Abstract
Phthalates, a ubiquitous class of environmental chemicals, may interfere with typical reproductive hormone production both in utero and in adulthood. Although they are best known as anti-androgens, increasingly, evidence suggests that phthalates, particularly di-2-ethylhexyl phthalate (DEHP), may also suppress estrogen production. Given that both androgens and estrogens are essential for sexual function, particularly sexual interest, it is plausible that adult exposure to phthalates alters sexual function. To this end, we used data from 360 women participating in a pregnancy cohort study (the Study for Future Families) to examine whether urinary phthalate metabolite concentrations were associated with two dimensions of self-reported sexual dysfunction in the months prior to conception: lack of sexual interest and vaginal dryness. Women in the highest quartile of urinary concentrations of mono-2-ethyl-5-hydroxyhexyl phthalate, a DEHP metabolite, had 2.58 (95% CI 1.33, 5.00) times the adjusted odds of reporting that they almost always or often lacked interest in sexual activity, and results were similar for mono-2-ethyl-5-oxohexyl phthalate (aOR: 2.56, 95% CI 1.32, 4.95), another DEHP metabolite. Self-reported vaginal dryness was not associated with any phthalate metabolite concentration. This study is novel in its focus on sexual function in relation to environmentally relevant (rather than occupational) exposure to phthalates in adult women and these preliminary findings merit replication in a large, prospective study. Better understanding how adult exposure to phthalates may affect reproductive health, including sexual function, is of public health interest given that virtually all Westerners are exposed to phthalates.
Keywords: Phthalates, Endocrine disruptors, Environmental chemicals, Sexual interest, Sexual dysfunction
Introduction
In humans and other primates, reproductive hormones are essential drivers of sexual motivation and interest; in the absence of androgens, sexual interest decreases sharply (Dixson, 1993; Kwan et al., 1983; Resko and Phoenix, 1972; Schenck and Slob, 1986; Wallen, 2001). In hypogonadal men, testosterone administration elicits increased sexual arousal and enjoyment (O’Connor et al., 2004; Skakkebaek et al., 1981; Wang et al., 2004) whereas experimentally-induced hypogonadism reduces sexual function (which can then be restored by testoster one administration) (Schmidt et al., 2009). More recent work on hypogonadism suggests that not only is testosterone important, but estradiol also contributes to the restoration of sexual function in men (Finkelstein et al., 2013). In females, the hormonal correlates of sexual interest have been more complicated to unravel. The dramatic changes in reproductive hormone concentrations across the menstrual cycle make it difficult to determine the precise role that individual hormones play in sexual interest; however, the precipitous drop in sexual interest in ovariectomized women (and its restoration with combined estradiol and testosterone therapy) demonstrates the central role of sex steroid hormones in female sexual motivation (Sherwin and Gelfand, 1987; Sherwin et al., 1985).
Despite the preponderance of evidence demonstrating that reproductive hormones are essential for sexual interest and motivation, there has been very little research on the extent to which exposure to endocrine-disrupting chemicals (EDCs) in adulthood may affect sexual interest and behavior in humans and other primates. EDCs can act through multiple mechanisms including mimicking endogenous hormones or blocking hormone production. Of particular relevance to sexual function are EDCs that alter reproductive hormone concentrations. Evidence from numerous animal species shows that EDC exposure (experimental or environmental) can exert widespread effects on sexual behavior, impairing species- and sex-typical proceptive and receptive behaviors (reviewed in Blocker and Ophir, 2013).
Exposure to synthetic EDCs in the modern world is ubiquitous (Woodruff et al., 2011), nevertheless, there has been little research on EDCs and sexual function in humans, and most of it focuses on supra-normal exposures in men. Loss of male sexual interest and motivation has been linked to high exposure to insecticides and delousing agents believed to be EDCs (Brody and Loriaux, 2003; Munk and Nantel, 1977; Sonnenschein and Soto, 1998). Similarly male workers occupationally exposed to bisphenol A (BPA), a weakly estrogenic EDC, reported lower sexual desire and, more generally, greater sexual dysfunction, than controls (Li et al., 2010a, 2010b). To our knowledge only one study has reported on sexual function in relation to environmentally relevant levels of EDC exposure, finding that urinary BPA concentrations were inversely associated with level of self-reported sex drive and ejaculation strength (Li et al., 2010b). Additional research on the relationship between exposure to environmentally-relevant concentrations of EDCs and sexual function is needed, as is work on whether EDCs affect sexual function in women, a heretofore unstudied question (Lara et al., 2012).
Phthalates are a class of EDCs that may be particularly relevant in this context. They are used in the production of a wide range of consumer products including foodstuffs, personal care products, pharmaceuticals, vinyl flooring, electronics, and pesticides. Exposure is virtually ubiquitous in Western populations (Koch and Calafat, 2009; Woodruff et al., 2011) and phthalates’ anti-androgenic properties have been widely documented (Foster, 2006; Gray et al., 2000; Swan et al., 2005). In adult men and peripubertal boys, phthalate metabolite concentrations are inversely associated with serum free testosterone concentrations and the free androgen index as well as estradiol concentrations (Duty et al., 2005; Ferguson et al., 2014; Meeker et al., 2009; Meeker and Ferguson, 2014; Mendiola et al., 2012; Pan et al., 2006). Little is known about how phthalate exposure affects hormone activity in women. We found that in pregnant women, urinary concentrations of metabolites of several phthalates (including di-2-ethylhexyl phthalate (DEHP)) were associated with lower testosterone and estradiol concentrations (Sathyanarayana et al., 2014) and more recently, in the National Health and Nutrition Examination Survey (NHANES), associations were found between certain phthalate metabolites and testosterone levels in women, particularly among the 40–60 year old age group (Meeker and Ferguson, 2014). Evidence from animal models echoes these results and suggests that certain phthalates, such as DEHP, are not only anti-androgenic, but may alter activity of other hormones including estradiol, progesterone, and thyroid hormones (Davis et al., 1994; Harris et al., 1997; Huang et al., 2007; Jobling et al., 1995; Lovekamp and Davis, 2001; Meeker et al., 2007; Meeker and Ferguson, 2011; Treinen et al., 1990).
Given these multiple lines of evidence suggesting that adult exposure to phthalates may alter typical reproductive hormone activity in humans, we used data from a large pregnancy cohort study to explore the extent to which phthalate exposure is also related to sexual function. Our primary analyses examine the relationship between phthalate exposure and self-reported a lack of interest in sexual activity in adult women, and we hypothesize that urinary concentrations of phthalate metabolites (particularly DEHP metabolites) are inversely associated with self-reported interest in sexual activity. Secondarily, we examine whether phthalate metabolite concentrations are associated with a second aspect of women’s sexual dysfunction, vaginal dryness.
Methods
Study population
Pregnant women and their partners were recruited into the Study for Future Families (SFF) from four cities around the United States (Los Angeles, CA, Minneapolis, MN, Columbia, MO, and Iowa City, IA) from 1999–2002. To be eligible, couples had to be age 18 or older, have a non-medically assisted pregnancy, have no major threat to the pregnancy, be receiving prenatal care at one of the participating obstetric clinics, and speak English or Spanish. Subjects completed questionnaires and a subset gave blood and urine samples during the same visit. Eligibility for inclusion in the current analyses included having completed a questionnaire and given a urine sample which was analyzed for phthalate metabolites. Human subject committees at the participating institutions approved the study and all subjects signed informed consent prior to participating in any study activities.
Questionnaires
During the pregnancy, subjects completed an extensive questionnaire including items on demographics, lifestyle, and reproductive history. They were asked a short series of questions on sexual health. In particular, they were asked the question “In the three months before your current pregnancy, how often did you have difficulty with the following?” and then were given items on “lack of interest in sex” and “vaginal dryness”. For each item, subjects chose from the following possible responses: almost always, often, sometimes and almost never. In addition, the questionnaire included items on age, race and ethnicity, parity, educational attainment, medication use, and stress, among other things. Stress was assessed through a set of items on stressful life events (SLEs) occurring in the previous three months. Items were derived from two widely-used questionnaires (Dohrenwend et al., 1978; Holmes and Rahe, 1967), and included: job loss or unemployment (self or partner); serious injury or illness (self or partner); death of a close family member (i.e. parent, child, sibling); divorce, separation, or serious relationship difficulties with one’s partner; serious legal or financial problems (self or partner); or other major life events (write-in option) (Barrett et al., 2013).
Phthalate metabolite measurements
All subjects collected urine in polypropylene cups, after which the urine was aliquoted and frozen at −80 °C. Samples from subjects who continued to participate in the study postnatally (n = 380) were subsequently analyzed for urinary phthalate metabolite concentrations at the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, which had no access to subjects’ data or identifiers. The samples were analyzed according to a modification of a previously published method (Silva et al., 2004). In short, the assays entailed enzymatic hydrolysis of phthalate metabolites from their conjugated form, followed by automated on-line solid-phase extraction, separation with high-performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry (Silva et al., 2004). Nine phthalate metabolites were quantified simultaneously: monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), monomethyl phthalate (MMP), monobenzyl phthalate (MBzP), monoisobutyl phthalate (MiBP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP). Limits of detection (LODs) were in the low nanogram per milliliter range. To improve precision and accuracy, isotopically labeled internal standards and conjugated internal standards were used. Between-day relative standard deviations were less than 10% and quality control samples and laboratory blanks were used to assess performance. At the time of phthalate metabolite measurements, creatinine was measured, but more recently, we also measured specific gravity (SpG) using a hand-held refractometer (National Instrument Company, Inc., USA) after thawing the urine that had been stored frozen. Deionized water was used to calibrate the refractometer prior to each measurement at ambient temperature. All collection materials used in the study were determined to be phthalate-free by the CDC.
Statistical analysis
We conducted univariate analyses to examine the distribution of values for all relevant variables. Following standard protocols for handling data that are not highly skewed, phthalate metabolite concentrations below the LOD were assigned a value of the LOD divided by the square root of 2 (Hornung and Reed, 1990). Less than 15% of samples had concentrations below the LOD for any of metabolite measured, with the exception of MMP, for which over 50% of concentrations were below the LOD. For this reason, we elected not to include MMP in further analyses. To account for variation in urine dilution, we adjusted all concentrations for specific gravity (SpG) using the formula: Pc = P [(1.01 − 1)/SpG − 1)]. In this formula, Pc is the SpG-adjusted phthalate concentration (ng/ml), P is the measured phthalate concentration (ng/ml), 1.01 is the mean SpG for all SFF samples, and SpG is the specific gravity of the individual urine sample (Boeniger et al., 1993). An additional DEHP metabolite, mono(2-ethyl-5-carboxyheptyl phthalate), or MECPP, was not standardly measured by the CDC at the time and thus was not available in this data set. For each metabolite, we dichotomized concentrations into high (>75th percentile) or low (≤75th percentile).
We derived a number of variables based on the questionnaire data. Responses to the sexual function questions were dichotomized to compare subjects who answered “almost always” or “often” to subjects who answered “sometimes” or “almost never”. We selected a number of covariates a priori for inclusion in models. These included age (continuous), highest level of educational attainment (less than college/college or higher), race (white/non-white), parity (parous/nulliparous), current anti-depressant use (any/none), and stress (0–1 SLEs/2 or more SLEs).
We then conducted a series of bivariate analyses including correlations and t-tests to examine the relationships between the variables of interest. Finally, we fit logistic regression models in order to examine the odds of sexual dysfunction in relation to categorical concentration for each metabolite (high/low). In our primary model, a lack of interest in sexual activity was the outcome, and in our secondary models, the outcome was vaginal dryness. Sensitivity analyses were conducted including gestational age at questionnaire completion as a covariate. Model assumptions of linearity and normally distributed error with constant variance were checked. In each model, we checked for outliers and influential points, however none were identified. All analyses were conducted in SAS Version 9.3 (SAS Institute, Cary, NC, USA).
Results
In total, 954 women completed the questionnaires and gave urine samples at a mean gestational age of 26 weeks (min–max: 7–41 weeks). Urine samples were analyzed for phthalate metabolite concentrations in the 380 women who continued with longitudinal follow-up after their infants were born. Here we report on a subset of those (n = 360) with complete data for all covariates (Table 1). Overall, the sample was mostly white and non-Hispanic (84%) and well educated (93% completed at least some college). Forty-eight percent of women had had at least one previous liveborn child. Of the subjects included in the current analyses, 46 (13%) reported that they almost always or often lacked interest in sexual activity in the months prior to pregnancy. In addition, 37 women (10%) reported problems with vaginal dryness in the months prior to pregnancy. The majority of subjects (78%) reported experiencing zero or one stressful life event during pregnancy and only 5% was using anti-depressants. Compared to women who completed the questionnaires but were lost to follow-up, subjects in the current study were more educated, older, and more likely to be white. The two groups did not differ in anti-depressant use or self-reported life events stress (not shown).
Table 1.
Characteristics of the study population stratified by self-reported lack of interest in sexual activity in the months prior to pregnancy (n = 360).
| Characteristic | Almost always or often lacked interest in sexual activity (n = 46)
|
Rarely or never lacked interest in sexual activity (n = 314)
|
p-Valuea | All (n = 360)
|
|---|---|---|---|---|
| N (%) | N (%) | N | ||
| Age (years) | ||||
| <20–30 | 24 (14) | 149 (86) | 0.63 | 173 |
| >30–42 | 22 (12) | 165 (88) | 187 | |
| Race | ||||
| White, non-Hispanic | 44 (15) | 258 (85) | 0.02 | 302 |
| Non-white | 2 (3) | 56 (97) | 58 | |
| Educational attainment | ||||
| Less than college | 2 (8) | 24 (92) | 0.55 | 26 |
| College or more | 44 (13) | 290 (87) | 334 | |
| Employment status | ||||
| Employed | 38 (13) | 257 (87) | 1.00 | 295 |
| Not employed | 8 (12) | 57 (88) | 65 | |
| Parity | ||||
| Parous | 30 (18) | 141 (82) | 0.01 | 171 |
| Nulliparous | 16 (8) | 173 (92) | 189 | |
| Life stressors during pregnancy | ||||
| 0–1 | 38 (13) | 248 (87) | 0.69 | 286 |
| 2+ | 8 (11) | 66 (89) | 74 | |
| Current use of anti-depressants | ||||
| Yes | 4 (24) | 13 (76) | 0.25 | 17 |
| No | 42 (12) | 301 (88) | 343 | |
Two-sided Fisher’s Exact Test examining lack of interest in sexual activity in relation to subject demographics.
Of the phthalate metabolites measured in the urine samples, MEP was present in the highest concentrations (geometric mean: 119 μg/l) and MiBP concentrations were the lowest (geometric mean: 3.14 μg/l; Table 2). For most of the metabolites measured, over 90% of women had detectable concentrations. The exceptions were MEHP and MiBP, which were present in only 77% and 74% of women, respectively. In bivariate analyses (Table 1), women who were parous were more likely to report low interest in sexual activity than nulliparous women. White women were more likely to express low interest in sexual activity than non-white women. There were no other notable bivariate relationships between the outcomes of interest and the covariates.
Table 2.
Distribution of specific-gravity corrected phthalate metabolite concentrations in μg/l (n = 360).
| Phthalate (abbreviation) | Metabolite (abbreviation) | Geometric mean | Percentiles
|
LOD (% detected) | ||
|---|---|---|---|---|---|---|
| 25% | 50% | 75% | ||||
| Di-2-ethylhexyl phthalate (DEHP) | Mono-2-ethylhexyl phthalate (MEHP) | 4.08 | 1.84 | 3.97 | 9.09 | 0.981 (77) |
| Mono-2-ethyl-5-oxohexyl phthalate (MEOHP) | 13.12 | 8.40 | 13.84 | 23.62 | 1.068 (97) | |
| Mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) | 14.66 | 7.29 | 12.57 | 20.50 | 0.954 (98) | |
| Di-n-butyl phthalate (DBP) | Mono-n-butyl phthalate (MBP) | 18.72 | 11.73 | 18.77 | 30.53 | 1.071 (98) |
| Diethyl phthalate (DEP) | Mono-ethyl phthalate (MEP) | 118.64 | 46.66 | 105.91 | 293.97 | 1.0 (99) |
| Di-isobutyl phthalate (DIBP) | Mono-isobutyl phthalate (MiBP) | 3.14 | 1.73 | 3.10 | 5.60 | 1.038 (82) |
| Butylbenzyl phthalate (BBzP) | Mono-benzyl phthalate (MBzP) | 9.21 | 4.64 | 9.71 | 18.54 | 1.0 (96) |
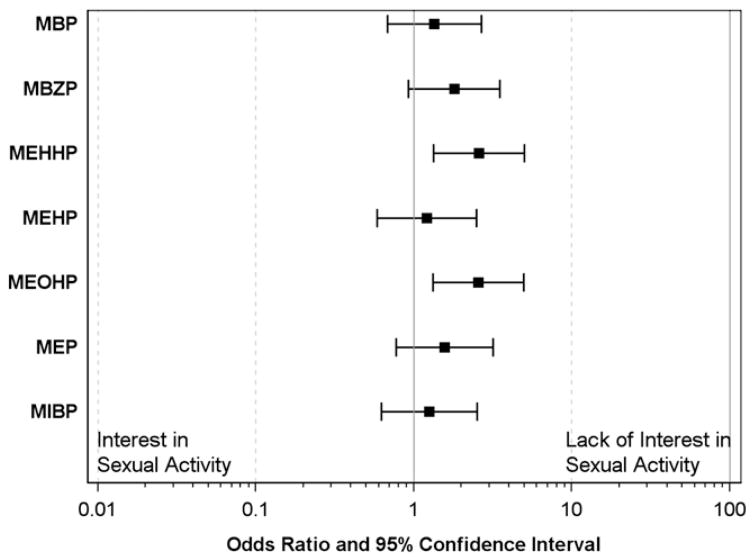
In our primary multivariable analyses, compared to women in the lowest three concentration quartiles, women in the highest quartile of MEOHP had 2.56 times the odds of reporting a lack of interest in sex (95% CI 1.32, 4.95) (Fig. 1). Similar results were seen for MEHHP (OR: 2.58, 95% CI 1.33, 5.00), another DEHP metabolite. The associations between another metabolite, MBzP, and a lack of interest in sexual activity were similar (OR: 1.80, 95% CI 0.92, 3.50). The same pattern was observed across all metabolites measured, although associations were weaker (Table 3, Fig. 1). In our secondary models, we examined phthalate metabolite concentrations in relation to odds of self-reported vaginal dryness during intercourse. For most metabolites, higher concentration was associated with increased odds of vaginal dryness, however these relationships were weak and included the null value. In the sensitivity analyses, the inclusion of gestational age as a covariate did not change estimates (not shown).
Fig. 1.
Odds ratios (and 95% confidence intervals) for lack of interest in sexual activity in women in the highest concentration quartile of phthalate metabolite as compared to women in the lowest three concentration quartiles (n = 360).
Table 3.
Adjusted odds of sexual dysfunction among women in the highest concentration quartile of phthalate metabolite compared to women in the lowest three concentration quartiles (n = 360).a
| Phthalate metabolite | Lack of sexual interest OR (95% CI) |
Vaginal dryness OR (95% CI) |
|---|---|---|
| MEHP | 1.20 (0.58, 2.48) | 0.93 (0.41, 2.08) |
| MEOHP | 2.56 (1.32, 4.95) | 1.80 (0.86, 3.74) |
| MEHHP | 2.58 (1.33, 5.00) | 1.72 (0.83, 3.59) |
| MBP | 1.35 (0.68, 2.68) | 1.21 (0.55, 2.65) |
| MEP | 1.57 (0.78, 3.17) | 1.04 (0.40, 2.35) |
| MiBP | 1.25 (0.62, 2.51) | 0.91 (0.40, 2.11) |
| MBzP | 1.80 (0.92, 3.50) | 1.13 (0.51, 2.48) |
Adjusted for age, education level, race, stress, parity, and use of anti-depressants.
Discussion
In the current analyses, we found inverse associations between phthalate metabolite concentrations (particularly of the DEHP metabolites, MEHHP and MEOHP) and interest in sexual activity in reproductive age women. Women in the highest quartiles of MEHHP and MEOHP concentrations had over 2.5 times the odds of reporting a lack of interest in sexual activity compared to women with lower metabolite concentrations. These findings are a novel contribution to this literature in several respects. First, our results are from a population with environmentally relevant, rather than occupational, phthalate exposure. Phthalate metabolite concentrations measured in subjects in this study are similar to those reported in NHANES during the same time period, suggesting that they approximate typical exposures in American women (Silva et al., 2004). This stands in striking contrast to the previous work on EDCs and sexual function, which has typically focused on the supra-normal occupational levels. Second, our study focused on females, who have not been studied in this context previously. Finally, to our knowledge, our study is the first to focus on phthalates, a class of chemicals believed to act upon multiple hormone pathways, making them a prime candidate for study in this context.
The anti-androgenic properties of phthalates have been widely studied and to the extent that interest in sexual activity in women is dependent on circulating androgen concentrations, this may be one potential explanatory mechanism for our findings. Indeed, hormone replacement including testosterone appears to restore interest in sexual activity in post-menopausal women to some extent, particularly in combination with estrogen therapy (Alexander et al., 2004; Braunstein et al., 2005; Davis et al., 2008). However, more recently, research examining sexual interest and daily hormonal changes within menstrual cycles has indicated that estradiol, not testosterone, may be most important in driving sexual motivation. Sexual interest and behavior in women and other female primates tend to peak around ovulation, which is when both testosterone and estradiol levels are highest (Bullivant et al., 2004; Diamond and Wallen, 2011; Stanislaw and Rice, 1988; Wallen and Zehr, 2004; Wilcox et al., 2004). Across the menstrual cycle, sexual interest is linked to changes in daily estradiol concentrations, and no associations with testosterone are found after adjusting for estradiol levels (Roney and Simmons, 2013). This fits with the extensive data from other mammals showing that estradiol, not testosterone, promotes female-initiated sexual behavior (Rissman, 1991; Wallen and Zehr, 2004; Zehr et al., 1998).
Although phthalates are best known as anti-androgens, they may interfere with estradiol production as well, suggesting an alternative mechanism by which they may suppress sexual interest in women. In animal models, adult exposure to DEHP results in smaller granulosa cells, lower estradiol levels, and ultimately, disrupted estrous cycling and ovulation (Davis et al., 1994). These results confirm in vivo work showing decreased estradiol production in ovarian cultures derived from DEHP-treated rodents (Laskey and Berman, 1993). This reduced estradiol production may be due to suppressed transcription of the aromatase enzyme in granulosa cells (Lovekamp and Davis, 2001) and/or by increased activity of 17β-hydroxysteroid dehydrogenase type IV, the enzyme responsible for converting estradiol to estrone (Fan et al., 1998). In humans, much less is known about how phthalates may disrupt estradiol production. Several epidemiological studies have linked phthalates to estrogen-sensitive health outcomes (including breast cancer, fibroids, endometriosis, and IVF outcomes) as well, further supporting the possibility that phthalate exposure is associated with altered estradiol levels (Lopez-Carrillo et al., 2010; Souter et al., 2013; Upson et al., 2013; Weuve et al., 2010). In a subset of the current cohort, we found that DEHP exposure was inversely associated with testosterone and estradiol levels during pregnancy (Sathyanarayana et al., 2014). Unfortunately, because these hormone concentrations were measured during pregnancy, a period of dramatic hormonal change, they cannot shed light on variation in sexual interest prior to pregnancy, the focus of the current analyses (not shown). Additional work is needed to investigate the extent to which phthalates and other EDCs may influence sexual function by altering sex steroid concentrations.
In our analyses, phthalate metabolite concentrations were not associated with the second measure of sexual dysfunction, vaginal dryness. Vaginal dryness may have many causes, but among the most common one may be urogenital atrophy, changes in the vagina frequently linked to the decline in estrogen levels at menopause. Vaginal dryness is a common side-effect in women taking anti-estrogenic medications or undergoing medical treatments that lower estrogen levels (Bachmann and Nevadunsky, 2000). The condition is often successfully treated with systemic or local estrogen administration, further suggesting the central role that estrogens play in the disorder (Lynch, 2009). To the extent that phthalates interfere with estrogen production, we would have expected to find an association with vaginal dryness in our population. For most phthalate metabolites, women with higher concentrations had increased odds of reporting problems with vaginal dryness. It is possible that we would have been able to detect statistically significant associations with a larger sample size (only 37 women reported this issue). However in pre-menopausal women, vaginal dryness may have a number of other, non-hormonal causes, including exposure to irritants, medication use, and stress (Basson, 2006). Although we attempted to adjust for several of these factors, there may have been considerable outcome misclassification, given that only hormone-dependent vaginal dryness is likely to be of relevance in this context.
Our study had several limitation of note. The major limitation of our study is the lack of concordance between the timing of exposure assessment and outcome measurement. Recognizing that pregnancy often brings changes in sexual interest and activity, we queried subjects retrospectively about sexual function in the three months prior to pregnancy (rather than at the time of questionnaire completion, typically during mid-late pregnancy), however because women were recruited during pregnancy, no urine samples are available from the pre-conception period. Thus temporally, our exposure measurement comes from samples obtained after the period of exposure assessment and a single spot sample may not be representative of levels over a longer period (Adibi et al., 2008; Braun et al., 2012; Irvin et al., 2010). Similarly, for reasons of convenience and expense, it is common practice in large epidemiological studies (such as SFF) to assess phthalate exposure based on concentrations measured in a single urine sample, however it would be preferable to collect and analyze multiple samples in future work. In addition, we do not have data on pre-pregnancy body mass index (BMI), which could be a potential confounder given that obesity has been associated with higher phthalate metabolite concentrations as well as sexual dysfunction (Buser et al., 2014; Kolotkin et al., 2012). However, in our other work, we have found that in adult women, the normal range of variation in BMI is not associated with phthalate metabolite concentrations (unpublished). It remains possible that there is another, un-identified factor related to both phthalate exposure and sexual interest for which we have not accounted, or alternatively, that the associations found are spurious. Our self-reported questionnaire data on sexual function may also be subject to recall bias and our subjects, who were mostly well-educated, Caucasian women, may not be representative of American women in general. Indeed, because of the longitudinal nature of the current study, the sample available for these analyses was not representative of the original recruited subject pool in race, age, and education level. A shorter study on this topic could help avoid this differential loss to follow-up.
Use of more extensive, validated scales indexing sexual activity and interest (with a partner as well as solo), relationship quality and satisfaction, depression, and use of medications (particularly anti-depressants) would be valuable in future work. Given these limitations, we view these results as preliminary and in need of confirmation in prospectively designed studies. This may be an interesting and novel direction for future research on the reproductive health consequences of adult exposure to environmental chemicals as well as the underlying causes of sexual dysfunction. Our results also point to a need for additional research examining the extent to which adult exposure to phthalates affects reproductive hormone profiles, particularly in cycling women.
Acknowledgments
We wish to acknowledge the SFF study team and the families who participated in the study. In addition, we acknowledge A. Calafat, M. Silva, E. Samandar, J. Preau, and J. Reidy for measuring the concentrations of phthalate metabolites. Funding for SFF was provided by the following grants from the National Institutes of Health R01ES09916, M01-RR00400, M01RR0425, and UL1TR000124. Funding for the current analyses was provided by K12 ES019852-01 and supported by P30 ES001247.
Contributor Information
Emily S. Barrett, Email: Emily_barrett@urmc.rochester.edu.
Lauren E. Parlett, Email: lauren.parlett@jhu.edu.
Christina Wang, Email: wang@labiomed.org.
Erma Z. Drobnis, Email: drobnisE@health.missouri.edu.
J. Bruce Redmon, Email: redmo001@umn.edu.
Shanna H. Swan, Email: shanna.swan@mssm.edu.
References
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, Hauser R. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JL, Kotz K, Dennerstein L, Kutner SJ, Wallen K, Notelovitz M. The effects of postmenopausal hormone therapies on female sexual functioning: a review of double-blind, randomized controlled trials. Menopause. 2004;11:749–765. doi: 10.1097/01.gme.0000142887.31811.97. [DOI] [PubMed] [Google Scholar]
- Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician. 2000;61:3090–3096. [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Sathyanarayana S, Liu F, Redmon JB, Wang C, Swan SH. Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiol Behav. 2013;114–115:14–20. doi: 10.1016/j.physbeh.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson R. Clinical practice. Sexual desire and arousal disorders in women. N Engl J Med. 2006;354:1497–1506. doi: 10.1056/NEJMcp050154. [DOI] [PubMed] [Google Scholar]
- Blocker TD, Ophir AG. Cryptic confounding compounds: a brief consideration of the influences of anthropogenic contaminants on courtship and mating behavior. Acta Ethologica. 2013;16 doi: 10.1007/s10211-012-0137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582–1589. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- Brody SA, Loriaux DL. Epidemic of gynecomastia among Haitian refugees: exposure to an environmental antiandrogen. Endocr Pract. 2003;9:370–375. doi: 10.4158/EP.9.5.370. [DOI] [PubMed] [Google Scholar]
- Bullivant SB, Sellergren SA, Stern K, Spencer NA, Jacob S, Mennella JA, McClintock MK. Women’s sexual experience during the menstrual cycle: identification of the sexual phase by noninvasive measurement of luteinizing hormone. J Sex Res. 2004;41:82–93. doi: 10.1080/00224490409552216. [DOI] [PubMed] [Google Scholar]
- Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int J Hyg Environ Health. 2014;217(16):687–694. doi: 10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128:216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 2008;61:17–26. doi: 10.1016/j.maturitas.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Wallen K. Sexual minority women’s sexual motivation around the time of ovulation. Arch Sex Behav. 2011;40:237–246. doi: 10.1007/s10508-010-9631-2. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Sexual and aggressive behaviour of adult male marmosets (Callithrix jacchus) castrated neonatally, prepubertally, or in adulthood. Physiol Behav. 1993;54:301–307. doi: 10.1016/0031-9384(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: the Peri Life Events Scale. J Health Soc Behav. 1978;19:205–229. [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. Phthalate exposure and reproductive hormones in adult men. Hum Reprod. 2005;20:604–610. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Fan LQ, Cattley RC, Corton JC. Tissue-specific induction of 17 beta-hydroxysteroid dehydrogenase type IV by peroxisome proliferator chemicals is dependent on the peroxisome proliferator-activated receptor alpha. J Endocrinol. 1998;158:237–246. doi: 10.1677/joe.0.1580237. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Peterson KE, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, Tellez-Rojo MM, Meeker JD. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol. 2014;47:70–76. doi: 10.1016/j.reprotox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. (discussion 181–145) [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22:2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Irvin EA, Calafat AM, Silva MJ, Aguilar-Villalobos M, Needham LL, Hall DB, Cassidy B, Naeher LP. An estimate of phthalate exposure among pregnant women living in Trujillo, Peru. Chemosphere. 2010;80:1301–1307. doi: 10.1016/j.chemosphere.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103:582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotkin RL, Zunker C, Ostbye T. Sexual functioning and obesity: a review. Obesity (Silver Spring, Md) 2012;20:2325–2333. doi: 10.1038/oby.2012.104. [DOI] [PubMed] [Google Scholar]
- Kwan M, Greenleaf WJ, Mann J, Crapo L, Davidson JM. The nature of androgen action on male sexuality: a combined laboratory–self-report study on hypogonadal men. J Clin Endocrinol Metab. 1983;57:557–562. doi: 10.1210/jcem-57-3-557. [DOI] [PubMed] [Google Scholar]
- Lara LA, Duarte AA, Reis RM, Vieira CS, Rosa-e-Silva AC. Endocrine disrupters: potential risk factors affecting sexual function in both men and women. J Sex Med. 2012;9:941–942. doi: 10.1111/j.1743-6109.2011.02596.x. [DOI] [PubMed] [Google Scholar]
- Laskey JW, Berman E. Steroidogenic assessment using ovary culture in cycling rats: effects of bis(2-diethylhexyl)phthalate on ovarian steroid production. Reprod Toxicol. 1993;7:25–33. doi: 10.1016/0890-6238(93)90006-s. [DOI] [PubMed] [Google Scholar]
- Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, Wang J, Weng X, Ferber JR, Herrinton LJ, Zhu Q, Gao E, Checkoway H, Yuan W. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010a;25:519–527. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- Li DK, Zhou Z, Miao M, He Y, Qing D, Wu T, Wang J, Weng X, Ferber J, Herrinton LJ, Zhu Q, Gao E, Yuan W. Relationship between urine bisphenol-A level and declining male sexual function. J Androl. 2010b;31:500–506. doi: 10.2164/jandrol.110.010413. [DOI] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Hernandez-Ramirez RU, Calafat AM, Torres-Sanchez L, Galvan-Portillo M, Needham LL, Ruiz-Ramos R, Cebrian ME. Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect. 2010;118:539–544. doi: 10.1289/ehp.0901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172:217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Lynch C. Vaginal estrogen therapy for the treatment of atrophic vaginitis. J Women’s Health. 2009;18:1595–1606. doi: 10.1089/jwh.2008.1281. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect. 2011;119:1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J Clin Endocrinol Metab. 2014;99(11):4346–4352. doi: 10.1210/jc.2014-2555. (jc20142555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl. 2009;30:287–297. doi: 10.2164/jandrol.108.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Meeker JD, Jorgensen N, Andersson AM, Liu F, Calafat AM, Redmon JB, Drobnis EZ, Sparks AE, Wang C, Hauser R, Swan SH. Urinary concentrations of di(2-ethylhexyl) phthalate metabolites and serum reproductive hormones: pooled analysis of fertile and infertile men. J Androl. 2012;33:488–498. doi: 10.2164/jandrol.111.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk ZM, Nantel A. Acute lindane poisoning with development of muscle necrosis. Can Med Assoc J. 1977;117:1050–1054. [PMC free article] [PubMed] [Google Scholar]
- O’Connor DB, Archer J, Wu FC. Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab. 2004;89:2837–2845. doi: 10.1210/jc.2003-031354. [DOI] [PubMed] [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006;114:1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resko JA, Phoenix CH. Sexual behavior and testosterone concentrations in the plasma of the rhesus monkey before and after castration. Endocrinology. 1972;91:499–503. doi: 10.1210/endo-91-2-499. [DOI] [PubMed] [Google Scholar]
- Rissman EF. Evidence that neural aromatization of androgen regulates the expression of sexual behaviour in female musk shrews. J Neuroendocrinol. 1991;3:441–448. doi: 10.1111/j.1365-2826.1991.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm Behav. 2013;63:636–645. doi: 10.1016/j.yhbeh.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, Wang CW, Swan S. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction (Cambridge, England) 2014;147:401–409. doi: 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck PE, Slob AK. Castration, sex steroids, and heterosexual behavior in adult male laboratory-housed stumptailed macaques (Macaca arctoides) Horm Behav. 1986;20:336–353. doi: 10.1016/0018-506x(86)90042-5. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Steinberg EM, Negro PP, Haq N, Gibson C, Rubinow DR. Pharmacologically induced hypogonadism and sexual function in healthy young women and men. Neuropsychopharmacology. 2009;34:565–576. doi: 10.1038/npp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1987;49:397–409. doi: 10.1097/00006842-198707000-00009. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339–351. doi: 10.1097/00006842-198507000-00004. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Bancroft J, Davidson DW, Warner P. Androgen replacement with oral testosterone undecanoate in hypogonadal men: a double blind controlled study. Clin Endocrinol (Oxf) 1981;14:49–61. doi: 10.1111/j.1365-2265.1981.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. 1998;65:143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- Souter I, Smith KW, Williams PL, Ehrlich S, Hauser R. Association of urinary phthalate (UrP) metabolite concentrations with ovarian response and early in-vitro (IVF) outcomes. Hum Reprod. 2013;28:i35–i37. [Google Scholar]
- Stanislaw H, Rice FJ. Correlation between sexual desire and menstrual cycle characteristics. Arch Sex Behav. 1988;17:499–508. doi: 10.1007/BF01542338. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinen KA, Dodson WC, Heindel JJ. Inhibition of FSH-stimulated cAMP accumulation and progesterone production by mono(2-ethylhexyl) phthalate in rat granulosa cell cultures. Toxicol Appl Pharmacol. 1990;106:334–340. doi: 10.1016/0041-008x(90)90252-p. [DOI] [PubMed] [Google Scholar]
- Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, Holt VL. Phthalates and risk of endometriosis. Environ Res. 2013;126:91–97. doi: 10.1016/j.envres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Sex and context: hormones and primate sexual motivation. Horm Behav. 2001;40:339–357. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Wallen K, Zehr JL. Hormones and history: the evolution and development of primate female sexuality. J Sex Res. 2004;41:101–112. doi: 10.1080/00224490409552218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environ Health Perspect. 2010;118:825–832. doi: 10.1289/ehp.0901543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Hum Reprod. 2004;19:1539–1543. doi: 10.1093/humrep/deh305. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Maestripieri D, Wallen K. Estradiol increases female sexual initiation independent of male responsiveness in rhesus monkeys. Horm Behav. 1998;33:95–103. doi: 10.1006/hbeh.1998.1440. [DOI] [PubMed] [Google Scholar]