This phase I study evaluated the safety, tolerability, and pharmacokinetics of copanlisib, an intravenously administered pan-phosphatidylinositol 3-kinase inhibitor in patients with advanced solid tumors or non-Hodgkin's lymphoma. Copanlisib was well tolerated with a manageable safety profile, with anti-tumor activity in both advanced solid tumors and hematological malignancies.
Keywords: copanlisib, PI3K inhibitor, advanced cancer, non-Hodgkin's lymphoma
Abstract
Background
To evaluate the safety, tolerability, pharmacokinetics, and maximum tolerated dose (MTD) of copanlisib, a phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors or non-Hodgkin's lymphoma (NHL).
Patients and methods
Phase I dose-escalation study including patients with advanced solid tumors or NHL, and a cohort of patients with type 2 diabetes mellitus. Patients received three weekly intravenous infusions of copanlisib per 28-day cycle over the dose range 0.1–1.2 mg/kg. Plasma copanlisib levels were analyzed for pharmacokinetics. Biomarker analysis included PIK3CA, KRAS, BRAF, and PTEN mutational status and PTEN immunohistochemistry. Whole-body [18F]-fluorodeoxyglucose positron emission tomography (18FDG-PET) was carried out at baseline and following the first dose to assess early pharmacodynamic effects. Plasma glucose and insulin levels were evaluated serially.
Results
Fifty-seven patients received treatment. The MTD was 0.8 mg/kg copanlisib. The most frequent treatment-related adverse events were nausea and transient hyperglycemia. Copanlisib exposure was dose-proportional with no accumulation; peak exposure positively correlated with transient hyperglycemia post-infusion. Sixteen of 20 patients treated at the MTD had reduced 18FDG-PET uptake; 7 (33%) had a reduction >25%. One patient achieved a complete response (CR; endometrial carcinoma exhibiting both PIK3CA and PTEN mutations and complete PTEN loss) and two had a partial response (PR; both metastatic breast cancer). Among the nine NHL patients, all six with follicular lymphoma (FL) responded (one CR and five PRs) and one patient with diffuse large B-cell lymphoma had a PR by investigator assessment; two patients with FL who achieved CR (per post hoc independent radiologic review) were on treatment >3 years.
Conclusion
Copanlisib, dosed intermittently on days 1, 8, and 15 of a 28-day cycle, was well tolerated and the MTD was determined to be 0.8 mg/kg. Copanlisib exhibited dose-proportional pharmacokinetics and promising anti-tumor activity, particularly in patients with NHL.
ClinicalTrials.gov
NCT00962611; https://clinicaltrials.gov/ct2/show/NCT00962611.
introduction
The phosphatidylinositol 3-kinase (PI3K)/serine/threonine kinase (AKT)/mammalian target of rapamycin (mTOR) pathway regulates cellular growth and survival, and is constitutively activated in many cancers [1, 2]. PTEN negatively regulates the PI3K/AKT/mTOR pathway, although PTEN activity is frequently lost in cancer, leading to constitutive pathway activation and tumorigenesis [3]. Over-activation of PI3K signaling by receptor tyrosine kinase activation or mutation of PI3K isoforms is also observed in many cancers [4]. Several PI3K inhibitors, most of which are orally administered, either with broad activity against all PI3K isoforms or selectivity for specific isoforms, are in development or have been recently approved for clinical use in a range of solid tumors, and lymphomas [5–13].
Copanlisib (BAY 80-6946; Bayer Pharma AG, Berlin, Germany) is a novel, intravenous, potent, highly selective, pan-class I PI3K inhibitor with preferential activity against the p110α and p110δ isoforms, compared with the p110β and p110γ isoforms (IC50 values of 0.5, 0.7, 3.7, and 6.4 nmol/l, respectively) [14]. Copanlisib has demonstrated potent anti-tumor and pro-apoptotic activity in various tumor cell lines and xenograft models [15].
Herein, we report on the safety, tolerability, pharmacokinetics, and maximum tolerated dose (MTD) of intravenous copanlisib in patients with advanced solid tumors. Evaluations included biomarkers, pharmacodynamic parameters, and tumor response. Copanlisib was further evaluated in predefined cohorts of patients with non-Hodgkin's lymphoma (NHL) and solid tumors, the latter including patients with tumors bearing PIK3CA mutations, and a cohort of patients with solid tumors and type 2 diabetes mellitus.
patients and methods
study design
The primary objective of this study was to determine the safety, tolerability, pharmacokinetics, and MTD of copanlisib administered once weekly for 3 weeks every 28 days as a 1-h intravenous infusion in patients with advanced solid tumors and, in the MTD expansion cohort, NHL. The secondary objectives were to: evaluate biomarkers, pharmacodynamics, and tumor response in patients treated with copanlisib; assess, in the MTD cohort, indications of tumor-specific pharmacodynamic effect and signs of clinical benefit in a patient population selected for high likelihood of PI3K pathway activation; and assess the safety of copanlisib treatment in patients with type 2 diabetes mellitus.
Patients received a single intravenous infusion of copanlisib over 1 h on days 1, 8, and 15 of a 28-day cycle. Patients fasted for 8 h before and 3 h after the start of copanlisib infusion on cycle 1, day 1, after which they received food. An initial accelerated titration design was used, with one patient assigned per dose level until the occurrence of predefined pharmacodynamic or safety events (increase in plasma glucose ≥50 mg/dl from baseline and/or increase in plasma insulin ≥2 times the baseline value within 2 h of completion of infusion, or copanlisib-related grade three toxicity, in one patient each). A modified 3 + 3 design was adopted thereafter, with three patients enrolled per dose level and three additional patients enrolled if a dose-limiting toxicity (DLT) was observed in the first three patients, or if there was evidence of tumor-specific pharmacodynamic effects, including changes in [18F]-fluorodeoxyglucose positron emission tomography (18FDG-PET) or tumor shrinkage in the first three patients. Dosing started at 0.1 mg/kg, escalating by 100% in successive cohorts until the observation of a DLT (see supplementary materials, available at Annals of Oncology online). The MTD was defined as the highest dose that could be given to six patients such that no more than one patient (<33%) experienced a DLT. Copanlisib was continued until disease progression or until the treating physician felt that the risk:benefit assessment was no longer favorable. In the interest of safety, patients in the expansion cohort of solid-tumor patients with type 2 diabetes mellitus were planned to be treated one dose level below the MTD.
patients
Patients aged ≥18 years with a histologically or cytologically confirmed diagnosis of an advanced and/or refractory non-hematologic malignancy were eligible. Patients in the MTD expansion phase had to have a histologic diagnosis of either NHL (6–12 patients) or a solid tumor (24 patients). At least eight patients with solid tumors bearing PIK3CA mutations were to be enrolled in the MTD expansion phase. An additional expansion cohort of six diabetic patients with solid tumors was enrolled to evaluate the safety of administration of copanlisib to patients with type 2 diabetes mellitus. Further eligibility criteria and exclusion criteria are provided in the supplementary materials, available at Annals of Oncology online.
assessments
Following screening (see supplementary materials, available at Annals of Oncology online), assessments included tumor assessment, evaluation of urine and serum ketones, and measurement of hemoglobin A1c and plasma and blood glucose levels. Safety [including adverse events (AEs)] was assessed at planned times during each cycle and during follow-up. Serial plasma samples for single-dose and steady-state pharmacokinetic evaluation of copanlisib were collected on days 1 and 15 of cycle 1, after the start of copanlisib infusion; additional samples were collected on cycle 3, day 15 from patients in the MTD expansion phase. Plasma glucose was measured pre-dose on cycle 1, day 1 and at each pharmacokinetic assessment. Plasma insulin was measured pre-dose on cycle 1, day 1 and at 1, 2, and 3 h after the start of copanlisib infusion. As elevated blood glucose was expected based on the known profile of PI3K/AKT pathway inhibitors [7, 16–18], the use of short-acting insulin was recommended for the management of blood glucose >200 mg/dl post-infusion for non-diabetic patients. Whole-body 18FDG-PET scans were carried out at baseline and at 48–72 h after the first dose to assess early pharmacodynamic effects of copanlisib. Response was assessed by investigator assessment using modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 in patients with solid tumors and by investigator assessment using the International Working Group response criteria for lymphoma in NHL patients [19]. Biomarker assessments, along with statistical analysis, are described in the supplementary materials, available at Annals of Oncology online.
results
baseline demographics and disease characteristics
Sixty-eight patients were enrolled; 57 received treatment and 11 did not complete screening. Seventeen patients with solid tumors were enrolled in the dose-escalation cohorts (Table 1): one at 0.1 mg/kg; three at 0.2 mg/kg; three at 0.4 mg/kg; seven at 0.8 mg/kg; and three at 1.2 mg/kg. Twenty-five patients were enrolled in the solid-tumor expansion cohort (0.8 mg/kg), nine in the NHL expansion cohort [0.8 mg/kg; six with follicular lymphoma (FL) and three with diffuse large B-cell lymphoma (DLBCL)], and six patients in the diabetic cohort (0.4 mg/kg).
Table 1.
Baseline patient demographics and disease characteristics
| Dose escalation (n = 17) | Expansion cohorts |
Total (n = 57) | |||
|---|---|---|---|---|---|
| Solid tumors 0.8 mg/kg (n = 25) |
NHL 0.8 mg/kg (n = 9) |
Diabetic 0.4 mg/kg (n = 6) |
|||
| Median age, years (range) | 62 (35–86) | 61 (33–81) | 72 (40–84) | 67 (46–71) | 65 (33–86) |
| Males, n (%) | 7 (41) | 4 (16) | 4 (44) | 5 (83) | 20 (35) |
| ECOG performance status, n (%) | |||||
| 0 | 5 (29) | 7 (28) | 2 (22) | 4 (67) | 18 (32) |
| 1 | 11 (65) | 15 (60) | 5 (56) | 2 (33) | 33 (58) |
| 2 | 1 (6) | 3 (12) | 2 (22) | 0 | 6 (11) |
| Any prior systemic therapy, n (%) | 16 (94) | 24 (96) | 9 (100) | 6 (100) | 55 (97) |
| Median number of prior systemic therapies (range) | 3 (0–7) | 4 (0–14) | 3 (1–8) | 4 (2–6) | 4 (0–14) |
| Prior therapies, n (%) | |||||
| Rituximaba | 1 (6) | 0 | 9 (100) | 0 | 10 (18) |
| Bendamustine | 0 | 0 | 4 (44) | 0 | 4 (7) |
| PI3K/mTOR inhibitor | 0 | 2 (8) | 2 (22) | 0 | 4 (7) |
| Prior radiotherapy, n (%) | 9 (53) | 13 (52) | 3 (33) | 2 (33) | 27 (47) |
| Cancer diagnosis, n (%) | |||||
| Breast | 0 | 16 (64) | 0 | 0 | 16 (28) |
| NHL | 0 | 0 | 9 (100) | 0 | 9 (16) |
| Uterine | 2 (12) | 3 (12) | 0 | 0 | 5 (9) |
| Gastric/esophageal | 3 (18) | 2 (8) | 0 | 1 (17) | 6 (11) |
| Bladder | 1 (6) | 2 (8) | 0 | 1 (17) | 4 (7) |
| Pancreatic | 3 (18) | 0 | 0 | 0 | 3 (5) |
| Ovarian | 0 | 2 (8) | 0 | 1 (17) | 3 (5) |
| NSCLC | 1 (6) | 0 | 0 | 2 (33) | 3 (5) |
| Melanoma | 1 (6) | 0 | 0 | 0 | 1 (2) |
| Colon | 1 (6) | 0 | 0 | 1 (17) | 2 (4) |
| Other | 5 (29) | 0 | 0 | 0 | 5 (9) |
aOne patient in cohort 4 with a solid tumor had received prior rituximab because of follicular lymphoma diagnosed several years after the solid tumor but also before study entry (second tumor; allowed in study protocol).
ECOG, Eastern Cooperative Oncology Group; mTOR, mammalian target of rapamycin; NHL, non-Hodgkin's lymphoma; NSCLC, non-small-cell lung cancer; PI3K, phosphatidylinositol 3-kinase.
The median age was 65 years and 97% of patients had received prior systemic anti-neoplastic therapy (Table 1). Breast cancer (28%) and NHL (16%) were the most common malignancies. Seven NHL patients (78%) had advanced-stage disease at study entry (Ann Arbor stage III or IV) and all nine (100%) had received prior rituximab (Table 1). One diabetic patient was on insulin therapy at screening and all six received oral anti-diabetic treatment at study entry (four with metformin and two with glibenclamide).
Tumors harboring PI3K pathway activation [via PIK3CA mutation (detected in circulating tumor DNA and/or tumor tissue) and/or PTEN protein loss] were observed in 27 patients, 12 of whom had PIK3CA mutation and 19 had loss of or low (<5% of tumor cells) PTEN expression (Table 2). Eight patients had KRAS mutations and one patient had a BRAF mutation.
Table 2.
Baseline tumor molecular status
| Dose escalation | Solid-tumor expansion | NHL expansion | Diabetic | Total | |
|---|---|---|---|---|---|
| PIK3CA mutational status in ctDNAa, n (%) | |||||
| n | 17 | 25 | 9 | 6 | 57 |
| PIK3CA mutation | 0 | 7 (28) | 0 | 1 (17) | 8 (14) |
| PIK3CA wild type | 17 (100) | 18 (72) | 9 (100) | 5 (83) | 49 (86) |
| PIK3CA mutational status in tumor tissuea,b,c, n (%) | |||||
| n | 5 | 22 | 7 | 4 | 38 |
| PIK3CA mutation | 1 (20) | 8 (36) | 0 | 1 (25) | 10 (26) |
| PIK3CA wild type | 4 (80) | 14 (64) | 7 (100) | 3 (75) | 28 (74) |
| PIK3CA mutational status in ctDNA and/or tumor tissuea,b,c, n (%) | |||||
| n | 17 | 25 | 9 | 6 | 57 |
| PIK3CA mutation | 1 (6) | 10 (40) | 0 | 1 (17) | 12 (21) |
| PIK3CA wild type | 16 (94) | 15 (60) | 9 (100) | 5 (83) | 45 (79) |
| PTEN expression status by IHC in tumor tissued, n (%) | |||||
| n | 18 | 7 | 3 | 28 | |
| PTEN loss | ND | 9 (50) | 1 (14) | 2 (67) | 12 (43) |
| Low PTEN | ND | 3 (17) | 4 (57) | 0 | 7 (25) |
| No PTEN loss | ND | 6 (33) | 2 (29) | 1 (33) | 9 (32) |
| PI3K activation statusa,b,c, n (%) | |||||
| n | 17 | 25 | 9 | 6 | 57 |
| Activation | 1 (6) | 18 (72) | 5 (56) | 3 (50) | 27 (47) |
| No activation detected | 16 (94) | 7 (28) | 4 (44) | 3 (50) | 30 (53) |
| KRAS mutational status in ctDNA and/or tumor tissuea,b, n (%) | |||||
| n | 17 | 22 | 5 | 6 | 50 |
| KRAS mutation | 3 (18) | 2 (9) | 0 | 3 (50) | 8 (16) |
| KRAS wild type | 14 (82) | 20 (91) | 5 (100) | 3 (50) | 42 (84) |
| BRAF mutational status in ctDNA and/or tumor tissuea,b, n (%) | |||||
| n | 17 | 22 | 5 | 6 | 50 |
| BRAF mutation | 1 (6) | 0 | 0 | 0 | 1 (2) |
| BRAF wild type | 16 (94) | 22 (100) | 5 (100) | 6 (100) | 49 (98) |
aMutational status was assessed by BEAMing (Sysmex Inostics GmbH, Hamburg, Germany) and/or next-generation sequencing of DNA isolated from tumor tissue, and/or by BEAMing of circulating tumor DNA.
bNext-generation sequencing was not carried out on samples from the dose-escalation cohorts.
cCases with mutations detected by one test but not the other were designated mutant.
dPTEN loss: 0% of tumor cells staining positive for PTEN by IHC; low PTEN: 1% to <5% staining positive; no PTEN loss: ≥5% staining positive.
BEAMing, beads, emulsions, amplification, and magnetics technology; ctDNA, circulating tumor DNA; IHC, immunohistochemistry; ND, not determined; NHL, non-Hodgkin's lymphoma; PI3K, phosphatidylinositol 3-kinase.
copanlisib treatment and MTD
Patients received a median of two treatment cycles (range, 1–49) of copanlisib, and the median duration of treatment was 6 weeks (range, 0.1–2051). Patients in the solid-tumor expansion cohort received a median of 2 treatment cycles (range, 1–14) and patients with NHL received 10 (range, 1–49); one patient with FL was treated for 47 months, and as of 26 February 2016, the last patient with FL went off study after having been treated for >43 months.
One patient (male, 65 years old) with metastatic colon carcinoma in the 1.2 mg/kg dose-escalation cohort experienced multiple DLTs of grade 3 increased alanine aminotransferase, grade 4 increased aspartate aminotransferase, grade 4 hyperglycemia with lactic acidosis, anion gap and increased serum ketone, and grade 3 left ventricular systolic dysfunction, starting on the day of the first copanlisib infusion. Copanlisib was discontinued and the cardiac dysfunction resolved within 7 days. Due to the nature and severity of the events at the 1.2 mg/kg dose seen for this patient, this dose was considered toxic and the MTD was defined as 0.8 mg/kg copanlisib. Two other patients were concurrently enrolled in the 1.2 mg/kg cohort, one of whom had not yet received the planned dose and one who had received a single dose at 1.2 mg/kg with no DLTs. Both patients then received 0.8 mg/kg during the first treatment cycle. There were no DLTs in the 0.8 mg/kg dose-escalation cohort. Grade 3 or 4 drug-related events reported in the 34 patients of the expansion cohorts at 0.8 mg/kg, and which would have fulfilled the DLT criteria used in the dose-escalation phase, did not lead to permanent discontinuation of copanlisib (Table 3).
Table 3.
Summary of adverse events possibly related to study drug
| n (%) | Dose-escalation cohorts |
Expansion cohorts |
Total (n = 57) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 0.1 mg/kg (n = 1) |
Cohort 2 0.2 mg/kg (n = 3) |
Cohort 3 0.4 mg/kg (n = 3) |
Cohort 4 0.8 mg/kg (n = 7) |
Cohort 5 1.2 mg/kg (n = 3) |
Solid tumors 0.8 mg/kg (n = 25) |
NHL 0.8 mg/kg (n = 9) |
Diabetic 0.4 mg/kg (n = 6) |
|||||||||||
| Any drug-related AEa | 1 (100) | 3 (100) | 2 (67) | 7 (100) | 3 (100) | 20 (80) | 8 (89) | 5 (83) | 49 (86) | |||||||||
| Drug-related AEs of grade 3 or 4 | ||||||||||||||||||
| Grade 3 | 0 | 2 (67) | 1 (33) | 4 (57) | 2 (67) | 12 (48) | 5 (56) | 3 (50) | 29 (51) | |||||||||
| Grade 4 | 0 | 0 | 0 | 0 | 1 (33) | 0 | 1 (11) | 0 | 2 (4)b | |||||||||
| Drug-related AEs in ≥10% of patients | All | Grade 3 | All | Grade 3 | All | Grade 3 | All | Grade 3 | All | Grade 3 | All | Grade 3 | All | Grade 3 | All | Grade 3 | All | Grade 3c |
| Hyperglycemia | 0 | 0 | 1 (33) | 0 | 1 (33) | 0 | 7 (100) | 4 (57) | 3 (100) | 1 (33) | 15 (60) | 8 (32) | 8 (89) | 3 (33) | 1 (17) | 1 (17) | 36 (63) | 17 (30) |
| Nausea | 0 | 0 | 3 (100) | 0 | 0 | 0 | 1 (14) | 0 | 1 (33) | 0 | 7 (28) | 0 | 7 (78) | 0 | 2 (33) | 0 | 21 (37) | 0 |
| Hypertension | 0 | 0 | 0 | 0 | 1 (33) | 1 (33) | 1 (14) | 0 | 0 | 0 | 5 (20) | 2 (8) | 3 (33) | 3 (33) | 2 (33) | 2 (33) | 12 (21) | 8 (14) |
| Diarrhea | 0 | 0 | 2 (67) | 1 (33) | 0 | 0 | 1 (14) | 0 | 0 | 0 | 2 (8) | 0 | 3 (33) | 0 | 1 (17) | 0 | 9 (16) | 1 (2) |
| Rash/desquamation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (24) | 2 (8) | 2 (22) | 1 (11) | 1 (17) | 1 (17) | 9 (16) | 4 (7) |
| Vomiting | 0 | 0 | 1 (33) | 0 | 0 | 0 | 1 (14) | 0 | 1 (33) | 0 | 3 (12) | 0 | 1 (11) | 0 | 0 | 0 | 7 (12) | 0 |
| Fatigue | 0 | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (8) | 0 | 3 (33) | 0 | 0 | 0 | 6 (11) | 0 |
| Oral cavity mucositis | 0 | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (8) | 0 | 3 (33) | 0 | 0 | 0 | 6 (11) | 0 |
aAll AEs were defined according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
bThe two drug-related AEs of grade 4 as worst grade were hyperglycemia and increased aspartate aminotransferase in one patient in the 1.2 mg/kg cohort (dose-limiting toxicities).
cDrug-related AEs of grade 3 or 4 occurring in <10% of patients treated at the 0.8 mg/kg dose level were: two cases of drug-related grade 3 non-infectious pneumonitis in two patients with NHL; one case of grade 3 hypoxia (reported concomitantly with one of the cases of non-infectious pneumonitis in a patient with NHL); grade 3 decreased white blood cell count with fever ≥38.5°C in one patient with NHL; one case of grade 4 increased amylase in a patient with NHL; grade 3 anemia in one patient with NHL; grade 3 dyspnea and dizziness, both in one solid-tumor patient (infusion reaction); and grade 3 chest pain in one solid-tumor patient.
AE, adverse event; NHL, non-Hodgkin's lymphoma.
safety
All patients experienced at least one treatment-emergent AE, regardless of causality (supplementary Table S1, available at Annals of Oncology online). AEs possibly related to the study drug occurred in 49 patients (86%) (Table 3). The most common (≥20%) drug-related AEs (all grades) included hyperglycemia (63%), nausea (37%), and hypertension (21%) (Table 3). The most common drug-related grade 3 AEs were hyperglycemia (30%), hypertension (14%), and rash (7%). Grade ≥3 diarrhea occurred in one patient; one case of grade 2 colitis (not drug-related) occurred in the 1.2 mg/kg cohort in a patient with a history of radiation enteritis. Grade 4 thrombocytopenia occurred in one patient but was not considered drug-related. Two patients (4%) experienced a total of three drug-related grade 4 AEs: hyperglycemia and increased aspartate aminotransferase in one patient (DLTs) and elevated serum amylase in another patient. Laboratory aspartate aminotransferase and alanine aminotransferase findings are summarized in the supplementary materials, available at Annals of Oncology online.
There were 41 grade 3 AEs and 1 grade 4 AE regardless of causality, of which the most common (≥5%) was hyperglycemia (32% grade 3; 2% grade 4) (supplementary Table S1, available at Annals of Oncology online). There were seven (12%) grade 5 AEs, none of which was considered drug-related (see supplementary Table S2 for details, available at Annals of Oncology online). There were two cases of drug-related grade 3 non-infectious pneumonitis and one case of grade 3 viral pneumonitis not considered drug-related; further details are provided in the supplementary materials, available at Annals of Oncology online. Serious drug-related AEs occurred in six patients (11%): left ventricular systolic dysfunction (grade 3 DLT), chest pain, hypertension, and hyperglycemia (in one patient each), and pneumonitis (in two patients).
Dose modifications (delays, interruptions, and reductions) caused by drug-related AEs occurred in 14 patients (25%). The primary reasons for discontinuation included disease progression, as assessed by radiological measurement (n = 26) and clinical progression (n = 12). Four patients discontinued because of AEs. There was one drug-related AE leading to permanent discontinuation (the DLT of left ventricular systolic dysfunction). No patient discontinued the study because of hyperglycemia, and elevated blood glucose was not observed upon discontinuation of copanlisib.
Peak plasma glucose values were observed 5–8 h after the start of copanlisib infusion on cycle 1, day 1 followed by a decline to baseline levels by the time of the next infusion (supplementary Figure S1A, available at Annals of Oncology online). Thirty-three of the 51 non-diabetic patients (65%) received at least 1 dose of short-acting insulin to manage blood glucose values >200 mg/dl (per protocol). There was no tendency for increased pre-dose glucose values over time, and no patients developed diabetic ketoacidosis during the study.
A similar transient pattern of elevated blood glucose post-infusion was seen for the cohort of six diabetic patients treated with 0.4 mg/kg copanlisib, all of whom received insulin following the first copanlisib infusion. AE incidence in this small cohort was similar to that seen in non-diabetic patients; there were four drug-related grade 3 AEs in three patients: hypertension in two patients, and hyperglycemia and rash/desquamation in one patient each.
Hemoglobin A1c levels changed only modestly over the course of copanlisib treatment (supplementary Table S3, available at Annals of Oncology online). Post-infusion increases in blood pressure peaked at 1–2 h, and resolved within 24 h post-infusion (supplementary Figure S1B and Table S4, available at Annals of Oncology online).
pharmacokinetics
Following administration, copanlisib typically reached maximum plasma concentration (Cmax) between 0.5 and 1 h (Table 4), followed by a rapid, multiphasic, then slow decline in the plasma concentration–time profiles up to 168 h (Figure 1). Copanlisib geometric mean Cmax and area under the curve from time zero to 25 h after the start of infusion [AUC(0–25)] increased dose-proportionally between 0.1 and 1.2 mg/kg, with moderate to high variability, with coefficients of variation of ∼30–50%. At the MTD (0.8 mg/kg), inter-patient variability was moderate to high (73% for Cmax; 47% for AUC). The terminal half-life was 38.2 h (coefficient of variation 43%), and no accumulation was observed after once-weekly administration (Table 4; Figure 1). The trough levels of copanlisib on cycle 1, day 8 were 4.92 µg/l (range, 2.74–23.0).
Table 4.
Copanlisib pharmacokinetic parameters in cycle 1
| Dose-escalation cohorts |
Expansion cohorts |
MTD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 0.1 mg/kg (n = 1) |
Cohort 2 0.2 mg/kg (n = 3) |
Cohort 3 0.4 mg/kg (n = 2) |
Cohort 4 0.8 mg/kg (n = 7) |
Cohort 5 1.2 mg/kg (n = 3) |
Solid tumors 0.8 mg/kg (n = 25) |
NHL 0.8 mg/kg (n = 9) |
Diabetic 0.4 mg/kg (n = 6) |
0.8 mg/kg combined (n = 41) | |
| Day 1 | |||||||||
| Cmax, µg/l (%)a | 32.9 | 79.8 (38.0) | 143–144 | 293 (16.5) | 616–739b,c | 537 (82.7) | 402 (58.5) | 138 (36.7) | 454 (73.3) |
| tmax, h (range)a | 0.5 | 1.1 (0.5–1.1) | 0.5–1.1 | 1.1 (0.8–1.2) | 0.6–1.3b,c | 1.0 (0.5–3.0) | 1.0 (0.5–1.0) | 1.0 (1.0–1.2) | 1.0 (0.5–3.0)d |
| AUC(0–25), µg × h/l (%)a | 170 | 384 (29.4) | 348–556 | 1595 (25.6) | 2508–3821b,c | 1689 (46.7) | 1353 (36.8) | 607 (43.6) | 1593 (41.9) |
| AUC(0–tlast), µg × h/l (%)a | 254 | 472 (22.0) | 722–1449 | 2943 (45.9) | 4088–5234b,c | 2918 (47.2) | 2713 (50.3) | 1070 (66.2) | 2876 (46.5) |
| tlast, h (range)a | 48.6 | 48.4 (25.0–49.0) | 167.8–170.2 | 142.5 (25.0–192.0) | 29.3–167.2b,c | 118.5 (46.4–194.2) | 167.0 (47.3–191.9) | 49.0 (48.9–167.4) | 142.5 (25.0–194.2)e |
| t1/2, h (%) | NC | NC | 60.6f | 42.3 (28.6)g | 33.0f | 36.9 (47.8)h | 38.9 (41.3)i | 34.4 (138)b | 38.2 (42.5)j |
| Day 15 | |||||||||
| Cmax, µg/l (%)a | 37.6 | 89.4 (26.5) | 70.1–193 | 370 (29.8)k | – | 477 (76.4)l | 507 (52.2)i | 151 (23.4) | 463 (64.4)m |
| tmax, h (range)a | 0.5 | 1.0 (1.0–1.2) | 0.5–1.1 | 1.0 (0.5–1.1)k | – | 1.0 (0.5–1.3)l | 0.8 (0.5–1.0)i | 1.0 (0.5–1.1) | 1.0 (0.5–1.3)m |
| AUC(0–25), µg × h/l (%)a | 175 | 453 (14.4) | 324–803 | 1484 (22.3)k | – | 1525 (39.7)n | 1761 (46.3)i | 687 (36.0) | 1569 (38.4)o |
aGeometric mean (% coefficient of variation) given for n ≥ 3, range given for n = 2, and individual data given for n = 1.
bn = 2.
cErroneous dosing in one of the three patients in the 1.2 mg/kg cohort.
dn = 34.
en = 7.
fn = 1.
gn = 4.
hn = 16.
in = 8.
jn = 28.
kn = 6.
ln = 22.
mn = 36.
nn = 21.
on = 35.
AUC(0–25), area under the curve from time zero to 25 h after start of infusion; AUC(0–tlast), area under the curve from time zero to the last time point above the lower limit of quantification; Cmax, maximum drug concentration; MTD, maximum tolerated dose; NC, not calculated; NHL, non-Hodgkin's lymphoma; t1/2, half-life associated with the terminal slope; tlast, time zero to the last time point above the lower limit of quantification; tmax, time to maximum drug concentration.
Figure 1.
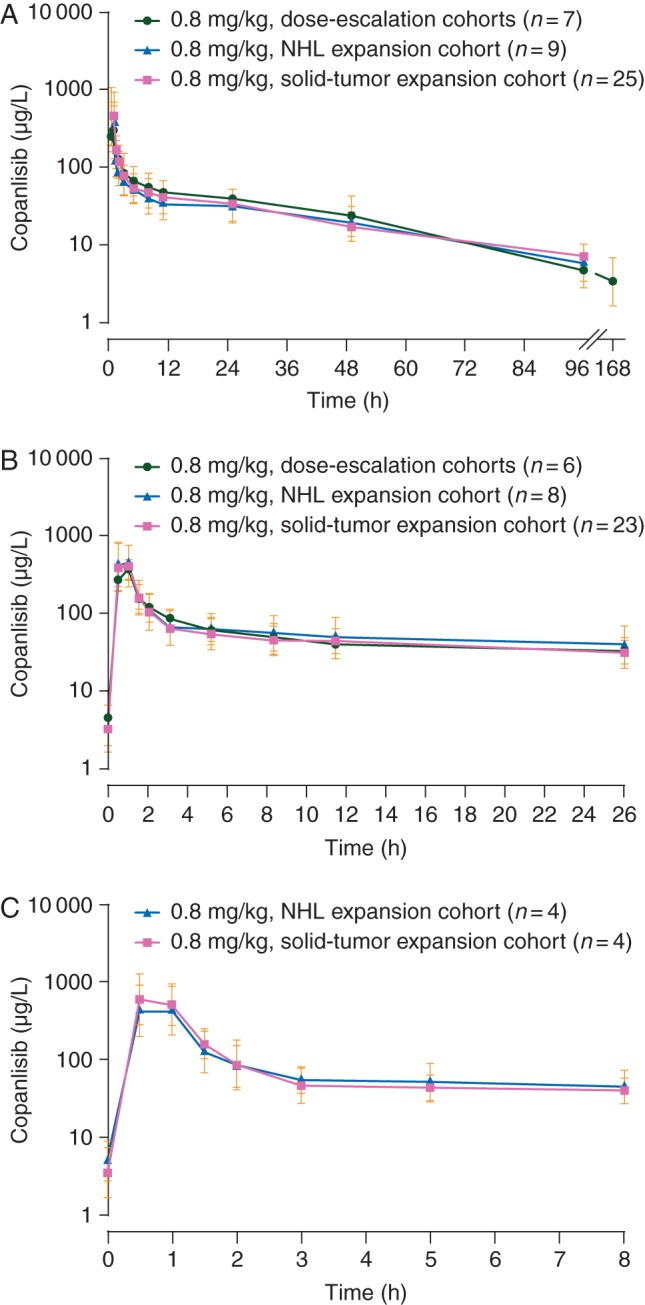
Geometric mean (standard deviation) plasma concentration–time profiles for copanlisib at the maximum tolerated dose expansion dose (0.8 mg/kg) on cycle 1 (at the 96-h time point, samples were collected 72–120 h following the start of infusion), day 1 (A), cycle 1, day 15 (B), and cycle 3, day 15 (C). NHL, non-Hodgkin's lymphoma.
pharmacodynamics
Fifty-three patients (93%) experienced a predefined pharmacodynamic effect following the first copanlisib infusion. Copanlisib exposure [AUC(0–25)] correlated with plasma glucose levels; an almost linear increase in maximum changes from baseline was observed up to a plasma AUC(0–25) of ∼1000 µg/l, with increasing variability at higher exposure levels (supplementary Figure S2, available at Annals of Oncology online).
Paired 18FDG-PET scans were evaluable in 21 non-diabetic patients. A weak correlation between exposure and change in tumor FDG uptake from baseline to cycle 1, days 3 and 4 was seen in 19 of 21 patients (supplementary Figure S3, available at Annals of Oncology online). Seven patients (33%) showed a reduction of >25% in FDG uptake between baseline and days 3 or 4, indicating target modulation. However, the number of patients with solid tumors having an early FDG-PET response (four patients) was too low to allow a correlation with RECIST response.
response
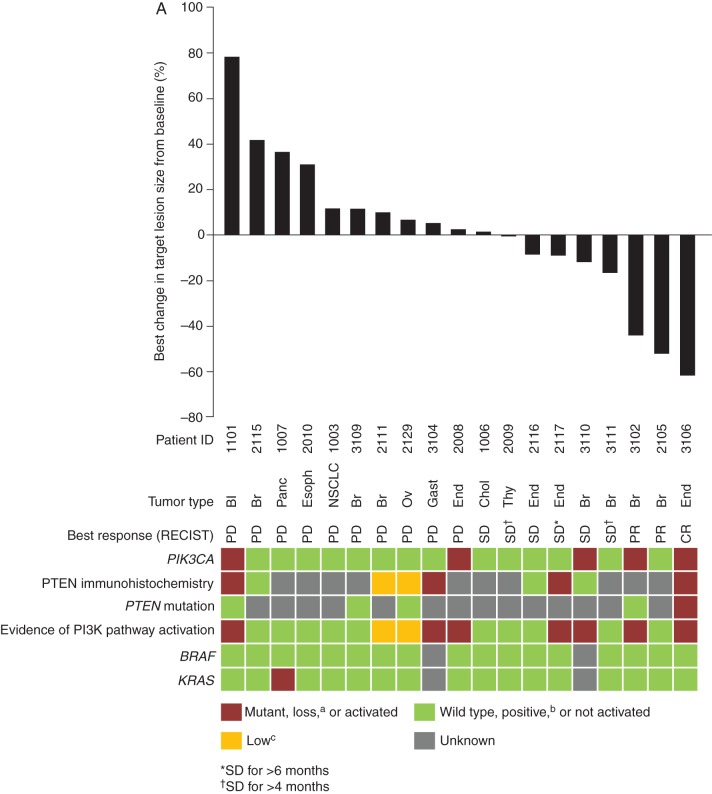
Of the 48 patients with solid tumors (dose-escalation, MTD expansion, and diabetic cohorts combined), clinical responses were observed only in patients from the MTD expansion cohort. One patient (2%) had a complete response (CR), 2 (4%) had a partial response (PR; 1 confirmed, 1 unconfirmed), 15 (31%) achieved stable disease, and 15 (31%) had disease progression by investigator assessment, according to RECIST version 1.1. Seven patients (15%) had disease progression by clinical judgment and eight patients (17%) were not assessed.
The patient with a CR had endometrial carcinoma with mutations in PIK3CA and PTEN, and complete PTEN protein loss, and achieved a CR after 10 cycles (Figure 2A). Details of specific mutations are presented in supplementary Table S5, available at Annals of Oncology online. The patient with a confirmed PR had estrogen receptor-/progesterone receptor-positive, HER2-negative, PIK3CA wild-type breast cancer; the patient with an unconfirmed PR had estrogen receptor-/progesterone receptor-positive, HER2-positive breast cancer, with a PIK3CA mutation (PTEN status unavailable for both patients). Of the 15 patients with stable disease, 6 had stable disease ≥6 cycles, including: 1 patient with endometrial carcinoma in the 0.4 mg/kg cohort who experienced stable disease (>25% tumor reduction) for 8 cycles and had PIK3CA wild-type, KRAS-mutant status and unknown PTEN status; 1 patients with PTEN loss (with endometrial cancer, treated at 0.8 mg/kg); and 1 patient with breast cancer and mutated PIK3CA (PTEN unknown) treated at 0.8 mg/kg. Of the 10 patients without complete PTEN loss, none had objective response or stable disease for >4 cycles.
Figure 2.
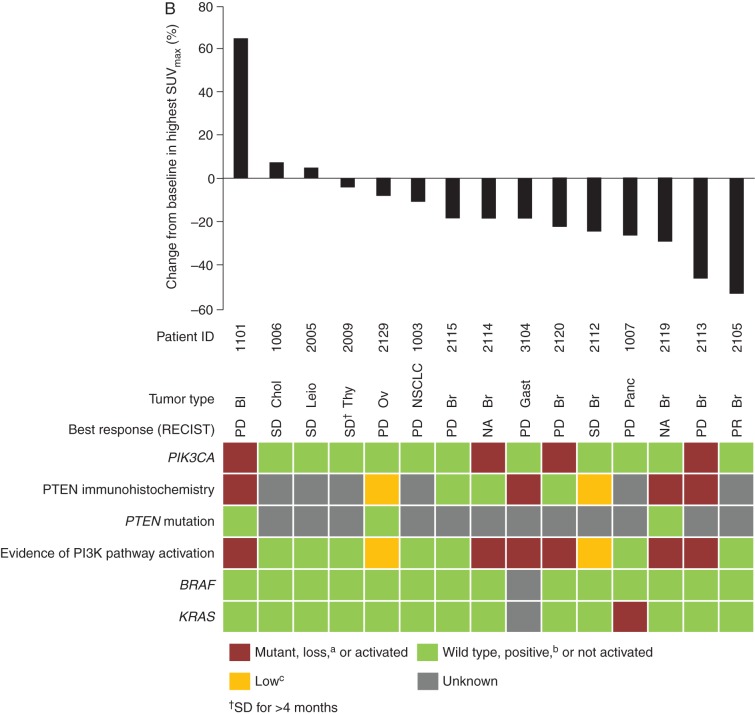
Radiologic response to copanlisib with corresponding status of tumor PIK3CA mutation, PTEN protein loss by immunohistochemistry, and PTEN mutation (A) and metabolic response to copanlisib (percentage change from baseline in SUVmax from 18FDG-PET) with corresponding status of tumor PIK3CA, PTEN, and KRAS alteration (B), in patients with solid tumors, and radiologic response to copanlisib with corresponding status of tumor PIK3CA mutation, PTEN protein loss by immunohistochemistry, and PTEN mutation in patients from the NHL expansion cohort (C), treated with 0.8 mg/kg copanlisib. aLoss defined as 0% of tumor cells staining positive for PTEN by immunohistochemistry; bpositive defined as ≥5% of tumor cells staining positive for PTEN by immunohistochemistry; clow defined as 1% to <5% of tumor cells staining positive for PTEN by immunohistochemistry. 18FDG-PET, [18F]-fluorodeoxyglucose positron emission tomography; Bl, bladder cancer; Br, breast cancer; Chol, cholangiocarcinoma; CR, complete response; DLBCL, diffuse large B-cell lymphoma; End, endometrial carcinoma; Esoph, esophageal cancer; FL, follicular lymphoma; Gast, gastric cancer; NA, not available; NHL, non-Hodgkin's lymphoma; NSCLC, non-small-cell lung cancer; Ov, ovarian cancer; Panc, pancreatic cancer; PD, progressive disease; PI3K, phosphatidylinositol 3-kinase; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SUVmax, maximum standardized uptake value; Thy, thyroid carcinoma.
Of the 14 patients with solid tumors who were treated at the MTD (0.8 mg/kg) with at least 1 response assessment carried out and PTEN immunohistochemistry data available, 7 had complete PTEN loss, of whom 3 (43%) had either CR, PR, or stable disease lasting ≥6 cycles. Seven patients did not have PTEN loss, of whom none had CR, PR, or stable disease (P = 0.192 compared with PTEN loss group). No clear relationship was identified between PIK3CA mutational status (P = 1.0) and disease control (CR, PR, or durable stable disease).
Change in 18FDG-PET uptake did not have any apparent relationship to PI3KCA mutational status, PTEN expression level, PI3K pathway activation, or BRAF/KRAS mutational status (Figure 2B). One patient with breast cancer with an objective response showed early changes in PET imaging that preceded the objective response.
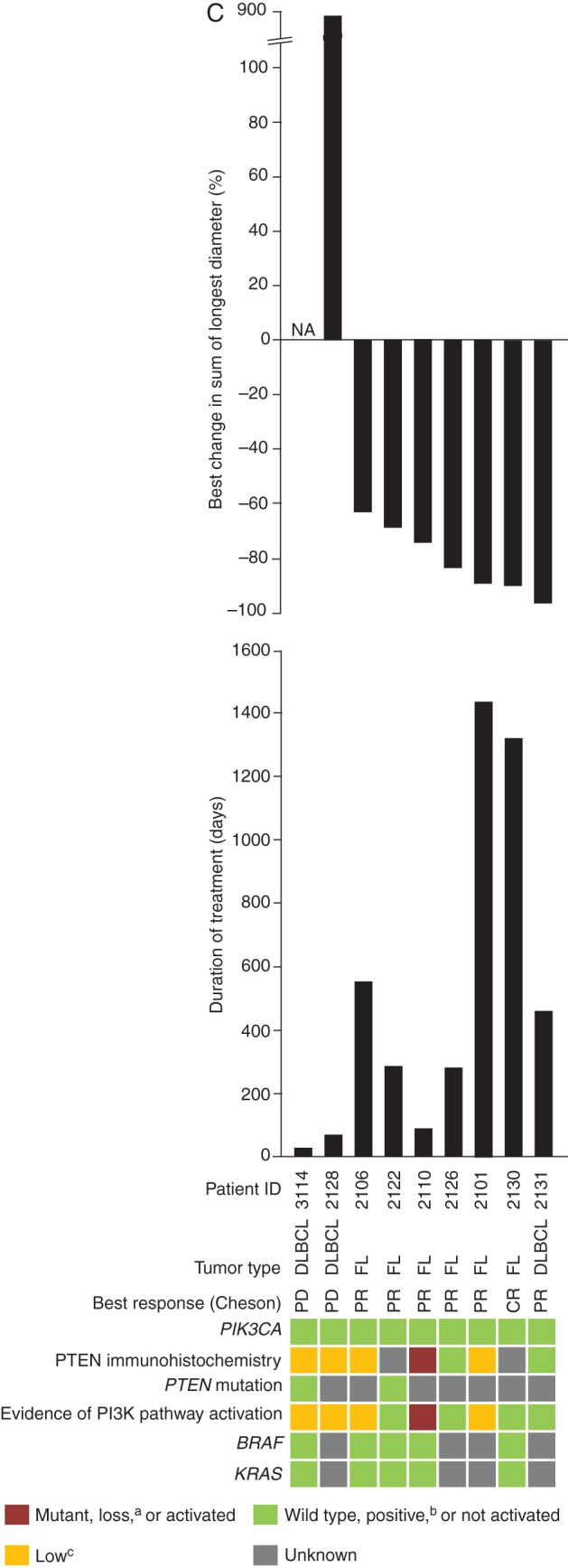
All nine patients with NHL were evaluable for response by investigator assessment using the International Working Group criteria [19]. One FL patient achieved a CR as best response, six patients had PR as best response (five with FL and one with DLBCL), and two patients with DLBCL had disease progression (Figure 2C); post hoc follow-up radiologic review carried out for eight of the nine NHL patients with radiology imaging determined that two FL patients had achieved a CR. In addition, two FL patients had long-term durable responses (Figure 2C), one of whom was on treatment for ∼4 years before coming off study because of disease progression; the other patient was still on treatment as of 19 January 2016 (>3 years).
All seven NHL responders had PI3KCA wild-type status; by immunohistochemistry, one patient had complete PTEN loss, two had low PTEN expression, two had positive PTEN expression, and two had unknown PTEN expression (Figure 2C). Both patients with disease progression had low PTEN expression.
discussion
This first-in-human phase I trial established that copanlisib can be safely administered intravenously to patients with advanced malignancies. The MTD for copanlisib was 0.8 mg/kg once weekly, and copanlisib was generally well tolerated in a small cohort of six diabetic patients treated at 0.4 mg/kg (highest dose tested). The most common drug-related AEs were metabolic (hyperglycemia), gastrointestinal (nausea and diarrhea), and cardiovascular (hypertension). Drug-related pneumonitis was reported in two patients, consistent with the known safety profile of mTOR/PI3K pathway inhibitors [20]. No severe metabolic complications were reported. Most AEs were managed with dose modifications, and there was only one drug-related AE leading to permanent discontinuation [left ventricular systolic dysfunction (DLT)], which resolved after discontinuation.
Blood glucose elevations have been reported with other PI3K/AKT/mTOR inhibitors [7, 17] and are a likely on-target class effect via inhibition of insulin signaling [21, 22], and were thus extensively monitored here in this first-in-human study. Blood glucose values typically peaked 5–8 h after the start of copanlisib infusion, and declined to pre-diabetic levels 24–48 h after infusion and to normal levels before the start of the next infusion. The transient hyperglycemia was not a DLT for patients treated at the MTD of 0.8 mg/kg infused weekly, 3 weeks on and 1 week off. The extent to which food intake confounded post-infusion blood glucose values was not ascertained. The incidence of drug-related all-grade (63%) and grade 3 (29%) hyperglycemia was higher with copanlisib than reported in other phase I studies with pan-class I PI3K inhibitors [7, 9, 11] (all-grade: 7–37%; grade 3: <10%), but such differences could be related to the routes of administration, the timing of blood sampling [9, 11], or using gradings not taken from the National Cancer Institute Common Terminology Criteria for Adverse Events [7]. Overall, no long-term consequences of the transient hyperglycemia were apparent; the mean hemoglobin 1Ac levels were broadly unchanged. Likewise, treatment of six diabetic patients with 0.4 mg/kg copanlisib appeared to be feasible.
The incidence and severity of gastrointestinal toxicities due to copanlisib were low; nausea was the most common drug-related gastrointestinal toxicity (37%; all events grade ≤2) and diarrhea was less common (all-grade: 16%; grade ≥3: 2%). Elevated aminotransferase was primarily an incidental laboratory toxicity finding and was mostly grade 1; grade ≥3 in five patients. For orally administered PI3K inhibitors, the reported incidence of all-grade and grade ≥3 diarrhea ranged from 32% to 92% [5–9] and 9% to 20% [5, 6, 8], respectively, while the reported incidence of grade ≥3 elevations in aminotransferases was up to 25%, and serious gastrointestinal toxicity, such as colitis, was reported in 4% of patients [5, 6]. Although these toxicities are considered class effects of PI3K inhibitors [6, 11, 16, 23], their reduced incidence and severity seen with copanlisib suggests that the route of administration may play a role, and that higher concentrations in the gut, and possible first-pass metabolism, could be dose-limiting for oral agents. Consistent with this profile, intravenous administration of a dual PI3K/mTOR inhibitor with pan-PI3K inhibitory activity has demonstrated low incidence of both diarrhea (14%, grade 1 only) and elevated aminotransferase grade ≥3 (5–7%) [11].
A transient increase in blood pressure and return to baseline within 24 h was seen following copanlisib infusion, and overall incidence of drug-related all-grade and grade 3 hypertension was 21% and 14%, respectively. Hypertension was not reported as an AE for other pan-PI3K inhibitors [7, 11], including the intravenous pan-PI3K/mTOR inhibitor [9]. Frequent monitoring, as used here in a first-in-human study, may account for some of the reported differences, particularly for an intravenous agent [11]. The rapid onset and preclinical evidence of peripheral vasoconstriction following copanlisib administration in laboratory animals (Bayer, data on file) would suggest a direct effect on the vasculature, possibly involving dysregulation of endothelial-derived vasoconstrictors and vasodilators [24–27]. Acute elevation of insulin in the presence of PI3K blockade may also be involved [27, 28].
There is increasing evidence in animal models that complete transient target inhibition with intermittent dosing of PI3K inhibitors may be equally effective as or more effective than continuous dosing regimens with less toxicity and chronic feedback reactivation [15, 29–32]. Copanlisib displayed a dose-proportional increase in exposure, up to and including at the MTD (0.8 mg/kg). It is interesting to note that at the MTD, Cmax levels of unbound copanlisib (∼150 nM) would be expected to greatly exceed the in vitro cellular IC90 for all PI3Kα and PI3Kδ isoforms (Paul et al., submitted), resulting in complete inhibition of PI3K signaling, whereas 1 week after copanlisib administration, the concentration of unbound copanlisib in the blood (1.6 nM; range, 0.9–7.6) would be at or below the in vitro cellular IC50 values but well below the IC90 values and thus consistent with a transient inhibition mechanism of action. The early concentration–time profile and the long plasma half-life (∼38 h) indicate a wide tissue distribution of copanlisib and, together with the observed lack of accumulation between days 1 and 15, support once-weekly intravenous administration. Recent analyses indicate that an intermittent dose schedule of 60 mg weekly on days 1, 8, and 15 of a 28-day cycle was likely to achieve a similar risk:benefit ratio as 0.8 mg/kg weight-based dosing [33], and fixed dosing is being explored in ongoing studies.
Shortly after administration, copanlisib exposure positively correlated with plasma glucose levels, and 18FDG-PET data revealed a decrease in tumor glucose uptake post-infusion in most patients studied, irrespective of PI3KCA mutational status, PTEN expression level, PI3K pathway activation, or BRAF/KRAS mutational status. These preliminary data suggest rapid onset of copanlisib pharmacodynamic activity (48–72 h post-infusion), suggestive of copanlisib-mediated inhibition of tumor PI3K signaling. The use of maximum standardized uptake as a measure of tumor metabolic burden has been previously described as a prognostic marker in various tumor types, including NHL [34, 35], and may indicate pharmacodynamic and anti-tumor activity [36]. Although the 18FDG-PET sample size was relatively low (n = 21), the pharmacodynamic data may support the use of hyperglycemia as a biomarker for anti-tumor activity in patients with solid tumors and NHL; one patient with an objective response had early changes in PET imaging.
Most patients had tumors that were wild-type for PI3KCA, although PTEN loss or low expression was commonly observed; 47% had PI3K pathway activation (via either PIK3CA mutation or PTEN loss), similar to that reported (46%) in a previous study of PI3K inhibition in patients with advanced solid tumors [7]. As an objective of this study, a minimum number of patients with PI3KCA mutations were enrolled in the solid-tumor MTD expansion phase. Copanlisib response may be enhanced among patients with PTEN loss, although there was no apparent association between PI3KCA mutational status alone and objective tumor response (n = 22) or 18FDG-PET response (n = 15) to copanlisib, and conclusive interpretations are difficult based on the relatively small number of patients assessed.
There were two PRs (metastatic breast cancer) and one CR for an overall response rate of 6% for patients with solid tumors. The CR was observed in a patient with endometrial cancer and tumor PI3K pathway activation.
The response rate in NHL patients, and particularly the 100% response rate for FL, was promising compared with previous reports for other PI3K inhibitors in patients with indolent NHL [5, 6, 37], and consistent with preclinical activity of copanlisib in vitro and in vivo against lymphoid cells [38]. Two FL patients received long-term treatment with copanlisib, one of whom achieved a CR. The clinical activity of copanlisib is being further explored for indolent NHL [Phase III: NCT02369016 (monotherapy), NCT02367040 (in combination with rituximab), NCT02626455 (in combination with standard immunochemotherapy: either rituximab with bendamustine, or rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone/prednisolone)] and aggressive NHL [Phase II: NCT02455297 (monotherapy)].
Finally, pan-class PI3K inhibitors administered intravenously once weekly may have attractive properties [11], including improved patient adherence [39] and potentially less gastrointestinal toxicity (diarrhea, colitis, nausea, vomiting, and aminotransferase elevations) [5–9], compared with orally administered isoform-selective and pan-class I PI3K inhibitors. Reports also indicate that the severity of gastrointestinal AEs with oral agents may increase over time [5, 8], suggesting cumulative toxicity, and the resulting dose interruptions and/or dose reductions may negatively impact on clinical efficacy. Greater PI3K pathway inhibition may also be possible with intravenous compared with oral agents, through both higher peak and sustained drug concentrations. Pan-PI3K inhibitors are amenable to combination with other targeted agents to overcome resistance [40] and can be more active than isoform-selective agents [41]. Evidence exists for functional redundancy among PI3K isoforms, and residual activity via unblocked isoforms may permit cell survival [42]; this is an area of active investigation with copanlisib.
In summary, copanlisib administered intravenously on an intermittent dose schedule on days 1, 8, and 15 of a 28-day cycle in the 0.1–0.8 mg/kg (MTD) dose range was generally safe and well tolerated, with a dose-proportional pharmacokinetic profile supportive of weekly dosing. The incidence and severity of gastrointestinal toxicity were relatively low. For patients with type 2 diabetes mellitus, a dose of 0.4 mg/kg copanlisib appeared equally safe. Copanlisib showed promising anti-tumor pharmacodynamic activity and preliminary clinical benefit in patients with a range of advanced solid tumors and in patients with NHL, including FL and DLBCL. Further studies of copanlisib safety and efficacy are warranted.
funding
This work was supported by Bayer Pharma AG. Editorial assistance was funded by Bayer HealthCare Pharmaceuticals, Inc. No grant number applies.
disclosure
AP, LJA, and RKR: research funding, Bayer Pharma AG; AWT: consultant role and board memberships, Bayer Pharma AG; CP, MJ, and CX: employees, Bayer HealthCare Pharmaceuticals, Inc.; SR and IG: employees, Bayer Pharma AG. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
Medical writing assistance was provided by Tanja Torbica, PhD, and Louise Picken, PhD, at Complete HealthVizion (Macclesfield, Cheshire, UK), based on detailed discussion and feedback from all the authors. Assistance for pharmacokinetics was provided by Susanne Reschke and Michaela Damaske of Bayer Pharma AG (Berlin, Germany). We thank Dr Ron Dubowy for his contributions to the design and implementation of this clinical study.
references
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008; 27: 5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther 2009; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saal LH, Johansson P, Holm K et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA 2007; 104: 7564–7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015; 15: 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flinn IW, Kahl BS, Leonard JP et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 2014; 123: 3406–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopal AK, Kahl BS, de Vos S et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014; 370: 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendell JC, Rodon J, Burris HA et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 2012; 30: 282–290. [DOI] [PubMed] [Google Scholar]
- 8.Brown JR, Davids MS, Rodon J et al. Phase I trial of the pan-PI3K inhibitor pilaralisib (SAR245408/XL147) in patients with chronic lymphocytic leukemia (CLL) or relapsed/refractory lymphoma. Clin Cancer Res 2015; 21: 3160–3169. [DOI] [PubMed] [Google Scholar]
- 9.Sarker D, Ang JE, Baird R et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2015; 21: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro GI, Rodon J, Bedell C et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2014; 20: 233–245. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro GI, Bell-McGuinn KM, Molina JR et al. First-in-human study of PF-05212384 (PKI-587), a small-molecule, intravenous, dual inhibitor of PI3K and mTOR in patients with advanced cancer. Clin Cancer Res 2015; 21: 1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flinn I, Oki Y, Patel M et al. A Phase 1 evaluation of duvelisib (IPI-145), a PI3K-δ,γ inhibitor, in patients with relapsed/refractory iNHL. Blood (ASH Annual Meeting Abstracts) 2014; 124: abstr 802. [Google Scholar]
- 13.Horwitz SM, Porcu P, Flinn I et al. Duvelisib (IPI-145), a phosphoinositide-3-kinase-δ,γ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Blood (ASH Annual Meeting Abstracts) 2014; 124: abstr 803. [Google Scholar]
- 14.Haike K, Stasik E, Soujon M et al. Molecular mechanisms supporting inhibition of PI3K isoforms by copanlisib in blocking B-cell signaling and tumor cell growth in diffuse large B-cell lymphoma. Poster 48 presented at the 2014 1st American Society of Hematology Meeting on Lymphoma Biology, Colorado Springs, Colorado, USA, 10–13 August. [Google Scholar]
- 15.Liu N, Rowley BR, Bull CO et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther 2013; 12: 2319–2330. [DOI] [PubMed] [Google Scholar]
- 16.Tabernero J, Rojo F, Calvo E et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a Phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008; 26: 1603–1610. [DOI] [PubMed] [Google Scholar]
- 17.Yap TA, Yan L, Patnaik A et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 2011; 29: 4688–4695. [DOI] [PubMed] [Google Scholar]
- 18.Busaidy NL, Farooki A, Dowlati A et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol 2012; 30: 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 20.Borders EB, Bivona C, Medina PJ. Mammalian target of rapamycin: biological function and target for novel anticancer agents. Am J Health Syst Pharm 2010; 67: 2095–2106. [DOI] [PubMed] [Google Scholar]
- 21.Foukas LC, Claret M, Pearce W et al. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 2006; 441: 366–370. [DOI] [PubMed] [Google Scholar]
- 22.Knight ZA, Gonzalez B, Feldman ME et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 2006; 125: 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soria JC, LoRusso P, Bahleda R et al. Phase I dose-escalation study of pilaralisib (SAR245408, XL147), a pan-class I PI3K inhibitor, in combination with erlotinib in patients with solid tumors. Oncologist 2015; 20: 245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J Biol Chem 2001; 276: 30392–30398. [DOI] [PubMed] [Google Scholar]
- 25.Quaschning T, Voss F, Relle K et al. Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J Am Soc Nephrol 2007; 18: 730–740. [DOI] [PubMed] [Google Scholar]
- 26.Ha JM, Kim YW, Lee DH et al. Regulation of arterial blood pressure by Akt1-dependent vascular relaxation. J Mol Med (Berl) 2011; 89: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 27.Symons JD, McMillin SL, Riehle C et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 2009; 104: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q, Gao F, Ma XL. Insulin says NO to cardiovascular disease. Cardiovasc Res 2011; 89: 516–524. [DOI] [PubMed] [Google Scholar]
- 29.Will M, Qin AC, Toy W et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov 2014; 4: 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W, Hosford SR, Dillon LM et al. Strategically timing inhibition of phosphatidylinositol 3-kinase to maximize therapeutic index in estrogen receptor alpha-positive, PIK3CA-mutant breast cancer. Clin Cancer Res 2016; 22: 2250–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toska E, Baselga J. Pharmacology in the era of targeted therapies: the case of PI3K inhibitors. Clin Cancer Res 2016; 22: 2099–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudson K, Hancox UJ, Trigwell C et al. Intermittent high-dose scheduling of AZD8835, a novel selective inhibitor of PI3Kα and PI3Kδ, demonstrates treatment strategies for PIK3CA-dependent breast cancers. Mol Cancer Ther 2016; 15: 877–889. [DOI] [PubMed] [Google Scholar]
- 33.Reif S, Ahsman M, Jentsch G et al. Use of a population pharmacokinetic approach and time-to-event analysis to support the clinical recommendation of a flat dosing of copanlisib in cancer patients. Clin Pharmacol Ther 2016; 99: S5–S107.26803826 [Google Scholar]
- 34.Kim MH, Lee JS, Mok JH et al. Metabolic burden measured by 18F-Fluorodeoxyglucose positron emission tomography/computed tomography is a prognostic factor in patients with small cell lung cancer. Cancer Res Treat 2014; 46: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi C, Kanoun S, Berriolo-Riedinger A et al. Interim 18F-FDG PET SUVmax reduction is superior to visual analysis in predicting outcome early in Hodgkin lymphoma patients. J Nucl Med 2014; 55: 569–573. [DOI] [PubMed] [Google Scholar]
- 36.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009; 9: 550–562. [DOI] [PubMed] [Google Scholar]
- 37.Bartlett NL, LaPlant BR, Qi J et al. Ibrutinib monotherapy in relapsed/refractory follicular lymphoma (FL): preliminary results of a Phase 2 consortium (P2C) trial. Blood (ASH Annual Meeting Abstracts) 2014; 124: 800. [Google Scholar]
- 38.Glauer J, Pletz N, Schön M et al. A novel selective small-molecule PI3K inhibitor is effective against human multiple myeloma in vitro and in vivo. Blood Cancer J 2013; 3: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danesi R, Boni JP, Ravaud A. Oral and intravenously administered mTOR inhibitors for metastatic renal cell carcinoma: pharmacokinetic considerations and clinical implications. Cancer Treat Rev 2013; 39: 784–792. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien NA, McDonald K, Tong L et al. Targeting PI3K/mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT. Clin Cancer Res 2014; 20: 3507–3520. [DOI] [PubMed] [Google Scholar]
- 41.Sweetlove M, Wrightson E, Kolekar S et al. Inhibitors of pan-PI3K signaling synergize with BRAF or MEK inhibitors to prevent BRAF-mutant melanoma cell growth. Front Oncol 2015; 5: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foukas LC, Berenjeno IM, Gray A et al. Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc Natl Acad Sci USA 2010; 107: 11381–11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.