Dynamic contrast-enhanced ultrasonography is a functional technique enabling quantitative assessment of solid tumor perfusion in metastatic patients treated with antiangiogenic therapies. In this study, we show that the mean transit time evaluated by DCE-US at day 7 may be used as a vascular normalization biomarker to predict the outcome of metastatic patients treated with bevacizumab.
Keywords: dynamic contrast-enhanced ultrasonography (DCE-US), imaging biomarker, early evaluation, bevacizumab, breast cancer, colon cancer
Abstract
Background
Dynamic contrast-enhanced ultrasonography (DCE-US) has been used for evaluation of tumor response to antiangiogenic treatments. The objective of this study was to assess the link between DCE-US data obtained during the first week of treatment and subsequent tumor progression.
Patients and methods
Patients treated with antiangiogenic therapies were included in a multicentric prospective study from 2007 to 2010. DCE-US examinations were available at baseline and at day 7. For each examination, a 3 min perfusion curve was recorded just after injection of a contrast agent. Each perfusion curve was modeled with seven parameters. We analyzed the correlation between criteria measured up to day 7 on freedom from progression (FFP). The impact was assessed globally, according to tumor localization and to type of treatment.
Results
The median follow-up was 20 months. The mean transit time (MTT) evaluated at day 7 was the only criterion significantly associated with FFP (P = 0.002). The cut-off point maximizing the difference between FFP curves was 12 s. Patients with at least a 12 s MTT had a better FFP. The results according to tumor type were significantly heterogeneous: the impact of MTT on FFP was more marked for breast cancer (P = 0.004) and for colon cancer (P = 0.025) than for other tumor types. Similarly, the differences in FFP according to MTT at day 7 were marked (P = 0.004) in patients receiving bevacizumab.
Conclusion
The MTT evaluated with DCE-US at day 7 is significantly correlated to FFP of patients treated with bevacizumab. This criterion might be linked to vascular normalization.
AFSSAPS No
2007-A00399-44.
introduction
Recently, targeted agents have significantly improved outcomes across a wide range of solid tumors [1]. Progression-free survival (PFS) and overall survival (OS) are often used to assess treatment efficacy. However, these criteria require a long duration of patient observation and thus other surrogate end points might be preferred. Tumor response measures such as Response Evaluation Criteria in Solid Tumors (RECIST) [2] have proven to be of limited value in assessing response to antiangiogenic agents [3].
Vascular endothelial growth factor (VEGF) has an important role in tumor progression and can be stopped by antiangiogenic treatment [4]: the anti-VEGF receptor tyrosine kinase inhibitors and the monoclonal antibodies against VEGF (anti-VEGF).
Dynamic contrast-enhanced ultrasonography (DCE-US) is a technique using Doppler ultrasound with perfusion software and contrast injection [5, 6]. It enables quantitative assessment of solid tumor perfusion using a mathematical model to analyze raw linear ultrasound data. A large multicenter prospective study including 539 patients was conducted to evaluate the utility of DCE-US for predicting treatment efficacy with antiangiogenic therapies in patients with solid tumors [7]. The best criterion correlated to freedom from progression (FFP) was the ratio between the area under the curve (AUC) at day 30 and at baseline [8]. The best cut-off point corresponded to a decrease of 40% in the AUC between baseline and day 30.
The objective of the present study was to identify an earlier biomarker (before day 30) in patients treated with antiangiogenic treatments.
materials and methods
study design
Details of the study methodology have been reported previously [7]. To summarize, 19 centers across France participated in a prospective study, including patients with metastatic breast cancer, metastatic melanoma, metastatic colon cancer, gastrointestinal stromal tumor (GIST), metastatic renal cell carcinoma (RCC), or primary hepatocellular carcinoma (HCC). Patients were enrolled in a clinical trial of antiangiogenic-based therapy or were otherwise eligible for an approved antiangiogenic treatment.
Patients were not included if they were younger than 18 years or had heart failure, or if the tumor could not be evaluated by the method (tumor not accessible to ultrasonography or not visually estimated vascularized at baseline DCE-US examination by the radiologist). For each patient, one tumor was studied and was selected as follows: the tumor size had to be larger than 2 cm and the percentage of necrosis in B-Mode <50% of the total tumor volume.
All patients provided written informed consent, either specific to this study or in the context of a clinical trial. The study was approved by the ethics committee of each institution and was declared to the French Commission Nationale Informatique et Liberté (CNIL declaration No. 912346).
DCE-US technique and quantification
DCE-US was carried out with an Aplio® sonograph (Toshiba, Puteaux, France) according to a standardized procedure [7]. DCE-US examination started with an intravenous bolus injection of 4.8 ml of SonoVue® (Bracco S.P.A., Milan, Italy). Tumor vascularization was evaluated visually by the radiologist: if it was <50% at baseline, the patient was excluded. A 3 min perfusion curve was recorded just after injection of the contrast agent. A quantitative analysis of perfusion curve was carried out with CHI-Q software (Toshiba, Puteaux, France). Each time–intensity curve was modeled using a mathematical model (patent PCT/IB2006/003742) to determine seven DCE-US criteria: four related to blood volume (peak intensity, AUC, area under the wash-in, and area under the wash-out); two related to blood flow (time to peak intensity, and slope of the wash-in); and the mean transit time (MTT). The quality of DCE-US was expressed with a score of 0–5 defined according to six criteria: the tumor size (>2 cm = 1), the motion (intensive tracking not required = 1), the ROI contour (clear borders = 1), the loss of target (the target was not lost for <20 s = 1), the wash-in (total data acquisition during the wash-in period = 1), and VRI window adapted (VRI window adapted to the lesion size = 1) [7]. Examinations with score 0, corresponding to very poor quality, were excluded (3% of all examinations).
assessments
DCE-US was carried out at baseline and 7 days after treatment. For each DCE-US examination, we modeled the tumor perfusion curve with the seven DCE-US criteria mentioned above. For each patient and for each criterion, the ratio of day 7 value over the baseline value was calculated.
The duration of follow-up was estimated in accordance with Schemper's method [9]. Patients were to be followed up with a computed tomography (CT) scan every 2 months for 1 year or until death. Progression was assessed in accordance with RECIST. Patients who died because of their malignancy without a documented progression were considered as in progression and the date of progression equal to the date of death. Patients who stopped the treatment because of toxicity were censored when the treatment stopped, and patients who died without progression were censored at the date of death. The main end point was FFP, defined as the time between the DCE-US examination and the date of progression.
analyses
The relationship between each criterion and FFP was analyzed at baseline and at day 7. The relationship between change from baseline to day 7 in each criterion was also tested. Thus, seven criteria were tested at three time-points, leading to 21 criteria/time-point combinations. For each combination, we tested the linear trend between the criterion value (without specifying a cut-off value) and the FFP. In order to account for multiple testing, each test was considered as significant when the P value was ≤0.0024 which corresponds to the P value with the Bonferroni correction (0.05/21).
Criteria/time points with the strongest correlation with FFP were further analyzed through a systematic search to identify the best cut-off point for each. The best single cut-off point was that with the lowest P value for association with FFP. Correlation between criteria and OS was studied after the best cut-off point had been estimated.
The impact on FFP of the best combination cutoff point/criteria was further investigated by testing the heterogeneity [10, 11] of the association between the criteria and FFP according to the type of tumor and to the type of treatment. The heterogeneity test was based on the logrank statistics. In order to find the categories contributing the most to heterogeneity, we estimated the heterogeneity after each category had been removed. The category for which the decrease in heterogeneity was the more marked was considered as the category contributing the most to heterogeneity. This process was repeated until the heterogeneity became not significant. Survival curves are presented in the subgroups that contribute the most to the heterogeneity.
Statistical analyses were carried out using SAS® software version 9.4 (SAS, Cary, NC). All statistical tests were two-sided, and the significance level was 0.05 unless otherwise specified.
results
A total of 539 patients were enrolled in the study between October 2007 and March 2010. Five hundred and twenty-one patients had baseline evaluation and 462 patients had day 7 evaluation. Raw data were absent for 52 patients at baseline and for 53 patients at day 7. Thus, data were present at day 7 for 409 patients. Quality was insufficient for six patients at baseline (1.3% of the examinations with available raw data) and for eight patients at day 7 (2.0%). The quality of the data was considered good for 463 patients at baseline and for 401 patients at day 7.
Patient characteristics are described in Table 1. The overlap between treatment and cancer types is described in supplementary table S1, available at Annals of Oncology online. The median follow-up was 20 months.
Table 1.
Characteristics of the patients at inclusion, and of the patients with raw data and with good quality data at baseline and at day 7
| Patients included (n = 539) | Patients with raw data and good quality data at baseline (n = 463) | Patients with raw data and good quality data at day 7 (n = 401) | |
|---|---|---|---|
| Sex | |||
| Male | 337 (62.5) | 285 (61.6) [−2] | 264 (65.8) [−4] |
| Female | 202 (37.5) | 178 (38.4) [−1] | 137 (34.2) [—] |
| Agea | |||
| 21–49 | 121 (22.4) | 110 (23.8) [—] | 92 (22.9) [—] |
| 50–88 | 416 (77.2) | 352 (76.2) [−2] | 309 (77.1) [−4] |
| Tumor type | |||
| Renal cell carcinoma | 157 (29.1) | 141 (30.5) [—] | 123 (30.7) [—] |
| Hepatocellular carcinoma | 107 (19.8) | 84 (18.1) [−1] | 79 (19.7) [−2] |
| Colorectal carcinoma | 67 (12.4) | 58 (12.5) [−2] | 46 (11.5) [—] |
| Breast cancer | 61 (11.3) | 49 (10.6) [—] | 38 (9.5) [—] |
| Melanoma | 52 (9.7) | 50 (10.8) [—] | 46 (11.5) [−2] |
| Gastrointestinal stromal tumor | 52 (9.7) | 43 (9.3) [—] | 41 (10.2) [—] |
| Other | 43 (8.0) | 38 (8.2) [—] | 28 (7.0) [—] |
| Antiangiogenic treatment | |||
| Sorafenib (NEXAVAR) | 166 (30.8) | 138 (29.8) [−1] | 124 (30.9) [−2] |
| Sunitinib (SUTENT) | 144 (26.7) | 116 (25.1) [—] | 103 (25.7) [—] |
| Bevacizumab (AVASTIN) | 128 (23.7) | 121 (26.1) [−1] | 95 (23.7) [—] |
| Imatinib (GLIVEC) | 44 (10.6) | 40 (8.6) [—] | 40 (10.0) [−1] |
| Other | 57 (8.2) | 48 (10.4) [−1] | 39 (9.7) [−1] |
aAge was missing for two patients at baseline; one of these two patients has good quality data at baseline.
[ ] Patients not included in the analyses of time to progression.
At baseline, there was no significant association between any of the DCE-US criteria values and FFP. At day 7, among the seven DCE-US criteria, the MTT was the only criterion correlated to FFP (P = 0.002, Table 2). The ratio between day 7 and baseline was not significant for any of criteria. We carried out supplementary analyses following the remark of a reviewer who questioned the reality of the biological effect since the ratio between day 7 and baseline was not significant. In these analyses that were not pre-specified in the analysis plan, the difference between day 7 and the baseline was highly significant for MTT (P = 0.0003).
Table 2.
Significance level (P value) of the association between criteria values used as continuous variables and freedom from progression at baseline and day 7
| Variable | Baseline | Day 7 | Ratio day 7/baseline | Difference day 7 − baseline |
|---|---|---|---|---|
| Area under the curve | NS | NS | NS | NS |
| Area under the wash in | NS | NS | NS | NS |
| Area under the wash out | NS | NS | NS | NS |
| Mean transit time | NS (0.071) | 0.002 | NS (0.20) | 0.0003 |
| Peak intensity | NS | NS | NS | NS |
| Slope | NS | NS | NS | NS |
| Time to peak | NS (0.048) | NS (0.023) | NS (0.88) | 0.028 |
The analyses of the difference between day 7 and baseline were not pre-specified in the analysis plan; they are added following a reviewer's question comment on the biological effect of the treatment.
Not significant (NS) corresponds to P values >0.05.
The italics value indicates, P value significant after Bonferroni correction.
The best cut-off point identified for the MTT at day 7 in the total population was 12 s. This cut-off point was associated with FFP (P = 0.002, Figure 1): tumors with an MTT of at least 12 s had a better FFP. The association with OS was not significant.
Figure 1.
Freedom from progression in patients defined by dynamic contrast-enhanced ultrasonography mean transit time <12 s at day 7. The first panel corresponds to the overall population; the second panel to patients treated with bevacizumab; the last two panels to patients with colon and breast cancers.
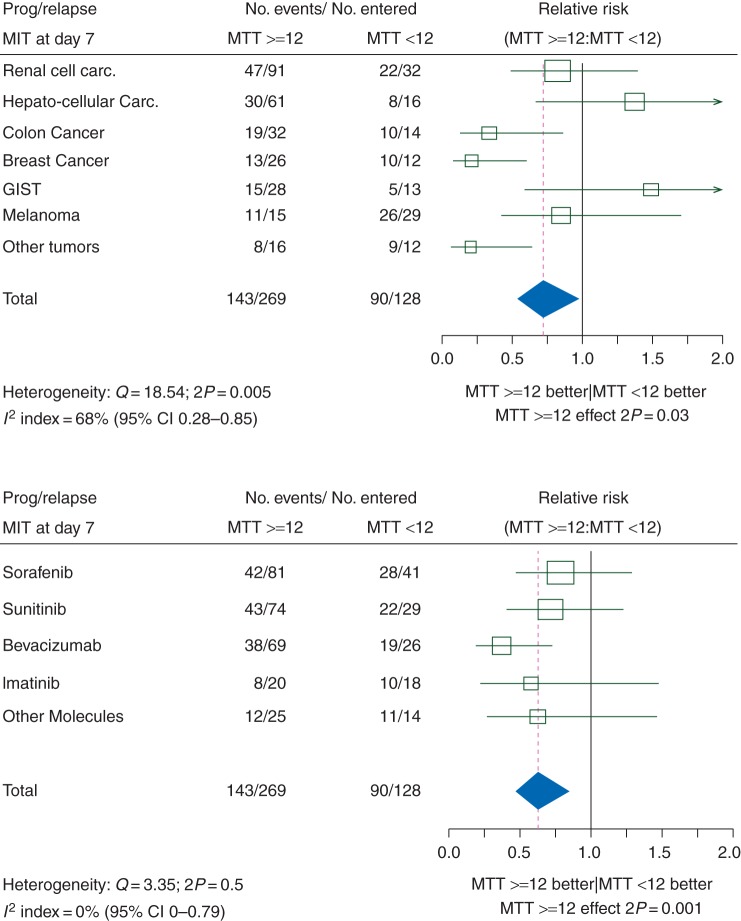
The impact of MTT at day 7 on FFP was significantly different according to tumor localization (heterogeneity test: P = 0.005, Figure 2). Breast cancer was the tumor type contributing the most to heterogeneity. Globally, colon, breast cancer and unclassified tumors each had a significantly better prognosis when the MTT was ≥12 s, while other tumors had either no relation between prognosis and MTT (renal carcinoma and melanoma) or had a non-significantly worse prognosis when MTT was ≥12 s (HCC, GIST).
Figure 2.
Impact of a dynamic contrast-enhanced ultrasonography mean transit time <12 s at day 7 on freedom from progression. The upper panel shows forest plots of relative risks according to tumor type. The lower panel shows forest plots of relative risks according to antiangiogenic therapy.
The heterogeneity according to the antiangiogenic treatment was not significant (P = 0.5). However, most of the patients with breast or colon cancers had been treated with bevacizumab (Avastin®, Roche, Basel, Switzerland). Moreover, bevacizumab was the only treatment associated with a significant link between MTT and FFP. Therefore, despite the non-significant heterogeneity of the correlation between MTT and FFP according to treatment, we present the FFP according to MTT in patients treated with bevacizumab.
Evaluations at day 7 with good quality data were available for 38 patients with breast cancer, 46 patients with colon cancer tumors, and a total of 95 patients treated with bevacizumab (Table 1). At day 7, FFP was significantly associated with MTT for patients treated with bevacizumab (P = 0.004), patients with breast cancer (P = 0.004), and those with colon cancers (P = 0.025) (Figure 1).
discussion
We identified MTT as an early DCE-US biomarker (at day 7) in patients treated with antiangiogenic treatments. Its impact is particularly marked in metastatic colon and breast cancer treated with bevacizumab. An MTT of at least 12 s is predictive of a better FFP.
The day 7 assessment with DCE-US technique has two interests. First, the day 7 evaluation is often used in our institution in phase I studies in which we evaluate toxicity. Thus, doses could be adapted to maintain efficacy even when doses are reduced. Also in this type of study, different types of tumors are included, and the oncologist could benefit from an early evaluation of the tumors for which the new drugs could be most efficient. Another occasion, in which the day 7 evaluation could be interesting, would be to improve the administration schedule of drugs by adapting in real time according to efficacy as detected by DCE-US [12].
Results for patients under bevacizumab were the most significant in this study. These results may be explained by the vascular normalization theory, established by R.K. Jain, who described that for tumors treated with bevacizumab, vessels become normalized during a short-lived period, a time window which lasts about 6 days [13]. During this normalization phase, excessive branching and shunts are reduced, leading to decreased hypoxia (improving oxygen delivery to the tumor [14]) and interstitial pressure. Radiation therapy and delivery of chemotherapeutic agents would therefore be more efficient [13]. One other monocentric study with HCC showed a correlation between increased MTT at day 7 and RECIST response (P = 0.03) [15].
Three main functional imaging techniques are available to evaluate patients treated with antiangiogenic treatments: DCE-US, dynamic contrast-enhanced computed tomography (DCE-CT), and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) [16]. Currently and to our knowledge, no multicentric studies have focused on the early evaluation (day 7) of patients treated with antiangiogenic treatments.
Concerning DCE-US, published monocentric studies have used quantitative analysis of tumor perfusion [15, 17]. A multicentric study identified the AUC at day 30 as a biomarker predictive of tumor progression in solid tumors treated with antiangiogenic therapies [8]. This biomarker is correlated to FFP, and the best criterion was the ratio between the AUC at day 30 and baseline. The best cut-off point was globally significant, with no significant heterogeneity across tumor types and treatments. Compared with other imaging techniques, DCE-US is less expensive (around $200) and does not expose to radiation. DCE-US can be used in patients with renal failure because of the pulmonary elimination of the contrast agent. The learning curve is relatively short, because radiologists are autonomous after having carried out 60 examinations [7]. In 2012, the indication of DCE-US for the evaluation of treatments was added to the World Federation for Ultrasound in Medicine and Biology (WFUMB) guidelines [18] in cooperation with the American Institute of Ultrasound in Medicine (AIUM) with a level of evidence A1b. Such guidelines also allow for standardized acquisition and post-processing protocols which will encourage dissemination of the technique.
There are some limits to our study. The main limit is that tumors had to be sufficiently vascularized before beginning therapy for the technique to be used. Indeed, if the signal is not high enough before therapy, changes cannot be detected. Since this was a non-inclusion criterion, it is not possible to evaluate which proportion of patients was excluded. More generally, limits to the DCE-US technique include the following: (i) it cannot be used for metastases in the lung or brain; (ii) it requires the selection of a single target tumor; (iii) one must wait 20 min before a second injection of the contrast agent; and (iv) the technique is approved only in adult patients.
DCE-US is a functional imaging technique that provides a criterion at day 7, the MTT, which could be used as a biomarker for vascular normalization to predict outcomes of metastatic patients treated with bevacizumab. The next step could be to validate this early DCE-US biomarker and its cut-off point in an expanded cohort. In order to decrease operator dependence, automatic tracking and/or three-dimensional quantification of the tumor could be useful.
funding
This study was supported by a grant from the French National Cancer Institute. Additional funding was provided by Toshiba and Bracco. No grant number is applicable.
disclosure
NL received a grant from the Institut National de Cancer (INCA) for another ongoing project; honoraria and lectures fees from Pfizer, Novartis, Hoffmann-La Roche, Bracco, and Toshiba. MK received a grant from Toshiba for a past project. VV received grants from the Cancer Institute as an Associate Investigator for an ongoing project, and from SIRTEX as a Principal Investigator for a study on liver radioembolization. OL received payment from Bracco for a past project to write educational material about contrast-enhanced ultrasound imaging of the liver. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Muriel Ducourtieux, Stana Agnes, Julien Pellier, Louis Chapotot, Julie Chevalier, and Florence Journeau for data collection and quality control as well as Dr Jean-Pierre Armand for his valuable advice on the preparation of the protocol. Final medical editing support was provided by S. Randall Thomas (IR4M, CNRS University Paris Sud, University Paris-Saclay).
references
- 1.Motzer RJ, Hutson TE, Tomczak P et al. . Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–124. [DOI] [PubMed] [Google Scholar]
- 2.Eisenhauer EA, Therasse P, Bogaerts J et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 3.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. Am J Roentgenol 2010; 194: 157–165. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 5.Lassau N, Lamuraglia M, Vanel D et al. . Doppler US with perfusion software and contrast medium injection in the early evaluation of isolated limb perfusion of limb sarcomas: prospective study of 49 cases. Ann Oncol 2005; 16: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 6.Eggermont AM. Evolving imaging technology: contrast-enhanced Doppler ultrasound is early and rapid predictor of tumour response. Ann Oncol 2005; 16: 995–996. [DOI] [PubMed] [Google Scholar]
- 7.Lassau N, Chapotot L, Benatsou B et al. . Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: the French multicenter Support for Innovative and Expensive Techniques Study. Invest Radiol 2012; 47: 711–716. [DOI] [PubMed] [Google Scholar]
- 8.Lassau N, Bonastre J, Kind M et al. . Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol 2014; 49: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 10.Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101–129. [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lassau N, Cosgrove D, Armand JP. Early evaluation of targeted drugs using dynamic contrast-enhanced ultrasonography for personalized medicine. Future Oncol 2012; 8: 1215–1218. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307: 58–62. [DOI] [PubMed] [Google Scholar]
- 14.Tolaney SM, Boucher Y, Duda DG et al. . Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci USA 2015; 112: 14325–14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassau N, Koscielny S, Chami L et al. . Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification—preliminary results. Radiology 2011; 258: 291–300. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor JP, Jayson GC. Do imaging biomarkers relate to outcome in patients treated with VEGF inhibitors? Clin Cancer Res 2012; 18: 6588–6598. [DOI] [PubMed] [Google Scholar]
- 17.Lassau N, Koscielny S, Albiges L et al. . Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res 2010; 16: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 18.Claudon M, Dietrich CF, Choi BI et al. . Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013; 34: 11–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.