Abstract
Purpose
We examined the effects of surgery type and adjuvant chemotherapy on change in early-stage breast cancer patients' quality of life (QOL) over time.
Methods
A cohort of 549 patients (33.5% ductal carcinoma in situ, 66.5% stages I/IIA) were interviewed a mean 6.1 weeks (Time1), and 6.2 (Time2), 12.3 (Time3), and 24.4 (Time4) months following definitive breast-conserving surgery (BCS) or mastectomy. QOL was measured using the total Functional Assessment of Cancer Therapy-Breast (FACT-B). Adjusting for demographic, psychosocial, and clinical variables, multiple linear regression models estimated the associations between QOL and each of surgery type, chemotherapy and their 2-way interaction at each interview. Adjusted Generalized Estimating Equation (GEE) models tested Time1-Time4 change in QOL.
Results
At T2, chemotherapy (P < 0.001) and BCS (P < 0.001) were independently associated with worse QOL in adjusted linear regression, and the adverse effect of chemotherapy was prominent among patients who received BCS compared with those who received mastectomy (Pinteraction = 0.031). In the GEE model, QOL significantly improved over time among patients who received BCS (Ptrend = 0.047), mastectomy (Ptrend = 0.024), and chemotherapy (Ptrend < 0.001) but not among patients who did not receive chemotherapy (Ptrend = 0.720). All patients completed adjuvant chemotherapy and radiation by Time3. Regardless of surgery type, patients receiving chemotherapy reported lower QOL following surgery, and QOL improved after completion of adjuvant treatment.
Conclusions
Chemotherapy had a short-term negative impact on QOL after definitive surgical treatment regardless of surgery type. QOL rebounded after completion of adjuvant treatment.
Keywords: Quality of life, Chemotherapy, Breast-conserving surgery, Mastectomy, Cohort study
Much of the increase in early-stage breast cancer incidence over the past several decades has resulted from widespread use of screening mammography and early detection of breast cancers, including ductal carcinoma in situ (DCIS, stage 0) and early-invasive breast cancer (EIBC, stages I/IIA).1 Although DCIS is clinically distinct from EIBC2–6 and offers excellent prognosis, DCIS and EIBC patients are offered similar surgical-treatment options (i.e., mastectomy or breast-conserving surgery [BCS] and radiation therapy, and hormone therapy, as indicated).7 The equivalence of BCS and mastectomy in prevention of DCIS/EIBC recurrence, morbidity, and mortality has been demonstrated.8–10 However, with growing numbers of breast cancer survivors enjoying longer life expectancy,11 quality of life (QOL) outcomes are increasingly important considerations when making treatment decisions.
Many studies (largely cross-sectional) have examined QOL outcomes after early-stage breast cancer treatment.12–21 Reports of equivalent QOL outcomes by surgery type depend on timing of the QOL assessments and whether physical or psychological aspects of QOL are being measured.13,22–31 Few longitudinal studies of early-stage breast cancer patients examined QOL changes over time,13,32,33 but these studies did not evaluate QOL improvement in association with surgery type, chemotherapy, and the surgery*chemotherapy interaction. In a large cohort study of same-aged women with and without breast cancer (controls), both DCIS and EIBC patients reported QOL improvements over 2-year follow-up, but DCIS patients reached QOL levels reported by controls sooner than EIBC patients.32 Since DCIS patients do not receive chemotherapy, we hypothesized that the observed differences in QOL improvement between DCIS and EIBC patients32 might reflect adverse effects of chemotherapy on QOL among EIBC patients.34–36 Therefore, we examined whether and to what extent improvements in QOL were affected by surgery type, chemotherapy, and their interaction.
MATERIALS AND METHODS
Patients were recruited prospectively between October 2003 and June 2007 from the Siteman Cancer Center and Saint Louis University School of Medicine, both in St. Louis, MO.32 We included English-speaking patients who were at least 40 years old, diagnosed with first primary, pathology-confirmed stage 0-IIA breast cancers, no prior breast cancer history, had not received neoadjuvant chemotherapy, and did not demonstrate cognitive impairment on the Orientation-Memory-Concentration Test.37
Institutional Review Boards at both universities approved the study, and participants provided informed consent. We conducted four computer-assisted telephone interviews 4–6 weeks (Time1), 6 months (Time2), 1 year (Time3), and 2 years (Time4) following definitive surgical treatment, during which we collected demographic information and administered new and previously validated questionnaires to identify potential covariates of QOL. We collected data regarding pathological stage at diagnosis, definitive surgical treatment, and adjuvant therapies from the medical record.
At each interview, we measured QOL using the Functional Assessment of Cancer Therapy-Breast (FACT-B) Version 4.38 FACT-B total scores range from 0 to 144, with higher scores reflecting better QOL. Based on previous work,32 we included the following covariates of QOL in our analysis. The 19-item Medical Outcomes Study (MOS) Social Support Survey39 measures perceived availability of social support; higher scores indicate greater perceived availability of social support, if needed. Using a validated interview measure of comorbidity,40 we computed a weighted index score using the Charlson Comorbidity Index algorithm;41 higher scores indicate more severe comorbidity. A history of depression was determined by an affirmative response to either, “Has a doctor ever told you that you had depression?” or “Have you ever been treated for depression with medication or psychotherapy?” We also included a validated measure of surgical-side-effects severity experienced in the past month,42,43 with higher mean scores indicating more severe side effects from surgery and lymph-node excision. We also collected demographic information, including age, marital status, education, race, and height and weight to compute body mass index (BMI).
Data for receipt of adjuvant chemotherapy, radiotherapy, and hormone therapy were obtained from patients at each interview and confirmed by the medical record. Stage at diagnosis determined by surgical pathology (stages 0, I and IIA) and type of definitive surgical treatment (BCS, mastectomy) also were collected from the medical record.
Data Analysis
Using IBM SPSS Statistics, Release 21.0.0.2 (IBM Corporation, 2012), we identified covariates of QOL at Time1, using analysis of variance (ANOVA) grouping by each demographic and clinical categorical variable and Pearson product-moment correlations between QOL and each continuous variable. We used chi-square tests to examine associations among categorical variables of interest.
Using SAS version 9.3 (SAS Institute, Cary, NC), we performed separate linear regression analyses with Time1, Time2, Time3, and Time4 data to assess the associations of surgery type and chemotherapy with QOL at each interview, controlling for covariates associated with QOL at Time1. To determine if the effect of chemotherapy on QOL differed between patients who received BCS and mastectomy, an interaction term between surgery type and chemotherapy was included in the models. We used the GENMOD procedure in SAS to fit the generalized estimating equations (GEE) to compare changes in QOL over the 2-year follow-up (Time1-Time4) according to surgery type and chemotherapy, adjusted for selected covariates. The GEE model accounts for correlations among repeated measurements from each study participant and allows for inclusion of all available data. An unstructured correlation was specified to model the correlation of responses from each participant. The interactions of surgery type and chemotherapy with time since definitive surgery were included in the model to evaluate whether QOL changed over time in a different way between patients who received BCS and mastectomy and who did and did not receive chemotherapy. We tested these interaction effects using the CONTRAST statement in PROC GENMOD. Two-sided P values < 0.05 were considered statistically significant.
RESULTS
We invited 772 early-stage breast cancer patients to participate in the parent QOL study and enrolled 549 (71%). There were no significant differences between patient participants and non-participants by pathological stage (P = 0.837), surgery type (BCS vs. mastectomy; P = 0.095), or marital status (married vs. unmarried; P = 0.072). However, patient participants were younger, on average, than non-participants (mean [SD], 58.3 [10.6] vs.60.6 [12.6]; P = 0.011) and were more likely to be white (79.2% vs. 63.8%; P < 0.001).
Patient characteristics by surgery type and chemotherapy are shown in Table 1. Patients completed four interviews a mean (SD) 6.1 (2.5) weeks and 6.2 (0.4), 12.3 (0.4), and 24.5 (0.5) months following definitive surgical treatment. Retention remained high with 514 patients (93.6%) completing all four interviews, and these patients were not significantly different from those who dropped out after the first interview in terms of age, education, or race (each P > 0.05). However, a greater proportion of patients who dropped out had never been married (20.0% vs. 8.8%, P = 0.019) and reported lower QOL at Time1–Time3 (each P < 0.01) compared with patients who completed all four interviews.
TABLE 1.
Patient characteristics at first interview (except as noted), by surgery type and receipt of chemotherapya
| BCS | Mastectomy | P value | Chemotherapyb | No chemotherapy | P value | |
|---|---|---|---|---|---|---|
| n = 356 | n = 193 | n = 136 | n = 413 | |||
| Age, mean (SD) | 59.7 (10.8) | 55.9 (9.8) | < 0.001 | 53.3 (7.3) | 60.0 (11.0) | < 0.001 |
| Body mass index, mean (SD)c | 28.8 (6.8) | 27.8 (6.7) | 0.101 | 28.8 (6.9) | 28.4 (6.7) | 0.513 |
| Surgical-side-effects severity, mean (SD) | 1.4 (0.5) | 2.2 (0.8) | < 0.001 | 1.9 (0.8) | 1.6 (0.7) | < 0.001 |
| Comorbidity, mean (SD) | 0.6 (0.9) | 0.5 (1.0) | 0.347 | 0.5 (0.8) | 0.6 (1.0) | 0.226 |
| Social Support, mean (SD) | 4.5 (0.6) | 4.5 (0.7) | 0.898 | 4.5 (0.7) | 4.5 (0.6) | 0.832 |
|
| ||||||
| Race | 0.102 | 0.095 | ||||
| White, n (%) | 292 (82.0) | 147 (76.2) | 102 (75.0) | 337 (81.6) | ||
| Non-white, n (%) | 64 (18.0) | 46 (23.8) | 34 (25.0) | 76 (18.4) | ||
| Marital status | 0.040 | 0.039 | ||||
| Married/Member of unmarried couple, n (%) | 203 (57.0) | 130 (67.4) | 82 (60.3) | 251 (60.8) | ||
| Widowed, n (%) | 55 (15.4) | 15 (7.8) | 9 (6.6) | 61 (14.8) | ||
| Divorced/separated, n (%) | 63 (17.7) | 31 (16.1) | 30 (22.1) | 64 (15.5) | ||
| Never been married, n (%) | 35 (9.8) | 17 (8.8) | 15 (11.0) | 37 (9.0) | ||
| Education | 0.337 | 0.179 | ||||
| < High school graduate, n (%) | 25 (7.0) | 18 (9.3) | 7 (5.1) | 36 (8.7) | ||
| At least high school graduate, n (%) | 331 (93.0) | 175 (90.7) | 129 (94.9) | 377 (91.3) | ||
| History of depression | 0.413 | 0.845 | ||||
| Yes, n (%) | 124 (34.8) | 74 (38.3) | 50 (36.8) | 148 (35.8) | ||
| No, n (%) | 232 (65.2) | 119 (61.7) | 86 (63.2) | 265 (64.2) | ||
| Pathologic stage | < 0.001 | < 0.001 | ||||
| 0, n (%) | 111 (31.2%) | 73 (37.8%) | 0 (0.0%) | 184 (44.6%) | ||
| I, n (%) | 203 (57.0%) | 79 (40.9%) | 73 (53.7%) | 209 (50.6%) | ||
| IIA, n (%) | 42 (11.8%) | 41 (21.2%) | 63 (46.3%) | 20 (4.8%) | ||
| Radiation therapy d | < 0.001 | 0.498 | ||||
| Yes, n (%) | 332 (93.3) | 18 (9.3) | 90 (66.2) | 260 (63.0) | ||
| No, n (%) | 24 (6.7) | 175 (90.7) | 46 (33.8) | 153 (37.0) | ||
| Endocrine therapy e | < 0.001 | < 0.310 | ||||
| Yes, n (%) | 250 (70.2) | 94 (48.7) | 82 (60.3) | 262 (63.4) | ||
| No, n (%) | 104 (29.2) | 96 (49.7) | 54 (39.7) | 146 (35.4) | ||
| Unknown, n (%) | 2 (0.6) | 3 (1.6) | 0 (0.0) | 5 (1.2) | ||
BCS breast-conserving surgery, SD standard deviation
Tests of significance were one-way analysis of variance for continuous variables and chi-square tests for categorical variables
Numbers shown are for patients who received adjuvant chemotherapy at any time after enrollment; 59 (43.3% of 136) early-invasive breast cancer patients reported taking chemotherapy at the first interview
Body mass index was not calculated for 3 women lacking height and/or weight data
Numbers shown are for patients who received radiation therapy at any time after enrollment; 130 patients reported receipt of radiation therapy at the first interview
Numbers shown are for patients who received endocrine therapy at any time after enrollment; 96 patients reported taking endocrine therapy at the first interview
At Time1, 130 (23.7%) of 549 patients reported receiving radiotherapy, and 59 (10.7%) reported receiving chemotherapy. At Time2, 321 (59.8%) of 537 patients reported receiving radiotherapy, and 132 (24.6%) reported receiving chemotherapy. All patients who received chemotherapy had initiated treatment by Time2; all patients who received chemotherapy and/or radiotherapy had completed these adjuvant treatments by Time3. Receipt of hormone therapy was reported by 17% (96/549) of patients at Time1, 47.5% (255/537) at Time2, 58.9% (311/528) at Time3, and 58.0% (298/514) at Time4.
Associations between QOL and factors measured at Time1 are shown in Tables 2 and 3. All variables significantly associated with QOL at Time1 were included as covariates in the multivariable regression and GEE models. We included receipt of radiation therapy and hormone therapy as covariates, because they were reported to be associated with QOL during and after treatment.44,45
TABLE 2.
Unadjusted mean (SD) FACT-B total scores at each of the four interviews (Time1–Time4) after definitive surgical treatment, for each demographic and clinical covariate of interest at Time1
| Time1 covariates | Time1 | P value | Time2 | P value | Time3 | P value | Time4 | P value |
|---|---|---|---|---|---|---|---|---|
| n = 549 | n = 536a | n = 527b | n = 514 | |||||
| Race | 0.024 | 0.003 | 0.001 | < 0.001 | ||||
| White | 116.2 (18.4) | 119.1 (18.7) | 118.9 (16.3) | 122.1 (16.5) | ||||
| Non-white | 111.5 (23.4) | 112.6 (23.7) | 112.4 (21.2) | 113.1 (26.0) | ||||
| Marital status | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Married/member of an unmarried couple | 117.8 (17.0) | 119.8 (17.5) | 120.9 (14.6) | 123.6 (15.8) | ||||
| Widowed | 122.4 (15.9) | 124.3 (14.3) | 119.00 (17.0) | 123.1 (16.0) | ||||
| Divorced/separated | 105.6 (22.8) | 109.0 (24.0) | 108.5 (21.2) | 110.3 (23.7) | ||||
| Never been married | 107.7 (24.4) | 111.4 (25.9) | 110.8 (21.0) | 112.2 (25.1) | ||||
| Education | 0.003 | 0.005 | 0.003 | 0.001 | ||||
| < High school graduate | 106.8 (22.0) | 109.2 (24.8) | 109.8 (17.7) | 111.2 (23.2) | ||||
| At least high school graduate | 116.0 (19.2) | 118.5 (19.3) | 118.3 (17.3) | 121.1 (18.4) | ||||
| History of depression | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Yes | 106.8 (20.6) | 109.9 (23.4) | 112.0 (20.2) | 113.4 (22.4) | ||||
| No | 120.1 (17.2) | 122.2 (16.1) | 120.7 (15.0) | 124.1 (15.7) | ||||
| Pathologic stage | < 0.001 | < 0.001 | 0.003 | 0.006 | ||||
| 0 | 117.9 (18.3) | 120.0 (18.3) | 119.7 (16.8) | 122.5 (17.4) | ||||
| I | 116.0 (18.9) | 118.6 (19.3) | 118.0 (16.7) | 120.7 (17.6) | ||||
| IIA | 107.1 (22.4) | 109.9 (23.5) | 111.7 (20.6) | 114.2 (25.5) | ||||
| Surgery type | 0.001 | 0.031 | 0.011 | 0.094 | ||||
| BCS | 117.3 (18.2) | 119.2 (18.99) | 119.1 (15.8) | 121.4 (20.0) | ||||
| Mastectomy | 111.7 (21.4) | 115.3 (21.3) | 115.0 (20.1) | 118.4 (20.8) | ||||
| Radiation therapy c | 0.136 | 0.324 | 0.234 | 0.320 | ||||
| Yes | 116.2 (18.3) | 118.4 (19.0) | 118.3 (16.6) | 121.00 (18.00) | ||||
| No | 113.6 (21.6) | 116.7 (21.4) | 116.4 (18.9) | 119.2 (20.8) | ||||
| Chemotherapy c | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Yes | 106.4 (21.8) | 109.2 (23.5) | 112.8 (20.0) | 114.9 (23.1) | ||||
| No | 118.2(17.9) | 120.6 (17.7) | 119.2 (16.3) | 122.1 (17.1) | ||||
| Endocrine therapy d | 0.200 | 0.101 | 0.309 | 0.472 | ||||
| Yes | 117.7 (15.8) | 121.0 (16.2) | 119.5 (13.9) | 121.9 (16.2) | ||||
| No | 114.9 (20.3) | 117.3 (20.5) | 117.5 (17.9) | 120.3 (19.6) |
SD standard deviation, FACT-B Functional Assessment of Cancer Therapy-Breast, Time1 first interview (4–6 weeks), Time2 second interview (6 months), Time3 third interview (1 year), Time4 fourth interview (2 year), BCS breast-conserving surgery
Although 537 women completed Time2, one participant refused to answer the FACT-B during the Time2 interview
Although 528 women completed Time3, one participant had incomplete FACT-B data and could not be included in the Time3 analysis
Obtained from the medical record
Based on patient's self-reported receipt of adjuvant hormone therapy at each interview, confirmed by the medical record
TABLE 3.
Pearson product-moment correlations between FACT-B total scores at each interview (Time1–Time4) after definitive surgical treatment and each covariate of interest measured at first interview (Time1)
| Time1 | P value | Time2 | P value | Time3 | P value | Time4 | P value | |
|---|---|---|---|---|---|---|---|---|
| Time1 covariates | n = 549 | n = 536a | n = 527b | n = 514 | ||||
| Age | .300 | < 0.001 | .258 | < 0.001 | .179 | < 0.001 | .177 | < 0.001 |
| Body mass index c | −.156 | < 0.001 | −.126 | < 0.004 | −.222 | < 0.001 | −.165 | < 0.001 |
| Surgical-side-effects severity | −.467 | < 0.001 | −.419 | < 0.001 | −.385 | < 0.001 | −.363 | < 0.001 |
| Comorbidity | −.112 | 0.009 | −.129 | 0.003 | −.134 | 0.002 | −.130 | 0.003 |
| Social support | .440 | < 0.001 | .427 | < 0.001 | .365 | < 0.001 | .376 | < 0.001 |
FACT-B Functional Assessment of Cancer Therapy-Breast, Time1 first interview (4–6 weeks), Time2 second interview (6 months), Time3 third interview (1 year), Time4 fourth interview (2 year)
Although 537 women completed Time2, one participant refused to answer the FACT-B during the interview
Although 528 women completed Time3, one participant had missing FACT-B data and could not be included in the analysis at this point in time
Three women lacked data to compute body mass index
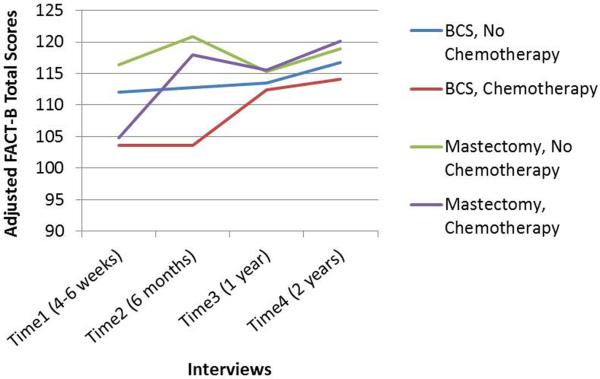
We used linear regression models adjusted for covariates to test the differences in QOL (least squares means) by surgery type and receipt of chemotherapy at each interview (Fig. 1). At Time1, BCS (vs. mastectomy; P = 0.014) and chemotherapy (vs. no chemotherapy; P < 0.001) were associated with worse QOL, but the surgery*chemotherapy interaction effect was not significant (P = 0.468). At Time2, BCS (P < 0.001) and chemotherapy (P = 0.001) were associated with worse QOL, and the adverse effect of chemotherapy on QOL was more prominent among patients who received BCS than mastectomy (Pinteraction = 0.031). At Time3, neither surgery type nor chemotherapy was significantly associated with QOL, but at Time4, BCS was associated with worse QOL (P = 0.005).
FIG. 1.
Quality of life over 2-year follow-up of early-stage breast cancer patients, by surgery type (breast-conserving surgery [BCS] or mastectomy) and receipt of chemotherapy. Least squares means, shown here, were derived from four separate linear regression models (run separately for each of the four interviews after definitive surgical treatment). Each model was adjusted for the following covariates: age, race, education, marital status, body mass index, social support, comorbidity, history of depression, surgical-side-effects severity, cancer stage, and receipt of radiation and endocrine therapy.
Because women with DCIS in the absence of invasive disease typically do not receive chemotherapy, we ran models without them. Results were similar to the models with DCIS patients, except that only chemotherapy was associated with worse QOL at Time1 (P < 0.001) and the surgery*chemotherapy interaction effect was attenuated at Time2 (Pinteraction = 0.215).
We used GEE models to evaluate the trends of QOL over the 2-year follow-up by surgery type and chemotherapy. Although it appeared that QOL in patients who received BCS and chemotherapy took longer to recover compared with patients in the other three groups (Fig. 1), the GEE 3-way interaction (surgery type*chemotherapy*time) was not significant (P = 0.86), indicating the change in QOL over time among patients receiving chemotherapy was not modified by surgery type. In Table 4, GEE models evaluating 2-way interactions (chemotherapy*time and surgery*time) showed QOL improved among patients who received BCS and mastectomy; the improvement in each group was comparable (Pdifference = 0.440). QOL also improved significantly in patients who received chemotherapy, but not in patients who did not receive chemotherapy. Excluding DCIS patients, the 3-way interaction in the GEE model still was not significant (P = 0.96); QOL significantly improved for patients who received chemotherapy and mastectomy but not for patients who received BCS or who did not receive chemotherapy.
TABLE 4.
Generalized Estimating Equation (GEE) models testing differences in change in total FACT-B scores per 6 months after definitive surgical treatment over 2-year follow-up, by surgery type and receipt of chemotherapya
| Mean change (confidence limits) | P for trend | P for difference in trend | |
|---|---|---|---|
| Model with DCIS patients | |||
| Surgery type | 0.440 | ||
| BCS | 0.38 (0.01, 0.75) | 0.047 | |
| Mastectomy | 0.64 (0.09, 1.20) | 0.024 | |
| Chemotherapy | < 0.001 | ||
| No | 0.06 (−0.28, 0.40) | 0.720 | |
| Yes | 2.02 (1.35, 2.70) | < 0.001 | |
|
| |||
| Model without DCIS patients | |||
| Surgery type | 0.271 | ||
| BCS | 0.28 (−0.20, 0.76) | 0.256 | |
| Mastectomy | 0.80 (0.01, 1.59) | 0.046 | |
| Chemotherapy | < 0.001 | ||
| No | −0.18 (−0.66, 0.31) | 0.480 | |
| Yes | 1.77 (1.07, 2.47) | < 0.001 | |
FACT-B Functional Assessment of Cancer Therapy-Breast, BCS breast-conserving surgery
GEE models examining change in QOL by surgery type and chemotherapy were adjusted for covariates: age, race, education, marital status, BMI, social support, comorbidity, history of depression, surgical-side-effects severity, cancer stage, receipt of radiation therapy, and receipt of endocrine therapy
DISCUSSION
Poorer QOL outcomes in EIBC patients at 2-year follow-up compared with DCIS patients were previously reported.32 Since DCIS patients did not receive chemotherapy, we hypothesized that EIBC patients' poorer QOL might reflect adverse effects of chemotherapy on QOL among EIBC patients. Here, we ran regression models with and without DCIS patients and found similar results; but, in the model without DCIS patients, only chemotherapy was associated with worse QOL at Time1, and the surgery*chemotherapy interaction effect was attenuated at Time2. The adverse effect of chemotherapy on QOL was more prominent among patients who received BCS compared with mastectomy. These results support our hypothesis and contribute substantively to the literature regarding QOL change in association with surgical and adjuvant treatment regimens for early-stage breast cancer.
Earlier studies indicated equivalent QOL outcomes in woman who underwent BCS versus mastectomy.22–31 However, many of these studies either did not examine the impact of adjuvant therapy on QOL23–27,29–31 or measured only anxiety and depression,22 which are important affective components of QOL, but do not measure other aspects of QOL that could be affected by surgery type. Other studies reported a slight advantage in QOL outcomes in women who underwent BCS versus mastectomy;31,46–49 but some studies were retrospective49 or cross-sectional31,46 by design, precluding examination of potential improvements in QOL over time. These design differences alone could account for the discrepant results reported in the literature.
The effect of surgery type on QOL also might depend on the timing of QOL assessments following surgery.13,47 Although we found a significant effect of surgery type on QOL six months following definitive surgical treatment, adjusting for demographic, psychosocial, and clinical covariates, the change pattern in QOL over time did not differ by surgery type (Table 4), suggesting that QOL gains two years following definitive surgical treatment were equivalent between BCS and mastectomy.
We and others36,50 observed a short-term adverse effect of chemotherapy on QOL within two years of breast cancer surgery. Chemotherapy also has been reported to predict impaired QOL in 5- and 10-year survivors.51,52 QOL following specific adjuvant treatment regimens has been described in longitudinal studies with patients receiving radiation,44,45,53 chemotherapy,54 radiation and chemotherapy,55,56 and endocrine therapy.57–60 However, unlike these studies, we included an adjuvant chemotherapy*time interaction term in our analysis—in relation to receipt of BCS or mastectomy as well—allowing us to determine if, and how, the change in QOL over time differed by receipt of chemotherapy. Women receiving chemotherapy demonstrated a significant rate of improvement in QOL within the first year, which was not observed in patients who did not receive chemotherapy, as women who received chemotherapy reported poorer QOL at Time1 than women who did not receive chemotherapy. In addition, this higher rate of improvement among women who received chemotherapy occurred at different times depending upon surgery type, as most women who received mastectomy had not received radiation, and most women who received BCS received radiation as standard of care.
To examine the trend of QOL over time, we adjusted the GEE model for whether or not a patient received radiation therapy over the study period. Although change in QOL over the 2-year follow-up among patients receiving chemotherapy was not modified by surgery type, we could not account for the timing of initiation or duration of radiation from the medical record to determine the potential cumulative effect of radiation for those patients who received BCS. These treatment factors, however, may not entirely explain the associations observed between QOL and either surgery type or adjuvant chemotherapy. Psychosocial factors (e.g., social support52 or a history of depression26,61) might explain fluctuations in QOL over time. Further research is warranted, because we controlled for these factors and yet observed small (albeit not minimally important62) declines in QOL between Time2 and Time3 in both mastectomy groups but not in the BCS groups (Fig. 1).
The longitudinal design and high retention of patients are study strengths. But participation and retention rates were higher for white and for married patients—potential sources of selection bias. Generalizability of our findings might be limited by recruitment from a National Cancer Institute-designated comprehensive cancer center and another academic medical center in the same city. Our sample was representative of the racial/ethnic distribution of breast cancer patient population in our catchment area (21%), but 95% of non-white participants were Black, limiting generalizability to other non-white racial/ethnic groups. Findings also might not be generalizable to patients with more advanced disease or patients younger than 40 years of age who are more likely to present with aggressive disease.63
In addition, each surgery*chemotherapy treatment interaction group yielded smaller numbers, thereby diminishing the power to detect significant differences among the various treatment combinations after completion of adjuvant treatments at Time3 and Time4. Finally, although we did not include breast reconstruction and type of lymph node biopsy (sentinel lymph node biopsy versus axillary lymph node dissection) in the multivariate models, we controlled for surgical-side-effects severity42,43 to account for the negative effects of lymphedema and other surgical side effects42,43 on QOL.64–66
In conclusion, we demonstrated that patients who received chemotherapy reported poorer QOL in the first year after surgery, but QOL rebounded within months of treatment completion regardless of surgery type. While BCS predicted worse QOL at Time2, potentially due to the additional receipt and timing of adjuvant radiation therapy44 and type of chemotherapy,36 the pattern of change in QOL over 2-year follow-up did not differ significantly by surgery type. The relative effects of chemotherapy and radiation on changes in QOL over time remain unknown.
Synopsis.
We examined the effects of surgery and adjuvant chemotherapy on quality of life (QOL) over 2-year follow-up in early-stage breast cancer patients. Chemotherapy negatively affected QOL shortly after surgery, regardless of surgery type, but QOL rebounded after adjuvant therapy completion.
ACKNOWLEDGMENTS
This study was supported by grants from the National Cancer Institute (NCI) and Breast Cancer Stamp Fund (R01 CA102777; PI: Jeffe, DB) and the NCI Cancer Center Support Grant (P30 CA091842; PI: Eberlein, T) to the Alvin J. Siteman Cancer Center. Contents of this paper are solely the responsibility of the authors; the funders did not influence the study design, data collection, analysis, or manuscript preparation. Views expressed herein do not necessarily represent the official views of the NCI or Breast Cancer Stamp Fund.
We thank our participants, the interviewers, and the Siteman Cancer Center's Health Behavior, Communication, and Outreach Core for data management services. We also thank the physicians who helped us recruit their patients for this study, including Drs. Barbara Monsees, Jill Dietz, Julie Margenthaler, Virginia Herrmann, Timothy Eberlein, Matthew Ellis, Imran Zoberi, Marie Taylor, Michael Naughton, Antonella Rastelli, Donald Lombardi, Cynthia Ma, Loren Michel, and Rama Suresh at Washington University School of Medicine and Dr. Eddie Hsueh and Pam Hunborg, RN, at Saint Louis University School of Medicine.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
REFERENCES
- 1.Virning BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 2.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. The Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 3.Morrow M, Strom E, Bassett L, et al. Standard for the management of ductal carcinoma in situ of the breast (DCIS) CA: A Cancer Journal for Clinicians. 2002;52(5):256–276. doi: 10.3322/canjclin.52.5.256. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein MJ, Barth A, Poller DN, et al. Ten year results comparing mastectomy to excision and radiotherapy for ductal carcinoma in situ of the breast. Eur J Cancer. 1995;31A(9):1425–1427. doi: 10.1016/0959-8049(95)00283-o. [DOI] [PubMed] [Google Scholar]
- 5.Bartelink H, Horiot J, Poortmans P, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 6.Elder E, Kennedy C, Gluch L, et al. Patterns of breast cancer relapse. European Journal of Surgical Oncology. 2006;32:922–927. doi: 10.1016/j.ejso.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized study comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 9.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98(4):697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 11.President's Cancer Panel, US Department of Health and Human Services, National Institutes of Health, National Cancer Institute [Accessed December 3, 2014];Living beyond cancer: finding a new balance. Available at: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp03-04rpt/Survivorship.pdf.
- 12.Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results in Cancer Research. 1998;152:396–411. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- 13.Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29(9):1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amichetti M, Caffo O, Arcicasa M, et al. Quality of life in patients with ductal carcinoma in situ of the breast treated with conservative surgery and postoperative irradiation. Breast Cancer Research and Treatment. 1999;54(2):109–115. doi: 10.1023/a:1006125602353. [DOI] [PubMed] [Google Scholar]
- 15.Claus E, Petruzella S, Carter D, Kasl S. Quality of life for women diagnosed with breast carcinoma in situ. J Clin Oncol. 2006;24(30):4875–4881. doi: 10.1200/JCO.2005.05.2290. [DOI] [PubMed] [Google Scholar]
- 16.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16(2):501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 17.Klein D, Mercier M, Abeilard E, et al. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Research and Treatment. 2011;129:125–134. doi: 10.1007/s10549-011-1408-3. [DOI] [PubMed] [Google Scholar]
- 18.Paskett E, Alfano C, Davidson M, et al. Breast cancer survivors' health-related quality of life. Cancer. 2008;113(11):3222–3230. doi: 10.1002/cncr.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paskett ED, Herndon JE, Day JM, et al. Applying a conceptual model for examining health-related quality of life in long-term breast cancer survivors: CALGB study 79804. Psychooncology. 2008;17:1108–1120. doi: 10.1002/pon.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trentham-Dietz A, Sprague B, Klein R, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Research and Treatment. 2008;109(2):379–387. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gestel YR, Voogd AC, Vingerhoets AJ, et al. A comparison of quality of life, disease impact and risk perception in women with invasive breast cancer and ductal carcinoma in situ. Eur J Cancer. 2007;43(3):549–556. doi: 10.1016/j.ejca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575–580. doi: 10.1136/bmj.301.6752.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz PA, Schag CA, Lee JJ, Polinsky ML, Tan SJ. Breast conservation versus mastectomy: is there a difference in psychological adjustment or quality of life in the year after surgery? Cancer. 1992;69:1729–1738. doi: 10.1002/1097-0142(19920401)69:7<1729::aid-cncr2820690714>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Pozo C, Carver CS, Noriega V, et al. Effects of mastectomy versus lumpectomy on emotional adjustment to breast cancer: a prospective study of the first year postsurgery. J Clin Oncol. 1992;10(8):1292–1298. doi: 10.1200/JCO.1992.10.8.1292. [DOI] [PubMed] [Google Scholar]
- 25.Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998;16(2):487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 26.Shimozuma K, Ganz PA, Petersen L, Hirji K. Quality of life in the first year after breast cancer surgery: Rehabilitation needs and patterns of recovery. Breast Cancer Research and Treatment. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- 27.Janni W, Rjosk D, Dimpfl T, et al. Quality of life influenced by primary surgical treatment for stage I-III breast cancer: Long-term follow-up of a matched-pair analysis. Ann Surg Oncol. 2001;8(6):542–548. doi: 10.1007/s10434-001-0542-2. [DOI] [PubMed] [Google Scholar]
- 28.Nissen MJ, Swenson KK, Ritz LJ, Farrell JB, Sladek ML, Lally RM. Quality of life after breast carcinoma surgery: a comparison of three surgical procedures. Cancer. 2001;91(7):1238–1246. [PubMed] [Google Scholar]
- 29.Nano MT, Grantly G, Kollias J, Bochner MA, Malycha P, Winefield HR. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ Journal of Surgery. 2005;75:940–947. doi: 10.1111/j.1445-2197.2005.03517.x. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy F, Harcourt D, Rumsey N, White P. The psychosocial impact of ductal carcinoma in situ (DCIS): a longitudinal prospective study. Breast. 2010;19(5):382–387. doi: 10.1016/j.breast.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 31.He Z-Y, Tong Q, Wu S-G, Li F-Y, Lin H-X, Guan X-X. A comparison of quality of life and satisfaction of women with early-stage breast cancer treated with breast conserving therapy vs. mastectomy in southern China. Supportive Care in Cancer. 2012;20(10):2441–2449. doi: 10.1007/s00520-011-1364-9. [DOI] [PubMed] [Google Scholar]
- 32.Jeffe DB, Pérez M, Liu Y, Collins KK, Aft RL, Schootman M. Quality of life changes over time in women diagnosed with ductal carcinoma in situ, early-stage invasive breast cancer, and age-matched controls. Breast Cancer Research and Treatment. 2012;134:379–391. doi: 10.1007/s10549-012-2048-y. PMCID: PMC3448489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauzier S, Maunsell E, Levesque P, et al. Psychological distress and physical health in the year after diagnosis of DCIS or invasive breast cancer. Breast Cancer Research and Treatment. 2010;120:685–691. doi: 10.1007/s10549-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 34.Ganz PA. Late effects of cancer and its treatment. Seminars in Oncology Nursing. 2001;17:241–248. doi: 10.1053/sonu.2001.27914. [DOI] [PubMed] [Google Scholar]
- 35.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Research and Treatment. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 36.Ganz PA, Land SR, Geyer CE, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29(9):1110–1116. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration test of cognitive impairment. American Journal of Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 38.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality of life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 39.Sherbourne CD, Stewart AL. The MOS social support survey. Social Science & Medicine. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 40.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 42.Pérez M, Liu Y, Schootman M, et al. Changes in sexual problems over time in women with and without early-stage breast cancer. Menopause. 2010;17(5):924–937. doi: 10.1097/gme.0b013e3181d5dd26. PMCID: PMC 2939280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins K, Liu Y, Schootman M, et al. Effects of breast cancer surgery and surgical side effects on body image over time. Breast Cancer Research and Treatment. 2011;126(1):167–176. doi: 10.1007/s10549-010-1077-7. PMCID: PMC3265936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260–2266. [PubMed] [Google Scholar]
- 45.Garsa AA, Ferraro DJ, DeWees TA, et al. A Prospective Longitudinal Clinical Trial Evaluating Quality of Life After Breast-Conserving Surgery and High-Dose-Rate Interstitial Brachytherapy for Early-Stage Breast Cancer. International Journal of Radiation Oncology Biology Physics. 2013;87(5):1043–1050. doi: 10.1016/j.ijrobp.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Härtl K, Janni W, Kastner R, et al. Impact of medical and demographic factors on long-term quality of life and body image of breast cancer patients. Ann Oncol. 2003;14(7):1064–1071. doi: 10.1093/annonc/mdg289. [DOI] [PubMed] [Google Scholar]
- 47.Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. The breast journal. 2004;10(3):223–231. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- 48.Arndt V, Stegmaier C, Ziegler H, Brenner H. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus mastectomy: a population-based study. Journal of Cancer Research and Clinical Oncology. 2008;134(12):1311–1318. doi: 10.1007/s00432-008-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han J, Grothuesmann D, Neises M, Hille U, Hillemanns P. Quality of life and satisfaction after breast cancer operation. Archives of Gynecology and Obstetrics. 2010;282(1):75–82. doi: 10.1007/s00404-009-1302-y. [DOI] [PubMed] [Google Scholar]
- 50.Taira N, Shimozuma K, Shiroiwa T, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Research and Treatment. 2011;128:735–747. doi: 10.1007/s10549-011-1631-y. [DOI] [PubMed] [Google Scholar]
- 51.Casso D, Buist DS, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health and Quality of Life Outcomes. 2004;2:25. doi: 10.1186/1477-7525-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 53.Versmessen H, Vinh-Hung V, Van Parijs H, et al. Health-related quality of life in survivors of stage I-II breast cancer: randomized trial of post-operative conventional radiotherapy and hypofractionated tomotherapy. BMC Cancer. 2012;12:495–511. doi: 10.1186/1471-2407-12-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arora NK, Gustafson DH, Hawkins RP, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma: a prospective study. Cancer. 2001;92(5):1288–1298. doi: 10.1002/1097-0142(20010901)92:5<1288::aid-cncr1450>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 55.Browall M, Ahlberg K, Karlsson P, Danielson E, Persson LO, Gaston-Johansson F. Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. European Journal of Oncology Nursing. 2008;12(3):180–189. doi: 10.1016/j.ejon.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Noal S, Levy C, Hardouin A, et al. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2011;81(3):795–803. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 57.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA: The Journal of the American Medical Association. 2006;295(23):2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 58.Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 59.Takei H, Ohsumi S, Shimozuma K, et al. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04) Breast Cancer Research and Treatment. 2012;133(1):227–236. doi: 10.1007/s10549-011-1943-y. [DOI] [PubMed] [Google Scholar]
- 60.van Nes JG, Fontein DB, Hille ET, et al. Quality of life in relation to tamoxifen or exemestane treatment in postmenopausal breast cancer patients: a Tamoxifen Exemestane Adjuvant Multinational (TEAM) Trial side study. Breast Cancer Research and Treatment. 2012;134(1):267–276. doi: 10.1007/s10549-012-2028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. Br Med J. 2005;330:702–705. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. Journal of Clinical Epidemiology. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Gajdos C, Tartter PI, Bleiweiss IJ. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Annals of Surgery. 1999;230(5):692–696. doi: 10.1097/00000658-199911000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Temple LK, Baron R, Cody HS, III, et al. Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 women. Ann Surg Oncol. 2002;9(7):654–662. doi: 10.1007/BF02574481. [DOI] [PubMed] [Google Scholar]
- 65.Kootstra JJ, Hoekstra-Weebers JEHM, Rietman JS, et al. A Longitudinal Comparison of Arm Morbidity in Stage I-II Breast Cancer Patients Treated with Sentinel Lymph Node Biopsy, Sentinel Lymph Node Biopsy Followed by Completion Lymph Node Dissection, or Axillary Lymph Node Dissection. Ann Surg Oncol. 2010;17(9):2384–2394. doi: 10.1245/s10434-010-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langer I, Guller U, Berclaz G, et al. Morbidity of Sentinel Lymph Node Biopsy (SLN) Alone Versus SLN and Completion Axillary Lymph Node Dissection After Breast Cancer Surgery: A Prospective Swiss Multicenter Study on 659 Patients. Annals of Surgery. 2007;245(3):452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]