SUMMARY
BACKGROUND
The association between thrombophilia and deep venous thrombosis associated with central venous catheter (CADVT), the most important pediatric risk factor for thrombosis, is unclear in children. Pediatric studies with small sample sizes report conflicting results. We sought to evaluate whether among children with central venous catheter, thrombophilia increases the risk of CADVT.
MATERIALS AND METHODS
We systematically searched MEDLINE, EMBASE, Web of Science, Cochrane Central Register for Controlled Trials, PubMed and reference lists for controlled studies published from inception of the database until September 2015. Included were studies of children <21 years old with central venous catheter who were systematically tested for thrombophilic traits commonly screened for in clinical practice. Pooled prevalence and odds ratio (pOR) of CADVT with thrombophilia were estimated using random effects model.
RESULTS
We analyzed 16 cohort studies with 1,279 children, 277 of whom had CADVT, and with 12 traits tested. There was significant heterogeneity in the included studies. Presence of ≥1 trait was associated with CADVT (pOR: 3.20; 95% confidence interval [CI]: 1.56–6.54). Although the prevalence of most traits was <0.10, children with protein C deficiency, elevated factor VIII and factor V Leiden mutation had increased prevalence of CADVT. The association with thrombophilia seems stronger with symptomatic (pOR: 6.71; 95% CI: 1.93–23.37) than asymptomatic CADVT (pOR: 2.14; 95% CI: 1.10–4.18).
CONCLUSIONS
Based on the low prevalence of specific traits, relatively weak association with CADVT, and limitations of included studies, we cannot recommend routine testing of thrombophilias in children with CADVT.
Keywords: central venous catheter, factor V Leiden, factor VIII, protein C deficiency, venous thromboembolism
INTRODUCTION
Deep venous thrombosis (DVT) is a multifactorial disease. In children, central venous catheter (CVC)-associated DVT (CADVT) accounts for >85% of DVT [1]. The pathophysiology of pediatric CADVT is poorly understood [2]. Without doubt, the CVC itself, causing endothelial damage and obstruction to flow, is thought to be the most potent factor [3]. However, not all children with CVC develop CADVT. We have shown that CADVT occurs in only 20% of children with CVC who underwent active radiologic surveillance for DVT [4]. Because of complications associated with CADVT, such as pulmonary embolism, paradoxical embolic stroke, blood stream infection and loss of venous access, identification of risk factors for CADVT in children may improve our understanding of the pathophysiology of CADVT, aid in risk-stratification and may even provide future targets for prevention [3].
Thrombophilia is a hypercoagulable state that increases the propensity to the development of DVT [5]. Inherited thrombophilic traits are uncommon and occur in <10% of the general population [6]. However, in its presence, Young et al showed that the risk of a venous thromboembolic event increases by 3- to 9-fold in children depending on which trait is present [7]. It is unclear whether thrombophilias confer additional risk of DVT in children with CVC. Pediatric studies report conflicting results, likely due to small sample sizes, variations in which tests were performed, inconsistencies in the definition of CADVT, differences in underlying diagnoses, and perhaps most importantly, the lack of control populations [5, 7]. As a consequence, the rationale of diagnostic evaluation for thrombophilia, which has become standard practice in many pediatric centers, remains uncertain for children with CADVT [5, 8]. In this systematic review, we sought to evaluate whether among children with CVC, thrombophilia increases the risk of CADVT.
PATIENTS AND METHODS
The study protocol was registered with the PROSPERO database (Registry Number: CRD42015027517) [9]. The findings were reported according to the Meta-Analyses and Systematic Review of Observational Studies guideline [10].
Eligibility Criteria
We included studies with concurrent controls (i.e., cross-sectional, case-control, cohort, and randomized controlled trials) that recruited children <21 years old with any type of CVC. Only articles in which children were systematically evaluated for CADVT and ≥1 thrombophilic trait were included. We included the most common clinically tested inherited traits (factor V [factor V Leiden; G1691A], methylenetetrahydrofolate reductase [MTHFR; C665C→T or C1286A→C] or prothrombin [G20210A] gene mutations), as well as traits that may be inherited or acquired (protein C, protein S or antithrombin deficiency; elevated lipoprotein(a), homocysteine or factor VIII level; or, presence of antiphospholipid antibody, including anticardiolipin antibody and lupus anticoagulant) [2, 5]. In studies with multiple citations, including abstracts and unpublished articles, we only included the article with the most complete results. For studies with children and adults, we analyzed the data from children.
Data Sources and Searches
We searched MEDLINE (OvidSP), EMBASE (OvidSP), Web of Science (Thomson Reuters), Cochrane Central Register for Controlled Trials (Wiley Online) and PubMed (unindexed materials only) for studies from inception of the database until September 2015. We searched the Conference Proceedings Citation Index via Web of Science (Thomson Reuters) for conference abstracts. With a medical research librarian (DH), 2 authors (SNV and EVSF) developed, refined and performed the search. The search strategy used controlled vocabulary words and synonymous free text words to capture the concepts of venous thrombosis, catheter and thrombophilia (supplemental material). It was limited to the age group of 0-24 but was not limited by language of publication. We also hand searched reference lists and review articles for additional citations.
We hierarchically identified eligible studies. Independently and blindly, 2 authors (SNV and RP) reviewed the titles and abstracts for exclusion, then reviewed the full texts of the remaining articles to finalize the list. A third author (EVSF) resolved conflicts in opinion.
Data Abstraction and Definitions
Data was abstracted by 3 authors (SNV, RP and EVSF) independently. Conflicts in opinion were resolved by discussion. We abstracted data on the included studies, children enrolled, traits and CADVT. In studies in which the mean age of the children enrolled were not reported, we estimated the mean age from the reported age ranges. Authors were contacted for missing data. The data we presented may be different from the publications because of clarifications from the authors.
A positive trait was defined as per the included studies, except that heterozygous and homozygous factor V Leiden and prothrombin gene mutations were combined to define a positive trait in our analyses [7]. In addition, only homozygous or compound heterozygous mutations in the MTHFR gene were considered positive. We used the definition of CADVT in the included studies. Typically, it is a thrombus extending from the CVC into the lumen of the deep vein where the CVC was inserted that was diagnosed radiologically [4]. It can be symptomatic if with signs and symptoms consistent with DVT or CVC dysfunction, or asymptomatic if detected only through active radiologic surveillance. In some of the studies, symptomatic CADVT was the only outcome while in other studies with active radiologic surveillance, data on symptomatic CADVT could be abstracted.
Assessment of Risk of Bias
Risk of bias was independently assessed by 2 authors (SNV and EVSF) with conflicts of opinion resolved by discussion. Publication bias was assessed graphically and quantitatively [11]. Qualitatively, the risk of selection, performance, detection, attrition and reporting bias was categorized as low, high or unclear, as modified from the Cochrane Collaboration’s tool for randomized trials [12]. Risk of bias was unclear if the available data was insufficient to determine the risk. A study was considered low risk of selection bias if ≥75% of eligible children participated [13]. The risk of attrition bias was considered low if ≥95% of enrolled children were assessed for CADVT [14].
Data Synthesis and Analysis
The presence of CADVT at any time during the study period was the primary outcome measure with presence of ≥1 trait the primary exposure of interest. Our unit of analysis was the individual child. The pooled prevalence of a trait and CADVT, and the pooled odds ratio (pOR) of CADVT with thrombophilia were calculated using the method of DerSimonian and Laird [15]. We used the random effects model because, a priori, we hypothesized that there were significant differences in the study design and population, and definition of exposure and outcome among studies. We assessed statistical heterogeneity with the I2 statistic, which is the percentage of total variation across studies that is due to heterogeneity rather than chance [16]. Meta-regression was performed to adjust for potential confounding [17].
In the secondary analyses, we stratified children based on trait, symptomatic vs. asymptomatic CADVT, and underlying medical diagnosis. We classified asymptomatic CADVT as no CADVT using data from studies with symptomatic CADVT only and those with active radiologic surveillance. We also performed the analysis excluding asymptomatic CADVT using only the data from studies with active radiologic surveillance. To estimate the proportion of CADVT in children with CVC that can be attributed to thrombophilia assuming that the association was causal, we calculated the population attributable risk, which combined the prevalence of the exposure (pooled prevalence of the trait) and its strength of association with the outcome (pOR of CADVT) [18, 19]. We conducted sensitivity analyses for missing data by searching for scenarios that minimized and maximized the pOR.
All statistical analyses were performed using Stata 14.1 (StataCorp, College Station, TX). We determined statistical significance at a 2-sided P<0.05.
RESULTS
Description of Included Studies
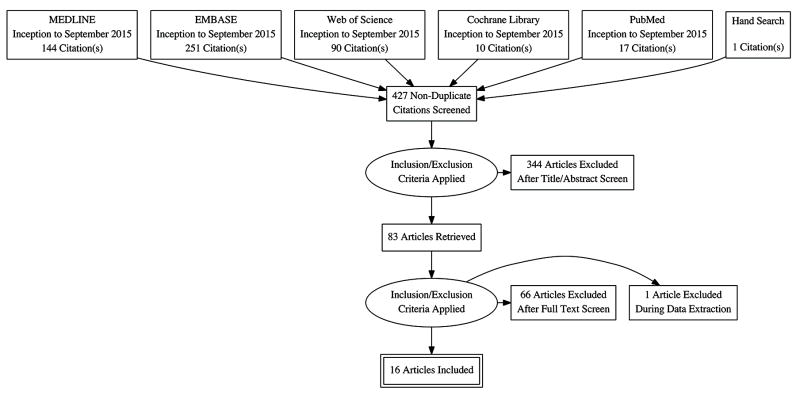
We obtained 426 non-duplicate citations from the electronic databases (Figure 1). Hand searching yielded 1 additional unique citation. Studies were excluded based on study design (n=113), age of the study population (n=40), absence of CVC (n=233), lack of systematic testing for thrombophilia (n=152) or absence of CADVT as outcome (n=246). We excluded 1 study during data extraction because we were unable to obtain data in children despite attempts to contact the authors [20]. A total of 15 prospective and 1 retrospective cohort studies with 1,279 children were analyzed (Table 1) [21-36]. There were 9 studies with 629 (49.2%) children with malignancy and 2 with 230 (18.0%) children with cardiac disease [21-25, 28, 29, 31-34]. A total of 3 studies enrolled children with mixed diagnoses that could not be further categorized into specific diagnoses [27, 30, 35]. The mean age of children enrolled ranged from 0 to 17.7 years while the proportion of male children enrolled ranged from 52.8% to 72.2%.
Figure 1.
Study selection
Table 1.
Characteristics of included studies
| Author (year) [ref] | Study design |
Study population |
No. of children |
Mean age in years |
No. of males |
Thrombophilia trait tested |
Imaging modality |
Symptomatic CADVT only |
No. with thrombophilia |
No. with CADVT |
|---|---|---|---|---|---|---|---|---|---|---|
| Nowak-Gottl (1997) [21] | Prospective cohort | Malignancy, cardiac | 163 | 3.1 | NR | APLA, AT, FVL, LPA, PC, PS | Echo, US | No | 17 | 18 |
| Knöfler (1999) [22] | Prospective cohort | Malignancy | 77 | 8.0 | NR | AT, FVL, HC, LPA, PC, PS, PT | US, venography | No | 16 | 11 |
| Wermes (1999) [23] | Prospective cohort | Malignancy | 133 | 6.0 | 77 | FVL, MTHFR, PT | CTV, MRV, US, venography | Yes | 30 | 6 |
| Ruud (2002) [24] | Prospective cohort | Malignancy | 41 | 7.9 | 24 | APLA, AT, FVL, LPA, HC, PC, PS, PT | US | No | 12 | 18 |
| Mitchell (2003) [25] | Prospective cohort | Malignancy | 60 | 5.3 | NR | APLA, FVL, PT | Echo, MRV, US | No | 10 | 22 |
| Finkelstein (2004) [26] | Prospective cohort | Thalassemia | 23 | 13.2 | NR | APLA, AT, FVL, LPA, MTHFR, PC, PS, PT | Echo, US, venography | No | 9 | 8 |
| Dubois (2007) [27] | Prospective cohort | Mixed | 214 | 6.8 | 113 | FVL, PT | Venography, US | No | 9 | 20 |
| Farinasso (2007) [28] | Prospective cohort | Malignancy | 56 | 5.0 | 32 | APLA, FVIII, FVL, HC, LPA, MTHFR, PC PS, PT | CTV, US | No | 19 | 41 |
| Lipay (2007) [29] | Retrospective cohort | Malignancy | 89 | 10.2 | 63 | APLA, FVL, MTHFR, PT | US | Yes | 9 | 9 |
| Turebylu (2007) [30] | Prospective cohort | Mixed | 27 | 0 | NR | FV, MTHFR, PT | US | No | 7 | 6 |
| Chung (2008) [31] | Prospective cohort | Malignancy | 36 | 9.1 | 26 | AT, FVL, MTHFR, PC, PS, PT | Venography | Yes | 2 | 3 |
| Rask (2011) [32] | Prospective cohort | Hemophilia | 20 | 5.5 | NR | FVL, PT | US, venography | No | 3 | 6 |
| Albisetti (2013) [33] | Prospective cohort | Malignancy | 114 | 6.0 | 64 | AT, FVL, LPA, PC, PS, PT | MRV | No | 38 | 45 |
| Thom (2014) [34] | Prospective cohort | Cardiac | 90 | 2.7 | 56 | AT, FVL, HC, LA, LPA, MTHFR, PC, PS, PT | Echo, US, venography | No | 34 | 25 |
| Faustino (2015) [35] | Prospective cohort | Mixed | 83 | 5.8 | 49 | FVIII, FVL, PT | US | No | 14 | 37 |
| Munck (2015) [36] | Prospective cohort | Cystic fibrosis | 53 | 17.7 | NR | APLA, AT, FVL, PC, PS, PT | US | No | 8 | 2 |
NR – not reported; CADVT – central venous catheter-associated deep venous thrombosis; APLA – antiphospholipid antibody; AT – antithrombin deficiency; FVIII – elevated factor VIII; FVL – factor V Leiden mutation; HC – homocysteinemia; LPA – elevated lipoprotein(a); MTHFR – methylene tetrahydrofolate reductase gene mutation; PC – protein C deficiency; PS – protein S deficiency; PT – prothrombin mutation; CTV – computed tomography venography; echo – echocardiography; MRV – magnetic resonance venography; US – ultrasonography
The included studies tested for a total of 12 traits with a median of 6 (range: 2-11) (Table 1). Of 11 studies that tested for potentially acquired traits, only 5 repeated testing to confirm the diagnosis [21, 22, 24, 25, 31].
CADVT was diagnosed radiologically using 4 different modalities (Table 1). A total of 3 studies had symptomatic CADVT only as outcome [23, 29, 31]. Data on symptomatic CADVT was available from 7 studies with active radiologic surveillance [21, 22, 28, 30, 34-36].
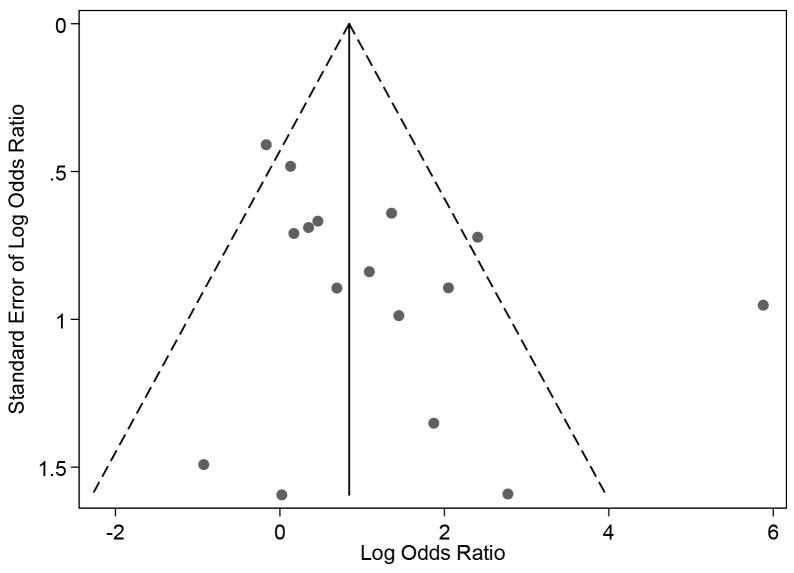
There was no significant publication bias in the included studies (bias: -23.3, P=0.63; Figure 2). Qualitatively, the risk of bias was generally low (Table 2). However, we were unable to assess the risk of selection and detection biases in a number of studies due to insufficient data. We initially assessed 4 studies to be at high risk of reporting bias because of missing data needed to categorize children with respect to the presence of thrombophilia [24, 28, 29, 36]. In 3 of these, the required data was provided by the authors minimizing the risk of bias [28, 29, 36].
Figure 2.
Funnel plot for publication bias
Table 2.
Risk of bias assessment
| Author (year) [ref] | Low participation rate (selection bias) | Blinding of personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|
| Nowak-Gottl (1997) [21] | ? | − | ? | − | − |
| Knöfler (1999) [22] | ? | − | ? | − | − |
| Wermes (1999) [23] | ? | − | ? | − | − |
| Ruud (2002) [24] | − | − | − | − | + |
| Mitchell (2003) [25] | − | − | − | − | − |
| Finkelstein (2004) [26] | ? | − | ? | − | − |
| Dubois (2007) [27] | + | − | − | + | − |
| Farinasso (2007) [28] | ? | − | ? | − | − |
| Lipay (2007) [29] | ? | − | ? | − | − |
| Turebylu (2007) [30] | + | − | − | − | − |
| Chung (2008) [31] | + | − | ? | + | − |
| Rask (2011) [32] | − | − | ? | − | − |
| Albisetti (2013) [33] | + | − | − | − | − |
| Thom (2014) [34] | ? | − | − | − | − |
| Faustino (2015) [35] | + | − | − | − | − |
| Munck (2015) [36] | − | − | − | − | − |
− low risk of bias;
+ high risk of bias;
? bias unclear
Prevalence of Thrombophilia in Children with CVC
A total of 237 children had ≥1 trait for a pooled prevalence of 0.20 (95% confidence interval [CI]: 0.14–0.26; Table 3). There was significant heterogeneity among studies (I2=86.7%). The prevalence of thrombophilia in each study was related to the number of traits tested (coefficient: 0.03; P=0.02) but not the year of publication (coefficient: 0.004; P=0.42).
Table 3.
Summary of effects
| Thrombophilia trait | No. of children (studies) | Prevalence | Odds Ratio | Population attributable risk (95% CI) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Point estimate (95% CI) | I2 | Point estimate (95% CI) | I2 | |||
| ≥1 trait | 1,279 (16) | 0.20 (0.13–0.26) | 86.7% | 3.20 (1.56–6.54) | 69.6% | 0.31 (0.10–0.53) |
| Elevated factor VIII | 139 (2) | 0.11 (0.06–0.17) | 93.4% | 3.29 (1.02–10.60) | 0% | 0.20 (0–0.51) |
| Methylene tetrahydrofolate reductase gene mutation | 448 (7) | 0.09 (0.05–0.14) | 58.9% | 2.37 (1.00–5.60) | 0% | 0.11 (0–0.29) |
| Protein C deficiency | 643 (9) | 0.02 (0–0.04) | 33.6% | 6.83 (1.35–34.49) | 39.9% | 0.10 (0.01–0.40) |
| Elevated lipoprotein(a) | 537 (7) | 0.11 (0.04–0.20) | 87.8% | 1.81 (0.72–4.58) | 39.7% | 0.08 (-0.03–0.28) |
| Factor V Leiden mutation | 1,274 (16) | 0.03 (0.02–0.05) | 56.3% | 2.99 (1.14–7.83) | 48.1% | 0.06 (0–0.17) |
| Protein S deficiency | 640 (9) | 0.02 (0–0.05) | 68.9% | 2.87 (0.78–10.60) | 18.8% | 0.04 (0–0.16) |
| Elevated homocysteine | 223 (4) | 0.06 (0–0.16) | 84.0% | 1.68 (0.28–10.05) | 0% | 0.04 (-0.05–0.35) |
| Prothrombin mutation | 1,102 (15) | 0.02 (0.01–0.03) | 0% | 2.12 (0.82–5.46) | 16.6% | 0.02 (0–0.08) |
| Antiphospholipid antibody | 567 (8) | 0.02 (0–0.05) | 77.9% | 2.11 (0.70–6.36) | 0% | 0.02 (-0.01–0.10) |
| Antithrombin deficiency | 590 (8) | 0 (0–0.01) | 36.0% | 0.98 (0.15–6.31) | 0% | 0 (0–0) |
CI – confidence interval
Elevated factor VIII defined as >180% (pooled prevalence: 0.11; 95% CI: 0.06–0.17; I2=93.4%) and elevated lipoprotein(a) (pooled prevalence: 0.11; 95% CI: 0.04–0.20; I2=87.8%) were the most common traits (Table 3) [28]. The other traits were uncommon with pooled prevalence <0.10 for each trait.
The pooled prevalence of ≥1 trait in studies with symptomatic CADVT was 0.21 (95% CI: 0.15–0.26; I2=76.9%) when asymptomatic CADVT was classified as no CADVT. When asymptomatic CADVT was excluded, the pooled prevalence of ≥1 trait was 0.20 (95% CI: 0.12-0.29; I2=77.5%).
Association between Thrombophilia and CADVT
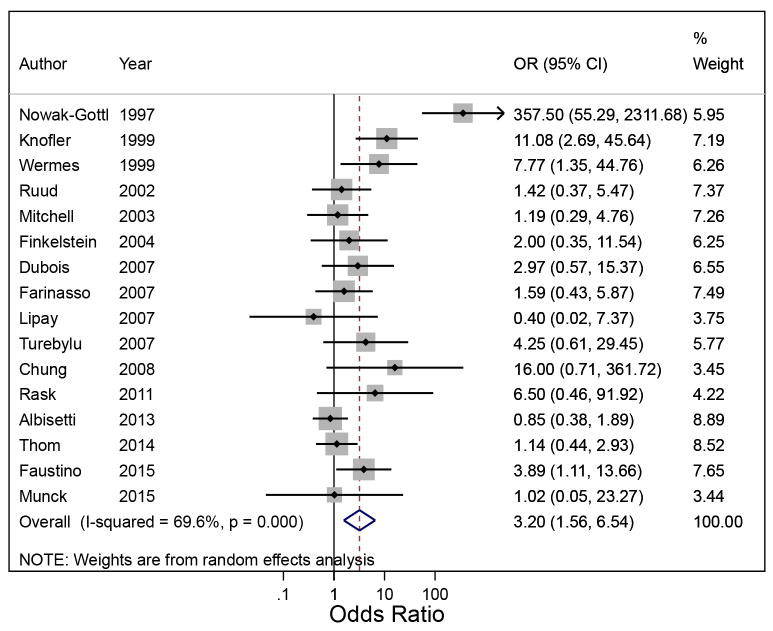
A total of 277 children had CADVT for a pooled prevalence of 0.23 (95% CI: 0.15–0.34; I2=93.4%; Table 3). Presence of ≥1 trait was associated with CADVT (pOR: 3.20; 95% CI: 1.56–6.54; I2=69.6%; Figure 3). The OR in each study was not associated with the year of publication (coefficient: -7.00; P=0.07). The population attributable risk of CADVT due to the presence of ≥1 trait was 0.31 (95% CI: 0.10–0.53).
Figure 3.
Random effects meta-analysis showing the individual and pooled odds ratio of central venous catheter-associated deep venous thrombosis with ≥1 thrombophilic trait
Protein C deficiency, elevated factor VIII, and factor V Leiden mutation were associated with CADVT (Table 3). The pOR of CADVT for each trait was 6.83 (95% CI: 1.35–34.49; I2=39.9%), 3.29 (95% CI: 1.02–10.60; I2=0%), and 2.99 (95% CI: 1.14–7.83; I2=48.1%), respectively. The population attributable risk of CADVT due to protein C deficiency was 0.10 (95% CI: 0.01–0.40), 0.20 (95% CI: 0–0.51) for elevated factor VIII, and 0.06 (95% CI: 0–0.17) for factor V Leiden mutation. The other traits were not associated with CADVT.
In the study by Ruud et al, 12 children were positive for 15 traits (protein C deficiency – 1, protein S deficiency – 3, presence of antiphospholipid antibody – 2, elevated lipoprotein(a) – 4, and elevated homocysteine – 5), which we were unable to categorize with respect to the presence of CADVT [24]. In our sensitivity analyses, the pOR of CADVT with protein C deficiency ranged from 4.67 (95% CI: 0.97–22.52; I2=44.5%) to 6.35 (95% CI: 1.59–25.39; I2=28.5%), while that for protein S deficiency ranged from 2.08 (95% CI: 0.53–8.11; I2=34.2%) to 3.30 (95% CI: 1.02–10.66; I2=12.8%). The pOR of CADVT did not significantly change with the other traits.
In studies with symptomatic CADVT, the pooled prevalence of CADVT was 0.05 (95% CI: 0.03–0.08; I2=68.0%) when asymptomatic CADVT was classified as no CADVT. The pOR of symptomatic CADVT with ≥1 trait in these studies was 6.71 (95% CI: 1.93–23.37; I2=71.2%). When asymptomatic CADVT was excluded, the pooled prevalence of symptomatic CADVT was 0.06 (95% CI: 0.02–0.11; I2=72.6%) with a pOR of symptomatic CADVT of 14.93 (95% CI: 1.44–154.58; I2=81.5%). In comparison, the pOR of asymptomatic CADVT was 2.14 (95% CI: 1.10–4.18; I2=9.3%).
Thrombophilia was associated with CADVT in children with malignancy (pOR of CADVT with ≥1 trait: 2.73; 95% CI: 1.14–6.56; I2=62.8%), but not with other diagnoses. The pOR of CADVT in children with the other diagnoses ranged from 1.02 to 25.32.
DISCUSSION
In this systematic review of 16 studies including 1,279 children with CVC, we report that the presence of ≥1 thrombophilic trait is associated with CADVT. This suggests that inherited and/or acquired prothrombotic abnormalities do, in fact, increase the risk of CADVT in children with CVC. Protein C deficiency, elevated factor VIII and factor V Leiden mutation were all associated with CADVT. The association between thrombophilia and symptomatic CADVT seems stronger than with asymptomatic CADVT.
Protein C can decrease, while factor VIII can increase, with inflammation [37]. Although protein C deficiency and elevated factor VIII can be inherited, we were unable to distinguish between inherited and acquired abnormalities in the levels of these factors because repeat testing after the acute illness was not consistently performed. It is also unclear whether the association between abnormalities in the levels of these factors represent a direct prothrombotic effect, or simply a marker of other risk factors, such as inflammation, because of inconsistencies in the timing of the tests. In contrast, the association between factor V Leiden mutation and CADVT is likely causal. Factor V Leiden mutation, which is present in 3-7% in the general population, is the most common inherited risk factor for venous thromboembolism [6]. Similar to our findings, it was found to be associated with venous thromboembolism in children with and without risk factors for DVT, other than CVC [7]. Furthermore, in adults with cancer, only factor V Leiden mutation has been shown to be associated with CADVT [38].
The strength of association between some traits and CADVT in our meta-analysis seems weaker than that with any venous thromboembolic event, regardless of its association with CVC, in the meta-analysis by Young et al [7]. For example, the pOR of any event with antithrombin deficiency is 9.44 compared with 0.98 for CADVT in our meta-analysis. A similar pattern may also be found for protein S deficiency (pOR of 5.77 vs. 2.87 in our meta-analysis), but not necessarily for other traits, such as protein C deficiency (pOR of 7.72 vs. 6.83 in our meta-analysis) and factor V Leiden (pOR of 3.77 vs. 2.99 in our meta-analysis). These comparisons suggest that the presence of a CVC may modify the effect of some, but not all, traits on the development of CADVT, such that the contribution of some traits on the development of DVT may be less in children with CVC.
The majority of studies included in our meta-analysis performed active radiologic surveillance. As expected, most CADVT were asymptomatic [39]. The apparent differential strength of association of thrombophilia with symptomatic and asymptomatic CADVT suggests that CADVT is more likely to be symptomatic with thrombophilia. Because signs and symptoms of DVT are related to clot burden, clot burden may mediate this differential effect [28].
Our findings do not conclusively define the association between thrombophilia and CADVT in children. The pOR of CADVT in our meta-analysis is imprecise with wide intervals. Some of the estimates, particularly for protein C and S deficiencies, are unstable with significant changes in the pOR with reclassification of small numbers of children. There is also significant heterogeneity among studies even within those with specific traits, same definition of CADVT and diagnoses. Because of the low prevalence of each trait, achieving adequate sample size in a homogeneous population is challenging when designing and conducting studies on thrombophilia in children. Traits may be prioritized for future studies using the population attributable risks that we estimated.
At present, we cannot recommend routine clinical testing of thrombophilias in children with CADVT. Testing should be considered when it is likely to influence management, such as in children presenting with purpura fulminans and in those with a high prevalence of inherited thrombophilia [8, 40]. The prevalence of each trait in our meta-analysis is low, its association with CADVT is relatively weak and there are significant limitations in our estimates. There is insufficient evidence in children and adults to support the use of these tests to inform management decisions [3, 40]. Clinical risk factors alone are sufficient to determine the duration and intensity of treatment of DVT and testing for thrombophilia to determine duration of treatment is not cost-effective [3, 41]. There are also no evidence-based guidelines for thromboprophylaxis in children or adults based primarily on the presence of thrombophilia [2, 38, 40].
Other limitations need to be considered. Significant heterogeneity in a number of our analyses, despite stratification, suggests unaccounted factors, which would include the child’s age, treatments received, characteristics of the CVC, definition of a positive trait, and imaging modality used to diagnose CADVT [4, 5]. In the absence of available data, we were unable to adjust for these factors. We were also unable to study the association between thrombophilia and CADVT at different age groups. Individual participant data meta-analysis would have provided significant insight into the association between thrombophilia and CADVT with adjustment for these potential confounders [42]. The diagnostic accuracy in detecting DVT varies among the different imaging modalities [43]. We likely underestimated the true prevalence of ≥1 trait because not all studies tested for all traits. With nearly half of the children analyzed diagnosed with a malignancy, our estimates may have been biased towards the results from these children, limiting the generalizability of our findings. We were underpowered to detect associations between some of the traits and CADVT, and in children with specific diagnoses. The low prevalence of the traits and the relatively weak associations contributed to the lack of statistical power. Other limitations of observational studies, such as biases and measurement errors, should be considered in interpreting our results.
CONCLUSION
Thrombophilia confers additional risk of CADVT in children with CVC. Protein C deficiency, elevated factor VIII and factor V Leiden mutation are associated with CADVT. CADVT maybe more likely to be symptomatic with thrombophilia. Because of the low prevalence of each trait, relatively weak association with CADVT, and limitations in our estimates and the included studies, we cannot recommend routine testing for thrombophilias in children with CADVT. Further studies on homogenous study populations will be challenging to conduct because of the large numbers of children needed to achieve adequate statistical power. If performed, traits may be prioritized using the population attributable risks that we estimated.
Supplementary Material
ESSENTIALS.
It is unclear if thrombophilia increases the risk of catheter-associated thrombosis in children.
We conducted a meta-analysis on thrombophilia and pediatric catheter-associated thrombosis.
Presence of ≥1 trait confers additional risk of venous thrombosis in children with catheters.
Limitations of included studies preclude us from recommending routine thrombophilia testing.
Acknowledgments
This work was supported in part by awards from the National Institutes of Health (T32 HD068201) to. R. Pierce, and the American Heart Association (Award Number 14CRP20490002) to. E.V.S. Faustino.
Footnotes
S. Neshat-Vahid contributed substantially to the concept and design, acquisition and interpretation of data, critical writing, and final approval of the manuscript. R. Pierce contributed substantially to the acquisition of data, revision of intellectual content, and final approval of the manuscript. D. Hersey contributed substantially to the concept and design, acquisition of data, revision of intellectual content, and final approval of the manuscript. L.J. Raffini contributed substantially to the design, interpretation of data, revision of intellectual content, and final approval of the manuscript. E.V.S. Faustino contributed substantially to the concept and design, acquisition and interpretation of data, critical writing, and final approval of the manuscript.
References
- 1.Monagle P, Adams M, Mahoney M, Ali K, Barnard D, Bernstein M, Brisson L, David M, Desai S, Scully MF, Halton J, Israels S, Jardine L, Leaker M, McCusker P, Silva M, Wu J, Anderson R, Andrew M, Massicotte MP. Outcome of pediatric thromboembolic disease: A report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47:763–6. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Raffini L, Thornburg C. Testing children for inherited thrombophilia: More questions than answers. Br J Haematol. 2009;147:277–88. doi: 10.1111/j.1365-2141.2009.07820.x. [DOI] [PubMed] [Google Scholar]
- 3.Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (9) 2012;141:e737S–801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidal E, Sharathkumar A, Glover J, Faustino EV. Central venous catheter-related thrombosis and thromboprophylaxis in children: A systematic review and meta-analysis. J Thromb Haemost. 2014;12:1096–109. doi: 10.1111/jth.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raffini L. Thrombophilia in children: Who to test, how, when, and why? Hematology Am Soc Hematol Educ Program. 2008:228–35. doi: 10.1182/asheducation-2008.1.228. [DOI] [PubMed] [Google Scholar]
- 6.Middeldorp S. Is thrombophilia testing useful? Hematology Am Soc Hematol Educ Program. 2011;2011:150–5. doi: 10.1182/asheducation-2011.1.150. [DOI] [PubMed] [Google Scholar]
- 7.Young G, Albisetti M, Bonduel M, Brandao L, Chan A, Friedrichs F, Goldenberg NA, Grabowski E, Heller C, Journeycake J, Kenet G, Krumpel A, Kurnik K, Lubetsky A, Male C, Manco-Johnson M, Mathew P, Monagle P, van Ommen H, Simioni P, Svirin P, Tormene D, Nowak-Gottl U. Impact of inherited thrombophilia on venous thromboembolism in children: A systematic review and meta-analysis of observational studies. Circulation. 2008;118:1373–82. doi: 10.1161/CIRCULATIONAHA.108.789008. [DOI] [PubMed] [Google Scholar]
- 8.Holzhauer S, Goldenberg NA, Junker R, Heller C, Stoll M, Manner D, Mesters R, Krumpel A, Stach M, Nowak-Gottl U. Inherited thrombophilia in children with venous thromboembolism and the familial risk of thromboembolism: An observational study. Blood. 2012;120:1510–5. doi: 10.1182/blood-2012-01-405514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst Rev. 2012;1:2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: A survey of practice. Am J Epidemiol. 2006;163:197–203. doi: 10.1093/aje/kwj036. [DOI] [PubMed] [Google Scholar]
- 14.Schulz KF, Grimes DA. Sample size slippages in randomised trials: Exclusions and the lost and wayward. Lancet. 2002;359:781–5. doi: 10.1016/S0140-6736(02)07882-0. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–82. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 18.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–41. [PubMed] [Google Scholar]
- 19.Daly LE. Confidence limits made easy: Interval estimation using a substitution method. Am J Epidemiol. 1998;147:783–90. doi: 10.1093/oxfordjournals.aje.a009523. [DOI] [PubMed] [Google Scholar]
- 20.Abdelkefi A, Ben Romdhane N, Kriaa A, Chelli M, Torjman L, Ladeb S, Ben Othman T, Lakhal A, Guermazi S, Ben Hassen A, Ladeb F, Ben Abdeladhim A. Prevalence of inherited prothrombotic abnormalities and central venous catheter-related thrombosis in haematopoietic stem cell transplants recipients. Bone Marrow Transplant. 2005;36:885–9. doi: 10.1038/sj.bmt.1705156. [DOI] [PubMed] [Google Scholar]
- 21.Nowak-Gottl U, Dubbers A, Kececioglu D, Koch HG, Kotthoff S, Runde J, Vielhaber H. Factor V Leiden, protein C, and lipoprotein (a) in catheter-related thrombosis in childhood: A prospective study. J Pediatr. 1997;131:608–12. doi: 10.1016/s0022-3476(97)70071-4. [DOI] [PubMed] [Google Scholar]
- 22.Knofler R, Siegert E, Lauterbach I, Taut-Sack H, Siegert G, Gehrisch S, Muller D, Rupprecht E, Kabus M. Clinical importance of prothrombotic risk factors in pediatric patients with malignancy - Impact of central venous lines. Eur J Pediatr. 1999;158(Suppl 3):S147–50. doi: 10.1007/pl00014342. [DOI] [PubMed] [Google Scholar]
- 23.Wermes C, von Depka Prondzinski M, Lichtinghagen R, Barthels M, Welte K, Sykora KW. Clinical relevance of genetic risk factors for thrombosis in paediatric oncology patients with central venous catheters. Eur J Pediatr. 1999;158(Suppl 3):S143–6. doi: 10.1007/pl00014341. [DOI] [PubMed] [Google Scholar]
- 24.Ruud E, Holmstrom H, Natvig S, Wesenberg F. Prevalence of thrombophilia and central venous catheter-associated neck vein thrombosis in 41 children with cancer - A prospective study. Med Pediatr Oncol. 2002;38:405–10. doi: 10.1002/mpo.10062. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell LG, Andrew M, Hanna K, Abshire T, Halton J, Anderson R, Cherrick I, Desai S, Mahoney D, McCuster P, Wu J, Dahl G, Chait P, de Veber G, Lee KJ, Mikulis D, Ginsberg J, Way C Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase Group. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: Results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer. 2003;97:508–16. doi: 10.1002/cncr.11042. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein Y, Yaniv I, Berant M, Zilber R, Garty BZ, Epstein O, Lahav J, Tamary H. Central venous line thrombosis in children and young adults with thalassemia major. Pediatr Hematol Oncol. 2004;21:375–81. doi: 10.1080/08880010490457042. [DOI] [PubMed] [Google Scholar]
- 27.Dubois J, Rypens F, Garel L, David M, Lacroix J, Gauvin F. Incidence of deep vein thrombosis related to peripherally inserted central catheters in children and adolescents. CMAJ. 2007;177:1185–90. doi: 10.1503/cmaj.070316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farinasso L, Bertorello N, Garbarini L, Gajno TM, Barisone E, Artesani L, Valori A, Giacchino M, Pastore G, Saracco P. Risk factors of central venous lines-related thrombosis in children with acute lymphoblastic leukemia during induction therapy: A prospective study. Leukemia. 2007;21:552–6. doi: 10.1038/sj.leu.2404560. [DOI] [PubMed] [Google Scholar]
- 29.Lipay NV, Dmitriev VV, Borisenok MB. Thrombotic complications during cancer treatment in children. Exp Oncol. 2007;29:231–35. [PubMed] [Google Scholar]
- 30.Turebylu R, Salis R, Erbe R, Martin D, Lakshminrusimha S, Ryan RM. Genetic prothrombotic mutations are common in neonates but are not associated with umbilical catheter-associated thrombosis. J Perinatol. 2007;27:490–5. doi: 10.1038/sj.jp.7211786. [DOI] [PubMed] [Google Scholar]
- 31.Chung BH, Ma ES, Khong PL, Chan GC. Inherited thrombophilic factors do not increase central venous catheter blockage in children with malignancy. Pediatr Blood Cancer. 2008;51:509–12. doi: 10.1002/pbc.21617. [DOI] [PubMed] [Google Scholar]
- 32.Rask O, Bjorgell O, Wattsgard C, Ljung R. High frequency of asymptomatic thrombosis associated with central venous lines in children with haemophilia. J Thromb Haemost. 2011;9:462–3. [Google Scholar]
- 33.Albisetti M, Kellenberger CJ, Bergstrasser E, Niggli F, Kroiss S, Rizzi M, Schmugge M. Port-a-cath-related thrombosis and postthrombotic syndrome in pediatric oncology patients. J Pediatr. 2013;163:1340–6. doi: 10.1016/j.jpeds.2013.06.076. [DOI] [PubMed] [Google Scholar]
- 34.Thom K, Male C, Mannhalter C, Quehenberger P, Mlczoch E, Luckner D, Marx M, Hanslik A. No impact of endogenous prothrombotic conditions on the risk of central venous line-related thrombotic events in children: Results of the KIDCAT study (KIDs with Catheter Associated Thrombosis) J Thromb Haemost. 2014;12:1610–5. doi: 10.1111/jth.12699. [DOI] [PubMed] [Google Scholar]
- 35.Faustino EV, Li S, Silva CT, Pinto MG, Qin L, Tala JA, Rinder HM, Kupfer GM, Shapiro ED Northeast Pediatric Critical Care Research Consortium. Factor VIII may predict catheter-related thrombosis in critically ill children: A preliminary study. Pediatr Crit Care Med. 2015;16:497–504. doi: 10.1097/PCC.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munck A, Kheniche A, Alberti C, Hubert D, Martine RG, Nove-Josserand R, Pin I, Bremont F, Chiron R, Couderc L, Dalphin ML, Darviot E, Delaisi B, Dominique S, Durieu I, Fanton A, Fayon M, Gerardin M, Ginies JL, Giraut C, Grenet D, Guillot M, Huet F, Le bourgeois M, Murris-Epin M, Ramel S, Sardet A, Sermet-Gaudelus I, Varaigne F, Wanin S, Weiss L, Hurtaud MF. Central venous thrombosis and thrombophilia in cystic fibrosis: A prospective study. J Cyst Fibros. 2015;14:97–103. doi: 10.1016/j.jcf.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Kenet G, Strauss T, Kaplinsky C, Paret G. Hemostasis and thrombosis in critically ill children. Semin Thromb Hemost. 2008;34:451–8. doi: 10.1055/s-0028-1092875. [DOI] [PubMed] [Google Scholar]
- 38.Boersma RS, Hamulyak K, Cate HT, Schouten HC. Congenital thrombophilia and central venous catheter-related thrombosis in patients with cancer. Clin Appl Thromb Hemost. 2010;16:643–9. doi: 10.1177/1076029610371471. [DOI] [PubMed] [Google Scholar]
- 39.Faustino EV, Spinella PC, Li S, Pinto MG, Stoltz P, Tala J, Card ME, Northrup V, Baker KE, Goodman TR, Chen L, Silva CT. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J Pediatr. 2013;162:387–91. doi: 10.1016/j.jpeds.2012.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baglin T, Gray E, Greaves M, Hunt BJ, Keeling D, Machin S, Mackie I, Makris M, Nokes T, Perry D, Tait RC, Walker I, Watson H British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149:209–20. doi: 10.1111/j.1365-2141.2009.08022.x. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien SH, Smith KJ. Using thrombophilia testing to determine anticoagulation duration in pediatric thrombosis is not cost-effective. J Pediatr. 2009;155:100–4. doi: 10.1016/j.jpeds.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Saber W, Moua T, Williams EC, Verso M, Agnelli G, Couban S, Young A, De Cicco M, Biffi R, van Rooden CJ, Huisman MV, Fagnani D, Cimminiello C, Moia M, Magagnoli M, Povoski SP, Malak SF, Lee AY. Risk factors for catheter-related thrombosis (CRT) in cancer patients: A patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9:312–9. doi: 10.1111/j.1538-7836.2010.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell LG, Goldenberg NA, Male C, Kenet G, Monagle P, Nowak-Gottl U Perinatal and Paediatric Haemostasis Subcommittee of the SSC of the ISTH. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost. 2011;9:1856–8. doi: 10.1111/j.1538-7836.2011.04433.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.