Abstract
Purpose
Urologic chronic pelvic pain syndromes (UCPPS) have refractory bladder or pelvic pain as their dominant symptom, which has been attributed to changes in the central nervous system caused by a chronic barrage of noxious stimuli. We developed a novel challenge protocol that induces bladder distention in study participants to reproduce pain and urinary symptoms, and tested to see whether it could discriminate between persons with UCPPS-like symptoms and asymptomatic controls.
Methods
We recruited 10 female twin pairs who were discordant for UCPPS-like symptoms. Before scanning, each twin urinated to completion, then consumed 500 cc of water. Each twin was scanned with our resting state functional magnetic resonance imaging (rsfMRI) protocol immediately, and approximately 50 minutes after consumption. Time series were extracted from the right and left periaqueductal gray (PAG) and right and left amygdala subregions. We conducted a repeated-measures, two-sample t-test to assess differences in connectivity between symptomatic and asymptomatic twins before and after bladder distention.
Results
Group-by-condition interaction effects were found from the PAG to the right cerebellum VIIIa, amygdala, right premotor cortex/supplementary motor area, and insular cortex; and between the amygdala and the frontal pole/medial orbital frontal cortex, hypothalamus, insular cortex, thalamus, and anterior cingulate cortex.
Conclusions
These findings demonstrate that our non-invasive bladder distention protocol can detect differences in the processing of urinary sensation between twins discordant for lower urinary tract pain.
Keywords: resting state fMRI, urologic chronic pelvic pain syndrome, interstitial cystitis, twins
Introduction
Urologic chronic pelvic pain syndromes (UCPPS), also known as interstitial cystitis/bladder pain syndrome and chronic prostatitis/male chronic pelvic pain syndrome, have bladder or pelvic pain as their dominant symptom 1. Pain is typically compounded by bladder distention and is associated with lower urinary tract (LUT) symptoms such as frequency and urgency. While some patients experience mild symptoms of relatively short duration that respond to current treatments, a significant proportion have refractory symptomatology2.
A key finding in chronic pain research, including studies of UCPPS, is the identification of central nervous system (CNS) changes that result in altered pain perception 3-6. Such alteration is believed to reflect central sensitization that originates in the hyperexcitability of dorsal horn neurons in the spinal cord 7. CNS alterations can amplify and spread pain through increased central neural connections, triggering sensory neurons to release substances that cause peripheral hyperexcitability in adjoining sensory nerves. However, the mechanisms by which CNS processes relate to symptom chronicity or localization in UCPPS remain uncertain. Because the defining criterion for UCPPS is chronic pain, the concept of CNS alteration, including changes in processing sensory information, is critical to understanding these conditions.
Growing recognition of the importance of the CNS in pain maintenance has led to an explosion of structural and functional neuroimaging investigations of chronic pain 8. Preliminary studies indicate that CNS changes are present in persons with UCPPS, as measured by fMRI9-11. Neuroimaging studies in other non-urologic chronic pain syndromes have also returned evidence for CNS changes12-14. What is unique to UCPPS is that pain can be reliably provoked or exacerbated with bladder distention. If the bladder could be distended in an experimental situation, this would provide an opportunity to examine brain changes in patients not only at rest, but with provocation of urinary and pelvic pain symptoms.
During the first phase of the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network (www.mappnetwork.org), we developed a novel, non-invasive challenge protocol to reproduce urinary and bladder pain symptoms through provoked diuresis. Our intention was to develop a testing paradigm to compare CNS changes before and after the provocation of urinary tract symptoms. This proof of principle study was conducted to demonstrate the feasibility of this protocol, both to elicit symptoms as well as to identify brain activity differences between women with and without UCPPS-like symptoms.
Materials and Methods
Overview
We measured changes in brain activity by performing resting-state fMRI (rsfMRI) of participants with an empty bladder, and after they consumed 500 cc of water. In this exploratory study, we used a region of interest (ROI) approach to investigate potential CNS mediators of UCPPS by focusing on brain areas associated with bladder and LUT function, pain modulation, and emotional regulation. Control of the bladder and LUT depends on proper functioning of brain areas that regulate the micturition reflex15-16. Brain areas common to both the micturition reflex and pain modulation include the nucleus raphe magnum, nucleus locus coeruleus alpha, and periaqueductal gray (PAG)17. We selected the PAG as one ROI, since it is involved in both micturition and pain modulation. We also sought to identify changes in functional connectivity from the amygdala, a component of the limbic system that may be involved in suppressing unpleasant sensations provoked by bladder filling, such as urgency16. By “connectivity” we refer to a covariance measurement of synchronous brain activity in distinct brain areas, as detected by rsfMRI.
Participant Selection
The Institutional Review Board at our medical center approved this study protocol. From the University of Washington Twin Registry18, we recruited 10 female twin pairs (5 monozygotic, 5 dizygotic; N=20) who were discordant for UCPPS-like symptoms on a screening questionnaire. This community-based recruitment of twins occurred within a larger study of hereditary and environmental factors in UCPPS. To be identified as being affected by UCPPS-like symptoms, participants were required to give a positive response to the following question: “Do you experience unpleasant sensations of pain, pressure, or discomfort that you believe to be related to the bladder and/or pelvic region, associated with lower urinary tract symptoms?” The twins with UCPPS-like symptoms did not have a physician-based diagnosis of UCPPS, and thus we use the term “UCPPS-like symptoms”. Twin pairs were scanned consecutively on the same day in a randomly assigned order. The average age of participants was 36.8 years (range 24-66).
rsfMRI Behavioral Task
Participants underwent rsfMRI at the beginning and end of a protocol that was designed to provoke bladder symptoms. To start, each participant urinated to completion, then consumed 500 cc of water, and immediately entered the scanner. The first rsfMRI scan (identified as “pre”) began about 15 minutes after consuming the water. Each participant was instructed to relax and lie still with her eyes closed. After about 30 minutes of structural scans, rsfMRI imaging was repeated about 50 minutes after consuming the water, while bladder distention was in progress (identified as “post”). Pain and urgency ratings were verbally reported at five time points throughout the protocol through a two-way communication system in the magnet, using Likert scales for urgency (range 1-7) and pain (range 0-10). The first time point was immediately after drinking water and before entering the scanner, the second time point was immediately before the first rsfMRI (pre) scan, and the fifth/last time point was immediately before the second rsfMRI (post) scan.
rsfMRI Data Acquisition
MRIs and rsfMRIs were collected on a 3T Phillips Achieva MR system (Philips Medical Systems, Best, Netherlands) using a 32-channel SENSE head coil. A T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) structural image was collected (TR = 7.7 ms; TE = 3.7 ms; flip angle = 9; FOV = 220 mm; matrix 200×200; 180 slices; acquisition voxel size (mm) = 1.00/1.00/1.00; reconstruction voxel size (mm) 0.86/0.86/1.00; TFE shots = 144; TFE durations = 1633.0; inversion delay (TI) 823.8 ms; slice orientation axial, fold-over direction RL). Two identical rsfMRI series of whole-brain T2*-weighted images were acquired by using a single-shot gradient-recalled echo planar imaging sequence (TR = 2000 ms; TE = 28 ms; flip angle = 76°; FOV = 220 mm) with a matrix size of 64 × 64 (in-plane resolution = 3.4375×3.4375 mm). Thirty-eight axial slices (slice thickness = 3.5 mm, 0 mm gap) were acquired sequentially during each image volume. Five dummy scans followed by 300 volumes were collected while the participant rested (approximately 10 minutes).
rsfMRI Processing and Statistical Analysis
Preprocessing of rsfMRI data was performed by using the Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library (FSL) version 4.1.7 (http://www.fmrib.ox.ac.uk/fsl/), and Analysis of Functional NeuroImages (http://afni.nimh.nih.gov/afni/). Our preprocessing pipeline consisted of artifact de-noising with Multivariate Exploratory Linear Optimized Decomposition into Independent Components19 and motion correction20, along with brain extraction21. We used 3dDespike to remove spike artifacts, and we performed physiological correction by filtering signal from the cerebrospinal fluid, filtering motion parameters, and when applicable, using single-point motion regressors22. We performed temporal smoothing with a high-pass filter of sigma = 100 s and spatial smoothing with full width half maximum = 5 mm.
Head motion during the rsfMRI scans was quantified and compared between groups using FSL's motion correction software. No significant between-group differences were found.
Seed Point Times Series Extraction
Time series were extracted from the PAG and the amygdala. PAG ROIs were hand-drawn on the Montreal Neurological Institute 152 (MNI152) standard template brain by one of the investigators (KRM). Amygdala ROIs were defined by using probabilistic maps of cytoarchitectonic boundaries23. ROI masks were transformed from MNI space into native space, then timeseries were extracted from the right and left laterobasal (LB), centromedial (CM), and superficial (SF) amygdala subregions24. Individual fMRI data were then registered to the MPRAGE and warped to the MNI152 standard image with an affine transformation.
Data Analysis
Time series statistical analyses were conducted by using FMRIB's Improved Linear Model with local autocorrelation correction25. Four, first level analyses were run on each resting state scan: the right and left PAG and the right and left amygdala. For the right and left amygdala, all three side-specific subregional time series were included simultaneously in the first-level model (CM, LB, SF). However, condition effects for the amygdala analyses were based on LB subregion (controlling for the CM and SF timeseries), because of the LB's strong association with emotional perception and sensory processing26.
A whole-brain analysis of group-wise effects was conducted by using FMRI's Local Analysis of Mixed Effects (FLAME), a method for modeling and estimating the random-effects component of the inter-session mixed-effects variance. Bladder distention was the experimental condition, and level of functional connectivity was the outcome. The latter was derived through four time series statistical analyses (right PAG, left PAG, right LB amygdala, and left LB amygdala) using FILM with local autocorrelation correction25. To test for differences in connectivity between cases and controls before and after bladder distention, we conducted a repeated-measures, two-sample t-test. All analyses used an ROI approach to correct for multiple comparisons. Our a priori ROIs included brain regions with afferent and efferent connections to the PAG27 and brain regions involved in micturition28 and in pain and sensory processing29-31. Statistical corrections for multiple comparisons were conducted by using cluster-thresholding based on Markov Chain Monte Carlo sampling set at z > 2.3 (voxel height) and p < .05 (cluster extent), ROI volume corrected.
Results
Pain and Urgency Measurements
Between-group differences in self-reported pain and urgency levels were tested by using paired-samples t-tests. The symptomatic cohort reported significantly more pain than the controls at time points 2-5 (Table 1, Supplementary Figure 1), confirming that the task resulted in provocation of pain. In contrast, the same cohort reported significantly more urgency than the controls only at the second time point (immediately before the first rsfMRI). These differences between pain and urgency reflect the fact that the control cohort reported almost no pain, yet they experienced levels of urgency similar to those of the case cohort as the scanning session progressed.
Table 1.
Paired-samples t-tests between cases and controls for pain and urgency ratings
| Paired Differences | p-values (2-tailed) | |||
|---|---|---|---|---|
| Mean* | Std. Deviation | Std. Error Mean | ||
| Pain 1 | 0.4 | 0.97 | 0.31 | 0.223 |
| Pain 2 | 0.5 | 0.85 | 0.27 | 0.096 |
| Pain 3 | 1.9 | 1.45 | 0.46 | 0.002 |
| Pain 4 | 2.44 | 1.94 | 0.65 | 0.005 |
| Pain 5 | 3 | 2.65 | 1 | 0.024 |
| Urgency 1 | 0.1 | 0.32 | 0.1 | 0.343 |
| Urgency 2 | 0.4 | 0.52 | 0.16 | 0.037 |
| Urgency 3 | 0.9 | 2.03 | 0.64 | 0.193 |
| Urgency 4 | 0.56 | 1.59 | 0.53 | 0.325 |
| Urgency 5 | 0.43 | 1.4 | 0.53 | 0.448 |
A positive mean difference score corresponds to a higher rating by cases than by controls.
rsfMRI Results
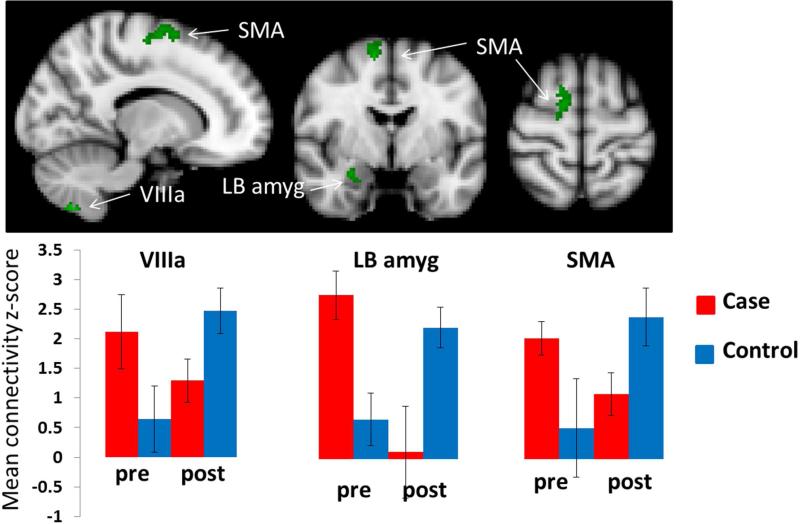
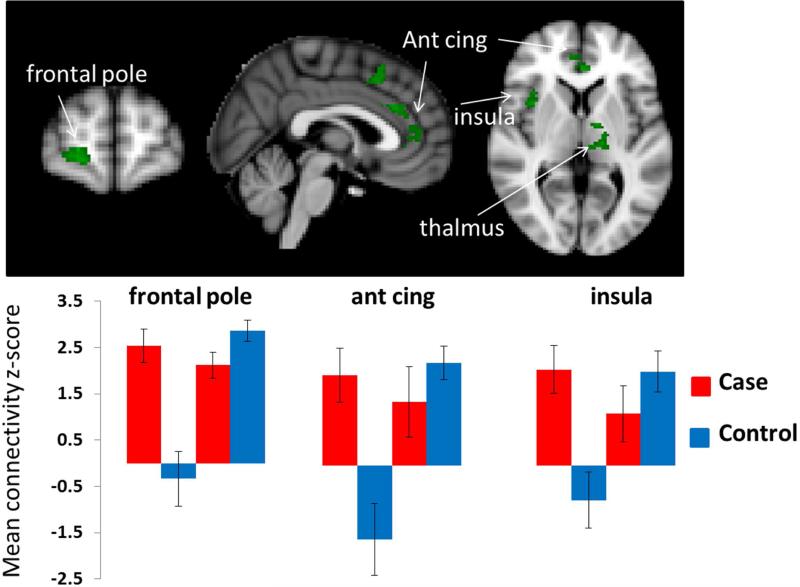
The difference in connectivity between scans performed before and after bladder distention depended significantly on group in the following regions: between the right PAG and the right cerebellum VIIIa, right amygdala, and right supplementary motor area (SMA)(Figure 1 and Table 2), and between and the left PAG and the left and right LB amygdala and right insular cortex. In addition, we found significant interaction effects between the right LB amygdala seed region and the right frontal pole/medial orbital frontal cortex, anterior cingulate cortex, right insular cortex, left thalamus, and hypothalamus (Figure 2 and Table 2). (See also Supplementary Tables 1 and 2, Supplementary Figure 2.)
Figure 1.
Significant group-by-condition interaction effects from the right PAG seed region. Significant clusters are shown in green. The bar graphs in the lower panel show the basis of the interaction effect in the cerebellar, amygdala, and supplementary motor area (SMA) clusters. Bar graphs were created by extracting the average z-score and the standard deviation of the voxels within the significant interaction cluster before and after bladder distention for each cohort separately.
Table 2.
Brain regions showing significant interaction effect. The effect on brain connectivity was dependent on clinical status (control vs. case). Results in italics indicate trend effects of p < .1 and >.05.
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Seed Region | Brain region | vox | p value | z-max | z-max x (mm) | z-max y (mm) | z-max z (mm) |
| Right PAG | R Cerebellum VIIIac | 54 | .0479 | 3.66 | 14 | −64 | −56 |
| R Cerebellum VIIIbc | 52 | .0531 | 3.08 | −18 | −48 | −60 | |
| R laterobasal amygdalaa | 28 | .0383 | 3.1 | 28 | −4 | −18 | |
| R SMA | 157 | .0284 | 3.72 | 12 | −4 | 62 | |
| Left PAG | L laterobasal amygdalaa | 21 | .0454 | 2.87 | −22 | −4 | −18 |
| R laterobasal amygdalaa | 24 | .0439 | 3.01 | 22 | −4 | −22 | |
| R insulab/OFCd | 95 | .0361 | 3.73 | 30 | 20 | −16 | |
| Right Amygdala | R medial OFCb/frontal poled | 153 | .0274 | 3.46 | 20 | 62 | 0 |
| hypothalamus | 2 | .0094 | 2.53 | −8 | −4 | −4 | |
| R insulad | 166 | .0087 | 3.26 | 40 | 8 | −12 | |
| L thalamusd (ventral lateral nucleusb) | 164 | .0107 | 3.19 | −12 | −6 | 4 | |
| R SMA | 106 | .0692 | 3.39 | 4 | 18 | 46 | |
| L anterior cingulateb,d | 156 | .0160 | 3.31 | −2 | 36 | 2 | |
| R anterior cingulateb,d | 132 | .0254 | 3.21 | 4 | 34 | 28 | |
Brain regions labled according the (a)Juelich Histological Atlas, (b)Talaraich Atlas, the (c)Cerebellar Atlas in MNI space after normalization in FLIRT, and (d)Harvard-Oxford Cortical Structural Atlas.
R = right, L = left. PAG = periaqueductal gray, SMA = supplemental motor area, OFC = orbital frontal cortex.
Figure 2.
Significant group-by-condition interaction effects from the right LB seed region. Significant clusters are shown in green. The bar graph in the lower panel shows the basis of the interaction effect in the frontal pole/medial orbital frontal cortex, anterior cingulate, and right anterior insular clusters. Bar graphs were created by extracting the average z-score and the standard deviation of the voxels within the significant interaction cluster before and after bladder distention for each cohort separately.
Discussion
Our study used an innovative neural systems approach to investigate whether women with UCPPS-like symptoms showed differences in functional connectivity associated with the natural provocation of bladder symptoms. Reliable replication of symptoms across participants with simultaneous neuroimaging is an extremely powerful method to investigate CNS mechanisms that might perpetuate UCPPS symptoms. No previous studies of UCPPS have attempted to provoke symptoms and measure brain activity before and after symptoms manifest. A major strength of our method of provocation is its simplicity, as it does not involve bladder catheterization32. Catheterization complicates study logistics and its effects on a hypersensitive urinary tract can lead to confounding symptoms, such as artificially-induced bladder sensations and detrusor contractions, which can be reflected in the imaging.
In our study, participants drank 500 cc of water immediately before rsfMRI scanning, with the first scan occurring about 15 minutes after consumption and the second about 50 minutes after. As expected, this approach revealed the significant modulation of neural circuitry associated with naturally provoked bladder symptoms in our control cohort. These results indicate that our protocol is a robust, non-invasive way to investigate the neural correlates of micturition. Notably, connectivity in the control cohort increased between the right PAG and the cerebellar and cortical regions involved in sensorimotor planning, as well as from the LB amygdala to brain regions including the anterior cingulate, insula, somatosensory cortex, premotor cortex, thalamus, and medial prefrontal cortex.
Although our sample was small, we found preliminary evidence that female twins with UCPPS-like symptoms show abnormal patterns of connectivity associated with bladder distention, compared to their healthy twin. We found significant interaction effects in connectivity with the premotor cortex, insula, thalamus, anterior cingulate, and medial prefrontal cortex. Connectivity from both the PAG and the LB amygdala was significantly higher in symptomatic twins than in controls before bladder distention. As illustrated in Figures 1 and 2, the symptomatic twin cohort showed no significant change in connectivity associated with bladder filling. Instead, connectivity in these twins was elevated following water consumption and remained at approximately the same level during the post-bladder distention scan. In contrast, connectivity in the control twins showed a marked increased after bladder distention.
This pattern of results preliminarily indicates that CNS changes are present in symptomatic twins, but not in their asymptomatic co-twins, and that these changes are characterized by chronic, enhanced connectivity that is not significantly changed by bladder distention. This finding comports with other studies of autonomic nervous system changes in UCPPS, suggesting that UCCPS patients at rest have a relatively high sympathetic nervous system discharge, so that the usual autonomic changes resulting from provocation are no longer evident33-34. These alterations in the autonomic nervous system (measured as changes in heart rate variability) in UCPPS patients have also been shown to be associated with changes in connectivity35.
The only exception to this pattern was connectivity between the PAG and the left LB amygdala, which was elevated at baseline in symptomatic twins but reduced by the time of the post-distention scan. It is possible that this pattern of changes in PAG/left amygdala connectivity is associated with anticipatory processing of an aversive event 36.
Strengths and Weaknesses
Limitations of this pilot study include its small sample size, which necessitates validation of our protocol by future investigations. However, an advantage of twin studies is that by controlling for genetic variability and common familial exposures and experiences, differences between symptomatic and control twin cohorts can been seen even with small sample sizes. We note that the degree of bladder distention occurring in each person over time is variable. However, neural pathways should be activated even with disparate degrees of sensory experience, so this variation is unlikely to markedly affect connectivity. To address physiological variability in distention and pain would require catheterizing participants and instilling fluid until they reported a predetermined level of urgency or pain (e.g., 8 on a 10-point scale). This method poses its own problems, such as instilling differing amounts of fluid and introducing catheter-induced bladder irritation. Because such an approach would substantially confound imaging results, we believe that our protocol is preferable. Future studies might include non-invasive methods to measure bladder volume at different time points.
Conclusions
This study demonstrates the feasibility of an imaging protocol that uses natural diuresis to provoke bladder symptoms. The same protocol can be used to demonstrate brain connectivity linked to urinary urgency, pelvic pain, and altered neurophysiology in UCPPS. By demonstrating CNS alterations in these chronic pain conditions, findings from such investigations can potentially inform novel approaches to managing UCPPS.
Supplementary Material
Acknowledgements
We wish to thank the twins for taking part in the University of Washington Twin Registry and for their time and enthusiasm for this project. We also acknowledge Raymond Harris, PhD, for writing assistance and Paul Robinson, for his assistance with data preprocessing and the creation of the figures.
Funding Source: NIH U01 DK082325-05
Key to Abbreviations
- UCPPS
urologic chronic pelvic pain syndromes
- rsfMRI
resting state functional magnetic resonance imaging
- PAG
periaqueductal gray
- LUT
lower urinary tract
- CNS
central nervous system
- ROI
region of interest
- LB
laterobasal
- SMA
supplementary motor area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kartha GK, Kerr H, Shoskes DA. Clinical phenotyping of urologic pain patients. Current opinion in urology. 2013;23:560. doi: 10.1097/MOU.0b013e3283652a9d. [DOI] [PubMed] [Google Scholar]
- 2.Clemens JQ, Clauw DJ, Kreder K, et al. Comparison of Baseline Urological Symptoms in Men and Women in the MAPP Research Cohort. J Urol. 2015;193:1554. doi: 10.1016/j.juro.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 4.Yang CC, Lee JC, Kromm BG, et al. Pain sensitization in male chronic pelvic pain syndrome: why are symptoms so difficult to treat? J Urol. 2003;170:823. doi: 10.1097/01.ju.0000082710.47402.03. [DOI] [PubMed] [Google Scholar]
- 5.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 6.Lai HH, Gardner V, Ness TJ, et al. Segmental Hyperalgesia to Mechanical Stimulus in Interstitial Cystitis/Bladder Pain Syndrome: Evidence of Central Sensitization. J Urol. 2013 doi: 10.1016/j.juro.2013.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DA, Gracely RH. Biology and therapy of fibromyalgia. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res Ther. 2006;8:224. doi: 10.1186/ar2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MC, Tracey I. Imaging pain: a potent means for investigating pain mechanisms in patients. Br J Anaesth. 2013;111:64. doi: 10.1093/bja/aet174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192:947. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmer MA, Chanda ML, Parks EL, et al. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer MA, Huang L, Martucci K, et al. Brain white matter abnormalities in female interstitial cystits/bladder pain syndrome: a MAPP Network neuroimaging study. J Urol. 2015;194:118. doi: 10.1016/j.juro.2015.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen KB, Srinivasan P, Spaeth R, et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. 2013;65:3293. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puri BK, Jakeman PM, Agour M, et al. Regional grey and white matter volumetric changes in myalgic encephalomyelitis (chronic fatigue syndrome): a voxel-based morphometry 3 T MRI study. Br J Radiol. 2012;85:e270. doi: 10.1259/bjr/93889091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krhut J, Tintera J, Holy P, et al. A preliminary report on the use of functional magnetic resonance imaging with simultaneous urodynamics to record brain activity during micturition. J Urol. 2012;188:474. doi: 10.1016/j.juro.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 17.Mennini T, Testa R. Are descending control pathways of the lower urinary tract and pain overlapping systems? Cent Nerv Syst Agents Med Chem. 2010;10:113. doi: 10.2174/187152410791196387. [DOI] [PubMed] [Google Scholar]
- 18.Afari N, Noonan C, Goldberg J, et al. University of Washington Twin Registry: construction and characteristics of a community-based twin registry. Twin Res Hum Genet. 2006;9:1023. doi: 10.1375/183242706779462543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson M, Bannister PR, Brady JM, et al. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemieux L, Salek-Haddadi A, Lund TE, et al. Modelling large motion events in fMRI studies of patients with epilepsy. Magn Reson Imaging. 2007;25:894. doi: 10.1016/j.mri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 24.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolrich MW, Ripley BD, Brady M, et al. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 26.Sah P, Faber ES, Lopez De Armentia M, et al. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 27.Linnman C, Moulton EA, Barmettler G, et al. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage. 2012;60:505. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature reviews. Neuroscience. 2008;9:453. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in Cognitive Sciences. 2008;12:306. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Price DD. Psychological and Neural Mechanisms of the Affective Dimension of Pain. Science. 2000;288:1769. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Pu Y, Gao J-H, et al. The human red nucleus and lateral cerebellum in supporting roles for sensory information processing. Hum Brain Mapp. 2000;10:147. doi: 10.1002/1097-0193(200008)10:4<147::AID-HBM10>3.0.CO;2-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tadic SD, Tannenbaum C, Resnick NM, et al. Brain responses to bladder filling in older women without urgency incontinence. Neurourol Urodyn. 2013;32:435. doi: 10.1002/nau.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yilmaz U, Liu YW, Berger RE, et al. Autonomic nervous system changes in men with chronic pelvic pain syndrome. J Urol. 2007;177:2170. doi: 10.1016/j.juro.2007.01.144. [DOI] [PubMed] [Google Scholar]
- 34.Williams DP, Chelimsky G, McCable NP, et al. Effects of chronic pelvic pain on heart rate variability in women. J Urol. 2015 May 9;:S0022–5347(15)03909-9. doi: 10.1016/j.juro.2015.04.101. doi: 10.1016/j.juro.2015.04.101. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thayer JF, Ahs F, Fredrikson M, et al. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Mackiewicz KL, Sarinopoulos I, Cleven KL, et al. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci U S A. 2006;103:14200. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.