Abstract
Background
Primary and secondary progressive multiple sclerosis (MS), collectively called progressive multiple sclerosis (PMS), is characterized by gradual progression of disability. The current anti-inflammatory treatments for MS have little or no efficacy in PMS in the absence of obvious active inflammation. Optimal biomarkers for phase II PMS trials is unknown. Ibudilast is an inhibitor of macrophage migration inhibitor factor and phosphodiesterases-4 and -10 and exhibits possible neuroprotective properties. The goals of SPRINT-MS study are to evaluate the safety and efficacy of ibudilast in PMS and to directly compare several imaging metrics for utility in PMS trials.
Methods
SPRINT-MS is a randomized, placebo-controlled, phase II trial of ibudilast in patients with PMS. Eligible subjects were randomized 1:1 to receive either ibudilast (100 mg/day) or placebo for 96 weeks. Imaging is conducted every 24 weeks for whole brain atrophy, magnetization transfer ratio, diffusion tensor imaging, cortical brain atrophy, and retinal nerve fiber layer thickness. Clinical outcomes include neurologic disability and patient reported quality of life. Safety assessments include laboratory testing, electrocardiography, and suicidality screening.
Results
A total of 331 subjects were enrolled, of which 255 were randomized onto active study treatment. Randomized subjects were 53.7% female and mean age 55.7 (SD 7.3) years. The last subject is projected to complete the study in May 2017.
Conclusion
SPRINT-MS is designed to evaluate the safety and efficacy of ibudilast as a treatment for PMS while simultaneously validating five different imaging biomarkers as outcome metrics for use in future phase II proof-of-concept PMS trials.
Keywords: Clinical Trial, Ibudilast, Progressive Multiple Sclerosis, Magnetic Resonance Imaging
Background
Multiple sclerosis (MS) is a chronic demyelinating disease affecting the brain, spinal cord, and optic nerves. The precise etiology of MS is still unknown, although several pathological processes including inflammation, demyelination, and axonal damage contribute to the disease manifestations. MS most commonly starts as an episodic disorder called relapsing remitting MS (RRMS), with the majority of untreated patients eventually developing gradually progressive disability, which marks the secondary progressive form of MS (SPMS).1 About 15% of patients do not have initial phase of episodic relapses and instead present with gradually progressive disability, which is a form of MS called primary progressive MS (PPMS). Together, SPMS and PPMS comprise progressive multiple sclerosis (PMS) which affects about 1 million people worldwide.2
While there are multiple disease modifying therapies (DMT) for RRMS, no effective therapy is currently available for PMS in the absence of obvious active inflammation. Treatment of PMS is therefore primarily limited to symptomatic and supportive care, making PMS a significant unmet clinical need in neurologic care.
Ibudilast is a small molecule phosphodiesterase inhibitor which is currently approved in Japan and other Asian countries for treatment of asthma and post-stroke symptoms at a 20–30 mg/day dosage. While ibudilast is not yet approved outside of Asia, development is ongoing for neurological conditions including MS, neuropathic pain, and drug addictions.3–6 Ibudilast penetrates the CNS well and selectively inhibits the pro-inflammatory cytokine macrophage migration inhibitory factor (MIF)7 and certain cyclic nucleotide phosphodiesterases at clinically relevant plasma and CNS concentrations.8 Both systems have been implicated in neurodegeneration and disease progression in animal models and their inhibition leads to neuroprotective effects.9–15 The potential neuroprotective effects of ibudilast were suggested in a phase II trial in RRMS, where it slowed the progression of brain atrophy and decreased the proportion of gadolinium-enhancing lesions converting into T1 black holes.3 Taken together, the potential neuroprotective effects of ibudilast makes it an attractive candidate therapy for PMS.
Development of effective therapies for PMS has been limited by the lack of validated biomarkers for use in Phase II trials. Currently, whole brain atrophy (WBA) is the most widely utilized outcome measure for phase II PMS trials.16,17 WBA can be detected at all stages of MS disease and represents a summation of the destructive pathologic processes in MS. Many studies show significant correlations between WBA and overall clinical disability, cognitive impairment,18–22 depression,23 fatigue,24,25 and quality of life.26,27 However, WBA is a relatively crude measure of overall brain injury, lacking granularity to characterize localized injury.
Better metrics of MS injury are needed to screen potential therapies for PMS. Candidate markers should correlate with tissue injury, be dynamic over the course of disease, and be easily implemented in a standardized fashion in multi-centered clinical trials. Potential metrics include a few novel MRI measures (magnetization transfer ratio (MTR), diffusion tensor imaging (DTI), and cortical atrophy) and optical coherence tomography (OCT), which is a non-invasive imaging tool for measuring retinal nerve fiber layer (RNFL) thickness in the retina.
Methods
The NeuroNEXT 102 (NN102)/Secondary and Primary pRogressive Ibudilast NeuroNEXT Trial in Multiple Sclerosis (SPRINT-MS) is a randomized, placebo-controlled, Phase II clinical trial evaluating the effect of ibudilast and assessing the utility of other imaging biomarkers in PMS.
Study Organization
The clinical trial is a collaborative study conducted by the Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT). Started in 2011, NeuroNEXT is an initiative of the National Institute of Neurological Disorders and Stroke (NINDS) designed to accelerate development of therapies for neurological diseases through partnerships with academic institutions, non-profit organizations, and industry. The core of NeuroNEXT comprises a Clinical Coordinating Center (CCC, which is located at the Massachusetts General Hospital, Boston, MA), a Data Coordinating Center (DCC, which is located at the University of Iowa, Iowa City, IA), and 25 academic medical centers across the US. Central to the function of NeuroNEXT is external peer-review to ensure high quality scientific rigor; centralized ethics oversight, which is provided through the Central Institutional Review Board (CIRB) at Massachusetts General Hospital; a single master clinical trial agreement for each participating clinical site, through which all NeuroNEXT trials at that site are contracted; and operational support by the NINDS. Details about NeuroNEXT structure have been published previously.28 Funding for the SPRINT-MS trial is primarily through a competitive peer-reviewed grant issued by the NINDS, with additional funding provided by the National MS Society and Medicinova, which holds intellectual property rights to ibudilast.
Overall Trial Design
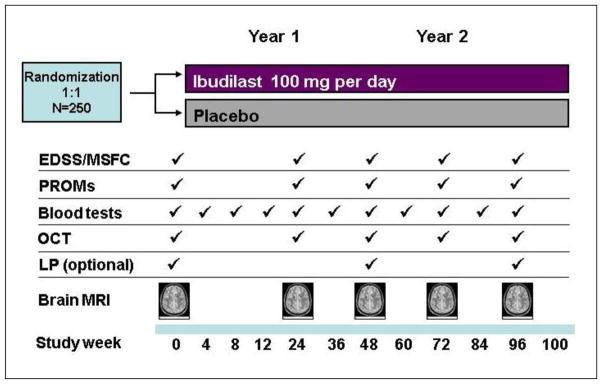
The primary objective of SPRINT-MS is to evaluate the safety, tolerability, and activity of ibudilast (100 mg/day taken orally) compared to placebo in subjects with PMS. The trial is designed as a multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Participants recruited into the study were required to have a confirmed diagnosis of either PPMS or SPMS. Concomitant treatment with injectable MS therapies interferon-β1 or glatiramer acetate (any of each) were allowed. A total of approximately 250 male and female subjects from 21 to 65 years old, inclusive, were planned to be randomized to ibudilast or placebo. The study consisted of a screening phase during which subjects were assessed for eligibility (Table 1) over 45 days, followed by a 96 week treatment phase where eligible subjects were randomized to one of two treatment groups: ibudilast 100 mg/d in two or three divided doses, or matching placebo, in a 1:1 ratio. Efficacy is assessed every 24 weeks using imaging and clinical assessments (Figure 1). All subjects were invited to participate in optional annual lumbar punctures to collect cerebrospinal fluid (CSF) for biomarker assessments.
Table 1.
Eligibility Criteria
Inclusion Criteria:
Exclusion Criteria:
|
Figure 1.
A schematic of events scheduled at each study visit in the SPRINT-MS trial.
Trial Governance
The study was proposed by the PI (RJF) to the NeuroNEXT network and subsequently underwent review by the NeuroNEXT network, NINDS Extramural Science Committee, and external peer review. The study protocol was developed by a Protocol Working Group, which included a patient advocate (includes RJF, CSC, MEC, ECK, KB, DE, RC). Following funding, a Protocol Steering Committee (RJF, CSC, MEC, TG, AG, ECK, KM, MM, RC, and RN) was formed and maintained for the duration of the trial, which also included the patient advocate. The NeuroNEXT Executive Committee and NINDS program staff provide ongoing external oversight to the study. Of the 25 network sites comprising NeuroNEXT, 23 requested to participate in the trial, with only the two pediatric sites not participating. Some NeuroNEXT sites comprise multiple medical centers. In total, 27 NeuroNEXT medical centers requested to participate in the study, along with Cleveland Clinic as an ad hoc participating center.
Ibudilast Dose Rationale
The dosage of 100 mg/day (50 mg orally twice a day, but could be spread to across three dosing intervals if needed for tolerability) of ibudilast was chosen based on both preclinical and clinical data. In vitro studies on microglial-induced neuronal cell death and MIF inhibition have shown a dose-dependent neuroprotective role for ibudilast.7,29 A dose-dependent benefit of ibudilast was observed in a study of experimental autoimmune encephalomyelitis in Dark August rats,30 rat nerve ligation and spinal cord injury models,31 and cerebral aneurysm induction models.15 Furthermore, a dose-dependent benefit of ibudilast was observed on brain atrophy and T1 hole conversion in a Phase II RRMS trial using 30–60 mg/d.16 Completed phase 1 clinical trials in pain and substance dependence have demonstrated good tolerability of 80–100 mg/d with no significant safety signals at this dose (NCT01217970, NCT01389193, NCT02025998).32 To improve tolerability and reduce adverse effects, a 8 week dose titration was outlined, with allowance to modify daily dose to 60mg/d, 80 mg/d, and 100mg/d over the first 8 weeks at the treating neurologist’s discretion. After 8 weeks of treatment, the subject was required to maintain a stable dose of ibudilast/placebo. Temporary study medication suspensions were allowed over the course of the study as thought appropriate by the treating neurologist and were also prescribed for specific laboratory abnormalities.
Eligibility
Inclusion criteria specified male or female subjects age 21 to 65 (inclusive), diagnosis of either PPMS or SPMS according to the 2010 International Panel Criteria,33 on either no MS disease modifying therapies or receiving glatiramer acetate (GA) or interferon beta (IFNβ-1a or IFNβ-1b); able to walk 25 feet either with or without an assistive device (Expanded Disability Status Scale [EDSS] 6.5 or less), and having clinical evidence of disease progression over the previous two years as measured by increase in EDSS, or 20% slowing in either 25-foot timed walk, or 9-hole peg test. For EDSS progression, retrospective documentation according to patient history or clinical notes was allowed. Full inclusion and exclusion criteria are outlined in Table 1. Written informed consent was obtained from all participants.
Intervention
After a screening phase of up to 45 days, the double-blind treatment phase consisted of a baseline visit followed by 11 scheduled clinic visits. Subjects who met eligibility were randomized 1:1 to either ibudilast 100mg/d or placebo (matching capsules). Randomization was stratified according to disease status (primary or secondary progressive MS) and by use of immunomodulating therapy (yes or no). A list of random treatment assignments was generated for each strata using randomized block methods. Block sizes of four and six were randomly chosen and within each block treatment assignments were randomly generated in a 1:1 fashion. Kits of study medication (both placebo and ibudilast) were assigned random ID numbers and linked to treatment assignment. Only unblinded study statisticians and IT personnel at the DCC and personnel at the central pharmacy had access to the treatment assignments and medication kit ID numbers. All other study personnel and subjects were blinded to treatment assignments.
Following a 4- to 8-week dose titration, subjects will continue on study medication for a total of 96 weeks. Safety assessments are conducted every 4 weeks for the first 12 weeks, then every 12 weeks for the remainder of the study, plus a safety visit 4 weeks after treatment completion. Clinical and imaging efficacy assessments are conducted every 24 weeks. The schedule of events is shown in Figure 1 and a list of scheduled assessments is shown in Table 2.
Table 2.
Schedule of Assessments
| Tests and Evaluations | Screening Visit | Baseline Visit 4 | Week 4 ± 5 days | Week 8 ± 5 days | Week 12 ± 5 days | Week 24 ± 5 days | Week 36 ± 5 days | Week 48 ± 5 days | Week 60 ± 5 days | Week 72 ± 5 days | Week 84 ± 5 days | Week 96 ± 5 days | Week 100 follow-up (± 5days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Visit Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Informed consent | X | ||||||||||||
| Inclusion/exclusion criteria | X | X | |||||||||||
| Medical and MS history | X | ||||||||||||
| Physical examination | X | X | X | X | X | X | X | X | X | X | X | X | |
| Randomization | X | ||||||||||||
| Body height | X | ||||||||||||
| Body weight | X | X | X | X | X | X | X | X | X | X | X | X | |
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Interval history | X | X | X | X | X | X | X | X | X | X | X | X | |
| Adverse event review | X | X | X | X | X | X | X | X | X | X | X | X8 | |
| Concomitant meds | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Relapse assessment | X | X | X | X | X | X | X | X | X | X | X | ||
| Cognitive test (SRT)7 | X | X | X | X | X | X | |||||||
| Brief Pain Inventory (BPI) | X | X | X | X | X | X | X | X | X | X | X | X | |
| Short Form-36 Health Survey (SF-36) | X | X | X | X | X | X | |||||||
| Multiple Sclerosis Impact Scale (MSIS-29) | X | X | X | X | X | X | |||||||
| EuroQol 5 Dimensions (EQ-5D) | X | X | X | X | X | X | |||||||
| Beck Depression Inventory-Fast Screen (BDI-FS) | X | ||||||||||||
| Suicide Behaviors Questionnaire-revised (SBQ-R) | X | X | X | X | X | X | X | X | X | X | X | X | |
| Clinical labs (chemistry, hematology, urinalysis) | X | X | X | X | X | X | X | X | X | X | X | X | |
| Lipid Profile | X | X | X | ||||||||||
| Serum samples (biomarkers) | X | X | X | X | |||||||||
| Serum pregnancy test9 | X | X | X | X | X | X | X | X | X | X | X | ||
| Urine pregnancy test | X | ||||||||||||
| Plasma for biomarkers and PK | X10 | X | X | X | |||||||||
| ECG | X | X | X | X | X | X | X | X | X | X | X | ||
| Multiple Sclerosis Functional Composite (MSFC) | X | X | X | X | X | X | |||||||
| Expanded Disability Status Scale (EDSS)1 | X | X | X | X | X | X | |||||||
| Brain MRI | X5 | X5 | X5 | X5 | |||||||||
| Optical Coherence Tomography (OCT) | X | X | X | X | X | ||||||||
| Lumbar Puncture (optional)3 | X | X | X | ||||||||||
| Study Drug Dispensing6 | X | X | X | X | |||||||||
| Study Drug Accountability | X | X | X | X | X | X | X | X | X | X |
EDSS must be performed by a neurologist blinded to treatment assignment.
The baseline MRI must be approved by the MRI Reading Center before randomization.
If performed, the lumbar puncture must be done after the brain MRI.
The Baseline visit must occur within 45 days following the Screening visit.
Postpone MRI until 1 month after completion of any unscheduled corticosteroid treatment.
Study drug will be taken twice daily in the morning and evening. The first dose will be taken the evening of the baseline visit.
Cognitive tests include the Symbol Digit Modalities Test (the only CORE CDE cognitive test) and the Selective Reminding Test.
For subjects who are no longer taking study drug and are being followed on a semi-annual basis (Wk 24, 48, 72, 96), AEs will not be collected post study drug discontinuation. Existing AEs will be followed until the AE resolves or stabilizes.
If a pregnancy is discovered between regularly scheduled study visits, subjects should return for an unscheduled visit and a pregnancy test should be obtained. Once confirmed, the patient should be discontinued from the study and study drug should be returned.
At screening, plasma samples required for biomarkers ONLY. Plasma PK aliquot is omitted.
Study Objectives and Endpoints
The primary objectives are to evaluate the safety, tolerability and activity of ibudilast (100 mg/d) versus placebo administered orally in subjects with PMS. The primary outcome is change in whole brain atrophy as measured by Brain Parenchymal Fraction (BPF) over 96 weeks (Table 3).34
Table 3.
Endpoints and Measures.
| Endpoints | Measure |
|---|---|
| Primary Endpoint | |
| Whole Brain Atrophy (WBA) | Brain parenchymal fraction (BPF) analysis by MRI |
| Main Secondary Endpoints | |
| Diffusion Tensor Imaging (DTI) | DTI in descending pyramidal tracts |
| Magnetization Transfer Ratio (MTR) | MTR in the normal-appearing brain tissue |
| Optical Coherence Tomography (OCT) | Mean peripapillary retinal nerve fiber layer thickness |
| Cortical atrophy | Cortical Longitudinal Atrophy Detection Algorithm |
| Additional Secondary Endpoints | |
| Inflammatory Disease Activity |
|
| Disability |
|
| Cognitive Impairment |
|
| Quality of Life |
|
| Neuropathic Pain |
|
| Tertiary Endpoints | |
| Activity of Ibudilast at 48 weeks |
|
| Activity of Ibudilast at 96 weeks |
|
The main secondary outcomes are the activity of ibudilast at 96 weeks using advanced imaging measures DTI, MTR, OCT, and cortical atrophy. Additional secondary outcomes include MRI measures of inflammatory disease activity (T2 lesions and T1 black holes), clinical disability, cognitive impairment, Quality of Life, and neuropathic pain (Table 3). Two standard, validated measures of clinical disability - the Expanded Disability Status Scale (EDSS) and the modified 4-parameter Multiple Sclerosis Functional Composite (MSFC-4) - were chosen to evaluate potential clinical efficacy as well as to provide clinical measures for validation of the advanced imaging metrics. The MSFC-4 components in this trial are 25-foot timed walk, 9-hole peg test, Symbol Digit Modality Test (SDMT, which replaced the Paced Auditory Serial Addition Test previously used in the MSFC35), and the 2.5% low contrast visual acuity test.36 Cognitive function is assessed using the SDMT, which measures sustained attention/information processing speed, and the Selective Reminding Test (SRT), which measures episodic verbal memory. Since ibudilast may have beneficial effects on pain, and neuropathic pain is common in MS, the Brief Pain Inventory (BPI) is also included. A blinded rater who is separate from the treating neurologist, performs EDSS. EDSS certification was provided by NeuroStatus (Basel, Switzerland), and MSFC/SDMT certification and SRT training were provided by Cleveland Clinic.
Ideally, a Phase II trial in PMS should be conducted over a shorter interval than 2 years. Accordingly, tertiary objectives include the assessment of BPF, DTI, MTR, OCT, and cortical atrophy at 48 weeks. Additional advanced imaging assessments will also be analyzed at 96 weeks (Table 3). The optimal brain atrophy measurement tool is not known. Therefore, whole brain atrophy using both BPF and SIENA will be assessed and compared. Exploratory analyses will evaluate pharmacokinetics (PK) of ibudilast using a population PK approach. Serum, plasma, and (in a subset of subjects who consented) CSF will be evaluated for biomarkers (i.e., neurofilament light chain) of both ibudilast activity and MS disease progression.
Imaging
The primary and main secondary outcomes for the study are imaging, which requires standardized image acquisition. Standard protocols were developed and implemented for MRI and OCT acquisition. Imaging is obtained during the screening period and then every 24 weeks over the course of the 96-week study, for a total of five time points.
MRI
All MRIs are conducted using contemporary Siemens (Trio or Skyra) or GE (version 12X or higher) 3T systems. The image acquisition includes 3D spoiled gradient-recalled echo; proton density weighted and T2 weighted 2D turbo/fast spin-echo; 2D T2-weighted FLAIR; 3D spoiled gradient-recalled echo with selective excitation, with and without magnetization transfer pulse; 64-direction high angular resolution diffusion imaging (twice refocused spin echo, single-shot EPI readout for Siemens Trio; Monopolar Plus for Siemens Skyra; Stejskal-Tanner single-shot EPI readout for GE). Gadolinium is not used both to allow more time for advanced imaging modalities and because it doesn’t add significantly to therapeutic outcome measures in PMS beyond new or enlarging T2 lesions.
Scans are transmitted from each clinical site via a secure server to the primary imaging coordinating center (NeuroRx, Montreal, Canada), where overall MRI and MTR quality control is conducted. Images are then transmitted to the atrophy and DTI laboratories (Cleveland Clinic, Cleveland, OH), where additional quality control assessment is conducted. Common reasons to reject a scan include incorrect MRI acquisition parameters, incorrect head angle, and motion.
Scanner performance is monitored using monthly scans of the Biomedical Informatics Research Network (BIRN) phantom using an abbreviated imaging protocol. Imaging physicists visited every imaging site prior to subject enrollment to review the study scanning protocol and phantom scan acquisition. In addition to analysis of monthly phantom scans, quality assurance assessments are conducted on every study subject scan to ensure adequate acquisition of high quality images. Image acquisition and quality assurance is being overseen by a collaboration of three image analysis coordinating centers.
Optical Coherence Tomography
OCT is being obtained from all enrolled subjects using Zeiss Cirrus or Heidelberg Spectralis instruments. Sites are required to provide OCT consistently on the same instrument for the duration of the study. Five OCT scans are planned for each subject, at screening, week 24, week 48, week 72 and week 96. OCT analysis is being coordinated through a central OCT reading center, with specifications on agreement among certified graders who select from all available individual scans at each time point. Peripapillary and Macular scans are obtained using Optic Disc Cube 200 × 200 and Macular Cube 512 × 128 respectively on Cirrus instruments, and Glaucoma RNFL 768 × 496, Axonal RNFL-N 1536 × 496, and Posterior Pole 61 × 768 on Spectralis instruments. Scan quality parameters are as specified by the OSCAR-1B criteria and those scans with artifacts due to eye movement or blinking are excluded. The main OCT outcome is change in pRNFL thickness over the course of the trial. In addition, group analysis of total macular volume and ganglion cell/inner plexiform thickness (initial subanalysis only on scans from Zeiss Cirrus, followed by platform-agnostic segmentation in all subjects) is also planned.
Safety Assessments
Inclusion and exclusion criteria provided initial steps to ensure enrollment of only MS subjects who are otherwise relatively healthy. Screening evaluation included review of medical history, physical examination, laboratory testing, ECG, and administration of the Beck Depression Inventory Fast Screen (BDI-FS) to assess for severe depression. During the course of the trial, safety is assessed through interval clinical history, physical examination, laboratory testing, ECG, and suicidality screening. Safety assessments related to MS include neurologic assessments using the EDSS and MSFC, and clinical assessment of brain MRIs by a blinded central neuroradiologist. The treating neurologist is notified if ≥ 5 new or enlarging T2 lesions are observed by the central neuroradiologist.
Safety oversight is provided by an NINDS-appointed Independent Medical Monitor (IMM), an NINDS-appointed Data Safety Monitoring Board (DSMB), and the NeuroNEXT Central IRB. After 30 patients had been enrolled for at least 30 days, and again after 60 patients had been enrolled for at least 60 days, and at quarterly intervals thereafter, the IMM reviews pooled (i.e., blinded to treatment) safety data provided by the DCC. At approximately six-month intervals and additionally when considered necessary, the DSMB meets to review blinded safety data, including adverse events and serious adverse events (SAE). Alerts are provided to the treating neurologist for laboratory, ECG, suicidality, and MRI outcomes.
Statistical Methods
Analysis Populations
The population of inference is based on the modified Intent-to-Treat (mITT) principle, meaning that all randomized subjects who receive at least one dose of study medication and have at least one efficacy assessment in the double-blind phase are analyzed. Subjects will be analyzed based on the treatment to which they are randomized. Within this population, the Per Protocol (PP) Population includes all mITT subjects who satisfy the following conditions: receive assigned study medication as randomized, have 75% –125% compliance, and have no major protocol deviations that would be expected to impact data integrity, determined by a blinded data review.
Endpoint Analysis
All imaging endpoints will be statistically evaluated using linear mixed models (LMM)37 focusing on change over time. The primary analysis will be conducted using a mITT analysis. Since this is a phase II proof of concept study, the threshold for statistical significance will be set at the 0.10 level. To account for baseline imbalance due to randomization vagaries, the baseline group means (intercepts) in the statistical analysis will be constrained to be equal. Sensitivity analyses of brain atrophy will be conducted using brain atrophy as measured by SIENA and adjustment for covariates with potential impact on atrophy that were unbalanced among treatment groups.
The utility of advanced imaging metrics for measuring efficacy of putative PMS therapies will be identified using data collected simultaneously from the same patients over two years. A direct comparison of different atrophy measures, MTR, DTI, and OCT will be made for variability, sensitivity to change over time, cost, ease of implementation, and correlation with clinical measures. The clinical measures will include disability and patient-reported outcomes. In addition, an exploratory analysis will be conducted to correlate imaging changes over the initial 6–12 months with clinical changes over 2 years. A multivariate version of the LMM will be used to simultaneously model two response variables.38 This will allow the statistical comparison of the slopes, for example, of brain atrophy and a patient-reported outcome to see if they are changing similarly over time. Through these analyses, recommendations regarding the most robust and practical outcome for implementation in future phase II trials in PMS will be made, acknowledging that different PMS therapies may affect advanced imaging metrics differently.
Interim and Safety Analysis
One interim efficacy analysis will be performed for this study when half the subjects have completed 96 weeks follow-up. The Lan-DeMets spending function approach with O’Brien-Fleming stopping boundaries along with a formal futility assessment will be used.39,40 If predictive power is below 20% at the time of the interim analysis, then the trial will be stopped for futility.
The main assessment of safety will involve a comparison of treatment-related SAE’s across the two treatment groups. The percentage of subjects who experience any treatment-related SAE in each group will be assessed by using a chi-square test, and the rates of treatment-related SAE’s will be assessed using a Poisson regression model. Laboratory assessments over time across the two groups will be evaluated using a LMM. Adverse events will be further summarized by severity and by relationship to study drug. The summary will be limited to treatment-emergent AEs.
The safety analyses will be conducted using the mITT principle. Adverse events (AEs), discontinuation due to AEs, and serious adverse events (SAE) will be summarized by presenting, for each treatment group, the number and percentage of subjects with any AE, and AEs by system organ class and preferred term.
Sample Size Justification
Estimated required sample for the primary objective was computed based on published studies and our own pilot data using analytic formulas for LMM analysis.41 Using a different atrophy metric and only 2 time points, the ibudilast RRMS Phase 2 trial observed a 33%–36% slowing in brain atrophy.3 The pilot data (considered the control group) consisted of N = 36 RR- and SPMS participants with up to 3 annual BPF measures from the same 3T scanner at the Cleveland Clinic. A treatment effect of 30%–50% reduction in atrophy progression was assumed, which is similar to a previous sample size estimation study.42 A 10% drop-out rate was assumed and sample sizes were inflated by this percentage. Based on these assumptions, a sample size of N =125 subjects per treatment arm provides power close to 80% for effects of 33% or larger (assuming a type I error rate of 0.10).
Results
Study Enrollment and Randomization
The study was registered on ClinicalTrials.gov on 29 October 2013 (NCT01982942). CIRB approval for the final protocol with FDA required amendments was obtained on 8 October 2013. CIRB approval for the first site was obtained on 4 November 2013 and for the final site on 19 June 2014, a delay due to difficulties in bringing the site’s scanner into acceptable performance.
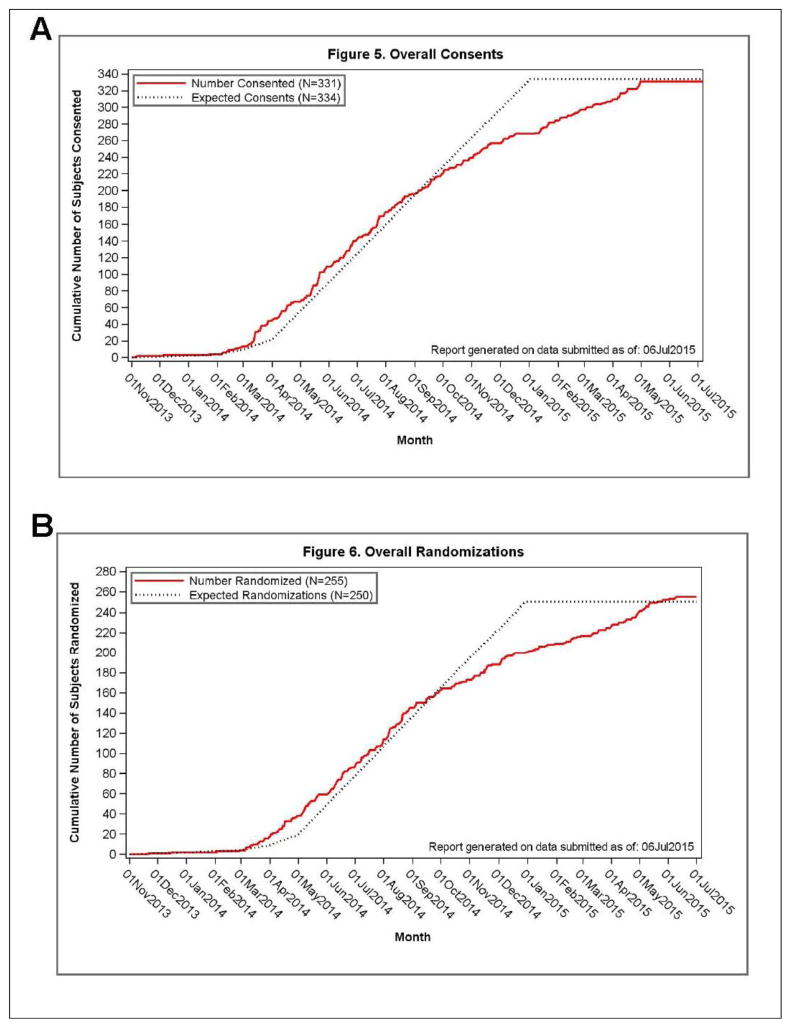
The first subject was enrolled on 5 November 2013, and randomized on 21 November 2013. The last study subject was enrolled on 30 April 2015 and randomized on 9 June 2015. Subjects were recruited from the MS practices at the individual study sites. In addition, the study was promoted by the National MS Society (NMSS) and National Public Radio (NPR). Study sites enrolled 3 to 27 subjects, and randomized 2 to 22 subjects. Average subject enrollment was 0.74 subjects per site per month over the course the study, according to each site’s specific open enrollment period (Figure 2).
Figure 2.
Overall rate of enrollment (A) and randomization (B) in the SPRINT-MS trial.
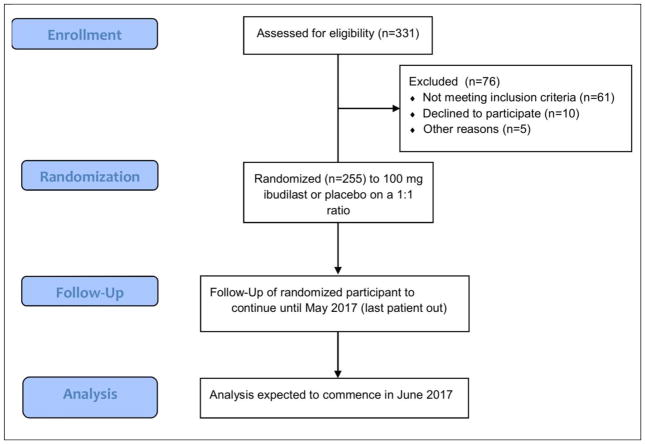
Of 331 subjects enrolled, 76 failed screening (Figure 3, Table 4). Almost half failed screening due to laboratory abnormalities. Twelve (15.8%) failed screening because of inadequate baseline MRI (typically due to excessive motion). As an imaging-driven trial, with primary and most of the main secondary outcomes derived from MRI, inadequate baseline MRI was included in the exclusion criteria to help ensure interpretable outcome data. Seven (9.2%) failed screening due to severe depression as measured by the BDI-FS.
Figure 3.
SPRINT-MS trial flow diagram (per CONSORT specifications).
Table 4.
Main reasons for screen failure
| Abnormal laboratory results | 44.7% |
| Unacceptable quality MRI | 15.8% |
| Unwilling to comply with study procedures | 13.1% |
| No documented disease progression | 11.8% |
| Recent corticosteroid use | 10.5% |
| Alcoholism or Depression | 9.2% |
| Other reasons | 6.6% |
Note: total is >100% because some subjects had more than one reason
255 subjects were randomized onto active treatment or placebo. Randomized subjects were 53.7% female, mean age 55.7 y (±7.3), and mean EDSS 5.1 (±1.2) (Table 5). Randomized subjects were evenly split between PPMS and SPMS. 80 subjects (31.4%) were receiving treatment with IFNβ1 or glatiramer acetate at the time of screening. 90 of the randomized subjects (35.3%) opted to undergo lumbar puncture for the CSF sub-study. The last patient is projected to finish in May 2017
Table 5.
Baseline Characteristics of SPRINT-MS and comparison with other contemporary progressive MS trials
| Characteristics | SPRINT-MS | INFORMS43 | EXPAND44 | ORATORIO45 | ASCEND46–48 |
|---|---|---|---|---|---|
| Experimental Therapy | Ibudilast | Fingolimod | Siponimod | Ocrelizumab | Natalizumab |
| Date completed or published | (ongoing) | 2014 | (ongoing) | 2015 | 2015 |
| Inclusion Criteria | |||||
| Trial Population | SPMS and PPMS | PPMS | SPMS | PPMS | SPMS |
| Disease Duration | Unspecified | 2–10 years | Unspecified | 15 years | Unspecified |
| Evidence of disability progression | 2 years; EDSS, 25FW or 9HPT | 2 years | 2 years; EDSS | Not available | 1 year |
| Age | 21–65 years | 25–65 years | 18–60 years | 18–55 years | 18–58 years |
| Other | positive brain MRI | positive brain MRI; spinal cord MRI or CSF | evidence of progression to progressive stage 6 months prior to study start | ≥ 2 pyramidal function score; positive oligoclonal bands | MSSS ≥ 4; onset of SPMS 2 years prior |
| EDSS | 3.0 – 6.5 | 3.5–6.0 | 3.0 – 6.5 | 3.0 – 6.5 | 3.0 – 6.5 |
| Baseline Characteristics | |||||
| No. of subjects | 255 | 823 | 1651 | 732 | 889 |
| Male | 118 (46.3) | 425 (51.6%) | 660 (40%) | 371 (50.7%) | 340 (38.2) |
| Female | 137 (53.7) | 398 (48.4%) | 989 (60%) | 361 (49.3%) | 549 (61.8) |
| Age Mean (SD) | 55.6 (7.3) | 48.5 (8.4) | 48.0 (7.9) | 44.6 (NA) | 47.2 (7.59) |
| SPMS Number (%) | 122 (47.84) | Not applicable | 100 % SPMS | Not applicable | 100% SPMS |
| PPMS Number (%) | 133 (52.16) | 100 % PPMS | Not applicable | 100 % PPMS | Not applicable |
| Disease Duration Mean (SD) | 11.88 (9.05) | 5.8 (2.4) | 16.8 (8.4) | 6.48 (3.89) | 16.5 (7.7) |
| EDSS Mean (SD) | 5.14 (1.15) | 4.67 (1.03) | 5.4 (1.1) | 4.7 (1.17) | 5.6 (0.9) |
| 25 FW Mean (SD) | 16.55 (22.13) | 9.08 (6.87) | 17.4 (28.2) | Not available | 13.47 (7.96) |
| 9HPT (SD) Dominant Hand Mean | 34.08 (28.15) | 28.65 (14.62) | 34.2 (32.7) | Not available | 34.86 (25.53) |
| MRI | |||||
| T2 lesion volume mm3, mean | 10,350 (11,140) | 9,794.5 (11,943.48) | 15,322 (16,062) | 12,100 | 11,920 |
| Normalized brain volume (SD) | 0.8042 BPF (0.0295) | 1491.4 cm3 (mean) (85.5) | NA | 1464.99 cm3 (mean) (85.96) | 1423.94 cm3 (mean) (83.26) |
Discussion
The SPRINT-MS study was cooperatively developed and implemented by the NeuroNEXT network and is the first therapeutic trial to be conducted by the network. NeuroNEXT aims to accelerate the development of neurologic therapies through the efficient and effective conduct of clinical trials. Key aspects of NeuroNEXT include the prequalification of clinical sites, centralized ethics oversight, and master clinical trial agreements. Importantly, each site voluntarily opted to participate in the trial, affirmatively stating their interest and ability to enroll and conduct the trial at their site.
Study start-up in SPRINT-MS was very successful. Site surveys for general interest, clinical capabilities, and imaging facilities were conducted prior to funding submission using the network-funded infrastructure at each site. These surveys provided a clear understanding of site interest and capacity to conduct the trial. Centralized IRB oversight accelerated timely ethics approval at each site and overall trial start-up. All of the sites were academic medical centers, which traditionally have resisted centralized ethics oversight. However, each institution agreed to participate in central IRB prior to involvement in NeuroNEXT.49 Delays in overall site activation were mostly due to problems with MRI certification, which was considered a worthy delay to help ensure the highest quality imaging data.
Site enrollment varied from 3 to 27 subjects, and randomization varied from 2 to 22 per site. Recruitment assistance by patient advocacy organizations such as the National MS Society and media such as National Public Radio seemed helpful in supporting recruitment although the relative effectiveness of these efforts is not known. Nonetheless, enrollment of 0.74 subjects per site per month over the course of the study allowed for complete enrollment over 18 months. Enrollment rates from other MS studies are not easily obtained, although this rate appears faster than most comparable MS trials.
The baseline characteristics of the SPRINT-MS trial are comparable to other PMS trials in many ways (Table 5). The sex distribution is less heavily weighted towards women than what is seen across MS in general, but is similar to that seen in progressive MS cohorts,50,51 including many other PMS trials (Table 5). The EDSS range for inclusion criteria was similar to other studies and resulted in an average EDSS that was similar to many PMS trials.
The maximum allowed age of 65 years is similar to many of the older PMS studies, but older than many of the more recent PMS trials. For example, ORATORIO, ASCEND, and EXPAND capped enrollment to between 55 and 60 years. Some trials limited disease duration. For example, INFORMS limited disease duration to 2–10 years, and ORATORIO to 5–15 years. As a result, the average age at enrollment in SPRINT-MS is 55.8, which is quite a bit older than the 44.6 to 48.5 years in other contemporary PMS trials. The mean disease duration in the SPRINT-MS trial is about 12 years, which is higher than that reported in INFORMS and ORATORIO. However, when compared to ASCEND and EXPAND (neither of which specified disease duration in their enrollment criteria), the mean duration for SPRINT-MS is about 5 years shorter. Although average EDSS is similar to other trials, the baseline functional performance as measured by 25FW and 9HPT is on the slower end of all of the listed trials. The impact of studying an older and somewhat more impaired PMS patient population is not known, but may make it more difficult to detect a treatment effect by ibudilast due to normal aging and accumulated MS disease injury.
SPRINT-MS is designed to evaluate the safety and efficacy of ibudilast as a treatment for PMS while simultaneously validating five different imaging biomarkers as outcome metrics for use in PMS trials. Progress in development of an effective therapy for PMS has suffered from both a lack of a viable clinical therapeutic candidate and absence of a validated biomarker for use in proof-of-concept clinical trials. Preliminary clinical studies indicate that ibudilast may be a promising therapy for PMS, prompting the implementation of this multicenter phase II trial. This trial also provides an ideal platform to compare the advanced imaging metrics whole brain atrophy, MTR, DTI and cortical atrophy, as well as OCT, and evaluate their correlation with validated clinical measures. To our knowledge, the SPRINT-MS trial is the first to use all five imaging metrics simultaneously in all patients enrolled in a multi-center clinical trial. The large sample size and 2 years of follow-up will provide an opportunity to conduct validation studies of the clinical relevance of changes in these imaging metrics. Comparing different imaging metrics simultaneously may identify the best modality for future PMS trials. The data may also identify a composite measure of multiple imaging modalities. However, there may not be a single ideal imaging modality for all PMS therapies, as different mechanisms of action may be measured differently across these imaging modalities.
The SPRINT-MS trial will also provide significant insights regarding the logistical challenges of implementing a highly advanced imaging protocol in a multi-centered clinical trial. In the past, metrics such as MTR and DTI have not been used routinely in multicenter studies, presumably due to the complexity involved in standardizing acquisition protocols, conducting ongoing QA assessments of scanner performance, and performing a robust centralized analysis. Additionally, high angular diffusion imaging is performed differently on each MRI platform, making the design of a harmonized imaging protocol challenging. This implementation has benefited from our previous experience harmonizing DTI acquisition across different imaging platforms.52,53 OCT data from different platforms also faces similar challenges in image acquisition and the combining of results across platforms, although previous studies have identified solutions to these challenges.54
An important aspect of the SPRINT-MS study is that while it is a 96 week trial, clinical and imaging endpoint assessments are conducted at 24, 48, and 72 weeks. These periodic assessments will allow us to determine the earliest time when treatment effects can be observed. Even if there is no beneficial effect of ibudilast, we can still utilize this dataset to conduct sample size estimates with each imaging modality for application to future PMS clinical trials. Correlating the five imaging outcome metrics with validated measures of clinical disability such as the EDSS and MSFC will not only allow us to assess the concurrent validity of each metric, but also their predictive validity.
Conclusions
The SPRINT-MS trial will evaluate both the efficacy and safety of ibudilast, as well as directly compare five imaging metrics for use in Phase 2 trials of progressive MS. The SPRINT-MS trial will advance how clinical trials in PMS are conducted. Defining validated and robust imaging measures for PMS that are more sensitive and reliable than conventional MRI will potentially result in more rapid trial completion with fewer participants. A similar need for robust biomarkers exists for other neurodegenerative diseases, including Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and Huntington’s disease. Imaging metrics validated in this trial could potentially be used to characterize tissue injury in these other neurological conditions as well. By directly comparing leading candidate measures of brain tissue integrity, we will advance our ability to screen putative neuroprotective and neurorepairative therapies for potential efficacy through small, focused clinical trials, with potential applications across a broad range of neurodegenerative disorders.
Acknowledgments
This study was funded by the NINDS (U01NS082329) and NMSS (RG 4778-A-6). Ibudilast study drug was provided at no cost by MediciNova Inc. The NeuroNEXT Network is supported by the NINDS (Central Coordinating Center: U01NS077179, Data Coordinating Center: U01NS077352). We thank Dr. Elizabeth McNeil, the former program manager at NINDS, for her steady support through the trial development and start-up process. This study was made possible by the dedication and support of people with MS who volunteered to participate as well as their families and friends who support their involvement in this study.
Abbreviations
- BPF
brain parenchymal fraction
- CCC
clinical coordinating center
- CIRB
Central Institution Review Board
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DCC
data coordinating center
- DMT
disease modifying therapy
- DTI
diffusion tensor imaging
- EAE
experimental autoimmune encephalomyelitis
- EDSS
expanded disability status scale
- FDA
Food and Drug Administration
- GA
glatiramer acetate
- IFN
interferon
- MIF
migration inhibitory factor
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- MTR
magnetization transfer ratio
- NINDS
National Institute of Neurological Disorders and Stroke
- OCT
optical coherence tomography
- PI
principal investigator
- RNA
ribonucleic acid
- RNFL
retinal nerve fiber layer
- RRMS
relapsing-remitting multiple sclerosis
- PMS
progressive multiple sclerosis
- PPMS
primary progressive multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
- WBA
whole brain atrophy
Author Disclosures
Dr. Fox has received consulting fees from Biogen Idec, GlaxoSmithKline, Novartis, Questcor, Teva, and Xenoport; research support from Novartis. Dr. Coffey has received consulting fees from ZZ Biotech, LLC. Dr. Cudkowicz has received consulting fees from Astra Zenica, Cytokinetics, Biohaven, Denali, Biogen-Idec, and Genentech. Dr. Goodman has received received consulting fees from Acorda Therapeutics, Actelion, Biogen-Idec, Genzyme-sanofi, GW Pharma, Mylan, Novartis, Teva, and Vaccinex for consulting services; research support from Acorda Therapeutics, Avanir, Biogen Idec, EMD Serono, Genzyme-sanofi, Novartis, Ono, Roche, Sun, Takeda, and Teva. Dr. Klawiter has received consulting fees from Genentech, Biogen-Idec and Mallinckrodt; research support from Roche, Biogen, Atlas 5D and EMD Serono. Dr. Naismith has received consulting fees from Alkermes, Acorda, Biogen-Idec, Genentech, Genzyme, EMD Serono, Novartis, Pfizer and Questcor; and speaking fees from Acorda, Biogen-Idec and Genzyme. Dr. Bermel has received consulting fees from Biogen, Novartis, Genentech, and Genzyme; research support from Biogen and Novartis. Dr. Long has received consulting fees from Neurophage Inc., Azevan Inc. and Roche Pharma. Dr. Agius has received consulting fees from Acorda Therapeutics, Bayer, Biogen, Genzyme, MedImmune, Novartis and Teva NeuroScience. Dr. Bashir has received research support from Novartis, BiogenIdec, and MedImmune. Dr. Cohen has received consulting fees from EMD-Serono, Genentech, Genzyme, Novartis and Teva; research support from Biogen-Idec, Hoffman La Roche, Genentech and Novartis. Dr. Coyle has received consulting fees from AbbVie, Accordant, Acorda, Bayer, Biogen-Idec, Genentech/Roche, Genzyme/Sanofi, Mallinckrodt, Novartis, Serono, Teva; research support from Actelion, Genentech/Roche, NINDS, Novartis, and Opexa. Dr. Dewitt has received consulting and speaking fees from Novartis and Teva. Dr. Flores has received speaking feas from Biogen and Teva; served on the advisory board for Biogen. Dr. Goldman has received consulting fees from Genzyme, Sarepta, Acorda, Novartis and Biogen-Idec; research support from Novartis and Biogen. Dr. Jubelt has received speaking fees from CMEducation Resources LLC and Prime Education Inc; grant support from BiogenIdec, Sanofi-Aventis, Genzyme, Novartis, Grifols and Receptos. Dr. Lynch has received support from by Biogen, Teva, Novartis, Opexa, Genzyme, Roche, Genentech, Sun Pharma and Acorda. Dr. Miravalle received consulting fees from Bayer, Genzyme, Questcor/Mallinckrodt, Biogen-Idec, Consortium of MS Centers, Rocky Mountain MS Center and Genentech. Dr. Moses has received consulting fees from Biogen-Idec, Teva Neuroscience, EMD-Serono, Medimmune, Novartis, Genzyme and Bayer; speaking fees from Biogen-Idec, Teva Neuroscience, EMD Serono, Bayer and Genzyme. Dr. Ontaneda has received consulting fees from Genzyme, Novartis, Teva, and Mallinckrodt; and research funding from the National Institutes of Health, Genzyme, and Novartis. Dr. Perumal has received consulting fees from Biogen-Idec and Genzyme; speaking fees from Biogen-Idec, Teva, Genzyme and Acorda; research support from Genzyme. Dr. Racke has received consulting fees from EMD Serono, Genentech/Roche, Biogen-Idec, Novartis and Teva Neuroscience. Dr. Repovic served as advisor for Anvil biosciences, Biogen, EMD Serono, Genzyme/Sanofi, Novartis and Teva; speaker bureau member for Biogen, EMD Serono, Genzyme/Sanofi, Novartis, Pfizer and Teva; and received research support from Novartis. Dr. Riley has received consulting fees from Biogen-Idec, Genzyme, Novartis and Teva. Dr. Severson has received consulting fees from Biogen-Idec, Genentech and Novartis; speaking fees from Foundation of Neurologic Diseases and MS cure fund. Dr. Weinstock-Guttman has received consulting and speaking fees from Biogen Idec, Teva Neuroscience, EMD Serono, Novartis, Genzyme, Sanofi and Genentech; research support from Biogen Idec, Teva Neuroscience, EMD Serono, Novartis, Genzyme, Sanofi, Genentech and Mallinckrodt Pharmaceuticals, Inc; serves as an editorial board memeber for BMJ Neurology, Journal of International MS and CNS Drugs. Dr. Yadav has received consulting fees from Biogen-Idec and Genentech; research support from the Department of Veterans Affairs, NIH, National Multiple Sclerosis Society (NMSS) Foundation, Nancy Davis Center Without Walls Foundation, and Biogen-Idec; salary support by the Tykeson Family through a Term Professorship in Wellness Research. Dr. Zabeti has received speaking fees from Acorda, Biogen, and Genzyme/Sanofi; research support from Actelion, Genentech/Roche, Novartis, and Opera. Drs. Conwit, Delgado, Giesser, Lava, Natarajan, Ramachandran, Shinnar, Suski, Mr. Gleason, Mr. Yankey, Ms. Ashokkumar, Ecklund, Koepp, McGovern, Skaramagas and Thornell report no competing interests.
Footnotes
Author Contributions
RJF contributed to study conception and design; protocol writing; trial start-up, implementation, management and oversight; manuscript preparation and review. RJF, CSC, MEC, TG, AG, ECK, KM, MM, RC, and RN are members of protocol steering committee. CSC, DE, MK, JL and JY are DCC staff and contributed to study start-up; various DCC tasks, oversight of DCC; statistical analysis; manuscript review. MEC, MM, AA and BT are CCC staff and contributed to study start-up, various CCC tasks, oversight of CCC; manuscript review. TG is patient advocate and member of protocol steering committee and contributed to manuscript review. RB contributed towards OCT imaging oversight and manuscript review. SR contributed to manuscript preparation and review. SN and TS assist RJF in study management; manuscript preparation, review. AG, ECK, RN, MA, KB, BC, PC, DD, SD, AF, BG, MG, BJ, NL, SL, AM, HM, DO, JP, MR, PR, CR, SS, SS, VS, BWG, VY and AZ are site PIs and reviewed the manuscript. All authors read and approved the final version of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cottrell DA, Kremenchutzky M, Rice GP, Hader W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 6. Applications to planning and interpretation of clinical therapeutic trials in primary progressive multiple sclerosis. Brain. 1999;122:641–7. doi: 10.1093/brain/122.4.641. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed 17 February, 2016];International Progressive MS Alliance: Facts and Figures. 2015 at http://www.progressivemsalliance.org/progressive-ms/facts-and-figures/
- 3.Barkhof F, Hulst HE, Drulovic J, Uitdehaag BM, Matsuda K, Landin R. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74:1033–40. doi: 10.1212/WNL.0b013e3181d7d651. [DOI] [PubMed] [Google Scholar]
- 4.Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–904. doi: 10.1517/14656560903426189. [DOI] [PubMed] [Google Scholar]
- 5.Ledeboer A, Jekich BM, Sloane EM, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–98. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–50. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- 7.Cho Y, Crichlow GV, Vermeire JJ, et al. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A. 2010;107:11313–8. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson LC, Hastings SF, McPhee I, et al. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Chen RW, Williams AJ, Liao Z, Yao C, Tortella FC, Dave JR. Broad spectrum neuroprotection profile of phosphodiesterase inhibitors as related to modulation of cell-cycle elements and caspase-3 activation. Neurosci Lett. 2007;418:165–9. doi: 10.1016/j.neulet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Inacio AR, Ruscher K, Leng L, Bucala R, Deierborg T. Macrophage migration inhibitory factor promotes cell death and aggravates neurologic deficits after experimental stroke. J Cereb Blood Flow Metab. 2011;31:1093–106. doi: 10.1038/jcbfm.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manrique-Hoyos N, Jurgens T, Gronborg M, et al. Late motor decline after accomplished remyelination: impact for progressive multiple sclerosis. Ann Neurol. 2012;71:227–44. doi: 10.1002/ana.22681. [DOI] [PubMed] [Google Scholar]
- 12.Nakamizo T, Kawamata J, Yoshida K, et al. Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res. 2003;71:485–95. doi: 10.1002/jnr.10483. [DOI] [PubMed] [Google Scholar]
- 13.Bhargava P, Mowry EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14:492. doi: 10.1007/s11910-014-0492-2. [DOI] [PubMed] [Google Scholar]
- 14.Fox RJ, Thompson A, Baker D, et al. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult Scler. 2012;18:1534–40. doi: 10.1177/1352458512458169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yagi K, Tada Y, Kitazato KT, Tamura T, Satomi J, Nagahiro S. Ibudilast inhibits cerebral aneurysms by down-regulating inflammation-related molecules in the vascular wall of rats. Neurosurgery. 2010;66:551–9. doi: 10.1227/01.NEU.0000365771.89576.77. discussion 9. [DOI] [PubMed] [Google Scholar]
- 16.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–66. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 17.Chataway J, Alsanousi A, Chan D, et al. The MS-STAT trial: high dose simvastatin demonstrates neuroprotection without immune-modulation in secondary progressive multiple sclerosis (SPMS) - a phase II trial. ECTRIMS; 2012; Lyon, France. 2012. [Google Scholar]
- 18.Benedict RH, Carone DA, Bakshi R. Correlating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosis. J Neuroimaging. 2004;14:36S–45S. doi: 10.1177/1051228404266267. [DOI] [PubMed] [Google Scholar]
- 19.Edwards SG, Liu C, Blumhardt LD. Cognitive correlates of supratentorial atrophy on MRI in multiple sclerosis. Acta Neurol Scand. 2001;104:214–23. doi: 10.1034/j.1600-0404.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 20.Hohol MJ, Guttmann CR, Orav J, et al. Serial neuropsychological assessment and magnetic resonance imaging analysis in multiple sclerosis. Arch Neurol. 1997;54:1018–25. doi: 10.1001/archneur.1997.00550200074013. [DOI] [PubMed] [Google Scholar]
- 21.Lazeron RH, Boringa JB, Schouten M, et al. Brain atrophy and lesion load as explaining parameters for cognitive impairment in multiple sclerosis. Mult Scler. 2005;11:524–31. doi: 10.1191/1352458505ms1201oa. [DOI] [PubMed] [Google Scholar]
- 22.Rao SM, Glatt S, Hammeke TA, et al. Chronic progressive multiple sclerosis. Relationship between cerebral ventricular size and neuropsychological impairment. Arch Neurol. 1985;42:678–82. doi: 10.1001/archneur.1985.04060070068018. [DOI] [PubMed] [Google Scholar]
- 23.Feinstein A, Roy P, Lobaugh N, Feinstein K, O’Connor P, Black S. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology. 2004;62:586–90. doi: 10.1212/01.wnl.0000110316.12086.0c. [DOI] [PubMed] [Google Scholar]
- 24.Marrie RA, Fisher E, Miller DM, Lee JC, Rudick RA. Association of fatigue and brain atrophy in multiple sclerosis. J Neurol Sci. 2005;228:161–6. doi: 10.1016/j.jns.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Tedeschi G, Dinacci D, Lavorgna L, et al. Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci. 2007;263:15–9. doi: 10.1016/j.jns.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Janardhan V, Bakshi R. Quality of life and its relationship to brain lesions and atrophy on magnetic resonance images in 60 patients with multiple sclerosis. Arch Neurol. 2000;57:1485–91. doi: 10.1001/archneur.57.10.1485. [DOI] [PubMed] [Google Scholar]
- 27.Rudick RA, Cutter G, Baier M, et al. Use of the Multiple Sclerosis Functional Composite to predict disability in relapsing MS. Neurology. 2001;56:1324–30. doi: 10.1212/wnl.56.10.1324. [DOI] [PubMed] [Google Scholar]
- 28.The Lancet N. NeuroNEXT: accelerating drug development in neurology. Lancet Neurol. 2012;11:119. doi: 10.1016/S1474-4422(12)70008-X. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno T, Kurotani T, Komatsu Y, et al. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–11. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto T, Sakoda S, Fujimura H, Yanagihara T. Ibudilast, a phosphodiesterase inhibitor, ameliorates experimental autoimmune encephalomyelitis in Dark August rats. J Neuroimmunol. 1999;95:35–42. doi: 10.1016/s0165-5728(98)00251-3. [DOI] [PubMed] [Google Scholar]
- 31.Ledeboer A, Liu T, Shumilla JA, et al. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2006;2:279–91. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson AN, Wright M, Birath JB, et al. Ibudilast may reduce inattention associated wtih methampheramine use. Annual Meeting of the College on Problems of Drug Dependence; 2013; San Diego, CA, United States. 2013. [Google Scholar]
- 33.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher E, Cothren RM, Tkach JA, Masaryk TJ, Cornhill JF. Knowledge-based 3D segmentation of MR images for quantitative MS lesion tracking. SPIE Medical Imaging. 1997;3034:599–610. [Google Scholar]
- 35.Drake AS, Weinstock-Guttman B, Morrow SA, Hojnacki D, Munschauer FE, Benedict RH. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: replacing the PASAT with the Symbol Digit Modalities Test. Mult Scler. 2010;16:228–37. doi: 10.1177/1352458509354552. [DOI] [PubMed] [Google Scholar]
- 36.Balcer LJ, Baier ML, Cohen JA, et al. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003;61:1367–73. doi: 10.1212/01.wnl.0000094315.19931.90. [DOI] [PubMed] [Google Scholar]
- 37.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 38.Fieuws S, Verbeke G. Joint modelling of multivariate longitudinal profiles: pitfalls of the random-effects approach. Stat Med. 2004;23:3093–104. doi: 10.1002/sim.1885. [DOI] [PubMed] [Google Scholar]
- 39.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–52. doi: 10.1002/sim.4780131308. discussion 53–6. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 41.Yi Q, Panzarella T. Estimating sample size for tests on trends across repeated measurements with missing data based on the interaction term in a mixed model. Control Clin Trials. 2002;23:481–96. doi: 10.1016/s0197-2456(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 42.Altmann DR, Jasperse B, Barkhof F, et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology. 2009;72:595–601. doi: 10.1212/01.wnl.0000335765.55346.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016 doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 44.Kappos L, Bar-Or A, Cree B, et al. Siponimod (BAF312) for the treatment of secondary progressive multiple sclerosis (SPMS): Baseline characteristics of the EXPAND study population. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2015; Barcelona, Spain. 2015. p. Poster 649. [Google Scholar]
- 45.Montalban X, Hemmer B, Rammohan K, et al. Baseline Demographics and Disease Characteristics from ORATORIO, a Phase III Trial Evaluating Ocrelizumab in Patients with Primary Progressive Multiple Sclerosis. American Academy of Neurology (AAN); 2015; Washington, D.C., USA. 2015. p. Poster P7.017. [Google Scholar]
- 46.Mikol D, Freedman MS, Goldman MD, et al. ASCEND study of natalizumab efficacy on reducing disability in patients with secondary progressive multiple sclerosis: baseline demographics and disease characteristics. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2013; Copenhagen, Denmark. 2013. p. P1087. [Google Scholar]
- 47.Kapoor R, Arnold DL, Miller A, et al. Gray Matter Volume Correlates with Information Processing as Measured by the Symbol Digit Modalities Test (SDMT) but not with Physical Disability as Measured by the Expanded Disability Status Scale (EDSS) in Patients with Secondary Progressive Multiple Sclerosis (SPMS): Analysis of Baseline Correlations from the ASCEND Natalizumab Study. American Academy of Neurology; 2015; Washington, D.C. USA. 2015. p. P6.143. [Google Scholar]
- 48.Mikol D, Goldman MD, Hartung H, et al. The 9-Hole Peg Test (9HPT) Has a Stronger Correlation Than the Expanded Disability Status Scale (EDSS) With Patient-Reported Upper Extremity Impairment as Assessed Using Abilhand in Patients with Secondary Progressive Multiple Sclerosis (SPMS): Analysis of Baseline Data From the Ascend Study. American Academy of Neurology (AAN); 2015; Washington, D.C. USA. 2015. p. P7.222. [Google Scholar]
- 49.Hudson KL, Collins FS. Bringing the Common Rule into the 21st Century. N Engl J Med. 2015;373:2293–6. doi: 10.1056/NEJMp1512205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marrie RA, Yu N, Blanchard J, Leung S, Elliott L. The rising prevalence and changing age distribution of multiple sclerosis in Manitoba. Neurology. 2010;74:465–71. doi: 10.1212/WNL.0b013e3181cf6ec0. [DOI] [PubMed] [Google Scholar]
- 51.Giovannoni G. Advances in Clinical Neuroscience and Rehabilitation. Wiltshire: Whitehouse Publishing; 2012. Primary Progressive Multiple Sclerosis. [Google Scholar]
- 52.Fox RJ, Sakaie K, Lee JC, et al. A validation study of multicenter diffusion tensor imaging: reliability of fractional anisotropy and diffusivity values. AJNR Am J Neuroradiol. 2012;33:695–700. doi: 10.3174/ajnr.A2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnotta VA, Matsui JT, Liu D, et al. Brain Connect. 2012. Multi-Center Reliability of Diffusion Tensor Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warner CV, Syc SB, Stankiewicz AM, et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS One. 2011;6:e22947. doi: 10.1371/journal.pone.0022947. [DOI] [PMC free article] [PubMed] [Google Scholar]