Abstract
Background and aims
Previous experiments suggest that both increased endothelial cell apoptosis and endothelial surface glycocalyx shedding could play a role in the endothelial dysfunction and inflammation of athero-prone regions of the vasculature. We sought to elucidate the possibly synergistic mechanisms by which endothelial cell apoptosis and glycocalyx shedding promote atherogenesis.
Methods
4- to 6-week old male C57Bl/6 apolipoprotein E knockout (ApoE−/−) mice were fed a Western diet for 10 weeks and developed plaques in their brachiocephalic arteries.
Results
Glycocalyx coverage and thickness were significantly reduced over the plaque region compared to the non-plaque region (coverage plaque: 71±23%, non-plaque: 97±3%, p= 0.02; thickness plaque: 0.85±0.15 μm, non-plaque: 1.2±0.21 μm, p= 0.006). Values in the non-plaque region were not different from those found in wild type mice fed a normal diet (coverage WT: 92±3%, p= 0.7 vs. non-plaque ApoE−/−, thickness WT: 1.1±0.06 μm, p= 0.2 vs. non-plaque ApoE−/−). Endothelial cell apoptosis was significantly increased in ApoE−/− mice compared to wild type mice (ApoE−/− :64.3±33.0, WT: 1.1±0.5 TUNEL-pos/cm, p= 2×10−7). The number of apoptotic endothelial cells per unit length was 2 times higher in the plaque region than in the non-plaque region of the same vessel (p= 3×10−5). Increased expression of matrix metalloproteinase 9 co-localized with glycocalyx shedding and plaque buildup.
Conclusions
Our results suggest that, in concert with endothelial apoptosis that increases lipid permeability, glycocalyx shedding initiated by inflammation facilitates monocyte adhesion and macrophage infiltration that promote lipid retention and the development of atherosclerotic plaques.
Keywords: atherosclerosis, glycocalyx, endothelial cell apoptosis, inflammation
Introduction
The accumulation of lipoproteins in the arterial wall is a hallmark of atherosclerosis [1]. Due to the clinical importance of atherosclerosis, the biomechanical, cellular, and molecular mechanisms underlying atherogenesis have been the focus of intense research. Atherosclerotic plaques build up at predictable arterial sites—vessel branches and curvatures—where blood flow is disrupted and recirculating flows exist, creating a biomechanical environment that extends lipid residence time, weakens the vascular wall against lipid infiltration, promotes inflammation, and sustains atherosclerotic plaque growth [2]. The flux of low density lipoprotein (LDL) into the arterial wall depends on both the plasma concentration and the vessel wall permeability [3]. A correlation between enhanced permeability to macromolecules and the localization of atherosclerotic plaques is well established. In experimental animals, the focal sites of predilection for either spontaneous or dietary-induced atherosclerosis display increased permeability to plasma proteins, most notably albumin, horseradish peroxidase and LDL [4, 5]. We [6, 7] and others [8, 9] have previously shown that the permeability of endothelial cell (EC) monolayers is highly correlated with their rate of apoptosis.
The endothelial glycocalyx (GCX) is made up largely of cell-linked glycosaminoglycans (GAGs) including heparan sulfate (HS), hyaluronic acid (HA), and chondroitin sulfate (CS)[10]. The association of altered GCX characteristics with atherosclerosis-prone locations in arteries was recognized in the early 1980s. Lewis et al. [11] observed the coronary arteries of White Carneau pigeons and noted that the GCX, as assessed by ruthenium red staining, was thinnest in areas with high disease predilection, and that upon cholesterol challenge, the GCX thickness was reduced in all arterial zones. More recently, van den Berg et al. [12] observed enhanced intimal accumulation of LDL in the internal carotid branch of mice where the HS and HA components of the GCX were thin, in comparison to the adjacent regions of the common carotid where LDL accumulation was reduced and HS and HA were thick.
Previous studies have shown that the local hemodynamics play a role in EC apoptosis, GCX shedding and inflammation. High levels of steady shear have been shown to reduce the rate of EC apoptosis [13, 14], while irregular flow conditions increase EC apoptosis [15]. Similarly, atheroprotective flow conditions increase the expression [16] and synthesis rate [17, 18] of GCX components compared to atheroprone flow. In addition, several studies have shown that disturbed flow leads to a pro-inflammatory EC gene expression [19, 20].
No study, however, has compared the association of GCX degradation, EC apoptosis and inflammation in atherosclerotic plaque formation. Thus a major objective of the present work is to understand the possibly synergistic mechanisms by which EC apoptosis, GCX shedding and inflammation promote atherogenesis. To address this objective, we studied high fat fed apolipolipoprotein E knockout (ApoE−/−) mice, a well-established animal model of atherosclerosis. We quantified the number of apoptotic ECs and measured the thickness and continuity of a major EC surface GAG, hyaluronic acid, in plaque and non-plaque regions of the brachiocephalic artery (BCA), using the descending aorta (DA) as a control region that did not develop plaques. In addition, we immunostained for matrix metalloproteinase 9 (MMP-9) and CD68 as markers of inflammation.
Materials and methods
Animals studies, euthanasia, and fixation
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of The City College of New York. Six male C57Bl/6 background ApoE−/− mice were obtained from Jackson Laboratories (Bar Harbor, Maine) at 6 weeks of age. The animals were fed a high fat diet (Dyets, Inc., Bethlehem, Pa), which was high in saturated fats and cholesterol, to induce atherosclerotic plaque formation in their proximal arteries. After 10 weeks on the high fat diet, the ApoE−/− mice were placed on a 5-hour fast. Following the fast, mice were anesthetized with 2% chloralose and 10% urethane in PBS at the initial dosage 7 μl/g and an additional 30 μl/dose when needed. A mid-line surgical incision was then made from the abdominal wall to thoracic wall of each mouse. The heart was exposed after cutting the diaphragm. A blood sample was obtained from the right atrium for future lipid and lipoprotein analysis. The inferior vena cava and right atrium were severed to drain the blood and 20 to 30 mL of PBS containing 1% BSA were pressure perfused from the left ventricle through the circulation system. The vessels were then pressure perfused with 20 to 30 mL of 2% paraformaldehyde in PBS. For reference, strain-, gender-, and age-matched wildtype (WT) C57Bl/6 mice fed a normal chow diet were also studied.
Vessel dissection, cryopreservation, and cryostat sectioning
After the blood was completely washed away and the mice were thoroughly fixed, their aortas were excised from the aortic root to the thoracic aorta. We also excised the brachiocephalic branch where disease commonly localizes and plaque rupture ultimately occurs [21, 22]. Excised vessels were frozen in a block of optimal cutting temperature (OCT) compound, stored at −80°C, and later sectioned (6μm thick sections) to expose the vessel wall cross-section using a cryostat set at −20°C. Six serial sections (36 μm apart) were placed on positively charged glass slides (Color Frost Plus, Fisher Scientific, Pittsburgh, PA). Sections mounted on glass slides were dried overnight before further processing or before storage with desiccate at −80°C for future use.
Staining
Apoptotic cells were identified by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) technique using the in situ cell dectection kit from Roche (Indianapolis, IN) according to the manufacturer’s instructions. Briefly, frozen sections were fixed in 4% PFA for 20 min, washed in PBS for 30 min, blocked with 3% H2O2 in methanol for 10 min, permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice, then incubated with the TUNEL reaction mixture for 1 hr at 37°C. After washing 3X in PBS, sections were counterstained with DAPI and mounted with Vectashield medium.
Platelet endothelial cell adhesion molecule (PECAM) was used to stain endothelial cells, and HA was stained as a marker of the GCX layer. In addition, vessels were stained for matrix MMP-9 and CD68 as markers of inflammation. For PECAM and HA staining, the sections were post fixed in 4% PFA for 10 minutes, washed 2X in PBS, and permeabilized with 0.3% Triton X-100 in PBS for 10 minutes. Antigen retrieval was performed by heating the sections in 10mM sodium citrate after which the slides were washed 2X with PBS and blocked with 10% goat serum for 1 hour. The slides were then incubated in 1:50 biotinylated hyaluronic acid binding protein (b-HABP, EMD Millipore, Billerica, MA) for 2 nights or in 1:100 rabbit polyclonal anti-PECAM for 1 night in a humidified chamber at 4°C. Following washing 5X in PBS, b-HABP was detected by incubation with 1:500 HRP-conjugated streptavidin and anti-PECAM was detected by incubation with 1:500 HRP-conjugated anti-rabbit secondary antibody, both from Jackson ImmunoResearch (West Grove, PA). The signal was amplified using the TSA Plus Cyanine 3 System (Perkin Elmer, Waltham, MA). Sections were counterstained with DAPI and mounted with Vectashield (Vector Labs, Burlingame, CA). For MMP-9 staining, the sections were post fixed in 4% PFA for 10 minutes and washed 2X in Tris-buffered Saline (TBS), followed by antigen retrieval by heating in Tris-EDTA (10mM Tris Base, 1mM EDTA, 0.05% Tween 20, ph 9.0). The slides were then washed in TBS-0.025% Triton X-100, blocked with 10% goat serum and 1% BSA for 2hrs, and incubated with primary rabbit anti-MMP-9 (1:1000, Abcam, ) overnight at 4°C. Anti-MMP-9 was detected by incubation with 1:500 HRP-conjugated anti-rabbit secondary antibody, and signal amplification was performed as above. For CD68 staining, the sections were post fixed in ice cold acetone for 10 minutes and washed in PBS containing 0.1% Tween 2X, followed by blocking in 4% rabbit serum for 10 minutes. The sections were then incubated with primary rat anti-mouse CD68 (1:250, AbD Serotec, Raleigh, NC) for 1hr at RT. Anti-CD68 was detected by incubation with biotinylated rabbit anti-rat secondary antibody (1:200, Vector Labs) for 10 minutes, followed by incubation with HRP-conjugated streptavidin and signal amplification as described above.
Lipids were stained after sections were post-fixed in 2% PFA for 10 minutes. Following a 30-second pre-incubation of the sections in 60% isopropanol, the sections were incubated for 25 minutes in 0.24% (w/v) Oil Red O dissolved in 60% isopropanol. Sections were counterstained using Mayer’s hemotoxylin and mounted with Vectashield medium.
Imaging and analysis
TUNEL positive cells were viewed and counted using a Nikon Eclipse TE2000-E or a Nikon TiE inverted microscope at 20x and 40x magnification. Images were taken with a CCD color camera on the Nikon TiE microscope at 20x magnification. The luminal perimeter of each vessel section was measured from the acquired images using NIH Image J software. Apoptosis was expressed as the number of TUNEL positive cells per unit length (in cm) overlying the plaque or non-plaque regions of each vessel section.
HA, MMP-9, and CD68 staining was visualized using a Zeiss LSM 510 Confocal Laser Scanning system at 10X and 63X/oil magnification. Glycocalyx (HA) thickness and coverage, and MMP-9 intensity were analyzed using NIH ImageJ software. HA continuity was measured using a grid to standardize measurements. A ratio of the number of grid-cells containing continuous HA staining to the total number of grid-cells covering the inner vessel wall was computed to find a percent coverage per vessel. Gycocalyx thickness was determined after calibration with a stage micrometer or calibration bar (for each objective lens). One transverse line was drawn on the HA layer and measured in each grid-cell, giving approximately 10 measurements per image or section and 60 measurements from each of three glass slides for the vessel of interest. MMP-9 intensity was quantified by selecting a region of interest (ROI) containing only the luminal layer of endothelial cells. The MMP-9 intensity was expressed as the fraction of the ROI area having a value above the background intensity threshold, which was defined by the method described in [23].
Lipid labeling was examined using a Zeiss AxioImager, 5X and 10X objectives, and an Olympus DP72 digital camera to record images in color. Atherosclerotic plaque size, based on Oil red O staining, was quantified using NIH Image J software.
Lipid and lipoprotein analyses
Blood samples were obtained from the right atrium. Plasma cholesterol levels were determined using colorimetric enzymatic assays from Wako Diagnostics (Richmond, VA). Total cholesterol was measured with the Total Cholesterol-E assay; HDL cholesterol was measured with the HDL Cholesterol-E assay; LDL cholesterol was measured with the L-type LDL-C cholesterol assay.
Statistical analysis
Student’s t-test was used to compare differences between two groups. When applicable, because of non-normality of the data, non-parametric tests were used. For comparison of MMP-9 intensity data, 1-way ANOVA with Tukey’s method to correct for multiple comparisons was used. GraphPad Prism was used to perform statistical analysis and to generate data plots.
Results
General pathological findings
Mean serum total cholesterol concentrations were significantly higher in the ApoE−/− high fat fed mice than in the WT normal diet fed mice (Table 1). Total cholesterol concentration was 65.1±1.8 mg/dL in WT mice and increased significantly by 12-fold to 811.6±53.6 in ApoE−/− mice. HDL cholesterol decreased significantly from 31.5±4.6 mg/dL in WT to 6.5±0.7 mg/dL in ApoE−/−. LDL cholesterol significantly increased from 25.1±2.5 mg/dL in WT to 412.6±14.6 mg/dL in ApoE−/−. The amount of cholesterol contained in other lipid fractions such as VLDL, triglycerides and chylomicrons was estimated by subtracting HDL and LDL cholesterol from the total cholesterol. The concentration of cholesterol in these fractions increased from 8.4±7.8 mg/dL in WT to 392.6±40.9 mg/dL in ApoE−/−.
Table 1.
Mean blood cholesterol concentration in wild type mice fed a normal chow diet and ApoE−/− mice fed a western diet. Numbers in parenthesis represent the percent of total cholesterol. p<0.0002 vs. wild type, for all types of cholesterol. n=4 for wild type; n=6 for ApoE−/−
| Total cholesterol (mg/dL) |
HDL (mg/dL) |
LDL (mg/dL) |
VLDL/Tri/CH (mg/dL) |
|
|---|---|---|---|---|
| Wild type | 65.1 ± 1.8 | 31.5 ± 4.6 | 25.1 ± 2.5 | 8.4 ± 7.8 |
| Normal diet | (48%) | (39%) | (13%) | |
|
| ||||
| ApoE −/− | 811.6 ± 53.6 | 6.5 ± 0.7 | 412.6 ± 14.6 | 392.6 ± 40.9 |
| Western diet | (1%) | (51%) | (48%) | |
Despite the systemically elevated levels of plasma cholesterol in the high fat fed mice, only the predictable vessel wall regions exhibited lipid retention and atherosclerotic plaque growth. After 10 weeks on the high fat diet, the ApoE−/− mice exhibited raised plaques that were visible as white streaks under the dissection microscope (Figure 1C). Plaques were visible in the expected areas: the lower curvature of the aortic arch and the outer wall of the bifurcation in the BCA. Wild type mice fed a normal chow diet did not show any lesions (Figure 1B). In the present work, we studied lesions in the BCA and used sections of the descending thoracic aorta that were free of intercostals arteries as a non-diseased control area (Figure 1A).
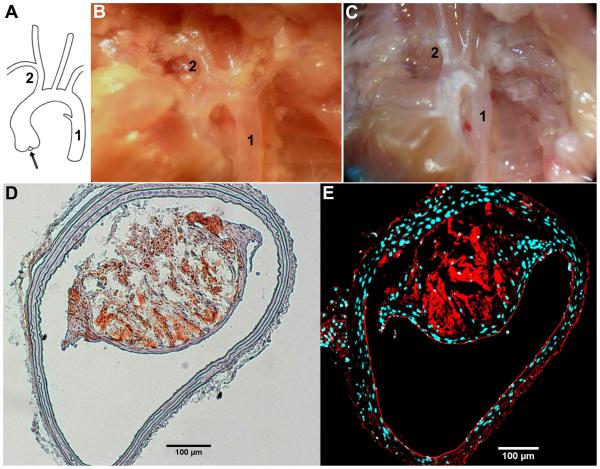
Fig. 1. Dissection of vessels from normal diet fed wild type mouse and high fat fed ApoE−/− mouse.
(A) Schematic showing flow direction (arrow) of blood, 1% BSA, or 2% paraformaldehyde from the heart/aortic root and through the proximal branches of the vascular tree. (B) Picture of dissected wild type mouse on a normal diet, with clear vessels. (C) Picture of dissected ApoE−/− mouse on a high fat diet, with plaque filled (white streaks) vessels. The dissected vessels of interest are denoted by numbers 1 and 2, where 1 is the descending aorta (DA) and 2 is the brachiocephalic artery (BCA). (D) Representative image of Oil Red O stained lipid-laden plaque from the BCA of a high fat diet fed ApoE−/− mouse. (E) BCA of the same mouse immunostained for PECAM (red) demonstrates that, although a large plaque is present, endothelial cell coverage on the inner vessel wall can be seen on both the non-plaque and plaque regions. The staining also shows increased intra-plaque neo-vascularization. DAPI (blue) labels cell nuclei.
Cryosections of the BCA were stained with Oil Red O to visualize the lipid filled plaques (Figure 1D). The average area of the plaques in ApoE−/− mice was found to be 82.2±18.6 × 103 μm2, and occupying an average of 33% of the vessel luminal area, similar to previous studies [24]. PECAM immunostaining was performed to confirm the presence of an intact endothelial layer in both the plaque and non-plaque regions of the vessel (Figure 1E).
Apoptosis in the BCA
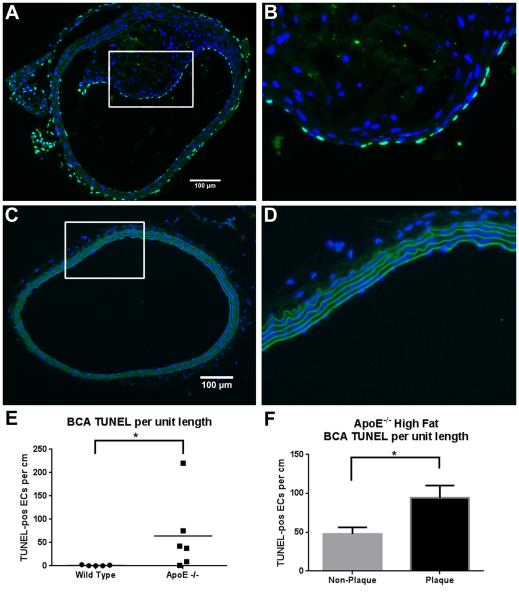
Figures 2A-2D show representative images of TUNEL staining in the BCA of ApoE−/− and WT mice. We counted apoptotic cells (bright green FITC label) and measured the perimeter of the lumen to determine the number of apoptotic ECs per unit length in each section. The number of TUNEL positive cells varied widely from animal to animal in the ApoE−/−, ranging from 1.1 to 220.2 TUNEL-pos/cm. In the WT mice the number of TUNEL positive cells ranged from 0.23 to 2.60 The ApoE−/− mice BCA had an average of 64.3±33.0 TUNEL-pos/cm, significantly higher than the average in the WT BCA of 1.1±0.5 TUNEL-pos/cm (Figure 2E).
Fig. 2. Apoptosis in the BCA.
Cross-sections shown in images A-D were stained for apoptosis using TUNEL. Cell nuclei are stained with DAPI (blue), elastin sheets auto-fluoresce in green, and apoptotic cells are labeled with FITC-TUNEL (bright green). (A) 20X image of the BCA obtained from a high fat fed ApoE−/− mouse, and (B) zoomed image of the boxed area in (A) showing elevated apoptosis. (C) 20X image of the BCA obtained from a normal diet fed wildtype mouse, and (D) zoomed image of the boxed area in (C) showing no sign of apoptosis. (E) TUNEL-positive ECs per cm in the in the wildtype vs. ApoE−/− (including plaque and non-plaque areas). N=86 sections from 6 animals for ApoE−/−, N=73 sections from 5 animals for wild type (data points represent the mean of each animal); *p<0.0001 by non-parametric Mann-Whitney test. (F) TUNEL-positive ECs per cm in plaque and non-plaque areas of the ApoE−/− BCA. N=86 sections; *p<0.0001 by paired t-test.
To determine whether apoptosis was occurring preferentially in the endothelial cells covering the plaque area, we measured the length of the plaque and non-plaque areas, and calculated the number of TUNEL-positive cells per unit length in each area. The non-plaque area had 47.95±8.34 TUNEL-pos/cm, while the number increased by 2-fold in the plaque area (94.38 + 15.8 TUNEL-pos/cm; Figure 2F). This increase in apoptosis was found to be highly significant by paired t-test (p<0.0001).
Apoptosis in the DA
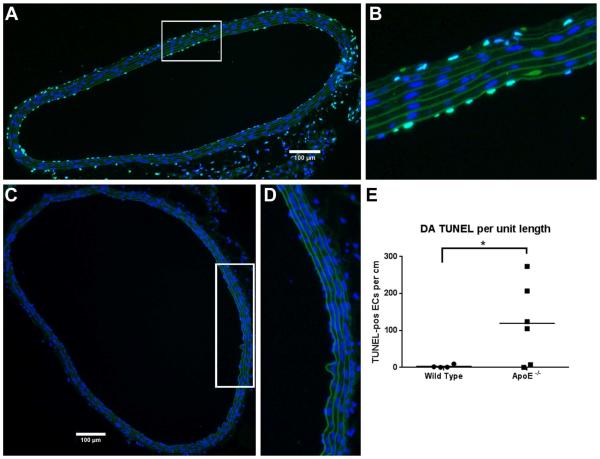
Figures 3A-3D shows representative images of TUNEL staining in the DA of ApoE−/− and WT mice. In WT mice, endothelial apoptosis in the DA ranged from 0.33 to 9.6 with an average of 3.1±2.2 TUNEL-pos/cm. In the ApoE−/− mice, the DA had average endothelial apoptosis of 119.5±44.1, 38 times higher than in the WT (Figure 3E). As with the values in the BCA, the number of TUNEL positive cells varied widely from animal to animal ranging from 0.26 to 273.7 TUNEL-pos/cm.
Fig. 3. Apoptosis in DA.
Cross-sections shown in images A-D were stained for apoptosis using TUNEL. Cell nuclei are stained with DAPI (blue), elastin sheets auto-fluoresce in green, and apoptotic cells are labeled with FITC-TUNEL (bright green). (A) 20X image of the DA obtained from a high fat fed ApoE−/− mouse, and (B) zoomed image of the boxed area in (A) showing elevated apoptosis. (C) 20X image of the DA obtained from a normal diet fed wild type mouse, and (D) zoomed image of the boxed area in (C) showing no sign of apoptosis. (E) TUNEL-positive ECs per cm in the wild type vs. ApoE−/−. N=97 sections from 6 animals for ApoE−/−, N=67 sections from 4 animals for wild type (data points represent the mean of each animal); *p<0.0001 by non-parametric Mann-Whitney test.
HA staining in the BCA
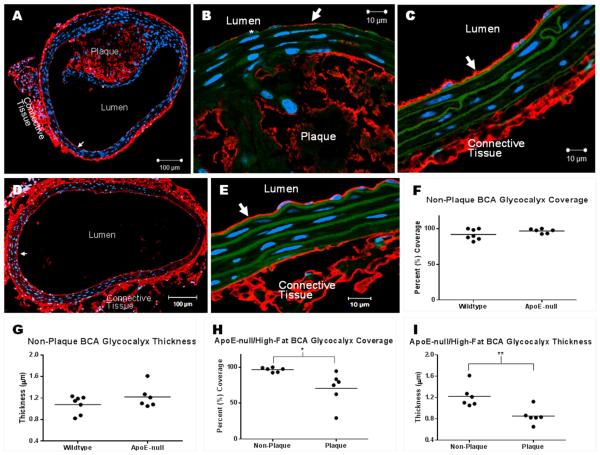
Figure 4 shows representative images of HA staining in the BCA of ApoE−/− and WT mice. In the ApoE−/−, the GCX can be seen overlying the non-plaque regions (Figure 4C) and it is degraded in the areas overlying the plaque (Figure 4B). GCX coverage was found to be 97±3% in the non-plaque regions of the ApoE−/− BCA, and was significantly decreased to 71±23% in the plaque regions (Figure 4H). Similarly, GCX thickness was 1.2±0.21 μm in the non-plaque regions and was significantly decreased to 0.85±0.15 μm in the plaque region (Figure 4I).
Fig. 4. GCX coverage and thickness in BCA sections stained with HABP to label the HA GCX component.
(A) 10X image of a BCA with plaque, obtained from a high fat fed ApoE−/− mouse. GCX can be seen overlying non-plaque tissue (arrow) and is degraded overlying the plaque (star). (B) 63X/oil image of degraded GCX over plaque. Star indicates location of degraded GCX; arrow points to remaining GCX. (C) 63X/oil image of intact GCX (arrow) over the non-plaque region of diseased vessel. (D) 10X image of a healthy BCA without plaque and with fully intact GCX (arrow), obtained from a normal diet fed wildtype mouse. (E) 63X/oil image of intact GCX (arrow) in healthy vessel. (F-I) GCX quantification taken from 6-7 mice per condition. Mean comparisons were performed using unpaired t-tests. *p < 0.05; **p < 0.01.
In the WT mice (Figures 4D and E) the GCX appears fully intact. GCX coverage was 92±3% in the WT BCA, and the thickness was 1.1±0.06 μm. These values were not significantly different from the values on the non-plaque regions of the ApoE−/− mice (Figures 4F and G).
HA staining in the DA
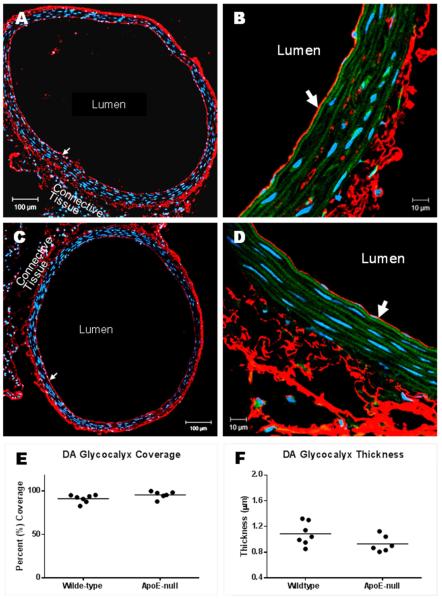
Figure 5 shows representative images of HA staining in the DA of ApoE−/− and WT mice. GCX appears intact for both groups in the athero-protected DA (Figures 5A-D). GCX coverage was found to be 91±2% in the WT and 96±2% in the ApoE−/−. GCX thickness was found to be 1.1±0.07 μm in the WT and 0.93±0.05 μm in the ApoE−/−. Neither value was found to be significantly different between the two groups (Figures 5E and F).
Fig. 5. GCX coverage and thickness in DA sections stained with HABP to label the HA GCX component.
(A) 10X image of a DA obtained from a high fat fed ApoE−/− mouse. The GCX coats the entire inner vessel wall (arrow). (B) 63X/oil image of intact GCX shown in A; arrow points to the GCX. (C) 10X image of GCX (arrow) on DA obtained from a normal diet fed wildtype mouse. (D) 63X/oil image of intact GCX (arrow) shown in C. (E, F) GCX quantification taken from 6-7 mice per condition. Mean comparisons were performed using unpaired t-tests.
Inflammatory marker staining
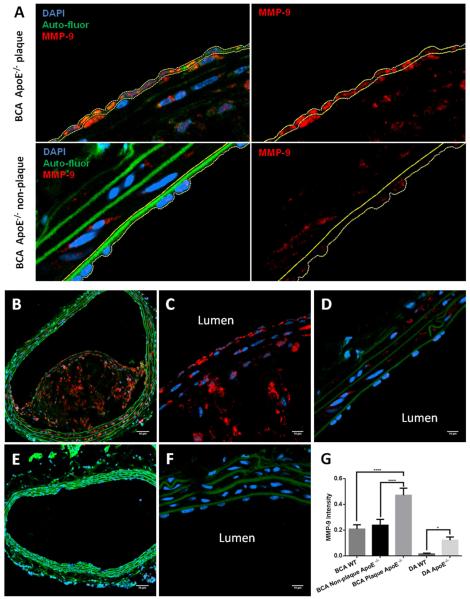
A typical ROI selection for MMP-9 intensity quantification is shown in Figure 6A for the plaque (top panels) and non-plaque (bottom panels) regions of the BCA. The ROI was selected in the merged image (left panels) to include the endothelial cell layer only, and quantified in the red channel (MMP-9; right panels). Figures 6B-6G show representative images of MMP-9 immunostaining in the BCA of ApoE−/− and WT mice. MMP-9 is present throughout the plaque in the ApoE−/− mouse (Figures 6B and C) while it is markedly decreased in the non-plaque area of the ApoE−/− mouse as well as in the WT mouse (Figures 6D-F). DA sections from WT and ApoE−/− mice were also immunostained for MMP-9 (images not shown). Figure 6G shows the results of the quantification of MMP-9 intensity in all the vessels studied. There was no significant difference in MMP-9 intensity between the BCA in WT mice, and the non-plaque areas of the ApoE−/− mice. In the plaque area of the ApoE−/− BCA, MMP-9 intensity was 2-fold higher than in both the WT BCA and non-plaque areas of the ApoE−/− BCA. MMP-9 intensity in the WT DA was found to be the lowest of all the vessels examined. Intensity in the WT DA was 8-fold lower when compared to the ApoE−/− DA, and 13-fold lower when compared to the WT BCA. Immunostaining of CD68 in DA and BCA sections from ApoE−/− mice (Supplemental Figure 1) shows a pattern similar to MMP-9, i.e., strong staining in the plaques and little or no staining in the non-plaque areas.
Fig. 6. MMP-9 staining in BCA sections.
(A) Selection of the region of interest (ROI) for MMP-9 quantification in the endothelial layer. ROI was traced (yellow line) in the merged image (left panels) and quantified in the red channel (MMP-9; right panels). Typical selection areas are shown for the plaque (top panels) and non-plaque (bottom panels) areas of the ApoE−/− BCA. (B) 20X image of BCA with plaque, obtained from a high fat fed ApoE−/− mouse. MMP-9 staining (red) is seen throughout the plaque. (C) 63X/oil image of MMP-9 stain in the plaque. (D) 63X/oil image of MMP-9 stain in the non-plaque region of diseased vessel. (E) 20X image of a healthy BCA without plaque, obtained from a normal diet fed wildtype mouse, shows little MMP-9 staining. (F) 63X/oil image of MMP-9 stain in healthy vessel. (G) Quantification of MMP-9 intensity within the ROI as shown in (A). Mean comparisons were performed using 1-way ANOVA with Tukey’s method to correct for multiple comparisons. N=33 for BCA samples; N=71 for DA WT; N=54 for DA ApoE−/−. ****p<0.0001, *p<0.05. Scale bar = 50 μm for B and E; scale bar = 10 μm for C-D, F.
Discussion
In the present study, after 10 weeks on a high fat diet, all of the ApoE−/− mice exhibited prominent plaques in the BCA. Oil Red O staining indicated that there was substantial lipid accumulation in the plaques, but no observable Oil Red O staining in the non-plaque regions of the BCA or the DA. These plaques appeared to be typical of early to mid-stage disease progression (Fig. 1).
Transport of macromolecules across the endothelium can occur through three potential pathways [5]: 1) transcytosis in vesicles, 2) paracellular transport through the normal breaks in the tight junction strand, and 3) through the leaky junction associated with cells undergoing apoptosis or mitosis. It is unlikely that a normal junction break would permit the passage of LDL (22 nm diameter), even without the GCX as a molecular filter, because the widest part of the junction is expected to be about 20 nm [25]. Weinbaum et al. [9] were first to propose the leaky junction as the primary transport pathway for LDL. In vivo studies by Chien and coworkers [8, 26] supported the importance of the leaky junction pathway in LDL and albumin transport in the rat aorta. In vitro, our lab showed that the leaky junction is the dominant transport pathway for LDL [7], and that there is a strong correlation between EC apoptosis and LDL permeability [6]. Despite all the evidence, few studies have examined EC-specific apoptosis in relation to early atherosclerotic plaque development in vivo. Here we demonstrated that the number of apoptotic ECs per unit length is significantly higher in the plaque region of the BCA of ApoE−/− mice than in the non-plaque region of the same vessel (Figure 3F), which would be expected to result in higher permeability of the plaque region to macromolecules. Overall EC apoptosis rates (as a percentage of total ECs) were 18.2±8.5% in the BCA and 39.8±14.3% in the DA. These values, while extremely high, are in agreement with those reported in recent studies that examined the atheroprone aortic root of ApoE−/− mice fed a western diet for 6-8 weeks [27] [28]. Those studies also found a 15-fold increase in cleaved caspase-3 expression compared to WT mice. While an increase in apoptosis in atheroprone areas is expected [15], the most surprising result is that we observed EC apoptosis to be elevated everywhere in the ApoE−/− mice, including the athero-protected DA. To our knowledge, the present study is the first to look at EC apoptosis in non-plaque regions of the ApoE−/− model. We hypothesize that the systemic increase in EC apoptosis is due to the highly atherogenic lipid profile developed in the ApoE−/− mice on a Western diet. LDL and VLDL cholesterol, which have been shown to induce EC apoptosis [29], increased by 16- and 46-fold, respectively, while HDL cholesterol, which has been shown to inhibit EC apoptosis [30], decreased by nearly 5-fold (Table 1). It is also possible that increased EC apoptosis is inherent to the ApoE−/− model and the condition is exacerbated by the lipid profile. Further studies of EC apoptosis in ApoE−/− mice fed a normal diet as well as measurement of EC apoptosis at several time points after the start of the Western diet are necessary to establish the causes of systemic elevated apoptosis in the ApoE−/− model.
We observed significantly lower coverage and thickness of the glycocalyx (HA layer) in the plaque region of the BCA compared to the non-plaque region of the BCA or the DA in the ApoE−/− mouse, where these values were not different from values in the WT mouse (Figs. 4 and 5). This is consistent with the earlier observations of van den Berg et al. [31] that the glycocalyx was reduced in the disease prone sinus region of the mouse internal carotid. We also found that MMP-9 was significantly elevated in the intimal layer overlying the plaque in the BCA and, to a lesser degree, in the DA of the ApoE−/− and the BCA of the WT mouse.
There are several possible interpretations of our observations and their relevance to the development of atherosclerosis. First, the reduced glycocalyx and elevated MMP-9 in the BCA may be considered as indicators of local inflammation induced by the fluid mechanical environment. This interpretation is consistent with findings reported by Gouverneur and coworkers [18], which demonstrated that GCX components regrow at a higher rate in arteries exposed to healthy levels of vessel wall shear stress, compared to low levels of wall shear stress. Recent in vitro studies showed similar phenomena. Koo et al. [16] observed that an atheroprotective shear stress waveform increased the expression of heparan sulfate on HUVECs via upregulation of its syndecan-1 proteoglycan, while an atheroprone waveform had the opposite effect. The same group showed faster regrowth of heparan sulfate after enzymatic degradation under laminar shear compared to static conditions [17]. Therefore, the growth and shedding of GCX components are actively regulated by ECs in response to the local hemodynamics.
Furthermore, ECs exposed to disturbed flow upregulate their expression of important chemokines and chemokine receptors and activate the NF-κB signaling pathway [32] which mediates the inflammatory response [33, 34]. Magid et al [35] showed a 3-fold increase in MMP-9 protein and mRNA expression in ECs exposed to oscillatory shear stress compared to unidirectional shear, suggesting MMP-9 expression by ECs is sensitive to flow and may contribute to the initiating events in lesion development. Cui et al. [36] and Mulivor and Lipowsky [37] found that MMPs degrade glycocalyx while Lipowsky and Lescanic [38] described degradation of the glycocalyx by reactive oxygen species (ROS). Recent studies from Jo’s group [39] using an ApoE−/− mouse model with partial ligation of the left carotid artery to induce a reversing oscillatory flow field in the common carotid artery showed rapid development of atherosclerotic plaques due to a mechanism involving oscillatory shear-induced upregulation of microRNA 712 that suppresses tissue inhibitor of metalloproteinase 3, thus allowing increased MMP activity.
Second, a low level inflammation (MMP-9, Fig. 6) in all areas of ApoE−/− compared to WT mice may be considered an indicator of systemic inflammation induced by the elevated cholesterol. Higher serum levels of MMP-9 and lower levels of TIMP-1, an endogenous inhibitor of MMPs, were found in patients with familial hypercholesterolemia [40]. Um et al. [41] showed increased MMP-9 and MMP-1 mRNA expression in the aortas of rabbits fed a high cholesterol diet for 8 weeks. The GCX could also be affected by cholesterol levels indirectly by the activation of MMPs capable of cleaving GCX components [37, 42-45]. More directly the GCX is affected by the reduction in HDL levels (Table 1) because it can be in part regulated by cardioprotective [46, 47] sphingosine-1-phosphate (S1P). S1P is released from erythrocytes into plasma and then transported by albumin and HDL through plasma to ECs. On ECs S1P preserves the GCX by inducing synthesis and inhibiting shedding [45, 48, 49]. In our study, however, systemic inflammation alone (as in the DA) does not result in detectable degradation of the GCX or recruitment of monocytes/macrophages in ApoE−/− mice (Fig 5 and Supplemental Fig. 1).
Third, the diminished glycocalyx barrier can propagate further inflammation by providing circulating leukocytes and platelets with enhanced access to adhesion receptors on the endothelial surface thus recruiting them to the intima [50]. Reitsma et al. [51] found reduced GCX thickness and increased platelet adhesion in the carotid bifurcation of ApoE−/− mice fed a normal diet. Nagy et al. [52] showed that inhibiting endothelial hyaluronan synthesis with 4-methylumbelliferone reduced the GCX and resulted in increased leukocyte adhesion, macrophage retention, and accelerated plaque development in ApoE−/− mice fed a Western diet for 25 weeks. Similarly, Voyvodic et al. [53] showed increased leukocyte recruitment and inflammatory response in a syndecan-1 knockout mouse. Axelsson et al. [54] used a tissue-specific knockout of Hs2st to modify the structure of heparan sulfate in the ECs of a mouse by eliminating 2-O-sulfate groups. The modification resulted in increased neutrophil recruitment in response to inflammatory stimuli. Inflammatory cells recruited to the intima would be expected to release mediators, including ROS and MMPs [55-57] which are known to degrade the GCX [38, 56]. Therefore, by a positive feedback mechanism the initial flow-induced GCX degradation can lead to recruitment of inflammatory cells that further degrade the GCX and propagate the inflammatory response.
Degradation of the EC GCX is observed in many vascular-related diseases including diabetes and renal dysfunction [58]. Hyperglycemia resulted in a decrease in heparan sulfate content along with an attenuation of eNOS activation, hydraulic conductivity response, and cell alignment in an in vitro model of diabetes [59]. Forbes et al. [60] used an ApoE−/− mouse western diet with streptozotocin-induced diabetes and showed a 10-fold increase in plaque area in the athero-protected thoracic aorta compared to non-diabetic controls. Similarly, atherosclerosis in diabetic human subjects is not confined to areas of low shear and disturbed flow but found to be more uniformly distributed [61]. Increased GCX shedding was also observed in patients with chronic kidney disease [62] and dialysis patients [63], conditions that are associated with increased cardiovascular morbidity. These observations underscore the importance of the GCX in maintaining EC barrier function, limiting access to leukocytes that propagate inflammation, and promoting EC mechanotransduction mechanisms that protect against disease.
We note two unexpected results from our study: 1) apoptosis is high in the ApoE−/− DA and 2) there is a low level of inflammation (increased MMP-9) in this same vessel that is nonetheless higher than in the WT DA. We hypothesized above that both of these results can be attributed to systemic elevation of atherogenic lipid fractions (LDL, VLDL, etc.). Elevated apoptosis suggests increased permeability of the endothelium to LDL. Indeed it has been shown that the aorta in the high-fat fed ApoE−/− mouse model does experience increased solute permeability [64], consistent with increased apoptosis. However, the DA, away from intercostal ostia, remains protected from atherosclerosis. Our observation that the glycocalyx is not diminished in the DA provides a clue to explain these unexpected results. It suggests that even in the ApoE−/− animal on a high fat diet, the DA is a region of low enhancement of inflammation whereas the plaque region of the BCA that has a diminished glycocalyx experiences highly elevated inflammation. It is the combined effect of local inflammation, induced by disturbed flow in the BCA, along with systemic inflammation, induced by elevated cholesterol levels, which together produce the environment for LDL retention and plaque formation. Within this environment of enhanced inflammation, the GCX is diminished enough for inflammatory cells to be recruited. In addition to degrading the GCX, inflammatory mediators (e.g. ROS and MMPs) are capable of degrading HS proteoglycans [57] and other GAGs and proteins [43] present in the extracellular matrix. Such alterations of the extracellular matrix have been shown to increase retention of LDL in the subendothelial intima [65]. In addition, ROS and MMPs can modify LDL particles, increasing their affinity to matrix proteoglycans, and triggering their aggregation and fusion [66].
In contrast, the inflammatory phenotype induced by disturbed flow on the outer wall of the BCA is not present in the DA where inflammatory indicators (glycocalyx thinning and MMP-9 localization) are absent or present at low levels, but apoptosis is still high. We have previously shown that high apoptosis rates lead to an increase in water flux and LDL permeability of EC monolayers in vitro [6]. In the rabbit thoracic aorta, Tedqui and Lever [67] showed that, in vessels with a damaged endothelium, enhanced water flux led to lower albumin retention in the vessel wall. It is known that ECs not exposed to disturbed flow, such as the DA, upregulate genes that are anti-thrombotic, anti-inflammatory and anti-oxidant [68]. Thus, our results suggest that these athero-protected areas may still experience high permeability due to elevated apoptosis induced by systemic risk factors such as high cholesterol levels, but do not develop plaques because LDL is not retained in the subendothelial intima without local inflammation and is instead washed away by the increased water flux.
The main limitation of the present study is that it looks at a single time point. Further studies should consider analysis of earlier plaques to assess the time course of GCX thinning, apoptosis elevation and MMP-9 elevation. In addition, while the ApoE−/− model on a high fat diet provides accelerated development of plaques and has been extensively characterized, the cholesterol levels developed in this model are much higher than seen in humans. It may be useful to consider an alternative model such as the LDLR−/− mouse, which develops cholesterol levels closer to those observed in humans [21]. Lowering cholesterol levels and measuring GCX, EC apoptosis, and MMP-9 at several time points may help to further clarify the relative importance of systemic and local factors in atherogenesis.
In summary, our apoptosis, glycocalyx and MMP-9 data in the ApoE−/− mouse model suggest that systemic inflammation leading to elevated apoptosis and LDL permeability, in concert with flow-induced local inflammation leading to elevated MMP-9 localization to the intima and reduced glycocalyx thickness, facilitate monocyte/macrophage accumulation in the intima, and lead to increased LDL retention and ultimately plaque formation on the outer wall of the BCA.
Supplementary Material
Supplemental Figure 1: CD68 immunostaining in the BCA and DA of ApoE−/− mice. Cross-sections shown in images A-E were stained for CD68 (red) and cell nuclei (DAPI; blue). Elastin sheets auto-fluoresce in green. (A) 10X image of the BCA obtained from a high fat fed ApoE−/− mouse. CD68 is seen within the plaque. (B) 63X/oil image CD68 staining in the plaque region. (C) 63X/oil image of the non-plaque region of the diseased vessel. (D) 10X image of the DA obtained from a high fat fed ApoE−/− mouse. (E) 63X/oil image of the DA from high fat fed ApoE−/− mouse. Little to no CD68 staining is observed in the non-plaque regions of the ApoE−/− mouse (C-E).
Highlights.
Apoptosis is elevated systemically in the high fat fed ApoE−/− mouse model
GCX thickness and coverage are decreased in atheroprone region only (BCA)
Local and systemic inflammation together lead to LDL retention and plaque formation
Acknowledgments
Financial support
Research supported by NIH HL094889.
Drs. Tarbell, Cancel and Ebong report support from NIH grant HL094889 during the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Solomon Mensah has nothing to disclose.
Carly Hirshberg has nothing to disclose.
References
- 1.Santos-Gallego CG, Picatoste B, Badimon JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep. 2014;16(4):401. doi: 10.1007/s11883-014-0401-9. [DOI] [PubMed] [Google Scholar]
- 2.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79(3):703–61. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 4.Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng. 2002;30(4):430–46. doi: 10.1114/1.1467924. [DOI] [PubMed] [Google Scholar]
- 5.Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Annu Rev Biomed Eng. 2003;5:79–118. doi: 10.1146/annurev.bioeng.5.040202.121529. [DOI] [PubMed] [Google Scholar]
- 6.Cancel LM, Tarbell JM. The role of apoptosis in LDL transport through cultured endothelial cell monolayers. Atherosclerosis. 2010;208(2):335–41. doi: 10.1016/j.atherosclerosis.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancel LM, Fitting A, Tarbell JM. In vitro study of LDL transport under pressurized (convective) conditions. Am J Physiol Heart Circ Physiol. 2007;293(1):H126–32. doi: 10.1152/ajpheart.01188.2006. [DOI] [PubMed] [Google Scholar]
- 8.Lin S-J, Jan K-m, Chien S. Role of Dying Endothelial Cells in Transendothelial Macromolecular Transport. Arteriosclerosis. 1990;10:703–9. doi: 10.1161/01.atv.10.5.703. [DOI] [PubMed] [Google Scholar]
- 9.Weinbaum S, Tzeghai G, Ganatos P, Pfeffer R, Chien S. Effect of Cell Turnover and Leaky Junctions on Arterial Macromolecular Transport. American Journal of Physiology. 1985;248:H945–H58. doi: 10.1152/ajpheart.1985.248.6.H945. [DOI] [PubMed] [Google Scholar]
- 10.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259(4):339–50. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JC, Taylor RG, Jones ND, St Clair RW, Cornhill JF. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest. 1982;46(2):123–38. [PubMed] [Google Scholar]
- 12.van den Berg BM, Spaan JA, Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 2009;457(6):1199–206. doi: 10.1007/s00424-008-0590-6. [DOI] [PubMed] [Google Scholar]
- 13.Cho A, Mitchell L, Koopmans D, Langille BL. Effects of changes in blood flow rate on cell death and cell proliferation in carotid arteries of immature rabbits. Circ Res. 1997;81(3):328–37. doi: 10.1161/01.res.81.3.328. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Haendeler J, Rippmann V, Nehls M, Zeiher AM. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399(1-2):71–4. doi: 10.1016/s0014-5793(96)01289-6. [DOI] [PubMed] [Google Scholar]
- 15.Freyberg MA, Kaiser D, Graf R, Buttenbender J, Friedl P. Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem Biophys Res Commun. 2001;286(1):141–9. doi: 10.1006/bbrc.2001.5314. [DOI] [PubMed] [Google Scholar]
- 16.Koo A, Dewey CF, Jr., Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol. 2013;304(2):C137–46. doi: 10.1152/ajpcell.00187.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giantsos-Adams KM, Koo AJ, Song S, Sakai J, Sankaran J, Shin JH, et al. Heparan Sulfate Regrowth Profiles Under Laminar Shear Flow Following Enzymatic Degradation. Cell Mol Bioeng. 2013;6(2):160–74. doi: 10.1007/s12195-013-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med. 2006;259(4):393–400. doi: 10.1111/j.1365-2796.2006.01625.x. [DOI] [PubMed] [Google Scholar]
- 19.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99(2):315–27. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimbrone MA, Jr., Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 9-40. [DOI] [PubMed] [Google Scholar]
- 21.Santos-Gallego CG, Badimon JJ, Ibanez B. Modelos experimentales de aterosclerosis. Rev Esp Cardio Supl. 2013;13(E):3–12. [Google Scholar]
- 22.Bond AR, Jackson CL. The fat-fed apolipoprotein E knockout mouse brachiocephalic artery in the study of atherosclerotic plaque rupture. J Biomed Biotechnol. 2011;2011:379069. doi: 10.1155/2011/379069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng Y, Ebong EE, Fu BM, Tarbell JM. The structural stability of the endothelial glycocalyx after enzymatic removal of glycosaminoglycans. PLoS One. 2012;7(8):e43168. doi: 10.1371/journal.pone.0043168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams H, Johnson JL, Carson KG, Jackson CL. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22(5):788–92. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- 25.Fu BM, Weinbaum S, Tsay RY, Curry FE. A junction-orifice-fiber entrance layer model for capillary permeability: application to frog mesenteric capillaries. J Biomech Eng. 1994;116(4):502–13. doi: 10.1115/1.2895802. [DOI] [PubMed] [Google Scholar]
- 26.Chien S, Lin SJ, Weinbaum S, Lee MM, Jan KM. The role of arterial endothelial cell mitosis in macromolecular permeability. Adv Exp Med Biol. 1988;242:59–73. doi: 10.1007/978-1-4684-8935-4_8. [DOI] [PubMed] [Google Scholar]
- 27.Sun GB, Qin M, Ye JX, Pan RL, Meng XB, Wang M, et al. Inhibitory effects of myricitrin on oxidative stress-induced endothelial damage and early atherosclerosis in ApoE−/−mice. Toxicol Appl Pharmacol. 2013;271(1):114–26. doi: 10.1016/j.taap.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Guo X, Chen W. Inhibitory effects of oleoylethanolamide (OEA) on H(2)O(2)-induced human umbilical vein endothelial cell (HUVEC) injury and apolipoprotein E knockout (ApoE−/−) atherosclerotic mice. Int J Clin Exp Pathol. 2015;8(6):6301–11. [PMC free article] [PubMed] [Google Scholar]
- 29.Galle J, Schneider R, Heinloth A, Wanner C, Galle PR, Conzelmann E, et al. Lp(a) and LDL induce apoptosis in human endothelial cells and in rabbit aorta: role of oxidative stress. Kidney Int. 1999;55(4):1450–61. doi: 10.1046/j.1523-1755.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- 30.Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem. 2001;276(37):34480–5. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- 31.van den Berg BM, Spaan JA, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol. 2006;290(2):H915–20. doi: 10.1152/ajpheart.00051.2005. [DOI] [PubMed] [Google Scholar]
- 32.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101(41):14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33(16):5308–19. doi: 10.1093/nar/gki836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86(2):211–8. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magid R, Murphy TJ, Galis ZS. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress. Role of c-Myc. J Biol Chem. 2003;278(35):32994–9. doi: 10.1074/jbc.M304799200. [DOI] [PubMed] [Google Scholar]
- 36.Cui N, Wang H, Long Y, Su L, Liu D. Dexamethasone Suppressed LPS-Induced Matrix Metalloproteinase and Its Effect on Endothelial Glycocalyx Shedding. Mediators Inflamm. 2015;2015:912726. doi: 10.1155/2015/912726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation. 2009;16(8):657–66. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 38.Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res. 2013;90:80–5. doi: 10.1016/j.mvr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Son DJ, Kumar S, Takabe W, Kim CW, Ni CW, Alberts-Grill N, et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nenseter MS, Narverud I, Graesdal A, Bogsrud MP, Halvorsen B, Ose L, et al. Elevated serum MMP-9/TIMP-1 ratio in patients with homozygous familial hypercholesterolemia: effects of LDL-apheresis. Cytokine. 2013;61(1):194–8. doi: 10.1016/j.cyto.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Um MY, Hwang KH, Choi WH, Ahn J, Jung CH, Ha TY. Curcumin attenuates adhesion molecules and matrix metalloproteinase expression in hypercholesterolemic rabbits. Nutr Res. 2014;34(10):886–93. doi: 10.1016/j.nutres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, et al. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278(42):40764–70. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 43.Gronski TJ, Jr., Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, et al. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997;272(18):12189–94. doi: 10.1074/jbc.272.18.12189. [DOI] [PubMed] [Google Scholar]
- 44.Yu WH, Woessner JF., Jr. Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7) J Biol Chem. 2000;275(6):4183–91. doi: 10.1074/jbc.275.6.4183. [DOI] [PubMed] [Google Scholar]
- 45.Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306(3):H363–72. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badimon JJ, Santos-Gallego CG. HDL Dysfunction: Is the Answer in the Sphinx's Riddle? J Am Coll Cardiol. 2015;66(13):1486–8. doi: 10.1016/j.jacc.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Santos-Gallego CG, Vahl TP, Goliasch G, Picatoste B, Arias T, Ishikawa K, et al. Sphingosine-1-Phosphate Receptor Agonist Fingolimod Increases Myocardial Salvage and Decreases Adverse Postinfarction Left Ventricular Remodeling in a Porcine Model of Ischemia/Reperfusion. Circulation. 2016;133(10):954–66. doi: 10.1161/CIRCULATIONAHA.115.012427. [DOI] [PubMed] [Google Scholar]
- 48.Zeng Y, Liu XH, Tarbell J, Fu B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Exp Cell Res. 2015;339(1):90–5. doi: 10.1016/j.yexcr.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Zeng M, Fan J, Tarbell JM, Curry FR, Fu BM. Sphingosine-1-phosphate Maintains Normal Vascular Permeability by Preserving Endothelial Surface Glycocalyx in Intact Microvessels. Microcirculation. 2016;23(4):301–10. doi: 10.1111/micc.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipowsky HH. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann Biomed Eng. 2012;40(4):840–8. doi: 10.1007/s10439-011-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reitsma S, Oude Egbrink MG, Heijnen VV, Megens RT, Engels W, Vink H, et al. Endothelial glycocalyx thickness and platelet-vessel wall interactions during atherogenesis. Thromb Haemost. 2011;106(5):939–46. doi: 10.1160/TH11-02-0133. [DOI] [PubMed] [Google Scholar]
- 52.Nagy N, Freudenberger T, Melchior-Becker A, Rock K, Ter Braak M, Jastrow H, et al. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation. 2010;122(22):2313–22. doi: 10.1161/CIRCULATIONAHA.110.972653. [DOI] [PubMed] [Google Scholar]
- 53.Voyvodic PL, Min D, Liu R, Williams E, Chitalia V, Dunn AK, et al. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem. 2014;289(14):9547–59. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Axelsson J, Xu D, Kang BN, Nussbacher JK, Handel TM, Ley K, et al. Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood. 2012;120(8):1742–51. doi: 10.1182/blood-2012-03-417139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm. 2014;2014:694312. doi: 10.1155/2014/694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Golen RF, van Gulik TM, Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2012;52(8):1382–402. doi: 10.1016/j.freeradbiomed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng. 2014;16:505–32. doi: 10.1146/annurev-bioeng-071813-104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez-Quintero SV, Cancel LM, Pierides A, Antonetti D, Spray DC, Tarbell JM. High glucose attenuates shear-induced changes in endothelial hydraulic conductivity by degrading the glycocalyx. PLoS One. 2013;8(11):e78954. doi: 10.1371/journal.pone.0078954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53(7):1813–23. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- 61.Danese C, Vestri AR, D'Alfonso V, Deriu G, Dispensa S, Baldini R, et al. Do hypertension and diabetes mellitus influence the site of atherosclerotic plaques? Clin Ter. 2006;157(1):9–13. [PubMed] [Google Scholar]
- 62.Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234(2):335–43. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 63.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23(11):1900–8. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Zhu HQ, Wang F, Zhou Q, Gui SY, Wang Y. MicroRNA-1 prevents high-fat diet-induced endothelial permeability in apoE knock-out mice. Mol Cell Biochem. 2013;378(1-2):153–9. doi: 10.1007/s11010-013-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pillarisetti S, Paka L, Obunike JC, Berglund L, Goldberg IJ. Subendothelial retention of lipoprotein (a). Evidence that reduced heparan sulfate promotes lipoprotein binding to subendothelial matrix. J Clin Invest. 1997;100(4):867–74. doi: 10.1172/JCI119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oorni K, Pentikainen MO, Ala-Korpela M, Kovanen PT. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res. 2000;41(11):1703–14. [PubMed] [Google Scholar]
- 67.Tedgui A, Lever MJ. The interaction of convection and diffusion in the transport of 131I-albumin within the media of the rabbit thoracic aorta. Circ Res. 1985;57(6):856–63. doi: 10.1161/01.res.57.6.856. [DOI] [PubMed] [Google Scholar]
- 68.Topper JN, Cai J, Falb D, Gimbrone MA., Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93(19):10417–22. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: CD68 immunostaining in the BCA and DA of ApoE−/− mice. Cross-sections shown in images A-E were stained for CD68 (red) and cell nuclei (DAPI; blue). Elastin sheets auto-fluoresce in green. (A) 10X image of the BCA obtained from a high fat fed ApoE−/− mouse. CD68 is seen within the plaque. (B) 63X/oil image CD68 staining in the plaque region. (C) 63X/oil image of the non-plaque region of the diseased vessel. (D) 10X image of the DA obtained from a high fat fed ApoE−/− mouse. (E) 63X/oil image of the DA from high fat fed ApoE−/− mouse. Little to no CD68 staining is observed in the non-plaque regions of the ApoE−/− mouse (C-E).