Abstract
Background and Aims
Erectile dysfunction (ED) and atherosclerotic cardiovascular disease (ASCVD) share many common risk factors, and vascular ED is a marker for increased ASCVD risk. Low 25-hydroxyvitamin D [25(OH)D] concentrations have been associated with increased ASCVD risk, but less is known regarding the relationship of low 25(OH)D with ED. We determined whether 25(OH)D deficiency is associated with ED independent of ASCVD risk factors.
Methods
We performed cross-sectional analyses of 3,390 men aged ≥20 years free of ASCVD who participated in NHANES 2001-2004. Serum 25(OH)D was measured by the DiaSorin radioimmunoassay; deficiency was defined as levels <20 ng/mL (<50 nmol/L). Self-reported ED, assessed by a single validated question, was defined as men who reported being “never” or “sometimes able” to maintain an erection. We assessed the relationship between 25(OH)D deficiency and ED prevalence using adjusted Poisson regression methods.
Results
After accounting for NHANES sampling, the weighted prevalence of 25(OH)D deficiency and of ED were 30% and 15.2%, respectively. 25(OH)D levels were lower in men with vs. those without ED (mean 22.8 vs 24.3 ng/mL, respectively; p=0.0005). After adjusting for lifestyle variables, comorbidities, and medication use, men with 25(OH)D deficiency had a higher prevalence of ED compared to those with levels ≥30 ng/ml (Prevalence Ratio 1.30, 95% CI 1.08-1.57).
Conclusion
In this cross-sectional analysis of a representative sample of U.S. men, vitamin D deficiency was associated with an increased prevalence of ED independent of ASCVD risk factors. Additional research is needed to evaluate whether treating vitamin D deficiency improves erectile function.
Keywords: vitamin D deficiency, erectile dysfunction, cardiovascular disease, race/ethnicity
Graphical abstract
Introduction
Erectile dysfunction (ED) is a highly-prevalent disorder among adult men,1,2 affecting approximately 1 in 5 adult men in the United States (U.S.) and up to 80% of men aged 75 years and older.3 ED can result from psychogenic and organic (medical) causes, or a combination of both. Among the organic forms, vascular ED is the most common, predominantly caused by underlying atherosclerosis and/or endothelial dysfunction.4 Indeed, ED and atherosclerotic cardiovascular disease (ASCVD) share many common risk factors5,6 and ED has been independently associated with increased risk for future ASCVD events.7,8 Thus, ED can be used as a marker of cardiovascular disease and identify men at elevated ASCVD risk.9
Low levels of vitamin D, defined as serum 25-hydroxyvitamin D [25(OH)D] below 30 ng/ml, affect over two thirds of the US adult population10 and an estimated 1 billion individuals worldwide11. Low 25(OH)D levels are also associated with increased risk for clinical ASCVD events.12-15 Suboptimal vitamin D status is thought to influence ASCVD risk predominantly through established vascular risk factors, namely hypertension, diabetes, inflammation, and endothelial dysfunction.16 However, whether treating a low 25(OH)D state with adequate vitamin D supplementation can prevent ASCVD events is still unknown, and clinical trials testing this question are in progress.17
It has been postulated that low 25(OH)D may contribute to ED through several mechanisms such as endothelial dysfunction, inflammation, impaired glucose homeostasis, and atherosclerosis,18 similar to the mechanisms linking low vitamin D with ASCVD, but the link between 25(OH)D levels and ED is still uncertain. Little is also known about potential racial/ethnic differences in the association between 25(OH)D and ED, though there is some evidence that vitamin D deficiency, defined as 25(OH)D concentrations less than 20 ng/ml, is a stronger risk factor for diabetes,19 heart failure,20 and ASCVD13-15 in whites compared to blacks.
Using a nationally representative sample of U.S. men, we sought to determine whether vitamin D deficiency is associated with self-reported ED independent of lifestyle and vascular risk factors, and whether this association varies by race/ethnicity.
Materials and Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative survey of the non-institutionalized U.S. civilian population, obtained by using stratified multistage clustered probability samples with planned oversampling of certain age and minority groups.21 NHANES is conducted by the National Center of Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The NHANES study protocols were approved by the institutional review board of the NCHS, and written informed consent was obtained from all participants.
In NHANES, questionnaire data on ED and laboratory data on 25(OH)D were available only in the 2001-2004 cycles [n=3,943 men ≥20 years of age with both ED and 25(OH)D data]. We excluded 457 men with self-reported ASCVD, 13 men taking phosphodiesterase type 5 (PDE5) inhibitors, and 83 men with prior diagnosis of prostate cancer, resulting in a final sample size of 3,390 men. A participant flow diagram is outlined in Supplemental Figure 1.
Vitamin D status
Serum 25(OH)D was measured by the DiaSorin radioimmunoassay (RIA). We used the RIA-harmonized data that were calibrated to the NHANES III (1988-1994) DiaSorin RIA assay and corrected for laboratory drifts during 2003-2006 using a model based on RIA quality control pool data (data release November 2010).22
For the primary analysis, we categorized the serum 25(OH)D levels into three clinically relevant categories as endorsed by the Endocrinology Society Clinical Practice Guidelines:23 deficient (<20 ng/mL), intermediate (20 to <30 ng/mL), and optimal (≥30 ng/mL). As the Institute of Medicine has stated that levels ≥20 ng/ml (rather than ≥30 ng/ml) should be adequate enough for health,24 we also performed a sensitivity analyses dichotomizing study participants into vitamin D deficient and non-deficient using a cutoff of 20 ng/mL. To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
Erectile dysfunction
Self-reported ED was assessed by a single question from the Massachusetts Male Aging Study (MMAS):25 “How would you describe your ability to get and keep an erection adequate for satisfactory intercourse?” Response options were “never able”, “sometimes able”, “usually able”, and “almost or almost always able”. For our primary outcome definition, we defined ED as those who answered “sometimes able” or “never able.” In a sensitivity analysis, we re-defined ED more stringently to include only men who reported “never able” to maintain an erection. Men taking PDE5 inhibitors were excluded from the analyses (see above).
Other variables
The NHANES visit included an interview using standardized questionnaires administered by a trained interviewer and a physical examination at a mobile examination center, which included blood and urine collection. Self-reported race/ethnicity was categorized into 4 groups (Non-Hispanic White, Non-Hispanic Black, Mexican American or Hispanic, and Other Race - Including Multi-Racial). Smokers were categorized as never, former, and current. Physical activity (determined by questionnaire) was the frequency and duration of moderate to vigorous intensity leisure-time physical activities performed for at least 10 minutes at a time during the past month. We also created dichotomous (yes/no) variables for the use of anti-depressants medications and beta-blockers based on self-reported medication use.
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, the use of medications for high blood pressure, or a self-reported diagnosis of high blood pressure. Diabetes was defined as a serum level of glycated hemoglobin (HbA1c) ≥6.5%, the use of anti-diabetic medications, or a self-reported diagnosis of diabetes mellitus. Hypercholesterolemia was defined as a serum level of total cholesterol ≥240 mg/dL or the use of lipid lowering medications. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation,26 and categorized into 3 groups; ≥90, 60-89, and <60 mL/min/1.73 m2. High-sensitivity C-reactive protein (CRP) was measured per the standard NHANES laboratory protocol.21
Statistical analysis
We accounted for the complex multi-stage stratified sampling technique of NHANES by using the svy commands in Stata using the appropriate 4-years weights provided with the datasets. We presented the demographic and clinical characteristics among the analytic sample by the presence or absence of ED. Continuous variables were presented using means and standard errors, and we compared the two outcome groups using student’s t-tests. Categorical variables were presented using proportions, and we compared the outcome groups using the chi-squared test.
To evaluate the association between vitamin D status and the prevalence of ED, we used Poisson regression models to estimate prevalence ratios (PRs) and 95% confidence intervals (CI) of ED comparing participants with deficient and intermediate vitamin D levels to those with optimal levels as the reference category, as well as per a 10 ng/ml decrease in 25(OH)D concentrations. In a sensitivity model, we repeated the Poisson regression analysis using the dichotomous vitamin D deficiency variable (<20 vs. ≥20 ng/ml). We also evaluated non-linear dose-response relationships using adjusted restricted cubic splines.
Our models were progressively adjusted for different sets of potential confounders as follows: Model 1 was unadjusted; Model 2 was adjusted for demographic factors, including age and race/ethnicity; Model 3 was further adjusted for potential confounding lifestyle factors, including smoking, alcohol consumption, BMI, and physical activity; Model 4 was further adjusted for hypertension, diabetes, hypercholesterolemia, eGFR, and CRP (i.e. potential mediators of the association between vitamin D and ED); and Model 5 was further adjusted for medication use that might affect erectile function including antidepressants and beta-blockers.
Wald tests were used to test for interactions of vitamin D status categories with race/ethnicity in relation to ED by including cross-product terms in the models. Based on prior studies showing racial differences in the association of 25(OH)D with ASCVD risk,14,15 we decided a priori to present the results overall and stratified by race/ethnicity, regardless of the presence of significant interactions. We also checked for interactions by diabetes status, given the purported role of vitamin D deficiency and incident diabetes risk.19
We performed three additional sensitivity analyses. First, to minimize confounding by poor health status, we restricted our analysis to men reported a general health status of excellent, very good or good (n=2,792). Second, to see if testosterone (as a potential mediator) can explain the association between vitamin D and risk of ED, we restricted our analysis to those who also had measured serum levels of sex hormones (testosterone, androstanediol glucuronide, sex hormone binding globulin, and estradiol, n=562) and adjusted for sex hormones. Third, we redefined the ED using the more stringent definition of only those who reported “never able” to maintain an erection.
Two sided p-values <0.05 were considered statistically significant. The data were analyzed using Stata Software (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
There were 3,390 men included in our study population, with age ranging from 20 to 85 years; 775 men had ED and 1327 had 25(OH)D deficiency (<20 ng/ml). After accounting for the NHANES sampling design, the weighted prevalence of vitamin D deficiency and of ED were 30% and 15.2%, respectively. Table 1 shows the weighted unadjusted baseline characteristics of the men included in our analytic sample, stratified by the prevalence of ED. Serum 25(OH)D levels were significantly lower in men with vs. those without ED (mean 22.8 vs 24.3 ng/mL, p=0.0005). Men with ED were more likely to be older, have lower socio-economic status, report fair/poor self-reported general health status, be former smokers, and have higher BMI, less physical activity, higher CRP, and lower eGFR and more likely to have diabetes, hypertension, hypercholesterolemia, and use prescribed beta-blockers and anti-depressant medications than men without ED. We did not see racial/ethnic differences in the prevalence of ED.
Table 1. Baseline Demographic and Clinical Characteristics of Study Population, NHANES 2001-2004.
| ED | No ED | |
|---|---|---|
| (N=775) | (N=2,615) | |
| Age (years) | 58.41±0.57 | 40.51±0.3 |
| 25(OH)D ng/ml | 22.83±0.52 | 24.26±0.39 |
| Vitamin D categories, n (%) | ||
| >=30 ng/mla (n=607) | 124 (19.2) | 483 (25) |
| 20-30 ng/mla (n=1456) | 325 (44.8) | 1,131 (46.2) |
| <20 ng/mla (n=1327) | 326 (36.03) | 1,001 (28.9) |
| Prevalence of 25(OH)D deficiency <20 ng/mla (%) | 36.03 | 28.9 |
| Race/Ethnicity | ||
| Non-Hispanic White (%) | 73 | 73.4 |
| Non-Hispanic Black (%) | 8.8 | 9.6 |
| Mexican American or Other Hispanic (%) | 14.6 | 12.7 |
| Other Race - Including Multi-Racial (%) | 3.6 | 4.4 |
| Education | ||
| High School or less (%) | 51.5 | 41.5 |
| Annual Household Income | ||
| Less than $20,000 (%) | 19.1 | 11.8 |
| Self-reported General Health Status | ||
| Excellent, Very Good, Good | 74.2 | 89 .9 |
| Fair, Poor | 25.8 | 10.1 |
| BMI (kg/m2) | 29.0±0.33 | 27.83±0.13 |
| Physical activity (%) | 55.7 | 71.9 |
| Smoking | ||
| Current (%) | 25.6 | 25.5 |
| Former (%) | 43.3 | 24.9 |
| Never (%) | 32.1 | 46.7 |
| Alcohol use | 80.4 | 84.4 |
| CRP (mg/L) | 0.44±0.03 | 0.3±0.01 |
| eGFR (ml/min per 1.73 m2) | 84.5±0.81 | 97±0.58 |
| eGFR Categories | ||
| <60 ml/min per 1.73 m2 | 12.6 | 1.8 |
| 60-90 ml/min per 1.73 m2 | 47.9 | 33.6 |
| 90 ml/min per 1.73 m2 | 39.5 | 64.6 |
| Diabetes (%) | 22 | 5.5 |
| Hypertension (%) | 52.3 | 27.3 |
| Hypercholesterolemia (%) | 45.2 | 32 |
| Use of beta-blockers (%) | 13.1 | 3.4 |
| Use of anti-depressants (%) | 11.7 | 5.6 |
To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
All variables were statistically significantly different between men with ED vs. men without ED (p<0.05) except for race/ethnicity (p=0.4).
National Health and Nutrition Examination Survey = NHANES; Erectile Dysfunction = ED; 25-hydroxyvitamin D = 25(OH)D; Body Mass Index = BMI; C-Reactive Protein = CRP; Estimated Glomerular Filtration Rate = eGFR
The association of vitamin D (3 categories) with ED
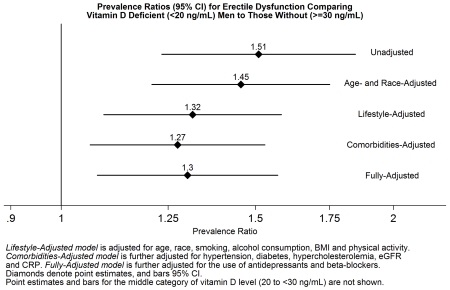
The unadjusted PRs (95% CI) for ED for comparing participants with intermediate and deficient vitamin D levels to those with optimal levels were 1.23 (0.99 to 1.51) and 1.51 (1.23 to 1.85), respectively (Table 2, Model 1). A similar pattern was observed after multivariable adjustment, although only the deficient category remained statistically significant in all models. In the fully adjusted model which adjusted for demographics, lifestyle factors, ASCVD risk factors, and medication usage (model 5), the PR of ED for comparing participants with deficient 25(OH)D levels to those with optimal levels was 1.30 (1.08 to 1.57).
Table 2. Prevalence Ratios (95% CI) of Prevalent ED by Three Vitamin D Categories (bold, denotes statistical significance), NHANES 2001-2004.
| N | ≥30 ng/mLa | 20-30 ng/mLa | <20 ng/mLa | per 10 ng/mLa Decrease in Vitamin D |
|
|---|---|---|---|---|---|
| Model 1b | 3,390 | 1 (Ref) | 1.23 (0.99-1.51) | 1.51 (1.23-1.85) | 1.20 (1.09-1.33) |
| Model 2c | 3,390 | 1 (Ref) | 1.14 (0.96-1.35) | 1.45 (1.21-1.75) | 1.17 (1.07-1.29) |
| Model 3d | 3,324 | 1 (Ref) | 1.11 (0.93-1.31) | 1.32 (1.09-1.58) | 1.12 (1.02-1.23) |
| Model 4e | 3,310 | 1 (Ref) | 1.10 (0.93-1.30) | 1.27 (1.06-1.53) | 1.11 (1.01-1.21) |
| Model 5f | 3,310 | 1 (Ref) | 1.13 (0.95-1.35) | 1.30 (1.08-1.57) | 1.12 (1.02-1.23) |
To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
Model 1 is unadjusted
Model 2 is adjusted for age and race/ethnicity
Model 3 is adjusted for Model 2 covariates and for smoking, alcohol consumption, BMI and physical activity
Model 4 is adjusted for Model 3 covariates and for hypertension, diabetes, hypercholesterolemia, eGFR and CRP
Model 5 is adjusted for Model 4 covariates and for the use of antidepressants and beta-blockers
National Health and Nutrition Examination Survey = NHANES; Erectile Dysfunction = ED; 25-hydroxyvitamin D = 25(OH)D; Body Mass Index = BMI; C-Reactive Protein = CRP; Estimated Glomerular Filtration Rate = eGFR
The association of vitamin D (2 categories) with ED
When categorizing vitamin D status in two categories (Supplemental Table 1), the unadjusted PR for ED comparing those with deficient to those with non-deficient levels was 1.32 (1.11 to 1.56). This association was slightly attenuated after adjusting for multiple lifestyle variables, comorbidities, and medication use [1.19 (1.02 to 1.39)].
The association of vitamin D (continuous) with ED
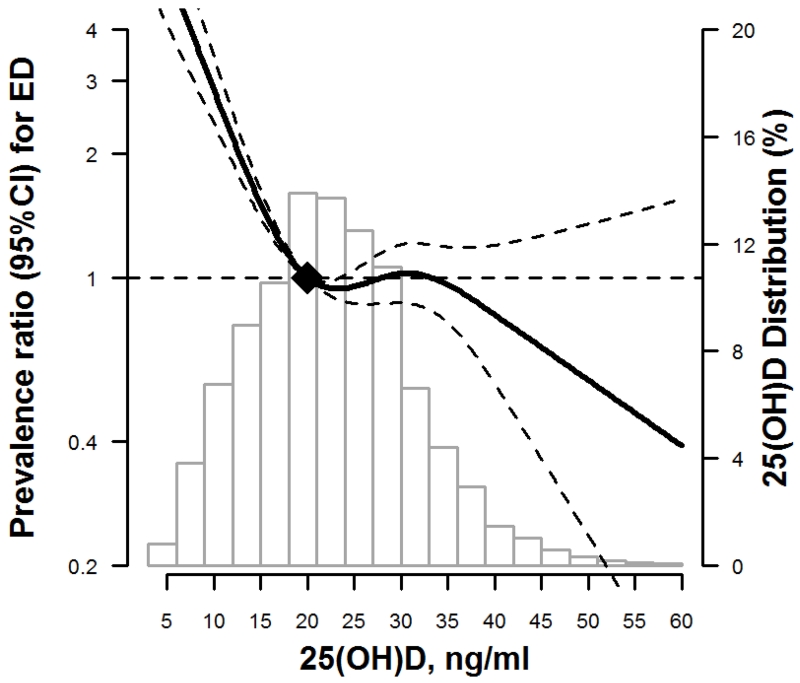
In fully-adjusted restricted cubic spline models, an increased risk for prevalent ED is seen for deficient levels of 25(OH)D <20 ng/ml, with a decreased prevalence of ED associated with 25(OH)D levels over 35 ng/ml (Figure 1).
Figure 1.
Restricted cubic spline of 25(OH)D and adjusted* prevalence ratio of ED, NHANES 2001-2004
* Curves represent adjusted prevalence ratio (solid line) and the 95% confidence intervals (dashed lines) based on restricted cubic splines for 25(OH)D level with knots at 10, 20, 30, 40 ng/ml. The reference values were set at 20 ng/ml. Model is adjusted for age, race, smoking, alcohol consumption, BMI, physical activity, hypertension, diabetes, hypercholesterolemia, eGFR, CRP, and the use of antidepressants and beta-blockers.
The association of vitamin D with ED by race/ethnicity
In race-stratified analysis, the fully adjusted PRs for ED comparing vitamin D deficient to optimal participants was qualitatively different in whites [1.36, (1.11 to 1.67)] compared to blacks [0.82 (0.38 to 1.79)], but the interaction by race was not statistically significant (p-interaction 0.2) [Table 3]. By diabetes status, the associations were qualitatively stronger in non-diabetic compared with diabetic men, but the interaction was also not statistically significant (data not shown).
Table 3. Adjusted Prevalence Ratios (95% CI) of Prevalent ED by Vitamin D and Race/Ethnic Categories in (bold, denotes statistical significance), NHANES 2001-2004 [n=3310 men].
| N | ≥30 ng/mLa |
20-30 ng/mLa | <20 ng/mLa | p b | |
|---|---|---|---|---|---|
| Non-Hispanic White | 1,740 | 1 (Ref) | 1.06 (0.85-1.33) | 1.36 (1.11-1.67) | 0.2 |
| Non-Hispanic Black | 619 | 1 (Ref) | 0.72 (0.36-1.44) | 0.82 (0.38-1.79) | |
| Mexican American or Hispanic |
845 | 1 (Ref) | 1.79 (1.14-2.80) | 1.47 (0.92-2.34) | |
| Other Race - Including Multi- Racial |
106 | 1 (Ref) | 1.65 (0.21-12.79) | 1.33 (0.20-9.04) |
To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
p for interaction
The prevalence of ED by race/ethnicity is as follows: 15.1% in Non-Hispanic Whites, 15.1% in Non-Hispanic Blacks, 17.1% in Mexicans/Hispanics, and 12.8% in other races.
Model is adjusted for age, smoking, alcohol consumption, BMI, physical activity, hypertension, diabetes, hypercholesterolemia, eGFR, CRP, and the use of antidepressants and beta-blockers.
National Health and Nutrition Examination Survey = NHANES; Erectile Dysfunction = ED; 25-hydroxyvitamin D = 25(OH)D; Body Mass Index = BMI; C-Reactive Protein = CRP; Estimated Glomerular Filtration Rate = eGFR
The association of vitamin D with ED among only men in good health
In sensitivity analyses restricted to the 2,792 men who reported a general health status of excellent, very good or good, the unadjusted PRs for ED comparing those with deficient to those with optimal vitamin D status was 1.38 (1.07 to 1.77), and associations remained similar and statistically significant in all other progressively adjusted models (Supplemental Table 2).
The association of vitamin D with ED adjusted for sex hormone levels
When we restricted our analysis to the 562 men with serum levels of sex hormones and adjusted for sex hormone levels, the association of vitamin D status with ED was even stronger (Supplemental Table 3), although given the smaller sample size, the 95% CI was much wider and not statistically significant.
The association of vitamin D with ED among only men with severe ED
Finally, using the more stringent definition of ED for those only “never able” to maintain an adequate erection, the adjusted PRs for the ED became stronger in all models [fully adjusted PR 1.80 (1.20 to 2.71), Table 4].
Table 4. Prevalence Ratios (95% CI) of Prevalent ED by Vitamin D Categories (ED redefined as only “never able” vs else) (bold, denotes statistical significance), NHANES 2001-2004 [n=3390 men].
| N | ≥30 ng/mLa | 20-30 ng/mLa | <20 ng/mLa | |
|---|---|---|---|---|
| Model 1 | 3,390 | 1 (Ref) | 1.49 (0.98-2.26) | 2.11 (1.42-3.14) |
| Model 2 | 3,390 | 1 (Ref) | 1.34 (0.92-1.96) | 2.03 (1.39-2.98) |
| Model 3 | 3,324 | 1 (Ref) | 1.31 (0.89-1.93) | 1.79 (1.18-2.70) |
| Model 4 | 3,310 | 1 (Ref) | 1.28 (0.87-1.90) | 1.75 (1.15-2.66) |
| Model 5 | 3,310 | 1 (Ref) | 1.34 (0.89-2.00) | 1.80 (1.20-2.71) |
To convert 25(OH)D levels to nmol/L from ng/ml, multiply by 2.496.
Model 1 is unadjusted
Model 2 is adjusted for age and race/ethnicity
Model 3 is adjusted for Model 2 covariates and for smoking, alcohol consumption, BMI and physical activity
Model 4 is adjusted for Model 3 covariates and for hypertension, diabetes, hypercholesterolemia, eGFR and CRP
Model 5 is adjusted for Model 4 covariates and for the use of antidepressants and beta-blockers
National Health and Nutrition Examination Survey = NHANES; Erectile Dysfunction = ED; 25-hydroxyvitamin D = 25(OH)D; Body Mass Index = BMI; C-Reactive Protein = CRP; Estimated Glomerular Filtration Rate = eGFR
Discussion
In this large representative sample of U.S. men free of self-reported clinical ASCVD, the prevalence of ED was higher in participants with 25(OH)D deficiency compared to those with optimal levels. The relationship between serum 25(OH)D and ED was approximately linear and was evident even after adjusting for potential confounders and ASCVD risk factors. Using a more stringent definition for severe ED (i.e. “never able”), associations were even stronger.
Only two prior studies, both of them small in size, have explored the relationship between vitamin D and ED. One study used penile echo-color-Doppler and classified 143 patients with ED as arteriogenic, borderline, non-arteriogenic ED.27 Vitamin D levels in arteriogenic ED patients were significantly lower than in non-arteriogenic ED patients. The prevalence of 25(OH)D deficiency (<20 ng/ml) in men with atherogenic and with non-atherogenic ED were 61 and 42%, respectively.27 Additionally, patients with 25(OH)D deficiency had lower scores on the International Index of Erectile Function (IIEF) and lower peak systolic velocity (PSV) in penile echo-color-Doppler measurements, indicating an arteriogenic-ED. Another study of 92 men with type 2 diabetes found a significant association of vitamin D with ED.28
In a recent state-of-the-art review article, Sorenson and Grant hypothesized that vitamin D deficiency could be a key contributor to ED18 and advocated that this hypothesis should be further tested through further observational and intervention studies. Our study provides observational evidence to support this hypothesis by demonstrating in a representative sample of the general population that 25(OH)D deficiency was associated with ED independent of potential confounders.
Several mechanisms could explain a biological relationship between vitamin D deficiency and ED. Vascular ED stems from endothelial dysfunction and/or atherosclerosis. Diabetes mellitus is a strong risk factor for both atherosclerosis and endothelial dysfunction,29 and diabetic men are 3 times more likely to have ED than non-diabetic men1. The association of 25(OH)D with ED and with ASCVD may be mediated by impaired glucose metabolism. Low vitamin D levels may impair β cell function (insulin secretion), and induce insulin resistance, glucose intolerance30,31 and incident type 2 diabetes19,32. We did not find a statistically significant interaction by diabetes status, but the association was qualitatively stronger among non-diabetics compared to diabetics, suggesting that once diabetes is already present, the association of 25(OH)D with ED is weakened.
Men with ED have an increased prevalence of endothelial dysfunction,33 and vitamin D may improve endothelial function.34 One mechanism linking low vitamin D levels with ED may be via reduced synthesis of nitric oxide. Secretion of nitric oxide is needed for relaxation of the smooth muscles of the corpora cavernosa and subsequent penile erection, and vitamin D may be a regulator of endothelial nitric oxide synthase.35 Vitamin D may also directly protect endothelial cells from oxidative stress.36
Deficient 25(OH)D levels may also contribute to ED through inflammation. Men with ED have increased levels of several pro-inflammatory cytokines including CRP37 (which we also found in the present study), tumor necrosis factor α (TNF-α),38 and endothelial cell adhesion molecules.39 Current evidence suggests that adequate circulating levels of 25(OH)D may be crucial for optimal anti-inflammatory response of immune cells,40 and vitamin D supplementation has anti-inflammatory effects.40,41
Arterial calcification is a marker of an increased risk for ED,42 and also has an inverse association with serum vitamin D concentrations.43,44 Subclinical atherosclerosis, as assessed by coronary artery calcification, may thus represent an intermediate phenotype between deficient 25(OH)D levels and clinical ASCVD events and deficient 25(OH)D with ED phenotype. However, subclinical atherosclerosis markers were not available in the NHANES cohort.
One of our key questions was whether there was heterogeneity of the association of 25(OH)D deficiency with ED by race/ethnicity. The prevalence of ED may vary by race/ethnicity,45 although this may be due in part to social/cultural differences in the reporting of ED. In our study, the prevalence of ED was generally similar among the race/ethnic groups, ranging from 13 to 17%. It is well established that U.S black adults have lower average levels of 25(OH)D compared to whites46 as their increased skin pigmentation blocks UVB-driven synthesis of vitamin D. We found that the association of 25(OH)D deficiency with ED was qualitatively stronger in whites than blacks, but the interaction by race/ethnicity was not statistically significant in our study, possibly because of limited power to evaluate interactions. Indeed, several studies have found a similar pattern of interaction by race/ethnicity with other types of ASCVD including heart failure,20 coronary heart disease,13,15 stroke,14 and diabetes.19 Racial differences in bioavailable vitamin D may underlie these associations.47
ED has been associated with low testosterone levels.48 However, our findings suggest that the link of 25(OH)D with ED appears to be independent of sex hormones. Although vitamin D is positively correlated with testosterone in men,49 our results were qualitatively stronger after adjustment for testosterone (PR 1.71 vs 1.30) and not weaker as would be anticipated by a mediator of the association. However, the results were no longer statistically significant in the sex hormone analysis given small sample size.
Our findings have potentially important health implications for men. 25(OH)D is an easy biomarker to screen for through simple commercially-available laboratory tests, and deficiencies can be treated with supplementation and/or modest sunlight exposure. Thus, if a causal relationship is confirmed in other studies (such as in randomized placebo-controlled interventional trials), treatment of vitamin D deficiency has the potential to improve erectile function through improvement of endothelial function, decreased atherosclerosis risk, and anti-inflammatory properties.
However, it is important to address some of the limitations in our findings. NHANES is a cross-sectional study; thus both reverse causation and residual confounding are of potential concern. To minimize reverse causation, we excluded men with known clinical vascular disease. While we adjusted for numerous potential confounding and mediating factors, residual confounding may still underlie the associations seen. Low 25(OH)D concentrations may simply be a potent marker of a poorer health state, although our findings were robust even in sensitivity analyses restricted to men reporting only good or excellent health. In addition, ED ascertainment in our study was self-reported and based on a single MMAS question. However this question has been shown to be a valid tool for ED assessment when compared to a clinical diagnosis by a trained urologist50 or to a more detailed questionnaire inventory,25 and we anticipate relatively little misclassification of ED status. Unfortunately, we are unable to determine the etiology of ED (pscyhogenic versus organic, and arteriogenic versus non- arteriogenic) to confirm our hypothesis that the association of 25(OH)D with ED is through a vascular mechanism. Finally, although 25(OH)D is widely accepted as the best biomarker for the assessment of total body vitamin D stores,23 1,25(OH)2D (calcitriol) is the activated form of vitamin D that binds to the vitamin D receptor and confers its biological effects; however we did not have measurement of 1,25(OH)2D in this study population.
Despite these limitations, our study has several strengths. NHANES 2001-2004 is a representative sample of the U.S. population that rigorously follows very well designed study protocols with extensive quality assurance and quality control. In addition, our findings were robust to a series of sensitivity analyses which confirmed our primary analysis.
In conclusion, this cross-sectional analysis of a representative sample of U.S. men showed that vitamin D deficiency was associated with an increased prevalence of ED independent of lifestyle factors, ASCVD risk factors, and sex hormones, through a dose-response relationship. Additional research is needed to evaluate if treating vitamin D deficiency, a potentially modifiable risk factor, could improve erectile function.
Supplementary Material
Highlights.
Both Vit D deficiency and ED have been linked to increased cardiovascular risk.
We found in a representative sample of U.S. men that both Vit D deficiency and ED were common, with prevalence of 30% and 15%, respectively.
We found Vit D deficiency was independently associated with an increased prevalence of ED after accounting for multiple lifestyle and vascular risk factors.
Additional research is needed to evaluate whether treating Vit D deficiency improves erectile function.
Acknowledgements
No additional acknowledgements
Financial Support: Dr. Michos is supported by a grant from the NIH/NINDS (R01NS072243) for vitamin D research and the Blumenthal Scholars Fund for Preventive Cardiology Research
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- ED
Erectile Dysfunction
- ASCVD
Atherosclerotic Cardiovascular Disease
- US
United States
- NHANES
National Health and Nutrition Examination Survey
- RIA
Radioimmunoassay
- PRs
Prevalence Ratios
- CI
Confidence Intervals
- PDE5
Phoshodiesterase type 5
- BMI
Body Mass Index
- CRP
C-Reactive Protein
- GFR
Glomerular Filtration Rate
- MMAS
Massachusetts Male Aging Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registry and website: Not applicable
Conflicts of Interest: The authors report no conflict of interest related to this work.
Author Contributions:
YMKF, EG, and EDM designed the research. YMKF analyzed the data under the supervision of EG, DZ, and EDM. YMKF wrote the first draft of the paper. DZ, RRK, MJB, DIF, SSM, PLL, KLB, EG, and EDM all reviewed manuscript and provided critical scientific input. YMKF and EDM had primary responsibility for final content. All authors approved of final manuscript draft.
References
- 1.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Grover SA, Lowensteyn I, Kaouache M, et al. The prevalence of erectile dysfunction in the primary care setting: importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166:213–9. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- 3.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Urologic Diseases in America P. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207–12. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
- 4.Shah NP, Cainzos-Achirica M, Feldman DI, et al. Cardiovascular Disease Prevention in Men with Vascular Erectile Dysfunction: The View of the Preventive Cardiologist. Am J Med. 2015 doi: 10.1016/j.amjmed.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 5.Kloner RA. Erectile dysfunction and cardiovascular risk factors. Urol Clin North Am. 2005;32:397–402. v. doi: 10.1016/j.ucl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328–38. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 8.Chung RY, Chan D, Woo J, et al. Erectile Dysfunction is Associated with Subsequent Cardiovascular and Respiratory Mortality in Cohort of 1,436 Chinese Elderly Men. J Sex Med. 2015;12:1568–76. doi: 10.1111/jsm.12918. [DOI] [PubMed] [Google Scholar]
- 9.Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–78. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Ginde AA, Liu MC, Camargo CA., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–29. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michos ED, Misialek JR, Selvin E, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis. 2015;241:12–7. doi: 10.1016/j.atherosclerosis.2015.04.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012;28:367–71. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310:179–88. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutsey PL, Michos ED. Vitamin D, calcium, and atherosclerotic risk: evidence from serum levels and supplementation studies. Curr Atheroscler Rep. 2013;15:293. doi: 10.1007/s11883-012-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313:1311–2. doi: 10.1001/jama.2015.1353. [DOI] [PubMed] [Google Scholar]
- 18.Sorenson M, Grant WB. Does vitamin D deficiency contribute to erectile dysfunction? Dermatoendocrinol. 2012;4:128–36. doi: 10.4161/derm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis JP, Michos ED, Selvin E, Pankow JS, Lutsey PL. Race, vitamin D-binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015;101:1232–40. doi: 10.3945/ajcn.115.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutsey PL, Michos ED, Misialek JR, et al. Race and Vitamin D Binding Protein Gene Polymorphisms Modify the Association of 25-Hydroxyvitamin D and Incident Heart Failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail. 2015;3:347–56. doi: 10.1016/j.jchf.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1. 2013:1–37. [PubMed] [Google Scholar]
- 22. at http://www.cdc.gov/nchs/nhanes/new_nhanes.htm.
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 24.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derby CA, Araujo AB, Johannes CB, Feldman HA, McKinlay JB. Measurement of erectile dysfunction in population-based studies: the use of a single question self-assessment in the Massachusetts Male Aging Study. Int J Impot Res. 2000;12:197–204. doi: 10.1038/sj.ijir.3900542. [DOI] [PubMed] [Google Scholar]
- 26.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barassi A, Pezzilli R, Colpi GM, Corsi Romanelli MM, Melzi d’Eril GV. Vitamin D and erectile dysfunction. J Sex Med. 2014;11:2792–800. doi: 10.1111/jsm.12661. [DOI] [PubMed] [Google Scholar]
- 28.Caretta N, de Kreutzenberg SV, Valente U, et al. Hypovitaminosis D is associated with erectile dysfunction in type 2 diabetes. Endocrine. 2016 doi: 10.1007/s12020-015-0851-z. [DOI] [PubMed] [Google Scholar]
- 29.Tousoulis D, Papageorgiou N, Androulakis E, et al. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013;62:667–76. doi: 10.1016/j.jacc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 30.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 31.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–8. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyton CC, Colaco MA, Kovell RC, Kim JH, Terlecki RP. Erectile Dysfunction is Predictive of Endothelial Dysfunction in a Well Visit Population. J Urol. 2016;195:1045–50. doi: 10.1016/j.juro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds JA, Haque S, Williamson K, Ray DW, Alexander MY, Bruce IN. Vitamin D improves endothelial dysfunction and restores myeloid angiogenic cell function via reduced CXCL-10 expression in systemic lupus erythematosus. Sci Rep. 2016;6:22341. doi: 10.1038/srep22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrukhova O, Slavic S, Zeitz U, et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol. 2014;28:53–64. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uberti F, Lattuada D, Morsanuto V, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab. 2014;99:1367–74. doi: 10.1210/jc.2013-2103. [DOI] [PubMed] [Google Scholar]
- 37.Chiurlia E, D’Amico R, Ratti C, Granata AR, Romagnoli R, Modena MG. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J Am Coll Cardiol. 2005;46:1503–6. doi: 10.1016/j.jacc.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 38.Carneiro FS, Webb RC, Tostes RC. Emerging role for TNF-alpha in erectile dysfunction. J Sex Med. 2010;7:3823–34. doi: 10.1111/j.1743-6109.2010.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bocchio M, Desideri G, Scarpelli P, et al. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004;171:1601–4. doi: 10.1097/01.ju.0000116325.06572.85. [DOI] [PubMed] [Google Scholar]
- 40.Calton EK, Keane KN, Newsholme P, Soares MJ. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS One. 2015;10:e0141770. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen N, Wan Z, Han SF, Li BY, Zhang ZL, Qin LQ. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients. 2014;6:2206–16. doi: 10.3390/nu6062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JH, Ngengwe R, Jones P, Tang F, O’Keefe JH. Erectile dysfunction as a coronary artery disease risk equivalent. J Nucl Cardiol. 2008;15:800–3. doi: 10.1007/BF03007361. [DOI] [PubMed] [Google Scholar]
- 43.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–60. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 44.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–12. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JF, Caan BJ, Sternfeld B, et al. Racial disparities in erectile dysfunction among participants in the California Men’s Health Study. J Sex Med. 2009;6:3433–9. doi: 10.1111/j.1743-6109.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- 46.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15 S5-97-101. [PubMed] [Google Scholar]
- 47.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novo S, Iacona R, Bonomo V, et al. Erectile dysfunction is associated with low total serum testosterone levels and impaired flow-mediated vasodilation in intermediate risk men according to the Framingham risk score. Atherosclerosis. 2015;238:415–9. doi: 10.1016/j.atherosclerosis.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Pilz S, Frisch S, Koertke H, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43:223–5. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 50.O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J Gen Intern Med. 2005;20:515–9. doi: 10.1111/j.1525-1497.2005.0076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.