% | 18.1% | 9.4% | |
| Cognitive Function, sp-MMSE Score | 13.4 (0.06) | 13.1 (0.08) | 14.2 (0.11) | <0.001 |
| Comorbid Health Conditions, % | ||||
| Hypertension | 44.2% | 46.9% | 38.2% | <0.001 |
| Diabetes | 11.9% | 14.8% | 5.5% | <0.001 |
| Hyperlipidemia | 32.0% | 29.8% | 36.9% | 0.001 |
| COPD | 12.4% | 15.1% | 6.6% | <0.001 |
| Cancer | 8.6% | 10.1% | 5.4% | <0.001 |
| Arthritis | 42.7% | 44.0% | 39.9% | 0.078 |
| Myocardial Infarction | 10.8% | 14.0% | 3.7% | <0.001 |
| Stroke | 5.1% | 6.3% | 2.3% | <0.001 |
| Heart Failure | 6.2% | 7.7% | 3.0% | <0.001 |
| Kidney Disease | 18.3% | 21.7% | 10.7% | <0.001 |
| Self-Rated Health, % | ||||
| Excellent | 14.3% | 12.1% | 19.2% | <0.001 |
| Very Good | 25.0% | 22.3% | 30.9% | |
| Good | 34.4% | 34.7% | 33.6% | |
| Fair | 20.2% | 22.8% | 14.3% | |
| Poor | 6.1% | 8.0% | 2.0% | |
| Hospitalization (≥1/year), % | 16.5% | 18.4% | 12.1% | <0.001 |
| Falls (≥1/year), % | 21.4% | 22.4% | 19.2% | 0.100 |
| Hemoglobin, g/dL | 14.0 (0.03) | 14.0 (0.05) | 14.0 (0.03) | 0.962 |
| Albumin, g/dL | 4.1 (0.01) | 4.0 (0.01) | 4.1 (0.01) | <0.001 |
| C-Reactive Protein, mg/dL | 0.52 (0.02) | 0.59 (0.02) | 0.37 (0.01) | <0.001 |
| Glycated Hemoglobin, % | 5.8 (0.02) | 5.9 (0.03) | 5.6 (0.03) | <0.001 |
| Insulin, pmol/L | 75.4 (1.52) | 80.2 (2.07) | 65.0 (1.54) | <0.001 |
| Glucose, mmol/L | 6.0 (0.04) | 6.2 (0.06) | 5.6 (0.05) | <0.001 |
| Creatinine, mg/dL | 1.2 (0.01) | 1.2 (0.01) | 1.1 (0.01) | <0.001 |
| Poor Balance (<10 s tandem balance), % | 26.9% | 32.0% | 15.9% | <0.001 |
| Gait Speed, meters/second | 0.76 (0.01) | 0.74 (0.01) | 0.82 (0.01) | <0.001 |
| Chair Rise Time, seconds | 13.4 (0.09) | 13.8 (0.11) | 14.5 (0.14) | <0.001 |
| Muscle Quality Index, watts | 120.2 (1.16) | 117.5 (1.37) | 126.2 (2.16) | 0.001 |
Note. SE: Standard error. sp-MMSE: short-portable version of the Mini-Mental State Examination.
Muscle Quality Index Characteristics
Among all participants, the median MQI was 110.1 W [Interquartile Range (IQR) 80.6–148.5]. The mean MQI was 117.5 W (SE: 1.37) among those who died, compared to 126.2 W (SE: 2.16) among those who were alive at the end of follow up (P<0.001). The MQI correlated with gait speed (r=0.39; P<0.0001).
Baseline Correlates of the Muscle Quality Index
Several demographic, clinical, biochemical, and physical function characteristics associated with MQI in univariable and multivariable regression analyses (Table 2). Select variables of interest that associated with MQI in the multivariable linear regression model included age, sex, smoking status, history of COPD or myocardial infarction, self-reported health, hemoglobin, and gait speed (all P<0.05). The multivariable regression model accounted for 38.8% of the variability in the MQI.
Table 2.
Correlates of muscle quality index in univariable and multivariable linear regression
| Univariable | Multivariable‡ | |||
|---|---|---|---|---|
|
|
||||
| Characteristic | beta | P-Value | beta | P-Value |
| Age* | −1.87 | <0.001 | −1.05 | <0.001 |
| Sex | ||||
| Male | 0.00 – Ref | 0.00 – Ref | ||
| Female | −54.47 | <0.001 | −47.78 | <0.001 |
| Race | ||||
| White | 0.00 – Ref | 0.00 – Ref | ||
| Black | −3.10 | 0.159 | 5.49 | 0.014 |
| Other | −34.4 | <0.001 | −33.65 | <0.001 |
| Smoking Status | ||||
| Never | 0.00 – Ref | 0.00 – Ref | ||
| Former | 20.23 | <0.001 | 0.23 | 0.922 |
| Current | −2.10 | 0.492 | −14.40 | <0.001 |
| Cognitive Function* | 1.48 | <0.001 | −0.24 | 0.417 |
| Comorbid Health Conditions | ||||
| Hypertension | 0.17 | 0.943 | 5.86 | 0.005 |
| Diabetes | −1.99 | 0.553 | −3.90 | 0.365 |
| Hyperlipidemia | −1.22 | 0.626 | −2.11 | 0.319 |
| COPD | −11.00 | 0.001 | −7.60 | 0.010 |
| Cancer | −3.92 | 0.321 | −0.30 | 0.923 |
| Arthritis | −10.54 | <0.001 | 2.47 | 0.243 |
| Myocardial Infarction | 0.11 | 0.975 | −7.71 | 0.023 |
| Stroke | −13.70 | 0.004 | −0.07 | 0.988 |
| Heart Failure | −1.52 | 0.745 | 4.43 | 0.373 |
| Kidney Disease | −8.01 | 0.005 | 2.46 | 0.379 |
| Self-Rated Health | ||||
| Excellent | 0.00 – Ref | 0.00 – Ref | ||
| Very Good | −10.41 | 0.015 | −3.80 | 0.424 |
| Good | −16.15 | <0.001 | −7.45 | 0.045 |
| Fair | −18.11 | <0.001 | −9.25 | 0.021 |
| Poor | −34.61 | <0.001 | −16.42 | 0.002 |
| Hospitalization | −4.47 | 0.146 | −1.88 | 0.491 |
| Falls | −11.73 | <0.001 | 0.23 | 0.919 |
| Hemoglobin† | 1.20 | <0.001 | 0.33 | <0.001 |
| Albumin† | 1.99 | <0.001 | −0.45 | 0.177 |
| C-Reactive Protein† | −0.30 | 0.001 | −0.11 | 0.213 |
| Glycated Hemoglobin† | −0.39 | 0.675 | 1.21 | 0.385 |
| Insulin† | 0.01 | <0.001 | 0.01 | <0.001 |
| Glucose† | 0.06 | 0.183 | −0.07 | 0.244 |
| Creatinine† | 2.36 | <0.001 | −0.06 | 0.804 |
| Poor Balance | −18.13 | <0.001 | −2.13 | 0.312 |
| Gait Speed† | 10.42 | <0.001 | 70.85 | <0.001 |
NOTE. SE: Standard error. Ref: Reference group. sp-MMSE: short-portable version of the Mini-Mental State Examination. Leg length and body mass were excluded from the regression models due to collinearity.
Beta value represents a 1.0 unit increase in the independent variable.
Beta value represents a 0.1 unit increase in the independent variable.
Multivariable model regression intercept: 189.73; R2=0.388.
Muscle Quality Index and Mortality
During a median 14.6 year follow up, 3,299 deaths occurred. Lower MQI was associated with higher mortality in all statistical models (Ptrend≤0.001). Compared to participants in the highest quintile of MQI, those in the lowest quintile of LEMP were 57% more likely to die [HR: 1.57 (95% CI: 1.23–1.99); Table 3]. These results remained unchanged after further adjustment for balance and gait speed [HR: 1.50 (95% CI: 1.15–1.96); Ptrend=0.001]. The MQI more accurately predicted mortality than the sit-to-stand test (C-statistic: 0.654 vs 0.624; P<0.001).
Table 3.
Association between muscle quality index and mortality
| Quintiles of Muscle Quality Index, Watts (W) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Ptrend | |
|
|
||||||
|
Full Sample (N=4,510)
|
||||||
| Median [IQR] (watts) | 57.3 [49.0–65.0] | 82.8 [76.8–88.7] | 105.4 [99.0–112.6] | 135.5 [126.5–144.9] | 186.1 [168.0–212.2] | |
| Median [IQR] Survival, Yrs. | 10.7 [6.25–18.8] | 13.4 [7.7–21.5] | 15.0 [8.7–21.9] | 15.9 [9.1–21.5] | 16.8 [9.8–22.7] | <0.001 |
| Model 1* | 1.92 (1.58–2.35) | 1.46 (1.21–1.75) | 1.27 (1.07–1.51) | 1.19 (1.01–1.39) | 1.00 – Ref | <0.001 |
| Model 2† | 1.90 (1.53–2.36) | 1.65 (1.36–1.98) | 1.47 (1.24–1.75) | 1.23 (1.05–1.44) | 1.00 – Ref | <0.001 |
| Model 3‡ | 1.57 (1.23–2.01) | 1.43 (1.16–1.75) | 1.23 (1.02–1.48) | 1.09 (0.92–1.30) | 1.00 – Ref | <0.001 |
| Model 4§ | 1.55 (1.20–1.99) | 1.43 (1.16–1.76) | 1.22 (1.01–1.47) | 1.13 (0.95–1.34) | 1.00 – Ref | <0.001 |
| Model 5|| | 1.50 (1.15–1.96) | 1.39 (1.11–1.73) | 1.21 (0.99–1.47) | 1.13 (0.95–1.34) | 1.00 – Ref | 0.001 |
|
| ||||||
|
Males Only (N=2,240)
|
||||||
| Median [IQR] (watts) | 81.4 [71.1–89.6] | 109.7 [102.9–115. | 131.5 [125.2–138.2] | 160.2 [151.9–170.0] | 208.7 [192.6–251.7] | |
| Median [IQR] Survival, Yrs. | 9.7 [4.1–15.0] | 9.7 [4.7–16.6] | 12.0 [7.0–19.0] | 16.2 [8.7–NR] | 16.7 [10.3–22.7] | <0.001 |
| Model 1* | 2.27 (1.72–3.01) | 2.07 (1.59–2.69) | 1.62 (1.27–2.07) | 1.12 (0.90–1.40) | 1.00 – Ref | <0.001 |
| Model 2† | 1.81 (1.39–2.37) | 1.73 (1.36–2.21) | 1.32 (1.04–1.68) | 1.05 (0.85–1.30) | 1.00 – Ref | <0.001 |
| Model 3‡ | 1.42 (1.05–1.90) | 1.46 (1.15–1.87) | 1.14 (0.88–1.47) | 0.92 (0.73–1.15) | 1.00 – Ref | <0.001 |
| Model 4§ | 1.44 (1.06–1.95) | 1.47 (1.15–1.89) | 1.20 (0.93–1.54) | 0.91 (0.72–1.14) | 1.00 – Ref | <0.001 |
| Model 5|| | 1.37 (1.00–1.87) | 1.42 (1.10–1.83) | 1.17 (0.91–1.50) | 0.89 (0.71–1.12) | 1.00 – Ref | 0.001 |
|
| ||||||
|
Females Only (N=2,270)
|
||||||
| Median [IQR] (watts) | 50.0 [43.4–54.9] | 68.9 [64.2–73.1] | 84.9 [80.6–89.3] | 104.1 [97.7–110.6] | 144.8 [130.5–165.4] | |
| Median [IQR] Survival, Yrs. | 10.0 [5.7–17.7] | 13.7 [8.2–NR] | 14.7 [9.2–NR] | 19.0 [11.7–NR] | 16.7 [12.3–NR] | <0.001 |
| Model 1* | 2.61 (1.98–3.43) | 1.60 (1.26–2.04) | 1.54 (1.23–1.93) | 1.06 (0.86–1.33) | 1.00 – Ref | <0.001 |
| Model 2† | 1.62 (1.23–2.14) | 1.33 (1.05–1.70) | 1.27 (1.03–1.58) | 1.00 (0.80–1.24) | 1.00 – Ref | <0.001 |
| Model 3‡ | 1.43 (1.05–1.93) | 1.20 (0.92–1.57) | 1.20 (0.95–1.51) | 0.87 (0.69–1.10) | 1.00 – Ref | 0.003 |
| Model 4§ | 1.33 (0.96–1.84) | 1.15 (0.87–1.52) | 1.17 (0.92–1.48) | 1.17 (0.66–1.07) | 1.00 – Ref | 0.013 |
| Model 5|| | 1.27 (0.89–1.83) | 1.12 (0.83–1.52) | 1.14 (0.88–1.48) | 0.83 (0.65–1.07) | 1.00 – Ref | 0.044 |
Note. NR: Not reached. Ref: Reference group.
Model 1 is adjusted for body mass.
Model 2 is adjusted for model 1, age and sex (except in sex-specific strata).
Model 3 is adjusted for model 2, race, leg length, smoking status, cognitive function, hypertension, diabetes, hyperlipidemia, COPD, cancer, arthritis, myocardial infarction, stroke, heart failure, kidney disease, self-rated health, hospitalization, falls, hemoglobin, albumin, C-reactive protein, glycated hemoglobin, insulin, glucose, and creatinine.
Model 4 is adjusted for model 3, and poor balance.
Model 5 is adjusted for model 4 and gait speed.
The tests for statistical interaction between muscle quality index and sex were as follows: Model 1, P<0.001; Model 2, P=0.003; Model 3, P<0.001; Model 4, P=0.002; Model 5, P=0.005.
Muscle Quality Index among Males versus Females
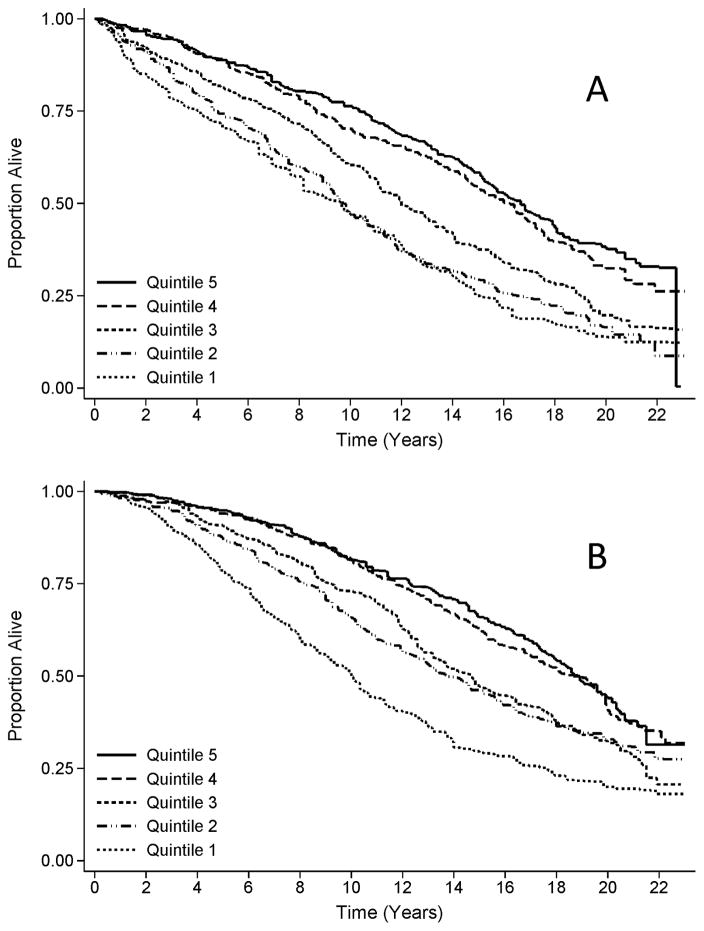
In exploratory multivariable-adjusted subgroup analyses (i.e., Model 5), the association between the MQI and mortality was stronger among males [highest versus lowest quintile of MQI, HR: 1.37 (95% CI: 1.00–1.87); Ptrend=0.001] compared to females [highest versus lowest quintile of MQI, HR: 1.27 (95% CI: 0.89–1.83); Ptrend=0.044; Pinteraction=0.005; Figure 1].
Figure 1.
Kaplan-Meier survival plot, stratified by quintile of muscle quality index for A) males and B) females
DISCUSSION
The principal finding of this study is that community-dwelling adults with a low MQI are more likely to die compared to those with a high MQI. This relationship was consistent in analyses that accounted for multiple prognostic variables including age, sex, comorbid health conditions, biochemical measures, balance, and gait speed. Adequate lower-extremity muscle quality is important to support many activities of daily living including walking, climbing stairs, and rising from a chair [20]. The MQI leverages anthropometric parameters and the sit-to-stand test. The sit-to-stand test is often familiar to older adults, appropriate for those with physical limitations, and can be implemented in settings with limited resources [7]. Our findings suggest that muscle quality, as defined by the MQI, may have important implications for longevity.
Our findings parallel other studies that have defined muscle quality as the ratio of muscle strength to muscle mass. Among 2,292 adults aged 70–79 years in the Health, Aging, and Body Composition (Health ABC) study, the ratio of knee-extensor strength to leg muscle mass was associated with a significant increase in the risk of mortality [HR: 1.34 (95% CI: 1.19–1.51)] [21]. Interestingly, in the Health ABC cohort, the relationship between lower-extremity muscle quality and mortality was stronger among males [HR: 1.39 (95% CI: 1.20–1.61)] compared to females [HR: 1.24 (95% CI: 1.00–1.52)]. The Health ABC study demonstrated that over three years males lose significantly more lower-extremity muscle mass, muscle strength, and consequently, muscle quality (all P<0.004) compared to females [6]. The reasons for differences in the loss of muscle quality between men and women are not entirely clear, but may be related baseline muscle characteristics, such that older adults with more muscle strength and mass at baseline have a greater opportunity for decline over time [6]. The observed prognostic difference of the MQI between males and females warrants further investigation. We hypothesize that the prognostic difference of the MQI between males and females may relate to neuro-hormonal and muscle composition differences such as pro-inflammatory cytokine concentrations and intramuscular adipose tissue infiltration [22,23]. These data underscore the importance of muscle quality, in addition to muscle mass, with advancing age.
We identified several demographic, clinical, biochemical, and physical characteristics associated with the MQI in cross-sectional multivariable linear regression analysis. Consistent with prior reports of muscle quality [6], older age and female sex were associated with a lower MQI. In addition, we identified several novel correlates of the MQI. These included race, smoking status, a prior diagnosis of hypertension, COPD, or myocardial infarction, self-rated health, hemoglobin, insulin, and gait speed. These variables accounted for 38.8% of the variability observed in the baseline MQI. These findings may provide mechanistic insight to the physiology that links the MQI to mortality. Other characteristics not included in our analysis that may account for additional variability in the MQI include genetic factors such as telomere length, muscle fiber distribution and innervation, and anabolic hormone concentrations such as testosterone [24]. The MQI may represent a surrogate measure of overall health, similar to that of handgrip strength [25]. Given the modest correlation between the MQI and gait speed (r=0.39), these two measures may quantify distinct aspects of lower-extremity physical function. As a surrogate of overall health, the MQI could be a sensitive measure, as it is directly influenced by muscle strength, muscle contractile properties such as force-velocity characteristics, muscle fiber type distribution, and neurologic innervation of muscle tissue. These factors are affected by aging and comorbidities [26–28]. Muscle quality is associated with physical function and mobility limitations [29,30]. Therefore the MQI may represent a measure that quantifies age-related deterioration in locomotion systems that are important for physical functioning and longevity. Additional research is necessary to understand how the MQI may complement other performance-based and self-reported measures of physical function [31].
Physical activity or exercise is an efficacious intervention to improve the MQI [7,32]. Among 25 older adults (average of 70.6±6.1 years), six-weeks of twice-weekly resistance exercise resulted in a clinically meaningful 41 (±19) W improvement in the MQI from baseline (P<0.001) [7]. This magnitude of exercise-induced improvement in the MQI would likely be sufficient to shift one quintile in our cohort. It is unknown if improving the MQI translates to a reduction in the risk of mortality (or prevention of other deleterious health outcomes). Contrary to expectations, six-weeks of detraining did not result in the regression of post-training values. These data indicate that short-term interventions may produce long-term benefits with respect to the MQI. These preliminary data warrant further investigation of the MQI as a study endpoint. It is unknown if longer duration of exercise interventions would yield larger improvements in the MQI. It is also unknown if aerobic exercise, such as brisk walking, would improve the MQI. Other modalities including hormonal, pharmacologic, and nutritional interventions warrant examination [32].
The main strength of this study is the large sample size that, based on the sampling design, is representative of the US population of community-dwelling midlife and older adults [9]. Participants in our sample represented a wide range of ages (from 60 to 90 years) and MQI values (from 14.5 to 625.3 W). The cohort had an extensive length of follow up (median 14.6 years) that allowed us to observe a high proportion of deaths, such that 73.1% of the cohort had died by the end of the follow up period. There are several limitations of this study. As a prospective cohort study we cannot interpret the association between the MQI and mortality as causal. We extensively adjusted for variables that are known or hypothesized to influence the MQI-mortality relationship. To our knowledge, this is the first cohort to examine the association between the MQI and mortality. Our findings should be replicated in external cohorts to confirm the relationship between the MQI and mortality. Future prospective cohort studies should examine the relationship between the MQI and other important intermediate health-related endpoints such as injurious falls, mobility disability, and institutionalization. Although our sample was population-based, the majority of participants were of white race. Future studies should consider examining characteristics of the MQI within cohorts with greater representation of non-white participants, as muscle characteristics may differ among ethnic populations [33]. Another limitation is that information on comorbid health conditions was self-reported. This likely underestimates the prevalence of comorbidities. For example, for each known, self-reported case of diabetes, there likely exists one unknown case [34].
CONCLUSIONS
In conclusion, our findings suggest that community-dwelling adults with a low MQI may be more likely to die compared to those with a high MQI. The prognostic impact of the MQI appears to differ between males and females. These data support the hypothesis that muscle quality has an important role in longevity. The MQI may represent a sensitive surrogate measure of overall health. Additional research examining the MQI is warranted.
Acknowledgments
FUNDING: Research reported in this publication was supported by the National Cancer Institute (F31-CA192560, R21-CA182726, U54-CA155850), National Heart, Lung, and Blood Institute (F31-HL127947) and the National Institute of Diabetes and Digestive and Kidney Diseases (F32-DK096758) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JCB, MOH, and MNH declare no conflicts of interest.
ABBREVIATIONS
- MQI
muscle quality index
- HR
hazard ratio
- CI
confidence interval
- SE
standard error
- W
watts
- NDI
National Death Index
Footnotes
Disclosures: The authors declare no conflicts of interest.
References
- 1.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan. 2014;3:9-2395-3-9. doi: 10.1186/2046-2395-3-9. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry BK, Carson RG. The consequences of resistance training for movement control in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:730–754. doi: 10.1093/gerona/59.7.m730. [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27:387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Barbat-Artigas S, Rolland Y, Zamboni M, Aubertin-Leheudre M. How to assess functional status: a new muscle quality index. J Nutr Health Aging. 2012;16:67–77. doi: 10.1007/s12603-012-0004-5. [DOI] [PubMed] [Google Scholar]
- 5.Takai Y, Ohta M, Akagi R, Kanehisa H, Kawakami Y, Fukunaga T. Sit-to-stand test to evaluate knee extensor muscle size and strength in the elderly: a novel approach. Journal of physiological anthropology. 2009;28:123–128. doi: 10.2114/jpa2.28.123. [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 7.Fragala MS, Fukuda DH, Stout JR, Townsend JR, Emerson NS, Boone CH, et al. Muscle quality index improves with resistance exercise training in older adults. Exp Gerontol. 2014;53:1–6. doi: 10.1016/j.exger.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Bohannon RW. Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills. 1995;80:163–166. doi: 10.2466/pms.1995.80.1.163. [DOI] [PubMed] [Google Scholar]
- 9.Plan and operation of the Third National Health and Nutrition Examination Survey 1988–94, Series 1: programs and collection procedures. Vital Health Stat. 1994;1(32):1–407. [PubMed] [Google Scholar]
- 10.Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39:719–734. doi: 10.1016/0021-9681(86)90155-4. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. The Third National Nutrition and Health Survey Linked Mortality File: Matching Methodology. Available from: http://www.cdc.gov/nchs/data/datalinkage/mort_calibration_study.pdf.
- 12.Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU. Health insurance and mortality in US adults. Am J Public Health. 2009;99:2289–2295. doi: 10.2105/AJPH.2008.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. NHANES III Questionnaires, Datasets and Related Documentation. Available from: http://www.cdc.gov/nchs/nhanes/nh3data.htm.
- 14.Obisesan TO, Obisesan OA, Martins S, Alamgir L, Bond V, Maxwell C, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2008;56:501–509. doi: 10.1111/j.1532-5415.2007.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. Laboratory Procedures Used for the Third National Health and Nutrition Exam Survey (NHANES III), 1988–1994. Available from: http://wonder.cdc.gov/wonder/sci_data/surveys/hanes/hanes3/type_txt/lab.asp.
- 16.Lacher DA, Hughes JP, Carroll MD. Estimate of biological variation of laboratory analytes based on the third national health and nutrition examination survey. Clin Chem. 2005;51:450–452. doi: 10.1373/clinchem.2004.039354. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 18.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 19.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 21.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 22.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 24.Welle S. Cellular and molecular basis of age-related sarcopenia. Canadian journal of applied physiology. 2002;27:19–41. doi: 10.1139/h02-002. [DOI] [PubMed] [Google Scholar]
- 25.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. The Lancet. 2015;18:266–73. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 26.Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–1679. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54:B207–B218. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 28.Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 29.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 30.Misic MM, Rosengren KS, Woods JA, Evans EM. Muscle quality, aerobic fitness and fat mass predict lower-extremity physical function in community-dwelling older adults. Gerontology. 2007;53:260–266. doi: 10.1159/000101826. [DOI] [PubMed] [Google Scholar]
- 31.Reuben DB, Seeman TE, Keeler E, Hayes RP, Bowman L, Sewall A, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59:1056–1061. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 32.Fragala MS, Kenny AM, Kuchel GA. Muscle Quality in Aging: a Multi-Dimensional Approach to Muscle Functioning with Applications for Treatment. Sports Medicine. 2015;45:641–658. doi: 10.1007/s40279-015-0305-z. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 34.Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, Sicree RA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25:829–834. doi: 10.2337/diacare.25.5.829. [DOI] [PubMed] [Google Scholar]