Abstract
Chronic infection with Helicobacter pylori is the strongest known risk factor for the development of gastric cancer. Saju et al. shed new light on mechanisms by which Epstein–Barr virus, a viral initiator of gastric cancer, potentiates the oncogenic effects of Helicobacter pylori in the stomach.
Worldwide, gastric cancer is the third leading cause of cancer-related mortality, accounting for >700,000 deaths each year1. The most common type of gastric cancer is adenocarcinoma, which can be stratified histologically into two distinct variants: diffuse-type gastric cancer, which consists of individually infiltrating neoplastic cells that do not form glandular structures; and intestinal-type adenocarcinoma, which progresses through a series of well-defined histological steps2. In 2014, a molecular classification of gastric cancer was developed through the Cancer Genome Atlas study that comprehensively analysed nearly 300 primary gastric adenocarcinomas and segregated tumours into four molecular subtypes: Epstein–Barr virus (EBV)-positive tumours; microsatellite unstable tumours; genomically stable tumours associated with diffuse-type histology; and intestinal-type tumours with chromosomal instability3. However, the degree to which these molecular classes overlap is unknown.
The strongest known environmental risk factor for developing gastric cancer is prolonged colonization with Helicobacter pylori, and microbial virulence constituents clearly modify disease risk4. H. pylori strains that possess the cag island — a type IV secretion system that translocates its substrate, CagA, into host cells — augment the risk of gastric cancer compared with cag− strains4. Following translocation, CagA becomes tyrosine phosphorylated at C-terminal glutamate-proline-isoleucine-tyrosine-alanine (EPIYA) motifs, endowing CagA with oncogenic activity5. In addition to H. pylori, EBV, a ubiquitous human herpes virus with oncogenic potential, is associated with the development of gastric adenocarcinoma, and has been linked to ~9% of gastric cancer cases worldwide6. An exciting new study by Saju et al.7 now describes a paradigm of how a bacterium and a virus collaborate to potentially exacerbate a disease they can each cause independently.
Specifically, Saju et al.7 report that a host phosphatase, SHP1 (also known as tyrosine-protein phosphatase non-receptor type 6 or PTPN6), interacts with H. pylori-delivered CagA and functions as a negative regulator of CagA phosphorylation. Using a combination of transient CagA transfections and co-culture of viable H. pylori cag+ strains with gastric epithelial cells, the researchers demonstrate that CagA forms a complex with SHP1, which was independent of CagA tyrosine phosphorylation status and did not involve CagA EPIYA phosphorylation motifs7. Glutathione S-transferase pull-down assays verified the direct interaction between CagA and SHP1, which required the N-terminal region of SHP1, and both the C-terminal and N-terminal regions of CagA7.
In terms of downstream effects, levels of phosphorylated CagA were decreased when SHP1 was ectopically overexpressed in host cells, which required SHP1 phosphatase activity. Conversely, levels of phosphorylated CagA were increased by knockdown of SHP1 (via small-interfering RNA), suggesting that SHP1 is a rheostat that tightly regulates the level of CagA tyrosine phosphorylation7. To definitively determine whether SHP1 is the mammalian phosphatase that dephosphorylates CagA, Saju et al.7 used an in vitro phosphatase assay whereby SHP1 was co-administered with tyrosine-phosphorylated CagA. Under these conditions, CagA was dephosphorylated by SHP1 and the active phosphatase domain was indispensible. Notably, under the same conditions, the related phosphatase SHP2 had no effect on the phosphorylation status of CagA but was instead, activated7. Collectively, these elegant experiments demonstrate that SHP1 is activated upon complex formation with CagA and this interaction induces CagA dephosphorylation, subsequently reducing the oncogenic potential of phosphorylated CagA7.
“… a paradigm of how a bacterium and a virus collaborate to potentially exacerbate a disease they can each cause independently”
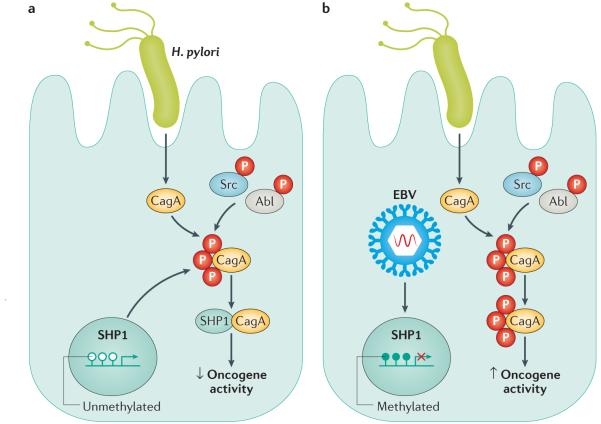
As co-infection with H. pylori and EBV is present in the majority of EBV-positive gastric cancer specimens, Saju et al.7 next demonstrated that EBV is capable of hyper-methylating the SHP1 promoter (FIG. 1), thus decreasing expression of SHP1 at both the RNA and protein level. Importantly, the oncogenic potential of phosphorylated CagA was enhanced in the presence of EBV, indicating a novel role for this virus in promoting pathways that might ultimately lead to gastric carcinogenesis7.
Figure 1. Co-infection with Helicobacter pylori and EBV in gastric epithelial cells promotes the oncogenic activity of CagA.
CagA is translocated into host cells by the H. pylori cag secretion system where it is tyrosine phosphorylated by Src and Abl kinases. a | In the absence of Epstein–Barr virus (EBV), SHP1 interacts with CagA and negatively regulates CagA phosphorylation. b | With concomitant EBV infection, the SHP1 promoter is hypermethylated and its expression is downregulated. This process results in elevated levels of oncogenic phosphorylated CagA.
Identification of synergy between H. pylori and EBV has important implications for managing patient populations and identifying individuals at high risk of developing gastric cancer. To date, it is not possible to accurately predict which H. pylori-infected individuals will develop this malignancy, and because the vast majority of infected individuals do not develop cancer, it is imperative to develop noninvasive tests with strong predictive potential. On the basis of this new study7, seropositivity for both H. pylori and EBV could help identify such a high-risk population. However, the molecular interaction between EBV and CagA is likely to be uncommon within the collective context of gastric adenocarcinoma cases. Only a minority of gastric cancers (<10%) harbour EBV and not all strains of H. pylori are CagA positive4. Nevertheless, these results invoke a novel working model for how bacteria and viruses might interact within and exogenous to the gastric epithelium.
In addition to bacterial–viral interactions, there is clearly precedence for microbial collaborations augmenting disease within the stomach. In germ-free mice that are monocolonized with H. pylori, gastric cancer develops at a markedly attenuated rate compared with mice that harbour a more complex gastric microbiota of at least three specific species of commensal bacteria: ASF356 Clostridium species, ASF361 Lactobacillus murinus and ASF519 Bacteroides species8,9. These effects could be magnified even further if progenitor cells are targeted by H. pylori; investigations have demonstrated that levels of CagA are increased in H. pylori-infected cancer stem-like cells when compared with infected differentiated cells10.
Taken together, the work of Saju and colleagues7 provides a fascinating example of how a virus and bacterial species collaborate within the gastric epithelium to exacerbate progression towards cancer. However, there are still many questions to address regarding the pathobiology of H. pylori and EBV infection, alone or in combination, and the mechanisms through which each pathogen promotes the cascade to carcinogenesis.
Footnotes
Competing interest statement
The authors declare no competing interests.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process — first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 3.The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amieva M, Peek RM., Jr Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller D, et al. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J. Clin. Invest. 2012;122:1553–1566. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein–Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saju PM-K, et al. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein–Barr virus. Nat. Microbiol. 2016;1:16026. doi: 10.1038/nmicrobiol.2016.26. [DOI] [PubMed] [Google Scholar]
- 8.Lofgren JL, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–220. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lertpiriyapong K, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014;63:54–63. doi: 10.1136/gutjnl-2013-305178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsugawa H, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]