Abstract
Despite flourishing interest in the topic of fatigue—as indicated by the many presentations on fatigue at the 2015 annual meeting of the American College of Sports Medicine—surprisingly little is known about its impact on human performance. There are two main reasons for this dilemma: (1) the inability of current terminology to accommodate the scope of the conditions ascribed to fatigue, and (2) a paucity of validated experimental models. In contrast to current practice, a case is made for a unified definition of fatigue to facilitate its management in health and disease. Based on the classic two-domain concept of Mosso, fatigue is defined as a disabling symptom in which physical and cognitive function is limited by interactions between performance fatigability and perceived fatigability. As a symptom, fatigue can only be measured by self-report, quantified as either a trait characteristic or a state variable. One consequence of such a definition is that the word fatigue should not be preceded by an adjective (e.g., central, mental, muscle, peripheral, and supraspinal) to suggest the locus of the changes responsible for an observed level of fatigue. Rather, mechanistic studies should be performed with validated experimental models to identify the changes responsible for the reported fatigue. As indicated by three examples (walking endurance in old adults, time trials by endurance athletes, and fatigue in persons with multiple sclerosis) discussed in the review, however, it has proven challenging to develop valid experimental models of fatigue. The proposed framework provides a foundation to address the many gaps in knowledge of how laboratory measures of fatigue and fatigability impact real-world performance.
Keywords: performance fatigability, perceived fatigability, endurance, critical power, multiple sclerosis
Despite a substantial literature on the influence of fatigue on human performance, relatively little progress has been made in translating this knowledge into practice. One of the key impediments has been the scope of its usage, with fatigue denoting reductions in physical and cognitive function that extend from an exercise-induced impairment of motor performance through to the sensations of tiredness and weakness that accompany some clinical conditions. As a consequence, knowledge on fatigue has become compartmentalized within such disciplines as clinical medicine, ergonomics, physiology, and psychology. The resulting fragmentation of work on fatigue has led to the absence of a consensus definition of fatigue, the emergence of terminology that tends to exclude individuals not familiar with a particular discipline, and the development of questionable experimental models.
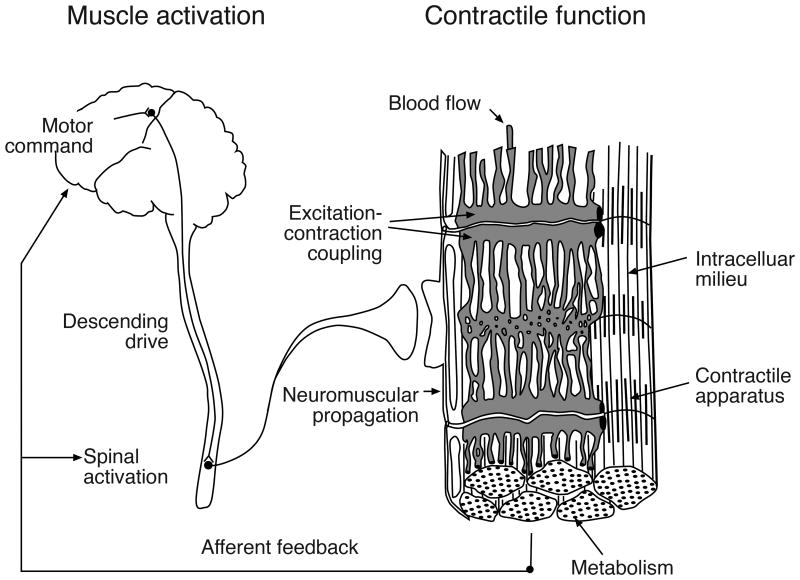
Contemporary research on the physiology of fatigue, for example, is often based on the Mosso dichotomy in which the phenomenon of force diminution is regarded as distinct from the sensations that arise from prolonged muscular activity (19). Mechanistic studies on the physiology of fatigue, therefore, have largely focused on identifying rate-limiting adjustments in central and peripheral processes that limit human performance (7) (Figure 1). Despite the many studies that have adopted the central-peripheral dichotomy, however, two key limitations with this approach have precluded the development of a consensus understanding on what causes fatigue.
Figure 1.
The physiological processes that can contribute to fatigue are classically categorized into two domains, those that establish the level of muscle activation (central) and those that influence contractile function (peripheral). Reprinted from Enoka (16).
The first limitation is the implicit assumption that the adjustments in neuromuscular activity needed to counteract an exercise-induced decrease in force capacity and thereby sustain task performance are independent from those involved in generating the accompanying sensations. Although it is possible to isolate the decline in contractile function during a fatiguing contraction by recording electrically evoked forces (6), adjustments in the activation signal discharged by motor neurons during a voluntary contraction begin before a detectable reduction in muscle force and are attributable to changes occurring within the muscle (11,20). Moreover, sensory feedback transmitted by group III/IV afferents can influence both the integration of synaptic input by spinal motor neurons and contribute to perceptions of pain (42). Such observations indicate that it is not possible to identify the etiology of fatigue by attempting to disentangle the decline in muscle force from sensations about fatigue, especially during long-lasting contractions (68).
The second limitation is that most of the physiological processes involved in performing a voluntary action, extending from the generation of the motor command down to the cross bridge cycle, can be challenged under appropriate experimental conditions and thereby contribute to the development of fatigue. For example, the decline in maximal voluntary contraction (MVC) force—a classic index of muscle fatigue—that develops during sustained low-intensity activity is largely attributable to a reduction in the activation signal generated by the nervous system, whereas the reduction in MVC force after high-intensity activity is more likely due to a decline in contractile function (68,72). Indeed, much of the literature on the physiology of fatigue is focused on exploiting a range of experimental protocols to quantify the capabilities of the neuromuscular system in various contexts (18,22,34,46,59). This work has clearly demonstrated that the rate-limiting adjustments during fatiguing contractions vary across conditions, which has become known as the task dependency of fatigue (19). A critical question that emerges from this work, however, is which of these impairments limit real-world performance?
The purpose of this brief review is to present a framework that can be used to evaluate the functional significance of fatigue-related adjustments. The framework comprises a taxonomy that can encompass the scope of the conditions ascribed to fatigue and a general approach to determine the influence of fatigue on human performance. To achieve this goal, we propose that fatigue be conceptualized as a disabling symptom in which physical and cognitive function is limited by interactions between performance fatigability and perceived fatigability.
Proposed Taxonomy
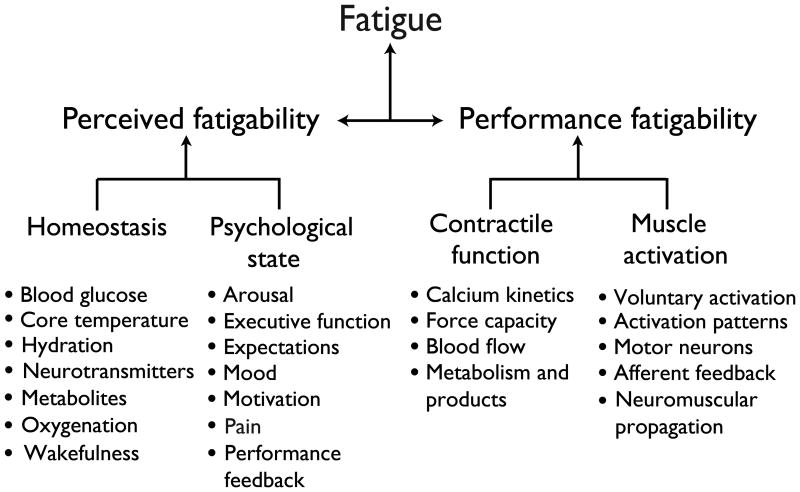
The original scheme proposed by Mosso remains relevant (Figure 1), but needs to be broadened to accommodate the contemporary scope of conditions ascribed to fatigue during human performance. As suggested in the taxonomy proposed by Kluger et al. (37), the concept of fatigue should acknowledge its two attributes: (1) performance fatigability – the decline in an objective measure of performance over a discrete period of time; and (2) perceived fatigability –changes in the sensations that regulate the integrity of the performer (Figure 2). Fatigue is defined in terms of fatigability to normalize the level of fatigue reported by an individual relative to the demands of the task that produces it. Individuals who are less fatigable, for example, are able to meet a greater demand to reach the same level of fatigue. When a person reports the level of fatigue during ongoing activity, therefore, the value at a specific time point will depend on the rates of change in its two attributes. As indicated in Figure 2, the attribute of performance fatigability depends on the contractile capabilities of the involved muscles and the capacity of the nervous system to provide an adequate activation signal derived from descending commands and afferent feedback for the prescribed task. In contrast, the attribute of perceived fatigability is derived from the initial value and the rate of change in sensations that regulate the integrity of the performer based on the maintenance of homeostasis and the psychological state of the individual.
Figure 2.
The proposed taxonomy suggests that fatigue be defined as a self-reported disabling symptom derived from two interdependent attributes: perceived fatigability and performance fatigability. Each of the attributes has two domains that themselves depend on the status of 4-7 modulating factors. The list of modulating factors is not intended to be all-inclusive, but rather it provides an initial set that can be expanded as new experimental findings emerge. By defining fatigue in terms of fatigability, the level of fatigue reported by an individual depends on the rates of change in the two attributes and thereby normalizes it to the demands of the task being performed. Adapted from Kluger et al. (37).
In contrast to performance fatigability, perceived fatigability can be assessed when a person is either at rest (21,23) or performing physical activity (57,62). Elevated perceived fatigability at rest is attributable to deviation of one or more of the modulating factors (e.g., core temperature, hydration, motivation, and pain) from normal baseline values. Conversely, perceived fatigability during onging activity is derived from rates of change in the modulating factors and are used to regulate the pace of the performance and thereby control the development of fatigue. Although the proposed scheme (Figure 2) suggests that the influence of psychological factors on fatigue is mediated via perceived fatigability, it remains to be determined whether or not psychological factors can have a direct effect on fatigue without involving perceived fatigability.
Most of the factors listed in Figure 2 are the familiar candidates that have been demonstrated to represent rate-limiting adjustments under selected conditions (17,24,38,50,52,58,68) (Figure 1). One of the unique features of the proposed scheme, however, is the explicit inclusion of psychological factors as significant contributors to the construct of fatigue. Although a potential role for psychological factors in the development of fatigue is occasionally acknowledged in studies on healthy individuals (43), they assume a more prominent role in the fatigue reported by various clinical cohorts. For example, the level of fatigue (Fatigue Severity Scale) reported by individuals with either multiple sclerosis or Parkinson's disease is significantly correlated with the level of depression (37) and represents a deviation in one of the modulating factors (mood) from normal baseline values. Similarly, lesions in the ascending reticular activating system suggest that reductions in arousal may mediate some aspects of fatigue in individuals with post-polio syndrome (37). Even in healthy individuals, however, psychological factors, such as performance feedback, can influence the average power produced by trained cyclists when performing a time trial as quickly as possible, which presumably coincides with maximal ratings of perceived exertion at the end of the task (66).
Although the taxonomy illustrated in Figure 2 lists many of the factors that can influence each attribute of fatigue (performance fatigability and perceived fatigability), the scheme acknowledges that most voluntary actions performed by humans involve significant interactions between the two domains. For example, several of the modulating factors that contribute to perceived fatigability, including the levels of blood glucose (49), core temperature (50), arousal (36), and mood (64), can all modulate the capacity of the individual to generate the required amount of voluntary activation, which is a factor that influences performance fatigability. Similarly, afferent feedback generated during high-intensity exercise can influence the adjustments required to maintain homeostasis and thereby contribute to perceived fatigability (33,59). The key feature of this scheme is that the level of fatigue experienced by an individual emerges from the many adjustments that occur in the modulating factors within and between each fatigability attribute. With this construct, fatigue is defined as a disabling symptom in which physical and cognitive function is limited by interactions between performance fatigability and perceived fatigability. As indicated in Figure 2, the level of fatigue experienced by an individual can be modulated by challenges to homeostasis, disturbances in the psychological state, reductions in contractile function, and limitations in the capacity to provide an adequate activation signal to the involved muscles.
According to the proposed definition, fatigue is a single entity that does not need to be modified by an accompanying adjective, such as central fatigue, mental fatigue, muscle fatigue, peripheral fatigue, physical fatigue, and supraspinal fatigue. Although the descriptors are often intended to imply the likely locus of the adjustments in the modulating factors that limit performance, the distinctions are too vague to be meaningful. Moreover, the literature on fatigue would be more coherent if the practice was to state the primary outcome variable and describe how it was influenced by the protocol, without suggesting that the study examined a particular type of fatigue.
Studies on performance fatigability, for example, should focus on the key outcome variable, which could include the duration that a task can be sustained (endurance time or time to task failure), the time it takes to complete a prescribed task (walking endurance, time trial), or the rate of change in such variables as MVC force, power production, the amplitude of evoked responses, reaction time, rating of perceived exertion, heart rate, mean arterial pressure, and core temperature. Critically, however, the outcome variable must be an ecologically valid measure of human performance, such as those specified in the NIH Toolbox, indices of athletic prowess, and standardized clinical assessments. Such an approach would facilitate the development of guidelines on how to manage fatigue in health and disease (3,37,77). An analogous construct has been proposed to monitor the fatigue experienced by athletes across a training cycle (26).
Human Performance and Fatigue
We now consider three examples of what we know about how fatigue influences human performance. Similar approaches have been used by other groups (24,38,63). In each of our examples, a performance criterion is identified and then what is known about the fatigue-related adjustments that limit this performance is discussed. In addition to providing a framework that can be extended to other activities, the examples included in the current review identify specific gaps in knowledge that need to be addressed in future work.
Walking Endurance of Healthy Adults
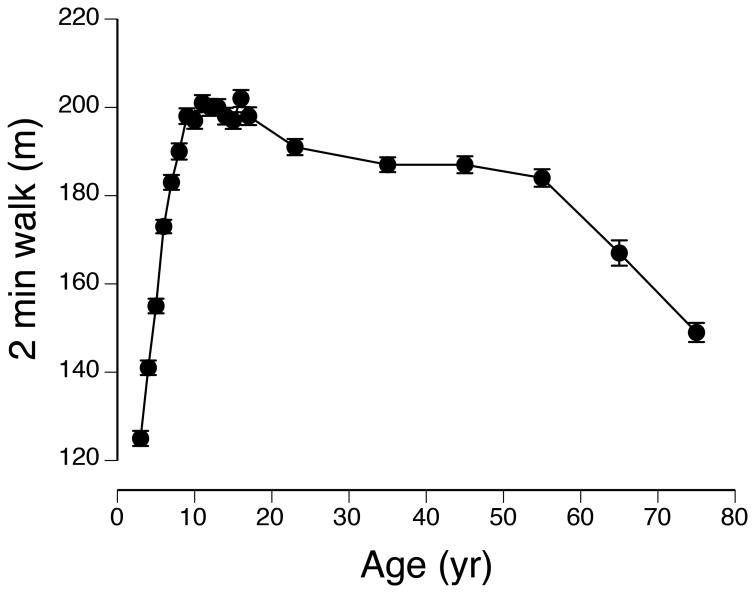
The National Institutes of Health (NIH) developed a multidimensional set of measures, known as the Toolbox, to provide brief yet comprehensive tests of neurological health and function across the lifespan. The NIH Toolbox has four domains: cognition, emotion, motor, and sensation (55). Motor function—the ability to perform physical tasks—is quantified with tests of endurance, locomotion, manual dexterity, non-vestibular balance, and strength. Endurance, which the Toolbox assesses as the distance that can be walked in 2 min, characterizes the global and integrated responses of the pulmonary, cardiovascular, and musculoskeletal systems. The distance that can be walked in 2 min varies across the lifespan (Figure 3) and walking endurance is reduced in old adults (65-102 yrs) who self-report elevated levels of fatigue (61,70). Moreover, time to complete another test of walking endurance (400 m) is a significant predictor of mortality in old adults (71).
Figure 3.
Normative data (mean ± SE) for the distance walked by individuals ranging in age from 3 yrs to 85 yrs. There were approximately 200 participants in each age group: 3, 4, 5,…16, 17, 18-29, 40-49, 50-59, 60, 69, 70-85 yrs. Note that the distance walked by the 5-yr group was similar to that for the oldest group (70-85 yrs). Data from Kallen et al. (31).
Given that walking endurance provides a clinically relevant measure of human performance, Justice et al. (30) compared the associations between walking endurance and measures of neuromuscular function in young (22 ± 4 yrs) and old (75 ± 6 yrs) adults. Of particular interest was the strength of the association between walking endurance (500-m walk) and a laboratory test of leg-muscle fatigability. Due to the significant loss of function in the dorsiflexor muscles of old adults (4,54,67), the test of fatigability involved supporting a submaximal mass (20% of the one-repetition maximum) with the dorsiflexor muscles for as long as possible while seated with the foot in a neutral position. The two groups (young and old) experienced a similar decline in maximal voluntary contraction (MVC) torque at task failure (∼23%), but endurance time for this particular fatiguing contraction was longer for the young adults (15.5 ± 0.9 min) than the old adults (8.9 ± 0.6 min) even though the young adults were stronger.
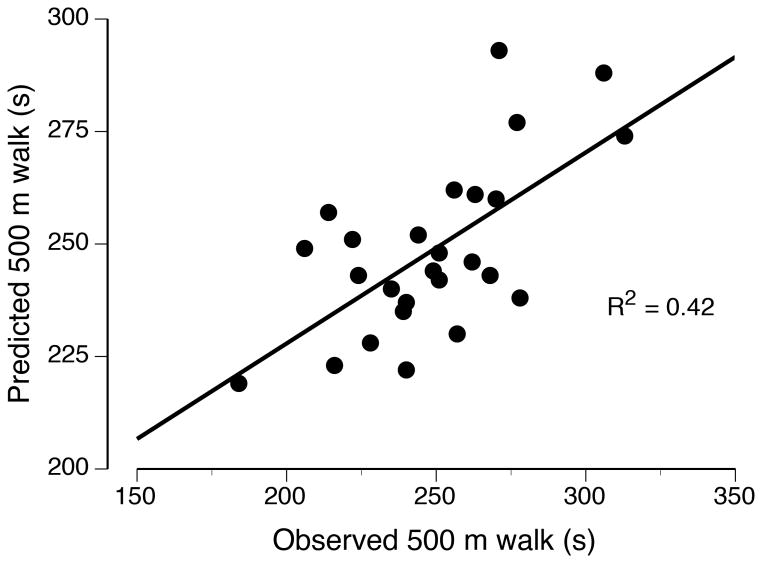
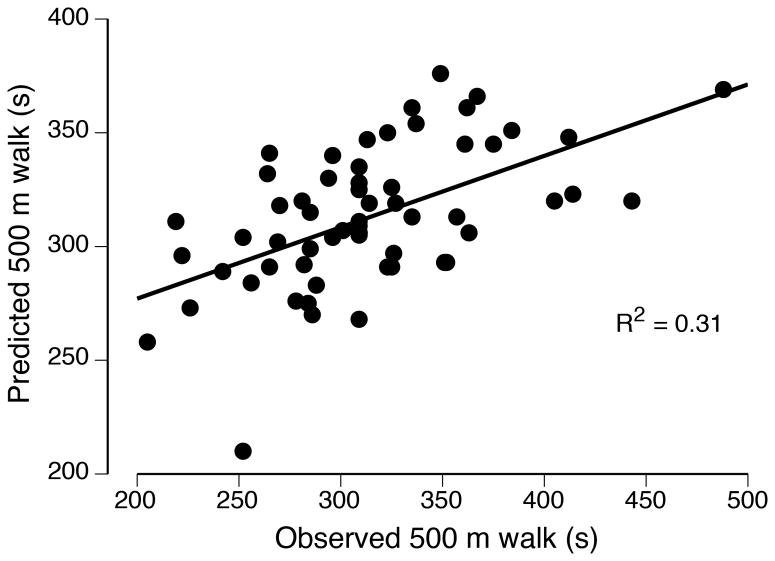
In a subsequent analysis of the data reported by Justice et al. (30), the outcome measures were entered into a stepwise, multiple-regression model to predict the variance in 500-m walk time for each group of participants. Separate models emerged for young and old adults, with more of the variance explained for young adults (R2 = 0.42) than for old adults (R2 = 0.31; Figure 4). The three explanatory variables in the model for old adults also emerged as predictors in the model for the young adults: MVC force for the knee flexors, 1-RM load for the dorsiflexors, and force steadiness when supporting a 20% 1-RM load with the dorsiflexors. The model for young adults included an additional two explanatory variables: endurance time for the measure of leg-muscle fatigability and dorsiflexor MVC force. These findings indicate that dorsiflexor fatigability during a sustained, submaximal contraction was moderately related to walking endurance for young adults, but not for old adults. Additional studies are needed to determine if more of the variance in walking endurance can be explained by other tests of performance fatigability, such as actions that engage multiple joints and those involving the stretch-shortening cycle (39).
Figure 4.
Associations between observed and predicted 500-m walk times for old (A) and young (B) adults. The relations were derived from a multiple-regression analysis of the data reported by Justice et al. (30).
In contrast to the absence of an association between walking endurance and the test of performance fatigability used by Justice et al. (30), another measure of fatigue has been found to be associated with walking endurance in old adults. In a sample of 1,155 adults (65-102 yrs), Vestergaard et al. (70) examined the association between fatigue and physical function by categorizing participants into those who self-reported elevated levels of fatigue and those who did not. Fatigue was assessed with two questions from the Center for Epidemiologic Studies-Depression Scale based on experience in the past week: (1) I feel that everything I did was an effort; (2) I could not get going. Possible answers were: (a) rarely (<1 day); (b) some of the time (1-2 days); (c) occasionally (3-4 days); or (d) all of the time (5-7 days). Those individuals who reported ≥3 days for either question were classified as fatigued, the prevalence of which was greater for women (29.1%) than for men (15.3%).
In general, the health behaviors and prevalence of clinical conditions was relatively similar for the two groups of participants for both sexes. The exceptions were that men who were classified as being fatigued, but not women, were more sedentary, had more comorbid conditions, reported a greater prevalence of coronary heart disease and chronic bronchitis/emphysema, and had greater levels of C-reactive protein in blood samples. Nonetheless, the fatigued participants of both sexes had weaker handgrip strength, greater activity limitations (Instrumented Activities of Daily Living), and worse walking endurance. For example, average speed during the 400-m walk was less for the fatigued groups (men: 1.29 and 1.22 m/s; women: 1.14 and 1.05 m/s) and the percentage of those individuals who could not complete the 400 m walk was greater for the fatigued groups (men: 5.9 and 21.0%; women: 6.9 and 14.8%). These findings indicate that an elevated trait level of fatigue is associated with reduced walking endurance (a measure of performance fatigability) in old adults.
Time Trials by Endurance Athletes
The peak power that a muscle can produce during sustained activity declines as the duration of the task increases. The relation between power production and task duration can be characterized with a hyperbolic function, with the asymptote corresponding to critical power and the curvature denoting the finite amount of work that can be performed above critical power at each level of power production (24,28). Critical power is located at the boundary between heavy and severe exercise, and has been characterized as the greatest exercise intensity at which the physiological adjustments can accommodate the challenge to intramuscular homeostasis. Exercise can be sustained for durations up to a maximum of ∼30 min when performed at intensities above critical power; that is, in the severe domain.
An individual's critical power, which provides a measure of performance fatigability, can be estimated with a single, 3-min test on a cycle ergometer in a laboratory (69). The test requires participants to cycle at a maximal cadence with the ergometer resistance set midway between the power outputs that correspond to the gas exchange threshold and maximal oxygen consumption as determined during a ramp protocol. The participant is required to achieve maximal power production quickly and to maintain as high a cadence as possible until told to stop. Critical power is quantified as the average power produced during the last 30 s of the test and the curvature of the hyperbolic function is estimated from the power-time integral above critical power.
The validity of the method as a measure of human performance was examined by comparing the ergometer-based estimate of critical power with the time it took 10 competitive cyclists to complete a 16.1 km time trial (8). Such time trials are performed at an intensity similar to critical power. The time trial was completed in 27.1 ± 1.2 min (mean ± SD) and critical power was estimated as 309 ± 34 W (4.20 ± 0.41 W/kg). Time trial performance was significantly correlated with critical power (r2 = –0.69, P < 0.01), indicating that faster times were associated with greater values for critical power. A similar correlation was found between time trial performance and total work (power-time integral) performed during the 3 min test. These findings indicate that the critical power protocol represents a valid model for mechanistic studies of performance fatigability during time trials, at least in highly trained individuals.
As one example of such a mechanistic study, Klass et al. (36) compared the influence of three drug conditions on the performance of 10 cyclists and triathletes on a time trial. The drug conditions, one of which was administered in each experimental session, comprised a placebo or a reuptake inhibitor for either noradrenaline (reboxetine) or dopamine (methylphenidate). The reuptake inhibitors result in each neurotransmitter lingering longer among synaptic contacts and thereby augmenting the actions it elicits. On each occasion, the participants performed a time trial in which they were required to complete a fixed amount of work (the equivalent of 30 min at 75% of maximal power production) on a cycle ergometer as quickly as possibly. The rating of perceived exertion (6-20 scale) was reported during the test. Before and after each time trial, contractions were evoked with transcranial magnetic stimulation and nerve-muscle stimulation to estimate the level of voluntary activation.
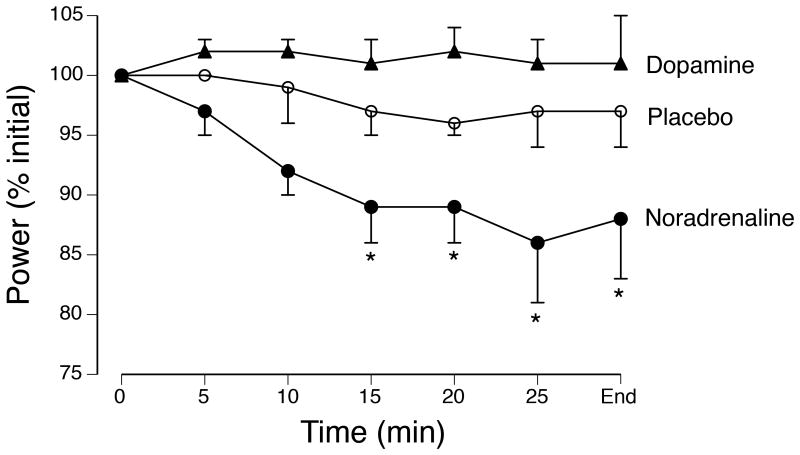
The average power produced during the time trial was depressed when noradrenaline reuptake was inhibited relative to the other two conditions (Figure 5). As a consequence, the time to complete the simulated time trial was similar for the placebo (30.8 ± 2.1 min) and the inhibition of dopamine reuptake (29.7 ± 1.4 min) conditions, but significantly longer for the noradrenaline condition (33.7 ± 3.6 min). Consistent with these results, the level of voluntary activation, as assessed with transcranial magnetic stimulation, did not differ from baseline values (∼97%) to those measured at 10 min after the time trial for either the placebo (95 ± 1.5%) or dopamine (95 ± 1.4%) groups, but was depressed for the noradrenaline group (89 ± 3.0%). In contrast, the rating of perceived exertion at the end of the time trial did not differ for the three groups (18.0 ± 1.4, 18.1 ± 1.1, and 18.7 ± 1.1, respectively). These results suggest that the average power produced during each time trial was regulated by adjustments that influence the rating of perceived exertion and that these adjustments were compromised when noradrenaline reuptake was inhibited relative to the other two conditions.
Figure 5.
Average power (% initial) produced by 8 trained cyclists during a ∼30-min time trial performed on three occasions. On each occasion, the participant was tested after oral ingestion of either placebo or one of two reuptake inhibitors (dopamine or noradrenaline). The goal on each occasion was to perform the prescribed amount of work as quickly as possible. *P < 0.05 relative to the initial level of power production. Data from Klass et al. (36).
The association between physiological adjustments and “volitional fatigue”—when ratings of perceived exertion were presumably maximal—during high-intensity exercise was demonstrated in another study when McKenna et al. (44) compared the influence of an antioxidant compound on cycling performance. The antioxidant administered during the study was N-acetylcysteine, which can influence the activity of Na+,K+ pumps in muscle and prolong the duration that high-intensity exercise can be sustained. In a cross-over design in which the influence of the drug was compared with saline, McKenna et al. (44) examined the performance and adjustments exhibited by eight endurance-trained athletes when they cycled on an ergometer for 45 min at 70% of peak oxygen consumption and then as long as possible at 92% of maximal oxygen consumption. The influence of the antioxidant compound was characterized by measuring maximal Na+,K+ pump activity in biopsy samples from vastus lateralis and the associated changes in plasma [K+].
Infusion of the antioxidant had a significant influence on maximal Na+,K+ pump activity, plasma [K+], and endurance time relative to the saline condition. The duration that the cyclists could sustain the cycling task was prolonged by 24 ± 8% for the antioxidant condition and this improvement in performance was accompanied by lesser changes in both maximal Na+,K+ pump activity and plasma [K+] when normalized to the amount of work that was performed. The attenuation of the decline in maximal Na+,K+ pump activity during the antioxidant condition suggests that the accumulation of reactive oxygen species contributes significantly to the inactivation of Na+,K+ pumps during high-intensity exercise. Because the task was performed for as long as possible for the two conditions, the results suggest the contraction-associated modulation of Na+,K+ pump activity can influence the neural pathways involved in generating the maximal level of tolerable exercise (76).
Taken together, these findings indicate that the physiological adjustments exhibited by endurance athletes during high-intensity exercise are strongly associated with ratings of perceived exertion. Key gaps in knowledge for this level of human performance include information about the relative significance of the different adjustments that contribute to the generation of perceived fatigability (1) and the extent to which tolerance of these perceptions can be improved with exercise interventions (29).
Fatigue in Multiple Sclerosis
Multiple sclerosis (MS) is an inflammatory disease that progressively impairs the ability of neurons to generate and conduct action potentials. Although the course of the disease varies among individuals, it invariably involves the development of difficulties with walking and elevated levels of fatigue (10,40,47). One large survey found that 74% of respondents (n = 9,077) reported severe fatigue, and worsening fatigue was associated with the development of limitations in walking (25).
Several studies have examined the association between the fatigue reported by individuals with MS and performance fatigability during standardized laboratory tests. In one such study, Steens et al. (64) compared the characteristics of 20 persons who had one form of the disease (relapsing-remitting MS) with a control group of age- and sex-matched individuals. Fatigue was assessed with the Fatigue Severity Scale (FSS), which is a validated and reliable instrument that quantifies the impact of fatigue on activities of daily living in individuals with MS (41). The FSS is a unidimensional scale that largely focuses on the physical aspects of fatigue. Performance fatigability was quantified as the decline in force during a sustained, isometric MVC with a hand muscle (first dorsal interosseus). This muscle was chosen because it is less likely than limb muscles to be impacted by the deconditioning associated with the relatively sedentary lifestyle of individuals with MS. In addition, mood was evaluated using the depression subscore of the Hospital Anxiety and Depression Scale questionnaire.
Participants in the MS group exhibited significantly greater levels of fatigue (FSS: 5.3 ± 0.9) and depression (4.8 ± 3.3) than those in the control group (2.9 ± 0.6 and 0.9 ± 1.0, respectively). There was a moderate association between FSS score and depression for the MS group (r2 = 0.36), but not for the control group. MVC force for the right hand muscle was not significantly different between groups, but when standardized as a Z score the MVC force was less for the MS group relative to the control group (–0.7 ± 1.0). Normalized MVC force (Z score) was significantly associated with the level of voluntary activation assessed during the MVCs for the MS group (r2 = 0.33), but not for the control group. The decline in force during the fatiguing contraction (sustained MVC for 124 s) was similar for the MS (61 ± 17%) and control (63 ± 12%) groups, and was significantly associated with MVC force (partial r2 = 0.15).
The assocation between FSS score and the measure of performance fatigability (decline in MVC force) was examined with a multiple-regression analysis. A significant amout of the variance in the FSS score for the participants in the MS group (R2 = 0.45), but not the control group, was explained with a model that included the measure of performance fatigability (partial r2 = 0.37) and normalized MVC score (partial r2 = 0.35). The model indicates that the individuals with MS who had greater FSS scores exhibited a greater amount of performance fatigability than would be expected on the basis of the normalized MVC score. However, more of the variance in FSS scores for the MS group was explained by including the depression score in the model (R2 = 0.77), which may have arisen from some of the FSS items concerning social aspects of fatigue.
In a subsequent study with similar groups of participants, Steens et al. (65) compared the adjustments in the activation signal generated by the nervous system during a fatiguing contraction with the hand muscle. The variance in performance fatigability (decline in MVC force) was explained by different outcome measures for the two groups of participants. Much of the variance for the decline in force exhibited by the control group was explained (R2 = 0.49) by the initial MVC force (strength) of the hand muscle. As reported in some other studies on the performance fatigability of healthy individuals (27), the strongest individuals were the most fatigable. Although the participants in the MS group experienced a similar reduction in force as the control group, the primary explanatory variable (R2 = 0.51) was the decline in voluntary activation as assessed by nerve-muscle stimulation (–20.1 ± 20.6%). The level of voluntary activation at the start of the fatiguing contraction did not differ between the two groups (∼90%) and declined only slightly by the end of the fatiguing contraction for the control group (–7.8 ± 11.8%). The regression model for the MS group indicated that those individuals with greater FSS scores experienced larger declines in voluntary activation.
To assess the cortical adjustments during the fatiguing contraction, Steens et al. (65) compared the changes in blood oxygen level-dependent (BOLD) contrast for the two groups of participants. As observed in other studies on healthy adults (53), the control group demonstrated a widespread increase in cortical activity during the fatiguing contraction despite a small decrease in voluntary activation and the progressive decline in maximal force. One potential explanation for the increase in cortical activity during fatiguing contractions is that it compensates for the reduction in excitation provided to spinal motor neurons (35,45). Nonetheless, the increase in cortical activity is not sufficient to overcome the adjustments responsible for the decrease in force during strong contractions. Consistent with this interpretation, participants in the MS group did not increase cortical activity and this was accompanied by a larger decline in voluntary activation. The similar decline in MVC force for the two groups, therefore, must be explained by other adjustments for the MS group. Indeed, the peak force elicited by a pair of stimuli—a measure of force capacity and the efficacy of excitation-contraction coupling—declined during the fatiguing contraction and was negatively related to the reduction in voluntary activation for the MS group (R2 = 0.25). Thus, greater decreases in voluntary activation were associated with lesser declines in the evoked twitch forces at rest.
Taken together, these findings indicate that the trait level of fatigue in persons with MS is associated with a measure of performance fatigability, the strength of the muscles involved in the fatiguing contraction, and the capacity to sustain a high level of voluntary activation during the fatiguing contraction. The caveat, however, is that these studies were performed on a hand muscle and future studies need to examine similar associations in limb muscles. In addition, little is known about the influence of fatigue and performance fatigability on mobility, such as walking endurance, in individuals with MS.
Translating Fatigue to Performance
By combining the proposed taxonomy (Figure 2) with the information presented in the preceding examples, we can complete the framework on how to translate knowledge on fatigue into practice. The foundation of this approach is the definition of fatigue as a symptom, which means it can only be measured by self-report. Classic measures of fatigue, such as the time to complete a prescribed task, the reduction in MVC force, and the decline in power production, are indices of performance fatigability, but do not provide a measure of the intensity of the symptom. Rather, the assessment of fatigue requires the individual to interpret relevant physiological and psychological factors by providing responses to standardized questions (3,5,26,56,75).
Trait and State Properties
As with many symptoms, fatigue can be assessed either as a trait characteristic or as a state variable. The trait level of fatigue represents the average amount of fatigue experienced during the preceding several days, whereas state measures reflect the rate of change in key adjustments during a fatiguing task. In the example of walking endurance in healthy adults, Vestergaard et al. (70) used a standardized instrument (Center for Epidemiologic Studies-Depression Scale) to determine the level of fatigue experienced by old adults during the preceding week. Such a measure indicates the trait level of fatigue, which Vestergaard et al. (70) used to assign the study participants to either a control group or a fatigue group. The relevance of the approach was underscored by finding significant differences in health status and performance criteria between the two groups of participants. Similarly, there are several instruments (e.g., Fatigue Severity Scale, Mobility-Tiredness Scale, Modified Fatigue Impact Scale, Multidimensional Fatigue Inventory, Recovery-Stress Questionnaire, Situational Fatigue Scale) that rely on self-reported responses about the preceding several days or weeks to quantify the trait level of fatigue experienced by individuals with various illnesses (3,5,37,47,64) and in athletes during a training cycle (26).
The measure of fatigue at a specific instant in time indicates the state level of fatigue. This can be accomplished by asking the performer to answer one or more questions about the level of fatigue “right now”. One instrument often used to assess the state level of fatigue is the visual analog scale, which can be anchored by “Fatigue is absent” on the left and “Most fatigue ever” on the right (12). Another option is to use the fatigue scale from the Profile of Mood States questionnaire, which has been demonstrated to provide a reliable and valid measure of the state level of fatigue across a wide range of cohorts (51). Participants are asked to provide a response on a 5-point scale (0 = not at all, 1 = a little, 2 = moderately, 3 = quite a bit, 4 = extremely) to five terms: Worn out, Fatigued, Exhausted, Sluggish, and Weary. The combined score indicates the state level of fatigue and can be compared at selected time points during a protocol. For example, Dishman et al. (14) used the Profile of Mood States fatigue scale to compare the influence of cycling exercise on the state level of fatigue in individuals who reported an elevated trait level of fatigue. Participants were assigned to either a 6-wk intervention (low- or moderate-intensity cycling) or a control group. The state level of fatigue was compared before and after cycling exercise at three time points during the 6-wk protocol. Participants in the low-intensity group, but not the other two groups (moderate-intensity exercise or control), exhibited less of an increase in the state level of fatigue after an exercise bout at weeks 3 and 6 of the study. These findings were interpreted to indicate that low-intensity exercise can be used to manage clinical symptoms and mood disorders, such as fatigue.
Alternatively, the state level of fatigue can be assessed with an instrument that is developed for a specific cohort. For example, Barry and colleagues devised a Fatigue and Energy Scale to measure the state level of fatigue after performing a bout of physical activity in individuals with chronic fatigue syndrome (32). One of the characteristics of the syndrome is the prolonged exacerbation of symptoms after performing physical activity. The approach involved convening focus groups comprising afflicted individuals to inform the development of a self-report instrument, examining the psychometric properties of the instrument in two case-control challenge studies, and assessing the ecological validity of the instrument. The resulting instrument (Fatigue and Energy Scale) comprised two domains—physical and mental fatigue—that each included three items. As intended, the Scale was able to capture the state level of fatigue reported by individuals with chronic fatigue syndrome after performing a bout of physical activity. With the increasing prevalence of fatigue being included in the diagnosis of various clinical conditions, there is an urgent need for similar instruments that can enable clinicians to quantify condition-specific levels of fatigue.
However, a word of caution is necessary about the use of self-report measures to assess the state level of fatigue in some cohorts, such as athletes and special operations forces personnel. Due to the competitive nature of these individuals, they are more likely than not to understate responses to questions about fatigue (1). Nonetheless, some assessment of fatigue is essential for these individuals to optimize the training load and lessen the likelihood of injuries and overtraining (26).
Experimental Strategies
To determine the influence of fatigue on human performance, the proposed approach involves three levels of analysis: (1) select a criterion measure of human performance that is modulated by fatigue; (2) identify a laboratory test that is a strong predictor of performance on the criterion measure; and (3) conduct mechanistic studies to determine the relative significance of the adjustments in the modulating factors (Figure 2) that limit performance on the laboratory test. Of the three examples discussed previously (walking endurance of healthy adults, time trials by endurance athletes, and fatigue in multiple sclerosis), most progress has been made on determining the influence of fatigue on the performance of endurance athletes during a time trial. The time it takes a trained cyclist to complete a time trial is reasonably correlated with the absolute critical power for the individual (8,9), which depends on the capacity of intramuscular adjustments to accommodate the challenge to homeostasis during high-intensity exercise (24,28,69). Given a specific critical power capability, however, performance during an individual time trial may be limited by factors that contribute to perceived fatigability, such as those that establish the performer's psychological state. Power production can be modulated, for example, by interventions that manipulate homeostasis and thereby alter ratings of perceived exertion (36,44). Future studies are needed to determine the relative significance of the various adjustments that influence ratings of perceived exertion.
In contrast, much less is known about how fatigue influences walking endurance of healthy adults and the fatigue reported by individuals with multiple sclerosis. Walking endurance, which is a strong predictor of health status (61,62,70), is reduced in old adults who exhibit an elevated trait level of fatigue, but there is not yet a validated test of fatigability that predicts performance on tests of walking endurance. Without an appropriate laboratory measure, it is not possible to identify the adaptations responsible for declines in walking endurance with advancing age and thereby develop appropriate countermeasures.
Although more is known about the influence of fatigue—as quantified with validated questionnaries—on motor function in persons with multiple sclerosis (13,48,74) than is known about walking endurance, significant gaps in knowledge remain. The elevated trait level of fatigue reported by individuals with relapsing-remitting MS is strongly associated with the strength of a hand muscle, the performance fatigability of the same hand muscle during a sustained maximal contraction, and perceived fatigability as indicated by a depression score (64,74). Despite comparable measures of performance fatigability (decline in MVC force), the explanatory variables were related to muscle strength for the control group and voluntary activation for the MS group (65). Additional studies are needed with this clinical cohort to examine such issues as the adjustments that limit voluntary activation during fatiguing contractions, the adjustments that establish performance fatigability on a laboratory test and how these relate to the trait level of fatigue, and the interaction between fatigue and limitations in mobility.
Some insight on these issues can be gleaned from randomized controlled trials of strength training performed by individuals with relapsing-remitting MS. Consistent with the finding that some of the variance in the trait level of fatigue (Fatigue Severity Scale) can be explained by the strength of a hand muscle (64), a 10-wk strength-training program that increased the strength and endurance of leg muscles also reduced the trait level of fatigue (Modified Fatigue Impact Scale) but did not improve walking endurance (2-min walk) (15). However, the decrease in fatigue was significantly correlated (r = 0.40) with a test of leg-muscle endurance, but not with the gains in muscle strength. Similar strength gains were achieved in another 12-wk intervention and these were accompanied by correlated (r = 0.30) reductions between the trait level of fatigue (Fatigue Severity Scale) and depression (Major Depression Inventory), but there was no association between the improvement in fatigue and increases in either muscle strength (knee extensors) or functional capacity (cumulative score based on chair-rise test, ascending-stairs test, 10-m walk test, and the 6-min walk test) (13). These findings suggest that some of the fatigue reported by individuals with relapsing-remitting MS can be explained by depression and the reduced capacity to sustain high levels of voluntary activation, and that the functional consequences are likely attributable to an inability to compensate for the disease-related decline in muscle activation.
Perspectives
Although there is widespread acceptance of the notion that fatigue can limit human performance, there are considerable gaps in knowledge on the underlying mechanisms and how these can be managed. This dilemma is largely attributable to the inability of current terminology to accommodate the scope of the conditions ascribed to fatigue and a paucity of valid experimental models. In an attempt to provide a more unifying rubric, it is proposed that fatigue be defined as a symptom in which physical and cognitive function is limited by interactions between performance fatigability and perceived fatigability. As a symptom, fatigue can only be measured by self-report, quantified as either a trait characteristic or a state variable. The trait level of fatigue represents the average amount of fatigue experienced during the preceding several days and depends on the absolute values of the modulating factors that contribute to perceived fatigability. In contrast, the state level of fatigue reflects the rate of change in the modulating factors that contribute to both performance and perceived fatigability during ongoing activity.
The two measures of fatigability (performance and perceived) normalize the observed fatigue to the demands associated with specific tasks. Less fatigable individuals, for example, are able to tolerate a greater demand to reach a given level of fatigue. Performance fatigability depends on the contractile capabilities of the involved muscles and the capacity of the nervous system to provide an adequate activation signal for the prescribed task. Perceived fatigability is derived from the sensations that regulate the integrity of the performer based on the maintenance of homeostasis and the psychological state of the individual. Perceived fatigability can be measured at rest or during physical activity, whereas performance fatigability is quantified as the rate of change in a criterion outcome due the adjustments made during a fatiguing task.
To determine how fatigue influences human performance, it is necessary to identify those modulating factors most responsible for establishing the levels of fatigability that contribute to the observed trait and state levels of fatigue. This can only be accomplished, however, after the development of a reliable laboratory test that is strongly associated with a criterion measure of human performance. Once an ecologically valid laboratory test has been determined, mechanistic studies can probe the critical adjustments and thereby suggest countermeasures to moderate the influence of the two attributes of fatigue on human performance. As indicated by the three examples discussed in this paper, much remains to be learned about how knowledge on fatigue can be translated into practice. The proposed framework provides a foundation to address these gaps in knowledge.
Acknowledgments
This work was partially supported by an award from National Institute of Child Health & Human Development (R03HD079508) to RME. The authors thank Andrew Jones, Benjamin Barry, Inge Zijdewind, Katrina Maluf, and Robyn Capobianco for comments on a draft of the manuscript.
References
- 1.Abbiss CR, Peiffer JJ, Meeusen R, Skorski S. Role of ratings of perceived exertion during self-paced exercise; what are we actually measuring? Sport Med. 2015;45:1235–1243. doi: 10.1007/s40279-015-0344-5. [DOI] [PubMed] [Google Scholar]
- 2.Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008;104:296–305. doi: 10.1152/japplphysiol.00908.2007. [DOI] [PubMed] [Google Scholar]
- 3.Avlund K. Fatigue in older populations. Fatigue: Biomed Health Behav. 2013;1:43–63. [Google Scholar]
- 4.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 5.Bennett BK, Goldstein D, Chen M, et al. Characterization of fatigue states in medicine and psychiatry by structured interview. Psychom Med. 2014;76:379–388. doi: 10.1097/psy.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 6.Bigland-Ritchie B, Furbush F, Woods JJ. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol. 1986;61:421–426. doi: 10.1152/jappl.1986.61.2.421. [DOI] [PubMed] [Google Scholar]
- 7.Bigland-Ritchie B, Jones DA, Hosking GP, Edwards RHT. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med. 1978;54:609–614. doi: 10.1042/cs0540609. [DOI] [PubMed] [Google Scholar]
- 8.Black MI, Durant J, Jones AM, Vanhatalo A. Critical power derived from a 3-min all-out test predicts 16.1-km road time-trial performance. Eur J Sport Sci. 2014;14:217–223. doi: 10.1080/17461391.2013.810306. [DOI] [PubMed] [Google Scholar]
- 9.Black MI, Jones AM, Bailey SJ, Vanhatalo A. Self-pacing increases critical power and improves performance during severe-intensity exercise. Appl Physiol Nutr Metab. 2015;40:662–670. doi: 10.1139/apnm-2014-0442. [DOI] [PubMed] [Google Scholar]
- 10.Bol Y, Duits AA, Hupperts RMM, Vlaeyen JWS, Verhey FRJ. The psychology of fatigue in patients with multiple sclerosis: a review. J Psychosom Res. 2009;66:3–11. doi: 10.1016/j.jpsychores.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol. 2001;534:903–912. doi: 10.1111/j.1469-7793.2001.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook DB, O'Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. NeuroImage. 2007;26:108–122. doi: 10.1016/j.neuroimage.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Dalgas U, Stenager E, Jakobsen J, et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler. 2010;16:1478–1484. doi: 10.1177/1352458509360040. [DOI] [PubMed] [Google Scholar]
- 14.Dishman RK, Thom NJ, Puetz TW, O'Connor PJ, Clementz BA. Effects of cycling exercise on vigor, fatigue, and electroencephalographic activity among young adults who report persistent fatigue. Psychophysiology. 2010;47:1066–1074. doi: 10.1111/j.1469-8986.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 15.Dodd K, Taylor N, Shields N, Prasad D, McDonald E, Gillon A. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis. Mult Scler. 2011;17:1362–1374. doi: 10.1177/1352458511409084. [DOI] [PubMed] [Google Scholar]
- 16.Enoka RM. Neuromechanics of Human Movement. 5th. Champaign, IL: Human Kinetics; 2015. p. 333. [Google Scholar]
- 17.Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol. 2011;21:208–219. doi: 10.1016/j.jelekin.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–12. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- 20.Farina D, Holobar A, Gazzoni M, Zazula D, Merletti R, Enoka RM. Adjustments differ among low-threshold motor units during intermittent isometric contractions. J Neurophysiol. 2009;101:350–359. doi: 10.1152/jn.90968.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fieo RA, Mortensen EL, Rantanen T, Avlund K. Improving a measure of mobility-related fatigue (The Mobility-Tiredness Scale) by establishing item intensity. J Am Geriatr Soc. 2013;61:429–433. doi: 10.1111/jgs.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 23.Glynn NW, Santanasto AJ, Simonsick EM, et al. The Pittsburgh Fatigability Scale for older adutls: development and validation. J Am Geriatric Soc. 2015;63:130–135. doi: 10.1111/jgs.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassi B, Rossiter HB, Zoladz JA. Skeletal muscle fatigue and decreased efficiency: two sides of the same coin? Exerc Sport Sci Rev. 2015;43:75–83. doi: 10.1249/JES.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 25.Hadjimichael O, Vollmer J, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008;6:100. doi: 10.1186/1477-7525-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halson SL. Monitoring training load to understand fatigue in athletes. Sports Med. 2014;44(Suppl 2):S139–144. doi: 10.1007/s40279-014-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter SK, Enoka RM. Sex differences in the fatigability of arm muscles depends on the absolute force during isometric contractions. J Appl Physiol. 2001;91:2686–2694. doi: 10.1152/jappl.2001.91.6.2686. [DOI] [PubMed] [Google Scholar]
- 28.Jones AM, Vanhatalo A, Burnley M, Morton RH, Poole DC. Critical power: implications for determination of VO2max and exercise tolerance. Med Sci Sports Exerc. 2010;42:1876–1890. doi: 10.1249/MSS.0b013e3181d9cf7f. [DOI] [PubMed] [Google Scholar]
- 29.Jones MD, Booth J, Taylor JL, Barry BK. Aerobic training increases pain tolerance in healthy individuals. Med Sci Sports Exerc. 2014;46:1640–1647. doi: 10.1249/MSS.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 30.Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol. 2014;55:92–101. doi: 10.1016/j.exger.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kallen B, Slotkin J, Griffith J, et al. NIH Toolbox technical manual. [Cited 2012 Jan 1]; Available from: www.nihtoolbox.org.
- 32.Keech A, Sandler CX, Vollmer-Coma U, Lloyd AR, Barry BK. Capturing the post-exertional exacerbation of fatigue following physical and cognitive challenge in patients with chronic fatigue syndrome. J Psychosom Res. 2015;79(6):537–49. doi: 10.1016/j.jpsychores.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy DS, Fitzpatrick SC, Gandevia SC, Taylor JL. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J Appl Physiol. 2015;118:408–418. doi: 10.1152/japplphysiol.00375.2014. [DOI] [PubMed] [Google Scholar]
- 34.Kent-Braun JA, Fitts RH, Christie A. Skeletal muscle fatigue. Compr Physiol. 2012;2:997–1044. doi: 10.1002/cphy.c110029. [DOI] [PubMed] [Google Scholar]
- 35.Klass M, Lévénez M, Enoka RM, Duchateau J. Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol. 2008;99:1096–1104. doi: 10.1152/jn.01252.2007. [DOI] [PubMed] [Google Scholar]
- 36.Klass M, Roelands B, Lévénez M, Fontenelle V, Pattyn N, Meeusen R, Duchateau J. Effects of noradrenaline and dopamine on supraspinal fatigue in well-trained men. Med Sci Sports Exerc. 2012;44:2299–2308. doi: 10.1249/MSS.0b013e318265f356. [DOI] [PubMed] [Google Scholar]
- 37.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knicker AJ, Renshaw I, Oldham AR, Cairns SP. Interactive processes link the multiple symptoms of fatigue in sport competition. Sports Med. 2011;41:307–328. doi: 10.2165/11586070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Komi PV. Stretch-shortening cycle: a powerful model to study normal and fatigued muscle. J Biomech. 2000;33:1197–1206. doi: 10.1016/s0021-9290(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 40.Kos D, Kerckhofs E, Nagels G, D'hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: a review of the literature. Neurorehabil. Neural Repair. 2008;22:91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- 41.Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RM. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci. 2013;331:102–107. doi: 10.1016/j.jns.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick A, Meijen C, Marcora S. Psychological determinants of whole-body endurance performance. Sports Med. 2015;45:99–1015. doi: 10.1007/s40279-015-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKenna MJ, Medved I, Goodman CA, et al. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009;587:5601–5612. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales-Alamo D, Losa-Reyna J, Torres-Peralta R, et al. What limits performance during whole body incremental exercise to exhaustion in humans? J Physiol. 2015;593(20):4631–48. doi: 10.1113/JP270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motl RM, McAuley E, Wynn D, Suh Y, Weikert M. Effects of change in fatigue and depression on physical activity over time in relapsing-remitting multiple sclerosis. Psychol Health Med. 2011;16:1–11. doi: 10.1080/13548506.2010.521569. [DOI] [PubMed] [Google Scholar]
- 48.Motl RM, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurosci. 2012;8:487–497. doi: 10.1038/nrneurol.2012.136. [DOI] [PubMed] [Google Scholar]
- 49.Nybo L. CNS fatigue and prolonged exercise: effect of glucose supplementation. Med Sci Sports Exerc. 2003;35:589–94. doi: 10.1249/01.MSS.0000058433.85789.66. [DOI] [PubMed] [Google Scholar]
- 50.Nybo L. Hyperthermia and fatigue. J Appl Physiol. 2008;104:871–878. doi: 10.1152/japplphysiol.00910.2007. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosomatic Res. 2004;57:435–441. doi: 10.1016/j.jpsychores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Place N, Yamada T, Bruton JD, Westerblad H. Muscle fatigue: from observation in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol. 2010;110:1–15. doi: 10.1007/s00421-010-1480-0. [DOI] [PubMed] [Google Scholar]
- 53.Post M, Steens A, Renken R, Mauritis NM, Zijdewind I. Voluntary activation and cortical activity during a sustained maximal contraction: an fMRI study. Hum Brain Mapp. 2009;30:1014–1027. doi: 10.1002/hbm.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power GA, Dalton BH, Rice CL, Vandervoort AA. Power loss is greater following lengthening contractions in old versus young women. Age (Dordr) 2012;34:737–750. doi: 10.1007/s11357-011-9263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(Suppl 3):S65–S75. doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt ME, Chang-Claude J, Vrieling A, Heinz J, Flesch-Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. J Cancer Surviv. 2012;6:11–19. doi: 10.1007/s11764-011-0197-3. [DOI] [PubMed] [Google Scholar]
- 57.Schnelle JF, Buchowski MS, Ikizler TA, Durkin DW, Beuscher L, Simmons SF. Evaluation of two fatigability severity measures in elderly adults. J Am Geriatr Soc. 2012;60:1527–1533. doi: 10.1111/j.1532-5415.2012.04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol. 2008;104:306–314. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]
- 59.Sidhu SK, Cresswell AG, Carroll TJ. Corticospinal responses to sustained locomotor exercises: moving beyond single-joint studies of central fatigue. Sports Med. 2013;43:437–449. doi: 10.1007/s40279-013-0020-6. [DOI] [PubMed] [Google Scholar]
- 60.Sidhu SK, Weavil JC, Venturelli M, et al. Spinal μ-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol. 2014;592:5011–5024. doi: 10.1113/jphysiol.2014.275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simonsick EM, Newman AB, Visser M, et al. Mobility limitation in self-described well-functioning older adults: importance of endurance walking test. J Gerontol A Biol Sci Med Sci. 2008;63:841–847. doi: 10.1093/gerona/63.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi: 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St Clair Gibson A, Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med. 2004;38:797–806. doi: 10.1136/bjsm.2003.009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steens A, de Vries A, Hemmen J, et al. Fatigue perceived by multiple sclerosis patients is associated with muscle fatigue. Neurorehabil Neural Repair. 2012;26:48–57. doi: 10.1177/1545968311416991. [DOI] [PubMed] [Google Scholar]
- 65.Steens A, Heersema DJ, Maurits NM, Renken RJ, Zijdewind I. Mechanisms underlying muscle fatigue differ between multiple sclerosis patients and controls: a combined electrophysiological and neuroimaging study. NeuroImage. 2012;59:3110–3118. doi: 10.1016/j.neuroimage.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 66.Stone MR, Thomas K, Wilkinson M, Jones AM, St Clair Gibson A, Thompson KG. Effects of deception on exercise performance: implications for determinants of fatigue in humans. Med Sci Sports Exerc. 2012;44:534–541. doi: 10.1249/MSS.0b013e318232cf77. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatric Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 68.Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104:542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- 69.Vanhatalo A, Doust JB, Burnley M. Determination of the critical power using a 3-min all-out cycling test. Med Sci Sports Exerc. 2007;39:548–555. doi: 10.1249/mss.0b013e31802dd3e6. [DOI] [PubMed] [Google Scholar]
- 70.Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol. 2009;64A:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vestergaard S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM. Characteristics of 400-meter walk test performance and subsequent mortality in older adults. Rejuven Res. 2009;12:177–184. doi: 10.1089/rej.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westerblad H, Bruton JD, Katz A. Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res. 2010;316:3093–3099. doi: 10.1016/j.yexcr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 73.Wolkorte R, Heersema DJ, Zijdewind I. Reduced dual-task performance in MS patients is further decreased by muscle fatigue. Neurorehabil Neural Repair. 2015a;29:424–435. doi: 10.1177/1545968314552529. [DOI] [PubMed] [Google Scholar]
- 74.Wolkorte R, Heersema DJ, Zijdewind I. Muscle fatigability during a sustain index finger abduction and depression scores are associated with perceived fatigue in patients with relapins-remitting multiple sclerosis. Neurorehabil Neural Repair. 2015b;29(8):796–802. doi: 10.1177/1545968314567151. [DOI] [PubMed] [Google Scholar]
- 75.Yang CM, Wu CH. The Situational Fatigue Scale: a different approach to measuring fatigue. Qual Life Res. 2005;14:1357–1362. doi: 10.1007/s11136-004-5680-0. [DOI] [PubMed] [Google Scholar]
- 76.Zénon A, Sidibé M, Olivier E. Disrupting the supplementary motor area makes physical effort appear less effortful. J Neurosci. 2015;35:8737–8744. doi: 10.1523/JNEUROSCI.3789-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zwarts MJ, Bleijenberg G, van Engelen BGM. Clinical neurophysiology of fatigue. Clin Neurophysiol. 2008;119:2–10. doi: 10.1016/j.clinph.2007.09.126. [DOI] [PubMed] [Google Scholar]