In patients with advanced melanoma in the CheckMate 066 study, baseline health-related quality of life (HRQoL) with nivolumab was maintained over time, with statistically significant and clinically meaningful improvements in some exploratory analyses, and no HRQoL improvements with dacarbazine. Added to the survival benefit of nivolumab, the benefit-to-risk ratio favors nivolumab over dacarbazine.
Keywords: advanced melanoma, health-related quality of life, nivolumab, programmed death-1 receptor
Abstract
Background
Nivolumab has shown significant survival benefit and a favorable safety profile compared with dacarbazine chemotherapy among treatment-naïve patients with metastatic melanoma in the CheckMate 066 phase III study. Results from the health-related quality of life (HRQoL) analyses from CheckMate 066 are presented.
Patients and methods
HRQoL was evaluated at baseline and every 6 weeks while on treatment using the European Organisation for Research and Treatment of Care (EORTC) Core Quality of Life Questionnaire (QLQ-C30) and the EuroQoL Five Dimensions Questionnaire (EQ-5D). Via a multi-step statistical plan, data were analyzed descriptively, cross-sectionally, and longitudinally, adjusting for baseline covariates, in patients having baseline plus ≥1 post-baseline assessment.
Results
Baseline-adjusted completion rates for all HRQoL questionnaires across treatment arms were 65% and 70% for dacarbazine and nivolumab, respectively, and remained similar throughout treatment. The mean baseline HRQoL scores were similar for patients treated with nivolumab and dacarbazine. Baseline HRQoL levels with nivolumab were maintained over time. This exploratory analysis showed a between-arm difference in favor of nivolumab on the EQ-5D utility index and clinically meaningful EQ-5D improvements from baseline at several time points for patients receiving nivolumab. Patients treated with nivolumab did not show increased symptom burden as assessed by the EORTC QLQ-C30. No HRQoL change was noted with dacarbazine patients up to week 43, although the high attrition rate after week 13 did not allow any meaningful analyses. Patients receiving nivolumab deteriorated significantly later than those receiving dacarbazine on several EORTC QLQ-C30 scales and the EQ-5D utility index.
Conclusions
In addition to prolonged survival, these exploratory HRQoL results show that nivolumab maintains baseline HRQoL levels to provide long-term quality of survival benefit, compared with dacarbazine in patients with advanced melanoma.
introduction
The treatment paradigm for advanced melanoma has changed dramatically in recent years with the approval of new agents in many countries, such as inhibitors of the programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways. PD-1 pathway inhibitors in particular have produced durable responses and have been proven to be tolerable in clinical trials in patients with advanced melanoma and other tumor types [1, 2]. The PD-1 inhibitor nivolumab was associated with a significant survival benefit and a favorable safety profile in a phase III study [CheckMate (Checkpoint pathway and nivolumab clinical trial evaluation) 066] versus dacarbazine chemotherapy in treatment-naïve patients with metastatic melanoma without a BRAF mutation [3]. In that study, the median overall survival (OS) was not reached with patients treated with nivolumab and was 10.8 months with patients treated with dacarbazine [hazard ratio (HR) for death, 0.42; 99.79% CI 0.25–0.73; P < 0.001] after a median follow-up of 16.7 months. In another phase III study (CheckMate 037), response rates improved in patients treated with nivolumab with disease progression following the CTLA-4 inhibitor ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor, when compared with chemotherapy [4]. Responses with nivolumab can be delayed up to ∼7 months after treatment initiation [4].
A frequent concern with immunotherapies is that their toxicity profile might diminish health-related quality of life (HRQoL), even when meaningful disease outcomes are observed. Given the increasing importance of considering HRQoL during treatment decision-making in oncology, the CheckMate 066 study incorporated these measures. Herein are presented results of prospectively collected analyses in CheckMate 066 that compared the impact of nivolumab and dacarbazine on HRQoL using reliable and validated patient-reported outcomes (PROs).
methods
study design
CheckMate 066 was a phase III, randomized, double-blind study comparing nivolumab 3 mg/kg every 2 weeks with dacarbazine 1000 mg/m2 of body surface area every 3 weeks in treatment-naïve patients who had metastatic melanoma without a BRAF mutation [3]. The primary end point was OS; secondary end points included progression-free survival (PFS), objective response rate as determined by RECIST version 1.1 [5], tumor PD-L1 expression, and European Organisation for Research and Treatment of Care (EORTC) Core Quality of Life Questionnaire (QLQ-C30); exploratory end points included EuroQoL Five Dimensions Questionnaire 3L (EQ-5D 3L) [3].
HRQoL assessment
HRQoL in CheckMate 066 was evaluated using the EORTC QLQ-C30 [6, 7] and the EQ-5D 3L [8], two PROs whose use has been well documented in advanced melanoma [9–15]. PRO data were captured electronically.
The EORTC QLQ-C30 is a validated, self-reported, 30-item, generic measure of HRQoL composed of a global health status/QoL, five functional (physical, role, emotional, social, and cognitive), and nine symptom or single-item (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties) questions. Raw scores were transformed to a linear scale ranging from 0 to 100, with higher scores representing better outcomes on the global health status/QoL and functioning scales and worse outcomes on the symptom and single-item scales. The minimally important difference (MID), which indicates clinically meaningful change, is a change in score of ≥10 [7]. This MID has been validated in four domains (physical functioning, emotional functioning, social functioning, and global QoL).
The EQ-5D 3L is a validated, self-reported, generic measure of HRQoL composed of the EQ-5D utility index and EQ visual analog scale (VAS) [8]. The EQ-5D utility index comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each having 3 assessment levels (no, some, or extreme problems). The EQ VAS evaluates the patient's self-rated health state on a 100-point vertical VAS (0, worst imaginable health state; 100, best imaginable health state). MID is a score difference of ≥0.08 for the EQ-5D utility index and of ≥7 for the EQ-5D VAS [16].
statistical analysis
HRQoL was assessed at baseline, every 6 weeks while on treatment, and at follow-up visits 1 and 2 (30 and 100–114 days, respectively, after discontinuing treatment). HRQoL was analyzed for all randomized patients who had baseline and ≥1 post-baseline assessments, regardless of timeframe. The adjusted questionnaire completion rate (defined as the proportion of patients who completed the questionnaire using the number of patients alive in the CheckMate 066 study at the particular time point as the denominator) was determined at each visit. Differences in PROs were assessed between- and within-treatment arms according to statistical significance (Wilcoxon signed-rank and Mann–Whitney–Wilcoxon tests at P = 0.05) and MID. To assess longitudinal changes from baseline within each treatment group and differences between treatment groups, a mixed-effect model repeated measures (MMRM) analysis was used that controlled for baseline covariates (PRO score, Eastern Cooperative Oncology Group performance status, region, PD-L1 status, lactate dehydrogenase level, gender, age, BRAF mutation status, and blinding status). The median time from randomization to first deterioration, as defined by the MID at the individual patient level for that scale, was estimated by the Kaplan–Meier method. A Cox proportional hazard regression model was used to compare the deterioration rates treating baseline HRQoL score as a covariate. Since patients in the dacarbazine arm were more likely to discontinue treatment earlier (due to toxicity, progressive disease, or consent withdrawal), a pattern mixture model (PMM) sensitivity analysis was conducted across treatments to adjust for early (last visit ≤19 weeks) or late dropout (last visit ≥25 weeks), thereby evaluating the effect of missing data on HRQoL results.
results
patients
A total of 418 patients were randomized to nivolumab (n = 210) or dacarbazine (n = 208) in Europe, Israel, Australia, Canada, and South America between January 2013 and January 2014 [3]. Baseline patient characteristics have been previously reported and were well balanced between the treatment groups [3].
HRQoL questionnaire completion rates
Questionnaires were completed over a maximum treatment period of 73 weeks in the nivolumab arm and 61 weeks in the dacarbazine arm, as well as at two follow-up visits after treatment was discontinued. The adjusted questionnaire completion rates for the EORTC QLQ-30 and EQ-5D at baseline were 70% for the nivolumab arm and 65% for the dacarbazine arm, and remained similar to baseline throughout treatment (supplementary Table S1, available at Annals of Oncology online). HRQoL analysis involving dacarbazine was difficult after week 13 due to small sample size attributed to high attrition rate (n ≤ 41).
descriptive and cross-sectional HRQoL analyses
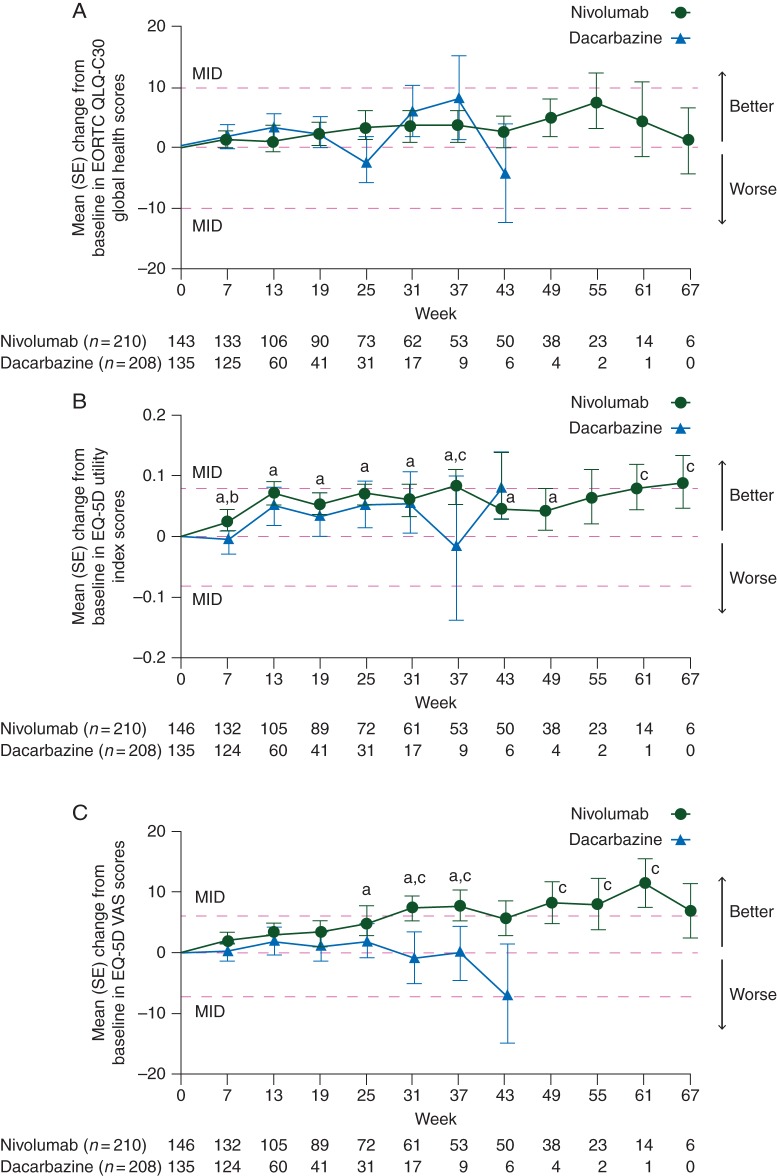
The mean (SD) EORTC QLQ-30 global health status/QoL scores at baseline were similar for patients treated with nivolumab [68.9 (20.2)] and for those treated with dacarbazine [66.2 (25.1)]. The mean changes from baseline in global health status/QoL scores that occurred, beginning at week 7, were modest with a trend toward improvement in both treatment groups, but these improvements were neither statistically significant nor clinically meaningful within each treatment group (Figure 1A). At week 25, there was a trend toward worsening in the dacarbazine group but not in the nivolumab group; however, the change from baseline was neither statistically significant nor clinically meaningful. There were also no significant differences between the treatment arms at any time point. In general, both EORTC QLQ-C30 functioning subscale and symptom mean scores remained relatively stable over time compared with baseline for both groups, with a few statistically significant and clinically meaningful changes.
Figure 1.
(A) Mean (SE) change from baseline in EORTC QLQ-C30 Global Health scores. Only time points where data are available for ≥5 patients in either treatment arm are plotted on the graph. MID consists of a change of ≥10. The mean baseline (SE) scores were 68.9 (20.2) for nivolumab and 66.2 (25.1) for dacarbazine. (B) Mean (SE) change from baseline in EQ-5D utility index scores. Only time points where data are available for ≥5 patients in either treatment arm are plotted on the graph. MID consists of a change of ≥0.08. The mean baseline (SE) scores were 0.778 (0.215) for nivolumab and 0.771 (0.310) for dacarbazine. (C) Mean (SE) change from baseline in EQ-5D VAS scores. Only time points where data are available for ≥5 patients in either treatment arm are plotted on the graph. MID consists of a change of ≥7. The mean baseline (SE) scores were 70.9 (19.9) for nivolumab and 69.1 (21.8) for dacarbazine. EORTC QLQ-C30, European Organisation for Research and Treatment of Care Core Quality of Life Questionnaire; EQ-5D, EuroQoL Five Dimensions Questionnaire; MID, minimally important difference; SE, standard error; VAS, visual analog scale. aP ≤ 0.05 versus baseline in the nivolumab arm. bP ≤ 0.05 for nivolumab versus dacarbazine arms. cExceeded MID.
The exploratory analysis mean (SD) EQ-5D utility scores were higher at baseline for patients treated with nivolumab [0.778 (0.215)] than for those treated with dacarbazine [0.711 (0.310)], and remained higher over time versus dacarbazine (Figure 1B). Significant improvements from baseline were observed for patients receiving nivolumab from week 7 (P = 0.011) through week 49 (P = 0.034). For patients receiving dacarbazine, there were no significant improvements from baseline at any time point. The only significant difference observed between treatment arms was at week 7 (P = 0.045), with improvement in nivolumab patients and deterioration in dacarbazine patients. Clinically meaningful improvements occurred in patients treated with nivolumab at weeks 37, 61, and 67.
Exploratory analysis mean (SD) EQ-5D VAS scores at baseline were similar for patients treated with nivolumab 70.9 (19.9) and those treated with dacarbazine 69.1 (21.8). Significant improvements from baseline were observed for patients receiving nivolumab at weeks 25, 31, and 37 (P ≤ 0.03; Figure 1C). Clinically meaningful improvements were noted for nivolumab patients at weeks 31, 37, 49, 55, and 61. No significant or clinically meaningful improvements from baseline were observed for dacarbazine patients. There were also no significant differences between the treatment arms at any time point.
MMRM analysis
In longitudinal modeling conducted across 61 weeks, significant improvements versus baseline were identified for patients treated with nivolumab in some PRO scales, including EORTC QLQ-C30 emotional functioning, nausea and vomiting, and insomnia; EORTC QLQ-C30 emotional functioning improved with dacarbazine (all P < 0.05; Table 1). None of these improvements were clinically meaningful based on MID. There were significant deteriorations from baseline in physical functioning in patients treated with nivolumab, and dyspnea in patients treated with dacarbazine. No significant differences were noted between the treatment arms for any of the EORTC QLQ-C30 functional domains or symptom/single-item scales.
Table 1.
Mixed-effects model for repeated measures results
| Domains/scalesa | Change from baseline, mean (SE) |
Difference in mean change (95% CI)b | |
|---|---|---|---|
| Nivolumab (n = 136) | Dacarbazine (n = 123) | ||
| EORTC QLQ-C30 functional domains | |||
| Global health status | 1.8 (1.8) | 0.9 (3.4) | 0.9 (−6.0, 7.8) |
| Physical functioning | −4.4 (1.6)c | −2.7 (2.6) | −1.7 (−7.1, 3.8) |
| Role functioning | −1.2 (2.3) | 3.6 (3.9) | −4.8 (−12.9, 3.2) |
| Emotional functioning | 6.3 (1.6)d | 5.3 (2.7)d | 1.0 (−4.5, 6.5) |
| Cognitive functioning | 0.4 (1.7) | 1.0 (3.2) | −0.7 (−7.2, 5.9) |
| Social functioning | −0.8 (2.0) | 0.3 (3.7) | −1.1 (−8.6, 6.3) |
| EORTC QLQ-C30 symptom/single-item scales | |||
| Fatigue | 2.0 (1.9) | 2.2 (3.3) | −0.2 (−6.9, 6.4) |
| Nausea and vomiting | −2.6 (1.0)d | 0.0 (1.7) | −2.6 (−6.0, 0.8) |
| Pain | −1.1 (2.5) | −1.6 (4.6) | 0.4 (−9.1, 9.9) |
| Dyspnea | 0.5 (2.1) | 7.4 (3.6)c | −6.9 (−14.2, 0.4) |
| Insomnia | −7.2 (2.2)d | −4.6 (4.0) | −2.6 (−10.7, 5.5) |
| Appetite loss | −3.6 (2.0) | 1.7 (3.5) | −5.2 (−12.2, 1.8) |
| Constipation | 0.1 (2.1) | 1.8 (3.9) | −1.7 (−9.7, 6.4) |
| Diarrhea | −0.5 (1.6) | −0.2 (2.9) | −0.3 (−6.4, 5.7) |
| Financial difficulties | 0.3 (2.2) | 0.8 (4.2) | −0.5 (−9.2, 8.2) |
| Nivolumab (n = 135) | Dacarbazine (n = 122) | ||
| EQ-5D utility index score | 0.040 (0.021) | 0.027 (0.038) | 0.013 (−0.065, 0.091) |
| EQ-5D VAS score | 2.2 (1.8) | 1.8 (3.4) | 0.4 (−6.6, 7.4) |
aHigher scores represent better outcomes on the EORTC QLQ-C30 functioning domains and worse outcomes on the EORTC QLQ-C30 symptom/single-item scales. Higher scores represent better outcomes on the EQ-5D utility index and EQ-5D VAS.
bA positive difference favors nivolumab on the EORTC QLQ-C30 functional domains and EQ-5D; negative difference favors nivolumab on the EORTC QLQ-C30 symptom scales. Differences in mean changes were not significant for all domains/scales.
cSignificant deterioration within arm (P < 0.05).
dSignificant improvement within arm (P < 0.05).
CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Care Core Quality of Life Questionnaire; EQ-5D, EuroQoL Five Dimensions Questionnaire; SE, standard error; VAS, visual analog scale.
time to deterioration
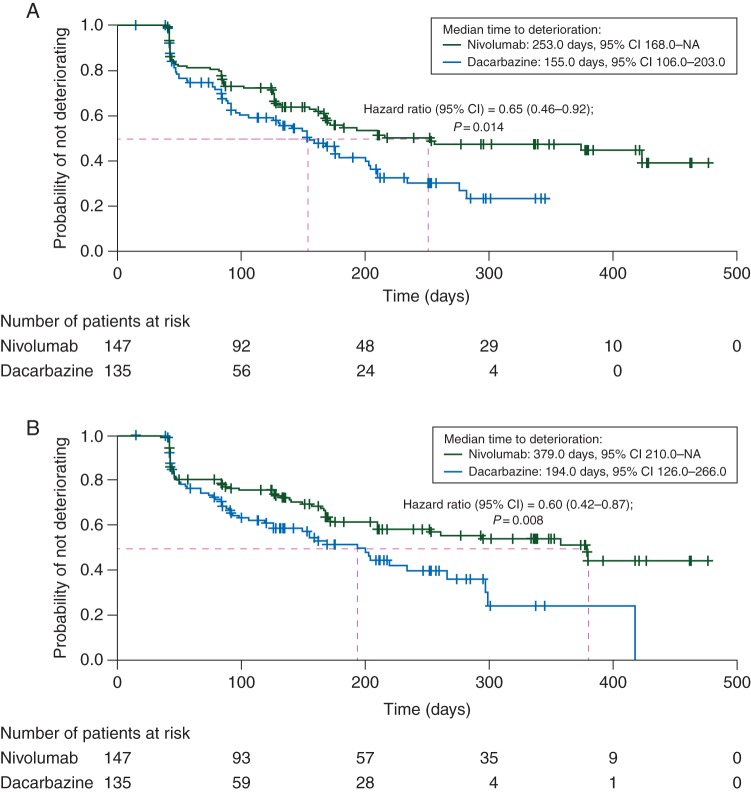
Patients treated with nivolumab were significantly less likely to deteriorate before those treated with dacarbazine for EORTC QLQ-C30 global health (Figure 2A), physical functioning (Figure 2B), role functioning, emotional functioning, cognitive functioning, social functioning, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, and constipation, as well as EQ-5D utility index (supplementary Table S2, available at Annals of Oncology online).
Figure 2.
(A) Time from randomization to first deterioration of EORTC QLQ-C30 global health status/QoL score based on MID. (B) Time from randomization to first deterioration of EORTC QLQ-C30 physical functioning score based on MID. EORTC QLQ-C30, European Organisation for Research and Treatment of Care Core Quality of Life Questionnaire; HR, hazard ratio; MID, minimally important difference.
PMM analysis
In a sensitivity analysis adjusting the MMRM models for early (last visit ≤19 weeks) or late dropout (last visit ≥25 weeks), no significant interactions for HRQoL between treatment and dropout were observed, apart from a significant interaction for the exploratory analysis EQ-5D VAS. Therefore, the missing data for the EQ-5D VAS may not be considered at random, indicating that the MMRM results might be biased and that missing data may have caused the positive nivolumab trend to be muted in the MMRM analysis for EQ-5D VAS.
discussion
Herein presented are the results of the prospectively collected analyses that assess the impact of the PD-1 inhibitor nivolumab on HRQoL in patients with advanced melanoma. In addition to the survival benefit seen with nivolumab when compared with dacarbazine in these treatment-naïve patients in the phase III CheckMate 066 study, baseline HRQoL levels with nivolumab were maintained over time, as assessed using validated self-reported questionnaires. Although only a single between-arm difference in favor of nivolumab was observed on the EQ-5D utility index at week 7, exploratory analyses showed significant and clinically meaningful EQ-5D improvements from baseline at several time points for patients receiving nivolumab. No statistically significant or clinically meaningful EQ-5D improvements from baseline were observed in patients treated with dacarbazine. An increased symptom burden was not observed with nivolumab, which is consistent with its adverse event profile in this trial [3]. In summary, no deterioration of HRQoL was identified with nivolumab. When added to the survival benefit of nivolumab, the benefit-to-risk ratio favors nivolumab over dacarbazine.
The PRO data were collected for the duration of treatment, for a period that exceeded 15 months in the nivolumab arm. Although HRQoL data are typically collected during a short timeframe in clinical studies, these analyses from CheckMate 066 examined long-term HRQoL. In addition, exploratory analyses revealed significant later time to deterioration or earlier time to improvement on several PRO scales in patients treated with nivolumab when compared with those treated with dacarbazine, confirming the superior benefit of nivolumab over dacarbazine in terms of not only survival but also quality of survival from the patient's perspective. These results suggest that patients receiving nivolumab for melanoma can expect to maintain their quality of life throughout treatment.
Although these findings provide insights into the impact of nivolumab on HRQoL, several factors limit data interpretation. First, the study used PROs that were not developed and validated specifically for melanoma. However, given their common use in melanoma clinical trials, these instruments were considered to be the most content valid among those currently available. Additionally, an MID of 10 points was used to identify clinically meaningful differences for all EORTC QLQ-C30 domains [7], similar to previous HRQoL work in this disease, but this MID has been validated in only four domains (physical functioning, emotional functioning, social functioning, and global QoL). Thus, the MID analysis for the other domains should be interpreted with caution. In addition, the MID for EQ-5D, although commonly used in clinical studies, has not been validated in melanoma patients. Because the analyses in this study were exploratory, P values have not been adjusted for multiplicity and must be interpreted with caution. In addition to the statistical analyses, differences should be assessed according to MID to identify clinically meaningful data. Finally, the high attrition rate in the dacarbazine arm, possibly due to death and/or disease progression (median PFS 2.2 versus 5.1 months with nivolumab) [3], limited the comparative analyses at later time points in the study. More specifically, there was a significant difference between early and late dropout between treatment arms for both the EORTC QLQ-C30 and EQ-5D instruments, indicating that patients in the dacarbazine arm were more likely to dropout earlier than those in the nivolumab arm. A significant interaction between dropout, treatment, and time was observed for the EQ-5D VAS according to a PMM that was used to test the impact of missing data on treatment results. It is possible that the missing data for the EQ-5D VAS may not be considered at random, indicating that the MMRM results might be biased.
This study presents the results from a multi-step analytical plan, considering data descriptively, cross-sectionally, and longitudinally, along with a PMM sensitivity analysis, so that conclusions are supported by a range of analytical methods. In CheckMate 066, nivolumab was associated with maintenance of HRQoL baseline levels over time, in addition to the survival benefits and a manageable adverse event profile. The HRQoL results presented for this study further support the clinical benefit of nivolumab in patients with advanced melanoma and show that nivolumab provides long-term quality of survival benefit in this population. In the future, the HRQoL analyses carried out here should be replicated for nivolumab versus other standards of care or emerging therapies and be stratified by patient subgroup characteristics (e.g. BRAF mutation and PD-L1 expression status) to further delineate the clinical value of nivolumab in advanced melanoma.
funding
This study was funded by Bristol-Myers Squibb.
disclosure
GVL: honoraria: Bristol-Myers Squibb, Merck, GlaxoSmithKline, and Roche; consulting or advisory role: Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche. VA: honoraria: Bristol-Myers Squibb, GlaxoSmithKline, and Merck Sharp & Dohme; consulting or advisory role: Bristol-Myers Squibb and Merck Sharp & Dohme; travel, accommodations, and expenses: Bristol-Myers Squibb and Roche. PAA: honoraria: Bristol-Myers Squibb, Roche-Genentech, and Ventana; research funding: Bristol-Myers Squibb, Roche-Genentech, and Ventana. CR: honoraria: Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche; consulting or advisory role: Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche. JCH: honoraria: Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme, and Roche; consulting or advisory role: Amgen and GlaxoSmithKline; speakers' bureau: Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme, and Roche; travel, accommodations, and expenses: Amgen and Bristol-Myers Squibb. PR: honoraria: Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and Roche; consulting or advisory role: Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, and Roche; speakers' bureau: Merck Sharp & Dohme, Novartis, and Pfizer; research funding: Bristol-Myers Squibb; travel, accommodations, and expenses: Novartis. KJS: honoraria: Bristol-Myers Squibb, Celgene, and Seattle Genetics; consulting or advisory role: Bristol-Myers Squibb and Seattle Genetics; research funding: Roche. FT: employment: Adelphi Values; research funding: Bristol-Myers Squibb (for Adelphi Values). CC: none. IG: stock or other ownership: Bristol-Myers Squibb. HBD: employment: Bristol-Myers Squibb; stock or other ownership: Bristol-Myers Squibb. IMW: employment: Bristol-Myers Squibb; stock or other ownership: Bristol-Myers Squibb. APA: employment: Flatiron Health, Inc; leadership: Flatiron Health, Inc, Athenahealth, Inc; stock or other ownership: Athenahealth, Inc; honoraria: Bristol-Myers Squibb, Helsinn, and Merck; consulting or advisory role: ACORN Research, Bristol-Myers Squibb; research funding: Bristol-Myers Squibb, Celgene, DARA Biosciences, Dendreon, GlaxoSmithKline, Helsinn, Kanglaite, and Pfizer.
Supplementary Material
acknowledgements
The authors wish to thank the patients and families for making this study possible, Lucinda Orsini at Bristol-Myers Squibb for her contributions to the study, and Mike DeRosa at Adelphi Values for leading the statistical analysis. Professional medical writing assistance was provided by Mark Palangio and medical editing assistance was provided by Artur Romanchuk of StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb. Results from this analysis were presented in part at the American Society of Clinical Oncology 2015 Annual Meeting; 29 May–2 June 2015; Chicago, IL, USA.
references
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33(17): 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin Oncol 2015; 42(3): 429–435. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372(4): 320–330. [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 6.Osoba D, Aaronson N, Zee B et al. Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer. The Study Group on Quality of Life of the EORTC and the Symptom Control and Quality of Life Committees of the NCI of Canada Clinical Trials Group. Qual Life Res 1997; 6(2): 103–108. [DOI] [PubMed] [Google Scholar]
- 7.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality of life scores. J Clin Oncol 1998; 16(1): 139–144. [DOI] [PubMed] [Google Scholar]
- 8.EuroQol Group. EQ-5D-3L User Guide. 2013. http://www.euroqol.org/about-eq-5d/publications/user-guide.html (10 November 2015, date last accessed).
- 9.Cornish D, Holterhues C, van de Poll-Franse LV, Coebergh JW, Nijsten T. A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol 2009; 20(Suppl 6): 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askew RL, Swartz RJ, Xing Y et al. Mapping FACT-melanoma quality-of-life scores to EQ-5D health utility weights. Value Health 2011; 14: 900–906. [DOI] [PubMed] [Google Scholar]
- 11.Revicki DA, van den Eertwegh AJ, Lorigan P et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes 2012; 10: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grob JJ, Amonkar MM, Martin-Algarra S et al. Patient perception of the benefit of a BRAF inhibitor in metastatic melanoma: quality-of-life analyses of the BREAK-3 study comparing dabrafenib with dacarbazine. Ann Oncol 2014; 25(7): 1428–1436. [DOI] [PubMed] [Google Scholar]
- 13.Schadendorf D, Amonkar MM, Milhem M et al. Functional and symptom impact of trametinib versus chemotherapy in BRAF V600E advanced or metastatic melanoma: quality-of-life analyses of the METRIC study. Ann Oncol 2014; 25(3): 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schadendorf D, Amonkar MM, Stroyakovskiy D et al. Health-related quality of life impact in a randomised phase III study of the combination of dabrafenib and trametinib versus dabrafenib monotherapy in patients with BRAF V600 metastatic melanoma. Eur J Cancer 2015; 51(7): 833–840. [DOI] [PubMed] [Google Scholar]
- 15.Schadendorf D, Dummer R, Hauschild A et al. Patient-reported outcomes (PROs) in KEYNOTE-002, a randomized study of pembrolizumab vs chemotherapy in patients (pts) with ipilimumab-refractory (IPI-R) metastatic melanoma (MEL). Presented at American Society for Clinical Oncology, 29 May–2 June 2015, Chicago, IL Poster 9040. [Google Scholar]
- 16.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.