Abstract
Object
To investigate white matter structural abnormalities using diffusion tensor imaging (DTI) in children with hydrocephalus before CSF diversionary surgery (including ventriculoperitoneal shunting and endoscopic third ventriculoscopy) and the course of recovery post-surgery in association with neuropsychological and behavioral outcome.
Methods
This was a prospective study that included 54 children with congenital hydrocephalus (21F/33M; age range: 0.03–194.5 months) who underwent surgery and 64 normal controls (30F/34M, age range: 0.30–197.75 months). DTI and neurodevelopmental outcome data were collected once in the control group and at pre-surgery, 3-month, and 12-month post-surgery in the patients. DTI measures, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) values were extracted from the genu of corpus callosum (gCC) and the posterior limb of internal capsule (PLIC). Group analysis was performed first cross-sectionally to quantify DTI abnormalities at three time points by comparing the controls and the patients group at the three time points separately. Longitudinal comparisons were conducted pairwise between different time points in patients whose data were acquired at multiple time points. Neurodevelopmental data were collected and analyzed using the Adaptive Behavior Assessment System, Second Edition (ABAS-II) and the Bayley Scales of Infant Development, Third Edition (Bayley-III). Correlation analyses were performed between DTI and behavioral outcomes.
Results
Significant DTI abnormalities were found in both the gCC (lower FA and higher MD, AD, and RD) and the PLIC (higher FA, lower AD and RD) at pre-surgery. The DTI measures in the gCC remained mostly abnormal at 3-month and 12-month post-surgery. The DTI abnormalities in the PLIC were significant in FA and AD at 3-month post-surgery but did nor persist when tested at 12-month post-surgery. Significant longitudinal DTI changes in the patients were found in the gCC between 3-month and 12-month post-surgery. In the PLIC, trend level longitudinal changes were found between pre-surgery and 3-month post-surgery as well as between 3-month and 12-month post-surgery. Significant correlation between DTI and developmental outcome were found at all three time points. Notably, significant correlation was found between DTI in the PLIC at 3-month post-surgery and developmental outcome at 12-month pots-surgery.
Conclusion
Our data showed significant WM abnormality based on DTI in both the gCC and the PLIC in children with congenital hydrocephalus before surgery and the abnormalities persisted in both the gCC and the PLIC at 3-month post-surgery. The DTI values remained significantly abnormal in the gCC at 12-month post-surgery. Longitudinal analysis showed signs of recovery in both WM structures between different time points. Combined with the significant correlation found between DTI and neuropsychological outcome, our study suggests that DTI can serve as a sensitive imaging biomarker for underlying neuroanatomical changes and post-surgical developmental outcome and even as a predictor for future outcomes.
Keywords: hydrocephalus, DTI, white matter injury, post-surgery, outcome
Introduction
For children with hydrocephalus, CSF diversionary surgery (including ventriculoperitoneal shunting and endoscopic third ventriculoscopy) is the standard of care that can relieve clinical signs and symptoms of enlarged ventricles and increased intracranial pressure (ICP). Surgery has significantly reduced morbidity and mortality.25 However, despite advances in technology, hydrocephalus remains an incurable lifelong disorder. The rapid post-op reversal of acute clinical symptoms does not correspond to a complete reversal of structural damage in the brain. Accumulating evidence has shown that there is no significant correlation between ventricle volume and neurocognitive outcome.11,30 The neuroanatomical change sustained prior to the surgery may remain or continue to progress, leading to long term behavioral and neuropsychological deficits.32 Data from both human and animal research have suggested that extensive areas of white matter fibers connecting various functionally important cortical and subcortical regions are vulnerable targets of injury as the result of ventricular enlargement and increased ICP in hydrocephalus.13,14,17,20,29,33,35
DTI is an advanced MR imaging technique that uses diffusion properties of water molecules as probes to examine tissue structure, revealing characteristics of its microscopic organization. Anisotropic diffusion properties, as measured by DTI, are strongly influenced by the micro-structural components of WM9 and provide a direct indication of the integrity of these components. Recent neuroimaging studies have shown that DTI can provide imaging biomarkers for hydrocephalus outcome.43 Cross-sectional studies have shown that DTI parameters can differentiate brain structures with hydrocephalus from normal controls in both human and animal studies.16,22,27,30,34,38,40–42,44 Initial longitudinal studies have shown that DTI parameters had variable degrees of tendency to return to a normal range after surgery.2,6,26,36 However, there is also strong evidence from both clinical and experimental studies suggesting that the damage to brain structures suffered before CSF diversion may be irreversible.5,12,13,15,19,29,31,32
Here we report the results of a prospective, longitudinal neuroimaging study combined with neuropsychological outcome assessment in pediatric patients with hydrocephalus. Data were acquired prior to CSF diversionary surgery, and again at 3-month and 12-month post-surgery. The overall goal of this study was to establish the WM anisotropic diffusion property derived from DTI as a marker for in vivo white matter damage and its course of recovery in children with hydrocephalus. This data allows us to have a better understanding of the variability in the outcomes and provide a non-invasive and quantifiable means to predict these outcomes. The following hypotheses were tested and confirmed: (1) Children with congenital hydrocephalus have abnormal anisotropic diffusion properties in WM prior to surgery; (2) The abnormal diffusion property in WM will recover with trends toward normal range after surgery; and (3) The WM anisotropic diffusion properties correlate significantly with outcome measures. The findings in this study provide a necessary step toward establishing DTI as a significant clinical tool in the management of hydrocephalic patients.
Methods and Material
Study Design and Participants
All participants were enrolled in a federally funded multi-center, prospective, longitudinal imaging study of pediatric hydrocephalus aimed at investigating neuroimaging and neurobehavioral outcomes at baseline prior to surgical management and at 3-month and 12-month post-surgery follow-up. Between Dec. 2009 and June 2014, a total of 146 children, including 74 children who were potential surgery candidates for hydrocephalus (age range 0.03–180.8 months) and 72 normal control children (age range 0.56–197.75 months) were recruited from the two participating hospitals, Cincinnati Children’s Hospital Medical Center (CCHMC) and St. Louis Children’s Hospital and Washington University (SLCH/WashU). Participants’ legal guardians gave written consent when enrolled into the study. Children older than 11 yrs also provided oral assent. The study was conducted according to IRB guidelines at both institutions.
For children in the hydrocephalus group, the inclusion criteria were as follows: age 0–18 yrs; referral to radiology for brain MRI for evaluation of hydrocephalus and later referral for CSF diversion surgery; hydrocephalus with ventriculomegaly; and no evidence of other medical diagnoses that would be a predisposition to him/her for adverse neurological outcomes (e.g., preterm birth with severe intraventricular hemorrhage, Dandy-Walker Syndrome, stroke, or spina bifida). Among the 74 patients with hydrocephalus, 20 were excluded from the study. Among these 20 patients, 11 were excluded because they did not receive surgical treatment for hydrocephalus, and 7 were excluded for failure to meet inclusion criteria. One family withdrew their consent to participate in the study. One additional child was excluded after being diagnosed with neurofibromatosis type 1. This resulted in a study population of 54 children (21F/33M; age range: 0.03–194.5 month) with congenital hydrocephalus for the final data analysis. Detailed demographic information and etiology are included in Table S1.
The children in the control group were recruited to match the hydrocephalus group in age and gender as closely as possible. They came from two sources: (1). Pediatric patients who were referred for clinical MRI which was evaluated as normal by clinical neuroradiologists. The inclusion criteria for the children in the control group from this source were: age 0–18 yrs; referral to radiology for non-specific symptoms not clearly related to a neurological disorder (e.g. headaches); diagnosed as having normal MRI; no clinical or radiographic history of neurological or psychological disorders prior to the scan; no evidence of any neurological disorders (e.g., epilepsy, stroke) or suggestion of white matter related brain pathology within three month follow up after the initial MRI, as reviewed with the patient’s medical record. (2). Healthy normal children recruited from the general healthy population. The inclusion criterion for children in the control group from this latter source was similar to those in the former “patient normal” subgroup. The MRI images from these healthy normal controls were acquired under research protocol on the same MRI scanners without sedation. Among the 72 normal participants initially enrolled, 1 child was excluded after the diagnosis of epilepsy. Four children were excluded because of the presence of abnormal MRI findings. Two children were disqualified for having standard scores below 70 in either ABAS-II and/or Bayley-III. One additional child was excluded for poor image quality. This left a normal control population 64 children (30F/34M, age range: 0.30 – 197.75 month) for further analysis.
Neuroimaging (MRI/DTI) and neuropsychological outcome data were acquired in children with hydrocephalus prior to the CSF diversionary surgery, 3-months post-surgery, and 12-month post-surgery. Neuroimaging data and neuropsychological data were acquired only once in children in the control group. Not all participants had data at all the time points, most often due to due to the short window between diagnosis and surgery. In addition, depending on the severity of hydrocephalus, some children’s ventricle enlargement was too severe to allow for reliable delineation of region of interest. The total number of children that generated useful neuroimaging and neuropsychological outcome data are listed in Table 1.
Table 1.
Number of datasets generated for different ROIs and outcome measures at different time points
| Control | HCP, pre-op | HCP, 3m post-op | HCP, 12m post-op | ||
|---|---|---|---|---|---|
| Neuroimaging – MRI/DTI | |||||
| gCC | 64 | 22 | 28 | 29 | |
| LPLIC | 64 | 22 | 29 | 30 | |
| Neuropsych – ABAS-II | |||||
| GAC | 53 | 26 | 43 | 28 | |
| Conceptual | 53 | 26 | 43 | 28 | |
| Social | 54 | 27 | 44 | 28 | |
| Practical | 54 | 27 | 41 | 28 | |
| Motor Scale | 48 | 23 | 40 | 23 | |
| Neuropsych – Bayley-III | |||||
| Social | 33 | 7 | 27 | 17 | |
| Cognitive | 40 | 6 | 34 | 19 | |
| Language | 40 | 5 | 34 | 19 | |
| Motor | 39 | 4 | 34 | 19 |
Note: ABAS-II: The Adaptive Behavior Assessment System, Second Edition; Bayley-III: The Bayley Scales of Infant Development, Third Edition; GAC: General Adaptive Composite; HCP: hydrocephalus; gCC: genu of corpus callosum; LPLIC: left posterior limb of internal capsule.
MRI Acquisition and Preprocessing
MRI data were all acquired on 1.5 Tesla scanners at either CCHMC (GE Signa, GE Healthcare, Milwaukee, Wisconsin) or SLCH/WashU (Siemens Avanto, Erlangen, Germany). A 15 direction diffusion-weighted spin-echo DTI sequence with single-shot EPI was used with the following specifications: FOV = 240 mm × 240 mm, matrix = 96 × 96, resolution = 2.5 mm × 2.5 mm × 2.5 mm, number of slices =76, TR/TE = 9400/93.2 msec; ASSET or IPAT factor = 2, number of averages = 2, b=1000 s/mm2. One additional image with no diffusion weighting (b=0) was also acquired. The MR compatibility across the two sites (two 1.5T GE Signa scanners from CCHMC and one 1.5T Siemens Avanto from SLCH) was established using both MR phantom (fBIRN phantom) and a traveling human phantom prior to the start of the subject enrollment. The compatibility was tested annually with the same MR phantom and human phantom on all scanners used in the study. In addition, ACR phantoms were also used for quality assurance within a week of subject scan to assure the stability of scanner performance.34,44,45
Image processing and analysis were all performed using the DTIStudio 3.02 software.28 Head motion and eddy current artifact were corrected using the automatic image registration method39 embedded in the DTIStudio software.28 An automatic bad slice detection method followed by additional visual inspection was performed to identify signal dropout on DWI images. DTI metrics including FA, MD, AD, and RD were calculated using the standard technique.7 Two region of interest (ROI) were examined in this study: genu of corpus callosum (gCC) and posterior limb of internal capsule (PLIC). The two ROIs were delineated based on the approach of Hermoye et al’s work in 200623 as well as in our previous work.2,40,44 It should be noted that, because shunt artifact was seen in post-surgery DTI images from many patients who were treated with adjustable valves (almost always on the right side), a bilateral DTI measurement in PLIC was not always feasible at all three time points. Therefore, DTI data from PLIC were extracted only from left hemisphere in the present study.
Ventricle Size Assessment
Ventricle size was quantified using the frontal and occipital horn ratio (FOHR)46 in the controls and the participants with hydrocephalus at all three time points.
Neuropsychological Outcomes
The Adaptive Behavior Assessment System, Second Edition (ABAS-II)21 and the Bayley Scales of Infant Development, Third Edition (Bayley-III)8 were included in the present study. Both tests were completed by pediatric neuropsychologists or by psychometrists supervised by pediatric neuropsychologists at either the Division of Developmental and Behavioral Pediatrics at CCHMC or the Department of Psychology at SLCH/WashU. The ABAS-II is a caregiver report form of adaptive skills comprised of an overall adaptive score, the General Adaptive Composite (GAC), and three subscales assessing Conceptual, Social, and Practical skills. It also includes a subtest assessing motor skills. The Bayley-III is a direct assessment tool that measures early developmental skills assessing Cognitive, Language, and Motor skills for patients between 0.5 to 42 months of age. The Bayley-III also includes a caregiver report measure of early social-emotional behavior.
Statistical Analysis
The developmental change in WM and DTI measures could significantly confound the results in the present study. Therefore, all the statistical analyses of DTI measures were corrected for age in the present study. We performed a regression analysis at each ROI for every DTI measure to remove the potential confounding effect of age. The residual values of the regression, defined as the difference between the actual DTI value from individual participant and the simulated normal DTI value at the corresponding age based on the regression, were then used to substitute for the raw DTI values in the subsequent analysis. The group differences of DTI between patients and controls were first tested cross-sectionally before surgery, 3-month post-surgery, and 12-month post-surgery separately using independent two-tailed unpaired t-test. The false discovery rate (FDR) method was applied at each time point to correct for potential false positive findings resulted from multiple comparisons of DTI measures and ROIs. Longitudinal comparisons of DTI abnormalities were tested between each pair of the three time points using paired t-test in children with hydrocephalus who had longitudinal data at the corresponding time points. Pearson correlation analysis was performed to test the association between DTI and neuropsychological outcome assessed at the same time and between DTI at 3-month post-surgery and neuropsychological outcome assessed at 12-month post-surgery. Due to the limitation in sample size in the longitudinal comparisons and correlation analyses, the statistical significance was reported without correction for multiple comparisons in these analyses.
Results
Ventricle size and Correlation with DTI measures and Neuropsychologial Outcomes at Pre-surgery, 3-month and 12-month Post-surgery
The ventricle size based on FOHR in the control group ranged from 0.197 to 0.375 (Mean±SD = 0.310±0.036). Compared to the controls, patients with hydrocephalus had significantly larger FOHR at pre-surgery (range = 0.331–0.777, Mean±SD = 0.555±0.102, p <0.0001) and at both 3-month post-surgery (range = 0.339 – 0.687, Mean±SD = 0.483 ± 0.090, p <0.0001) and 12-month post-surgery (range = 0.265 – 0.579; Mean±SD = 0.400 ± 0.089, p <0.0001). No significant correlation was found between FOHR and DTI measures or FOHR and neuropsychological outcome measures in any group or time point.
DTI Abnormalities in Children with Hydrocephalus at Pre-surgery, 3-month and 12-month Post-surgery (p-values corrected using FDR method)
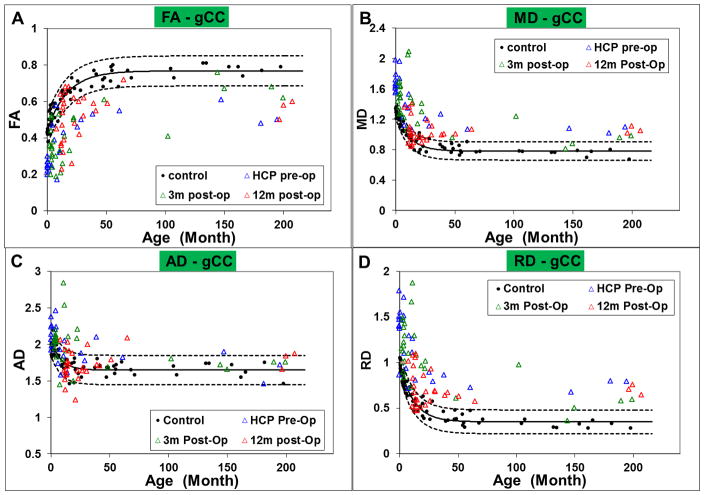
The ROI-based DTI measures in children with hydrocephalus at the pre-surgery, 3-month, and 12-month post-surgery are displayed in Figure 1 (for gCC) and Figure 2 (PLIC), both overlaid with the DTI values extracted from the same ROIs from the control group, indicated by the solid black line with the 95% prediction intervals for the normal control values shown by the dashed lines. As expected, there was a developmental change in the DTI values with age in both ROIs in the control group. The regression coefficients based on exponential model fit are included in Table 2.
Figure 1.
Scatter plot showing the group difference of A: FA; B: MD; C: AD; and D: RD in the gCC
Figure 2.
Scatter plot showing the group difference of A: FA; B: MD; C: AD; and D: RD in left PLIC
Table 2.
Results of regression analysis between normal DTI measures and age using an exponential model (Y = a*exp(−b*X+c))
| Regions | Regression coefficients
|
Exponential model fit
|
|||
|---|---|---|---|---|---|
| a | b | c | y-intercept | r2 | |
| gCC_FA | −0.3266 | 0.0588 | 0.7677 | 0.4411 | 0.9029 |
| gCC_MD | 0.5544 | 0.0937 | 0.7820 | 1.3364 | 0.9246 |
| gCC_AD | 0.4065 | 0.1215 | 1.6482 | 2.0545 | 0.6828 |
| gCC_RD | 0.6315 | 0.0829 | 0.3506 | 0.9821 | 0.9307 |
| LPLIC_FA | −0.2236 | 0.0748 | 0.5989 | 0.3753 | 0.8804 |
| LPLIC_MD | 0.3335 | 0.1816 | 0.7522 | 1.0875 | 0.9088 |
| LPLIC_AD | 0.2428 | 0.3218 | 1.3191 | 1.5618 | 0.6528 |
| LPLIC_RD | 0.3801 | 0.1414 | 0.4592 | 0.8391 | 0.9174 |
Note: gCC: genu of corpus callosum; LPLIC: left posterior limb of internal capsule; FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity. The regression model fit the data in all ROIs with a significance level of p<0.001.
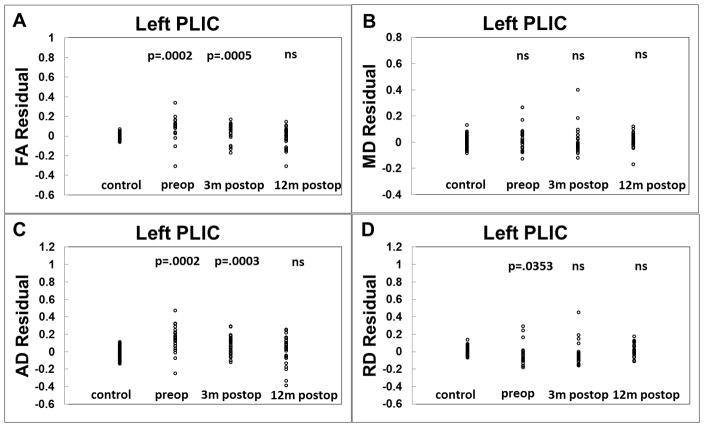
Quantitatively, at both pre-surgery and 3-month post-surgery, DTI parameters in the gCC in children with hydrocephalus were found to be significantly different from the controls characterized by lower FA (p=0.0008 at both time points), higher MD (p=0.0004 at both time points), higher AD (p=0.0095 at pre-surgery; p=0.0003 at 3-month post-surgery), and higher RD (p=0.0003 at pre-surgery; p=0.0002 at 3-month post-surgery). DTI parameters in the left PLIC in children with hydrocephalus were found to have significantly higher FA (p=0.0002 at pre-surgery, p=0.0005 at 3-month post-surgery), higher AD (p=0.0002 at pre-op; p=0.0003 at 3-month post-surgery), and lower RD at pre-surgery (p=0.0353) when compared with the controls. At 12-month post-surgery, DTI parameters in the gCC in children with hydrocephalus were found to have significantly lower FA (p=0.0008), higher MD (p=0.0004) and higher RD (p=0.0003). No significance in AD was found between children with hydrocephalus and the controls at 12-month post-surgery. In the left PLIC, no significant difference was found in any of the four DTI measures between children with hydrocephalus and the controls at the 12-month post-surgery time point.
Longitudinal Difference of DTI Abnormalities among Pre-surgery, 3-month, and 12-month Post-surgery
To perform longitudinal comparisons, we first quantified the DTI abnormalities as the difference between the actual DTI values in children with hydrocephalus and the normal DTI value at the corresponding age based on the regression of DTI data using the control datasets. Longitudinal DTI comparisons between different time points were made in those children with hydrocephalus who had multiple MRI/DTI datasets. As shown in Table 4, no longitudinal significant change in DTI abnormality (the difference between patient DTI and simulated normal DTI at the same age) between pre-surgery and 3-month post-surgery was found in any of the four DTI parameters in the gCC (n=10). Between 3-month post-surgery and 12-month post-surgery, the DTI abnormalities decreased significantly in FA (p=0.0417), MD (p=0.0049), and RD (p=0.0025) in the gCC (n=19). It should be noted that a decrease of DTI abnormalities means the DTI values from patients become closer to the normal simulated value as shown in Figure 5. Between pre-surgery and 12-month post-surgery, similar findings were observed in the gCC (n=11) with statistical significance in MD (p=0.0095) and RD (p=0.0002) or with trend level significance in FA (p=0.078). In the left PLIC, similar to the gCC, no statistically significant change was found in DTI abnormality in any of the four DTI parameters between pre-surgery and 3-month post-surgery. Between 3-month post-surgery and 12-month post-surgery, a trend level change in DTI abnormality was found in FA (p=0.0590) but not in the other three parameters. Between pre-surgery and 12-month post-surgery (Figure 6, Table 4), trend level change of DTI abnormalities was found in FA (p=0.0594), AD (p=0.0650), and RD (p=0.0717).
Table 4.
Longitudinal comparisons of DTI residual in children with HCP at three different time points
| Longitudinal Difference of DTI Residual | N | Mean | SD | SEM | Minimum | Median | Maximum | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| gCC | 3 month PostOp - PreOp | FA | 11 | −0.013 | 0.137 | 0.041 | −0.237 | 0.016 | 0.197 | ns |
| MD | 11 | 0.064 | 0.324 | 0.098 | −0.458 | 0.010 | 0.737 | ns | ||
| AD | 11 | 0.135 | 0.373 | 0.113 | −0.522 | 0.110 | 0.985 | ns | ||
| RD | 11 | 0.015 | 0.313 | 0.094 | −0.416 | −0.041 | 0.623 | ns | ||
| 12 month PostOp - 3 month PostOp | FA | 18 | 0.050 | 0.096 | 0.023 | −0.104 | 0.043 | 0.224 | 0.0418 | |
| MD | 18 | −0.228 | 0.300 | 0.071 | −1.040 | −0.247 | 0.160 | 0.0049 | ||
| AD | 18 | −0.273 | 0.565 | 0.133 | −2.035 | −0.137 | 0.270 | 0.0565 | ||
| RD | 18 | −0.237 | 0.284 | 0.067 | −0.935 | −0.224 | 0.186 | 0.0025 | ||
| 12 month PostOp - PreOp | FA | 10 | 0.031 | 0.046 | 0.015 | −0.054 | 0.025 | 0.101 | 0.0775 | |
| MD | 10 | −0.131 | 0.126 | 0.040 | −0.303 | −0.160 | 0.101 | 0.0095 | ||
| AD | 10 | −0.197 | 0.583 | 0.184 | −1.740 | −0.086 | 0.388 | ns | ||
| RD | 10 | −0.177 | 0.092 | 0.029 | −0.312 | −0.199 | −0.029 | 0.0002 | ||
| LPLIC | 3 month PostOp - PreOp | FA | 11 | −0.038 | 0.097 | 0.029 | −0.169 | −0.051 | 0.174 | ns |
| MD | 11 | −0.023 | 0.057 | 0.017 | −0.145 | −0.030 | 0.064 | ns | ||
| AD | 11 | −0.098 | 0.144 | 0.043 | −0.364 | −0.119 | 0.231 | 0.0470 | ||
| RD | 11 | 0.005 | 0.067 | 0.020 | −0.093 | 0.021 | 0.098 | ns | ||
| 12 month PostOp - 3 month PostOp | FA | 19 | −0.040 | 0.083 | 0.019 | −0.223 | −0.028 | 0.117 | 0.0525 | |
| MD | 19 | −0.004 | 0.098 | 0.022 | −0.353 | 0.023 | 0.094 | ns | ||
| AD | 19 | −0.047 | 0.159 | 0.036 | −0.512 | −0.029 | 0.219 | ns | ||
| RD | 19 | 0.029 | 0.100 | 0.023 | −0.251 | 0.030 | 0.207 | ns | ||
| 12 month PostOp - PreOp | FA | 11 | −0.096 | 0.150 | 0.045 | −0.365 | −0.065 | 0.146 | 0.0594 | |
| MD | 11 | 0.013 | 0.063 | 0.019 | −0.093 | 0.031 | 0.132 | ns | ||
| AD | 11 | −0.104 | 0.166 | 0.050 | −0.520 | −0.067 | 0.072 | 0.0650 | ||
| RD | 11 | 0.073 | 0.119 | 0.036 | −0.137 | 0.084 | 0.304 | 0.0717 |
Note: gCC: genu of corpus callosum; LPLIC:left posterior limb of internal capsule; FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity; SD: standard deviation; SEM: standard error mean;
Figure 5.
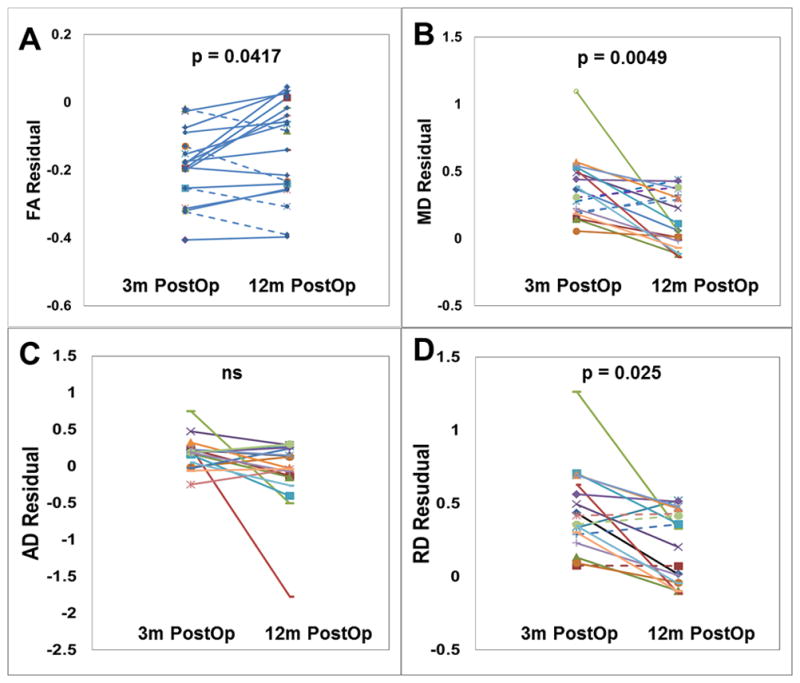
Longitudinal comparison of DTI residual in the gCC between 3-month post-surgery and 12-month post-surgery. A: FA; B: MD; C: AD; D: RD. All p values corrected using FDR method.
Figure 6.

Longitudinal comparison of DTI residual in left PLIC between pre-surgery and 12-month post-surgery. A: FA; B: MD; C: AD; D: RD
Abnormalities in Neuropsychological Outcomes at Pre-surgery, 3-month and 12-month Post-surgery (p-values corrected using FDR method)
A comprehensive analysis of neuropsychological measures in this cohort is provided elsewhere (Stephanie et al. under review). The results from the present sample are generally consistent with previous results and are summarized in Table 5. Briefly, in comparison to the controls, children with hydrocephalus had significantly (or at least with a trend) lower score in GAC, all the three subscale scores, and the motor skills at all three time points with the only exception being the Practical subscale at 3-month post-surgery based on the care giver assessment in ABAS-II tests. In Bayley test, children with hydrocephalus were found to have lower scores at trend level in Social Emotional skill at both post-surgery time points, and significantly lower scores in Cognitive, Language and Motor skills at all three time points.
Table 5.
Neuropsychological outcome measures in HCP at three different time points in comparison to the controls
| CTL | HCP, pre-op | HCP, 3m post-op | HCP, 12m post-op | |
|---|---|---|---|---|
| ABAS-II | ||||
| GAC | 101.6±14.2 | 89.1±16.9(p=0.0027) | 94.9±15.9 (p=0.0562) | 85.1±18.5 (p=0.0009) |
| Conceptual | 104.6±13.8 | 93.5±17.3(p=0.0065) | 99.3±15.9 (p=0.0981) | 92.4±17.7 (p=0.0012) |
| Social | 100.5±15.5 | 91.3±14.4(p=0.0179) | 96.2±15.0 (p=0.0954) | 90.3±17.5 (p=0.0097) |
| Practical | 99.5±14.4 | 91.9±15.2(p=0.0384) | 97.2±15.6 (ns) | 84.5±14.2 (p=0.0005) |
| Motor Scale | 10.1±2.9 | 7.9±3.9(p=0.0085) | 7.9±3.1 (p=0.0020) | 6.4±3.7 (p=0.0003) |
| Bayley-III | ||||
| Social | 103.6±14.9 | 92.9±21.0 (ns) | 95.9±15.6 (p=0.0826) | 93.5±23.8 (p=0.0707) |
| Cognitive | 99.0±9.8 | 89.2±15.0 (p=0.0434) | 82.6±17.2 (p=0.0009) | 84.2±17.5 (p=0.0002) |
| Language | 98.5±9.2 | 74.6±9.1 (p=0.0009) | 85.2±16.6 (p=0.0005) | 80.3±16.9 (p=0.0002) |
| Motor | 97.4±8.7 | 72.3±15.0 (p=0.0005) | 78.9±18.1 (p=0.0003) | 75.1±18.7 (p=0.0002) |
Note: ABAS-II: The Adaptive Behavior Assessment System, Second Edition; Bayley-III: The Bayley Scales of Infant Development, Third Edition; GAC: General Adaptive Composite; CTL: control; HCP: hydrocephalus; ns: not significant. All p-values corrected using FDR method.
Correlation of DTI with Neuropsychological Outcome Measures at the Time of Imaging
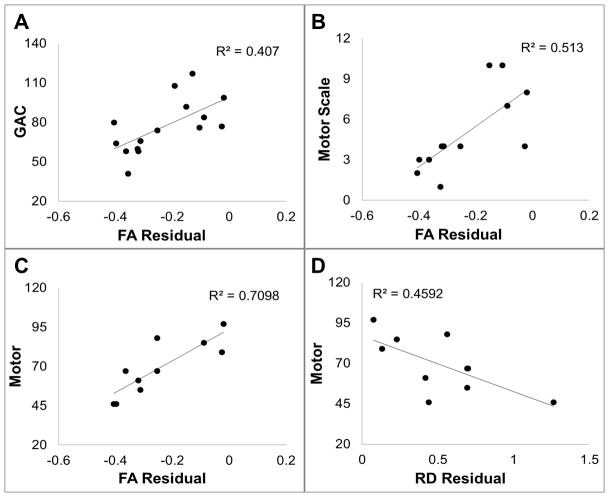
In children in the control group, no significant correlation was found between any DTI measures in the gCC or the left PLIC with any outcome measure in either ABAS-II or Bayley-III. In children with hydrocephalus at pre-surgery, the FA residual in gCC correlated positively with statistical significance with Motor Scale score in ABAS-II (r=0.79, n=10, p=0.007, Figure 7A). In children with hydrocephalus at 3-month post-surgery, FA residual in gCC correlated positively with statistical significance with GAC (r=0.43, n=26, p=0.028, Figure 7B), Motor Scale score (r=0.45, n=22, p=0.034) in ABAS-II and Language score (r=0.61, n=19, p=0.005, Figure 7C) in Bayley-III. RD in gCC was inversely correlated with Motor Scale score (r=−0.44, n=22, p=0.041) in ABAS-II. In children with hydrocephalus at 12-month post-surgery, FA in gCC correlated positively with Motor Scale score in ABAS-II (r=0.57, n=17, p=0.018) and Motor scores (r=0.72, n=14, p=0.004, Figure 7D) in Bayey-III.
Figure 7.
Correlation of DTI residual in the gCC with neuropsychological outcome assessed at the same time. A: FA residual in the gCC vs. ABAS-II Motor Scale at pre-surgery; B: FA residual in gCC vs. ABAS-II GAC at 3-month post-surgery; C: FA residual in the gCC vs. Bayley-III at 3-month post-surgery; D: FA residual in the gCC vs. Bayley-III motor at 12-month post-surgery.
Correlation of DTI with Future Neuropsychological Outcome
FA values in gCC measured at 3-month post-surgery was found to have a significant positive correlation with GAC (r=0.638, n=15, p=0.011, Figure 8A), Conceptual score (r=0.633, n=15, p=0.011), Practical score (r=0.633, n=15, p=0.011), and Motor Scale score (r=0.716, n=12, p=0.009, Figure 8B) in ABAS-II and Motor score (r=0.842, n=10, p=0.002, Figure 8C) in Bayley-III tested at 12-month post-surgery. RD in gCC measured at 3-month post-surgery was inversely correlated with the Motor Scale score (r=−0.605, p=0.037, n=12) in ABAS-II and Motor score (r=−0.678, n=10, p=0.031, Figure 8D) from in Bayley-III tested at 12-month post-surgery.
Figure 8.
Correlation of DTI residual in the gCC at 3-month post-surgery with neuropsychological outcome at 12-month post-surgery. A: FA residual in the gCC vs. ABAS-II GAC; B: FA residual in the gCC vs. ABAS-II Motor Scale; C: FA residual in the gCC vs. Bayley-III motor; D: RD residual in gCC vs. Bayley-III motor.
Discussion
The use of DTI for the non-invasive evaluation of clinical hydrocephalus was first reported by Assaf et al., in 2006.6 Since that time the evaluation of WM tracts to better understand pediatric hydrocephalus has been applied both prospectively and retrospectively in human and animal studies. Significant WM abnormalities in the corpus callosum and internal capsule, i.e., decreased FA in the corpus callosum and increased FA in the internal capsule (interpreted as disruption and compression, respectively) have been reported in our group as well as in the work of the others.3,6,22,40,44 Post CSF diversionary surgery recovery with a trend toward normalization has also been reported.2,6 It is interesting and worth noting that a different trend was identified when patients with benign external hydrocephalus were analyzed. Those patients also had various DTI abnormalities from initial screening MRIs. However, those with follow-up imaging detected no WM abnormalities without need of surgical intervention.37 More recently we reported preliminary findings suggesting a correlation between abnormal DTI values and motor deficits in a prospectively studied patient population (a subset of the patients in the present study at pre-operative stage44). Other groups have also reported that DTI parameters are sensitive in the study of hydrocephalus and suggest that DTI could serve as an important tool for quantitative assessment. Others have noted that DTI may offer a better association of WM injury and neurological deficits when compared to ventricular size.10,30
In the present study, DTI and neurophysiological outcomes were investigated in children with surgically treated congenital hydrocephalus in a prospective manner, first at pre-surgery, and then at 3 months and 12 months after surgery. The data collected in the present study supported the hypothesis that DTI measures at pre-op were abnormal in hydrocephalus in comparison to the age matched controls. This data is consistent with our previous reports as well as those of other groups.2,10.20,44 In general FA values are low in the genu of the corpus callosum and high in the posterior limb of internal capsule.
Persistence of DTI Abnormalities within 1 year After Surgery
It was hypothesized that there would be significant trends toward recovery in terms of DTI measures in the white matter at follow-up exams after the initial surgical procedure. However, this hypothesis was not proved to be entirely true. Qualitatively, abnormal DTI values seem to persist in many patients when they are assessed later in life (Figures 1&2). Cross-sectionally, the DTI in the corpus callosum of hydrocephalus patients demonstrated persistent abnormalities both at 3-month and 12-month follow-up when compared to normal controls. In contrast, the diffusion abnormalities in the PLIC were significant at 3-month post-surgery but the abnormalities were not appreciated at 12-month post-surgery (Figure 3&4). Longitudinally, the DTI abnormalities in hydrocephalus in the gCC (difference between control and hydrocephalus’s DTI measure in the gCC) were not significantly different between pre-surgery and 3-month post-surgery, suggesting a persistent WM injury within the 3 month time frame despite the decreased ventricle size and improved clinical symptoms. However, it was found that there was a significant longitudinal difference in all 4 DTI measures in gCC between 3-month and 12-month post-surgery with DTI values closer to normal at 12-month post-surgery. Significant or trend level differences in DTI abnormalities in the gCC between pre-surgery and 12-month post-surgery were also found with DTI values closed to the normal range at 12-month follow-up. In contrast, the DTI abnormality in hydrocephalus in the left PLIC (difference between control and hydrocephalus’s DTI measure in the left PLIC) appeared to recover sooner after the surgery based on the longitudinal comparisons.
Figure 3.
Cross-sectional comparisons of DTI residual in the gCC between controls and hydrocephalus patients at three different time points. A: FA; B: MD; C: AD; and D: RD. All p values corrected using FDR method.
Figure 4.
Cross-sectional comparisons of DTI residual in left PLIC between controls and hydrocephalus patients at three different time points. A: FA; B: MD; C: AD; and D: RD.
Correlation between DTI and Neuropsychological Outcome & Predictive Value of DTI for Future Outcome Measures
In the present study we reported correlations between DTI measures and neuropsychological outcomes in children with HCP. At all three time points, correlations were found between ABAS-II or Bayley-III in children with HCP and their DTI results in the gCC (in general, positively with FA, negatively with RD). The direction of correlation is in line with our hypotheses, i.e., with a structure with abnormally lower FA and/or higher MD (or AD or RD), a patient with HCP performs better in the neuropsychological assessment when his/her DTI measure in the gCC is closer to the normal range. Although there is no direct evidence based on underlying neuroanatomy, the correlation indicates that, in addition to the persistent abnormality in DTI at both 3-month and 12-month post-surgery, the association between neuroanatomy and outcome measures has not resolved within the time frame of this study (1 year post-surgery). In addition to the correlation between outcome measure and contemporaneous DTI measures, significant correlations were also found between DTI at 3-momnth post-surgery and outcome measures at 12-month post-surgery, suggesting that behavioral and neuropsychological outcome changes may be influenced by WM changes preceding the outcome measure. The predictive value of white matter integrity based on DTI for future outcome has been reported frequently in patient population such as preterm infants, stroke, TBI, and hypoxic-ischemic encephalopathy.1,4,18,24 The findings in the current study add new value to the field demonstrating the predictive and prognostic value of DTI at a time shortly after surgical treatment (i.e. 3-month post-surgery) for outcomes measures assessed at a later time (12-month post-surgery). Whether this relation remains for longer term prediction, and whether DTI at 12-month post-injury is a better predictor for future outcome are yet to be tested. However, our finding shows potential value of DTI in clinical application in the management of post-surgical hydrocephalus patients. Correlations were found between DTI at 3-momnth post-surgery and outcome measures at 12-month post-surgery.
DTI measures potentially can become a valuable tool for clinicians who follow hydrocephalus patients long term. The study presented here provides initial evidence that would allow the translation of non-invasively obtained radiographic information to follow temporal progression of WM integrity in children with hydrocephalus. We generated normative WM maturation curves based on DTI for specific regions and this potentially can be replicated in other major white matter regions or tracts of interest. Creation of such curves will allow us to objectively and non-invasively evaluate patients and eventually extrapolate to future neurocognitive outcomes. When followed longitudinally the imaging measures will serve to correlate functional outcomes the same way we currently follow head circumference measurements in infants and toddlers to predict normal development or alert us of potential impending clinical pathology.
Limitations
This study was designed as a prospective longitudinal clinical neuroimaging research project. Due to the logistical difficulty, it was often impractical to enroll and scan patients in a timely manner. Many patients enrolled initially in the study were not tested (MRI and neuropsychological testing) at all three time points, thus reducing the power in statistical analysis. Post-surgery follow-ups were designed at 3-month and 12-month after surgery. We found significant DTI abnormalities at pre-surgery and 3-month post-surgery in both the gCC and the left PLIC, and the abnormalities persisted in the gCC at 12-month after surgery. However, we were not able to extrapolate findings from the present study for the trajectory of further recovery after surgery. It would be interesting to follow these surgical patients for a longer period of time to determine when or if the abnormalities (DTI and neuropsychological outcomes) observed short term post-surgery will eventually normalize for all patients, and how the correlation between DTI tested at 3-month post-surgery and behavioral outcome tested at 12-month post-surgery will evolve temporally in these young patients. A third limitation is the lack of a more comprehensive test battery due to the age range in this patient population. In a recent study, it was found that the non-verbal performance in a cohort of school age children (6–16 yrs) with surgically treated hydrocephalus responded well to a short term OT intervention targeting the visual spatial, visual attention, and visual motor deficits, which are typical domains of long term deficits in this patient population. How we can merge the knowledge obtained from the present study in which most patients enrolled at 0–3 yrs and the other reports of long-term outcome in older children remains a challenge and warrants further investigation. Longer term follow up of this cohort would allow additional, quantitative neuropsychological scales to be tested to measure relative performance to the normal population.
Conclusion
This was the first prospective neuroimaging study that systemically investigated WM abnormalities based on DTI in children with hydrocephalus at pre- and post-surgery. We found (1) significant WM abnormalities at the acute stage prior to surgery; (2) longitudinal recovery with a trend toward normal range post-surgery; (3) persistent WM abnormalities in ROIs tested at 3-month and/or 12-month post-surgery; and (4) significant correlation between DTI and developmental outcome measures. These findings provide strong evidences that DTI is a sensitive imaging biomarker for WM abnormalities in pediatric hydrocephalus and potentially can serve as a clinical tool for diagnosis and prognosis in the management of this patient population.
Supplementary Material
Table 3.
Cross-sectional comparisons of DTI residual between normal controls and children with HCP at three different time points
| CTL | HCP | Group Difference | df | t | p* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | |||||||
|
| ||||||||||
| Controls vs. HCP at PreOp | gCC | FA | −0.0005 ± 0.0414 | −0.1743±0.1296 | 0.1738 | 0.1374 | 0.2102 | 84 | 9.4956 | 0.0008 |
|
| ||||||||||
| MD | 0.0006 ± 0.0709 | 0.3187±0.2065 | −0.3181 | −0.3771 | −0.2590 | 84 | 10.7109 | 0.0004 | ||
|
| ||||||||||
| AD | 0.0000 ± 0.1111 | 0.1008±0.2203 | −0.1008 | −0.1734 | 0.0281 | 84 | 2.7587 | 0.0095 | ||
|
| ||||||||||
| RD | 0.0008 ± 0.0687 | 0.4004±0.2904 | −0.3996 | −0.4767 | −0.3225 | 84 | 10.3054 | 0.0003 | ||
|
| ||||||||||
| LPLIC | FA | −0.0001 ± 0.0309 | 0.0828±0.1210 | −0.0829 | −0.1154 | −0.0504 | 84 | 5.0694 | 0.0002 | |
|
| ||||||||||
| MD | 0.0011 ± 0.0431 | 0.0227±0.0852 | −0.0228 | −0.0475 | 0.0019 | 84 | 1.8368 | ns | ||
|
| ||||||||||
| AD | −0.0000 ± 0.0624 | 0.1469±0.1476 | −0.1470 | −0.1919 | −0.1020 | 84 | 6.5021 | 0.0002 | ||
|
| ||||||||||
| RD | 0.0004 ± 0.0436 | −0.0388±0.1232 | 0.0392 | −0.0037 | 0.0747 | 84 | 2.1948 | 0.0353 | ||
|
| ||||||||||
| Controls vs. HCP at 3 month PostOp | gCC | FA | −0.0005 ± 0.0414 | −0.1948±0.1041 | 0.1942 | 0.1642 | 0.2243 | 90 | 12.8527 | 0.0008 |
|
| ||||||||||
| MD | 0.0006 ± 0.0709 | 0.3609±0.2486 | −0.3602 | −0.4271 | −0.2934 | 90 | 10.7034 | 0.0004 | ||
|
| ||||||||||
| AD | 0.0000 ± 0.1111 | 0.1850±0.2689 | −0.1850 | −0.2634 | −0.1066 | 90 | 4.6881 | 0.0003 | ||
|
| ||||||||||
| RD | 0.0008 ± 0.0687 | 0.4386±0.2734 | −0.4380 | −0.5102 | −0.3658 | 90 | 12.0531 | 0.0002 | ||
|
| ||||||||||
| LPLIC | FA | −0.0001 ± 0.0309 | 0.0430±0.0815 | −0.0431 | −0.0662 | −0.0199 | 91 | 3.7001 | 0.0005 | |
|
| ||||||||||
| MD | 0.0011 ± 0.0431 | 0.0107±0.0949 | −0.0109 | −0.0368 | 0.0152 | 91 | 0.8242 | ns | ||
|
| ||||||||||
| AD | −0.0000 ± 0.0624 | 0.0663±0.1007 | −0.0663 | −0.1002 | −0.0324 | 91 | 3.8833 | 0.0003 | ||
|
| ||||||||||
| RD | 0.0004 ± 0.0436 | −0.0264±0.1190 | 0.0267 | −0.0068 | 0.0060 | 91 | 1.5850 | ns | ||
|
| ||||||||||
| Controls vs. HCP at 12 month PostOp | gCC | FA | −0.0005 ± 0.0414 | −0.1437±0.1251 | 0.1432 | 0.1088 | 0.1777 | 91 | 8.2571 | 0.0008 |
|
| ||||||||||
| MD | 0.0006 ± 0.0709 | 0.1337±0.1716 | −0.1331 | −0.1829 | −0.0083 | 91 | 5.3101 | 0.0004 | ||
|
| ||||||||||
| AD | 0.0000 ± 0.1111 | −0.0644±0.3955 | 0.0628 | −0.0428 | 0.1684 | 91 | 1.1809 | ns | ||
|
| ||||||||||
| RD | 0.0008 ± 0.0687 | 0.2031±0.2007 | −0.2023 | −0.2579 | −0.1467 | 91 | 7.2219 | 0.0003 | ||
|
| ||||||||||
| LPLIC | FA | −0.0001 ± 0.0309 | 0.0089±0.0958 | 0.0088 | −0.0173 | 0.0350 | 92 | 0.6698 | ns | |
|
| ||||||||||
| MD | 0.0011 ± 0.0431 | 0.0113±0.0530 | −0.0114 | −0.0287 | 0.0058 | 92 | 1.3147 | ns | ||
|
| ||||||||||
| AD | −0.0000 ± 0.0624 | 0.0137±0.1531 | −0.0138 | −0.0578 | 0.0303 | 92 | 0.6198 | ns | ||
|
| ||||||||||
| RD | 0.0004 ± 0.0436 | 0.0090±0.0683 | −0.0087 | −0.0318 | 0.0145 | 92 | 0.7444 | ns | ||
Note: gCC: genu of corpus callosum; LPLIC:left posterior limb of internal capsule; FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity; CTL: control; HCP: hydrocephalus; SD: standard deviation; CI: confidence interval; df: degree of freedom; ns: not significant. p*: p-value corrected using FDR method.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (R01 NS066932. PIs: W. Y. and F. T. M.).
Grant Support: NIH/NINDS R01 NS066932
ABBREVIATIONS
- FA
fractional anisotropy
- MD
mean diffusivity
- AD
axial diffusivity
- RD
radial diffusivity
- ROI
region of interest
- gCC
genu of corpus callosum
- PLIC
posterior limb of internal capsule
- ABAS-II
Adaptive Behavior Assessment System, Second Edition
- Bayley III
Bayley Scale of Infant Development, Third Edition
- GAC
general adaptive composite
Footnotes
Authors Disclosure Statements
No competing financial interest exists for any of the authors.
References
- 1.Aeby A, De Tiège X, Creuzil M, David P, Balériaux D, Van Overmeire B, et al. Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. Neuroimage. 2013;78:145–151. doi: 10.1016/j.neuroimage.2013.03.076. [DOI] [PubMed] [Google Scholar]
- 2.Air EL, Yuan W, Holland SK, Jones BV, Bierbrauer K, Altaye M, et al. Longitudinal comparison of pre- and postoperative diffusion tensor imaging parameters in young children with hydrocephalus. J Neurosurg Pediatr. 2010;5:385–391. doi: 10.3171/2009.11.PEDS09343. [DOI] [PubMed] [Google Scholar]
- 3.Akbari SH, Limbrick DD, Jr, McKinstry RC, Altaye M, Ragan DK, Yuan W, et al. Periventricular hyperintensity in children with hydrocephalus. Pediatr Radiol. 2015;45:1189–1197. doi: 10.1007/s00247-015-3298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ancora G, Testa C, Grandi S, Tonon C, Sbravati F, Savini S. Prognostic value of brain proton MR spectroscopy and diffusion tensor imaging in newborns with hypoxic-ischemic encephalopathy treated by brain cooling. Neuroradiology. 2013;55:1017–1025. doi: 10.1007/s00234-013-1202-5. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama Y, Kinoshita Y, Yokota A, Hamada T. Neuronal damage in hydrocephalus and its restoration by shunt insertion in experimental hydrocephalus: A study involving the neurofilament-immunostaining method. J Neurosurg. 2006;104:332–339. doi: 10.3171/ped.2006.104.5.332. [DOI] [PubMed] [Google Scholar]
- 6.Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L. Diffusion tensor imaging in hydrocephalus: initial experience. AJNR Am J Neuroradiol. 2006;27:1717–1724. [PMC free article] [PubMed] [Google Scholar]
- 7.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 8.Bayley N. The Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: The Psychological Corporation; 2006. [Google Scholar]
- 9.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Sira L, Goder N, Bassan H, Lifshits S, Assaf Y, Constantini S. Clinical benefits of diffusion tensor imaging in hydrocephalus. J Neurosurg Pediatr. 2015;16:195–202. doi: 10.3171/2014.10.PEDS13668. [DOI] [PubMed] [Google Scholar]
- 11.Buckley RT, Yuan W, Mangano FT, Phillips JM, Powell S, McKinstry RC, et al. Longitudinal comparison of diffusion tensor imaging parameters and neuropsychological measures following endoscopic third ventriculostomy for hydrocephalus. J Neurosurg Pediatr. 2012;9:630–635. doi: 10.3171/2012.2.PEDS11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Bigio MR, Bruni JE. Periventricular pathology in hydrocephalic rabbits before and after shunting. Acta Neuropathol. 1988;77:186–195. doi: 10.1007/BF00687430. [DOI] [PubMed] [Google Scholar]
- 13.Del Bigio MR, Wilson MJ, Enno T. Chronic hydrocephalus in rats and humans: White matter loss and behavior changes. Ann Neurol. 2003;53:337–346. doi: 10.1002/ana.10453. [DOI] [PubMed] [Google Scholar]
- 14.Del Bigio MR, Zhang YW. Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol. 1998;154:157–169. doi: 10.1006/exnr.1998.6922. [DOI] [PubMed] [Google Scholar]
- 15.Eskandari R, McAllister JP, 2nd, Miller JM, Ding Y, Ham SD, Shearer DM, et al. Effects of hydrocephalus and ventriculoperitoneal shunt therapy on afferent and efferent connections in the feline sensorimotor cortex. J Neurosurg. 2004;101:196–210. doi: 10.3171/ped.2004.101.2.0196. [DOI] [PubMed] [Google Scholar]
- 16.Eskandari R, Abdullah O, Mason C, Lloyd KE, Oeschle AN, McAllister JP., 2nd Differential vulnerability of white matter structures to experimental infantile hydrocephalus detected by diffusion tensor imaging. Childs Nerv Syst. 2014;30:1651–1661. doi: 10.1007/s00381-014-2500-x. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher JM, McCauley SR, Brandt ME, Bohan TP, Kramer LA, Francis DJ, et al. Regional brain tissue composition in children with hydrocephalus. Relationships with cognitive development. Arch Neurol. 1996;53:549–557. doi: 10.1001/archneur.1996.00550060093022. [DOI] [PubMed] [Google Scholar]
- 18.Galanaud D, Perlbarg V, Gupta R, Stevens RD, Sanchez P, Tollard E, et al. Assessment of white matter injury and outcome in severe brain trauma: a prospective multicenter cohort. Anesthesiology. 2012;117:1300–1210. doi: 10.1097/ALN.0b013e3182755558. [DOI] [PubMed] [Google Scholar]
- 19.Hale PM, McAllister JP, 2nd, Katz SD, Wright LC, Lovely TJ, Miller DW, et al. Improvement of cortical morphology in infantile hydrocephalic animals after ventriculoperitoneal shunt placement. Neurosurgery. 1992;31:1085–1096. doi: 10.1227/00006123-199212000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Hannay HJ. Functioning of the corpus callosum in children with early hydrocephalus. J Int Neuropsychol Soc. 2000;6:351–361. doi: 10.1017/s1355617700633106. [DOI] [PubMed] [Google Scholar]
- 21.Harrison PL, Oakland T. Adaptive Behavior Assessment System. 2. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 22.Hasan KM, Eluvathingal TJ, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: A diffusion tensor tractography study of the association pathways. J Magn Reson Imaging. 2008;27:700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Hirai KK, Groisser BN, Copen WA, Singhal AB, Schaechter JD. Comparing prognostic strength of acute corticospinal tract injury measured by a new diffusion tensor imaging based template approach versus common approaches. J Neurosci Methods. 2015 Sep 16; doi: 10.1016/j.jneumeth.2015.09.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch JF. Surgery of hydrocephalus: Past, present and future. Acta Neurochir (Wien) 1992;116:155–160. doi: 10.1007/BF01540869. [DOI] [PubMed] [Google Scholar]
- 26.Jang SH, Ho Kim S. Diffusion tensor imaging following shunt in a patient with hydrocephalus. J Neuroimaging. 2011;21:69–72. doi: 10.1111/j.1552-6569.2009.00394.x. [DOI] [PubMed] [Google Scholar]
- 27.Jang SH, Choi BY, Chang CH, Jung YJ, Byun WM, Kim SH, et al. The effects of hydrocephalus on the periventricular white matter in intracerebral hemorrhage: a diffuser tensor imaging study. Int J Neurosci. 2013;123:420–424. doi: 10.3109/00207454.2012.763164. [DOI] [PubMed] [Google Scholar]
- 28.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Khan OH, Enno TL, Del Bigio MR. Brain damage in neonatal rats following kaolin induction of hydrocephalus. Exp Neurol. 2006;200:311–320. doi: 10.1016/j.expneurol.2006.02.113. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni AV, Donnelly R, Mabbott DJ, Widjaja E. Relationship between ventricular size, white matter injury, and neurocognition in children with stable, treated hydrocephalus. J Neurosurg Pediatr. 2015;16:267–274. doi: 10.3171/2015.1.PEDS14597. [DOI] [PubMed] [Google Scholar]
- 31.Lovely TJ, McAllister JP, 2nd, Miller DW, Lamperti AA, Wolfson BJ. Effects of hydrocephalus and surgical decompression on cortical norepinephrine levels in neonatal cats. Neurosurgery. 1989;24:43–52. doi: 10.1227/00006123-198901000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Mataro M, Junque C, Poca MA, Sahuquillo J. Neuropsychological findings in congenital and acquired childhood hydrocephalus. Neuropsychol Rev. 2001;11:169–178. doi: 10.1023/a:1012904907249. [DOI] [PubMed] [Google Scholar]
- 33.Olopade FE, Shokunbi MT, Sirén AL. The relationship between ventricular dilatation, neuropathological and neurobehavioural changes in hydrocephalic rats. Fluids Barriers CNS. 2012;9:19. doi: 10.1186/2045-8118-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopal A, Shimony JS, McKinstry RC, Altaye M, Maloney T, Mangano FT, Limbrick DD, Holland SK, Jones BV, Simpson S, Mercer D, Yuan W. White matter microstructural abnormality in children with hydrocephalus detected by probabilistic diffusion tractography. Am J Neuroradiol. 2013;34:2379–2385. doi: 10.3174/ajnr.A3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Röricht S, Meyer BU, Woiciechowsky C, Lehmann R. Callosal and corticospinal tract function in patients with hydrocephalus: a morphometric and transcranial magnetic stimulation study. J Neurol. 1998;245:280–288. doi: 10.1007/s004150050219. [DOI] [PubMed] [Google Scholar]
- 36.Scheel M, Diekhoff T, Sprung C, Hoffmann KT. Diffusion tensor imaging in hydrocephalus--findings before and after shunt surgery. Acta Neurochir (Wien) 2012;154:1699–706. doi: 10.1007/s00701-012-1377-2. [DOI] [PubMed] [Google Scholar]
- 37.Sun M, Yuan W, Hertzler DA, Cancelliere A, Altaye M, Mangano FT. Diffusion tensor imaging findings in young children with benign external hydrocephalus differ from the normal population. Childs Nerv Syst. 2012;28:199–208. doi: 10.1007/s00381-011-1651-2. [DOI] [PubMed] [Google Scholar]
- 38.Ware AL, Juranek J, Williams VJ, Cirino PT, Dennis M, Fletcher JM. Anatomical and diffusion MRI of deep gray matter in pediatric spina bifida. Neuroimage Clin. 2014;5:120–127. doi: 10.1016/j.nicl.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 40.Yuan W, Mangano FT, Air EL, Holland SK, Jones BV, Altaye M, et al. Anisotropic Diffusion Properties in Infants with Hydrocephalus: A Diffusion Tensor Imaging Study. AJNR Am J Neuroradiol. 2009;30:1792–1798. doi: 10.3174/ajnr.A1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan W, Deren KE, McAllister JP, Holland SK, Lindquist DM, Cancelliere A, Mason M, Shereen A, Hertzler DA, Altaye M, Mangano FT. Diffusion tensor imaging correlates with cytopathology in a rat model of neonatal hydrocephalus. Cerebrospinal Fluid Res. 2010;7:19. doi: 10.1186/1743-8454-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan W, McAllister JP, 2nd, Lindquist DM, Gill N, Holland SK, Henkel D, et al. Diffusion tensor imaging of white matter injury in a rat model of infantile hydrocephalus. Childs Nerv Syst. 2012;28:47–54. doi: 10.1007/s00381-011-1590-y. [DOI] [PubMed] [Google Scholar]
- 43.Yuan W, McAllister JP, Mangano FT. Neuroimaging of White Matter Abnormality in Pediatric Hydrocephalus. J Pediatr Neuroradiol. 2013;2:119–128. [Google Scholar]
- 44.Yuan W, McKinstry RC, Shimony JS, Altaye M, Powell SK, Phillips JM, et al. Diffusion tensor imaging properties and neurobehavioral outcomes in children with hydrocephalus. AJNR Am J Neuroradiol. 2013;34:439–445. doi: 10.3174/ajnr.A3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan W, Holland S, Shimony J, Jones B, Mangano F, Limbrick D, et al. Quality assurance in Multi-institution and Multi-scanner MRI Neuroimaging Research. IPR LONDON 2011 6th Congress and Exhibition of the joint Societies of Paediatric Radiology; London, UK. May 27–31, 2011. [Google Scholar]
- 46.O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: A linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245–249. doi: 10.1159/000028730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.