The optimal timing and sequencing of thoracic radiotherapy and chemotherapy, which is the standard treatment of ‘limited-stage’ small-cell lung cancer, has fuelled debate for many years. This individual patient data meta-analysis provides the best evidence of the beneficial effect of ‘earlier or shorter’ radiotherapy when chemotherapy is administered with good compliance.
Keywords: individual participant data meta-analysis, randomized clinical trials, thoracic radiotherapy, radiotherapy timing, small-cell lung cancer, chemotherapy compliance
Abstract
Background
Chemotherapy (CT) combined with radiotherapy is the standard treatment of ‘limited-stage’ small-cell lung cancer. However, controversy persists over the optimal timing of thoracic radiotherapy and CT.
Materials and methods
We carried out a meta-analysis of individual patient data in randomized trials comparing earlier versus later radiotherapy, or shorter versus longer radiotherapy duration, as defined in each trial. We combined the results from trials using the stratified log-rank test to calculate pooled hazard ratios (HRs). The primary outcome was overall survival.
Results
Twelve trials with 2668 patients were eligible. Data from nine trials comprising 2305 patients were available for analysis. The median follow-up was 10 years. When all trials were analysed together, ‘earlier or shorter’ versus ‘later or longer’ thoracic radiotherapy did not affect overall survival. However, the HR for overall survival was significantly in favour of ‘earlier or shorter’ radiotherapy among trials with a similar proportion of patients who were compliant with CT (defined as having received 100% or more of the planned CT cycles) in both arms (HR 0.79, 95% CI 0.69–0.91), and in favour of ‘later or longer’ radiotherapy among trials with different rates of CT compliance (HR 1.19, 1.05–1.34, interaction test, P < 0.0001). The absolute gain between ‘earlier or shorter’ versus ‘later or longer’ thoracic radiotherapy in 5-year overall survival for similar and for different CT compliance trials was 7.7% (95% CI 2.6–12.8%) and −2.2% (−5.8% to 1.4%), respectively. However, ‘earlier or shorter’ thoracic radiotherapy was associated with a higher incidence of severe acute oesophagitis than ‘later or longer’ radiotherapy.
Conclusion
‘Earlier or shorter’ delivery of thoracic radiotherapy with planned CT significantly improves 5-year overall survival at the expense of more acute toxicity, especially oesophagitis.
introduction
Small-cell lung cancer (SCLC) is a rapidly disseminating cancer so that its primary treatment is chemotherapy (CT), whatever the stage [1]. Approximately 25% of patients present with localized disease, formerly known as ‘limited-stage’ disease, now called stage I–IIIB [2]. It is well known that optimal survival is achieved when CT can be administered at the total intended dose and at the required intervals [1, 3]. Nevertheless, due to loco-regional failures after CT alone, the adjunction of thoracic radiotherapy was investigated. A worldwide meta-analysis showed that adding thoracic radiotherapy to CT improved long-term survival [4]. Concurrent use of CT, comprising cisplatin and etoposide, and thoracic radiotherapy has become the standard of care [1, 5, 6]. In non-progressing patients, this can be followed by prophylactic cranial irradiation, at the optimal dose of 25 Gy, as this treatment further prolongs survival [7, 8].
However, the optimal timing and sequencing of thoracic radiotherapy with CT has fuelled debate for many years. When all trials were pooled together, no survival gain was detected whether thoracic radiotherapy was delivered early with CT or later [9–12]. However, in trials where patients were treated with cisplatin-based CT at full dose, early administration of thoracic radiotherapy seemed to confer a long-term survival advantage. There is considerable variation in the definition of early or late radiotherapy: early radiotherapy was defined as starting before 9 weeks following the beginning of CT and before the third cycle of CT in two previous literature-based meta-analyses [12, 13], whereas a 30-day cut-off was used in other literature-based meta-analyses [9–11, 14] (supplementary Table S1, available at Annals of Oncology online for description of previous meta-analyses). One of these meta-analyses suggested that early delivery of thoracic radiotherapy yielded higher survival rates if all the intended cycles of CT could be administered [12], implying that the question of optimal radiotherapy timing and fractionation [15, 16] could only be addressed with precise information on individual patient compliance with CT administration. Such information can only be provided by an individual patient data (IPD) meta-analysis. We therefore undertook such a study, aiming at defining the best approach for combining thoracic radiotherapy with CT in stage I–IIIB SCLC.
materials and methods
The meta-analysis was carried out according to a pre-specified protocol that is available on the Gustave Roussy website (http://www.gustaveroussy.fr/sites/default/files/meta-analyses-protocol-rtt-sclc.pdf).
selection criteria and search strategy
To be eligible, trials had to compare two timing schedules of curative thoracic radiotherapy, i.e. earlier versus later within an individual trial in patients with limited-stage SCLC treated with chemo-radiotherapy. Our post hoc criterion to define early radiotherapy was similar to the one used by Fried et al. [13] and Spiro et al. [12]: radiotherapy should have been initiated before 9 weeks after randomization and before the third cycle of CT. Trials comparing two radiotherapy durations, i.e. a shorter versus a longer course within an individual trial with at least a two-week treatment difference observed between the two arms, were also eligible. In this article, we will use the term ‘earlier or shorter’ for arms where earlier and/or shorter radiotherapy was used and the term ‘later or longer’ for later and/or longer radiotherapy arms. Trials had to start after 1969 and to end before 2006 and be properly randomized. The planned CT schedule (drugs, doses, number of cycles) had to be the same in both arms, but radiotherapy modalities could be different. The total dose of radiotherapy had to be at least 30 Gy. Orthovoltage radiotherapy was an exclusion criterion. Eligible patients should have had a WHO (or equivalent) performance status of 0–2 and should not have received previous treatment of this cancer. To limit publication bias, we searched for both published and unpublished trials without language restriction (see supplementary Appendix S1, available at Annals of Oncology online for search strategy).
statistical analysis
We describe IPD collection and quality control in supplementary Appendix S2, available at Annals of Oncology online. The main end point was overall survival, and the secondary end points were progression-free survival and severe acute toxicities. Overall survival was defined as the time from randomization until death from any cause or the last follow-up for surviving patients. Progression-free survival was defined as the time from randomization until first progression or death from any cause, or the last follow-up for surviving patients without progression. We did not perform analyses on loco-regional control, cancer deaths and late toxicities due to lack of data. The median follow-up was estimated using the reverse Kaplan–Meier method [17].
We carried out all analyses on an intention-to-treat basis. Survival analyses were stratified by trial, and the log-rank expected number of deaths and variance were used to calculate individual and overall pooled hazard ratios (HRs) by the fixed-effect model [15]. A similar model was used to estimate odds ratios (ORs) for the comparison of toxicity between arms. χ2 tests and the I2 statistic were used to study heterogeneity between trials [18]. HRs were calculated using a DerSimonian–Laird random-effects model if heterogeneity had a P-value of <0.10 [19]. Stratified survival curves were estimated for control and experimental groups, using annual death rates and the pooled HR, and were used to estimate the absolute benefit at 3 and 5 years with their 95% CIs [20]. Five-year mean survival times, parameters commonly used in economic evaluation, were also estimated (supplementary Appendix S3, available at Annals of Oncology online) [21–23].
Subset analyses according to trial characteristics were preplanned. We investigated whether the treatment effect was dependent on any difference in the proportion of patients who were compliant with CT between the treatment arms within each trial. A patient was defined as compliant if he/she received 100% or more of the planned number of CT cycles, except for the CALGB8083 trial in which patients receiving six CT cycles or more were considered to be compliant. A trial was considered to be having different ‘between-arm’ compliance if the difference was ≥10% and to be having similar ‘between-arm’ compliance if it was <10% [12]. No other information on CT administration, such as the actual drug dose received or delays in CT administration, was available. χ2 tests for interaction or trend were used to assess treatment effects across trial subsets. Overall heterogeneity was decomposed into the sum of between-subset and residual (within-subset) heterogeneity: the lower the residual heterogeneity, the greater the overall heterogeneity of the treatment effect between trials was explained by the trial characteristic [24]. χ2 tests for interaction or trend were also used to test whether there was any evidence that a particular type of patient benefited more or less from ‘earlier or shorter’ radiotherapy according to predefined subgroups. If there was substantial overall heterogeneity, then subgroup analyses were planned within treatment categories. All P-values were two-sided. Analyses were carried out using SAS version 9.3.
role of the funding source
The funding sources had no role in the study design, data collection, data analysis, data interpretation or manuscript writing. B.L. and J.-P.P. had full access to all the raw data. The corresponding author had the final responsibility for the decision to submit for publication.
results
Twelve randomized trials [12, 16, 25–34] including 2668 patients were eligible. Data on nine trials and 2305 patients (86% of potentially eligible patients) were available for this IPD meta-analysis (supplementary Figure S1, available at Annals of Oncology online). Data from one trial were lost [32], and we did not succeed in contacting the investigator of two other trials [33, 34]. Table 1 depicts the nine trials included [12, 16, 25–31] and supplementary Table S2, available at Annals of Oncology online summarizes the trials with no available data. Four trials [16, 27, 30, 31] had different radiotherapy modalities between the two arms, including three trials [16, 30, 31] comparing shorter versus longer radiotherapy duration. Central randomization was used in all trials, except one that used sealed envelopes [25]. In total, out of the 80 patients initially excluded from the individual trial analyses, data concerning 75 patients were recovered. The median follow-up was 10 years without any difference between the treatment arms. Patient characteristics were well balanced between the two arms of the analysis (supplementary Table S3, available at Annals of Oncology online). Three trials [16, 26, 28] were categorized as having similar CT compliance in both arms, and they had a proportion of at least 79% of patients who were compliant with CT (i.e. receiving all their cycles) (supplementary Table S4, available at Annals of Oncology online). Five trials [12, 25, 27, 29, 31] had different CT compliance, with all of them exhibiting a lower compliance rate in the ‘earlier or shorter’ arm. For the CCWFU62286 trial, we had no data available on individual CT compliance neither in the patient-level data provided by the investigator nor in the publication [30]: the CCWFU62286 trial was thus excluded from the trial subset analysis based on CT compliance. In the ‘later or longer’ arm, 88% of patients started radiotherapy when compared with 93% in the ‘earlier or shorter’ arm (supplementary Table S5, available at Annals of Oncology online). Among the five trials [12, 25, 26, 27, 29] comparing earlier and later radiotherapy with individual data on radiotherapy compliance, the observed difference in median times between the two arms from randomization to the start of radiotherapy ranged from 63 to 93 days compared with 56 to 84 days for the planned difference (supplementary Table S6, available at Annals of Oncology online). There was also a significant association between individual RT compliance and CT compliance (Cochran–Mantel–Haenszel test stratified by trial: P < 0.0001). The more a patient was compliant with CT (i.e. receiving all their cycles), the more he/she was compliant with RT (i.e. receiving 90% of the total RT dose).
Table 1.
Description of trials
| Trials | Inclusion period | Start of thoracic radiation (day) | RT dose (Gy)/fraction/duration (weeks) | CT (mg/m2) | Number of CT cycles (before RT, during RT, after RT) | Number of patients randomized$a | Median follow-up (years) |
|---|---|---|---|---|---|---|---|
| Earlier versus later radiotherapy | |||||||
| CALGB8083 [25] | 1981–84 | EoS: Day 1 LoL: Day 64 |
50 Gy/24 fr/5 weeks | C: 1000 mg/m2, every 3 weeks V: 1.4 mg/m2, every 3 weeks E: 80 × 3 mg/m2 every 3 weeks Starting at cycle 7 for odd-numbered cycles: C: 1000 mg/m2, every 3 weeks V: 1.4 mg/m2, every 3 weeks A: 50 mg/m2, every 3 weeks |
About 26 cycles EoS: 2 cycles during RT, up to 24 cycles after RT LoL: 3 cycles before RT, 2 cycles during RT, up to 21 cycles after RT |
292 | 17.2 |
| BR.6 [26] | 1985–88 | EoS: Day 22 LoL: Day 106 |
40 Gy/15 fr/3 weeks | EoS: P: 25 mg/m2 × 3 days, weeks4,11,17 E: 100 mg/m2 × 3 days, weeks4,11,17 alternating with C: 1000 mg/m2, weeks1,8,14 A: 50 mg/m2, weeks1,8,14 V: 2 mg, weeks1,8,14 LoL: P: 25 mg/m2 × 3 days, weeks4,10,16 E: 100 mg/m2 × 3 days, weeks4,10,16 alternating with C: 1000 mg/m2, weeks1,7,13 A: 50 mg/m2, weeks1,7,13 V: 2 mg, weeks1,7,13 |
EoS: 6 cycles (1 before RT, 1 during RT, 4 after RT) LoL: 6 cycles (5 before RT, 1 during RT) |
332 | 11.2 |
| EORTC08877 [27] | 1989–95 | EoS: Day 43 LoL: Day 99 |
EoS: 12.5 Gy/5 fr/1 week + break 3 weeks + 12.5 Gy/5 fr/1 week + break 3 weeks + 12.5 Gy/5 fr/1 week + break 3 weeks + 12.5 Gy/5 fr/1 week LoL: 50 Gy/20 fr/4 weeks |
EoS: C: 1000 mg/m2, weeks1,5,9,13,17 A: 45 mg/m2 weeks1,5,9,13,17 E: 100 × 3 mg/m2 weeks1,5,9,13,17 LoL: C: 1000 mg/m2 weeks1,4,7,10,13 A: 45 mg/m2 weeks1,4,7,10,13 E: 100 × 3 mg/m2, weeks1,4,7,10,13 |
EoS: 5 cycles (1 before RT, 4 alternating with RTb) LoL: 5 cycles (5 before RT) |
349 | 7.2 |
| JCOG9104 [28] | 1991–95 | EoS: Day 2 LoL: Day 85 |
45 Gy/30 fr/3 weeks bid | EoS: P: 80 mg/m2, weeks1,5,9,13 E: 100 × 3 mg/m2, weeks1,5,9,13 LoL: P: 80 mg/m2, weeks1,4,7,10 E: 100 × 3 mg/m2, weeks1,4,7,10 |
EoS: 4 cycles (1 during RT, 3 after RT) LoL: 4 cycles (4 before RT) |
231 | 6.8 |
| LLCG93 [12] | 1993–99 | EoS: Day 22 LoL: Day 106 |
40 Gy/15 fr/3 weeks | P: 25 × 3 mg:m2, weeks4,10,16 E: 100 × 3 mg/m2, weeks4,10,16 alternating with C: 1000 mg/m2, weeks1,7,13 A: 50 mg/m2, weeks1,7,13 V: 2 mg, weeks1,7,13 |
EoS: 6 cycles (1 before RT, 1 during RT, 4 after RT) LoL: 6 cycles (5 before RT, 1 during RT) |
325 | 5.3 |
| HeCOG93 [29] | 1993–99 | EoS: Day 1 LoL: Day 57 |
45 Gy/30 fr/3 weeks bid | Cb: 6 AUC, weeks1,4,7,10,13,16 E: 100 × 3 mg/m2, weeks1,4,7,10,13,16 |
EoS: 6 cycles (1 during RT, 5 after RT) LoL: 6 cycles (3 before RT, 1 during RT, 2 after RT) |
81 | 11.8 |
| Trials | Inclusion period | Start of thoracic radiation (day) | RT dose (Gy)/ fraction/duration (weeks) | CT (mg/m2) | Number of CT cycles (before RT, during RT, after RT) | Number of patients randomizeda | Median follow-up (years) |
| Shorter versus longer radiotherapy duration | |||||||
| CCCWFU62286 [30] | 1987–92 | EoS: Day 1 LoL: Day 8 |
EoS: 50 Gy/25 fr/5 weeks LoL: 20 Gy/8 fr/2 weeks + break 1 week + 20 Gy/8 fr/2 weeks + break 1 week + 10 Gy/4 fr/1 week |
C: 750 mg/m2, weeks7,10,16 A: 60 mg/m2, weeks7,10,16 V: 2 mg, weeks7,10,16 alternating with P: 60 mg/m2, weeks1,4,13 E: 120 × 3 mg/m2, weeks1,4,13 |
EoS: 6 cycles (2 during RT, 4 after RT) LoL: 6 cycles (3 alternating with RT, 3 after RT) |
114 | 17.3 |
| 03PCL88 [31] | 1988–94 | EoS: Day 30 LoL: Day 36 |
EoS: 50 Gy/20 fr/5 weeks LoL: 20 Gy/8 fr/2 weeks + break 2 weeks + 20 Gy/8 fr/2 weeks + break 2 weeks + 15 Gy/6 fr/1.5 weeks |
C: 1000 mg/m2, weeks1,13,17,21 A: 45 mg/m2, weeks1,13,17,21 E: 150 × 2 mg/m2, weeks1,13,17,21 Alternating with C: 1000 mg/m2, weeks5,9 Vd: 3 mg/m2, weeks5,9 E: 150 × 2 mg/m2, weeks5,9 |
EoS: 6 cycles (2 before RT, 1 during RT, 3 after RT) LoL: 6 cycles (2 before RT, 2 alternating with RT, 2 after RT) |
164 | 6.5 |
| ECOG3588 [16] | 1989–92 | Both arms: Day 1 | EoS: 45 Gy/30 fr/3 weeks bid LoL: 45 Gy/ 25 fr/5 weeks |
P: 60 mg/m2, weeks1,4,7,10 E: 120 × 3 mg/m2, weeks1,4,7,10 |
4 cycles (2 during RT, 2 after RT) | 417 | 13.0 |
Trials are chronologically ordered within each category of trials (earlier versus later RT, and shorter versus longer RT).
bid, RT given twice a day; CT, chemotherapy; EoS, ‘earlier or shorter’ radiotherapy; fr, fraction; Gy, Gray; LoL, ‘later or longer’ radiotherapy; RT, radiotherapy; A, adriamycin; C, cyclophosphamide; Cb, carboplatin; E, etoposide; P, cisplatin; V, vincristine; Vd, vindesine; BR, bronchus; CALGB, Cancer and Leukaemia Group B; CCCWFU, Comprehensive Cancer Centre of Wake Forest University; ECOG, Eastern Cooperative Oncology Group; EORTC, European Organisation for Research and Treatment of Cancer; HeCOG, Hellenic Cooperative Oncology Group; JCOG, Japan Clinical Oncology Group; LLCG, London Lung Cancer Group; PCL, Petites Cellules Limitées.
aNumber of patients analysed equals the number of patients randomized, except for the HeCOG trial for which data on 81 patients were available out of the 86 randomized patients.
bPublication [27] stated that ‘RT started […] on the 14th day of the second and subsequent courses of chemotherapy in arm Earlier RT’.
overall survival and progression-free survival
In our main analysis, when all trials were pooled together, ‘earlier or shorter’ radiotherapy did not have a significant impact on overall survival compared with ‘later or longer’ radiotherapy (HR 0.99, 95% CI 0.91–1.08, P = 0.78) (supplementary Figure S2, available at Annals of Oncology online). Treatment effect heterogeneity was observed (P = 0.006, I2 = 63%). With a random-effects model, the HR was not significant (0.99, 0.85–1.15, P = 0.90).
Data on tumour progression were not available for two trials [27, 31], thus the progression-free survival analysis concerned only seven trials comprising 1764 patients and 1596 events. There was no significant impact of radiotherapy timing on progression-free survival (HR 0.93, 95% CI 0.84–1.02, P = 0.13) (supplementary Figure S3, available at Annals of Oncology online).
trial subsets
Table 2 shows the HRs for overall survival according to the different preplanned subsets analyses, described in supplementary Table S7, available at Annals of Oncology online, with overall between-trial heterogeneity decomposed into the sum of between-subset and residual (within-subset) heterogeneity. Trial subsets were in decreasing order of residual heterogeneity: the lower the residual heterogeneity for one trial subset, the greater the studied characteristic (CT compliance, RT dose per fraction, etc.) explained overall heterogeneity. In Table 2, between-subset heterogeneity was associated with an interaction test between the treatment received (‘earlier or shorter’ RT versus ‘later or longer’ RT) and the studied characteristic of the subset, and also with a trend test when the studied subset categories were ordinal (RT dose per fraction and RT overall treatment time). Five trial characteristics were found to be associated with an improvement in overall survival with ‘earlier or shorter’ radiotherapy (Table 2): similar CT compliance in both arms, a dose per fraction lower than 1.8 Gy, hyperfractionated radiotherapy, overall treatment time of less than 30 days and platin-based CT. It should be emphasized that trials using hyperfractionated radiotherapy delivered fractions of less than 1.8 Gy, and the overall treatment time was less than 30 days.
Table 2.
Effect of ‘earlier or shorter’ radiotherapy versus ‘later or longer’ radiotherapy on overall survival according to different trial subsets
| Trials characteristics | HR [95% CI]a | Heterogeneityb |
|
|---|---|---|---|
| Between-subset | Residual (or within-subset) | ||
| CT compliance between arms | |||
| Similar | 0.79 [0.69–0.91] | 19.5*** | 1.9 |
| Different | 1.19 [1.05–1.34] | ||
| RT dose per fraction | |||
| <1.8 Gy | 0.82 [0.71–0.96] | 7.5* (Ptrend = 0.02)c | 14.1* |
| 1.8–2.4 Gy | 1.11 [0.90–1.35] | ||
| >2.4 Gy | 1.06 [0.94–1.20] | ||
| Type of RT | |||
| Hyperfractionated | 0.82 [0.71–0.96] | 7.4** | 14.2* |
| Standard | 1.07 [0.96–1.19] | ||
| RT overall treatment time | |||
| ≤30 days in both arms | 0.89 [0.78–1.02] | 5.6 (Ptrend = 0.02)c | 16.0* |
| One arm ≤30 days, one >30 days | 0.99 [0.85–1.15] | ||
| >30 days in both arms | 1.16 [0.98–1.38] | ||
| Platin-based CT during RT in both arms | |||
| Yes | 0.89 [0.79–1.01] | 5.5** | 16.1* |
| No | 1.09 [0.97–1.24] | ||
| Concurrent CT in both arms | |||
| Yes | 0.95 [0.85–1.06] | 1.5 | 20.1** |
| No | 1.06 [0.92–1.22] | ||
| Same RT in the two arms | |||
| Yes | 0.96 [0.85–1.08] | 0.5 | 21.1** |
| No | 1.02 [0.90–1.16] | ||
CI, confidence interval; CT, chemotherapy; HR, hazard ratio; RT, radiotherapy.
aHazard ratio of death following ‘earlier or shorter’ versus ‘later or longer’ radiotherapy.
bTotal heterogeneity is the sum of between-subset and residual (within-subset) heterogeneity and is equal to 21.6 (analysis based on nine trials) except for CT compliance 21.4 (eight trials). The test associated with between-subset heterogeneity corresponds to the interaction test. The lower the residual heterogeneity, the greater was the overall heterogeneity of the treatment effect between trials explained by the trial characteristic.
cTest for trend.
*P < 0.05; **P < 0.01; ***P < 0.001.
The ‘between-arm’ CT compliance (number of cycles actually given) is the factor that best explained between-trial heterogeneity, i.e. with the lowest residual heterogeneity (Table 2).
CT compliance and overall survival
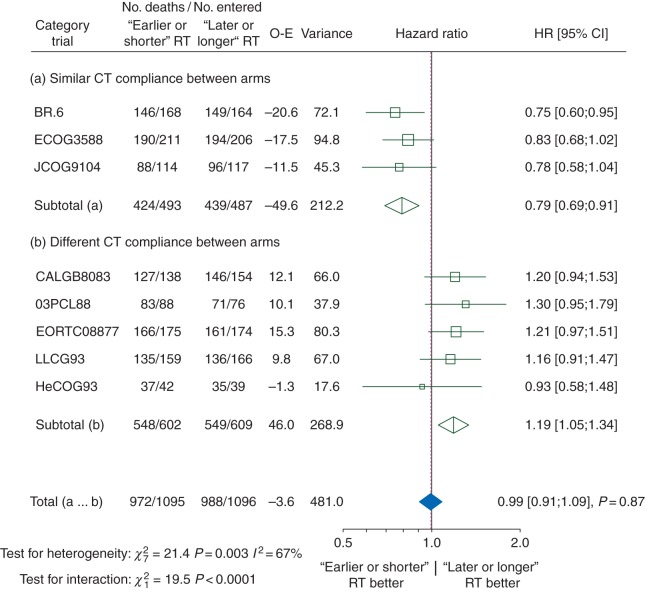
The HR for overall survival was significantly in favour of ‘earlier or shorter’ radiotherapy among trials in which the defined CT compliance was similar in both arms (Figure 1; HR 0.79, 95% CI 0.69–0.91) and in favour of ‘later or longer’ radiotherapy among trials with different CT compliance: (1.19, 1.05–1.34). There was a significant interaction between CT compliance and the treatment effect (interaction test, P < 0.0001). In trials with similar CT compliance in both arms, ‘earlier or shorter’ radiotherapy compared with ‘later or longer’ radiotherapy increased the absolute 3-year and 5-year overall survival rate by 5.7% (from 24.4% to 30.1%) and by 7.7% (from 16.5% to 24.2%), respectively (Figure 2). In trials with different CT compliance, ‘earlier or shorter’ radiotherapy decreased the absolute 3-year and 5-year overall survival rate, respectively, by 3.8% (from 16.1% to 12.3%) and 2.2% (from 10.5% to 8.3%) (Figure 2). In other words, ‘earlier or shorter’ radiotherapy extended the 5-year mean survival time by 4.2 months (95% CI 1.8–6.7) from 24.7 to 28.9 months in trials with similar CT compliance. In trials with different CT compliance, ‘earlier or shorter’ radiotherapy shortened the 5-year mean survival time by 3.1 months (95% CI 1.3–4.9) from 20.6 to 17.5 months.
Figure 1.
Effect of ‘earlier or shorter’ radiotherapy versus ‘later or longer’ radiotherapy on overall survival according to CT compliance. Each trial is represented by a square, the centre of which denotes the HR of death for that trial comparison, with the horizontal lines showing the 95% CIs. The size of the square is directly proportional to the amount of information contributed by the trial. The clear diamonds represent pooled HRs for the trial groups and the black diamond the overall HR, with the centre denoting the HR and the extremities the 95% CI. The fixed-effect model was used. Trials were chronologically ordered within each category of trials. Of note, data on CT compliance were not available for the CCCWFU62286 trial, which is thus not included in this analysis. CI, confidence interval; CT, chemotherapy; HR, hazard ratio; O-E, observed-expected; RT, radiotherapy.
Figure 2.
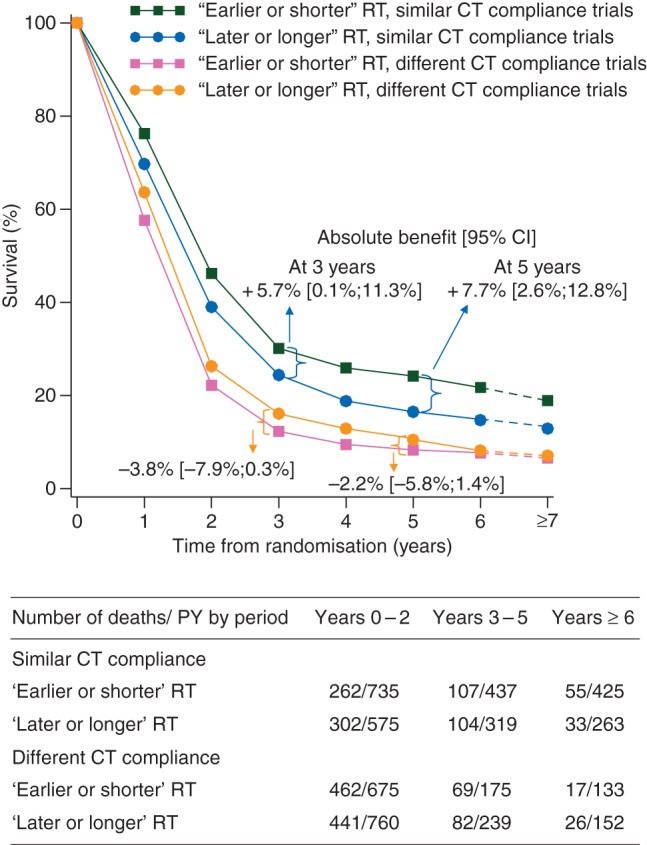
Survival curves for overall survival according to chemotherapy compliance. CI, confidence interval; CT, chemotherapy; HR, hazard ratio; PY, person-year; RT, radiotherapy.
compliance with CT and progression-free survival
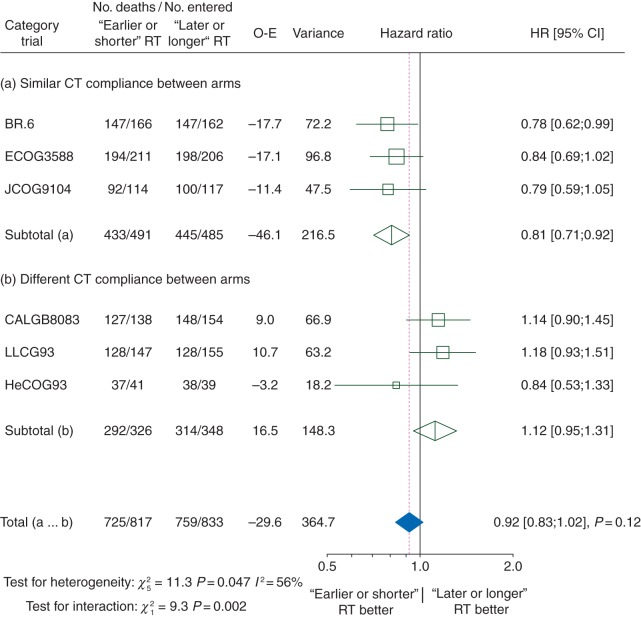
The HR for progression-free survival favours trials in which ‘earlier or shorter’ radiotherapy was delivered with similar CT compliance in both arms (HR for similar CT compliance: 0.81, 95% CI 0.71–0.92; for different CT compliance: 1.12, 0.95–1.31) (Figure 3). In trials in which CT compliance was similar, ‘earlier or shorter’ radiotherapy increased the 3-year progression-free survival rate by 6.3% (95% CI 1.0%–11.6%) and the 5-year progression-free survival rate by 5.6% (0.7%–10.5%) (supplementary Figure S4, available at Annals of Oncology online).
Figure 3.
Effect of ‘earlier or shorter’ radiotherapy versus ‘later or longer’ radiotherapy on progression-free survival according to chemotherapy compliance. Each trial is represented by a square, the centre of which denotes the HR of death or tumour progression for that trial comparison, with the horizontal lines showing the 95% CIs. The size of the square is directly proportional to the amount of information contributed by the trial. The clear diamonds represent pooled HRs for the trial groups and the black diamond the overall HRs, with the centre denoting the HR and the extremities the 95% CI. The fixed-effect model was used. CI, confidence interval; CT, chemotherapy; HR, hazard ratio; O-E, observed-expected; RT, radiotherapy.
compliance with CT and landmark analysis
As the observed effect of CT compliance may be due to early treatment interruption because of progression or death, a post hoc landmark analysis on the impact of individual CT compliance on overall survival and progression-free survival was carried out among patients who survived (or had no disease progression) for at least 120 days. This landmark was chosen because most of the patients finished their chemoradiation treatment at 120 days. Patients with good CT compliance, i.e. those receiving the planned total number of CT cycles, had higher overall survival and progression-free survival than those with poor CT compliance (HR 0.56, 95% CI 0.49–0.64 and 0.70, 0.59–0.83, respectively; supplementary Table S8, available at Annals of Oncology online).
subgroup analyses
When the two subsets of trials with similar and different CT compliance were considered separately, no variation in the treatment effect was seen according to age, sex or the performance status (supplementary Figure S5, available at Annals of Oncology online).
sensitivity analyses
Supplementary Table S9, available at Annals of Oncology online shows the results of preplanned sensitivity analyses after excluding some trials. The results were similar to those of the main analysis, in particular to those related to CT compliance.
toxicity
Three types of severe acute toxicities were significantly more frequent in patients receiving ‘earlier or shorter’ thoracic radiotherapy: neutrophil, oesophageal and cardiac toxicity (Table 3) [35]. The toxicity ORs according to trial subsets based on CT compliance are shown in supplementary Table S10, available at Annals of Oncology online. We did not perform analyses on late toxicities as IPD were available only for two trials [26, 27].
Table 3.
Acute toxicity according to radiotherapy arm
| Severe toxicity (grades 3–5) | Availability | Toxicity rate |
Results | P-value efficacy | I2 (%) | P-value heterogeneity | |
|---|---|---|---|---|---|---|---|
| Number of trials (patients) | ‘Later or longer’ RT | ‘Earlier or shorter’ RTa | OR [95% CI] | ||||
| Neutrophil | 6 (1,453) | 59 | 69 | 1.54 [1.19–2.00] | 0.001 | 79 | <0.001 |
| Haemoglobin | 6 (1,476) | 21 | 24 | 1.17 [0.91–1.52] | 0.22 | 31 | 0.21 |
| Platelets | 7 (1,817) | 18 | 21 | 1.22 [0.96–1.55] | 0.11 | 45 | 0.09 |
| Oesophageal | 8 (1,950) | 8 | 14 | 1.93 [1.45–2.56] | <0.001 | 45 | 0.08 |
| Pulmonary | 5 (1,207) | 4 | 6 | 1.50 [0.86–2.62] | 0.16 | 0 | 0.68 |
| Cardiac | 6 (1,648) | 1 | 3 | 3.12 [1.46–6.68] | 0.003 | 0 | 0.95 |
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, World Health Organization criteria or Eastern Cooperative Oncology Group Common Toxicity Criteria, depending on the trials. Severe toxicity was defined as grades 3–5 toxicity. Grade 5 was present only for pulmonary toxicity (n = 4) and cardiac toxicity (n = 1).
CI, confidence interval; OR, odds ratio of the ‘earlier or shorter’ RT arm compared with ‘later or longer’ RT arm; RT, radiotherapy.
aThe difference in the rate of toxicity between the two treatment arms was computed based on the rate in the ‘later or longer’ radiotherapy arm and the OR [35].
discussion
On the basis of this IPD meta-analysis of nine trials evaluating the optimal timing of thoracic radiotherapy in SCLC, overall there was no survival difference between ‘earlier or shorter’ and ‘later or longer’ thoracic radiotherapy (HR 0.99; P = 0.78). As individual trials favoured either ‘earlier or shorter’ or ‘later or longer’ thoracic radiotherapy, it seemed relevant to further analyse these data and perform a subset analysis focusing on CT compliance. For trials with different CT compliance, in which lower compliance was always observed in the ‘earlier or shorter’ arm, ‘earlier or shorter’ delivery had a deleterious effect on survival compared with ‘later or longer’ radiotherapy (HR 1.19, 95% CI 1.05–1.34). For trials that had similar (and good, i.e. at least 79% of compliant patients per arm) CT compliance, ‘earlier or shorter’ delivery of thoracic radiotherapy improved overall survival (HR 0.79, 95% CI 0.69–0.91). ‘Earlier or shorter’ thoracic radiotherapy, when delivered with similar and good CT compliance, yielded an absolute survival gain of 5.7% at 3 years and 7.7% at 5 years compared with ‘later or longer’ thoracic radiotherapy. Similar results were found for progression-free survival. We carried out sensitivity analyses by only taking into account trials in which patients received concomitant chemoradiation and trials that exclusively addressed the timing of thoracic radiotherapy in their design. In these sensitivity analyses, the survival gain of delivering ‘earlier or shorter’ thoracic radiotherapy with similar CT compliance remained significant (supplementary Table S9, available at Annals of Oncology online). Using a landmark analysis, it was possible to confirm with IPD that good CT compliance was associated with longer survival. Of note, there was a significant association at the patient level between RT compliance and CT compliance, which could explain our results.
Hyperfractionated accelerated radiotherapy also improved survival when delivered ‘earlier or shorter’, but this finding was driven by two large trials, JCOG9104 [28] and ECOG3588 [16], with good CT compliance. In the ECOG3588 trial [16], no dose adjustment was allowed for the first two cycles. Cisplatin-based CT seems to be more beneficial when combined with ‘earlier or shorter’ thoracic radiotherapy. Issues such as the total radiotherapy dose and the dose per fraction are more difficult to interpret, because they are tightly correlated (Tables 1 and 2).
‘Earlier or shorter’ thoracic radiotherapy was associated with a higher incidence of acute severe oesophagitis than ‘later or longer’ radiotherapy (OR 1.93 [1.45–2.56]), but had no consequence on compliance with either CT or radiotherapy. Mauguen et al. [15] also showed that hyperfractionated accelerated radiotherapy increased oesophageal toxicity. In this IPD meta-analysis, neutropenia was more frequent with ‘earlier or shorter’ radiotherapy (OR 1.54, 95% CI 1.19–2.00), and this effect was observed exclusively in trials with similar CT compliance (supplementary Table S10, available at Annals of Oncology online). Acute severe pulmonary toxicity was similar in ‘earlier or shorter’ or ‘later or longer’ thoracic radiotherapy groups, whereas acute severe cardiac toxicity was higher when ‘earlier or shorter’ radiotherapy was delivered (OR 3.12, 1.46–6.68). The latter finding should be interpreted with caution because it is based on only 26 cardiac events occurring in 1648 patients among whom this toxicity was documented.
The results of this IPD meta-analysis primarily reinforce the evidence that CT should be delivered as intended whenever possible [1, 36]. Cisplatin-based CT administered with good CT compliance appeared to be the best treatment when combined with ‘earlier or shorter’ thoracic radiotherapy, as all the three trials [16, 26, 28] with similar CT compliance used this regimen. This is in line with previous literature-based meta-analyses, [9–14] in particular that reported by Spiro et al. [12], which focused on CT compliance (supplementary Table S1, available at Annals of Oncology online). Interestingly, a recently published randomized trial [37], where all patients had early hyperfractionated radiotherapy given concomitantly with the first cycle of etoposide, showed a 5-year survival rate of 34.3%, which the authors attributed to better patient selection and radiotherapy quality control. It will be interesting to observe the results of the ongoing CALGB 30610 (NCT00632853) and the completed CONVERT (NCT00433563) randomized trials comparing early hyperfractionated radiotherapy to early standard radiotherapy with a higher total dose and concomitant cisplatin plus etoposide in both arms.
The present IPD meta-analysis has some shortcomings. First, the trials were conducted at a time when imaging was not as advanced as it is today. However, the observed 5-year survival rate of about 25%, when ‘earlier or shorter’ thoracic radiotherapy was combined with good CT compliance, remains among the best published results. These results continue to support their applicability today, as there has been no major change in the standard of care of SCLC (NCCN and ESMO guidelines) [6]. A recently published Korean phase III trial [38], which was not included in this meta-analysis as it was closed to accrual in 2010 (supplementary Table S2, available at Annals of Oncology online), showed a similar 5-year survival rate of approximately 24%. This trial did not show a significant difference in terms of overall survival between the two arms (HR 0.93, 0.67–1.29), but the study included only 222 patients. Second, data were not available for two other trials [32, 34] (supplementary Table S2, available at Annals of Oncology online). However, when we included these three trials for which we have only published data (two in the similar CT compliance group [34, 38] and one in a different CT compliance group [32]) in a post hoc analysis, we found similar effects on overall survival (HR 0.81, 95% CI 0.72–0.90 versus 1.18, 1.06–1.32 for similar and different CT compliance subsets, respectively). Third, only the number of CT cycles administered were available, but not doses or delays in treatment. However, consistency across end points and between the main analysis and sensitivity analyses underscore the robustness of our results. Another limitation is that data on long-term toxicity were not available, but less toxicity would be expected with the newer radiotherapy techniques. Lastly, the quality of radiotherapy could not be addressed in this meta-analysis as it was not explored in the studies included.
To improve the still dismal prognosis of patients with stage I–IIIB SCLC, we postulate that the optimal treatment should be full dose but acceptable CT combined with ‘earlier or shorter’ thoracic radiotherapy (i.e. before 9 weeks), preferably within a short overall treatment time. Our IPD meta-analysis provides the best evidence of the beneficial effect of ‘earlier or shorter’ radiotherapy when CT is administered with good compliance.
funding
The meta-analysis was funded by the French National Cancer Institute (Programme Hospitalier de Recherche Clinique), the Ligue Nationale Contre le Cancer and partly by Sanofi-Aventis (unrestricted grants). The investigators meeting was also funded by Gustave Roussy, Lilly and Astra-Zeneca (unrestricted grants). No grant number is applicable.
disclosure
Consultant or advisory role: D.H.J., Peloton Therapeutics/miRNA Therapeutics; P.B., Merck Sharp Dohme/Bristol-Myers Squibb; L.S., Boehringer Ingelheim. Stock ownership: L.S., AstraZeneca. Honoraria: P.B., AstraZeneca/Verastem; L.S., Innate Pharma. Research funding: P.B., Merck Sharp Dohme/Bristol-Myers Squibb; L.S., Pfizer, AstraZeneca, Astex Pharmaceuticals. Travel, accommodations, expenses: P.B., Merck Sharp Dohme. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank the patients and the clinical investigators who took part in the trials and contributed to this research. The meta-analysis would not have been possible without their participation or without the collaborating institutions or groups that provided their trial data: The Alliance for Clinical Trials in Oncology (formerly Cancer and Leukemia Group B), Comprehensive Cancer Centre of Wake Forest University, ECOG-ACRIN Cancer Research Group (formerly Eastern Cooperative Oncology Group), European Organization for Research and Treatment of Cancer, Hellenic Cooperative Oncology Group, Japan Clinical Oncology Group, London Lung Cancer Group, National Cancer Institute of Canada Clinical Trial Group and ‘Petites Cellules’ Group. We are grateful to Lorna Saint Ange for editorial assistance.
appendix
RTT-SCLC Collaborative Group
Secretariat
D. De Ruysscher, C. Le Pechoux, B. Lueza, E. Paris, J.P. Pignon, M. Pijls-Johannesma, A.S. Veillard.
advisory board
Rodrigo Arriagada, Paul Baas, Hak Choy, Allan Price, Lesley Seymour.
investigators
Rodrigo Arriagada (Gustave Roussy, Villejuif, France; Karolinska Institutet, Stockholm, Sweden), Paul Baas (Netherlands Cancer Institute—Antoni van Leeuwenhoek Ziekenhuis, Amsterdam, The Netherlands), William Blackstock (Wake Forest University School of Medicine, Winston-Salem, NC, USA), Sylvie Chevret (CRESS—UMR 1153, Inserm, Paris Diderot University, Paris, France), Hak Choy (University of Texas Southwestern, Dallas, Texas, USA), Jeffrey Crawford (Duke University School of Medicine, Durham, NC, USA), Urania Dafni (University of Athens, Greece), Suzanne Dahlberg (Dana-Farber Cancer Institute, Boston, MA, USA), Dirk De Ruysscher (Maastricht University Medical Center, Maastricht, the Netherlands; KU Leuven, Leuven, Belgium), Allan Hackshaw (University College London, UK), Baktiar Hasan (EORTC Data Center, Brussels, Belgium), David H. Johnson (UT Southwestern University School of Medicine, Dallas, USA), Cécile Le Pechoux (Gustave Roussy, Villejuif, France), Bernard Lebeau (Hôpital St Antoine, Paris, France), James Lovato (Wake Forest University Health Sciences, Winston-Salem, NC, USA), Béranger Lueza (Gustave Roussy, Villejuif, France), Nevin Murray (British Columbia Cancer Agency, Vancouver, Canada), Mary O’Brien (Royal Marsden Hospital, London, UK), Emmanuelle Paris (Gustave Roussy, Villejuif, France), Jean-Pierre Pignon (Gustave Roussy, Villejuif, France), Madelon Pijls-Johannesma (MAASTRO Clinic, Maastricht, the Netherlands), Allan Price (University of Edinburgh, Edinburgh, UK), Stephen Spiro (University College London Hospital London, UK), Lesley Seymour (NCIC-CTG, Kingston, Ontario, Canada), Taro Shibata (JCOG Data Center, National Cancer Center Coordinating, Tokyo, Japan), Dimosthenis Skarlos (Metropolitan Hospital N. Faliro, Athens, Greece), Stephen Spiro (University College London Hospital, London, UK), Minoru Takada (Osaka Prefectural Habikino Hospital, Osaka, Japan), Anne-Sophie Veillard (Gustave Roussy, Villejuif, France) and Xiaofei Wang (Alliance Data and Statistical Center, NC, USA).
Contributor Information
Collaborators: on behalf of the RTT-SCLC Collaborative Group, D. De Ruysscher, C. Le Pechoux, B. Lueza, E. Paris, J.P. Pignon, M. Pijls-Johannesma, A.S. Veillard, Rodrigo Arriagada, Paul Baas, William Blackstock, Sylvie Chevret, Hak Choy, Jeffrey Crawford, Urania Dafni, Suzanne Dahlberg, Dirk De Ruysscher, Allan Hackshaw, Baktiar Hasan, David H. Johnson, Cécile Le Pechoux, Bernard Lebeau, James Lovato, Béranger Lueza, Nevin Murray, Mary O’Brien, Emmanuelle Paris, Jean-Pierre Pignon, Madelon Pijls-Johannesma, Allan Price, Stephen Spiro, Lesley Seymour, Taro Shibata, Dimosthenis Skarlos, Stephen Spiro, Minoru Takada, Anne-Sophie Veillard, and Xiaofei Wang
references
- 1.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011; 378: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Crowley J, Van Houtte P et al. , International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007; 2: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 3.Arriagada R, Le Chevalier T, Pignon JP et al. . Initial chemotherapy doses and survival in limited small cell lung cancer. N Engl J Med 1993; 329: 1848–1852. [DOI] [PubMed] [Google Scholar]
- 4.Pignon JP, Arriagada R, Ihde DC et al. . A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992; 327: 1618–1624. [DOI] [PubMed] [Google Scholar]
- 5.Stahel R, Thatcher N, Fruh M et al. . 1st ESMO Consensus Conference in lung cancer; Lugano 2010: small-cell lung cancer. Ann Oncol 2011; 22: 1973–1980. [DOI] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer, version 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf (29 October 2014, date last accessed).
- 7.Aupérin A, Arriagada R, Pignon JP et al. . Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med 1999; 341: 476–484. [DOI] [PubMed] [Google Scholar]
- 8.Le Péchoux C, Dunant A, Senan S et al. . Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009; 10: 467–474. [DOI] [PubMed] [Google Scholar]
- 9.Pijls-Johannesma MC, De Ruysscher D, Lambin P, Rutten I, Vansteenkiste JF. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev 2005; 1: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J et al. . Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 2006; 17: 543–552. [DOI] [PubMed] [Google Scholar]
- 11.Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J et al. . Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev 2007; 33: 461–473. [DOI] [PubMed] [Google Scholar]
- 12.Spiro SG, James LE, Rudd RM et al. . Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol 2006; 24: 3823–3830. [DOI] [PubMed] [Google Scholar]
- 13.Fried DB, Morris DE, Poole C et al. . Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol 2004; 22: 4837–4845. Erratum in: J Clin Oncol 2005; 23: 248. [DOI] [PubMed] [Google Scholar]
- 14.Huncharek M, McGarry R. A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist 2004; 9: 665–672. [DOI] [PubMed] [Google Scholar]
- 15.Mauguen A, Le Péchoux C, Saunders MI et al. . Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol 2012; 30: 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turrisi AT, Kim K, Blum R et al. . Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999; 340: 265–271. [DOI] [PubMed] [Google Scholar]
- 17.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic or immune therapy: 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 1992; 339: 1–15. [PubMed] [Google Scholar]
- 21.Wei Y, Royston P, Tierney JF, Parmar MKB. Meta-analysis of time-to-event outcomes from randomized trials using restricted mean survival time: application to individual participant data. Stat Med 2015; 34: 2881–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lueza B, Mauguen A, Pignon JP, Rivero-Arias O, Bonastre J. Difference in restricted mean survival time for cost-effectiveness analysis using individual patient data meta-analysis: evidence from a case study. PLoS One 2016; 11: e0150032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lueza B, Rotolo F, Bonastre J, Pignon JP, Michiels S. Bias and precision of methods for estimating the difference in restricted mean survival time from an individual patient data meta-analysis. BMC Med Res Meth 2016; 16: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311: 899–909. [PMC free article] [PubMed] [Google Scholar]
- 25.Perry MC, Herndon JE, Eaton WL et al. . Thoracic radiation therapy added to chemotherapy for small-cell lung cancer: an update of Cancer and Leukemia Group B Study 8083. J Clin Oncol 1998; 16: 2466–2467. [DOI] [PubMed] [Google Scholar]
- 26.Murray N, Coy P, Pater JL et al. . Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993; 11: 336–344. [DOI] [PubMed] [Google Scholar]
- 27.Gregor A, Drings P, Burghouts J et al. . Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: a European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study. J Clin Oncol 1997; 15: 2840–2849. [DOI] [PubMed] [Google Scholar]
- 28.Takada M, Fukuoka M, Kawahara M et al. . Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002; 20: 3054–3060. [DOI] [PubMed] [Google Scholar]
- 29.Skarlos DV, Samantas E, Briassoulis E et al. . Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: a randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG). Ann Oncol 2001; 12: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 30.Blackstock AW, Bogart JA, Matthews C et al. . Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: final report of a randomized phase III trial. Clin Lung Cancer 2005; 6: 287–292. [DOI] [PubMed] [Google Scholar]
- 31.Lebeau B, Urban T, Brechot JM et al. . A randomized clinical trial comparing concurrent and alternating thoracic irradiation for patients with limited small cell lung carcinoma. ‘Petites Cellules Group’. Cancer 1999; 86: 1480–1487. [PubMed] [Google Scholar]
- 32.Work E, Nielsen OS, Bentzen SM, Fode K, Palshof T. for the Aarhus Lung Cancer Group. Randomized study of initial versus late chest irradiation combined with chemotherapy in limited-stage small-cell lung cancer. Aarhus Lung Cancer Group. J Clin Oncol 1997; 15: 3030–3037. [DOI] [PubMed] [Google Scholar]
- 33.Park SK, Kim GH, Jeong SS et al. . The effects according to the timing of thoracic radiotherapy in limited stage small cell lung cancer. Tuberc Respir Dis 1996; 43: 903–915. [Google Scholar]
- 34.Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study. J Clin Oncol 1997; 5: 893–900. [DOI] [PubMed] [Google Scholar]
- 35.Stewart L, Parmar M. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993; 341: 25–28. [DOI] [PubMed] [Google Scholar]
- 36.Pelayo AM, Gallego RÓ, Bonfill CX, Agra VY. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev 2009; 4: 1–44. [DOI] [PubMed] [Google Scholar]
- 37.Kubota K, Hida T, Ishikura S et al. , for the Japan Clinical Oncology Group. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomised phase 3 study. Lancet Oncol 2014; 15: 106–113. [DOI] [PubMed] [Google Scholar]
- 38.Sun JM, Ahn YC, Choi EK et al. . Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol 2013; 24: 2088–2092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.