Abstract
Previous studies have demonstrated that most of the intraspecies variation in sensitivity to the toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), including suppression of antibody responses, in murine models is due to single nucleotide polymorphisms (SNPs) within the aryl hydrocarbon receptor (AhR) gene. The underlying reason for variation in sensitivity to TCDD-induced suppression of IgM responses among humans is not well understood, but is thought, in part, to be a result of different polymorphic forms of the AhR expressed by different individuals. In this study, the functional properties of six (P517S, R554K, V570I, V570I+P517S, R554K+V570I and P517S+R554K+V570I) human AhR variants were examined in the human B cell line, SKW 6.4. TCDD-induced Cyp1B1 and Cyp1A2 mRNA expression levels and Cyp1B1-regulated reporter gene activity, used for comparative purposes, were markedly lower in SKW cells containing the R554K SNP than in SKW-AHR+ (control AhR) cells. Furthermore, all AhR variants were able to mediate TCDD-induced suppression of the IgM response; however, a combined P517S+R554K+V570I variant partially reduced sensitivity to TCDD-mediated suppression of IgM secretion. Collectively, our findings show that the R554K human AhR SNP alone altered sensitivity of human B cells to TCDD-mediated induction of Cyp1B1 and Cyp1A2. By contrast, attenuation of TCDD-induced IgM suppression required a combination of all three SNPs P517S, R554K, and V570I.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; single nucleotide polymorphisms; B cells
INTRODUCTION
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a physicochemically stable, highly lipophilic environmental contaminant, generated mostly as a by-product of industrial processes and waste incineration. Biological and toxic effects of TCDD include induction of drug metabolizing enzymes, immune suppression, wasting syndrome and endocrine disruption (Pohjanvirta and Tuomisto, 1994; Poland and Knutson, 1982). Suppression of the immune system had been described in many animal species and is one of the most sensitive consequences of TCDD exposure (Holsapple et al., 1991; Sulentic and Kaminski 2011). Moreover, TCDD had been shown to directly and specifically target B cell function (Dooley and Holsapple, 1988).
Studies with AhR-deficient experimental models, including mice, demonstrated that most, if not all, of the toxic effects of dioxins are mediated by the aryl hydrocarbon receptor (AhR) (Harrill et al., 2015; Mimura and Fujii-Kuriyama, 2003). In the absence of ligand binding, AhR is localized to the cytosol in a complex with heat shock protein 90 (HSP90), X-associated protein 2 (XAP2), and telomerase binding protein (p23) (Carver et al., 1998; LaPres et. al., 2000). Upon ligand binding, the AhR translocates into the nucleus, where it heterodimerizes with AhR nuclear translocator (Arnt). AhR-Arnt heterodimers can bind dioxin responsive elements (DREs) located in the promoter region of TCDD responsive genes to influence transcription (Hankinson, 1995; Morel and Barouki, 1998; Senft et al., 2002).
The AhR signaling pathway is conserved broadly across species (Hahn, 2002); however, remarkable differences in species, strain and gender sensitivity to TCDD-mediated biological and toxicological effects exist (Bello et al., 2001; Enan et al., 1996; Kleeman et al., 1988). For example, the lethal dose 50% (LD50) for TCDD vary from 1μg/kg for guinea pig, the most sensitive animal species, to >5000 μg/kg for hamster, the most resistant (Poland and Knutson, 1982). Similarly, AhR affinity and sensitivity to TCDD is about 10-fold higher in mice expressing the AhRb1 allele as compared to mice that express the AhRd allele (Poland et al., 1994). The molecular basis for decreased affinity of the product of the AhRd allele for TCDD is a single nucleotide polymorphism (SNP) at the codon 375, which causes valine for alanine substitution in the ligand-binding domain (Poland and Glover, 1990).
Differences in sensitivity to TCDD-induced biological responses also occur in humans including Cyp1B1 induction and suppression of the IgM response (Catteau et al., 1995; Harper et al., 2002; Lu et al., 2010). These differences in human responsiveness to TCDD are likely due, in part, to SNPs within the AhR. Most of the known human AhR SNPs occur within exon 10, a region that encodes the transactivation domain of the receptor. Experimental evidence suggests that SNPs in transactivation domain may result in differentially recruited coactivator/corepressor complexes, and consequently differentially regulate gene expression (Flaveny et al, 2008; Flaveny et al, 2010). Indeed, previous studies characterizing polymorphisms at the codon 517, 554 and 570 led to complicated and conflicting interpretation of the functional effects of these polymorphisms (Wong et al., 2001b; Celius and Matthews, 2010). For instance, the codon 554 variant correlated with induced Cyp1A1 activity in one study (Smart and Daly, 2000) but not in the others (Cauchi et al., 2001; Kawajiri et al., 1995). The combined occurrence of polymorphisms at codons 554 and 570, or 554 and 517 produced an AhR that failed to induce Cyp1A1 mRNA expression (Wong et al., 2001b). Since these studies were performed in mouse hepatoma cells, it is unknown whether a similar observation would be made in human B cells.
The objective of the current studies was to investigate the influence of identified human AhR polymorphisms on the ability of the receptor to mediate well-established TCDD-induced biological responses including induction of drug metabolizing enzymes and suppression of the IgM response in human B cells. Here we describe SKW 6.4 B cell lines that were stably transduced to express either a control AhR (SKW-AHR+) or one of six different polymorphic forms of the human AhR (P517S, R554K, V570I, V570I+P517S, V570I+R554K, and V570I+R554K+P517S). The characterization of these polymorphisms within a specific cellular context provides new insights into the effects of structural variations in the human AhR on functional outcomes.
MATERIALS AND METHODS
Chemicals and reagents
TCDD in dimethyl sulfoxide (DMSO) (purity 99.1%) was purchased from AccuStandard Inc (New Haven, CT). DMSO and lipopolysaccharide (LPS) (Escherichia coli, catalog no. L2755-10MG) were purchased from Sigma-Aldrich (St. Louis, MO). The anti-human AhR antibody was purchased from eBioscience (catalog no. 14-9854-82, San Diego, CA). The anti-mouse immunoglobulin capture antibody and the horseradish peroxidase anti-mouse IgM detection antibody were purchased from Boehringer Mannheim (Indianapolis, IN) and Sigma-Aldrich, respectively. The pokeweed mitogen (PWM) and puromycin were purchased from Sigma-Aldrich (St. Louis, MO, Lot. O L8777-5MG and P8833).
Cell lines
The SKW 6.4 cell line has been previously characterized by Ralph et al. (1984) and was obtained from ATCC (American Type Culture Collection , Rockville,MD). Cells were maintained under standard conditions (5% CO2/95% air, 98% humidity, 37°C) in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% bovine calf serum (HyClone Laboratories, Logan, UT), 13.5 mM HEPES, 23.8 mM sodium bicarbonate, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate and 2.5 g/l dextrose. Cells were cultured in suspension in 25-cm2 flasks (Becton Dickinson, Plymouth, GB) at density of < l05 cells/ml.
HEK293T cells were used in the co-culture experiments to generate SKW-based cell lines that stably express different polymorphic forms of the AhR and during transient transfections with Cyp1B1-regulated luciferase reporter constructs. HEK293T cells were cultured in DMEM supplemented with 10% bovine calf serum (HyClone Laboratories, Logan, UT), 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate. Cells were kept in a thermo-regulated incubator at 37°C, 5% CO2 and had 80% confluence when used.
Animals
Specific pathogen-free, female C57BL/6 mice and Sprague-Dawley rats (5-8 weeks of age) were purchased from Charles River (Portage, MI). Animals were randomized, transferred to plastic cages containing sawdust bedding (five mice/3 rats per cage), and quarantined for 1 week. Animals were provided food (Purina certified laboratory chow) and water ad libitum. Animal holding rooms were kept at 21–24 °C and 40–60% humidity with a 12-h light/dark cycle. The Michigan State University Institutional Animal Care & Use Committee approved all experiments involving the use of animals.
Isolation of rat and mouse B cells
Mouse or rat B cells were isolated from spleens of female C57BL/6 mice or Sprague-Dawley rats, and were made into single-cell suspensions by passage through a 40 μm cell strainer (BD Biosciences, San Jose, CA). Negative selection of rat or mouse B cells was conducted using MACS Naive Rat B cell, or Mouse B Cell Isolation Kits following the manufacturer's protocols (Miltenyi Biotec, Auburn, CA) and as described previously (Lu et al., 2009). In all cases, the purity of isolated B cells was ≥ 95%.
Preparation of the luciferase reporter construct
pGL3-basic firefly luciferase reporter vector containing the 5'-flanking region from −1523 to +20 of the human Cyp1B1 gene was kindly provided by Dr. Weiguo Han of the Albert Einstein College of Medicine. The 5'-flanking region of the human Cyp1B1 and luciferase were transferred into the pLEX-MCS lentiviral vector (Thermo Scientific, Waltham, MA). Nucleotide sequences were confirmed by DNA sequencing analyses.
Luciferase assays
For transient transfections, HEK293T cells were plated at 1.2 × 106 cells/well in 6-well plates and transfected with 6 μg of the Cyp1B1-luciferase constructs using lentiviral packing mix (Open Biosystems, Huntsville, AL) according to the manufacturer's instructions. After a 16 h incubation, HEK293T cells were co-cultured with 2 × 106 SKW cells/well for an additional 24h. After 24-h of co-culture, SKW cells were separated from HEK293T cells and treated with vehicle (0.01% DMSO) or TCDD (30 nM) for 24 h. The cells were then washed with 1× phosphate-buffered saline and lysed with 1× reporter lysis buffer (Promega, Madison, WI). Samples were immediately frozen at −80°C. To measure luciferase enzyme activity, samples were thawed, and 20 μl of sample lysate was mixed with 100 μl of luciferase assay reagent (Promega, Madison, WI) using an autoinjector. Luciferase activity was measured by KC-4 automated microplate reader (Bio-Tek, Winooski, VT) and represented as relative light units. Luciferase activity was normalized to the amount of protein determined by Bradford reaction (Protein Assay Kit, Pierce). Results are shown as fold induction determined by normalizing activation of different groups against VH control.
Preparation of stably expressing AhR cell lines
HEK293T cells were used for the transfection of the recombinant control and polymorphic human AhR fused to GFP as described above. After a 48h co-culture incubation media was replaced, target cells were separated and cultured at 37°C to approximately 80% confluence. Cells were then passaged and selected using RPMI media containing 0.5 μg/mL puromycin. Clones of the SKW cells were established using cloning by limiting dilution for 2 weeks in culture RPMI media containing 0.5 μg/mL puromycin. Individual SKW clones were screened for AhR mRNA expression and clones, expressing AhR mRNA at levels similar to that of primary human B cells, were selected for further characterization.
pTRIPZ-AHR-GFP lentiviral vector production
Human AhR cDNA (cloned from HepG2 cells) was amplified by PCR from a previously described pSV-Sport1 vector (Dolwick et al., 1993) kindly donated by C. Bradfield. To generate the human AhR fused to GFP, AhR was amplified from pSV-Sport1 and sub-cloned into phCMV-C-GFP vector (Genlantis, San Diego, CA) using primers 5'-CCG TCG TCG ACT TAACCGG TCT GGG CAC CAT GA and 3'-CCC GGG CCC GCG GACGCGT CGA CTG CAG A. To generate a positive control vector containing only GFP, GFP cDNA was amplified by PCR from phCMV-C-GFP vector using the following primers 5'-CCG TCG TCG ACT TA ACCGGT CTC GAG CTC AAG CT and 3'- CCC GGG CCC GCG G ACGCGT CGA CTG CAG A. Underlined nucleotides denote restriction sites AgeI and MluI respectively. Each cDNA was amplified by PCR using Phusion Pfu enzyme (Takara, Mountain Viev, CA) following manufacturer's recommendations and sub-cloned in pZerO-blunt vector (Invitrogen, Grand Island, NY). After enzymatic restriction of pZerO-blunt vectors with AgeI and MluI, fragments were gel purified and extracted using the QIAquick gel extraction kit (QIAGEN, Valencia, CA) and ligated into the lentiviral inducible transfer vector pTRIPZ Tet-OnR (Open Biosystems, Lafayette, CO) using T4 ligase (NEB, Ipswich, MA). Sanger sequencing was used to verify nucleotide sequences.
Site-directed mutagenesis
Site-directed mutagenesis was performed using the Quik-Change II XL kit (Stratagene, Santa Clara, CA) as previously described (Scott et al., 2002). Briefly, a total of 125 ng of two degenerate complementary primers with mutant sequences (517 sense 5'-CAT GAG CAA ATT GAC CAG TCT CAG GAT GTG AAC TCA T-3’, antisense 5'-ATG AGT TCA CAT CCT GAG ACT GGT CAA TTT GCT CAT G-3’; 554 sense 5'-CCT AGG CAT TGA TTT TGA AGA CAT CAA ACA CAT GCA GAA TG-3’, antisense 5'-CAT TCT GCA TGT GTT TGA TGT CTT CAA AAT CAA TGC CTA GG-3’; 570 sense 5'-CAG AAA TGA TTT TTC TGG TGA GAT TGA CTT CAG AGA CAT TGA CTT-3’, antisense 5'-AAG TCA ATG TCT CTG AAG TCA ATC TCA CCA GAA AAA TCA TTT CTG-3’) and 80 ng of template pTRIPZ-AHR-GFP was used for PCR amplification. PCR conditions were as follows: 95°C for 30 s, 58°C for 1 min, and 68°C for 27min. After15 cycles, the PCR product was digested with 10 U of DpnI to cleave template DNA at 37°C for 2 h. PCR reaction was precipitated with ethanol and resuspended in 10ul of water. Half of the PCR product was used for transfections. Sanger sequencing was used to verify nucleotide sequences of the constructs.
Quantitative real-time PCR
Total RNA was isolated using the SV40 Total RNA Isolation System (Promega Corporation, Madison, WI) and RNA concentrations were quantified using a Nanodrop ND-1000 spectrophotometer (Wilmington, DE). Double stranded cDNA was synthesized using 1000 ng of total RNA using the Applied Biosystems high capacity cDNA reverse transcription kit (Foster City, CA). qRT-PCR was performed according to manufacturer's instructions using the Taqman Universal PCR Master Mix and Taqman gene expression assays for human Cyp1A2 (Hs01070374_m1), Cyp1B1 (Hs00164383_m1) and AhR (Hs00169233_m1). All qRT-PCR measurements were made on an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). The change in gene expression was calculated using the ΔΔCt method using 18S ribosomal RNA (4319413E) as an internal control (Livak et al., 2001). For statistical analysis, unpaired two-tailed Student's t-tests were performed between treatments and their corresponding controls.
Western blot analysis
Total protein samples were prepared and concentrations determined as previously described (Bradford et al., 1976; Lowry et al., 1951). Proteins were separated on 4–20% Nu-Page Bis-Tris gels (Bio-Rad Laboratories, Hercules, CA), transferred to nitrocellulose membranes, and probed with anti-AhR antibody (eBioscience, San Diego,CA). Western blots were visualized using ECL Western blotting substrate (Pierce, Rockford, IL).
IgM enzyme-linked immunosorbent assay (ELISA)
A detailed procedure for determination of human, mouse and rat IgM concentration can be found in Sulentic et al. (1998). In brief, cells were activated with 150 μg/ml lipopolysaccharide (LPS) or pokeweed mitogen (PWM), cell culture supernatants were collected 120h post-activation and analyzed by a kinetic colorimetric sandwich ELISA specific for human, mouse and rat IgM. The IgM concentration (ng/ml) in each sample was calculated based on the standard curve using the KC4 software (BioTek, Winooski, VT). The concentration of IgM/106 cells was calculated by dividing the concentration (ng/ml) by the number of viable cells.
Statistical analysis
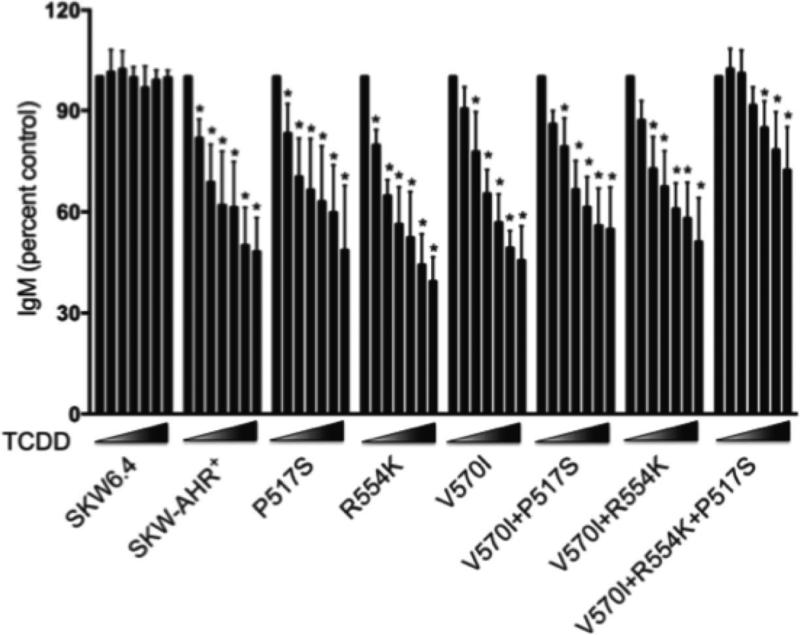
The mean ± S.E. was determined for each treatment group. Data were evaluated using a one-way ANOVA, and Dunnett's two-tailed t-test was used to compare treatment groups with the VH control when significant differences were observed. For data expressed as percent (Fig. 9), logarithmic transformation was conducted prior to statistical analysis as described previously (Kaplan et al., 2010). Statistical analyses were performed using GraphPad Prism version 4.0a for Macintosh OS X, GraphPad Software, San Diego, CA.
Figure 9.
Effects of TCDD on the LPS-induced IgM secretion in SKW clones. SKW cells (1×105/ml) were activated with LPS (150 μg/ml) and treated with 0.3, 1, 3, 10, 30 and 100 nM TCDD or vehicle (0.01% DMSO). Supernatants were harvested on day 5 of culture and analyzed for IgM by sandwich ELISA. Data were normalized to VH control (100%) and presented as percentage of control. The data are combined from six separate experiments with four experimental replicates per group. Statistical significance was determined using Dunnett's two-tailed t test; * represents values that are significantly different from VH control at p < 0.05.
RESULTS
Human SKW-AHR+ B cell line characterization
The AhR null human B cell line, SKW 6.4 was used to develop a series of clones that stably express either the control AhR (SKW-AHR+) or one of the known human AhR variants: P517S, V570I, and R554K. We included the combined R554K+V570I, V570I+ P517S double SNPs and V570I+ P517S+ R554K triple SNP, since it was previously reported that each failed to mediate TCDD induction of Cyp1A1. In addition, polymorphisms at the codon 517 were reported as only occurring in individuals who also carried alleles encoding lysine at codon 554 plus isoleucine at codon 570 (Wong et al., 2001a).
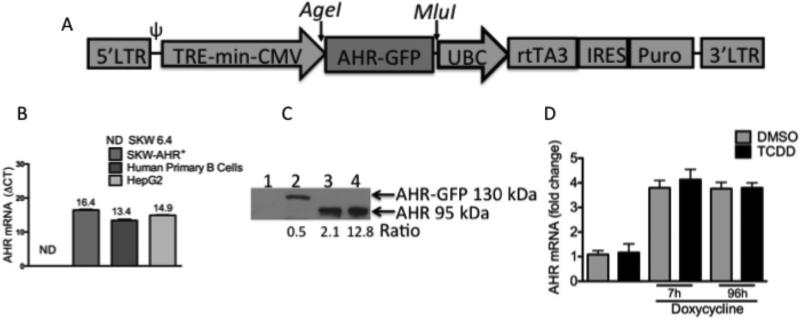
Cell lines of the lymphoid lineage are difficult to transfect efficiently and to establish long-lasting transgene expression using retroviruses, diethylaminoethyl dextran, or liposomes (Guven et al., 2005). Therefore, a doxycycline-inducible lentiviral vector system, pTRIPZ was used to establish all SKW clones (Fig. 1A). The SKW-AHR+ clone was chosen for preliminary investigations. RT-PCR was used to compare AhR mRNA expression levels between SKW 6.4, SKW-AHR+, HEPG2 and human primary B cells (Fig. 1B). SKW 6.4 cells express no detectable AhR mRNA, while SKW-AHR+, HEPG2 and human primary B cells express comparable levels of AhR mRNA. Western blot analysis was used to assess AhR protein levels in whole cell lysates obtained from the SKW 6.4, SKW-AHR+ ,HEPG2 and human primary B cells. Due to the very high levels of AhR expressed in HepG2 cells compared to B cells (primary and SKW-AHR+), it was necessary to load different amounts of cell lysate from the different cell preparations in order to allow for visualization of AhR on the same Western blot. Densitometry was performed on the AhR band and normalized to the amounts of cell lysate protein loaded into each lane. By doing so an estimate of the amount of AhR expressed in the various cell preparations is provided exhibiting the following rank order: HepG2>> primary B cells > SKW-AHR+. AhR protein was expressed at the expected molecular weight of AhR-GFP fusion protein (approximately 130 KDa) in the SKW-AHR+ and not in the SKW 6.4 cell line (negative control). HEPG2 and primary human B cells were used as positive controls and AhR protein was identified as expected at 95kDa (Fig. 1C). To test the stability of the AhR mRNA expression upon induction of AhR levels with doxycycline and TCDD treatment, SKW-AHR+ cells were treated with doxycycline (0.2ug/ml) and either vehicle (0.02% DMSO) or TCDD (30 nM). Samples for mRNA isolation were collected at 7h and 96h post-treatment and AhR mRNA levels were measured by real time RT-PCR. There was no significant difference in the AhR mRNA levels between VH and TCDD treated samples. As expected, doxycycline treatment resulted in approximately 3.5 fold increase of the AhR mRNA (Fig. 1D).
Figure 1.
A. Schematic representation of pTRIPZ vector for doxycycline-inducible transgene expression. In this vector, the expression of transgenic human aryl hydrocarbon receptor fused to green fluorescent protein (AHR-GFP) is under the control of human cytomegalovirus (CMV) constitutive promoter plus tetracycline response element (TRE) promoter, which can be activated by reverse tetracycline transactivator 3 (rtTA3) in the presence of doxycycline. pTRIPZ vector contains the 5’ and 3’ long terminal repeats (LTRs), packaging signal (ψ), internal ribosomal entry site (IRES) and puromycin selectable marker (Puro). B. Levels of the AhR gene expression in the SKW 6.4, SKW-AHR+, HepG2 cell lines and human primary B lymphocytes. Total RNA was extracted from untreated SKW 6.4, SKW-AHR+, HepG2, human primary B cells (1×106 cells/ml) and 500 ng of total RNA were analyzed by RT-PCR for AhR mRNA. Steady-state mRNA levels of the AhR were normalized to the endogenous 18S ribosomal RNA. AhR mRNA in the SKW 6.4 cells was not detected (ND). Representative of 3 independent experiments. Data are presented as the mean ± SD. C. Western blot analysis of the AhR protein expression. Whole cell lysates from the SKW 6.4 (60 μg), SKW-AHR+ (60 μg), HepG2 (3 μg) and human primary B cells (20 μg) were subjected to electrophoresis, blotted, and stained as described under Materials and Methods. Lane 1: SKW 6.4; lane 2: SKW-AHR+; lane 3: human primary B lymphocytes; lane 4: HepG2. Ratio was determined by dividing AhR protein density by the amount of cellular protein loaded per lane. One representative result from three independent experiments is shown. D. Inducibility and stability of the AhR mRNA in the absence and presence of doxycycline. SKW-AHR+ cells (1×106 cells/ml) were treated with vehicle (0.02 % DMSO) or TCDD (30 nM) in the presence of doxycycline (0.2 μg/ml), harvested at 7 h and 96 h of culture and analyzed for levels of AhR mRNA expression. AhR mRNA levels were normalized to the endogenous 18S ribosomal RNA. Data are presented as the mean ± SD from 3 independent experiments.
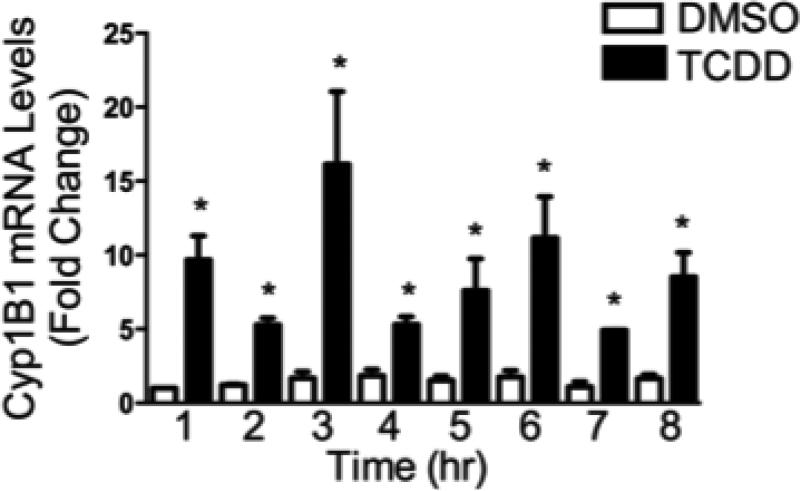
To investigate the transcriptional activity of the expressed AhR, SKW-AHR+ cells were treated with 30 nM TCDD or VH and incubated for 1, 2, 3, 4, 5, 6, 7, and 8h to obtain an expression kinetics profile for Cyp1B1 mRNA. As shown in figure 2, TCDD treatment rapidly induced Cyp1B1 mRNA levels in the SKW-AHR+ cells. TCDD-induced Cyp1B1 expression occurred as early as 1 h post TCDD treatment and remained elevated throughout the time course. Cyp1B1 mRNA was not induced in TCDD-treated AhR null SKW 6.4 cell line (data not shown). These results show that WT AhR retained its ability to transactivate Cyp1B1 gene expression in SKW-AHR+ cells with the GFP tag. Moreover, the magnitude of induction by TCDD of Cyp1B1 mRNA levels in SKW-AHR+ cells was relatively modest, compared to liver derived cells, and closely paralleled what we have previously reported in primary human peripheral blood B cells (Lu et al., 2010).
Figure 2.
Time-dependent induction of the Cyp1B1 gene expression by TCDD. The SKW-AHR+ cells (1×106 cells/ml) were treated with 30 nM of TCDD or 0.02% DMSO (VH) for indicated periods of time. Total RNA was isolated, and steady-state Cyp1B1 mRNA levels were measured by TaqMan qRT-PCR and normalized to the endogenous 18S ribosomal RNA. Data are presented as fold change compared to the VH control group at corresponding time point. The data are representative of two separate experiments with three experimental replicates per group. Statistical significance was determined using a two-way ANOVA and Dunnett's two-tailed t test; * represents values that are significantly different from naive at p < 0.05.
TCDD suppressed IgM secretion in human SKW-AHR+ but not SKW 6.4 B cells
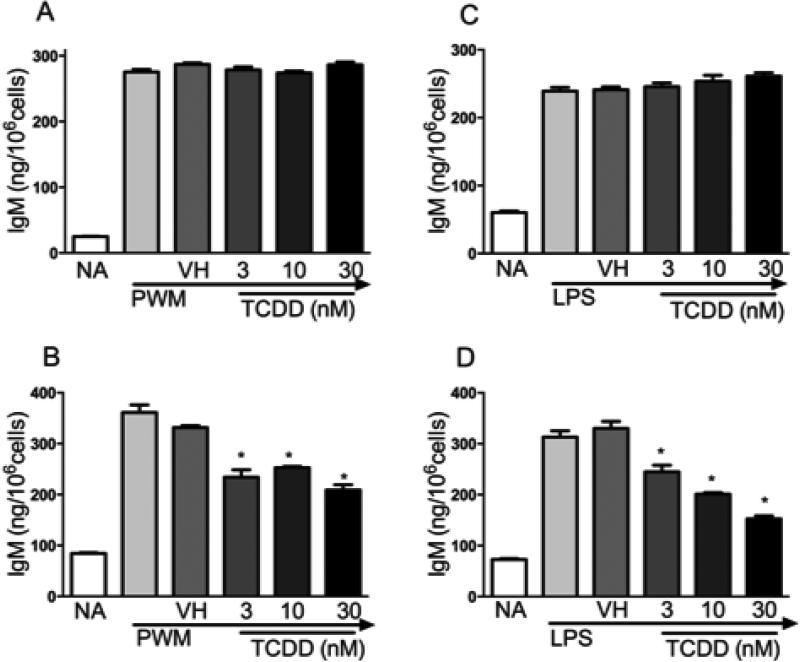
In primary human and mouse B cells, TCDD is well established to markedly suppress IgM secretory responses to lipopolysaccharide (LPS) and CD40 ligand activation (Dooley and Holsapple, 1988; Lu et al., 2010). To evaluate the sensitivity of SKW-AHR+ and SKW 6.4 cells to TCDD-mediated suppression of the IgM response, supernatant IgM was quantified by ELISA in the absence and presence of TCDD treatment, post LPS- and pokeweed mitogen (PWM) activation. TCDD (0, 3, 10, 30 nM) treatment resulted in a marked, concentration-related, suppression of LPS- and PWM-induced IgM secretion in the SKW-AHR+ (Fig. 3B and 3D) but not in the AhR-deficient SKW 6.4 cells (Fig. 3A and 3C).
Figure 3.
Effects of TCDD on LPS- and PWM-induced IgM secretion in the SKW 6.4 and SKW-AHR+ cells. A. SKW 6.4 cells (1×106 cells/ml) activated with PWM. B. SKW-AHR+ cells activated with PWM. C. SKW 6.4 cells activated with LPS and D. SKW-AHR+ cells activated with LPS. SKW cells (1×106 cells/ml) were activated with LPS or PWM (150 μg/ml) and treated with 3, 10, 30 nM TCDD or vehicle (0.01% DMSO). Supernatants were harvested on day 5 post LPS activation and the amount of secreted IgM was measured by sandwich ELISA. The data are representative of three separate experiments with 4 experimental replicates per group. Statistical significance was determined using Dunnett's two-tailed t test; * represents values that are significantly different from naive at p < 0.05.
Temporal effects of TCDD on IgM antibody response in the SKW-AHR+ , mouse and rat primary B cells
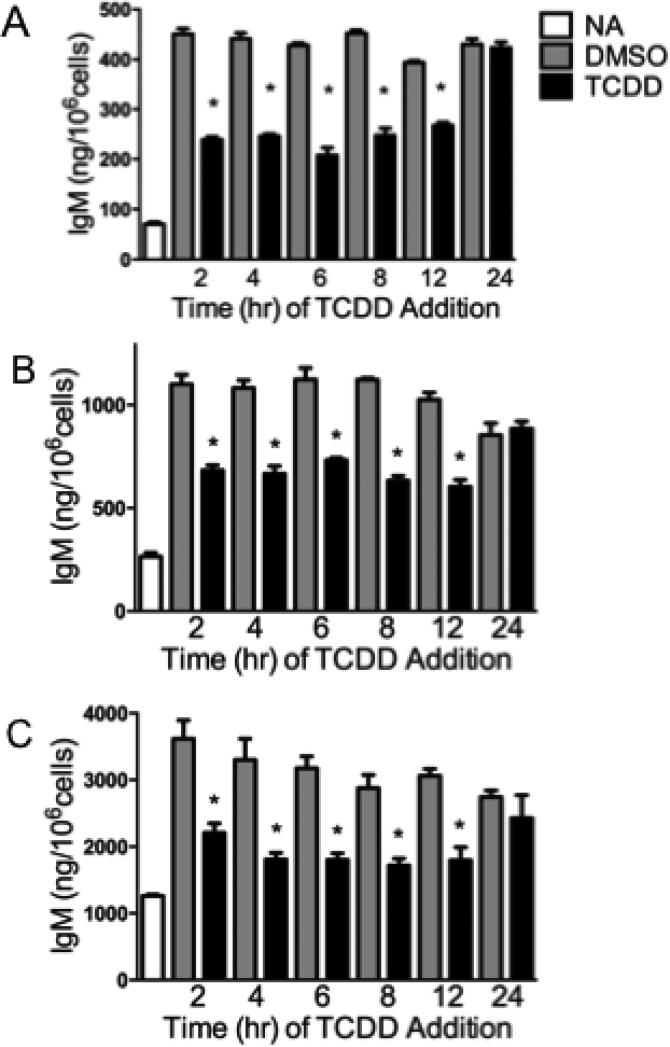
Previous studies have demonstrated that suppression of the antibody-forming cell response in splenocytes from C57BL/6 mice by TCDD occurred only when TCDD was added within the initial 24 h post activation (Tucker et al., 1986). In order to determine whether a window of sensitivity existed for the suppressive effects of TCDD on IgM secretion in SKW-AHR+ cells, time-of-addition studies were conducted. Additionally, studies were performed to compare SKW-AHR+ cells to well-characterized primary mouse and rat B lymphocytes. SKW-AHR+ cells were activated with pokeweed mitogen (PWM) and treated with 30 nM TCDD at various times after activation (2, 4, 6, 8, 12 and 24 h). In concordance with previous studies conducted in mouse primary splenocytes, TCDD was unable to suppress IgM production if added after the initial 24 h post activation in SKW-AHR+ (Fig. 4A). Moreover, TCDD must be added to the activated SKW-AHR+ cells within the initial 12 h of PWM stimulation to suppress the IgM secretion. Additionally, the window of sensitivity during which TCDD can suppress IgM secretion in primary isolated mouse and rat B lymphocytes is similar to that of SKW-AHR+. As shown in figures 4B and 4C, TCDD-induced suppression of IgM secretion in primary mouse and rat B cells is greatest during the initial 12 h period post activation. Taken together, the above findings confirm that SKW-AHR+, mouse and rat primary B cells exhibit a similar and narrow period of susceptibility for TCDD-mediated suppression of the IgM response. In addition, these findings suggest a common mechanism of action of IgM suppression by TCDD across all three species.
Figure 4.
Relationship between time of TCDD addition and in vitro IgM response. SKW-AHR+ (A), mouse (B) and rat (C) primary B cells were activated with PWM (150 μg/ml and 15 μg/ml respectively) and treated with 30 nM TCDD and/or vehicle (0.01% DMSO) at indicated time-points after activation. Supernatants were harvested at 120 h post PWM activation and the amount of secreted IgM was measured by sandwich ELISA. The data are representative of three separate experiments (one animal per experiment) with 4 experimental replicates per group. Statistical significance was determined using Dunnett's two-tailed t test; * represents values that are significantly different from naive at p < 0.05.
Effects of the AhR SNPs on the TCDD-mediated induction of Cyp1A2 and Cyp1B1 mRNA expression
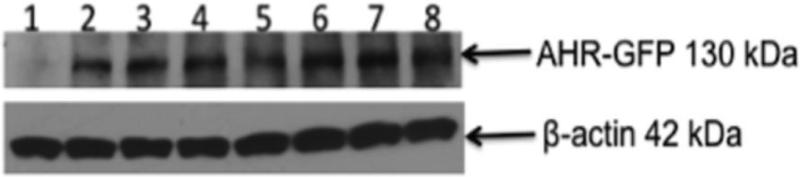
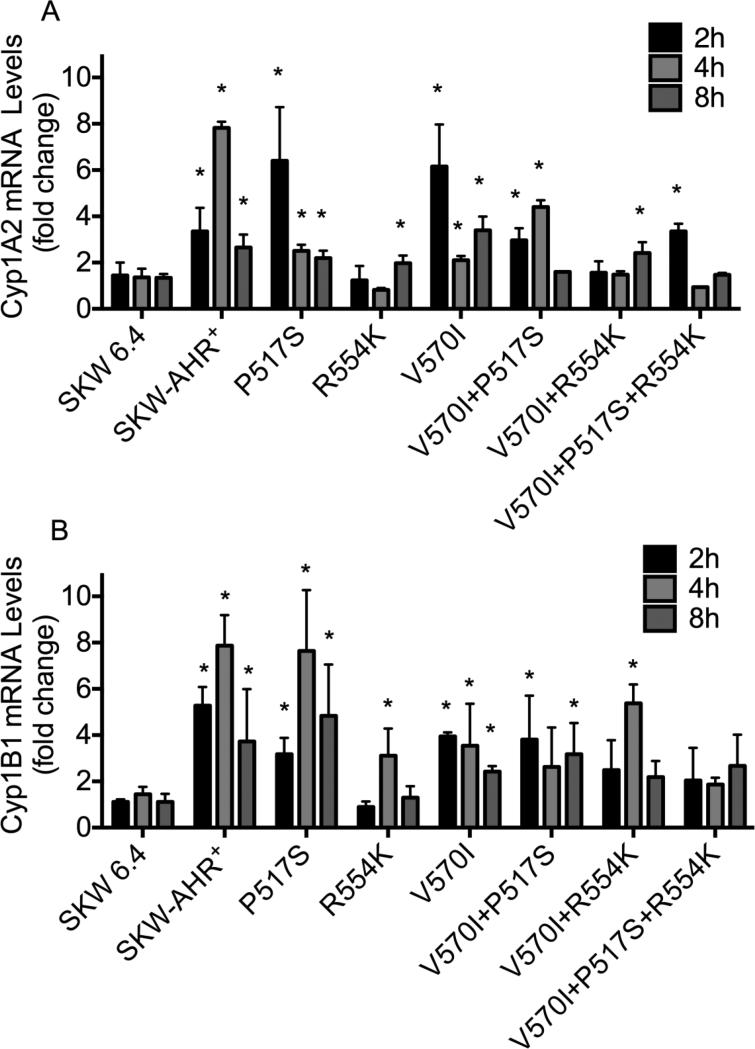
SKW 6.4 cells were transfected with AhR expression vectors to generate SKW clones that stably express P517S, R554K, I570V, P517S+I570V, R554K+I570V and P517S+R554K+I570V AhR variants. Western blot analysis showed that WT and variant AhR proteins are expressed at comparable levels across the SKW clones (Fig. 5). To investigate the transcriptional activity of the P517S, R554K, I570V, P517S+I570V, R554K+I570V and P517S+R554K+I570V variant AhRs, TCDD-mediated induction of Cyp1B1 and Cyp1A2 mRNA expression was measured by PCR. As shown in figure 6A, TCDD treatment resulted in 2-5-fold induction of the Cyp1A2 mRNA in WT, P517S, I570V and I570V+P517S clones at all time points. In contrast, R554K, R554K+I570V and P517S+R554K+I570V AhR variants had a decreased ability to mediate sustained activation of the Cyp1A2 mRNA at the time-points assessed when compared to the WT control. As expected, Cyp1A2 was not induced in the SKW 6.4 cells at any time-point. Similar findings were observed with R554K, R554K+I570V, and P517S+R554K+I570V AhR variants which displayed a decreased ability to up-regulate Cyp1B1 transcript levels (Fig. 6B). The data indicate that R554K AhR SNPs influence the transactivational activity of the AhR in human B lymphocytes, as suggested by a previous report (Wong et al., 2001b).
Figure 5.
Protein expression of the AhR variants in SKW clones. Equal amounts of whole cell lysates from the 1-SKW 6.4, 2-SKW-AHR+, 3-P517S, 4-V570I, 5-R554K, 6-R554K+V570I, 7-V570I+P517S and 8-V570I+P517S+R554K SKW cells were examined for AhR expression levels by Western Blotting. Cell lysate protein (60 μg) was loaded in each lane, resolved on 4-20% SDS-PAGE gel and probed with anti-AhR antibody. Results are representative of more than three separate experiments.
Figure 6.
Time-dependent induction of the Cyp1A2 (A) and Cyp1B1 (B) gene expression by TCDD in SKW clones. The SKW cells (5×106/ml) were treated with 30 nM of TCDD or 0.01% DMSO (VH) for the indicated periods of time. Total RNA was isolated, and steady-state Cyp1A2 and Cyp1B1 mRNA levels were measured by TaqMan qRT-PCR. Samples were normalized to the endogenous 18S ribosomal RNA, which served as loading control. Fold induction values were calculated relative to time-matched vehicle controls. The data are representative of two separate experiments with three experimental replicates per group. *, values significantly different (p<0.05) from time-matched vehicle controls.
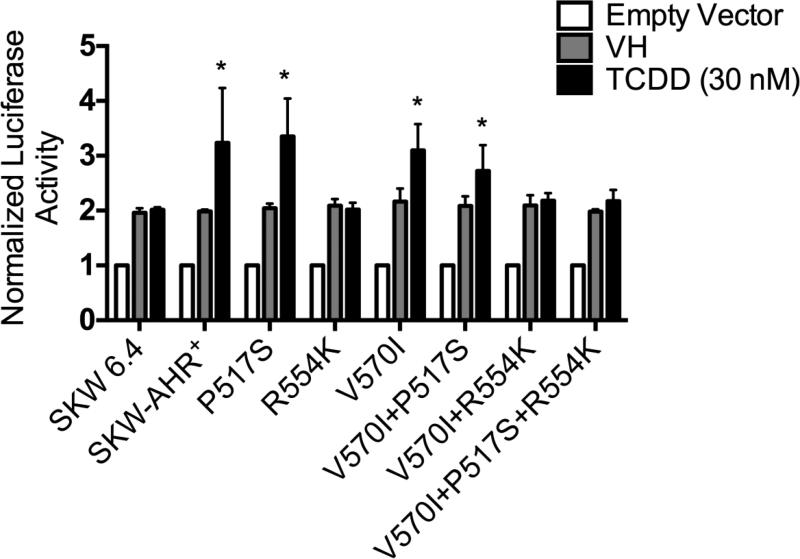
Effects of the AhR SNPs on the Cyp1B1-regulated reporter gene activity
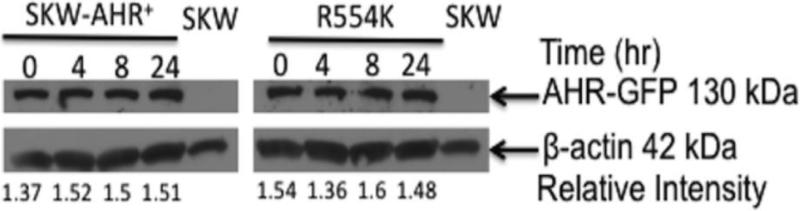
TCDD-mediated deregulation of gene expression involves the ability of AhR/ARNT complex to bind DRE elements in the regulatory regions of target genes (Okey, 2007). To determine whether any of the identified SNPs influence the ability of AhR to regulate Cyp1B1 levels, the SKW cell lines were transiently transfected with Cyp1B1-regulated reporter and treated with TCDD (30 nM) for 24h. A modest increase in Cyp1B1-regulated reporter gene activity was observed in SKW-AHR+, P517S, I570V and I570V+P517S but not in the R554K, R554K+I570V, and P517S+R554K+I570V (Fig. 7). To ensure that the loss of induction of the Cyp1B1 mRNA and Cyp1B1-regulated reporter gene activity was not a result of accelerated degradation of the polymorphic AhR, Western blot analysis was performed. Specifically, AhR protein was measured in the SKW-AHR+ and R554K cell lines at 0, 4, 8 and 24h post TCDD treatment. No difference in AhR protein levels or evidence of AhR degradation was observed after TCDD treatment in either of the two clones (Fig. 8). Thus, diminished CYP induction in the R554K expressing clones is not due to increased AhR degradation. Induction of Cyp1B1-regulated reporter gene activity was not observed in the SKW 6.4 cells, indicating that these responses require a functional AhR/ARNT signaling pathway.
Figure 7.
Ability of the WT AhR and AhR variants to mediate TCDD-induced Cyp1B1 reporter gene activity. SKW cells were transiently transfected with Cyp1B1 reporter plasmid and treated with 30 nM TCDD or VH (0.01% DMSO) for 24h. Luciferase enzyme activity was determined as described in the Materials and Methods section. Results are expressed as a fold change relative to empty vector treatment, which was set to one. n=4 for each treatment; results are combined from 4 independent experiments. Significance was determined by a one-way ANOVA followed by a Dunnett's post hoc test. A “*” denotes significance compared with the corresponding vehicle (VH) control at p < 0.05.
Figure 8.
Effects of the R554K SNP on the AhR protein stability. Time-dependent AhR protein degradation in SKW-AHR+ and R554K cells at 0, 4, 8 and 24h post TCDD treatment was examined by Western Blotting. Cell lysate protein (80 μg) was loaded in each lane, resolved on 4-20% SDS-PAGE gel and probed with anti-AhR antibody. Results are representative of more than three separate experiments.
Effects of AhR SNPs on sensitivity of the human B cells to TCDD-mediated suppression of the IgM secretion
To investigate the effects of a single P517S, R554K, I570V or a combination of P517S+I570V, R554K+I570V and P517S+R554K+I570V AhR SNPs on human B cell sensitivity to TCDD-mediated suppression of the IgM response, LPS-induced IgM secretion was measured by ELISA. To minimize the variability in background IgM levels among the SKW clones, the IgM response in all treatment groups was normalized to percent of the VH-treated control group for each SKW clone. The combined results from 6 independent experiments are presented in figure 9. TCDD at 1 nM significantly suppressed IgM secretion in SKW-AHR+, P517S, R554K, I570V, P517S+I570V and R554K+I570V SKW clones. Additionally, in all clones except for P517S+R554K+I570V, 30 and 100 nM TCDD suppressed the LPS-induced IgM response to approximately 50-60% of the VH control response. By contrast, only 10, 30 and 100 nM TCDD suppressed the IgM response in P517S+R554K+I570V clone, with maximum suppression of approximately 30% of VH control. No suppression of LPS-induced IgM secretion was observed in AhR-deficient SKW 6.4 cells at concentrations as high as 100 nM TCDD. These findings indicate that only a combination of all three AhR SNPs attenuate B cell sensitivity to TCDD-mediated suppression of the IgM response.
DISCUSSION
AhR genetic polymorphisms are associated with differences in sensitivity to toxic effects of dioxins in animal models (Moffat et al., 2007; Okey et al., 2005). Lu and colleagues (2010) reported that approximately one in six human donors showed no TCDD-induced suppression of B cell effector function. Subsequent sequencing of the AhR gene revealed that two out of the three non-responsive human donors had a polymorphism within their AhR, suggesting the possibility that AhR SNPs may play a role in human B cell sensitivity to immunotoxic effects of TCDD. To determine the influence of AhR SNPs on human B cell sensitivity to TCDD-induced suppression of the IgM response, the functional activity of three known (P517S, R554K, V570I) AhR variants, alone or in various combinations (V570I+P517S, V570I+R554K, and P517S+R554K+V570I), were examined in the human AhR null B lymphoblastiod cell line, SKW 6.4.
In a series of initial experiments, the control AhR, derived from HepG2 human hepatoma cells, was transduced into SKW 6.4 cells for the purpose of establishing AhR expressing SKW-AHR+ cells that would serve as a comparative control for investigating specific AhR SNPs. Characterization of the control SKW-AHR+ cell line showed the following important features. First, activation of SKW-AHR+ cells using the polyclonal activators, LPS and PWM, readily induced IgM secretion of similar magnitude in SKW 6.4 and SKW-AHR+ cells demonstrating that introduction of AhR expression did not suppresses or augment production of IgM compared to the parental line. Second, in spite of adding GFP to the C-terminus of the AhR, the AhR expressed by SKW-AHR+ was functional as evidenced by TCDD-mediated induction of metabolizing enzymes Cyp1B1 and Cyp1A2, and TCDD-induced suppression of the IgM response all of which did not occur in SKW 6.4 cells. Third, in time of addition experiments, the SKW-AHR+ cell line exhibited a discrete window of sensitivity to TCDD-induced suppression of the IgM response similar to that previously observed in primary mouse B lymphocytes and in the mouse B cell line, CH12.LX (Crawford et al., 2003; Tucker et al., 1986). Specifically, TCDD must be added to the cultured cells within the initial 12 h post activation to suppress IgM secretion. Taken together, the above findings show that the human AhR expressed in the SKW-AHR+ control line responds to TCDD in a manner similar to primary mouse and rat B cells as well as a previously characterized mouse B cell line.
In murine models AhR polymorphisms contribute to differences in intraspecies sensitivity to the toxic effects produced by TCDD (Okey et al., 2005). Previously, it was reported that differences in sensitivity to TCDD toxicity and induction of metabolizing enzymes in humans might be due to AhR polymorphisms (Micka et al., 1997). R554K is one of the most frequent (frequency of 21.7 %) and studied AhR polymorphism (Exome Variant Server, http://evs.gs.washington.edu/EVS/). Wong et al. (2001a) reported that P517S is in linkage disequilibrium (a nonrandom association of alleles at two or more loci) with R554K and V570I in individuals of African decent. All three polymorphisms are located in the transactivation domain of the AhR, and even though P517S and V570I are comparatively rare, frequencies of 0.24 % and 2.3 %, respectively, their combined expression might result in an AhR with distinct intrinsic transactivation potentials. Furthermore, Wong et al. (2001a) reported that three out of 339 (approximately 1.1 %) individuals screened expressed AhR heterozygous for all three variants. Current experimental findings suggest that the effects of the R554K AhR variant are controversial and require additional investigation. For example, Smart and Daly (2000) showed that the R554K SNP resulted in increased ability of the AhR to induce Cyp1A1 mRNA levels in human lymphocytes compared with the control AhR in response to 3-methylcholantrene (3MC) treatment. Conversely, Celius and Matthews (2010) found that R554K does not alter the ability of the AhR to transactivate Cyp1A1 and Cyp1B1 expression in Hepa1, MCF-7 or AHR100 cells after TCDD treatment. Koyano and coworkers (2005) reported that R554K SNP did not affect transactivation properties of AhR with beta-naphthoflavone (BNF), 3MC or omeprazole (OME) treatment in transiently transfected HeLa cells. However, a different study reported that the AhR variants with two SNPs R554K+V570I as well as the three SNPs K554R+V570I+P517S concomitantly expressed, failed to mediate TCDD-dependent induction of the Cyp1A1 mRNA in Hepa 1 cells (Wong et al., 2001b). The underlying reason for the differences among the studies is not well understood but might be due, in part, to intrinsic differences associated with assay conditions and cell models used. It is also noteworthy that the magnitude of induction by TCDD of Cyp1A2 and Cyp1B1 mRNA levels in SKW-AHR+ cell line, which was relatively modest compared to liver cells, closely paralleled what is typically observed in primary human peripheral blood B cells expressing lower levels of AhR (Lu et al., 2010).
Our studies are in agreement with Wong and coworkers (2001b) in that the R554K, R554K+V570I and K554R+V570I+P517S variants all exhibited attenuated induction of Cyp1A2, and Cyp1B1 mRNA levels and Cyp1B1-driven luciferase reporter in SKW cells by TCDD treatment. It is possible that the attenuation in Cyp1A2 and Cyp1B1 induction associated with the R554K polymorphic AhRs is due to a decrease in protein and mRNA stability; however, previous studies showed that this variant did not influence AhR mRNA and/or protein levels (Koyano et al., 2005). Additionally, it had been shown that polymorphisms in exon 10 of the AhR do not affect the ability of the receptor to bind ligands or DREs in vitro (Wong et al., 2001b). One possible interpretation for attenuated Cyp1A2 and Cyp1B1 inducibility in SKW cells that express R554K AhR is that SNPs within the transactivation domain interfere with the ability of the variant receptors to maintain necessary protein-protein interactions. To induce CYP1 gene expression, the AhR/Arnt heterodimer must bind DRE sequences, recruit and interact (via carboxyl terminus) with a large number of coactivators and mediators. For example, CREB binding protein (CBP), steroid receptor coactivator (SRC-1), the retinoblastoma protein Rb and multiple other protein coactivators have been shown to interact with the AhR/Arnt complex to mediate TCDD-induced reporter gene expression (Fujii-Kuriyama and Mimura, 2005). Considering a large number of protein-protein interactions are necessary to mediate upregulation of CYP1 metabolizing enzymes, it is plausible to speculate that R554K AhR variant might fail to recruit coactivators as efficiently as the control AhR. Additionally, R554K SNP may impact the stability of the AHR/ARNT heterodimer's interaction with DNA or decrease the AHR affinity for the necessary coactivators.
Multiple epidemiological studies have investigated the effects of the R554K polymorphism on susceptibility to disease in different human populations. The R554K variant was found to be associated with a decreased risk of developing male infertility (Safarinejad et al., 2013) and significantly lower levels of AhR, Arnt and Cyp1B1 mRNA expressed in white blood cells from the Caucasian population (Helmig et.al., 2011). No association was found between R554K and increased risk of non-Hodgkin's lymphoma (Ng et al., 2010). The R554K variant was found to be associated with a higher risk of coronary arterial disease in the Chinese population (Huang et al., 2015). Additionally, polymorphisms within AhR-interacting genes Ahrr, Hsp90, and AIP have been identified and could potentially contribute to physiological and health outcomes associated with TCDD-induced AhR activation (Cavaco et al., 2013; Rowlands et al., 2011; Urban et al., 2012).
In order to investigate the role of AhR SNPs on the sensitivity of the human B cells to TCDD-mediated IgM suppression, extensive concentration-response studies were conducted. TCDD suppressed IgM secretion in all SKW-based cell lines in a concentration-dependent manner, with 100nM TCDD suppressing the IgM response to approximately 50% of the VH control group. However, SKW-K554R+V570I+P517S expressing cells had a decreased sensitivity to TCDD-mediated IgM suppression as evidenced by a higher IC50 and an attenuated maximum level of suppression as demonstrated by only a 30% decrease in IgM secretion at 100nM TCDD, compared with vehicle control.
Our study has several limitations. Unlike previous studies, we used lentiviral transduction to establish clones that stably express different AhR variants. Genome-wide studies have shown that lentivirus randomly integrates into actively transcribed genes, possibly resulting in insertional mutagenesis potentially leading to changes in gene expression. Therefore, for each AhR SNP at least three clones were evaluated all of which exhibited a similar profile of activity. Additionally, we used the parental SKW 6.4 B cell line that, unlike naïve primary B cells, is intrinsically in the early stages of differentiation. Moreover, B cells have been previously reported to have a lower magnitude of TCDD-driven induction of Cyp1A1 and Cyp1B1 mRNA making it harder to differentiate between small differences in AhR variant activity (Lu et al., 2010).
In conclusion, we confirmed previous findings showing that the combined V570I+R554K variant exhibited attenuated induction of metabolizing enzymes in hepatocytes (Wong et al., 2001). Moreover, in leukocytes, all the AhR variants containing R554K polymorphisms had a reduced ability to support TCDD-mediated induction of Cyp1A2 and Cyp1B1. However, none of the individual polymorphisms attenuated suppression of the B cell IgM response. In fact, only all three SNPs in combination were able to partially attenuate suppression of the IgM secretion by TCDD, suggesting that that the effects of these SNPs might have a modest impact on the sensitivity of the human population to humoral immune suppression by TCDD. Until now, we have lacked an appropriate model to study the effects of different polymorphic forms of the human AhR on one of the most sensitive endpoints of TCDD exposure, suppression of B cell IgM secretion. The expression of different polymorphic forms of the human AhR in the human B cell line SKW 6.4 allowed the first opportunity to make direct functional comparisons between polymorphic receptor forms in a cell type- and species-specific manner yielding insights into aspects of the AhR biology that may contribute to differences in receptor function within and across species.
Highlights.
Mouse, rat and SKW-AHR+ B cells have a similar window of sensitivity to TCDD
R554K AhR SNP alters B cell sensitivity to TCDD-mediated Cyp1B1 and Cyp1A2 induction
Combination of P517S, R554K, and V570I SNPs attenuates TCDD-induced IgM suppression
ACKNOWLEDGMENTS
We thank Dr. Weiguo Han for providing the pGL3-basic vector containing Cyp1B1 promoter region used in this study.
FUNDING INFORMATION
This work was supported by the National Institute of Environmental Health Sciences [grant numbers P42 ES04911 and R01 ES002520].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bello SM, Franks DG, Stegeman JJ, Hahn ME. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: in vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci. 2001;60:77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carver LA, LaPres JJ, Jain S, Dunham EE, Bradfield CA. Characterization of the Ah receptor-associated protein, ARA9. J. Biol. Chem. 1998;273:33580–33587. doi: 10.1074/jbc.273.50.33580. [DOI] [PubMed] [Google Scholar]
- Catteau A, Douriez E, Beaune P, Poisson N, Bonaiti-Pellie C, Laurent P. Genetic polymorphism of induction of CYP1A1 (EROD) activity. Pharmacogenetics. 1995;5:110–119. doi: 10.1097/00008571-199504000-00008. [DOI] [PubMed] [Google Scholar]
- Cauchi S, Stucker I, Solas C, Laurent-Puig P, Cenee S, Hemon D, Jacquet M, Kremers P, Beaune P, Massaad-Massade L. Polymorphisms of human aryl hydrocarbon receptor (AhR) gene in a French population: relationship with CYP1A1 inducibility and lung cancer. Carcinogenesis. 2001;22:1819–1824. doi: 10.1093/carcin/22.11.1819. [DOI] [PubMed] [Google Scholar]
- Cavaco I, Hombhanje FW, Gil JP, Kaneko A. Frequency of the functionally relevant aryl hydrocarbon receptor repressor (AhRR) Pro185Ala SNP in Papua New Guinea. Drug Metab. Pharmacokinet. 2013;28:519–521. doi: 10.2133/dmpk.dmpk-13-sc-035. [DOI] [PubMed] [Google Scholar]
- Celius T, Matthews J. Functional analysis of six human aryl hydrocarbon receptor variants in human breast cancer and mouse hepatoma cell lines. Toxicology. 2010;277:59–65. doi: 10.1016/j.tox.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Crawford RB, Sulentic CE, Yoo BS, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p dioxin (TCDD) alters the regulation and posttranslational modification of p27kip1 in lipopolysaccharide-activated B cells. Toxicol. Sci. 2003;75:333–342. doi: 10.1093/toxsci/kfg199. [DOI] [PubMed] [Google Scholar]
- Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol. Pharmacol. 1993;44:911–917. [PubMed] [Google Scholar]
- Dooley RK, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: I. The role of the B lymphocyte. Immunopharmacology. 1988;16:167–180. doi: 10.1016/0162-3109(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Enan E, Overstreet JW, Matsumura F, VandeVoort CA, Lasley BL. Gender differences in the mechanism of dioxin toxicity in rodents and in nonhuman primates. Reprod. Toxicol. 1996;10:401–411. doi: 10.1016/0890-6238(96)83995-5. [DOI] [PubMed] [Google Scholar]
- Flaveny C, Reen RK, Kusnadi A, Perdew GH. The mouse and human Ah receptor differ in recognition of LXXLL motifs. Arch. Biochem. Biophys. 2008;471:215–223. doi: 10.1016/j.abb.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA, Perdew GH. Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicol. Sci. 2010;114:217–225. doi: 10.1093/toxsci/kfp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem. Biophys. Res. Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- Guven H, Konstantinidis KV, Alici E, Aints A, Abedi-Valugerdi M, Christensson B, Ljunggren HG, Dilber MS. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp. Hematol. 2005;33:1320–1328. doi: 10.1016/j.exphem.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem. Biol. Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Harper PA, Wong J, Lam MS, Okey AB. Polymorphisms in the human AH receptor. Chem. Biol. Interact. 2002;141:161–187. doi: 10.1016/s0009-2797(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Layko D, Nyska A, Hukkanen RR, Manno RA, Grassetti A, Lawson M, Martin G, Budinsky RA, Rowlands JC, Thomas RS. Aryl hydrocarbon receptor knockout rats are insensitive to the pathological effects of repeated oral exposure to 2,3,7,8-tetrachlorodibenzo p-dioxin. J. Appl. Toxicol. 2015 doi: 10.1002/jat.3211. doi: 10.1002/jat.3211. [DOI] [PubMed] [Google Scholar]
- Helmig S, Seelinger JU, Dohrel J, Schneider J. RNA expressions of AHR, ARNT and CYP1B1 are influenced by AHR Arg554Lys polymorphism. Mol. Genet. Metab. 2011;104:180–184. doi: 10.1016/j.ymgme.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Morris DL, Wood SC, Snyder NK. 2,3,7,8-tetrachlorodibenzo-p dioxin-induced changes in immunocompetence: possible mechanisms. Annu. Rev. Pharmacol. Toxicol. 1991;31:73–100. doi: 10.1146/annurev.pa.31.040191.000445. [DOI] [PubMed] [Google Scholar]
- Huang S, Shui X, He Y, Xue Y, Li J, Li G, Lei W, Chen C. AhR expression and polymorphisms are associated with risk of coronary arterial disease in Chinese population. Sci. Rep. 2015;5:8022. doi: 10.1038/srep08022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri K, Watanabe J, Eguchi H, Nakachi K, Kiyohara C, Hayashi S. Polymorphisms of human Ah receptor gene are not involved in lung cancer. Pharmacogenetics. 1995;5:151–158. doi: 10.1097/00008571-199506000-00003. [DOI] [PubMed] [Google Scholar]
- Kleeman JM, Olson JR, Peterson RE. Species differences in 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity and biotransformation in fish. Fundam. Appl. Toxicol. 1988;10:206–213. doi: 10.1016/0272-0590(88)90304-1. [DOI] [PubMed] [Google Scholar]
- Koyano S, Saito Y, Fukushima-Uesaka H, Ishida S, Ozawa S, Kamatani N, Minami H, Ohtsu A, Hamaguchi T, Shirao K, Yoshida T, Saijo N, Jinno H, Sawada J. Functional analysis of six human aryl hydrocarbon receptor variants in a Japanese population. Drug. Metab. Dispos. 2005;33:1254–1260. doi: 10.1124/dmd.105.004655. [DOI] [PubMed] [Google Scholar]
- LaPres JJ, Glover E, Dunham EE, Bunger MK, Bradfield CA. ARA9 modifies agonist signaling through an increase in cytosolic aryl hydrocarbon receptor. J. Biol. Chem. 2000;275:6153–6159. doi: 10.1074/jbc.275.9.6153. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu H, Crawford RB, North CM, Kaplan BL, Kaminski NE. Establishment of an immunoglobulin m antibody-forming cell response model for characterizing immunotoxicity in primary human B cells. Toxicol. Sci. 2009;112:363–373. doi: 10.1093/toxsci/kfp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Crawford RB, Suarez-Martinez JE, Kaplan BL, Kaminski NE. Induction of the aryl hydrocarbon receptor-responsive genes and modulation of the immunoglobulin M response by 2,3,7,8-tetrachlorodibenzo-p-dioxin in primary human B cells. Toxicol. Sci. 2010;118:86–97. doi: 10.1093/toxsci/kfq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micka J, Milatovich A, Menon A, Grabowski GA, Puga A, Nebert DW. Human Ah receptor (AHR) gene: localization to 7p15 and suggestive correlation of polymorphism with CYP1A1 inducibility. Pharmacogenetics. 1997;7:95–101. doi: 10.1097/00008571-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- Moffat ID, Roblin S, Harper PA, Okey AB, Pohjanvirta R. Aryl hydrocarbon receptor splice variants in the dioxin-resistant rat: tissue expression and transactivational activity. Mol. Pharmacol. 2007;72:956–966. doi: 10.1124/mol.107.037218. [DOI] [PubMed] [Google Scholar]
- Morel Y, Barouki R. Down-regulation of cytochrome P450 1A1 gene promoter by oxidative stress. Critical contribution of nuclear factor 1. J. Biol. Chem. 1998;273:26969–26976. doi: 10.1074/jbc.273.41.26969. [DOI] [PubMed] [Google Scholar]
- Ng CH, Janoo-Gilani R, Sipahimalani P, Gallagher RP, Gascoyne RD, Connors JM, Weber JP, Lai AS, Leach S, Le ND, Brooks-Wilson AR, Spinelli JJ. Interaction between organochlorines and the AHR gene, and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2010;21:11–22. doi: 10.1007/s10552-009-9429-5. [DOI] [PubMed] [Google Scholar]
- Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol. Sci. 2007;98:5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- Okey AB, Franc MA, Moffat ID, Tijet N, Boutros PC, Korkalainen M, Tuomisto J, Pohjanvirta R. Toxicological implications of polymorphisms in receptors for xenobiotic chemicals: the case of the aryl hydrocarbon receptor. Toxicol. Appl. Pharmacol. 2005;207:43–51. doi: 10.1016/j.taap.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol. Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- Poland A, Glover E. Characterization and strain distribution pattern of the murine Ah receptor specified by the Ahd and Ahb-3 alleles. Mol. Pharmacol. 1990;38:306–312. [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol. Pharmacol. 1994;46:915–921. [PubMed] [Google Scholar]
- Ralph P, Jeong G, Welte K, Mertelsmann R, Rabin H, Henderson LE, Souza LM, Boone TC, Robb RJ. Stimulation of immunoglobulin secretion in human B lymphocytes as a direct effect of high concentrations of IL 2. J. Immunol. 1984;133:2442–2445. [PubMed] [Google Scholar]
- Rowlands JC, Urban JD, Wikoff DS, Budinsky RA. An evaluation of single nucleotide polymorphisms in the human aryl hydrocarbon receptor-interacting protein (AIP) gene. Drug Metab. Pharmacokinet. 2011;26:431–439. doi: 10.2133/dmpk.dmpk-11-sc-013. [DOI] [PubMed] [Google Scholar]
- Safarinejad MR, Shafiei N, Safarinejad S. Polymorphisms in aryl hydrocarbon receptor gene are associated with idiopathic male factor infertility. Reprod. Sci. 2013;20:1423–1432. doi: 10.1177/1933719113488451. [DOI] [PubMed] [Google Scholar]
- Scott SP, Teh A, Peng C, Lavin MF. One-step site-directed mutagenesis of ATM cDNA in large (20kb) plasmid constructs. Hum. Mutat. 2002;20:323. doi: 10.1002/humu.9068. [DOI] [PubMed] [Google Scholar]
- Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, Kerzee JK, Uno S, Shertzer HG. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic. Biol. Med. 2002;33:1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- Smart J, Daly AK. Variation in induced CYP1A1 levels: relationship to CYP1A1, Ah receptor and GSTM1 polymorphisms. Pharmacogenetics. 2000;10:11–24. doi: 10.1097/00008571-200002000-00003. [DOI] [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol. Pharmacol. 1998;53:623–629. [PubMed] [Google Scholar]
- Sulentic CE, Kaminski NE. The long winding road toward understanding the molecular mechanisms for B-cell suppression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 120 Suppl. 2011;1:S171–191. doi: 10.1093/toxsci/kfq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AN, Vore SJ, Luster MI. Suppression of B cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol. Pharmacol. 1986;29:372–377. [PubMed] [Google Scholar]
- Urban JD, Budinsky RA, Rowlands JC. An evaluation of single nucleotide polymorphisms in the human heat shock protein 90 kDa alpha and beta isoforms. Drug Metab. Pharmacokinet. 2012;27:268–278. doi: 10.2133/dmpk.dmpk-11-sc-114. [DOI] [PubMed] [Google Scholar]
- Wong JM, Harper PA, Meyer UA, Bock KW, Morike K, Lagueux J, Ayotte P, Tyndale RF, Sellers EM, Manchester DK, Okey AB. Ethnic variability in the allelic distribution of human aryl hydrocarbon receptor codon 554 and assessment of variant receptor function in vitro. Pharmacogenetics. 2001;11:85–94. doi: 10.1097/00008571-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Wong JM, Okey AB, Harper PA. Human aryl hydrocarbon receptor polymorphisms that result in loss of CYP1A1 induction. Biochem. Biophys. Res. Commun. 2001;288:990–996. doi: 10.1006/bbrc.2001.5861. [DOI] [PubMed] [Google Scholar]