Abstract
Allogeneic hematopoietic stem cell transplantation and blood cell transfusions are commonly performed in patients with a variety of blood disorders. Unfortunately, these donor-derived cell therapies are constrained due to limited supplies, infectious risk factors, a lack of appropriately matched donors, and the risk of immunologic complications from such products. The use of autologous cell therapies has been proposed to overcome these shortcomings. One can derive such therapies directly from hematopoietic stem and progenitor cells of individuals, which can then be manipulated ex vivo to produce desired modifications or differentiated to produce a particular target population. Alternatively, pluripotent stem cells, which have a theoretically unlimited self-renewal capacity and an ability to differentiate into any desired cell type, can be used as an autologous starting source for such manipulation and differentiation approaches. In addition, such cell products can also be used as a delivery vehicle for therapeutics. In this review, we highlight recent advances and discuss ongoing challenges for the in vitro generation of autologous hematopoietic cells that can be used for cell therapy.
Introduction
In a number of blood disorders, hematopoietic stem cell (HSC) transplantation is currently the only available curative therapy. Approximately 50,000 HSC transplantation procedures are performed each year around the world [1]. However, a HSC transplant is generally not the first line of treatment for the majority of blood disorders. This is largely attributable to the numerous complications that can result from obtaining and transplanting HSCs from a donor (termed allogeneic transplantation) [1,2]. For example, an appropriate match does not exist for the majority of patients with blood disorders and even when a matched donor is identified, significant morbidity due to immunologic incompatibility between recipient and donor remains common [1,2]. An emerging alternative is the possibility of autologous transplantation, where HSCs or other cell sources derived from an individual are used as material for hematopoietic reconstitution of that individual, which can thereby avoid many of the complications inherent to allogeneic transplantation. Typically in autologous transplantation, stem cells are harvested and modulated ex vivo, the individual is subsequently conditioned with chemotherapy, and the harvested cells are transplanted to reconstitute the hematopoietic system. Unfortunately, this approach currently suffers from significant limitations, since an individual's HSCs often harbor the precise mutations that cause their blood disorder, particularly in genetic and malignant blood diseases [3].
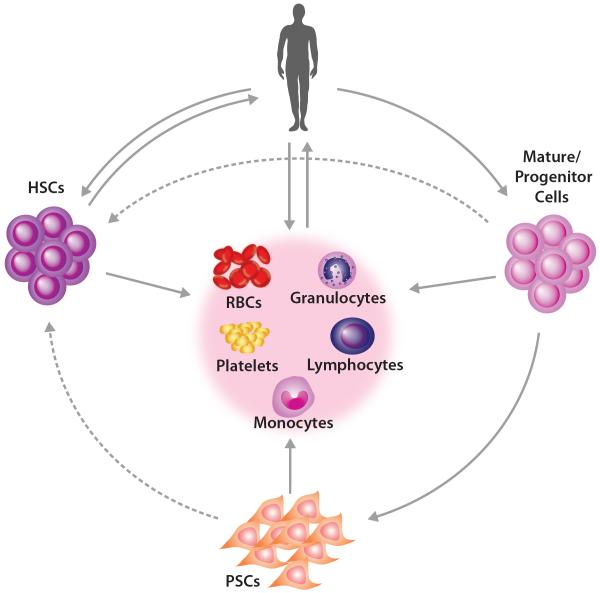
In many blood disorders, including sickle cell disease, thalassemia, bone marrow failure syndromes, and other chronic cytopenias, transfusion of specific blood cell components, such as red blood cells (RBCs) or platelets, is sufficient to confer clinical benefit [4,5]. Despite the prevalence of this procedure and the ready availability of donor-derived blood products, there are limitations including that the blood supply can be inadequate in certain circumstances and there can be transfusion-associated risks [6]. In addition, patients who frequently receive blood transfusions are at risk of developing alloimmunization, which presents a significant challenge to be able to obtain appropriately matched blood products [7]. Therefore, the development of alternative or complimentary approaches is under active investigation. One promising candidate approach is the generation of blood cells in vitro. In the case of blood cell transfusions, immature hematopoietic progenitors can be collected from a patient, expanded ex vivo, terminally differentiated (e.g. into RBCs), and ultimately transfused back into the same patient (Figure 1). Moreover, the ability to obtain large numbers of HSCs from patient-derived pluripotent stem cells (PSCs) or other autologous sources for use in hematopoietic transplantation is a major goal for the field of regenerative medicine. In this review, we discuss recent work that may advance the availability and clinical utilization of autologous blood cell therapies, while also highlighting challenges ahead to make the use of such therapies clinically feasible.
Figure 1. Strategies for producing and applying autologous blood cell therapies.
Autologous transplantation and transfusion cell sources can begin with the isolation of mature cells or progenitors, such as are found in peripheral blood mononuclear cells. Such mononuclear cells can either be directly differentiated toward mature blood cells or can be directly reprogrammed into desired cell types, including hematopoietic stem cells (HSCs). Pluripotent stem cells can be differentiated into downstream mature blood cells, including RBCs, platelets, monocytes, granulocytes, and lymphocytes, or potentially HSCs for autologous transplantation. The solid lines represent approaches that can currently be robustly performed, while the dashed lines represent approaches that are currently being developed or that will require further refinement.
Generation of blood cells ex vivo from hematopoietic stem and progenitor cells (HSPCs)
Hematopoiesis is continuously occurring to maintain the steady-state level of blood cells, and this process is regulated by the combination of various cytokines that direct self-renewal of stem cells and differentiation of progenitors [8,9]. Based on extensive studies of hematopoietic differentiation in vivo, this process has been recapitulated in vitro to produce multiple mature blood cell lineages, including RBCs, platelets, and neutrophils (Table 1) [10]. For example, the addition of erythropoietin (EPO) preferentially leads to erythroid-lineage commitment for RBC production, while the addition of thrombopoietin (TPO) preferentially results in megakaryocyte-lineage differentiation for platelet production [10]. Importantly, some in vitro-produced blood cells can circulate when transfused into human recipients. For example, a proof-of-principle experiment showed that in vitro-cultured RBCs could be successfully used for autologous transfusion and the transfused RBCs were maintained in the circulation of a recipient [11]. Although the differentiation of downstream hematopoietic cells has been well studied, a major challenge that remains to make such therapies clinically useful is the need to scale production in a cost-effective manner.
Table 1.
Summary of approaches for deriving HSPCs and mature blood cells.
| Blood product | Starting source | Approaches | References |
|---|---|---|---|
| RBCs | HSPCs | Cultures with cytokines, co-cultures, and/or genetic perturbations | [11,13–16,85–89] |
| RBCs | PSCs | Cultures with cytokines, co-cultures, and/or genetic perturbations | [12,13,16,28–31] |
| Platelets | HSPCs | Cultures with cytokines and/or co-cultures | [22–24,90,91] |
| Platelets | PSCs | Cultures with cytokines, co-cultures, and/or genetic perturbations | [32–36,44] |
| Platelets | PSCs | Bioreactors with cytokines | [25,26] |
| M, G, L | PSCs | Cultures with cytokines and/or co-cultures | [37–43] |
| HSPCs | HSPCs | Gene therapy (addition) | [92–94] |
| HSPCs | HSPCs | Genome editing | [66–69] |
| HSPCs | Endothelial cells | Direct reprogramming and co-cultures | [73–75] |
| HSPCs | PSCs | Cultures with cytokines, co-culture, and/or genetic perturbations | [80,81,95,96] |
| HSPCs | PSCs | By teratoma formation | [82,83] |
M = Monocytes; G = Granulocytes; L = Lymphocytes
A number of groups have developed culture systems that allow for the extended proliferation of lineage-restricted progenitors by perturbing regulatory genes (Table 1). For example, a few groups have shown that perturbing one or a combination of regulatory factors, including MYC, BCL-XL, HPV16-E6/E7, SOX2, TP53, and BMI1, allow the erythroblasts to stably proliferate for a few months while still retaining the ability to terminally mature to some extent [12–15]. Therefore, progenitors or immature precursors are expanded as much as possible before initiating terminal maturation. One limitation is that in most of these cases, the differentiation observed from such cells does not mimic the efficient terminal maturation seen with unperturbed primary hematopoietic progenitors. In addition to these methods, it has been shown that the suppression of SH2B3, a negative regulator of hematopoietic cytokine signaling, can significantly increase the production of RBCs derived from human HSPCs [16]. In contrast to the generation of immortalized cell lines, the method with SH2B3 suppression in HSPCs increases the yield of red cell production, while not perturbing and actually improving overall differentiation [16]. Given these significant advances in our understanding of the mechanisms governing erythroid self-renewal and differentiation, one logical next step would be to screen small molecules that can activate or inhibit those regulatory factors or other molecular pathways to establish long-term proliferating erythroblasts in vitro for future clinical use. Because RBCs have a average circulating lifespan of 120 days and are enucleate, the generation of RBCs in vitro would also allow for the use of RBCs as vehicles to deliver various molecules, such as therapeutics that have poor bioavailability or that need to be targeted to a particular tissue [17]. For example, it has been shown as a proof-of-principle that mouse RBCs can be engineered to express modified surface proteins that enable targeting to particular tissues and delivery of molecules via these engineered RBCs [18].
Recent advances have also allowed us to move toward improving upon and making large-scale production of platelets for autologous transfusion an achievable goal (Table 1). Megakaryocytes – the precursor cells giving rise to platelets - undergo endomitosis, a process of DNA replication without cytokinesis, prior to terminal maturation [19]. Mature megakaryocytes become polyploid and an individual mature megakaryocyte can release up to 11,000 platelets [19–21]. While in vitro culture with thrombopoietin (TPO) can result in megakaryocyte differentiation from HSPCs, co-culture with human telomerase catalytic subunit gene-transduced stromal cells and various cytokines can lead to large-scale and more robust generation of platelets from HSPCs [10,22–24]. Although megakaryopoiesis and thrombopoiesis can be recapitulated in vitro, currently employed culture methods result in the release of less than 50 platelets per megakaryocyte and only a fraction of cultured megakaryocytes can release platelets [21]. To address this challenge, a number of groups have developed bioreactors to mimic bone marrow microenvironments, which will be further discussed in the next section [10,21,25,26]. By using such approaches that more closely mimic endogenous physiologic conditions, the ability to more robustly produce terminally differentiated blood cells is likely to improve. In addition, a number of groups have been testing the function of platelets produced by such methods and the results obtained suggest that many functions are preserved in in vitro derived platelets, while some differences do exist [21,27]. This is an important area for future investigation, as improved protocols are developed to promote formation of fully functional platelets.
Production of blood cells from human PSCs
PSCs are attractive as a starting cell source for autologous blood cell products, since PSCs can be extensively expanded and manipulated in vitro, while maintaining genome integrity and self-renewal capability. Ever since the first hematopoietic progenitors were differentiated from human embryonic stem cells (ESCs), numerous protocols have been established for the differentiation of PSCs toward mature blood lineages, including erythroblasts, megakaryocytes/platelets, mast cells, macrophages, granulocytes, NK cells, B-lymphoid cells, and T-lymphoid cells (Table 1) [16,28–43]. Similar to the clinical production of RBCs ex vivo, the key hurdle that must be overcome for these stem cell-derived cell therapies is to generate mature blood products that function similar to their endogenous counterparts and in a scalable/ cost-effective manner. Low or reduced quality in vitro output is particularly a problem for platelet production, as well as for other cell types. Unlike in vivo thrombopoiesis where a single megakaryocyte generates thousands of platelets, in vitro-generated megakaryocytes generally produce fewer than 50 platelets, making it difficult to adequately achieve the 3 × 1011 platelets that compose a single platelet unit for typical transfusion purposes [21]. One solution to the problem of low in vitro platelet yield is to generate megakaryocyte progenitors that are themselves immortalized and thereby allow for increased expansion at the early progenitor stage (Table 1). Expandable megakaryocyte progenitors derived from human PSCs can be established by overexpressing MYC, BMI1, and BCL-XL [34]. In addition, researchers have achieved a high yield of megakaryocytes and platelets from mouse PSCs by temporally repressing Gata1 or from human PSCs by simultaneously overexpressing GATA1, FLI1, and TAL1 during the early stages of differentiation [32,44]. As far as can be assessed with currently available approaches, platelets produced in vitro share similar characteristics with native endogenously-derived platelets, but some differences exist [32]. In addition to the generation of immortalized megakaryocyte progenitors from PSCs, platelet generation with bioreactors is another promising area (Table 1). This method both increases the percent of megakaryocytes that can form platelets and mimics the bone marrow microenvironment, triggering the release of an increased number of platelets from megakaryocytes [10,21,25,26]. Such bioreactors have also been suggested to be extremely useful for in vitro production of other blood cells, as well [45].
One of the remaining concerns regarding the use of PSC-derived blood cells is the challenge of obtaining terminally differentiated cells that are similar to their adult counterparts, as opposed to those produced at earlier stages of development. During embryonic development, there are transient waves of hematopoietic progenitors, termed primitive and definitive erythroid-myeloid progenitors (EMP), that contribute to blood production prior to the emergence of HSCs [46]. Currently, most culture systems can recapitulate primitive and definitive EMP waves of hematopoiesis, but not the final wave involving HSCs, which all adult hematopoietic cells are derived from [47]. Since blood cells derived from the definitive EMP wave, especially for RBCs, share many similarities with HSC-derived blood cells, it will be important to identify discriminatory surface markers for definitive and primitive blood cells. This distinction is key, because even though some primitive RBCs can enucleate similar to definitive RBCs, they are substantially larger in size and have altered cell surface protein expression, potentially resulting in an impaired ability to circulate, and therefore may not ideal candidates for use as autologous cell therapies [48]. While ontogenic differences in RBCs have been well-studied, recently it has been shown that neonatal and adult platelets display distinct characteristics that may impact their function [49,50]. For example, it has been shown that fetal megakaryocytes generally have lower ploidy, with poorer generation of platelets as compared to those from adults [49–51]. Therefore, the low yield of platelets from megakaryocytes derived from human PSCs may be due to the recapitulation of embryonic/ fetal hematopoiesis, rather than a specific defect in the function of these cells. One future direction that avoids this ontogenic problem would be to first obtain true definitive HSPCs from PSCs, and directly differentiate these more developmentally mature progenitors into downstream progeny (Figure 1). An alternative approach is to use transcription factor-mediated reprogramming to promote more developmentally mature blood cell formation. For example, adult-type globin gene expression in erythroid cells can be achieved through the transduction of globin regulatory transcription factors, including BCL11A, KLF1, and MYB [52–56].
While enucleated blood cells can easily be used for transfusion purposes, since they do not pose concerns over genotoxicity, nucleated blood cells can also be derived from PSCs and may be useful as a source for cell replacement therapies. For example, granulocyte transfusion can be valuable to deal with complications in patients with neutropenia [57]. However, obtaining adequate numbers of neutrophils can be a challenge and there can be issues due to immunologic incompatibility. Therefore, differentiation of human PSCs toward neutrophils and transfusion back into the same patient could overcome these limitations. In addition, T lymphocytes, particularly when modified using chimeric antigen receptors or other targeting approaches, can be valuable to direct immunologic attacks again cancerous cells [58–60]. While protocols for mature T lymphocyte production from PSCs need to be further developed and improved, if mature T cell subtypes could be obtained from PSCs that can readily be manipulated in vitro, such cells may allow for dramatic improvements for immune cell therapies in patients with cancer refractory to standard therapies.
Autologous transplantation of HSCs
While numerous blood disorders can be cured with HSC transplantation, its application is often precluded by the lack of human leukocyte antigen (HLA)-matched donors [1]. Similar to the production of blood products, autologous approaches where the genetic lesion is corrected ex vivo, known as gene therapy, have been proposed for HSC transplantation and are currently undergoing clinical trials (Table 1) [61,62]. In this process, HSPCs are harvested, the mutation is corrected in a subset of these cells by introduction of exogenous genes (e.g. the gene mutated in a particular disorder) by viral or other methods, and the corrected cells are transplanted back into a patient [61,62]. However, the success of gene therapy has been limited due to safety issues with viral vectors and complications of insertional mutagenesis [62]. Moreover, the observation that HSCs tend to differentiate rather than undergo self-renewal over long periods of in vitro culture coupled with the low efficiency of viral transduction further hinders the successful correction of the underlying mutation in the majority of progenitor cells [61,63,64]. CRISPR-Cas9 and other versatile genome editing tools hold substantial promise to be able to enable more precise gene correction or modification than has been possible using traditional gene therapy approaches, which have primarily relied on exogenous gene addition (Table 1) [65]. However, precise correction of mutations in HSPCs remains extremely inefficient, often results in disruptive mutations instead of the desired changes, and the editing occurs preferentially in progenitors rather than long-term engrafting stem cells [66–69]. Indeed, attempts to correct a sickle cell mutation primarily resulted in gene disruption and the approach was extremely inefficient [69]. It is likely that additional advances, such as the use of genome editing tools that do not rely upon DNA breakage could allow more precise and efficient editing approaches [70]. Therefore, an alternative approach is to use genome editing of differentiated blood cells and then convert these cells into HSCs through reprogramming approaches, as has been achieved in mice [71,72]. While such reprogramming has not been achieved yet in humans, it is likely that important advances will be made in the near future. Recent groundbreaking studies have also succeeded in producing engraftable human hematopoietic cells reprogrammed from endothelial cells with a group of transcription factors, including FOSB, GFI1, RUNX1, and SPI1, followed by the co-culture with vascular niche cells (Table 1) [73]. However, these reprogramming methods remain inefficient and require co-culture approaches that may be challenging to translate to clinical settings. With additional follow up studies and improvement of such approaches, it may be feasible in the future to produce HSCs from endothelial or other patient-derived cells (Figure 1). Indeed, recent studies have shown that more mature hematopoietic progenitors can be obtained through such reprogramming approaches [56,73–75].
As an alternative approach, the concept of obtaining PSCs derived from a patient, correcting the underlying mutations found in these cells with genome editing tools, selecting clones with desired modifications, and differentiating cells derived from specific clones to become transplantable HSCs has tremendous potential and is a holy grail of regenerative medicine, since this same approach could be applied to derive any tissue of interest in theory (Figure 1) [76]. However, there are several issues that must be surmounted in order for PSC-derived HSCs to become clinically useful. First, it is not currently possible to convert PSCs to long-term engrafting HSCs, although important advances are being made. Advances in understanding normal human development, where the first transplantable HSCs can be detected around gestational day 30–35 in the aorta [77,78], may lead to improved methods for deriving HSCs. During gestation, HSC emergence occurs in a limited time window from a unique endothelial population known as the hemogenic endothelium (HE) [79]. Recent studies have shown that similar transitions can be achieved from PSC-derived cells, which suggests that we are getting closer to recapitulating the process of de novo HSC formation in vitro.
Soon after the initial derivation of human embryonic stem cells (ESCs) and the generation of human induced pluripotent stem cells (iPSCs), a number of laboratories have attempted to differentiate these cells toward the hematopoietic lineages [76]. Although different lines exhibit variation, multipotential hematopoietic progenitors defined by both surface markers and colony-forming ability have been successfully differentiated from human PSCs (Table 1) [76]. Nevertheless, the generation of engraftable and functional HSCs with the ability to reconstitute all blood lineages when transplanted into lethally irradiated immunodeficient recipient mice – the gold standard assay for human HSC activity that currently exists – has not yet been achieved. Enforced expression of specific transcription factors, including HOXA9, ERG, and RORA, or co-culture with vascular niche cells that activate Notch signaling encourages the production of hematopoietic progenitors from human PSCs and can allow short-term and low-level engraftment, although there is clear lineage-bias in such cells (Table 1) [80,81]. In addition, some studies have shown that engraftable hematopoietic cells can be generated in vivo via teratoma formation from human PSCs (Table 1) [82,83]. Together, these studies suggest that human PSCs can indeed be differentiated toward HSC-like cells if the proper signals or microenvironmental cues are provided. Improving our understanding of the mechanistic regulation of the process of HSC formation from HE will likely provide important insight into how this process can be modulated in vitro.
Recently, cell surface markers have allowed for enrichment of HE cells with hematopoietic potential from other endothelial cell types derived from PSCs. The HE was shown to be enriched in the population of cells that are CD34+CD73−CD184−, whereas arterial and venous endothelium are separately defined as CD34+CD73medCD184+ and CD34+CD73highCD184−, respectively [84]. Using these surface markers, we can now enrich and perform functional studies of HE derived from PSCs. One important question to address using this system is whether engraftable HSCs can be generated from the derived HE or if the HE obtained with these approaches only has the potential to produce progenitors from the earlier embryonic waves of hematopoiesis. Understanding variation in timing, signaling pathways, and culture conditions will be important to be able to derive HSC-competent HE from PSCs. Such insight is likely to be gained through in depth studies of human hematopoietic development and the molecular regulation of this process.
Conclusions
Donor-dependent blood cell therapies are commonly used in clinical practice, but their potential is often limited for a variety of reasons, as we have discussed in this review. Many patients cannot find an appropriate donor source and enabling production of blood cells from any patient would increase the range of applications for cell therapies. Autologous blood cell therapies - both from terminally differentiated cells and from HSCs - have the potential to dramatically improve our ability to treat many devastating blood disorders (Figure 1). Indeed, there is already tremendous potential being shown from the field of gene therapy, where numerous blood disorders have been cured through such approaches. The field of PSC differentiation has rapidly developed, as has our understanding of human hematopoiesis and its molecular regulation. Nonetheless, we have a considerable amount to still learn and we also must identify strategies to harness this understanding to improve upon our ability to produce autologous blood cell therapies.
Highlights.
Mature blood cells can be produced ex vivo by recapitulating in vivo processes
Scalable blood cell generation is a critical step for obtaining transfusion products
HSCs generation has not yet been achieved, but advances are being made
Insight from human development will improve our ability to make blood cells in vitro
Acknowledgements
We would like to thank members of the Sankaran laboratory for valuable feedback. We thank T. DiCesare for assistance with producing illustrations. This work was funded by NIH grants R01 DK103794 and R33 HL120791 (to V.G.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure The authors have no relevant conflicts of interest to disclose.
References
- 1.Copelan EA. Hematopoietic Stem-Cell Transplantation. N. Engl. J. Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Weisdorf DJ, Billett AL, Hannan P, Ritz J, Sallan SE, Steinbuch M, et al. Autologous Versus Unrelated Donor Allogeneic Marrow Transplantation for Acute Lymphoblastic Leukemia. Blood. 1997;90:2962–8. [PubMed] [Google Scholar]
- 3.Sankaran VG, Weiss MJ. Anemia: progress in molecular mechanisms and therapies. Nat. Med. 2015;21:221–30. doi: 10.1038/nm.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou ST. Transfusion therapy for sickle cell disease: a balancing act. ASH Educ. Program Book. 2013;2013:439–46. doi: 10.1182/asheducation-2013.1.439. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet Transfusion: A Clinical Practice Guideline From the AABBPlatelet Transfusion: A Clinical Practice Guideline From the AABB. Ann. Intern. Med. 2015;162:205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 6.Migliaccio AR, Palis J. Blood in a dish: In vitro synthesis of red blood cells. Drug Discov. Today Dis. Mech. 2011;8:e3–8. doi: 10.1016/j.ddmec.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–37. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene. 2007;26:6715–23. doi: 10.1038/sj.onc.1210756. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–91. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmins NE, Nielsen LK. Blood cell manufacture: current methods and future challenges. Trends Biotechnol. 2009;27:415–22. doi: 10.1016/j.tibtech.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Giarratana M-C, Rouard H, Dumont A, Kiger L, Safeukui I, Le Pennec P-Y, et al. Proof of principle for transfusion of in vitro–generated red blood cells. Blood. 2011;118:5071–9. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirose S, Takayama N, Nakamura S, Nagasawa K, Ochi K, Hirata S, et al. Immortalization of Erythroblasts by c-MYC and BCL-XL Enables Large-Scale Erythrocyte Production from Human Pluripotent Stem Cells. Stem Cell Rep. 2013;1:499–508. doi: 10.1016/j.stemcr.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurita R, Suda N, Sudo K, Miharada K, Hiroyama T, Miyoshi H, et al. Establishment of Immortalized Human Erythroid Progenitor Cell Lines Able to Produce Enucleated Red Blood Cells. PLOS ONE. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Shah S, Wang J, Ye Z, Dowey SN, Tsang KM, et al. Extensive Ex Vivo Expansion of Functional Human Erythroid Precursors Established From Umbilical Cord Blood Cells by Defined Factors. Mol. Ther. 2014;22:451–63. doi: 10.1038/mt.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim AR, Olsen JL, England SJ, Huang Y-S, Fegan KH, Delgadillo LF, et al. Bmi-1 Regulates Extensive Erythroid Self-Renewal. Stem Cell Rep. 2015;4:995–1003. doi: 10.1016/j.stemcr.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giani FC, Fiorini C, Wakabayashi A, Ludwig LS, Salem RM, Jobaliya CD, et al. Targeted Application of Human Genetic Variation Can Improve Red Blood Cell Production from Stem Cells. Cell Stem Cell. 2015 doi: 10.1016/j.stem.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by Mother Nature. Expert Opin. Drug Deliv. 2010;7:403–27. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Kundrat L, Pishesha N, Bilate A, Theile C, Maruyama T, et al. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10131–6. doi: 10.1073/pnas.1409861111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machlus KR, Italiano JE. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013;201:785–96. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman RM, Airo R, Pollack S, Crosby WH. Circulating Megakaryocytes and Platelet Release in the Lung. Blood. 1965;26:720–31. [PubMed] [Google Scholar]
- 21.Sim X, Poncz M, Gadue P, French DL. Understanding platelet generation from megakaryocytes: implications for in vitro–derived platelets. Blood. 2016;127:1227–33. doi: 10.1182/blood-2015-08-607929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zauli G, Vitale M, Falcieri E, Gibellini D, Bassini A, Celeghini C, et al. In vitro senescence and apoptotic cell death of human megakaryocytes. Blood. 1997;90:2234–43. [PubMed] [Google Scholar]
- 23.Matsunaga T, Tanaka I, Kobune M, Kawano Y, Tanaka M, Kuribayashi K, et al. Ex Vivo Large-Scale Generation of Human Platelets from Cord Blood CD34+ Cells. STEM CELLS. 2006;24:2877–87. doi: 10.1634/stemcells.2006-0309. [DOI] [PubMed] [Google Scholar]
- 24.Proulx C, Boyer L, Hurnanen DR, Lemieux R. Preferential Ex Vivo Expansion of Megakaryocytes from Human Cord Blood CD34+-Enriched Cells in the Presence of Thrombopoietin and Limiting Amounts of Stem Cell Factor and Flt-3 Ligand. J. Hematother. Stem Cell Res. 2003;12:179–88. doi: 10.1089/152581603321628322. [DOI] [PubMed] [Google Scholar]
- 25.Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, et al. Platelet bioreactor-on-a-chip. Blood. 2014;124:1857–67. doi: 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa Y, Nakamura S, Nakajima M, Endo H, Dohda T, Takayama N, et al. Two differential flows in a bioreactor promoted platelet generation from human pluripotent stem cell-derived megakaryocytes. Exp. Hematol. 2013;41:742–8. doi: 10.1016/j.exphem.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Hayes V, Jarocha D, Sim X, Harper DC, Fuentes R, et al. Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: a cautionary tale. Blood. 2015;125:3627–36. doi: 10.1182/blood-2014-08-593053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorn I, Klich K, Arauzo-Bravo MJ, Radstaak M, Santourlidis S, Ghanjati F, et al. Erythroid differentiation of human induced pluripotent stem cells is independent of donor cell type of origin. Haematologica. 2015;100:32–41. doi: 10.3324/haematol.2014.108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivier EN, Qiu C, Velho M, Hirsch RE, Bouhassira EE. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp. Hematol. 2006;34:1635–42. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Lapillonne H, Kobari L, Mazurier C, Tropel P, Giarratana M-C, Zanella-Cleon I, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95:1651–9. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang C-J, Mitra K, Koya M, Velho M, Desprat R, Lenz J, et al. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PloS One. 2011;6:e25761. doi: 10.1371/journal.pone.0025761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau T, Evans AL, Vasquez L, Tijssen MR, Yan Y, Trotter MW, et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat. Commun. 2016;7:11208. doi: 10.1038/ncomms11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Q, Shabrani N, Thon JN, Huo H, Thiel A, Machlus KR, et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 2014;3:817–31. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, et al. Expandable Megakaryocyte Cell Lines Enable Clinically Applicable Generation of Platelets from Human Induced Pluripotent Stem Cells. Cell Stem Cell. 2014;14:535–48. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Gaur M, Kamata T, Wang S, Moran B, Shattil SJ, Leavitt AD. Megakaryocytes derived from human embryonic stem cells: a genetically tractable system to study megakaryocytopoiesis and integrin function. J. Thromb. Haemost. JTH. 2006;4:436–42. doi: 10.1111/j.1538-7836.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 36.Takayama N, Nishikii H, Usui J, Tsukui H, Sawaguchi A, Hiroyama T, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zúñiga-Pflücker JC, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–35. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Kovarova M, Latour AM, Chason KD, Tilley SL, Koller BH. Human embryonic stem cells: a source of mast cells for the study of allergic and inflammatory diseases. Blood. 2010;115:3695–703. doi: 10.1182/blood-2009-08-237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uenishi G, Theisen D, Lee J-H, Kumar A, Raymond M, Vodyanik M, et al. Tenascin C Promotes Hematoendothelial Development and T Lymphoid Commitment from Human Pluripotent Stem Cells in Chemically Defined Conditions. Stem Cell Rep. 2014;3:1073–84. doi: 10.1016/j.stemcr.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lachmann N, Ackermann M, Frenzel E, Liebhaber S, Brennig S, Happle C, et al. Large-scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep. 2015;4:282–96. doi: 10.1016/j.stemcr.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermanson DL, Bendzick L, Pribyl L, McCullar V, Vogel RI, Miller JS, et al. Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. STEM CELLS. 2016;34:93–101. doi: 10.1002/stem.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carpenter L, Malladi R, Yang C-T, French A, Pilkington KJ, Forsey RW, et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117:4008–11. doi: 10.1182/blood-2010-08-299941. [DOI] [PubMed] [Google Scholar]
- 43.Kambal A, Mitchell G, Cary W, Gruenloh W, Jung Y, Kalomoiris S, et al. Generation of HIV-1 Resistant and Functional Macrophages From Hematopoietic Stem Cell–derived Induced Pluripotent Stem Cells. Mol. Ther. 2011;19:584–93. doi: 10.1038/mt.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noh J-Y, Gandre-Babbe S, Wang Y, Hayes V, Yao Y, Gadue P, et al. Inducible Gata1 suppression expands megakaryocyte-erythroid progenitors from embryonic stem cells. J. Clin. Invest. 2015;125:2369–74. doi: 10.1172/JCI77670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Migliaccio AR, Whitsett C, Papayannopoulou T, Sadelain M. The potential of stem cells as an in vitro source of red blood cells for transfusion. Cell Stem Cell. 2012;10:115–9. doi: 10.1016/j.stem.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGrath K, Palis J. Ontogeny of erythropoiesis in the mammalian embryo. Curr. Top. Dev. Biol. 2008;82:1–22. doi: 10.1016/S0070-2153(07)00001-4. [DOI] [PubMed] [Google Scholar]
- 47.McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, et al. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11:1892–904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Handel B, Prashad SL, Hassanzadeh-Kiabi N, Huang A, Magnusson M, Atanassova B, et al. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood. 2010;116:3321–30. doi: 10.1182/blood-2010-04-279489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrer-Marin F, Stanworth S, Josephson C, Sola-Visner M. Distinct differences in platelet production and function between neonates and adults: implications for platelet transfusion practice. Transfusion (Paris) 2013;53:2814–21. doi: 10.1111/trf.12343. [DOI] [PubMed] [Google Scholar]
- 50.Sola-Visner M. Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. ASH Educ. Program Book. 2012;2012:506–11. doi: 10.1182/asheducation-2012.1.506. [DOI] [PubMed] [Google Scholar]
- 51.Ma DC, Sun YH, Chang KZ, Zuo W. Developmental change of megakaryocyte maturation and DNA ploidy in human fetus. Eur. J. Haematol. 1996;57:121–7. doi: 10.1111/j.1600-0609.1996.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 52.Trakarnsanga K, Wilson MC, Lau W, Singleton BK, Parsons SF, Sakuntanaga P, et al. Induction of adult levels of β-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL. Haematologica. 2014;99:1677–85. doi: 10.3324/haematol.2014.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sankaran VG, Orkin SH. The switch from fetal to adult hemoglobin. Cold Spring Harb. Perspect. Med. 2013;3:a011643. doi: 10.1101/cshperspect.a011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basak A, Hancarova M, Ulirsch JC, Balci TB, Trkova M, Pelisek M, et al. BCL11A deletions result in fetal hemoglobin persistence and neurodevelopmental alterations. J. Clin. Invest. 2015;125:2363–8. doi: 10.1172/JCI81163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perkins A, Xu X, Higgs DR, Patrinos GP, Arnaud L, Bieker JJ, et al. Krüppeling erythropoiesis: an unexpected broad spectrum of human red blood cell disorders due to KLF1 variants. Blood. 2016;127:1856–62. doi: 10.1182/blood-2016-01-694331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capellera-Garcia S, Pulecio J, Dhulipala K, Siva K, Rayon-Estrada V, Singbrant S, et al. Defining the Minimal Factors Required for Erythropoiesis through Direct Lineage Conversion. Cell Rep. [Internet] 2016 doi: 10.1016/j.celrep.2016.05.027. [cited 2016 Jun 6];0. Available from: http://www.cell.com/article/S2211124716305952/abstract. [DOI] [PMC free article] [PubMed]
- 57.Drewniak A, Kuijpers TW. Granulocyte transfusion therapy: randomization after all? Haematologica. 2009;94:1644–8. doi: 10.3324/haematol.2009.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: Engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–7. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 59.Sadelain M, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 60.Themeli M, Rivière I, Sadelain M. New Cell Sources for T Cell Engineering and Adoptive Immunotherapy. Cell Stem Cell. 2015;16:357–66. doi: 10.1016/j.stem.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bank A. Hematopoietic stem cell gene therapy: selecting only the best. J. Clin. Invest. 2003;112:1478–80. doi: 10.1172/JCI20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Booth C, Gaspar HB, Thrasher AJ. Treating Immunodeficiency through HSC Gene Therapy. Trends Mol. Med. 2016;22:317–27. doi: 10.1016/j.molmed.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Sauvageau G, Iscove NN, Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–32. doi: 10.1038/sj.onc.1207942. [DOI] [PubMed] [Google Scholar]
- 64.Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann. N. Y. Acad. Sci. 2012;1266:138–50. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 65.Cox DBT, Platt RJ, Zhang F. Therapeutic Genome Editing: Prospects and Challenges. Nat. Med. 2015;21:121–31. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genovese P, Schiroli G, Escobar G, Di Tomaso T, Firrito C, Calabria A, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–40. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee Y-L, Kim KA, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 2007;25:1298–306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 68.Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010;28:839–47. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoban MD, Cost GJ, Mendel MC, Romero Z, Kaufman ML, Joglekar AV, et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood. 2015;125:2597–604. doi: 10.1182/blood-2014-12-615948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–4. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riddell J, Gazit R, Garrison BS, Guo G, Saadatpour A, Mandal PK, et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014;157:549–64. doi: 10.1016/j.cell.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanna J, Wernig M, Markoulaki S, Sun C-W, Meissner A, Cassady JP, et al. Treatment of Sickle Cell Anemia Mouse Model with iPS Cells Generated from Autologous Skin. Science. 2007;318:1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 73.Sandler VM, Lis R, Liu Y, Kedem A, James D, Elemento O, et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511:312–8. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pereira C-F, Chang B, Qiu J, Niu X, Papatsenko D, Hendry CE, et al. Induction of a Hemogenic Program in Mouse Fibroblasts. Cell Stem Cell. 2013;13:205–18. doi: 10.1016/j.stem.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pereira C-F, Chang B, Gomes A, Bernitz J, Papatsenko D, Niu X, et al. Hematopoietic Reprogramming In Vitro Informs In Vivo Identification of Hemogenic Precursors to Definitive Hematopoietic Stem Cells. Dev. Cell. 2016;36:525–39. doi: 10.1016/j.devcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slukvin II. Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013;122:4035–46. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tavian M, Hallais MF, Péault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Dev. Camb. Engl. 1999;126:793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- 78.Ivanovs A, Rybtsov S, Welch L, Anderson RA, Turner ML, Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med. 2011;208:2417–27. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat. Immunol. 2008;9:129–36. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doulatov S, Vo LT, Chou SS, Kim PG, Arora N, Li H, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13:459–70. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gori JL, Butler JM, Chan Y-Y, Chandrasekaran D, Poulos MG, Ginsberg M, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J. Clin. Invest. 2015;125:1243–54. doi: 10.1172/JCI79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol. Ther. J. Am. Soc. Gene Ther. 2013;21:1424–31. doi: 10.1038/mt.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amabile G, Welner RS, Nombela-Arrieta C, D'Alise AM, Di Ruscio A, Ebralidze AK, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121:1255–64. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ditadi A, Sturgeon CM, Tober J, Awong G, Kennedy M, Yzaguirre AD, et al. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat. Cell Biol. 2015;17:580–91. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu J, Liu J, Xue F, Halverson G, Reid M, Guo A, et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121:3246–53. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Migliaccio G, Sanchez M, Masiello F, Tirelli V, Varricchio L, Whitsett C, et al. Humanized culture medium for clinical expansion of human erythroblasts. Cell Transplant. 2010;19:453–69. doi: 10.3727/096368909X485049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neildez-Nguyen TMA, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana M-C, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat. Biotechnol. 2002;20:467–72. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 88.Leberbauer C, Boulmé F, Unfried G, Huber J, Beug H, Müllner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105:85–94. doi: 10.1182/blood-2004-03-1002. [DOI] [PubMed] [Google Scholar]
- 89.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat. Biotechnol. 2006;24:1255–6. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 90.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85:402–13. [PubMed] [Google Scholar]
- 91.Cramer EM, Norol F, Guichard J, Breton-Gorius J, Vainchenker W, Massé JM, et al. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997;89:2336–46. [PubMed] [Google Scholar]
- 92.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 93.Hacein-Bey-Abina S, Pai S-Y, Gaspar HB, Armant M, Berry CC, Blanche S, et al. A Modified γ-Retrovirus Vector for X-Linked Severe Combined Immunodeficiency. N. Engl. J. Med. 2014;371:1407–17. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 2014;32:554–61. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi K-D, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells Dayt. Ohio. 2009;27:559–67. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]