Abstract
Background
In a previously reported CoFAR study, 55 subjects with egg allergy underwent randomized, placebo-controlled egg oral immunotherapy (eOIT). Active treatment induced desensitization in most and sustained unresponsiveness (SU) in a smaller subset. We hypothesized that component-resolved analysis of IgE, IgG4, IgA, IgA1, and IgA2 may identify potential biomarkers of SU in OIT subjects.
Methods
Longitudinal samples for 51 egg-allergic subjects (37 active, 14 placebo) were available. Egg white- (EW), ovalbumin- (OVA), and ovomucoid (OVM)-specific levels of IgA, IgA1, and IgA2 were quantified by ELISA. IgE and IgG4 to these antigens were quantified using ImmunoCAP®. Clinical responders achieved SU to egg; all others were considered non-responders. Between-group comparisons were made amongst active and placebo, as well as responders and non-responders.
Results
No placebo subjects achieved responder status. Through month 48, among the 37 active subjects, baseline IgE-OVM was lower in responders (median 3.97 kU/L, n=19) than non-responders (10.9 kU/L, n=18, p=0.010). Logistic regression analysis revealed lower baseline IgE-EW (p = 0.038), IgE-OVM (p = 0.032) and a higher IgG4:IgE-OVM ratio (p=0.013) were associated with clinical response. Relative increases in IgG4-EW, IgA-EW and IgA2-EW were greater in responders (p= 0.024, 0.024, 0.029, respectively). Ratios of IgG4:IgE, IgA:IgE, IgA2:IgE for EW and IgA:IgE for OVA were significantly elevated among responders (p = 0.004, 0.009, 0.028, 0.008, respectively).
Conclusions
Increased IgG4-EW, IgA-EW and IgA2-EW during eOIT are associated with clinical response to eOIT. Lower pre-treatment IgE-EW and IgE-OVM are also associated with SU. Future studies are needed to evaluate and validate these potential biomarkers.
Keywords: Keywords: component testing, egg allergy, food allergy, IgA, oral immunotherapy
Introduction
Food allergy affects approximately 8% of children in the United States (1). Young children are disproportionately affected, with prevalence estimates exceeding 10% in some regions (2). Epidemiologic studies suggest that up to 2.5% of all children are allergic to hen's egg and it is the most common food sensitization at one year of life (3-5). Egg allergy resolves in about half of children by 6 years and two-thirds of children by age 16 (6, 7). However, egg allergy is a risk factor for the development of other food allergies, atopic dermatitis and asthma (8-10). In addition, difficulty associated with egg avoidance has generated intense interest in investigational therapies.
The Consortium of Food Allergy Research (CoFAR) investigators previously reported the results of a multicenter, double-blind, randomized, placebo-controlled trial investigating the safety and effectiveness of egg oral immunotherapy (eOIT) in children (11). After 10 months of treatment, we demonstrated that 55% of egg-allergic subjects were desensitized; whereas, none of the subjects on placebo passed an oral food challenge (OFC). At 22 months, the rate of desensitization among subjects receiving eOIT increased to 75%. Importantly, after discontinuation of eOIT for 4-6 weeks, 28% of the subjects in the OIT group tolerated an egg OFC. These individuals were considered to have sustained unresponsiveness (SU). Subjects without SU at 22 months were maintained on OIT with subsequent assessments at 36 and 48 months, demonstrating an increase in the proportion of subjects with SU to 50% at month 48 (12).
Limited mechanistic data have been published from large, prospective, controlled, OIT trials to identify predictors and biomarkers associated with SU. The purpose of this study was to determine whether we could detect such biomarkers in the serum of children undergoing eOIT. Because allergenic foods are complex, heterogeneous substances consisting of multiple proteins, component resolved diagnostic (CRD) tools have been developed to more precisely identify the antigenic targets of IgE. For example, the heat-stable, component protein ovomucoid has been identified as the dominant egg white allergen (13). Some evidence suggests allergen-specific (sIgE) testing for ovomucoid may discriminate egg-tolerant from egg-allergic subjects (14) and, we hypothesized that those with lower ovomucoid-specific IgE are more likely to respond to eOIT.
In addition to sIgE, other immunoglobulin classes and subclasses are likely important for the development of SU. Egg-specific IgG4 levels tend to rise in subjects undergoing eOIT (11); moreover, IgE:IgG4 ratios have been used to predict clinical reactivity to baked egg (15). IgA and its subtypes, IgA1 and IgA2, may also play a role as increases in antigen-specific IgA are seen in the saliva of subjects who respond favorably to peanut sublingual immunotherapy (SLIT) (16). Similarly, increases in antigen-specific IgA2 have been observed in the serum of subjects receiving subcutaneous immunotherapy (SCIT) for grass pollen allergy (17). Given these observations, we hypothesized that component resolved analysis of the serologic parameters IgA, IgA1, IgA2, IgE, and IgG4 would identify potential biomarkers of SU.
Methods
Clinical Protocol Summary
The clinical study protocol and results of the first 24 months of the clinical trial (NCT00461097) have been described previously (11). Fig E1 (Online Repository) is a CONSORT flow diagram detailing outcomes of subjects through month 48 (12). Briefly, egg-allergic subjects receiving eOIT, continued open label treatment up to 48 months or until they achieved SU. All subjects continuing eOIT in the active treatment arm underwent a 10g OFC at 36 months and 48 months to monitor for desensitization. If a child passed the desensitization OFC, a subsequent OFC paired with a 10 g open egg feeding was performed 4-6 weeks later to assess for SU. Subjects who achieved SU were allowed to incorporate egg into the diet and were followed until the end of the study. The primary clinical efficacy outcome of the study was the percentage of subjects achieving SU by 48 months.
Subjects
Forty subjects were originally randomized to eOIT; 20 achieved SU and were classified as clinical responders (n=19 with samples available for analysis). All others in the active treatment arm were classified as non-responders (n=18 with samples available for analysis). Longitudinal blood samples were collected over a 4-year period at 3, 10, 22, 36 and 48 months. Plasma samples were kept frozen at -80°C until analysis and assigned a unique identifier. Laboratory personnel were blinded during the analysis until the data were finalized.
IgA Measurement
Egg white (EW)-, ovalbumin (OVA)-, and ovomucoid (OVM)-specific IgA, IgA1 and IgA2 were measured by ELISA. OVA (albumin from chicken egg white; Grade V, >98% pure based on SDS-PAGE) and OVM (trypsin inhibitor from chicken egg white; Type III-O, free of ovoinhibitor, >99% pure based on SDS-PAGE) were purchased from Sigma-Aldrich. Egg proteins were coated on Immulon 4HBX microtiter plates (Thermo Fisher Scientific) at concentrations of 20 (EW), 5 (OVA), and 5 (OVM) μg/ml for one hour at 37°C. Standard curve wells were coated with mouse anti-human IgA1/IgA2 capture antibody (BD, diluted 1:250). Plates were then blocked with a PBS solution containing 0.05% Tween-20 and 2% BSA for one hour at 37°C. Diluted plasma samples were added and left to bind egg antigens overnight at 4°C. Detection was performed with the following antibodies diluted in blocking solution: goat anti-human IgA-HRP antibody (Southern Biotech, diluted 1:1000); mouse anti-human IgA1-HRP antibody (Southern Biotech, diluted 1:1000); mouse anti-human IgA2-HRP antibody (Southern Biotech, diluted 1:1500). The plates were then incubated at 37°C for one hour.
The detection system was developed with TMB substrate (KPL) for 10 minutes, acidified with a TMB stop solution (KPL); and then read on an ELISA plate reader to determine optical density (O.D.). Optical densities were compared to a standard curve and converted to weight per volume concentrations. A vast majority of the samples were diluted 1:10 in blocking solution. Samples out of range of the standard curve were diluted further until an O.D. < 4 was obtained.
IgE and IgG4 Measurement
EW-, OVA- and OVM-specific IgE and IgG4 were measured in plasma using the ImmunoCAP 100 instrument (Phadia AB) according to the manufacturer's instructions.
Statistical Analysis
Subjects were grouped according to treatment arm and responder status. Wilcoxon ranked-sum test (unadjusted for multiplicity of tests) was used to compare median immunoglobulin measurements between non-responders and responders at baseline. Correlations at baseline were determined by generating Spearman's rank correlation coefficients. Logistic regression analysis was used to identify predictors of clinical responses to eOIT. Values were log-transformed given highly skewed distributions. Mean values for each serologic parameter and log-transformed ratios of IgG4:IgE, IgA:IgE, IgA1:IgE and IgA2:IgE for EW, OVA, and OVM were compared among responders and non-responders (and placebo where noted) using a repeated-measurement analysis with an unstructured covariance matrix. Endpoint-to-baseline ratios were compared using the same repeated-measurement analysis. Interactions between visit and responder status were considered. If significant, the interaction p-value was reported. If not, the interaction term was removed and the main effect p-value was considered. Given the exploratory nature of the analysis, adjustment for multiple comparisons was not performed and a p-value of <0.05 was considered significant.
Results
Baseline comparisons of groups according to clinical outcomes
We examined baseline serologic parameters to identify possible predictors of SU. Responders had lower median baseline IgE levels to all antigens measured. Between-group comparisons of untransformed baseline values demonstrated that the median baseline IgE-OVM values were significantly greater in non-responders than responders (10.9 vs. 3.97 kU/L, p=0.011) For IgE-EW, this difference did not reach statistical significance (20.03 vs. 9.59 kU/L p = .052). Despite the general trend for responders to have lower IgE levels, these parameters had large standard deviations (Table 1 and Figure 1). In addition to having greater baseline IgE levels, non-responders were distributed over a much wider range. IgA and IgA1 varied more among responders, whereas this pattern was reversed for IgA2.
TABLE 1. Baseline analysis of serologic parameters between groups.
| Non-responders | Responders | Placebo | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N=18 | N=19 | N=14 | ||||
| Median | SD | Median | SD | Median | SD | |
| IgE_EW | 20.03 | 74.26 | 9.59 | 19.00 | 22.04 | 23.18 |
| IgE_OVA | 14.90 | 288.79 | 8.40 | 15.54 | 8.15 | 18.75 |
| IgE_OVM | 10.90 | 63.84 | 3.97 | 9.03 | 3.78 | 22.46 |
| IgG4_EW | .59 | 2.93 | .40 | 7.46 | 0.66 | 1.73 |
| IgG4_OVA | .47 | 2.79 | .48 | 5.05 | 0.63 | 1.36 |
| IgG4_OVM | 0.07 | 0.59 | .11 | 2.63 | 0.14 | 0.89 |
| IgA_EW | 23.21 | 35.46 | 11.20 | 84.66 | 16.29 | 52.24 |
| IgA_OVA | 15.69 | 21.75 | 7.70 | 58.62 | 12.81 | 33.02 |
| IgA_OVM | 3.54 | 14.80 | 2.32 | 185.17 | 1.2 | 28.07 |
| IgA1_EW | 49.56 | 65.82 | 32.43 | 197.92 | 44.11 | 97.41 |
| IgA1_OVA | 25.14 | 38.00 | 17.70 | 114.36 | 16.41 | 69 |
| IgA1_OVM | 0.01 | 27.54 | 0.01 | 432.02 | 11.19 | 42.94 |
| IgA2_EW | 6.30 | 26.06 | 4.38 | 6.78 | 7.31 | 3.29 |
| IgA2_OVA | 2.24 | 11.77 | 1.73 | 2.98 | 3.07 | 2.63 |
| IgA2_OVM | .42 | 2.98 | .01 | 3.21 | 0.01 | 1.59 |
IgA, IgA1 and IgA2 levels are reported in ng/ml. IgE is reported as kU/L and IgG4 is reported as μg/ml. Statistically significant differences between groups are highlighted in red.
p=0.010,
p = 0.052 Exact 2-sided Wilcoxon unadjusted for multiplicity of tests in the Journal of Allergy and Clinical Immunology(12).
FIGURE 1.
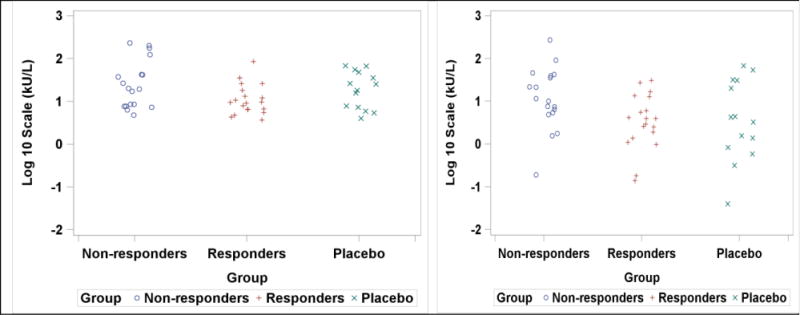
Comparison of log transformed IgE-EW and IgE-OVM values between groups at baseline. Panel A depicts differences among groups for log IgE-EW. Panel B shows differences for log IgE-OVM. Statistical comparisons of baseline median values performed using exact 2-sided Wilcoxon unadjusted for multiplicity of tests.
Logistic regression models using log-adjusted baseline values suggest lower baseline IgE-EW (p = 0.038 AUC 0.69 (0.51,0.86 95% CI)), IgE-OVM (p = 0.032, AUC 0.74 (0.58, 0.91 95% CI)), and higher IgG4:IgE for OVM (p = 0.013, AUC 0.70 (0.51, 0.89 95% CI)) were potential predictors of clinical response. Odds ratios for response per log increase of IgE-EW and IgE-OVM were 0.178 (95%CI 0.032, 0.906) and 0.28 (95%CI 0.088, 0.895), respectively. No regression models had multiple significant parameters and no other differences in baseline serologic parameters were significant.
To better characterize the relationships between baseline immunoglobulin measurements, we examined Spearman's rank correlation coefficients (Table 2). This allowed us to determine correlations between EW and is component proteins for IgE, IgG4, IgA, IgA1 and IgA2. In addition, we were able to define correlations between specific immunoglobulin classes/subclasses. Positive correlations (rs>0.50) existed between EW-, OVA- and OVM-specific levels of each immunoglobulin class/subclass. We also found positive correlations between IgG4 and IgA/IgA1 to all antigens.
TABLE 2. Baseline Spearman's rank correlation (rs) of serologic parameters.
| IgA | IgA | IgA | IgA1 | IgA1 | IgA1 | IgA2 | IgA2 | IgA2 | IgE | IgE | IgE | IgG4 | IgG4 | IgG4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EW | OVA | OVM | EW | OVA | OVM | EW | OVA | OVM | EW | OVA | OVM | EW | OVA | OVM | |
| IgA-EW | 1 | 0.76 | 0.72 | 0.90 | 0.76 | 0.64 | 0.61 | 0.47 | 0.38 | 0.39 | 0.30 | 0.24 | 0.35 | 0..35 | 0.24 |
| IgA-OVA | 1 | 0.93 | 0.79 | 0.85 | 0.83 | 0.48 | 0.50 | 0.46 | 0.51 | 0.39 | 0.36 | 0.41 | 0.42 | 0.32 | |
| IgA-OVM | 1 | 0.77 | 0.90 | .89 | 0.43 | 0.49 | 0.48 | 0.47 | 0.40 | 0.30 | 0.46 | 0.47 | 0.35 | ||
| IgA1-EW | 1 | 0.89 | 0.80 | 0.64 | 0.55 | 0.50 | 0.35 | 0.26 | 0.27 | 0.44 | 0.42 | 0.29 | |||
| IgA1-OVA | 1 | 0.93 | 0.52 | 0.53 | 0.56 | 0.45 | 0.37 | 0.30 | 0.52 | 0.49 | 0.40 | ||||
| IgA1-OVM | 1 | 0.43 | 0.50 | 0.54 | 0.47 | 0.39 | 0.27 | 0.51 | 0.49 | 0.37 | |||||
| IgA2-EW | 1 | 0.78 | 0.66 | 0.24 | 0.21 | 0.24 | 0.54 | 0.49 | 0.41 | ||||||
| IgA2-OVA | 1 | 0.86 | 0.31 | 0.25 | 0.19 | 0.53 | 0.49 | 0.34 | |||||||
| IgA2-OVM | 1 | 0.30 | 0.23 | 0.23 | 0.58 | 0.53 | 0.49 | ||||||||
| IgE-EW | 1 | 0.84 | 0.60 | 0.16 | 0.22 | 0.20 | |||||||||
| IgE-OVA | 1 | 0.56 | 0.25 | 0.17 | 0.16 | ||||||||||
| IgE-OVM | 1 | 0.23 | 0.22 | 0.38 | |||||||||||
| IgG4-EW | 1 | 0.98 | 0.90 | ||||||||||||
| IgG4-OVA | 1 | 0.76 | |||||||||||||
| IgG4-OVM | 1 |
rs values reported. Colored text denotes strong positive correlations (rs>0.50) between immunoglobulin classes (red), subclasses (blue) and antigens (green). Absolute correlations greater than .30 have unadjusted p-values of 0.05; those greater than 0.5 have unadjusted p-values <0.001.
Immunoglobulin trends during OIT
We measured the longitudinal production of IgE, IgG4, IgA, IgA1 and IgA2 to EW, OVA, and OVM to determine whether specific patterns emerged distinguishing responders from non-responders. Plasma samples from subjects receiving placebo were only available during the first 24 months of the study. First, we examined trends between groups to guide the analysis (Figure 2). Overall, we observed similar trends when these immunoglobulins were measured to EW or its component antigens, OVA and OVM. Generally, IgE increased among subjects on OIT during the first three months of the study. Declines in IgE were subsequently noted in these subjects; whereas, IgE for subjects on placebo remained constant. The IgE differences between the active treatment and placebo groups were statistically significant for all antigens (interaction p: IgE-EW=0.0001, IgE-OVA=0.005, IgE-OVM=0.0004) with significant interactions indicating differences between groups were not constant over time.
FIGURE 2.
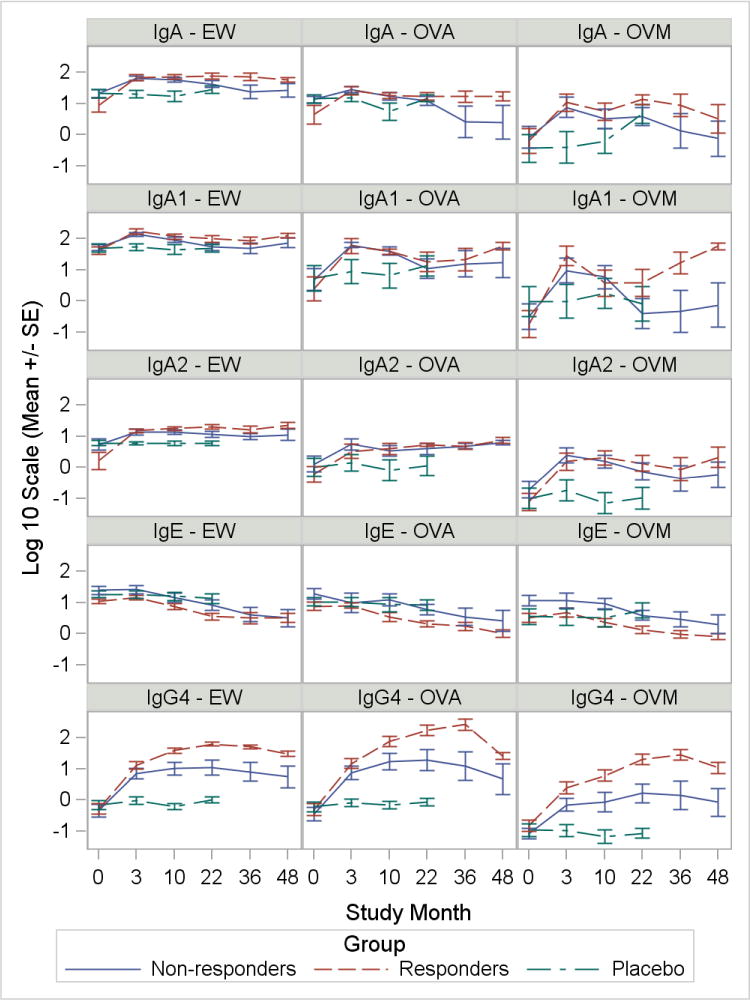
Immunoglobulin trends for antigen-specific IgE, IgG4, IgA, IgA1, and IgA2 among groups. Responder status defined as passing a DBPCFC after at least 4 weeks off therapy at 22, 36 or 48 months. Graphs show logarithmic mean and SEM for each immunoglobulin measurement. Horizontal axis depicts time in months during the clinical trial.
In contrast, IgG4 levels increased in subjects receiving active treatment in comparison to the placebo group (interaction p<0.0001 for all antigens). These increases were most pronounced among responders. IgG4 levels for all antigens rose sharply at 3 months and levels continued to increase until approximately 36 months. Parallel to IgG4, increases in IgA (interaction p: IgA-EW=0.004, IgA-OVA=0.031, IgA-OVM=0.008), IgA1 (interaction p: IgA1-EW=0.005, IgA1-OVA=0.010, IgA1-OVM=0.013) and IgA2 (interaction p: IgA2-EW=0.047, IgA2-OVA=0.023, IgA2-OVM=0.001) were observed in subjects receiving OIT. These increases were not observed in subjects on placebo. Responders demonstrated sustained increases in IgA, IgA1, and IgA2 to all antigens. Interestingly, IgA1-OVM at 36 and 48 months showed a large degree of separation between responders and non-responders. Although increases in IgA and its subtypes mirrored increases in IgG4, differences between responders and non-responders were not as pronounced.
IgG4, IgA and IgA2 differ between responders and non-responders during OIT
We noted a large degree of variation in the baseline immunoglobulin measurements of subjects (Table 1). We considered endpoint-to-baseline ratios of log-transformed immunoglobulin measurements for each time point. This allowed us to compare relative increases for each of the serologic parameters (Figure 3). Repeated measurement analysis of IgG4, IgA, and IgA2 to EW revealed significant differences between responders and non-responders. Greater increases were observed among responders for IgG4-EW (interaction p= 0.024), IgA-EW (interaction p=0.024), IgA2-EW (main effect p=0.029) and IgG4-OVM (interaction p = 0.025). Combining endpoint to baseline ratios for IgG4-EW and IgA-EW had an additive effect in discriminating responders and non-responders (IgG4-EW+IgA-EW, interaction p < 0.001). Statistically significant interactions indicate that the difference between responders and non-responders is not constant over time.
FIGURE 3.
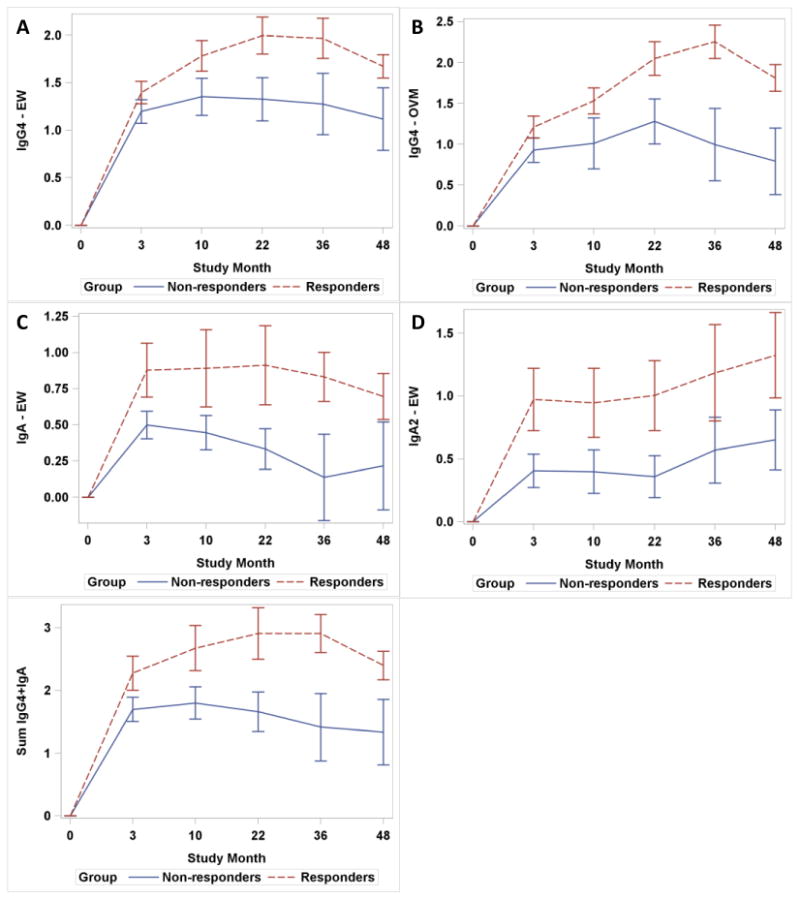
Trends in endpoint-to-baseline log ratios between responders and non-responders. Graphs show logarithmic mean and SEM of endpoint-to-baseline ratios for serologic parameters with significant differences between responders and non-responders. Relative increases in the IgG4-EW (A), IgG4-OVM (B), IgA-EW (C), IgA2-EW (D), and the sum of IgG4-EW and IgA-EW (E) are depicted.
Ratios of IgG4, IgA and IgA2 to IgE distinguish responders and non-responders
To further investigate differences between responders and non-responders receiving active treatment, we performed repeated measurement analysis of ratios of IgG4, IgA, IgA1, and IgA2 to IgE for EW, OVA and OVM (Figure 4). All ratios were increased among responders (main effect p < 0.05). Significant time by responder group interactions were noted for the following ratios: IgG4:IgE-EW (interaction p = 0.004), IgA:IgE-EW (interaction p = 0.009), IgA2:IgE-EW (interaction p = 0.028), IgA:IgE-OVA (interaction p = 0.008), with significant interactions indicating differences between groups were not constant over time. To assess the sensitivity of the results, samples for subjects who had been off treatment for greater than 6 months were removed (n=15) and the models were refit. IgG4 and IgA1 results were consistent with the full analysis while IgA and IgA2 ratios were typically not significant at the 0.05 level after removal of the samples. It is unclear, however, whether the change in significance was due an exposure effect or the loss of samples from the analysis.
FIGURE 4.
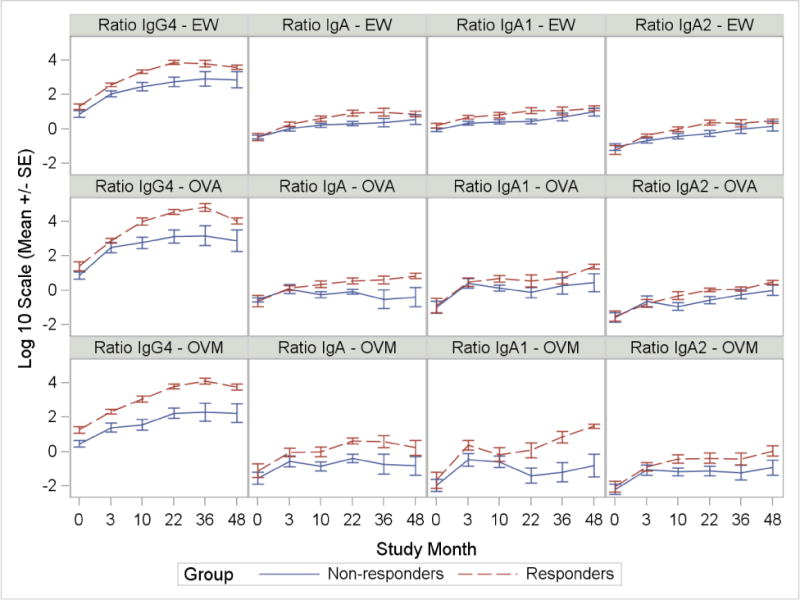
Log ratios of IgA, IgA1, IgA2 and IgG4 to IgE for EW, OVA and OVM in responders versus non-responders. Graphs show logarithmic mean and SE for ratios with significant differences between responders and non-responders. Ratios of IgG4:IgE, IgA:IgE, IgA2:IgE to EW and IgA:IgE to OVA have significant time by responder group interactions.
Discussion
Over the last decade, researchers have performed clinical trials investigating the safety and efficacy of OIT as a treatment modality to desensitize children allergic to common food allergens (18). Once desensitized, an allergic individual can ingest relatively large quantities of the allergenic food if regular maintenance dosing is continued (11, 19, 20); notwithstanding, the permanence of immunologic changes induced by OIT remains in question (21, 22). While OIT is a promising proactive therapeutic approach for food allergies, it is critical to define markers to distinguish subjects who have developed SU from those only transiently desensitized. Our findings suggest that subjects who develop SU have lower IgE-OVM levels, and increased IgG4:IgE-OVM ratios at baseline During OIT, they show greater relative increases in IgG4, IgA and IgA2 during OIT and have increased ratios of IgG4, IgA and IgA2 to IgE. These parameters may distinguish individuals who respond favorably to OIT and provide unique insights into its immunoglobulin-mediated mechanisms.
The immunologic changes induced by OIT have been described in several studies (16, 23-27) and generally include: (1) an initial rise in specific-IgE followed by a decrease below baseline levels (26); (2) a decrease in skin prick test wheal diameter; (3) increases in antigen-specific IgG4; (4) decreased ex vivo basophil activation upon antigenic stimulation; (5) alterations in T cell responses such as increased regulatory T cells and decreased Th2 cytokine production. Recently, Gorelik et al (27) reported that baseline basophil CD63 expression, histamine release and IL-4 production significantly correlate with SU in subjects undergoing OIT. While these changes are informative and provide some insight into the mechanisms of OIT, specific biomarkers associated with SU have not been rigorously examined in large, controlled OIT trials. We previously assessed egg-specific IgE and IgG4 and found that, at 10 months, SU correlated with egg-specific IgG4 levels but not egg-specific IgE (11). In the present study, we hypothesized that component-resolved analysis of IgE, IgG4, IgA, IgA1, and IgA2 would identify possible predictors of SU at baseline or changes in these serologic markers during the course of eOIT indicative of SU.
The increase in SU from 28% at 22 months to 50% at 48 months suggests that longer duration of treatment is valuable for some patients. In our study, we found that lower baseline log IgE-EW and log IgE-OVM were associated with SU at Year 4. No significant differences were found between groups for IgG4, IgA, IgA1, or IgA2 at baseline. Vickery et al. (22) reported similarly that IgE levels to peanut and the major allergens, Ara h 1 and Ara h 2, were lower at baseline in a subset of subjects that developed SU after peanut OIT. Longitudinally, responders tended to have lower IgE values to EW, OVA and OVM in comparison to non-responders (Figure 2), although statistically significant differences were not observed.
Despite reaching statistical significance (p< 0.05), we did not derive predictive thresholds for baseline IgE-EW or IgE-OVM as the large variability of baseline values and low AUCs (IgE-EW= 0.69 and IgE-OVM= 0.74) from the logistic regression model indicated limited predictive value. Importantly, some subjects with low IgE did not develop SU at 48 months (Figure 1). Utilizing epitope arrays with peptides from ovomucoid to determine levels of IgE binding to various peptides may be a more definitive approach. Bioinformatics analysis of epitope recognition patterns has successfully distinguished subjects who are sensitized, but tolerant, from those who are peanut-allergic (28).
According to our results, component testing may enhance our predictive ability to identify OIT responders. For example, IgE-OVM was a slightly better predictor of SU than IgE-EW at baseline and was significantly elevated in responders when compared to non-responders (Figure 1). Moreover, the IgG4:IgE-OVM ratio was significantly higher among responders; whereas, this was not true for EW or OVA. Nonetheless, we generally observed that immunoglobulin levels to EW and its component proteins (OVA, OVM) correlated strongly at baseline (Table 2) and similar trends during eOIT were observed across antigens for IgE, IgG4, IgA, IgA and IgA2 (Figure 2). Recent studies of both egg (29) and peanut (22) OIT suggest that baseline IgE to whole food protein are better predictors of response to OIT.
We corroborate previous findings that antigen-specific IgG4 increases in subjects receiving OIT in comparison to placebo. IgG4 has functional capacity to inhibit IgE-mediated immune function. For example, peanut-specific IgG inhibits antigen-IgE-FcεRII complex formation in a facilitated antigen binding (FAB) inhibition assay (22, 23). More recently, IgG and IgG4 in plasma from subjects on OIT have been shown to inhibit IgE-mediated basophil activation (30). Despite this, reports from previous OIT trials have not found correlations of IgG4 levels with clinical outcomes of SU (11, 22, 26). Given wide variation in subjects' immunoglobulin responses to OIT, we demonstrate that relative, rather than absolute, increases in IgG4 distinguish responders from non-responders. These increases may reflect changes in T cell responses driving IgG4 production, such as increased IL-10 secretion, possibly from regulatory T cells (24). IL-10-secreting B cells (regulatory B cells) may also play a role (31). Future studies should compare the inhibitory function of IgG4 in the plasma of responders and non-responders, providing an opportunity to correlate the functional effects of IgG4 on effector cell activity with clinical outcomes.
As the most abundant immunoglobulin in the human body, IgA is well known for its role in mucosal immunity and is particularly important for antigen exclusion in the gastrointestinal tract (32). Increases in serum IgA2-EW have been associated with development of natural tolerance in egg allergy (33) and antigen-specific serum IgA has been shown to increase in SCIT with aeroallergens (17). We found that similar to SCIT, EW-, OVA- and OVM-specific IgA, IgA1 and IgA2 increased with OIT compared to placebo. Interestingly, we noted significant differences between responders and non-responders in the relative increases of IgA-EW and IgA2-EW, but not IgA1-EW (Figure 3). The fact that relative increases in IgA1 were not significantly associated with responder status is intriguing and may be because serum IgA2 better correlates with IgA production at mucosal surfaces. Pilette et al. (17) demonstrated that antigen-specific IgA2 levels increased approximately 5-fold during aeroallergen SCIT whereas specific-IgA1 increased to a far lesser extent. This may be due to preferential changes in class switch recombination in IgE-producing B cells given the IgA2 (α2) H chain gene, in contrast to IgA1 (α1), is situated downstream of the IgE (ε) gene. Indeed, IgA2 levels in serum correlate with increases in TGF-beta from nasal biopsies (17, 21), indicating that systemic measurement of IgA2 may reflect changes in local compartments. Mucosal, antigen-specific IgA concentrations correlate with clinical outcomes, as increases in salivary IgA are greater in peanut allergic subjects achieving desensitization after 12 months of peanut SLIT (16).
While serum IgA2 may more accurately reflect local IgA concentrations, serum IgA itself may play an important role in preventing food-induced reactions. In mouse models of egg allergy, serum IgA, not secretory IgA in the gut lumen, is essential in protecting mice from anaphylaxis after oral administration of egg (34). We found in the majority of responders that antigen-specific serum IgA, IgA1, and IgA2 production was elevated and sustained throughout 48 months. In contrast, the non-responders had initial increases, which tended to decrease by 48 months. Examining longitudinal trends, the absolute levels of IgA1-OVM remained elevated at 48 month in responders, but had returned to near-baseline levels in the non-responders by 36 months. A recent study by Dodev et al. (35) showed that antibodies of different classes with the same specificity, including IgA, inhibit allergen-dependent IgE activity. Consequently, we hypothesize sustained, elevated levels of IgA-EW, IgA1-OVM, and IgA2-EW may prevent reactions during OFC's. Future studies should examine antigen-specific IgA levels in stool and saliva of subjects undergoing OIT to evaluate whether the systemic IgA increases we measured in plasma are correlated with local mucosal levels. Investigation of antigen-specific IgA secreting B-cell populations may also distinguish responders from non-responders as our data suggest that EW-specific, IgA-secreting B cells may more readily undergo apoptosis.
In summary, we report for the first time that egg- and component-specific IgA, IgA1 and IgA2 levels in plasma increase during eOIT and that relative increases in IgG4, IgA and IgA2 to EW and OVM are greater in responders than in non-responders. Ratios of IgG4, IgA and IgA2 to IgE may be useful in determining clinical response to egg OIT. Together these findings suggest that these IgG4 and IgA may be functionally protective after OIT is stopped. Ultimately, detailed mechanistic studies that link together T cell phenotypes, immunoglobulin levels, and effector cell functions will be key to advancing the field of food allergy immunotherapy.
Supplementary Material
Acknowledgments
Joseph E. Jones, Thermo Fisher Scientific
Footnotes
Author contributions: A.W.B., B.P.V., S.M.J., R.A.W., S.H.S., R.W.L., D.Y.M., H.A.S. collected the clinical specimens. M.K., B.L.W., B.P.V and A.W.B designed the study. M.K, B.L.W. and K.A.O. developed the ELISA for measurement of IgA. B.L.W., K.O. and M.K. performed the experiments. B.L.W., M.K., P.D., A.K.H and D.S. analyzed the data. All of the authors interpreted the data. B.L.W. and M.K. drafted the manuscript. All of the authors have critically revised and approved the final version of the manuscript.
References
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–76. doi: 10.1016/j.jaci.2011.01.039. e1-2. [DOI] [PubMed] [Google Scholar]
- 3.Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69(8):992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 4.Xepapadaki P, Fiocchi A, Grabenhenrich L, Roberts G, Grimshaw KE, Fiandor A, et al. Incidence and natural history of hen's egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy. 2016;71(3):350–7. doi: 10.1111/all.12801. [DOI] [PubMed] [Google Scholar]
- 5.Alduraywish SA, Lodge CJ, Vicendese D, Lowe AJ, Erbas B, Matheson MC, et al. Sensitization to milk, egg and peanut from birth to 18 years: A longitudinal study of a cohort at risk of allergic disease. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016;27(1):83–91. doi: 10.1111/pai.12480. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133(2):492–9. doi: 10.1016/j.jaci.2013.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120(6):1413–7. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Soderstrom L, Lilja G, Borres MP, Nilsson C. An explorative study of low levels of allergen-specific IgE and clinical allergy symptoms during early childhood. Allergy. 2011;66(8):1058–64. doi: 10.1111/j.1398-9995.2011.02578.x. [DOI] [PubMed] [Google Scholar]
- 9.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131(1):135–43. doi: 10.1016/j.jaci.2012.09.015. e1-12. [DOI] [PubMed] [Google Scholar]
- 10.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126(4):798–806. doi: 10.1016/j.jaci.2010.07.026. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367(3):233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SM, Burks AW, Wood RA, Fleischer DM, Sicherer SH, Henning AK, et al. Long-Lasting Egg Consumption in Egg Allergic Children Treated with Oral Immunotherapy (OIT): Follow-up from the Consortium of Food Allergy Research (CoFAR) Study J Allergy Clin Immunol. 2014;133(2):AB403. [Google Scholar]
- 13.Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994;93(6):1047–59. doi: 10.1016/s0091-6749(94)70054-0. [DOI] [PubMed] [Google Scholar]
- 14.Benhamou Senouf AH, Borres MP, Eigenmann PA. Native and denatured egg white protein IgE tests discriminate hen's egg allergic from egg-tolerant children. Pediatr Allergy Immunol. 2015;26(1):12–7. doi: 10.1111/pai.12317. [DOI] [PubMed] [Google Scholar]
- 15.Caubet JC, Bencharitiwong R, Moshier E, Godbold JH, Sampson HA, Nowak-Wegrzyn A. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J Allergy Clin Immunol. 2012;129(3):739–47. doi: 10.1016/j.jaci.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 16.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129(4):1159–62. doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007;178(7):4658–66. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 18.Kulis M, Wright BL, Jones SM, Burks AW. Diagnosis, management, and investigational therapies for food allergies. Gastroenterology. 2015;148(6):1132–42. doi: 10.1053/j.gastro.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008;122(6):1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62(11):1261–9. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 22.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–75. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. doi: 10.1016/j.jaci.2009.05.022. 300 e1-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133(2):500–10. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, et al. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy. 2012;42(8):1197–205. doi: 10.1111/j.1365-2222.2012.04028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131(1):128–34. doi: 10.1016/j.jaci.2012.10.048. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelik M, Narisety SD, Guerrerio AL, Chichester KL, Keet CA, Bieneman AP, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2015;135(5):1283–92. doi: 10.1016/j.jaci.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Bruni FM, Fu Z, Maloney J, Bardina L, Boner AL, et al. A bioinformatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129(5):1321–1328. doi: 10.1016/j.jaci.2012.02.012. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escudero C, Rodriguez Del Rio P, Sanchez-Garcia S, Perez-Rangel I, Perez-Farinos N, Garcia-Fernandez C, et al. Early sustained unresponsiveness after short-course egg oral immunotherapy: a randomized controlled study in egg-allergic children. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2015;45(12):1833–43. doi: 10.1111/cea.12604. [DOI] [PubMed] [Google Scholar]
- 30.Santos AF, James LK, Bahnson HT, Shamji MH, Couto-Francisco NC, Islam S, et al. IgG inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204–12. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev. 2005;206:64–82. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinou GN, Nowak-Wegrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA. Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: is there a role in tolerance induction? Pediatr Allergy Immunol. 2014;25(1):64–70. doi: 10.1111/pai.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, Finkelman FD. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol. 2011;127(4):982–9. doi: 10.1016/j.jaci.2011.01.034. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodev TS, Bowen H, Shamji MH, Bax HJ, Beavil AJ, McDonnell JM, et al. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015 doi: 10.1111/all.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


