Abstract
Background
Delirium is a common, morbid, and costly postoperative complication.. We aimed to identify blood-based postoperative delirium markers in a nested case control study of older surgical patients using a proteomics approach followed by enzyme-linked immunosorbent assay (ELISA) validation.
Methods and Materials
The Successful Aging after Elective Surgery Study enrolled dementia-free adults age ≥70 undergoing major scheduled non-cardiac surgery (N=566; 24% delirium). Plasma was collected at 4 timepoints: preoperatively (PREOP), post-anesthesia care unit (PACU), postoperative day 2 (POD2) and 1 month follow-up (PO1MO). Matched pairs were selected for the independent discovery (39 pairs) and replication cohorts (36 pairs), which were subsequently combined into the pooled cohort (75 pairs). iTRAQ-based relative quantitation mass spectrometry proteomics was performed to identify the strongest delirium-related protein, which was selected for ELISA validation. Using the ELISA results, statistical analyses using non-parametric signed-rank tests were performed in all cohorts examining the association between the identified protein and delirium.
Results
C-reactive protein (CRP) emerged from the proteomics analysis as the strongest delirium-related protein. ELISA validation confirmed that compared to controls, cases had significantly higher CRP levels (*p<.05, **p<.01) in the discovery, replication, and pooled cohorts at PREOP (median paired difference [mg/L] 1.97*, 0.29, 1.56**, respectively), PACU (2.83, 2.22*, 2.53**, respectively) and POD2 (71.97**, 35.18*, 63.76**, respectively), but not PO1MO (2.72, −0.66, 1.10, respectively).
Discussion
Elevated pre- and postoperative plasma levels of CRP were associated with delirium, suggesting that a pre-inflammatory state and heightened inflammatory response to surgery are potential pathophysiological mechanisms of delirium.
Keywords: Delirium, Postoperative, C-reactive protein, Proteomics, Inflammation, Case-control study
INTRODUCTION
Delirium affects 15–53% of older surgical patients (1–3), and has been associated with several poor outcomes, including longer hospital stay, greater postoperative complications, and higher rates of discharge to nursing homes (4–6). Delirium accounts for >$7 billion of Medicare hospital expenditures, with an annual estimated healthcare cost attributable to delirium ranging upwards of $164 billion (2). Despite our increasing understanding of the epidemiology and health costs of delirium, it remains a wholly clinical diagnosis with no established biomarkers to guide its diagnosis or management.
Several biological models of delirium have been proposed. These include: neuro-inflammation, neuro-aging, neuro-endocrine stress, neurotransmitter dysregulation, oxidative stress, sleep/wake dysregulation and network disconnectivity (7). The degree to which any or all of these pathophysiological mechanisms contribute to the occurrence of delirium among older postoperative patients remains unclear. The identification of a marker associated with delirium would advance our understanding of the pathophysiology of delirium and ultimately lead to new interventions that could improve patient outcomes.
Our current knowledge of the pathophysiology of delirium is largely based on cross-sectional studies, or small longitudinal studies examining a limited number of cerebrospinal fluid (CSF) and blood-based measures. To expand this line of investigation, we applied a global proteomics approach to identify the top candidate protein using plasma obtained at four serial timepoints from older patients undergoing major non-cardiac surgery. Our proteomics approach allowed for the examination of associations between multiple proteins and delirium longitudinally using a nested, matched, case-control design employed to increase statistical efficiency and address several confounders. We also used 2 independent cohorts to evaluate the reproducibility of our findings.
METHODS AND MATERIALS
Study Population
The Successful Aging after Elective Surgery (SAGES) Study is a prospective observational study designed to understand novel risk factors and long-term outcomes of delirium (8,9). SAGES enrolled patients aged 70 and older who were scheduled for major non-cardiac surgery (N=566), including total hip or knee replacement, cervical or lumbar laminectomy, abdominal aortic aneurysm repair, lower extremity vascular bypass, or colectomy. Patients received either general or spinal anesthesia. Additional major inclusion and exclusion criteria were previously published (8). Of note, patients underwent a detailed screening process to exclude dementia based on patient or family report of dementia diagnosis, medical record review, capacity assessment, and cognitive testing using the Modified Mini-Mental State (10). Additionally, patients underwent a neurocognitive battery at baseline, which was used to compute the General Cognitive Performance (GCP) summary measure (11). Comorbidities were identified from medical record review by trained physician abstractors and scored based on the Charlson index.
Specimen Collection
All patients underwent phlebotomy at four timepoints: preoperative (PREOP), postanesthesia care unit (PACU), postoperative day 2 (POD2), and 1 month post operation (PO1MO). Blood collection was incorporated into clinical blood draws taken in the pre-admitting testing center (PREOP), in the PACU, and on the surgical wards (POD2). The PO1MO blood was obtained either at the 30-day postoperative follow-up visit or by the study team in the patient’s home. During phlebotomy, mechanical disruption was minimized to prevent platelet activation or hemolysis. Blood was stored on ice in heparinized tubes until processing. During processing, low speed centrifugation (1500 g for 15 minutes at 4°C) was used to separate plasma from cellular material, and plasma was stored at −80°C until analyzed. Nearly all samples (99%) satisfied quality control standards, including ≤4 hours between blood draw and processing.
Delirium and Subsyndromal Delirium
Postoperative delirium was determined from daily interviews during hospitalization and a validated chart review method (12). Trained study staff conducted structured mental status examinations that tested attention, orientation, and memory. Delirium was assessed using the Confusion Assessment Method (CAM) diagnostic algorithm, which required the patient to have an acute onset of change or fluctuating mental status, inattention, and either disorganized thinking or altered level of consciousness (13). Presence of delirium by chart review was adjudicated by a minimum of two delirium experts, and discordance was resolved through consensus. Patients were considered delirious if delirium was present on either the CAM or the chart review method on any postoperative day; otherwise, patients were considered nondelirious (12).
If delirium was absent throughout the entire hospitalization, subsyndromal delirium was defined as: (1) an acute change in mental status or fluctuation, (2) ≥ 1 CAM core features (inattention, disorganized thinking, altered level of consciousness), and (3) ≥ 1 other CAM supporting features (disorientation, perceptual disturbance, delusion, psychomotor agitation, psychomotor retardation or inappropriate behavior).
Cohorts for Matching: Discovery, Replication, and Pooled Cohorts
Two cohorts from the overall sample of 566 patients were used to investigate the association between proteins and delirium. A discovery cohort of 39 matched pairs of delirium cases and no-delirium controls was identified from the first 272 participants. A replication cohort was generated from the remaining SAGES samples to identify 36 matched pairs of cases and controls (see (14) and Supplemental Figure S1 for additional details on the cohorts). We conducted a post hoc analysis using a pooled cohort that combined the discovery and replication cohorts (75 matched pairs).
Matching Variables
Cases and controls were matched on six variables that may influence the relationship between CRP and postoperative delirium: age within five years, baseline GCP within five points, and an exact match for sex, surgery type, presence of vascular comorbidity, and Apolipoprotein E (ApoE) ε4 carrier status. Vascular comorbidity was determined by the presence of any of the following Charlson diagnoses related to vascular disease: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, hemiplegia, diabetes, or diabetes with end organ damage. For ApoE genotyping, DNA was extracted from cellular material collected at PREOP and analyzed at the Partners Center for Personalized Medicine (Boston, MA). Since ApoE ε4 has been associated with Alzheimer’s Disease (15), we classified individuals with at least one ε4 allele as ApoE ε4 carriers.
Matching Procedure
Delirium cases were defined as participants with: (1) delirium on POD2, or (2) delirium on POD1 and subsyndromal delirium on POD2 or POD3. This definition was selected to ensure that blood collected on POD2 corresponded to the presence of delirium in all cases. Controls were defined as patients with no delirium and no subsyndromal delirium on any POD to ensure that controls did not include patients with some symptoms of delirium during study participation. Patients who did not fit the criteria for delirium cases or controls were ineligible for the matching procedure. For example, if the patient was only delirious on POD3, but not on any other day. This was done to ensure the POD2 blood was reflective of the delirious state. Also, anyone with subsyndromal delirium (without delirium) was ineligible for the match, since we aimed to strengthen the contrast between cases with delirium and controls without delirium and felt subsyndromal delirium would likely be an intermediate state. Additional criteria for inclusion in the matching procedure, which used the Optimal Matching Algorithm (16), required: sufficient blood sample volume and absence of moderate to severe hemolysis (see (14) and Supplemental Information for details).
Proteomics Analysis and Enzyme-Linked Immunosorbent Assay (ELISA) Validation
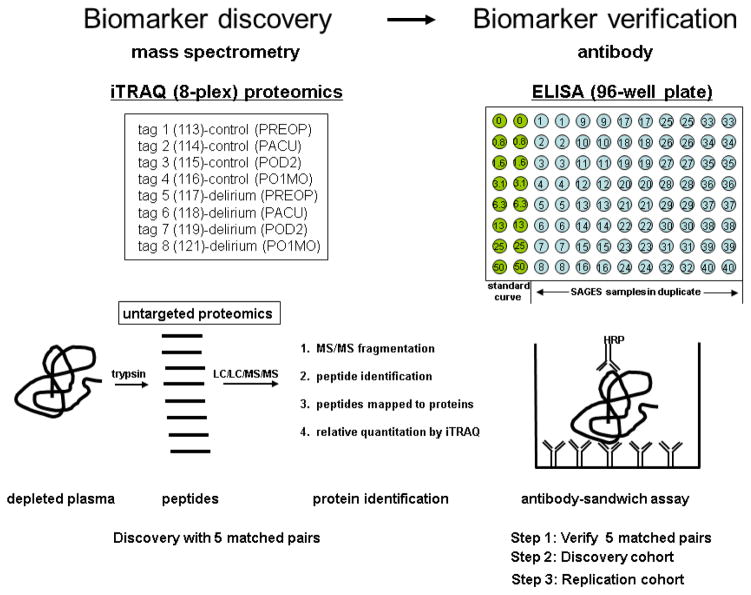
The initial proteomics phase used an iTRAQ-mass spectrometry assay to examine five sets of matched case-control plasma samples across the four timepoints in the discovery cohort (Figure 1). Relative quantitation proteomics was performed using the iTRAQ isobaric tagging system (17) following the manufacturer’s recommended protocol (AB-Sciex, Framingham, MA) (18). Using the 8-plex iTRAQ labels, each experiment examined one delirium case-control matched pair. The Protein Pilot 4.5b database search software calculated a relative fold change ratio of identified proteins across all eight iTRAQ labeled samples. The database identified approximately 100 proteins for each complete run of a matched case-control pair. Relative fold changes of each case to its matched control at each time point were calculated.
Figure 1. Protein identification and ELISA validation strategy: From iTRAQ mass spectrometry to antibody-based confirmation.
The plasma proteomics phase was performed using the 8-plex iTRAQ isobaric tagging system. Each matched pair (delirium case and no-delirium control) contained four timepoints (PREOP, PACU, POD2 and PO1MO) with one iTRAQ tag added per sample. In the biomarker validation stage, an antibody-based ELISA assay was employed. A typical 96-well plate set-up used in the CRP ELISA assay is depicted; forty samples in duplicate were assayed per plate and a standard curve was included on every plate. The diagram of a typical antibody-sandwich assay per well is depicted. Two different antibodies specific for the target protein must bind. PREOP= preoperative, PACU= postanesthesia care unit, POD2=postoperative day 2, PO1MO=1 month postoperation, ELISA=enzyme-linked immunosorbant assay
After analyzing five matched pairs using the iTRAQ system, we selected one protein that met the following two criteria for ELISA validation: 1) showed the strongest and most consistent differences at POD2, the timepoint when delirium was present, and 2) had differences at 1–2 additional timepoints. Once this protein for validation was identified, the protein levels were first confirmed in the initial five matched pairs using ELISA. If confirmed, the identified protein concentration was then measured by ELISA within the remaining discovery and replication cohorts (See Supplemental Information for additional information on the proteomics methodology and the ELISA assays.)
Statistical analysis
Since the protein concentration estimates and the differences between each of the matched pairs were non-normally distributed, all analyses were based on the median and statistical analyses were conducted using nonparametric tests. The univariate nonparametric signed-rank test was used to determine the median of the paired differences (MPD) at each timepoint. The signed-rank test ranks the absolute value of the paired difference, and the rank was multiplied by +1 if the difference was positive (case-control), -1 if negative, and 0 if there was zero difference. Summation of the signed ranks divided by the standard error approximates the z-statistic from which a standard normal distribution was used to calculate the p-value.
To address the clinical relevance of our findings, we used conditional logistic regression models to determine the predictive power estimates of CRP as a biomarker for delirium using c-statistics for each of the four timepoints.
Sensitivity Analysis
We conducted sensitivity analyses to determine potential confounding by additional factors that may influence the association between the identified protein and postoperative delirium (See Supplemental Information for detailed methods and results).
The SAS/STAT software version 9.3 was used for all analyses (SAS Institute, Cary, NC). Informed consent for study participation was obtained from all subjects according to procedures approved by the institutional review boards of Beth Israel Deaconess Medical Center and Brigham and Woman’s Hospital, the two surgical sites, and Hebrew SeniorLife, the study coordinating center, all located in Boston, MA.
RESULTS
Table 1 shows the sample characteristics of the matching variables stratified by cases and controls in the discovery, replication, and pooled cohorts. No significant differences between cases and controls were observed for any of the six matched variables, suggesting a successful matching procedure in all cohorts. Relative to the discovery cohort, cases and controls from the replication cohort did, however, have a greater prevalence of ApoE ε4 carriers and vascular comorbidity, and lower mean GCP.
Table 1.
Patient characteristics in the two matched cohorts (discovery and replication) and the pooled cohort
| Variable | Discovery (39 pairs) | Replication (36 pairs) | Pooled (75 pairs) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Delirium (n=39) | No Delirium (n=39) | Delirium (n=36) | No Delirium (n=36) | Delirium (n=75) | No Delirium (n=75) | |
|
| ||||||
| Age (M, SD) | 77.3 (5.0) | 76.8 (4.7) | 78.0 (4.4) | 77.6 (4.2) | 77.6(4.7) | 77.2(4.5) |
| Female (%) | 54 | 54 | 58 | 58 | 56 | 56 |
| GCP (M, SD) | 55.2 (5.6) | 56.4 (5.6) | 53.7 (5.0) | 54.6 (5.1) | 54.5(5.3) | 55.5(5.2) |
| Type of surgery (%) | ||||||
| Orthopedic | 92 | 92 | 83 | 83 | 88 | 88 |
| Vascular | 5 | 5 | 6 | 6 | 5 | 5 |
| Gastrointestinal | 3 | 3 | 11 | 11 | 7 | 7 |
| Vascular comorbidity (%) | 38 | 38 | 50 | 50 | 44 | 44 |
| ApoE ε4 carrier (%) | 13 | 13 | 28 | 28 | 20 | 20 |
GCP=general cognitive performance, a composite measure of neuropsychological measures reflecting cognitive domains vulnerable to delirium (11).
ApoE=Apolipoprotein E, a gene for which the presence of an ApoE ε4 allele (i.e., ApoE ε4 carrier) has been associated with increased risk of Alzheimer’s Disease (15).
Vascular comorbidity: present if patient had a myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, hemiplegia, diabetes, and diabetes with end organ damage.
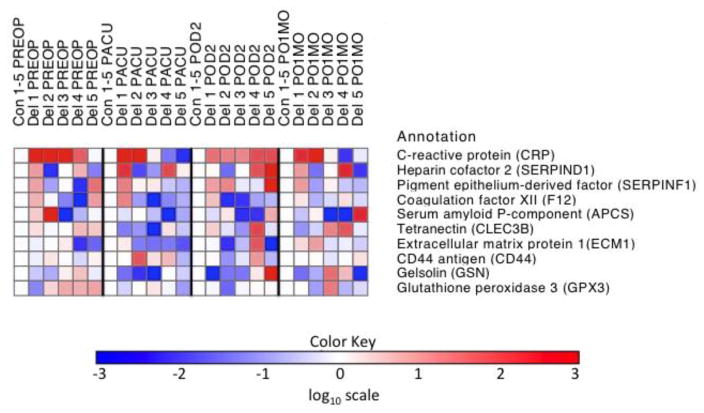
Expression levels of ten proteins identified across five matched pair sets at 4 timepoints are illustrated in Figure 2. Among all markers, C-reactive protein (CRP) was consistently identified in all matched pairs, with delirium cases yielding higher CRP levels than their matched controls at PREOP and POD2. Since CRP met our criteria for further ELISA validation (i.e., consistently elevated in delirium cases at POD2 and previous evidence for an association between CRP and delirium exists) (19,20), plasma CRP changes between cases and controls were then confirmed by ELISA (CRP Quantikine ELISA kit [catalog # DCRP00] from R&D Systems [Minneapolis, MN]). In the same sample of five matched pairs, the calculated fold-changes for CRP ELISA levels (Supplemental Table S2) closely mirror the pattern observed for CRP in the iTRAQ-MS assay at all four time points (Supplemental Table S1).
Figure 2. Heat map of iTRAQ relative quantitation for ten proteins in five matched case-control samples across four timepoints (PREOP, PACU, POD2 and PO1MO).
C-reactive protein and 9 other representative proteins commonly identified in the iTRAQ-mass spectrometry protein identification phase in the five matched case-control samples are presented. Red (and lighter shades) indicates that the protein increases in delirium cases versus matched no-delirium controls. Blue (and lighter shades) correspond to the protein decreasing in delirium samples. A white square means there was no significant detectable change in the protein levels in the case versus control. CRP is identified as a protein that increases in delirium samples at PREOP (4/5 matched pairs) and POD2 (5/5 matched pairs). The remaining nine proteins presented did not demonstrate consistent change at any timepoints. The log base 10 was used to generate the color key scale. CRP is the only protein in that demonstrates a specific and consistent directional change in relation to delirium. It is increased in its level in the delirious cases relative to matched controls, depicted by a red color. Several of the other proteins show differences between cases and controls, but they are a mixture of red and blue, so they go up or down in relation to their control condition and show no consistent pattern. PREOP= preoperative, PACU= postanesthesia care unit, POD2=postoperative day 2, PO1MO=1 month postoperation
Table 2 reports the MPD in plasma CRP levels between cases and controls measured using ELISA in the discovery, replication, and pooled cohorts for all 4 timepoints. In the discovery cohort, median CRP levels were significantly higher in cases relative to controls at PREOP (MPD=1.97 mg/l, p=0.02) and POD2 (MPD=71.97, p<.01), with results approaching significance at PACU (MPD=2.83, p=0.06) and PO1MO (MPD=2.72, p=0.06). In the replication cohort, cases had significantly higher CRP levels than controls at PACU (MPD=2.22, p=0.01) and POD2 (MPD=35.18, p=0.04).
Table 2.
Time-specific median of paired differences (MPD) of ELISA C-reactive protein concentrations between delirium cases and no-delirium controls at four timepoints in the discovery, replication, and pooled cohorts
| Time of Blood Draw | Discovery (39 pairs) | Replication (36 pairs) | Pooled(75 pairs) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| MPD (mg/L) | IQ rangea | P-value | MPD (mg/L) | IQ rangea | P-value | MPD (mg/L) | IQ rangea | P-value | |
| PREOP | 1.97 | (−1.02, 7.75) | 0.02 | 0.29 | (−1.68, 9.59) | 0.13 | 0.56 | (−1.61, 7.89) | <0.01 |
| PACU | 2.83 | (−2.29, 10.68) | 0.06 | 2.22 | (−0.91, 7.68) | 0.01 | 2.53 | (−1.57, 10.33) | <0.01 |
| POD2 | 71.97 | (5.05, 139.82) | <0.01 | 35.18 | (−30.42, 88.90) | 0.04 | 63.76 | (−22.29, 126.17) | <0.01 |
| PO1MO | 2.72 | (−1.85, 7.16) | 0.06 | −0.66 | (−3.83, 2.49) | 0.63 | 1.1 | (−3.17, 5.45) | 0.18 |
MPD=Median of paired differences (delirium case minus no-delirium control), ELISA=enzyme-linked immunosorbent assay, IQ=interquartile, PREOP= preoperative, PACU= postanesthesia care unit, POD2=postoperative day 2, PO1MO=1 month postoperation
p-values were obtained from nonparametric signed-tank test. Bold indicates significant at p<.05 level
IQ range: Not to be confused with a 95% confidence interval. A negative value for the lower bound number does not indicate a non-significant finding.
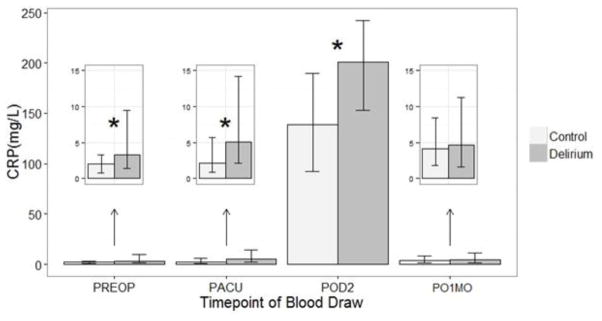
The pooled cohort yielded similar results to the separate analyses of the discovery and replication cohorts: median CRP levels were higher in cases relative to controls at PREOP (MPD=0.56, p<.01), PACU (MPD=2.53, p<.01), and POD2 (MPD=63.76, p<.01). Notably, due to improved statistical power of combining the 2 cohorts, more significant findings are seen in the pooled cohort. Figure 3 illustrates the median CRP concentrations by delirium status at the four timepoints in the pooled cohort (see Supplementary Figures S2A and S2B for graphs of CRP levels in the discovery and replication cohorts, respectively). With respect to the clinical relevance of our findings, the predictive power estimates (reported as c-statistics) of CRP as a biomarker for delirium were: PREOP 0.71, PACU 0.67, POD2 0.85, and PO1MO 0.68.
Figure 3. Bar graph of median C-reactive protein (CRP) concentrations by delirium status at four timepoints in the pooled cohort.
Based on signed-rank test. *p<.05. Vertical bars indicate 25th and 75th percentile values (in mg/L). PREOP= preoperative, PACU= postanesthesia care unit, POD2=postoperative day 2, PO1MO=1 month postoperation
Our sensitivity analysis indicated that the findings were robust after considering four additional potential confounders (preoperative connective tissue disease, anesthesia route, postoperative infectious complications, major postoperative complications, all obtained from medical record abstraction).
DISCUSSION
This study used a mass spectrometry-based proteomics approach followed by ELISA validation to identify and confirm proteins associated with postoperative delirium. We found evidence for the involvement of CRP in delirium: 1) strong and consistent associations of elevated CRP at POD2 in the discovery, replication, and pooled cohorts, and 2) elevations of CRP at PREOP (in the discovery and pooled cohorts) and PACU (in the replication and pooled cohorts). Taken together, these findings suggest the potential value of CRP as a peri-operative marker of delirium before surgery, immediately after surgery, and on POD2 at the peak prevalence of delirium.
Our study contributes important new information to the previous literature examining the relationship between CRP and delirium. Although the relationship between CRP and delirium has been reported as described below, none of these studies obtained true “pre-morbid” (or in the case of surgical patients, preoperative) levels of CRP since patients were enrolled after they were already ill. We are aware of only two other studies that examined the association between preoperative CRP levels and postoperative delirium (21,22). Both studies were conducted in small patient samples (68 and 65 patients, respectively) and neither observed an association between preoperative CRP and postoperative delirium.
Our observation of a significant association between preoperative CRP and postoperative delirium is particularly important in that it establishes CRP as a risk marker, though not necessarily for delirium. From a clinical standpoint, it means that CRP could be used to risk stratify patients before surgery, enabling proactive interventions. The moderately high predictive power of PREOP CRP provides further evidence for the importance of our novel finding of the association between preoperative CRP levels and postoperative delirium. From a pathophysiological standpoint, it suggests there are differences in the inflammatory activity of patients who develop delirium even prior to the surgical insult, a finding that will lead to new, important lines of investigation. Thus, our findings represent a major advance over our previous knowledge of an association between CRP levels measured concurrently with the presence of delirium.
In contrast with our preoperative CRP findings, which are novel, our findings at other timepoints after surgery align with previous studies reporting associations between elevated postoperative CRP levels and delirium. This was reported in small samples of postoperative patients (including hip and vascular surgery), patients admitted to the intensive care unit (ICU) and acutely admitted to medical wards, and stroke patients (23–31). In a study of patients age 65 and older undergoing hip surgery (emergency or planned), higher CRP levels 24 or 72 hours post-surgery were observed in patients with delirium relative to patients without delirium (25). Among patients in the ICU, CRP levels measured upon entry and levels 24 hours thereafter were associated with delirium incidence (29), with a similar relationship observed in mechanically ventilated critically ill patients (32). Importantly, these studies enrolled patients after they were already ill and therefore did not obtain a true “pre-morbid” baseline blood sample, or follow changes in CRP over time. Some studies, in contrast, have reported no association between CRP and delirium (33, 34). A study of 138 critically ill medical patients did not observe an association between CRP levels and delirium (35).
There are potential explanations for the disparate findings between our study and previous null associations. First is the relatively small sample size of cohort-based studies used in previous work. In contrast, our study used a nested, matched case-control design within a large cohort (N=566) that enhanced statistical efficiency. Second, many prior studies enrolled acutely ill patients, and/or did not focus on the older population (adults aged >18 years included). In comparison, our study screened relatively healthy older patients undergoing elective surgery and excluded patients with dementia. Our PREOP values were unique in representing a true baseline, before onset of any acute stressors, and elevated preoperative CRP in postoperative delirium has not previously been reported.
Circulating CRP, an acute-phase reactant, is one of the most commonly investigated biomarkers for inflammation and infection. While the etiology of the multifactorial delirium syndrome has not been well defined, the hypothesis of peripheral inflammatory processes predisposing and potentially playing a causative role in neuroinflammation and incidence of delirium has gained substantial traction over the last few years (36). Our proteomics approach followed by ELISA validation dovetails nicely with our previous work identifying elevated interleukin-6 (IL-6) at POD2 in delirious patients (14) and provides support for an inflammatory hypothesis of delirium. However, the current findings extend this work because we previously found IL-6 elevations only at POD2, while the present study found CRP elevations preoperatively. Our current findings suggest that a systemic pre-inflammatory state in at-risk patients predisposes patients to a heightened inflammatory response upon exposure to surgical stress. This notion is supported by the observation that inflammatory markers in blood increase during aging (37), thereby elevating the basal level of inflammation. This imbalance may ultimately result in alteration of the innate immune response in the central nervous system (CNS) and contribute to acute brain dysfunction.
Aside from CRP, other markers of inflammation have also been associated with delirium. CSF interleukin [IL]-1β and CSF:serum IL-1β ratio, for example, were higher in patients with incident delirium relative to patients without delirium (38). Similarly, other blood based markers of inflammation have been associated with delirium in critically ill patients (soluble tumor necrosis factor receptor (sTNFR)1 and sTNFR2 (39) and sTNFR1 (40), patients undergoing coronary-artery bypass graft surgery (IL-2 and TNF-α (41), and patients undergoing elective surgery (IL-6 (14)).
Our study had a number of notable strengths. First, we used a biorepository from the rigorously designed SAGES study, which incorporated state-of-the-art delirium measures and studied well-defined surgical cohorts enabling controls of many potential confounders, as evidenced in our matching procedures. Second, our incorporation of an ELISA validation based on the initial proteomics approach allowed for confirmation of initially identified proteins and speaks to the robustness of our findings. Third, the availability of multiple timepoints of blood draw, including PREOP, provided data which were largely absent from previous work given the nature of the study population (e.g., medical or ICU populations). Our study showed the longitudinal response of CRP to surgical stress that rose at PACU and peaked at POD2. This aligns with our knowledge of the time course of CRP, which typically increases two hours following the onset of inflammation and peaks after 48 hours, with a relatively short half-life of 19 hours (42). Interestingly, this peak in CRP at POD2 was in parallel with the presence of delirium. Fourth, our repeated analysis study design (discovery, replication, and pooled cohorts) provided protection against the detection of false positive markers. Lastly, our use of a nested case-control study within a large prospective cohort provides a strong and efficient study design to identify delirium-related proteins.
We note some study limitations. Our preliminary proteomics approach only allowed us to assess about 100 proteins consistently; many additional proteins exist that may be at or below the detectable range. However, this proteomics approach enabled us to examine multiple proteins at several timepoints in an unbiased fashion. One of the key benefits of this approach is that CRP emerged as the strongest delirium-associated protein from among many candidates. Additional, weaker protein correlates with delirium will be examined in future studies. Poljak and colleagues (43) employed a similar proteomics approach in examining cerebrospinal fluid (CSF) markers of delirium and found evidence that inflammation is a component of delirium in the proteins identified within two delirium cohorts. We acknowledge our use of only plasma measures and unavailability of CSF for studying proteins associated with delirium. It is also possible that some proteins may have degraded in storage, which would reduce our ability to detect them in this analysis. Since we did not collect information on liver enzyme levels, we were unable to explore the potential influence of liver function on the relationship between CRP and postoperative delirium, which may influence our findings since hepatic cells are the primary source of CRP.44 Lastly, the reported associations between CRP and delirium do not necessarily imply causation. Nonetheless, our findings have important implications particularly the newly reported association between preoperative CRP and postoperative delirium. This suggests that CRP may be a risk marker, though not necessarily specific for delirium. After further validation and refinement, CRP may be used clinically to risk stratify patients before surgery, enabling proactive and targeted intervention to prevent or ameliorate delirium.
In addition to these strengths and limitations, some methodological considerations warrant mention. First, our matched case-control design was selected to maximize efficiency or biomarker discovery for delirium. Unfortunately, such a design is not optimized to examine the relationship of CRP with any of the matching variables (ApoE, GCP), or variables related to the matching factors (neurocognitive tests). These will be examined in future studies that measure CRP across the entire cohort. The second consideration relates to the observed differences in the prevalence of vascular comorbidity and ApoE ε4 carrier status between the Discovery and Replication cohorts. These differences were not statistically significantly different between the two cohorts, thus they were likely due to chance and do not represent biases or flaws in our study design. Moreover, it is possible that (random) differences in prevalence of vascular comorbidity between the discovery and replication cohorts could explain the MPD differences in CRP levels between the delirium cases and controls. We explored this possibility by performing stratified analyses by vascular comorbidity status, and found that this did not alter our study findings. Therefore, we believe that the MPD differences are explained by other factors, including random variation. The third consideration relates to our use of heparinized plasma to conduct our iTRAQ and ELISA studies. We have noted that heparinized samples yield similar, and equally high quality results, as alternative anticoagulants (EDTA) for our proteomics analyses. Fourth, fasting was not required for all patients at all four time points of blood draw; however, PACU blood was by necessity fasting from the night prior to surgery and POD2 blood collected in the early morning and likely reflects fasting levels. Lastly, anesthetic agents were not addressed in our analysis; however, the surgical protocols are highly standardized at our two centers and therefore there is very little variation in anesthetic agent used from patient to patient for the same surgery type.
In summary, we found that in older patients undergoing major noncardiac surgery, CRP measured at several timepoints was associated with delirium. Compared to non-delirious patients, those with delirium had consistently significant elevations in CRP levels at POD2, with additional evidence for elevated levels at PREOP and PACU among delirious patients. Our data lend further support to a pathophysiological model of delirium in which heightened systemic inflammation and increased permeability of the blood brain barrier lead to neuroinflammation and potentially neuronal injury one proposed pathophysiological mechanism of delirium. If validated in future studies, preoperative CRP may identify patients at-risk for delirium, enabling proactive interventions, and postoperative CRP might provide a means of monitoring its course. From a pathophysiological standpoint, our findings suggest that differences exist in the inflammatory response of patients who develop postoperative delirium even prior to the surgical insult. This finding has the potential to lead to new studies to both better understand the role of inflammation in delirium and to design and target intervention strategies that modulate the inflammatory response to ameliorate this common, morbid, and costly syndrome.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by National Institute on Aging grants (T32AG023480, P01AG031720, K07AG041835, R01AG030618, K24AG035075) and the Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., Co-Trustee. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inouye SK. Delirium in older persons. N Engl J Med. 2008;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:2045. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120:807–813. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witlox J, Eurelings LS, de Jonhe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO, Jr, Fong TG, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13:818. doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt E, Saczynski J, Kosar C, Jones RN, Alsop DC, Fong TG, et al. The Successful Aging after Elective Surgery (SAGES) Study: Cohort description and data quality procedures. J Am Geriatr Soc. doi: 10.1111/jgs.13793. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 11.Jones RN, Rudolph JL, Inouye SK, Yang FM, Fong TG, Milberg WP, et al. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychology. 2010;32:1041–1049. doi: 10.1080/13803391003662728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saczynski JS, Kosar CM, Xu G, Puelle MR, Schmitt E, Jones RN, et al. A tale of two methods: Chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62:518–524. doi: 10.1111/jgs.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 14.Vasunilashorn SM, Ngo L, Inouye SK, Libermann TA, Jones RN, Alsop DC, et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J Gerontol A Biol Sci Medical Sci. 2015 doi: 10.1093/gerona/glv083. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Yeo SH, Park JM, Choi JY, Lee TH, Park SY, et al. Genetic markers for diagnosis and pathogenesis of Alzheimer's disease. Gene. 2014;545:185–193. doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR. Optimal matching for observational studies. J Am Stat Assoc. 1989;84:1024–1032. [Google Scholar]
- 17.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Afkarian M, Bhasin M, Dillon ST, Guerrero MC, Knowler WC, Thadhani R, Libermann TA. Optimizing a proteomics platform for urine biomarker discovery. Mol Cell Proteomics. 2010;9:2195–2204. doi: 10.1074/mcp.M110.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Pan L, Deng H, Ni H, Xu X. Prediction of delirium in critically ill patients with elevated C-reactive protein. J Crit Care. 2014;29:88–92. doi: 10.1016/j.jcrc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie CW, Newman TH, Leurent B, Sampson EL. The association between C-reactive protein and delirium in 710 acute elderly hospital admissions. Int Psychogeriatr. 2014;26:17–24. doi: 10.1017/S1041610213002433. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Hwang DS, Wang SK, Chee IS, Baeg S, Kim JL. Early assessment of delirium in elderly patients after hip surgery. Geropsychiatry. 2011;8:340–347. doi: 10.4306/pi.2011.8.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemstra AW, Kalisvaart KJ, Vreeswijk R, van Gool WA, Eikelenoom P. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int J Geriatr Psychiatry. 2008;23:943–948. doi: 10.1002/gps.2015. [DOI] [PubMed] [Google Scholar]
- 23.Beloosesky Y, Grinblat J, Pirotsky A, Weiss A, Hendel D. Different C- reactive protein kinetics in post-operative hip-fractured geriatric patients with and without complications. Gerontology. 2004;50:216–222. doi: 10.1159/000078350. [DOI] [PubMed] [Google Scholar]
- 24.Cerejeira J, Batista P, Nogueira V, Vaz-Serra A, Mukaetova-Ladinska EB. The stress response to surgery and postoperative delirium: evidence of hypothalamic- pituitary-adrenal axis hyperresponsiveness and decreased suppression of the GH/IGF-1 Axis. J Geriatr Psychiatry Neurol. 2013;26:185–194. doi: 10.1177/0891988713495449. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Hwang DS, Wang SK, Chee IS, Baeg S, Kim JL. Early assessment of delirium in elderly patients after hip surgery. Psychiatry Investig. 2011;8:340–347. doi: 10.4306/pi.2011.8.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macdonald A, Adamis D, Treloar A, Martin F. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing. 2007;36:222–225. doi: 10.1093/ageing/afl121. [DOI] [PubMed] [Google Scholar]
- 27.McGrane S, Girard TD, Thompson JL, Shintani AK, Woodworth A, Ely EW, Pandharipande PP. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. 2011;15:78. doi: 10.1186/cc10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie CW, Newman TH, Leurent B, Sampson EL. The association between C-reactive protein and delirium in 710 acute elderly hospital admissions. Int Psychogeriatr. 2014;26:717–724. doi: 10.1017/S1041610213002433. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Pan L, Deng H, Ni H, Xu X. Prediction of delirium in critically ill patients with elevated C-reactive protein. J Crit Care. 2014;29:88–92. doi: 10.1016/j.jcrc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Pol RA, van Leeuwen BL, Izaks GJ, Reijnen MM, Visser L, Tielliu IF, Zeebregts CJ. C-reactive protein predicts postoperative delirium following vascular surgery. Ann Vasc Surg. 2014;28:1923–1930. doi: 10.1016/j.avsg.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 31.McManus J, Pathansali R, Hassan H, Ouldred E, Cooper D, Stewart R, et al. The course of delirium in acute stroke. Age Ageing. 2009;38:385–389. doi: 10.1093/ageing/afp038. [DOI] [PubMed] [Google Scholar]
- 32.Tsuruta R, Nakahara T, Miyauchi T, Kutsuna S, Ogino Y, Yamamoto T, et al. Prevalence and associated factors for delirium in critically ill patients at a Japanese intensive care unit. Gen Hosp Psychiatry. 32:607–611. doi: 10.1016/j.genhosppsych.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Lemstra AW, Kalisvaart KJ, Vreeswijk R, van Gool WA, Eikelenboom P. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int J Geriatr Psychiatry. 2008;23:943–948. doi: 10.1002/gps.2015. [DOI] [PubMed] [Google Scholar]
- 34.van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, Pickkers P. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15:297. doi: 10.1186/cc10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girard TD, Ware LB, Bernard GR, Pandharipande PP, Thompson JL, Shintani AK, et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med. 2012;38:1965–1973. doi: 10.1007/s00134-012-2678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropatho. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 37.Nash SD, Cruickshanks KJ, Klein R, Klein BE, Nieto FJ, Chappell R, et al. Long-term variability of inflammatory markers and associated factors in a population- based cohort. J Am Geriatr Soc. 2013;61:1269–1276. doi: 10.1111/jgs.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cape E, Hall RJ, van Munster BC, de Vries A, Howie SE, Pearson A, et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1B in delirium after hip fracture. J Psychosom Res. 2014;77:219–25. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritter C, Tomasi C, Dal-Pizzol F, Pinto BB, Bollen Pinto B, Dyson A, et al. Inflammation biomarkers and delirium in critically ill patients. Crit Care. 2014;18:106. doi: 10.1186/cc13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girard TD, Ware LB, Bernard GR, Pandharipande PP, Thompson PP, Thompson JL, et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Int Care Med. 2012;28:1965–73. doi: 10.1007/s00134-012-2678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Raised IL-2 and TNF-[alpha] concentrations are associated with postoperative delirium in patients undergoing coronary-artery bypass graft surgery. Int Psychogeriatr. 2013;26:845–55. doi: 10.1017/S1041610213002378. [DOI] [PubMed] [Google Scholar]
- 42.Ablij HC, Meinders AE. C-reactive protein: history and revival. Eur J Intern Med. 2002;13:412–422. doi: 10.1016/s0953-6205(02)00132-2. [DOI] [PubMed] [Google Scholar]
- 43.Poljak A, Hill M, Hall RJ, MacLullich AM, Raftery MJ, Tai J, et al. Quantitative proteomics of delirium cerebrospinal fluid. Transl Psychiatry. 2014;4:477. doi: 10.1038/tp.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, et al. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2005;25:193–197. doi: 10.1161/01.ATV.0000148324.63685.6a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.