This is a phase II of 33 incurable adenoid cystic carcinoma patients treated with the tyrosine kinase inhibitor axitinib. Regression was achieved in 66.7% with three (9.1%) confirmed partial responses. Future work will focus on defining predictors of benefit and exploring drug combinations.
Keywords: axitinib, adenoid cystic carcinoma, MYB
Abstract
Background
Recurrent/metastatic adenoid cystic carcinoma (ACC) is an incurable disease with no standard treatments. The majority of ACCs express the oncogenic transcription factor MYB (also c-myb), often in the context of a MYB gene rearrangement. This phase II trial of the tyrosine kinase inhibitor (TKI) axitinib (Pfizer) tested the hypothesis that targeting pathways activated by MYB can be therapeutically effective for ACC.
Patients and methods
This is a minimax two-stage, phase II trial that enrolled patients with incurable ACC of any primary site. Progressive or symptomatic disease was required. Patients were treated with axitinib 5 mg oral twice daily; dose escalation was allowed. The primary end point was best overall response (BOR). An exploratory analysis correlating biomarkers to drug benefit was conducted, including next-generation sequencing (NGS) in 11 patients.
Results
Thirty-three patients were registered and evaluable for response. Fifteen patients had the axitinib dose increased. Tumor shrinkage was achieved in 22 (66.7%); 3 (9.1%) had confirmed partial responses. Twenty-five (75.8%) patients had stable disease, 10 of whom had disease stability for >6 months. The median progression-free survival (PFS) was 5.7 months (range 0.92–21.8 months). Grade 3 axitinib-related toxicities included hypertension, oral pain and fatigue. A trend toward superior PFS was noted with the MYB/NFIB rearrangement, although this was not statistically significant. NGS revealed three tumors with 4q12 amplification, producing increased copies of axitinib-targeted genes PDGFR/KDR/KIT. Two 4q12 amplified patients achieved stable disease for >6 months, including one with significant tumor reduction and the longest PFS on study (21.8 months).
Conclusions
Although the primary end point was not met, axitinib exhibited clinical activity with tumor shrinkage achieved in the majority of patients with progressive disease before trial enrollment. Analysis of MYB biomarkers and genomic profiling suggests the hypothesis that 4q12 amplified ACCs are a disease subset that benefit from TKI therapy.
introduction
Adenoid cystic carcinoma (ACC) is a malignant neoplasm that commonly arises from minor or major salivary glands, and more rarely from other sites. There are no standard treatments for incurable, recurrent/metastatic (R/M) ACC, as cytotoxic chemotherapy provides limited benefit. Axitinib (AG-013736) is a receptor tyrosine kinase inhibitor (TKI) of vascular endothelial growth factor receptors (VEFGRs) 1–3, KIT and platelet-derived growth factor receptors (PDGFRs) A/B, each of which may be critical for ACC pathogenesis. Multivariate analyses have shown that high VEGF expression in ACCs is an independent prognostic factor for survival [1]. KIT is highly expressed in >90% of ACCs, and is a hallmark of ACC histology [2]. Copy number analysis has uncovered recurrent gains at PDGF and PDGFR gene loci in ACC tumors [3, 4]. In the axitinib phase I trial, one of the three confirmed partial responses (cPRs) observed was in an ACC patient [5].
ACCs can be characterized by a unique t(6;9) translocation that creates a gene fusion of the MYB (also c-myb) and NFIB transcription factors, resulting in increased MYB expression [6, 7]. MYB is a bona fide oncogene in T-cell acute leukemia and is overexpressed in breast and colorectal cancers. In ACC, increased MYB transcriptional activity presumably drives overexpression of MYB-regulated genes, including VEGFA and KIT [7]. While ∼50% or more of ACCs are fusion-positive, ∼60%–70% of fusion-negative tumors also have elevated MYB, suggesting alternative mechanisms of activation [6]. Given MYB-dependent and -independent mechanisms of VEGFR/KIT/PDGFR activation and phase I evidence of clinical activity, we conducted a phase II trial to evaluate the efficacy of axitinib in patients with progressive, incurable ACC.
methods
study patients
Patients were required to have pathologically confirmed, incurable ACC (salivary or non-salivary primaries). RECIST version 1.1 measurable disease and evidence of disease progression (the presence of a new or progressive lesion on imaging carried out within 6 months of study enrollment and/or worsening disease-related symptoms) were required. All patients were treated at Memorial Sloan Kettering Cancer Center (MSKCC). The protocol (NCT01558661) was approved by the MSKCC Institutional Review Board (IRB). Written informed consent was obtained from all patients. See supplementary Figure S1, available at Annals of Oncology online, for complete protocol eligibility criteria.
study treatment
Patients were started on axitinib 5 mg oral twice daily (b.i.d.) (1 cycle = 4 weeks). Those without drug-related adverse events > grade 2 (CTCAE v4.0) for 2 weeks and blood pressure of <150/90 without antihypertensive medications were eligible for non-mandatory dose escalation to 7 mg b.i.d., and then 10 mg b.i.d. Dose reductions to 3 mg b.i.d. and 2 mg b.i.d. were allowed for toxicity. RECIST v1.1 tumor assessments were done at baseline and then every two cycles. After 10 months, assessments were done every three cycles. Patients remained on study until disease progression, unacceptable toxicity or withdraw of consent.
MYB immunohistochemistry
Paraffin sections were analyzed with the MYB antibody from Abcam (EP769Y). MYB quantification was assessed as previously published [8]: 2+ for strong staining in >50% of cancer cells, 1+ for weak or strong staining in <50% of the cells and 0 for <5% staining.
fluorescence in situ hybridization for MYB and NFIB rearrangements
Fluorescence in situ hybridization (FISH) was carried out on paraffin-embedded 5 µm sections utilizing custom probes developed from bacterial artificial chromosomes (BACs) covering and flanking the MYB and NFIB genes (see supplementary Table S1, available at Annals of Oncology online). Two hundred successive nuclei were examined. Detection of a sufficient break-apart signal was interpreted as a positive score.
next-generation sequencing
Ten cases were evaluated using the next-generation sequencing (NGS) assay MSK-IMPACT (Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets) after informed written consent to an IRB-approved study (NCT01775072). This assay is optimized for DNA from formalin-fixed, paraffin-embedded samples, and targets single-nucleotide variants (SNVs), indels and structural variants in 341 cancer-related genes, in addition to genome-wide copy number [9, 10]. One case was analyzed on the FoundationOne platform. Gene level copy number changes were calculated using segmented log-ratio values (Circular Binary Segmentation) of the tumor and normal sample. A test for significance was carried out on distance of the segment to zero log ratio. Log ratio of 1 and −1 were the thresholds for amplification and deletion, respectively.
statistical analysis
This was a single-arm, minimax two-stage phase II trial. The primary end point was best overall response (BOR) rate per RECIST version 1.1 criteria. In order to detect a difference between an unacceptable BOR rate of 5% and a desirable rate of 20% with a one-sided type I error of 10% and power of 90%, at least 1 response was required among the first 18 patients in the first stage within 10 cycles of treatment. If these criteria were met, then the study would accrue an additional 14 patients in the second stage. If >4 patients had a response out of a total of 32 enrolled, the regimen would be considered worthy of further investigation. Patients who received at least one dose of medication were included in the primary end point analysis. Patients who discontinued treatment without tumor assessment were classified as non-responders. The secondary end point was progression-free survival (PFS) estimated using the Kaplan–Meier methodology, with time origin at the start of the treatment, followed until progression of disease (PD) or death. Seven patients were censored up to the date of the last tumor assessment: five for withdrawal of consent, one for removal due to toxicity and one for development of inevaluable disease. The association between PFS and MYB biomarkers was evaluated by log-rank tests in an exploratory fashion. The 95% confidence intervals for proportions were calculated by the Clopper and Pearson method.
results
patient and disease characteristics
Between March 2012 and May 2013, 33 patients were enrolled: 18 in the first stage, 15 in the second. One patient enrolled in the second stage was determined to be ineligible and was replaced after two doses. This patient was considered evaluable for BOR, but not PFS. Patient characteristics are summarized in Table 1. Nearly all patients had distant metastatic disease (32/33 patients), and the majority had previously received systemic therapy [19/33 (57.6%)]. Six patients had previously been treated with antiangiogenesis agents. All patients had evidence of disease progression before study participation. All patients were started with axitinib at 5 mg orally b.i.d. Of 19 patients eligible for dose escalation, 15 had the dose increased: 6 patients to 7 mg b.i.d. and 9 increased two levels to 10 mg b.i.d.
Table 1.
Patient characteristics
| Patient characteristic | No. of patients (n = 33) |
|---|---|
| Age | |
| Median | 56 (range 39–78) |
| Sex | |
| Male | 18 (54.5%) |
| Female | 15 (45.5%) |
| ECOG performance status | |
| 0 | 3 (9.1%) |
| 1 | 22 (66.7%) |
| 2 | 8 (24.2%) |
| Primary tumor site | |
| Major salivary gland | 9 (27.2%) |
| Parotid | 1 |
| Submandibular | 7 |
| Sublingual | 1 |
| Minor salivary gland | 17 (51.5%) |
| Floor of mouth | 2 |
| Base of tongue | 6 |
| Hard palate | 5 |
| Paranasal sinus | 2 |
| Oral tongue | 1 |
| Oral cavity | 1 |
| Other | 7 (21.2%) |
| Lacrimal gland | 1 |
| Lung | 2 |
| Breast | 2 |
| Trachea | 1 |
| Unknown primary | 1 |
| Disease distribution | |
| Locoregional disease only | 1 (3.0%) |
| Distant metastases | 32 (97.0%) |
| Lung only | 9 |
| Liver | 8 |
| Peritoneum | 2 |
| Bone | 7 |
| Skin | 1 |
| Brain | 2 |
| Leptomeninges | 1 |
| Prior therapy | |
| Systemic therapy | 19 (57.6%) |
| For recurrent/metastatic ACC | 17 |
| As adjuvant therapy (w/o RT) | 1 |
| With radiation | 7 |
| Radiation | 31 (93.9%) |
| Axitinib dose | |
| Eligible for dose escalation | 19 (57.6%) |
| Dose escalated | 15 (45.5%) |
| 7 mg b.i.d. | 6 (18.2%) |
| 10 mg b.i.d. | 9 (27.3%) |
| Dose reduced | 11 (33.3%) |
| Without prior dose escalation (n = 18) | 7 (63.6%) |
| After dose escalation (n = 15) | 4 (26.7%) |
efficacy
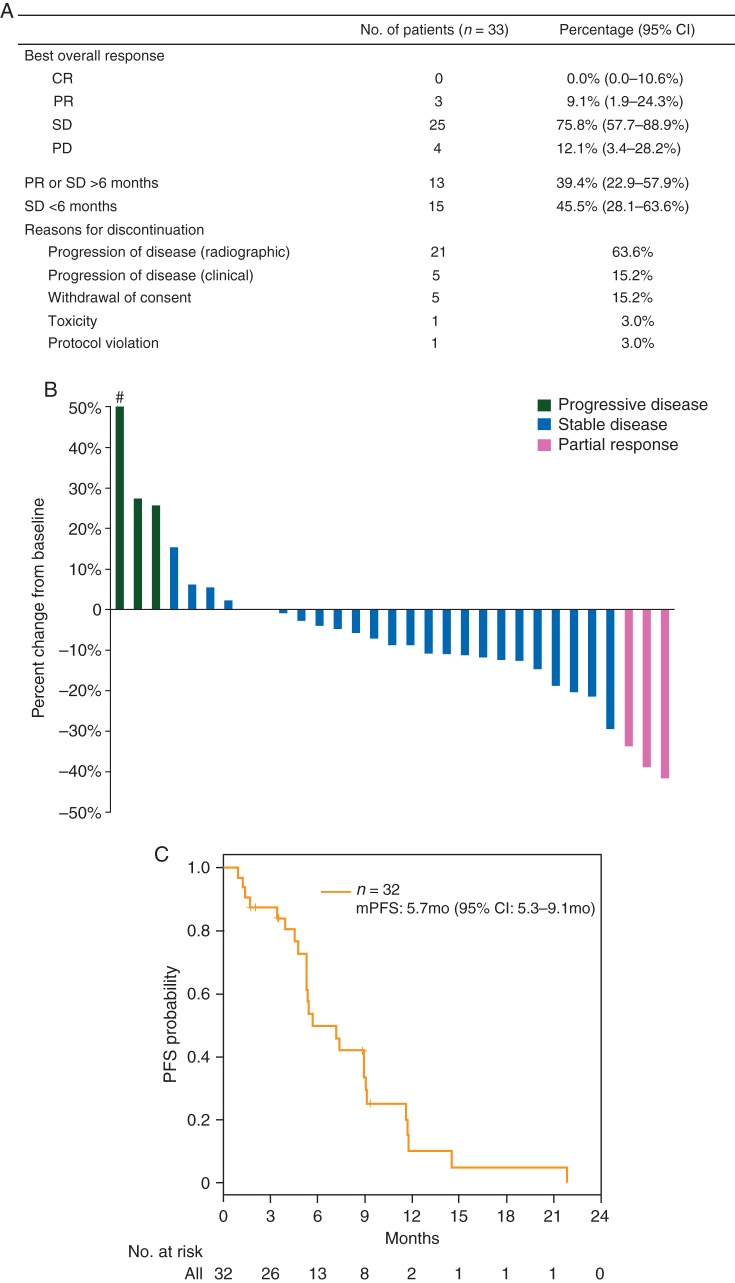
BOR outcomes are summarized in Figure 1A. The requirement that >1 confirmed responses be observed within 10 months of therapy among the first 18 patients was met, triggering enrollment of an additional 15. The majority of patients on trial experienced tumor shrinkage [22/33 (66.7%)] (Figure 1B), including three (9.1%) cPRs with durable benefit for more than 9 months (two with a PFS >11 months). Twenty-five (75.8%) patients had stable disease (SD), 10 for >6 months. Thirteen (40.63%) patients remained on axitinib for >6 months; two patients remained on axitinib for >1 year (14.5, 21.8 months) (supplementary Figure S2, available at Annals of Oncology online). Only four patients had PD as best response. The median PFS among 32 assessable patients was 5.7 months (95% CI: 5.3–9.1 months) (Figure 1C; median time on study was 5.3 months).
Figure 1.
Axitinib efficacy in incurable adenoid cystic carcinoma patients. (A) Summary of efficacy data. (B) Waterfall plot of maximum tumor reduction. Hash denotes that RECIST progression was due to the appearance of a new site of disease. (C) Kaplan–Meier curve for progression-free survival (PFS).
Of the three cPR patients, one was dose escalated, only later to be dose reduced for toxicity. All nine patients who underwent two dose escalations to 10 mg b.i.d. had SD as the best response. Two of the cPR patients had not previously received systemic therapy, while the other had been treated with a RAF inhibitor on a phase I trial.
toxicity and reasons for study removal
Treatment was well tolerated without any grade 4/5 toxicities attributable to axitinib. The most frequently reported axitinib-related toxicities were hypertension, fatigue, diarrhea, weight loss, anorexia, hand–foot syndrome, nausea, oral pain, myalgia, oral mucositis and liver function test elevations (supplementary Table S2, available at Annals of Oncology online). Grade 3 toxicities included hypertension (11), oral pain (4) and fatigue (2). Eleven (33.3%) patients required dose reduction, four of whom had the dose reduced after it had been escalated beyond 5 mg b.i.d. The most common reason for study discontinuation was radiographic PD [21 (63.6%)] (Figure 1A). Five (15.2%) were removed for clinical progression and one for toxicity (intolerable grade 2 fatigue). Three of the five patients who withdrew consent cited drug side-effects and poor quality of life as the reason.
analysis of MYB, NFIB status and clinical efficacy
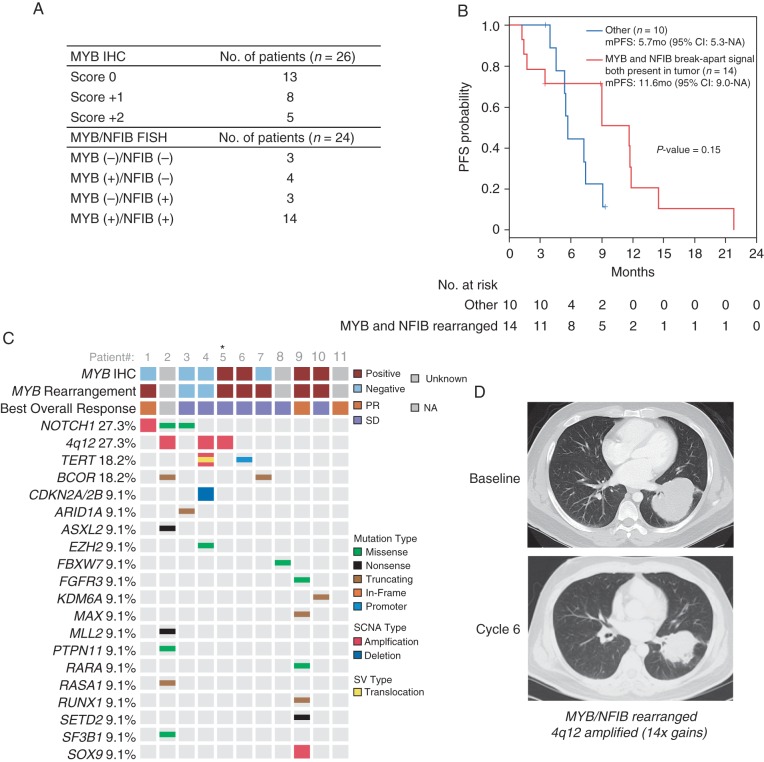
MYB immunohistochemistry (IHC) was carried out on tumors from 26 patients; half (13) had detectable MYB expression (Figure 2A). FISH for MYB and NFIB rearrangements carried out in tumors from 24 patients showed 14 (58.3%) harbored both MYB and NFIB rearrangements, while 3 were negative for rearrangement in either gene. Four had only the MYB break-apart signal, and three had only the NFIB break-apart signal. Of the 18 tumors with a MYB rearrangement (14 MYB+/NFIB+ and 4 MYB+/NFIB− tumors), 9 had detectable MYB protein by IHC (1+ or 2+). Among the three MYB−/NFIB− tumors, MYB IHC expression was absent in two and detectable in one.
Figure 2.
Adenoid cystic carcinoma (ACC) biomarkers/genomics and axitinib efficacy. (A) MYB immunohistochemistry and FISH for MYB and NFIB rearrangements. (B) Progression-free survival for the FISH detected MYB+/NFIB+ rearrangements and other FISH patterns (MYB+/NFIB−, MYB−/NFIB+, and MYB−/NFIB−). (C) Genetic alterations detected in ACC tumors from 11 enrolled study patients. Asterisk denotes the case that was profiled on the FoundationOne platform. (D) Significant response in a primary lung ACC tumor harboring both MYB/NFIB rearrangements and 4q12 amplification (14-fold amplification in PDGFRA/KDR/KIT).
No relationship between MYB expression by IHC and PFS was detected (mPFS: MYB+ 7.4 months, MYB− 7.2 months) (supplementary Figure S3, available at Annals of Oncology online). A longer mPFS [11.6 months (95% CI: 9.0–NA) versus 5.7 months (95% CI: 5.3–NA)] was observed in patients with MYB+/NFIB+ tumors compared with those with other FISH patterns (MYB+/NFIB−, MYB−/NFIB+ and MYB−/NFIB−), although the difference was not statistically significant (Figure 2B).
genomic analysis
NGS was carried out in 11 cases, including for the three patients with cPR (Figure 2C). The assessments were carried out on tissues obtained pre-axitinib in six cases (patients 3, 5, 6, 7, 9 and 10) and post-axitinib in five (patients 1, 2, 4, 8 and 11). The number of alterations discovered ranged from zero to eight. The most commonly detected were NOTCH1 alterations and 4q12 amplification (3 cases each; 27.3%) (Figure 2C). The 4q12 amplicon increases gene copy number for three molecular targets of axitinib: PDGFRA, KDR (VEGFR2) and KIT (2-, 9- and 14-fold gains). Two of the 4q12 amplified patients (4 and 5) were treated with axitinib for >1 day and achieved SD for >6 months, including a patient with lung ACC who experienced significant regression of the primary tumor and the longest PFS on study (21.8 months) (patient 5; Figure 2D). Patient 4 achieved a PFS of 7.2 months (12% regression), despite dose reduction to 3 mg b.i.d. after cycle 2. The twofold 4q12 amplification for this patient was detected in a post-axitinib tumor sample obtained after progression. Patient 4's tumor also harbored both telomerase reverse transcriptase (TERT) gene amplification and a novel t(20;5) translocation that produced a PRNP-TERT gene fusion in which the PRioN Protein (PRNP) gene promoter/5′UTR replaces the TERT promoter, suggesting that inappropriate TERT expression may also be critical to the oncogenic phenotype in this case.
discussion
The challenge of ACC trial design is reliably measuring drug activity in a patient population with a broad spectrum of disease aggressiveness. This challenge was addressed here by requiring disease progression before study entry and designating BOR as the primary end point. While this ACC trial successfully met the early efficacy signal for moving to the second stage, the 3 cPRs observed out of 33 total patients (9.1% response rate) fell just short of the pre-specified goal of at least 4 responders. Still, this trial was conducted in a progressive disease population, over half (57.6%) of whom had been previously treated with systemic therapy, and yet tumor shrinkage was still achieved in 22 of 33 (66.7%) and PR/SD for >6 months was observed in 13 (39.4%) (2 with cPR stayed on drug for >11 months). The 9.1% response rate reported here is comparable with the 0%–11% rates observed in several phase II trials of other multi-targeted TKIs tested in ACC, including sorafenib [11, 12] (VEGFR, PDGFR inhibitor), dovitinib [13, 14] (VEGFR, PDGFR, FGFR1-3, KIT inhibitor) and sunitinib (VEGFR, PDGFR, KIT, RET, FLT3 inhibitor) [15]; two of these studies were deemed positive for meeting a PFS primary end point [12, 14].
Identifying clinical and molecular markers that correlate to benefit is one strategy that would enhance the clinical utility of axitinib for ACC. This study concept was in part developed with the rationale that MYB is a central oncogenic driver that activates a number of signaling pathways targeted by axitinib. However, no association between clinical outcome and MYB expression by IHC was found. We did observe a longer mPFS among MYB+/NFIB+ patients relative to those with tumors harboring other FISH patterns (MYB+/NFIB−, MYB-/NFIB+ and MYB−/NFIB−), although the mechanistic basis for this remains unclear and the difference was not statistically significant. More recently, two groups published the observation that over one-third of t(6;9)-negative or MYB-negative ACCs harbor t(8;9) rearrangements resulting in high expression of another MYB family gene, MYBL1, producing a gene expression signature similar to that observed in MYB fusion tumors [16–18]. MYB status alone may be insufficient for delineating meaningful clinical subsets.
NGS of 11 cases identified 3 cases of 4q12 amplification, resulting in increased gene copy number of the axitinib targets PDGFRA/KDR/KIT. This 4q12 amplicon has been described in glioblastomas, malignant peripheral nerve sheath tumors and non-small-cell lung cancer with preclinical evidence linking it to susceptibility to TKIs [19]. Axitinib for two 4q12 amplified patients did produce tumor regression and SD for >6 months. While the twofold copy number increase for patient 4 was detected in a post-axitinib sample, the impressive degree of tumor regression and PFS (21.8 months) achieved in patient 5 (14-fold copy number increase) suggests the hypothesis that in both cases, 4q12 amplification denotes oncogenic dependence upon PDGFRA/KDR/KIT signaling and susceptibility to axitinib. Genomic analysis of primary ACC cases revealed this amplification was present in only 1 out of 55 (1.8%) tumors analyzed [20], raising the possibility that observing it in 3 of 11 (27.3%) cases here is an enrichment in more advanced disease. Ideally, future trials will enrich for 4q12 amplified patients to further evaluate TKI efficacy for this ACC subset and address how the degree of amplification may correlate to drug benefit. It bears highlighting that our study demonstrates that the presence of 4q12 amplification is not requisite for axitinib benefit as durable tumor regressions were achieved among those without this alteration. Additionally, the genomic analysis here is limited to a small number of patients, and there is a need to comprehensively investigate the utility of using profiling to identify predictors of benefit for axitinib in ACC patients.
NOTCH1 alterations in ACC are of increasing interest, and these were among the most common detected by NGS in this study. There is growing evidence that activating NOTCH pathway alterations are enriched in patients with more aggressive disease (higher grade tumors, liver/bone metastases, shorter survival) [21–23] and can be therapeutically targeted [24]. NOTCH1 amplification in patient 1 did correlate with atypically aggressive disease that included peritoneal metastases, and this patient experienced a cPR with a PFS of nearly 1 year (supplementary Figure S4, available at Annals of Oncology online), although the connection between NOTCH1 activation and axitinib susceptibility is not clear, since this alteration was detected in a post-axitinib specimen. The biologic significance of the other two cases of NOTCH1 alterations is unknown, given that both were located outside of the C-terminal heterodimerization and PEST domains in which activating mutations typically arise.
In conclusion, axitinib possesses activity against ACC, achieving tumor reductions in the majority of a clinically challenging recurrent/metastatic disease population. Biomarker and genomic analysis provided unique insights into the biologic landscape for a small cohort of incurable ACCs. There remains a need to more comprehensively incorporate molecular analyses in ACC investigations to inform how novel therapeutic approaches may be effectively developed.
funding
This investigator-initiated work was funded by the National Comprehensive Cancer Network and Pfizer. This research was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
disclosure
MGF is currently employed at Regeneron Pharmaceuticals. CSS is currently employed at Genentech/Roche. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Pfizer, Inc. All aspects of study conduct and data management were carried out by Memorial Sloan Kettering Cancer Center. NCCN and Pfizer reviewed and approved the decision to submit the manuscript for publication. Dr. Siraj Ali from Foundation Medicine contributed copy number information on the one case analyzed by the FoundationOne platform. We acknowledge the members of the Molecular Diagnostics Service in the Department of Pathology and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology. Dr. Ho is supported by the Geoffrey Beene Cancer Research Center at Memorial Sloan Kettering Cancer Center and Cycle for Survival.
references
- 1.Park S, Nam SJ, Keam B et al. . VEGF and Ki-67 overexpression in predicting poor overall survival in adenoid cystic carcinoma. Cancer Res Treat 2016; 48: 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett 2000; 154: 107–111. [DOI] [PubMed] [Google Scholar]
- 3.Bernheim A, Toujani S, Saulnier P et al. . High-resolution array comparative genomic hybridization analysis of human bronchial and salivary adenoid cystic carcinoma. Lab Invest 2008; 88: 464–473. [DOI] [PubMed] [Google Scholar]
- 4.Vekony H, Ylstra B, Wilting SM et al. . DNA copy number gains at loci of growth factors and their receptors in salivary gland adenoid cystic carcinoma. Clin Cancer Res 2007; 13: 3133–3139. [DOI] [PubMed] [Google Scholar]
- 5.Rugo HS, Herbst RS, Liu G et al. . Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 2005; 23: 5474–5483. [DOI] [PubMed] [Google Scholar]
- 6.Mitani Y, Li J, Rao PH et al. . Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: incidence, variability, and clinicopathologic significance. Clin Cancer Res 2010; 16: 4722–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson M, Andren Y, Mark J et al. . Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA 2009; 106: 18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West RB, Kong C, Clarke N et al. . MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol 2011; 35: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng DT, Mitchell TN, Zehir A et al. . Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015; 17: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrader KA, Cheng DT, Joseph V et al. . Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol 2016; 2: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locati LD, Bossi P, Civelli EM et al. . Sorafenib in recurrent and/or metastatic salivary gland carcinomas (RMSGCs): an investigator-initiated phase II trial (NCT01703455). J Clin Oncol 2013; 31 (Suppl): abstr 6020. [Google Scholar]
- 12.Thomson DJ, Silva P, Denton K et al. . Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck 2015; 37: 182–187. [DOI] [PubMed] [Google Scholar]
- 13.Dilon PM, Moskaluk CA, Fracasso PM et al. . Phase II study of dovitinib (TKI258) in patients with progressive metastatic adenoid cystic carcinoma. J Clin Oncol 2013; 31 (Suppl): abstr 6021. [Google Scholar]
- 14.Keam B, Kim SB, Shin SH et al. . Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer 2015; 121: 2612–2617. [DOI] [PubMed] [Google Scholar]
- 15.Chau NG, Hotte SJ, Chen EX et al. . A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol 2012; 23: 1562–1570. [DOI] [PubMed] [Google Scholar]
- 16.Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov 2016; 6: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao R, Cao C, Zhang M et al. . A unifying gene signature for adenoid cystic cancer identifies parallel MYB-dependent and MYB-independent therapeutic targets. Oncotarget 2014; 5: 12528–12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitani Y, Liu B, Rao P et al. . Novel MYBL1 gene rearrangements with recurrent MYBL1-NFIB fusions in salivary adenoid cystic carcinomas lacking t(6;9) translocations. Clin Cancer Res 2016; 22: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zietsch J, Ziegenhagen N, Heppner FL et al. . The 4q12 amplicon in malignant peripheral nerve sheath tumors: consequences on gene expression and implications for sunitinib treatment. PLoS One 2010; 5: e11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho AS, Kannan K, Roy DM et al. . The mutational landscape of adenoid cystic carcinoma. Nat Genet 2013; 45: 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drier Y, Cotton MJ, Williamson KE et al. . An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet 2016; 48: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrarotto R, Mitani Y, Cai Y, Diao L. Notch1 mutations to define a subgroup of adenoid cystic carcinoma (ACC): tumor stage, propensity to bone and liver metastasis, risk of relapse, and overall survival. J Clin Oncol 2015; 33 (Suppl): abstr 6081. [Google Scholar]
- 23.Stephens PJ, Davies HR, Mitani Y et al. . Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest 2013; 123: 2965–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoeck A, Lejnine S, Truong A et al. . Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov 2014; 4: 1154–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.