Abstract
The transverse carpal ligament (TCL) is a component of the flexor pulley system of the wrist, keeping the flexor tendons in place by resisting their volar displacement. The purpose of this study was to investigate the in vivo biomechanical interaction between the TCL and flexor tendons in response to tendon tensioning with the wrist at various postures. In eight healthy subjects, the flexor digitorum superficialis and profundus tendons were tensioned by isometrically applying loads (5, 10, and 15 N) to the index finger while the wrist posture was at 20° extension, neutral, 20° flexion, and 40° flexion. The TCL and flexor tendons were imaged at the distal carpal tunnel cross section using ultrasound. The volar-dorsal positions of the tendons, TCL arch height, and TCL-tendon distances were calculated. With increasing wrist flexion, the flexor tendons moved volarly, the TCL arch height increased, and the TCL-tendon distances decreased, indicating that the flexor tendons contacted the TCL and pushed it volarly. The TCL-tendon interaction was amplified by the combination of finger loading and wrist flexion. This study provides in vivo evidence of the biomechanical interaction between the TCL and flexor tendons. Repetitive TCL-tendon interactions may implicate the interacting tissues and the median nerve resulting in tissue maladaptation and nerve compression.
Keywords: carpal tunnel, flexor tendons, transverse carpal ligament, ultrasound imaging
1 Introduction
The carpal tunnel is a passageway within the wrist for the median nerve and flexor tendons. The tunnel is bordered laterally, dorsally, and medially by the carpal bones, and volarly by the transverse carpal ligament (TCL). Passing through the carpal tunnel are nine flexor tendons for the flexor pollicis longus of the first digit and the flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP) of the second through fifth digits. The digital flexor tendons facilitate joint motion by transmitting a force, from the flexor muscles to their bony attachments, about each joint. Tensioning of the flexor tendons results in their migration in the transverse plane of the carpal tunnel [1, 2], moving volarly with wrist flexion and dorsally with wrist extension [3, 4].
The flexor tendons can biomechanically interact with their surrounding structures when they move in the carpal tunnel. Previous studies have focused on the interaction between the flexor tendons themselves or between the tendons and the median nerve [5–11]. However, the interaction between the flexor tendons and the TCL remains unclear. The TCL provides mechanical advantages for the flexor tendons, acting as a pulley for the tendons during wrist flexion [12] and maintaining the moment arms of the flexor tendons at the wrist to prevent bowstringing [13]. The functional role of the TCL brings about a biomechanical interaction between the ligament and the tendons. Implications of this tissue interaction have been suggested by histological adaptation of carpal tunnel tissues [14] and by the altered movement of the flexor tendons after transection of the TCL in carpal tunnel release surgery [13, 15].
Tissue interactions inside the carpal tunnel may implicate the median nerve as a result of direct mechanical contact and/or tissue remodeling. As the flexor tendons move towards the TCL, they may compress the median nerve situated between the tendons and ligament. Furthermore, the repetitive TCL-tendon interactions may lead to maladaptation of the carpal tunnel structure and its contents, culminating in the onset of carpal tunnel syndrome. For example, carpal tunnel syndrome has been associated with pathological changes of the TCL [16–18] and tenosynovium [17, 19, 20].
Despite the clinical implication of the interactive carpal tunnel components, the explicit TCL-tendon interaction is unclear. Therefore, the purpose of this study was to investigate the in vivo biomechanical interaction between the TCL and flexor tendons in response to tensioning of the finger flexor tendons while the wrist assumed different postures. In this study, the biomechanical interaction was determined based on the relative positions of the tendons and the TCL; for example, an increased interaction would be indicated by the volar migration of the flexor tendons, increased TCL arch height, and decreased TCL-tendon distances. It was hypothesized that increased tendon tension and wrist flexion would result in an increased biomechanical interaction between the TCL and flexor tendons.
2 Methods
2.1 Human Subjects
Eight healthy, right-handed female subjects (age 26.9 ± 7.1 years) participated in this study. Individuals were excluded if they had any history of trauma or musculoskeletal/neuromuscular disorders affecting the right hand or wrist. The study procedures were approved by the local Institutional Review Board and written informed consent was provided prior to participation.
2.2 Experimental Setup
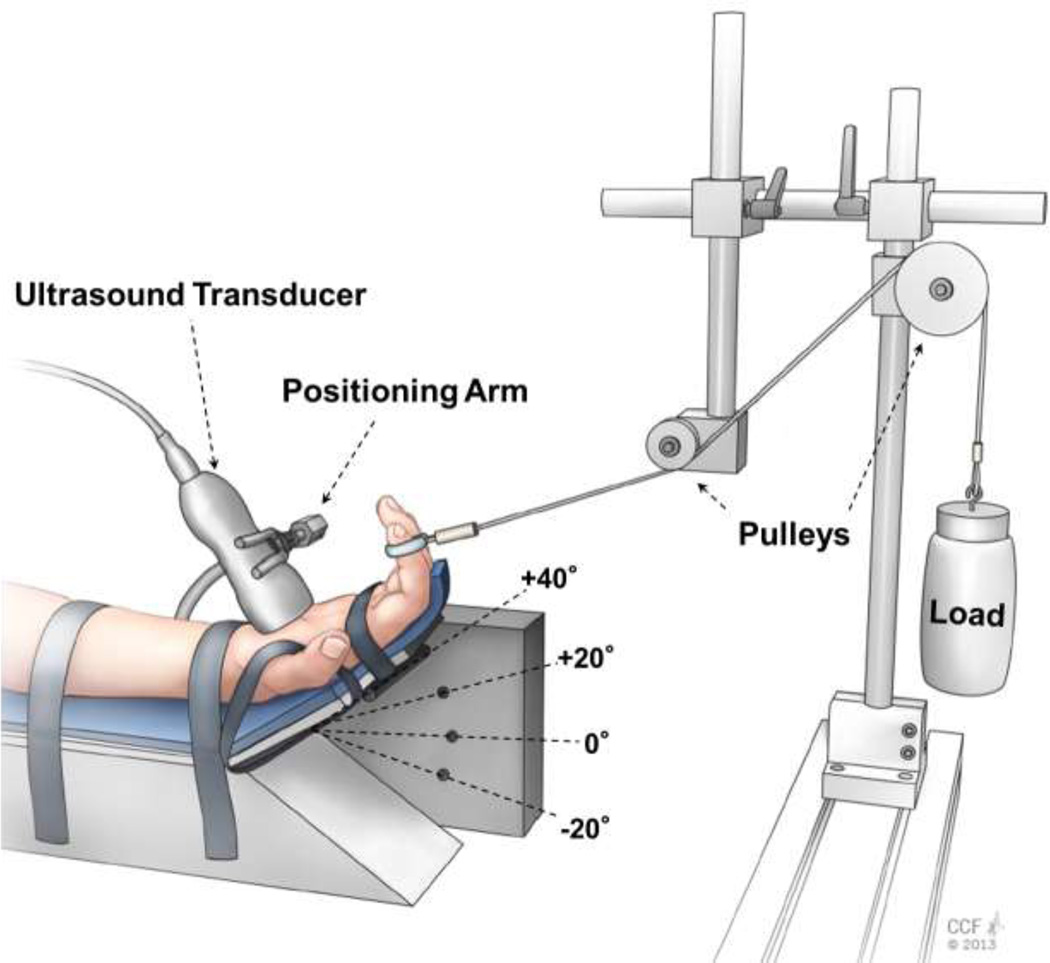
Each subject sat next to a testing table with the right shoulder abducted 30° and elbow flexed 90°. The right hand was supinated and secured to a custom apparatus that was designed to statically load the index finger while maintaining the wrist in various postures (Figure 1). The index, middle, ring, and little fingers were stabilized at the metacarpophalangeal joints and placed in a slightly flexed posture at the interphalangeal joints, while the thumb was abducted 30° in the palmar plane. The index finger was further positioned to ensure that its pad was perpendicular to the palm of the hand. A wire was looped around the index finger pad and redirected over the two adjustable pulleys of the custom apparatus. The wire’s line of action, from the index finger to the nearest pulley, was parallel to the palm of the hand.
Figure 1.
Experimental setup for ultrasound imaging at the distal carpal tunnel level while the wrist assumed a specific posture and the index finger was loaded
2.3 Experimental Procedures
The index finger of each subject was loaded to tension its FDS and FDP tendons while the wrist was positioned in 20° extension (−20°), neutral (0°), 20° flexion (+20°), and 40° flexion (+40°). The wrist postures were tested in a randomized order across subjects. Within each wrist posture the index finger was loaded, in randomized order, with 5 N, 10 N, and 15 N. The loads correspond to 10–50% of the maximal force of the index finger [31]. Each load was applied for 5 seconds via the wire looped around the index finger pad. For each posture-load condition, three trials were collected and a one-minute rest was provided between consecutive trials.
Ultrasound imaging of the distal carpal tunnel cross section was performed to capture the TCL and index finger flexor tendons under each posture-load condition. An ultrasound system (Acuson S2000 Siemens Medical Solutions USA, Mountain View, CA) with a linear array transducer (9L4, Siemens Medical Solutions USA, Mountain View, CA) was used with an imaging frequency of 7MHz, a depth of 3 cm, tissue harmonic imaging, and tissue equalization. A thick layer of ultrasound gel was placed on the palm of each subject at the distal carpal tunnel level; this thick gel layer was used to minimize the amount of force applied onto the palm by the transducer so as to avoid inadvertently influencing the morphology of the carpal tunnel. The transducer was oriented perpendicularly to the palm and adjusted so that the hook of the hamate, ridge of the trapezium, TCL, and index finger FDS and FDP tendons were clearly distinguishable on the ultrasound image. A positioning arm was used to stabilize the transducer’s position throughout the experiment. For each trial, a 10 second ultrasound video was captured. The ultrasound video was initiated when no load was applied to the index finger (0 N condition) and then 5-seconds after initiating the video the index finger was loaded with the predetermined load for 5 seconds. Ultrasound imaging was performed by a single operator (JLG) and the ultrasound videos were captured at a rate of 42 frames/sec.
2.4 Data Processing
Two image frames were extracted from each ultrasound video: (1) an image from the first 5 seconds of the video when the finger was unloaded (0 N condition) and (2) an image from the last 5 seconds of the video after the finger was loaded (5 N, 10 N, or 15 N condition). For each extracted image, the polygon tool in ImageJ (National Institutes of Health, Bethesda, MD) was used to manually trace the boundary of the FDS and FDP tendons of the index finger, and to determine the coordinates of each tendon’s centroid. Then, the volar and dorsal boundaries of the TCL were manually selected using the ImageJ multi-point tool to determine their respective coordinates. Furthermore, the coordinates of most volar points of hook of the hamate and ridge of the trapezium were identified.
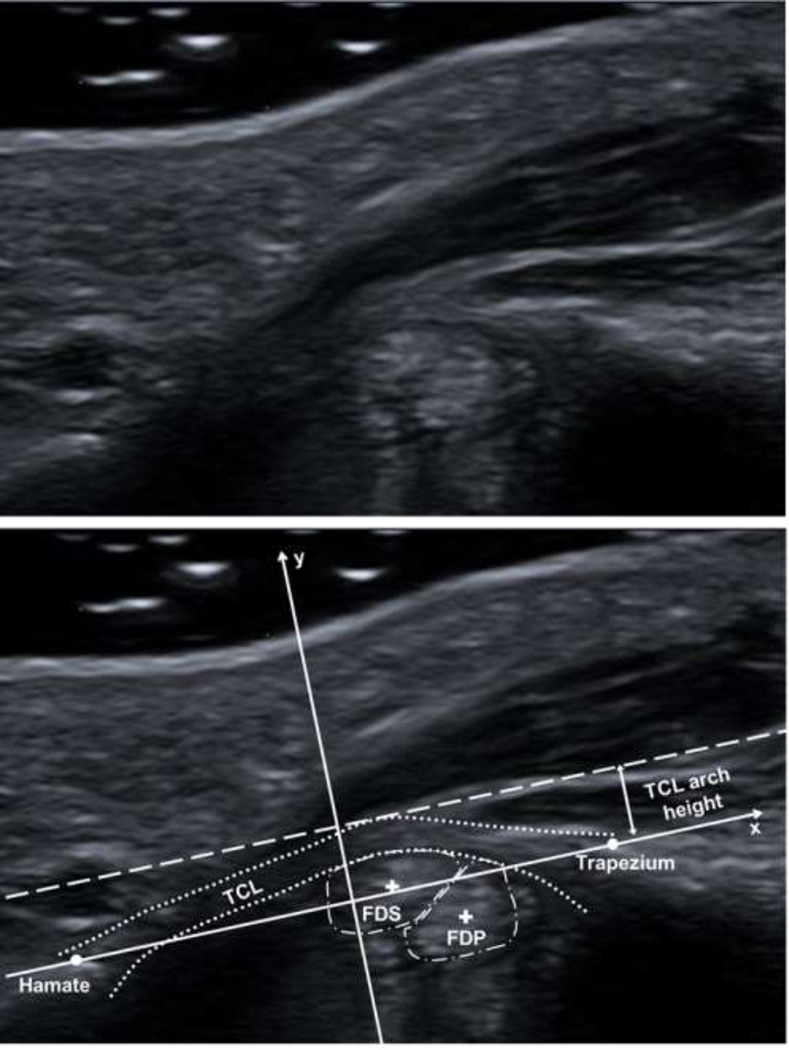
The obtained coordinates were used as inputs into a custom MATLAB program (Mathworks, Natick, MA). First, the program established an anatomical coordinate system whose x-axis (medial-lateral axis) was along the line connecting the hook of the hamate and ridge of the trapezium bony landmarks and whose y-axis (dorsal-volar axis) was the perpendicular bisector of the line between these two points (Figure 2). Then, the program transformed the coordinates identified in ImageJ to the defined anatomical coordinate system and calculated the outcome parameters. The outcome parameters were 1) FDS and FDP tendon positions, 2) TCL arch height, and 3) TCL-FDS and TCL-FDP distances. The FDS and FDP tendon positions were determined as the dorsal-volar location of each tendon’s respective centroid. The TCL arch height was defined as the distance between the apex of the TCL’s volar boundary and the x-axis. The TCL-tendon distance was calculated as the distance in the dorsal-volar direction between each tendon’s centroid and the dorsal boundary of the TCL.
Figure 2.
Unlabeled (top) and labeled (bottom) representative ultrasound images displaying the defined anatomical coordinate system (solid arrows), hook of the hamate, ridge of the trapezium, TCL (dotted line), index finger FDS and FDP tendons (dash-dot lines) and centroids (+), and TCL arch height
2.5 Statistical Analysis
Two-way (4 × 4) repeated measures ANOVAs were performed to determine the effects of wrist posture (−20°, 0°, +20°, +40°) and finger load (0 N, 5 N, 10 N, and 15 N) on the outcome parameters. Post-hoc Tukey’s tests were used for pair-wise comparisons. The significance level was α = 0.05. SigmaStat (v. 3.5, Systat Software Inc., San Jose, CA) was used to perform the statistical analyses.
3 Results
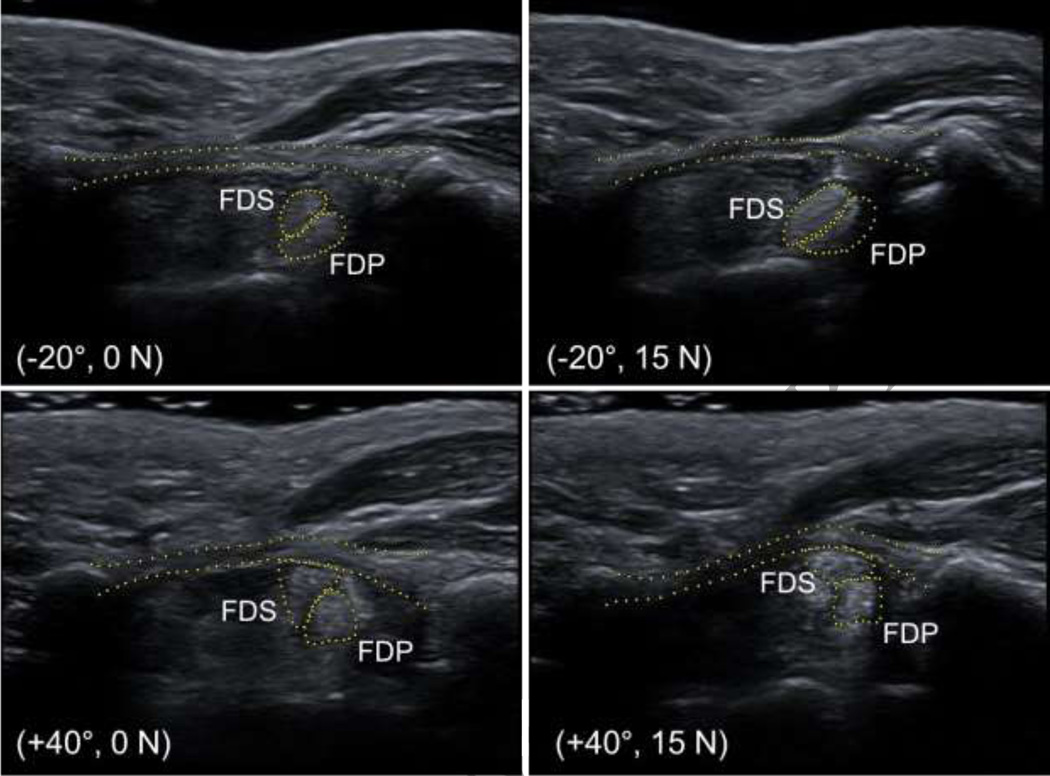
In general, wrist flexion caused the flexor tendons to move volarly, the TCL arch height to increase, and the TCL-tendon distances to decrease (Figure 3). Conversely, the flexor tendons moved dorsally with the wrist in extension. These phenomena were amplified when wrist flexion was combined with finger loading.
Figure 3.
Representative ultrasound images of the carpal tunnel for wrist postures of −20° and +40° under finger loads of 0 N and 15 N
3.1 Flexor Tendon Positions
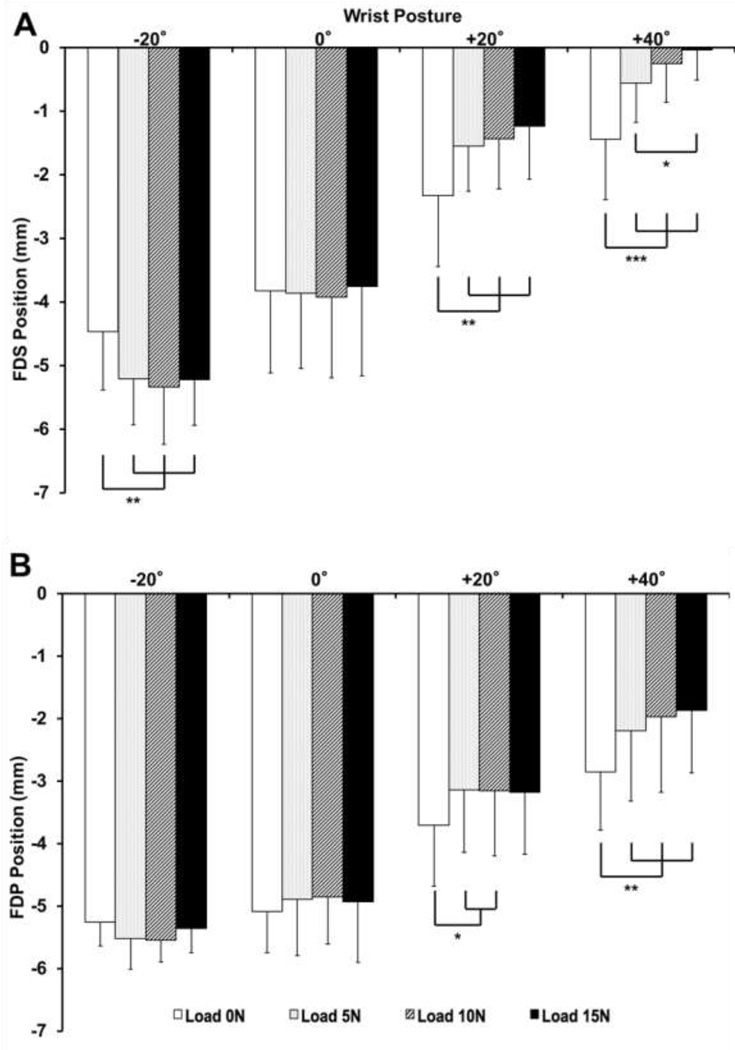
There was a statistical interaction between the factors of wrist posture and finger load on the position of the FDS tendon (p < 0.001). The FDS position was also significantly dependent on the main factors (p < 0.05). The FDS tendon moved dorsally in the extended wrist posture and volarly in the flexed postures, with increased movement as the finger load increased (Figure 4a). For the unloaded condition, the FDS tendon position at the +20° or +40° postures was significantly more volar than that at the −20° or 0° postures (p < 0.01). Under each finger load (5, 10, or 15 N) condition, pairwise comparisons showed that (a) the position of the FDS tendon at the +40° wrist posture was more volar than that at −20°, 0°, and +20° postures (p < 0.05), (b) the FDS tendon at the +20° posture was in a more volar position than that at −20° or 0° postures (p < 0.001), and (c) the FDS tendon at the 0° posture was in a more volar position than that at the −20° posture (p < 0.01).
Figure 4.
Dorsal-volar (+) positions of the FDS (A) and FDP (B) tendons for various wrist postures and finger loads (* p < 0.05, ** p < 0.01, and *** p < 0.001). Note: Statistical results for comparisons across loads within wrist posture are shown.
Similar to the FDS tendon, there was a statistical interaction between the wrist posture and finger load on the position of the FDP tendon (p < 0.001), as well as a main effect of each factor (p < 0.01). For the flexed postures of +20° or +40°, the position of the FDP tendon was dependent on finger loading (Figure 4b). For the 0 N and 5 N loads, the FDP tendon was significantly more volar at wrist postures of +20° and +40° relative to those of −20° and 0° (p < 0.01). For the 10 N load, pairwise comparisons showed that (a) the FDP tendon at the wrist posture of +40° was in a significantly more volar position than that at the −20°, 0°, and 20° postures (p < 0.05), and (b) the FDP tendon at the +20° posture was significantly more volar than that at the −20° and 0° postures (p < 0.001). For the 15 N load, the FDP tendon positions at all wrist postures were significantly different from each other (p < 0.01), except for those between the postures of −20° and 0°.
3.2 TCL Arch Height
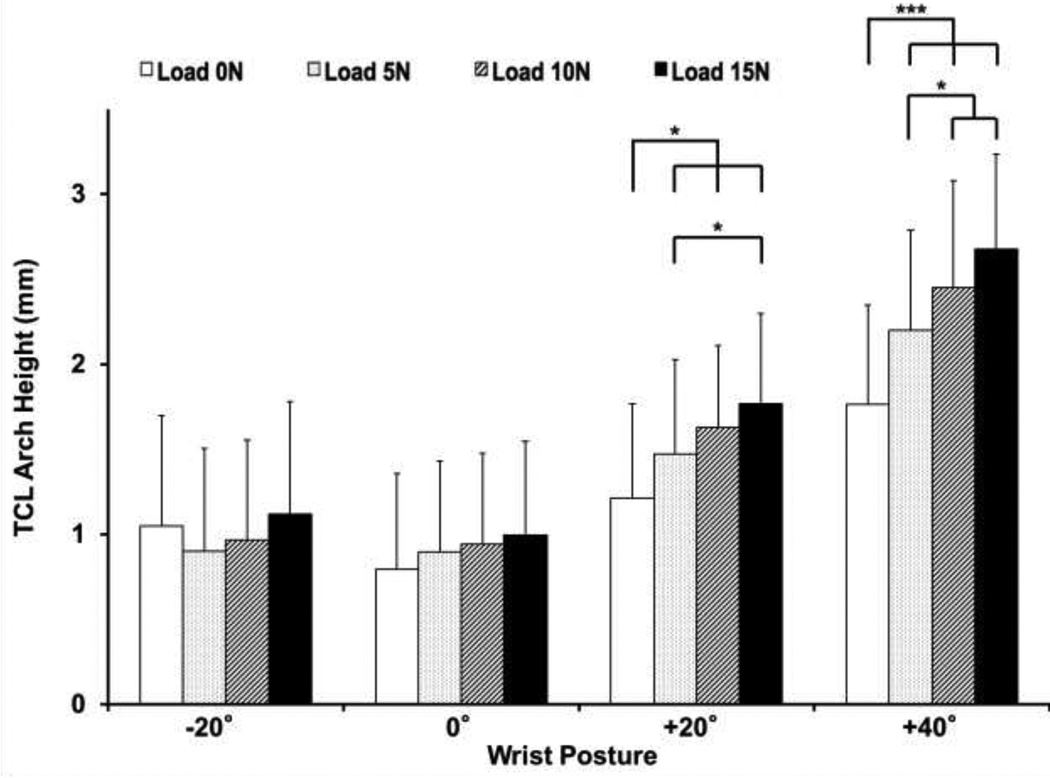
The TCL arch height was significantly affected by the factors of posture and load, as well as the posture-load interaction (p < 0.001; Figure 5). In general, the TCL arch height within a wrist posture increased with increased finger loading. At flexed wrist postures (+20° and +40°), the TCL arch heights under unloaded and all load (5, 10, and 15 N) conditions were significantly different from each other (Figure 5). For a given finger load, the TCL arch height increased as the wrist posture deviated from neutral toward flexion. For the unloaded condition, the TCL arch height at +40° (1.76 ± 0.59 mm) was significantly larger than those at −20° (1.05 ± 0.65 mm), 0° (0.79 ± 0.56 mm), and +20° (1.21 ± 0.55 mm) (p < 0.05). For each loaded condition (5, 10, or 15 N), the TCL arch height at the +40° posture was significantly larger than those at the −20°, 0°, and 20° postures (p < 0.01). Furthermore, the TCL arch height at the +20° posture was significantly larger than that at the −20° and 0° postures for each loaded condition (p < 0.01).
Figure 5.
TCL arch height for various wrist postures and finger loads (* p < 0.05 and *** p < 0.001). Note: Statistical results for comparisons across loads within wrist posture are shown.
3.3 TCL-Tendon Distance
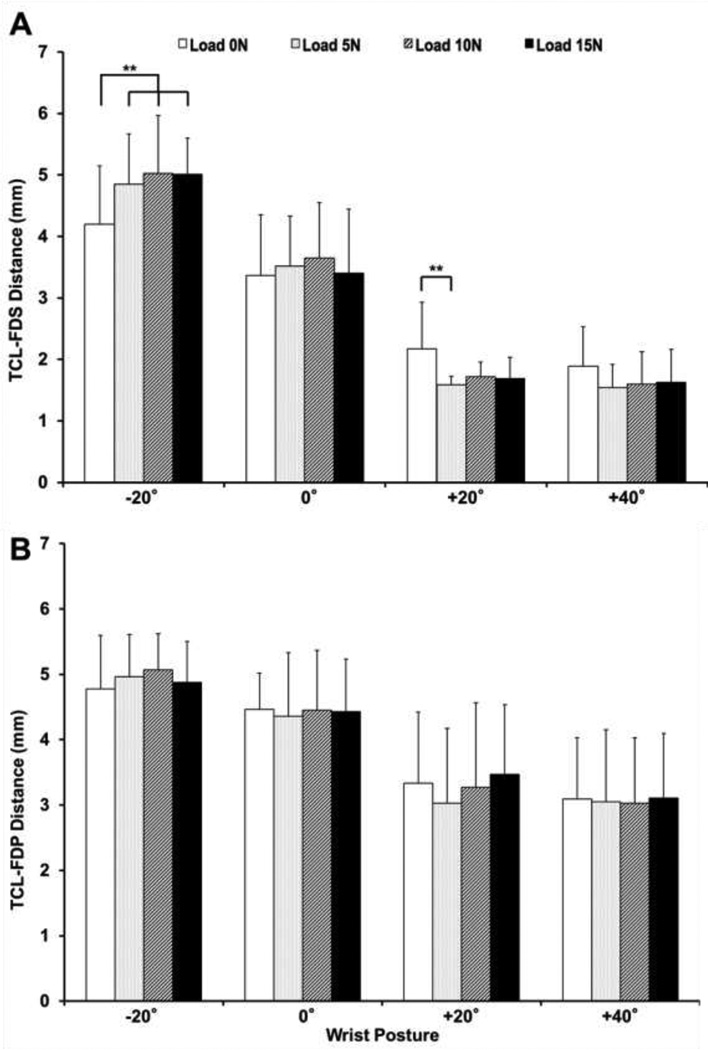
The distance between the TCL and FDS tendon was significantly dependent on the posture × load interaction (p < 0.001) and the factor of wrist posture (p < 0.001); there was no significant dependence on the factor of load (p = 0.85). See Figure 6a. The TCL-FDS distance was generally larger at the extended posture and smaller at the flexed postures with a specific finger load. For the unloaded condition, the TCL-FDS distance at the wrist postures of −20° and 0° was significantly larger than that at the +20° and +40° postures (p < 0.01). For each load (5, 10 or 15 N) condition, pairwise comparisons showed that (a) the TCL-FDS distance at the −20° posture was significantly larger than that at the 0°, +20° and +40° postures (p < 0.01), and (b) the TCL-FDS distance at the 0° wrist posture was significantly larger than that at the +20° and +40° postures (p < 0.001). However, the TCL-FDS distance at the +20° posture was not significantly different from that at the +40° posture (p = 0.841). The TCL-FDP distance was significantly dependent on the factor of wrist posture (p < 0.001), but not on load (p = 0.661) or the posture × load interaction (p = 0.751). See Figure 6b. Regardless of load condition, the TCL-FDP distance at the −20° posture was significantly greater than that at the +20° and +40° postures (p < 0.001). The TCL-FDP distance was significantly larger at the 0° wrist posture than the distance at the +20° and +40° postures (p < 0.01).
Figure 6.
TCL-FDS (A) and TCL-FDP (B) distances at various wrist postures and finger loads (** p < 0.01). Note: Statistical results for comparisons across loads within wrist posture are shown.
4 Discussion
In this study, we investigated the in vivo biomechanical interaction, i.e. contact and coupled movement, between the TCL and flexor tendons in the carpal tunnel resulting from wrist posture deviations and index finger loading. Using ultrasound imaging of the carpal tunnel, the TCL-tendon interaction was demonstrated by examining of the FDS and FDP tendon positions, the TCL arch height, and the TCL-tendon distances. We found that increased wrist flexion and tendon tension resulted in an increased interaction between the TCL and flexor tendons as indicated by the volar migration of the flexor tendons, increased TCL arch height, and decreased TCL-tendon distances.
The positions of the flexor tendons were strongly dependent on the interaction between wrist posture and finger load. As the wrist flexed, the flexor tendons moved towards their respective pulley mechanisms – the TCL at the flexed postures and the carpal bones at the extended posture. Finger loading together with wrist postural deviation amplified the movement of the flexor tendons. The flexor tendon movement presented in our study was consistent with previous studies in carpal tunnel dynamics demonstrating in vivo flexor tendon movement with wrist posture [3, 4] and in vitro tendon motion under tensioning [1].
In addition to tendon positional changes, we explicitly investigate positional changes between the TCL and flexor tendons. Functionally, the TCL keeps the tendons close to the wrist joint axis to maximize finger flexion with minimal tendon excursion [13]. This function is especially evident with bowstringing of the flexor tendons following carpal tunnel release surgery [13, 15], implying the existence of a biomechanical interaction between the flexor tendons and the TCL for the intact, native condition. The positional changes of the TCL and tendons suggest that the tendons contacted and imparted forces on the TCL resulting in their parallel movement. Previous studies have suggested that the height of the TCL during tendon loading is attributable to the increasing carpal tunnel contents, i.e. incursion of the lumbrical muscles into the carpal tunnel [6, 21, 22]. However, the findings of the current study indicate that the increase in TCL arch height with increased wrist flexion and finger loading was likely the result of a volarly directed force by the tendons to the TCL. The TCL arching mechanism by flexor tendons is also supported by a previous study demonstrating that the TCL arch height increases when a volarly directed force is applied to the TCL from within the carpal tunnel [23].
In this study, the TCL-tendon distances could be considered another main indicator of the TCL-tendon interaction. Generally, the TCL-FDS and TCL-FDP distances decreased with flexion of the wrist, i.e. the tendons moved towards the TCL. Interestingly for the flexed postures (+20° and +40°), the TCL-FDS distance at loads of 10 N and 15 N did not further decrease relative to that at the 5 N load. This plateau phenomenon indicates that the TCL and FDS tendon are contacting each other during tendon loading at certain wrist flexion postures, as evident from the tendon’s volar position with its close proximity to the TCL. Although the contact force between the TCL and tendons may increase with increased tendon loading and more deviated wrist flexion, the TCL-tendon distance does not further decrease because of the incompressibility of ligament-tendon tissues. Further evidence of tendon forces against the TCL is demonstrated by the parallel volar movement of the tendon and TCL arch height when the FDS tendon is tensioned and the wrist flexed.
The TCL-tendon interaction is clinically relevant to compression neuropathy of median nerve in the carpal tunnel. The median nerve in the carpal tunnel is typically positioned volar to the FDS tendon of the index finger and dorsal to the TCL [3, 4]. As the FDS tendon moves volarly during finger loading and/or wrist flexion, the tendon may compresses the median nerve against the TCL [4, 9, 11], potentially sandwiching the nerve and causing mechanical stress [3, 4]. Such repetitive stress on the median nerve may help to explain the increased incidence of median nerve compression neuropathy (i.e. carpal tunnel syndrome) in individuals whose occupations involve forceful use of fingers combined flexed wrist postures [24].
In addition to the direct mechanical insult of the median nerve, prolonged and repetitive biomechanical interactions among tissues may also lead to adaptation, and even maladaptation, of the interacting tissues. Tendons and ligaments are known to adapt to mechanical forces [25, 26], and such repetitive mechanical stress can lead to pathomorphological and pathomechanical changes to the tendons and the TCL [19, 27]. For example, studies have shown pathological changes of the TCL including its stiffening and hypertrophying [16–18, 20]. Hypertrophy of the TCL and/or tendons limits the available space within the carpal tunnel for the median nerve and promotes carpal tunnel stenosis, an etiological factor of carpal tunnel syndrome [28–30]. The findings from this study show that increased finger loading and the deviated posture of wrist flexion amplify the TCL-tendon interaction, thereby potentially increasing the risk of maladaptation. Therefore, repetitive TCL-tendon interactions could result in their maladaptation and eventually the onset of carpal tunnel syndrome.
There are a few limitations of this study. First, only female subjects were recruited to examine the tissue interaction between the TCL and flexor tendons; it is expected that this interaction is not gender dependent. Second, this study involved isometric loading of only the index finger. This specific loading condition permitted the well-controlled biomechanical investigation of loading and the resulting interaction effect between the tissues. More research is needed to understand the in vivo interaction between the TCL and flexor tendons during various activities of daily living that involve more complex and strenuous loading conditions (e.g. pinch and grasping). Third, other finger flexor tendons may have partially taken on the load during index finger loading due to enslaving effects of the extrinsic flexors of digits [31]. This load distribution was not monitored and therefore the contributions of the potentially enslaved tendons on the outcome measures are unknown. Fourth, discrete loads of 5 N, 10 N, and 15 N were used for all subjects. The loads were not adjusted based on physical characteristics (e.g. weight, strength) of the individual subjects because the overall purpose of the study was to quantify the TCL-tendon interaction at various wrist postures and loads.
In conclusion, this study demonstrated the biomechanical interaction between the TCL and flexor tendons in vivo. Wrist flexion and finger loading caused the flexor tendons to move volarly, the TCL arch height to increase, and the TCL-tendon distances to decrease. The TCL-tendon interaction may cause mechanical compression to the median nerve and morphological/mechanical maladaptations of the interacting tissues, potentially leading to conditions such as carpal tunnel syndrome.
Highlights.
Finger flexor tendons biomechanically interact with the transverse carpal ligament
In vivo tissue interaction is quantified using dynamic ultrasound imaging
Wrist flexion is associated with tendon displacements
Tendon-ligament interaction is increased by finger load with wrist flexion
Repetitive tendon-ligament interaction may lead to tissue maladaptation
Acknowledgments
The authors thank Raviraj Nataraj for assisting with data processing programs. Research reported in this publication was partially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01AR068278 and R21AR064957. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
Contributor Information
Joseph N. Gabra, Email: jgabra@gmail.com.
Joshua L. Gordon, Email: joshualgordon@gmail.com.
Tamara L. Marquardt, Email: marquat@ccf.org.
Zong-Ming Li, Email: liz4@ccf.org.
References
- 1.Agee JM, Maher TR, Thompson MS. Moment arms of the digital flexor tendons at the wrist: role of differential loading in stability of carpal tunnel tendons. J Hand Surg Am. 1998;23:998–1003. doi: 10.1016/S0363-5023(98)80006-3. [DOI] [PubMed] [Google Scholar]
- 2.Martin JR, Paclet F, Latash ML, Zatsiorsky VM. Changes in the flexor digitorum profundus tendon geometry in the carpal tunnel due to force production and posture of metacarpophalangeal joint of the index finger: an MRI study. Clin Biomech (Bristol, Avon) 2013;28:157–163. doi: 10.1016/j.clinbiomech.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keir PJ, Wells RP. Changes in geometry of the finger flexor tendons in the carpal tunnel with wrist posture and tendon load: an MRI study on normal wrists. Clin Biomech (Bristol, Avon) 1999;14:635–645. doi: 10.1016/s0268-0033(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 4.Zeiss J, Skie M, Ebraheim N, Jackson WT. Anatomic relations between the median nerve and flexor tendons in the carpal tunnel: MR evaluation in normal volunteers. AJR Am J Roentgenol. 1989;153:533–536. doi: 10.2214/ajr.153.3.533. [DOI] [PubMed] [Google Scholar]
- 5.Keir PJ, Wells RP, Ranney DA, Lavery W. The effects of tendon load and posture on carpal tunnel pressure. J Hand Surg Am. 1997;22:628–634. doi: 10.1016/S0363-5023(97)80119-0. [DOI] [PubMed] [Google Scholar]
- 6.Nadar MS, Dashti MH, Cherian J. Finger position alters the median nerve properties within the carpal tunnel: a pre-post MRI comparison study. PLoS One. 2013;8:e79273. doi: 10.1371/journal.pone.0079273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith EM, Sonstegard DA, Anderson WH., Jr Carpal tunnel syndrome: contribution of flexor tendons. Arch Phys Med Rehabil. 1977;58:379–385. [PubMed] [Google Scholar]
- 8.Ugbolue UC, Hsu WH, Goitz RJ, Li ZM. Tendon and nerve displacement at the wrist during finger movements. Clin Biomech (Bristol, Avon) 2005;20:50–56. doi: 10.1016/j.clinbiomech.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.van Doesburg MH, Yoshii Y, Villarraga HR, Henderson J, Cha SS, An KN, et al. Median nerve deformation and displacement in the carpal tunnel during index finger and thumb motion. J Orthop Res. 2010;28:1387–1390. doi: 10.1002/jor.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshii Y, Ishii T, Tung WL, Sakai S, Amadio PC. Median nerve deformation and displacement in the carpal tunnel during finger motion. J Orthop Res. 2013;31:1876–1880. doi: 10.1002/jor.22462. [DOI] [PubMed] [Google Scholar]
- 11.Yoshii Y, Villarraga HR, Henderson J, Zhao C, An KN, Amadio PC. Ultrasound assessment of the displacement and deformation of the median nerve in the human carpal tunnel with active finger motion. J Bone Joint Surg Am. 2009;91:2922–2930. doi: 10.2106/JBJS.H.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong TJ, Chaffin DB. An investigation of the relationship between displacements of the finger and wrist joints and the extrinsic finger flexor tendons. J Biomech. 1978;11:119–128. doi: 10.1016/0021-9290(78)90004-0. [DOI] [PubMed] [Google Scholar]
- 13.Kline SC, Moore JR. The transverse carpal ligament. An important component of the digital flexor pulley system. J Bone Joint Surg Am. 1992;74:1478–1485. [PubMed] [Google Scholar]
- 14.Armstrong TJ, Castelli WA, Evans FG, Diaz-Perez R. Some histological changes in carpal tunnel contents and their biomechanical implications. J Occup Med. 1984;26:197–201. [PubMed] [Google Scholar]
- 15.Netscher D, Mosharrafa A, Lee M, Polsen C, Choi H, Steadman AK, et al. Transverse carpal ligament: its effect on flexor tendon excursion, morphologic changes of the carpal canal, and on pinch and grip strengths after open carpal tunnel release. Plast Reconstr Surg. 1997;100:636–642. doi: 10.1097/00006534-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari FS, Della Sala L, Cozza S, Guazzi G, Belcapo L, Mariottini A, et al. High-resolution ultrasonography in the study of carpal tunnel syndrome. Radiol Med. 1997;93:336–341. [PubMed] [Google Scholar]
- 17.John V, Nau HE, Nahser HC, Reinhardt V, Venjakob K. CT of carpal tunnel syndrome. AJNR Am J Neuroradiol. 1983;4:770–772. [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto H, Miura T, Morizaki Y, Uehara K, Ohe T, Tanaka S. Comparative study on the stiffness of transverse carpal ligament between normal subjects and carpal tunnel syndrome patients. Hand Surg. 2013;18:209–214. doi: 10.1142/S0218810413500251. [DOI] [PubMed] [Google Scholar]
- 19.Schuind F, Ventura M, Pasteels JL. Idiopathic carpal tunnel syndrome: histologic study of flexor tendon synovium. J Hand Surg Am. 1990;15:497–503. doi: 10.1016/0363-5023(90)90070-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamagami T, Higashi K, Handa H, Minouchi K, Fujii M, Nishihara K, et al. Carpal tunnel syndrome: clinical experience of 61 cases. No Shinkei Geka. 1994;22:617–620. [PubMed] [Google Scholar]
- 21.Ham SJ, Kolkman WF, Heeres J, den Boer JA. Changes in the carpal tunnel due to action of the flexor tendons: visualization with magnetic resonance imaging. J Hand Surg Am. 1996;21:997–1003. doi: 10.1016/s0363-5023(96)80307-8. [DOI] [PubMed] [Google Scholar]
- 22.Horng YS, Chang HC, Lin KE, Guo YL, Liu DH, Wang JD. Accuracy of ultrasonography and magnetic resonance imaging in diagnosing carpal tunnel syndrome using rest and grasp positions of the hands. J Hand Surg Am. 2012;37:1591–1598. doi: 10.1016/j.jhsa.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 23.Li ZM, Tang J, Chakan M, Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng. 2009;131:081011. doi: 10.1115/1.3148469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11:343–358. doi: 10.1002/ajim.4700110310. [DOI] [PubMed] [Google Scholar]
- 25.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- 27.Mhanna C, Marquardt TL, Li ZM. Adaptation of the Transverse Carpal Ligament associated with Repetitive Hand Use in Pianists. PLOS ONE. 2016;11:e0150174. doi: 10.1371/journal.pone.0150174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelmers HJ. Primary carpal tunnel stenosis as a cause of entrapment of the median nerve. Acta Neurochir (Wien) 1981;55:317–320. doi: 10.1007/BF01808447. [DOI] [PubMed] [Google Scholar]
- 29.Papaioannou T, Rushworth G, Atar D, Dekel S. Carpal canal stenosis in men with idiopathic carpal tunnel syndrome. Clin Orthop Relat Res. 1992:210–213. [PubMed] [Google Scholar]
- 30.Moore JS. Biomechanical models for the pathogenesis of specific distal upper extremity disorders. Am J Ind Med. 2002;41:353–369. doi: 10.1002/ajim.10037. [DOI] [PubMed] [Google Scholar]
- 31.Li ZM, Latash ML, Zatsiorsky VM. Force sharing among fingers as a model of the redundancy problem. Exp Brain Res. 1998;119:276–286. doi: 10.1007/s002210050343. [DOI] [PubMed] [Google Scholar]