Abstract
Objective: Preliminary studies suggest that repetitive transcranial magnetic stimulation (rTMS) may be an effective and tolerable intervention for adolescents with treatment-resistant depression. There is limited rationale to inform coil placement for rTMS dosing in this population. We sought to examine and compare three localization techniques for coil placement in the context of an open-label trial of high-frequency rTMS for adolescents with treatment-resistant depression.
Methods: Ten adolescents with treatment-resistant depression were enrolled in an open-label trial of high-frequency rTMS. Participants were offered 30 rTMS sessions (10 Hz, 120% motor threshold, left 3000 pulses applied to the dorsolateral prefrontal cortex) over 6–8 weeks. Coil placement for treatment was MRI guided. The scalp location for treatment was compared with the locations identified with standard 5 cm rule and Beam F3 methods.
Results: Seven adolescents completed 30 rTMS sessions. No safety or tolerability concerns were identified. Depression severity as assessed with the Children's Depression Rating Scale Revised improved from baseline to treatment 10, treatment 20, and treatment 30. Gains in depressive symptom improvement were maintained at 6 month follow-up visits. An MRI-guided approach for coil localization was feasible and efficient. Our results suggest that the 5 cm rule, Beam F3, and the MRI-guided localization approaches provided variable scalp targets for rTMS treatment.
Conclusions: Open-label, high-frequency rTMS was feasible, tolerable, and effective for adolescents with treatment-resistant depression. Larger, blinded, sham-controlled trials are needed for definitive safety and efficacy data. Further efforts to understand optimal delivery, dosing, and biomarker development for rTMS treatments of adolescent depression are warranted.
Introduction
Major depressive disorder (MDD) is a common and impairing condition that often first presents in adolescence. Contemporary treatment approaches with psychotherapy, selective serotonin reuptake inhibitors (SSRIs), or their combination are often ineffective and do not engage relevant neurobiologic targets (March et al. 2004, 2007; Brent et al. 2008). For example, remission rates in clinical trials of adolescent MDD are ∼30% (Brent and Birmaher 2006; Walkup 2010). Suboptimal treatments contribute to a societal burden and functional impairment manifested by impaired academic performance, substance abuse, teen pregnancy, and increased risk for suicide. Safe, effective treatments informed by neuroscience are desperately needed (Brent and Birmaher 2006; Croarkin et al. 2010).
Repetitive transcranial magnetic stimulation (rTMS) has promise as a treatment for adolescent MDD (D'Agati et al. 2010; Donaldson et al. 2014). At present, four (rTMS) devices have United States Food and Drug Administration (FDA) clearance for the treatment of adult MDD. During rTMS sessions, a coil placed on the patient's scalp delivers magnetic pulses to the left dorsolateral prefrontal cortex (L-DLPFC), thereby inducing electrical currents in this region. The mechanism of action of rTMS likely involves alterations in the cortical inhibitory/excitatory balance or neuroplastic changes (Li et al. 2014). Nearly 3000 adult participants have been treated with rTMS in research protocols, whereas it is estimated that 18,000 patients have been treated clinically (Dunner et al. 2014). Unfortunately, systematic rTMS research in adolescent MDD is limited. A recent systematic review concluded that whereas rTMS may be a promising modality for adolescents with treatment-refractory depression, further work with consistent study designs is imperative (Donaldson et al. 2014). Existing studies suggest that rTMS is a safe acute treatment for adolescents. However, long-term follow-up data are lacking (Krishnan et al. 2015). In considering both adult and adolescent depression, there are many unanswered questions regarding the dosing and delivery of rTMS (Donaldson et al. 2014; Li et al. 2014; Mir-Moghtadaei et al. 2015).
One such area of inquiry focuses on the optimal procedure for coil localization prior to stimulus delivery for treatment (Fitzgerald et al. 2006, 2009; Mir-Moghtadaei et al. 2015). Prior therapeutic rTMS trials for adolescent depression utilized the “5 cm rule” technique for coil placement (Wall et al. 2011; Donaldson et al. 2014). First, the patient's contralateral abductor pollicis brevis (APB) muscle is monitored during motor cortical stimulation, thereby identifying the location producing maximum visually inspected contractions or motor-evoked potential amplitude. The treatment site is defined as 5 cm anterior in a parasagittal plane (O'Reardon et al. 2007; George et al. 2010). Prior adult work suggests that this pragmatic technique may fail to accurately locate the DLPFC in some patients when compared with MRI (George et al. 2010; Mir-Moghtadaei et al. 2015). The Beam F3 method was developed in 2009 with the aim of improving the interindividual reliability of rTMS coil placement in a cost-effective fashion (Beam et al. 2009). The F3 location of the international 10–20 electroencephalography (EEG) electrode placement method corresponds to the L-DLPFC. The Beam F3 method streamlines the 10–20 technique with three scalp measurements in cm (nasion to inion, left to right preauricular points, and head circumference) that are entered into a computer program. Computer-generated calculations provide two output measurements estimating the location of the F3 site in reference to the patient's vertex (Beam et al. 2009). Ongoing work with MRI or functional MRI-guided procedures for localization may produce more reliable procedures and optimize the clinical outcomes of rTMS treatments (Fitzgerald et al. 2009; Rusjan et al. 2010; Dunlop et al. 2015). Currently, however, these approaches are often impractical and cost prohibitive for clinical and research rTMS treatments. Further, a recent study of 100 adults suggested that the Beam F3 method reliably approximated MRI-guided coil placement. The authors argued that additional refinements of the Beam F3 approach may yield further improvements (Mir-Moghtadaei et al. 2015).
To our knowledge, no prior therapeutic trial of rTMS for adolescent depression has compared localization techniques (Croarkin et al. 2010; D'Agati et al. 2010; Donaldson et al. 2014). We sought to compare three localization techniques (the 5 cm rule, the Beam F3 method, and a systematic MRI-guided approach) in the context of an open-label trial of high-frequency rTMS for adolescents with recalcitrant depression. We hypothesized that the 5 cm and Beam F3 locations would vary. Secondarily, additional data and experience with open-label rTMS could inform the development of future randomized, sham-controlled studies and future clinical practice. We hypothesized that adolescents with treatment-resistant depression would tolerate and benefit from 30 sessions of MRI-guided, 10 Hz rTMS. Finally, we sought to describe the technique and implementation of an MRI method for localizing the DLPFC for adolescent rTMS dosing.
Methods
Study participants
Study procedures were approved by the Mayo Clinic Institutional Review Board (IRB) and a United States Food and Drug Administration Investigational Device Exemption (#G110091) was also obtained prior to enrollment of participants. All participants were evaluated by a board-certified child and adolescent psychiatrist (C.A.W. or P.E.C.). The initial evaluation included a semistructured diagnostic interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (KSADS-PL) (Kaufman et al. 1997). Inclusion criteria included active treatment for MDD based on Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM IV-TR) criteria (American Psychiatric Association 2000), a Children's Depression Rating Scale Revised (CDRS-R) (Poznanski et al. 1984) total score ≥40 (T score >63), and at least one prior failed antidepressant medication trial as defined by the Antidepressant Treatment History Form (ATHF) (Sackeim 2001). Further, inclusion criteria included active treatment with a selective serotonin reuptake inhibitor or serotonin and norepinephrine reuptake inhibitor at a stable and minimally effective dose (defined by ATHF) of at least 6 weeks. Participants were not eligible if there had been any change in psychotherapeutic treatment in the prior 4 weeks. Co-occurring dysthymic disorder, attention-deficit/hyperactivity disorder, and anxiety disorders were not exclusionary. Schizophrenia, schizoaffective disorder, bipolar spectrum disorders, substance abuse or dependence, somatoform disorders, dissociative disorders, posttraumatic stress disorder, obsessive-compulsive disorder, eating disorders, mental retardation, and pervasive developmental disorders were exclusionary. Patients with a suicide attempt in the past 6 months were not eligible for enrollment. All participants had a urine drug screen prior to rTMS treatment. All female participants had a urine pregnancy test. Participants continued approved antidepressant medications at a stable dose for the duration of the rTMS trial. Participants were allowed to take previously prescribed sleep aids during the trial. Stimulants, antipsychotics, tricyclic antidepressants, and bupropion were not permitted during the active treatment phase of the study (Pisani et al. 2002).
5 cm rule, Beam F3 and MRI guided localization of DLPFC
Participants were custom fitted with a swim cap in the TMS suite. Six locations (right helix of the external ear, left helix of the external ear, right supraorbital ridge, left supraorbital ridge, nasion, and inion) were noted on the swim cap to ensure reliable placement throughout localization sessions. The scalp locations of the APB and 5 cm site were found and traced on the swim cap with standard visualization of movement techniques published previously (O'Reardon et al. 2007; George et al. 2010). The F3 scalp location was determined and traced with the Beam F3 method. Participants wore this swim cap to the MRI suite for subsequent localization of the left DLPFC brain target (DBT) and the left DLPFC scalp target (DST). Eight fiducial markers were placed on the swim cap: Mid-frontal, ABP, F3, 5 cm, left mastoid, right mastoid, right parietal, and right frontal.
Scans for the MRI localization procedure were obtained with a GE 3.0 Tesla DV750 MRI scanner (GE Medical Systems, Waukesha, WI) running 22.0 software equipped with an eight channel head coil. Participants wore their swim caps during the MRI procedures. To minimize head movement, the participant's forehead was affixed with padding and adhesive tape, and neck support was provided as needed. T1-weighted structural images were acquired using a true-axial fast 3D-SPGR sequence (TR = 12.6 ms, TE = 5.6 ms, flip angle = 15 degrees, field of view (FOV) = 250 × 250 mm, slice = 1.5 mm, matrix = 512 × 512 pixels).
For localization of the left DLPFC, the “inferior plane” of the corpus callosum was identified as a line that abutted the inferior margins of the rostrum and splenium of the corpus callosum. Next, we prescribed and acquired a 20 mm thick coronal-oblique localizer slice exactly perpendicular to the inferior plane, such that the center of the localizer slice was placed exactly 10 mm anterior to the genu of the corpus callosum, and the posterior edge of the slice abutted the anterior margin of the rostrum of the corpus callosum. Next, on that localizer slice, we identified the deepest portion of the superior frontal sulcus (SFS), calling that point the DBT. For the rTMS treatment, we prescribed and acquired a 20 × 20 × 20 mm voxel with its center located exactly at the DBT (Fig. 1). Additional constraints were that 1) the superolateral corner of the voxel abutted, but did not include, the skull; 2) the medial margin of the voxel excluded the medial frontal cortex as feasible; and 3) the voxel was placed as superiorly as possible given constraints 1 and 2. During each scan, the T1-weighted anatomical images were transferred to a Medtronic Stealth Station S7 (Medtronic Navigation, Inc, Louisville, CO) equipped with an AxiEM frameless localization system running Synergy Cranial 2.2.6. This navigation system created a three-dimensional (3D) model of the brain, generating thin slices that were reformatted in any plane in real time. Using the above technique, a neuroradiologist (J.D.P.) identified a coronal localizing slice on the AxiEM Stealth System that best corresponded with the coronal localizer slice from the MR scanner, and marked the DBT on the image volume. After the MRI scan, each participant was co-registered with the Stealth System using all of the fiducial markers, including 5 cm, F3, and APB. Next, the Stealth technologist (L.M.H.) placed the system into “navigation mode,” and slowly swept the Stealth probe over the swim cap in a grid-like pattern until a point was found that had the shortest straight line distance to the DBT. This point was marked on the swim cap and labeled as the DST. This DST localizing process took ∼5 minutes to complete (Fig. 1).
FIG. 1.
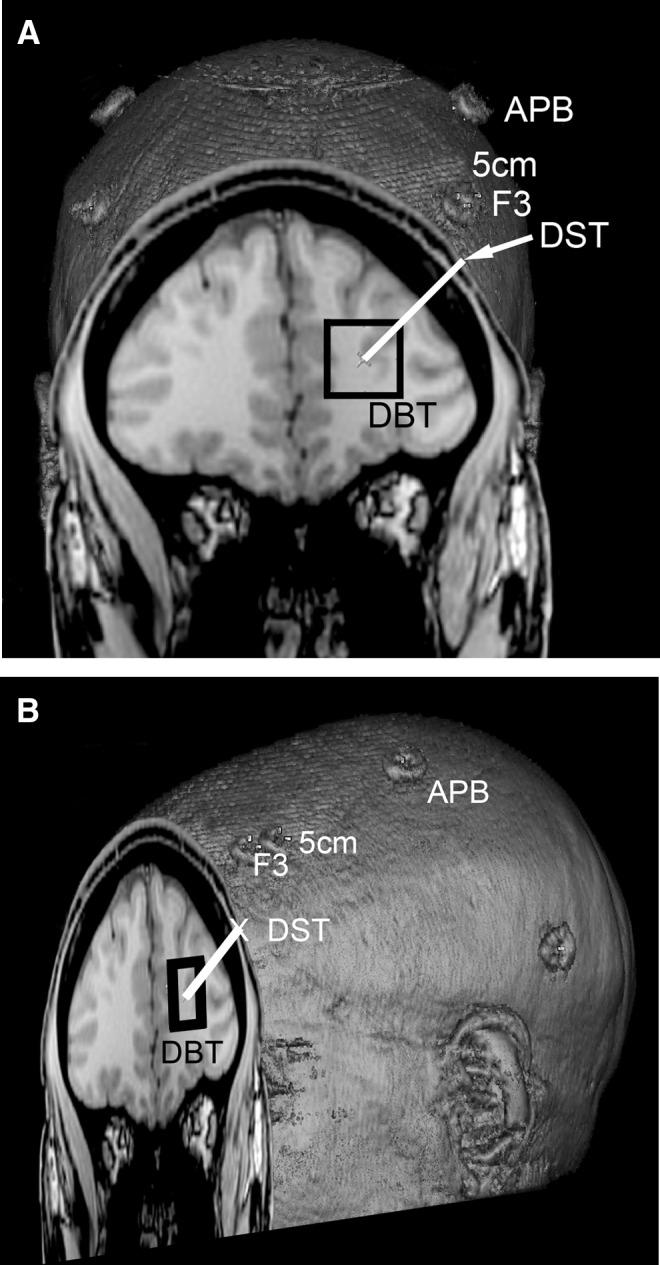
Stealth three-dimensional (3D) rendering of surface fiducial landmarks for a single subject. (A) Coronal view and (B) sagittal oblique view showing the locations of the abductor pollicis brevis (APB) point, the 5 cm rule point (5 cm) rostral/parasagittal from APB, the Beam F3 point (F3), the MRI localized dorsolateral prefrontal cortex (DLPFC) brain target (DBT), and the MRI localized DLPFC scalp target (DST).
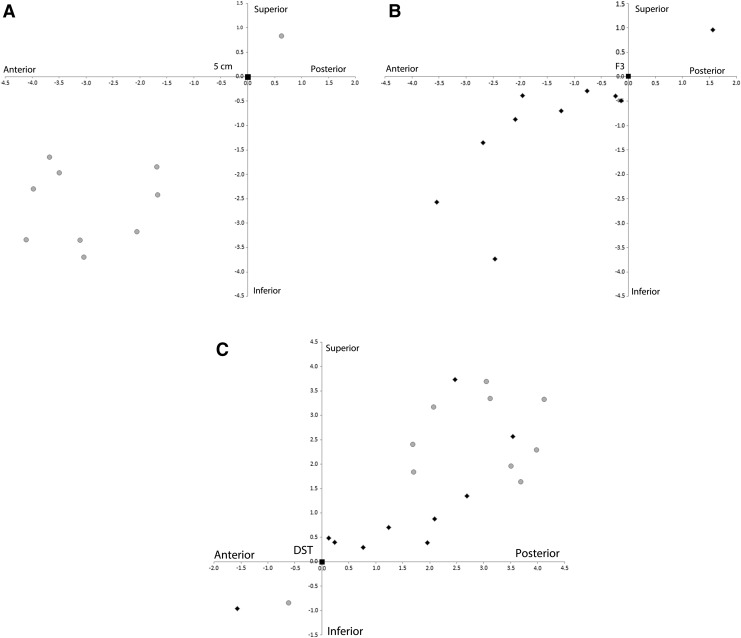
On the day of the MRI and co-registration or during a separate visit within 1 week, participants returned to the rTMS suite. While participants wore their custom swim cap, the locations of APB, 5 cm, and F3 were reconfirmed. Finally, the location of the DST was loaded and stored in the TMS system. All subsequent TMS treatments occurred with the TMS probe centered on the DST location. Euclidean distances between the DST to 5 cm location (Fig. 2a) and the DST to Beam F3 location (Fig. 2b) were measured with the Stealth System to compare potential discrepancies between these two commonly used targeting techniques for rTMS.
FIG. 2.
(A) Coordinates of the dorsolateral prefrontal cortex (DLPFC) scalp target (DST) points (gray circles) in cm from each of the 10 subjects relative to the aligned 5 cm rule point. Note that the majority of DST points are anterior and inferior to the 5 cm rule point. (B) Coordinates of the DST points (black diamonds) relative to the aligned Beam F3 point. Again, the majority of the DST coordinates are anterior and inferior to the F3 point. (C) Coordinates of the 5 cm rule points (gray circles) and Beam F3 points (black diamonds) relative to the aligned DST point. Note that the Beam F3 points are closer to the DST than the 5 cm rule points.
rTMS procedures
Participants wore ear protection during rTMS sessions to minimize the risk of auditory threshold changes. Thirty treatments delivered to the MRI-guided DST location were administered 5 days per week over 6–8 weeks. The 6–8 week range was specified to accommodate potential variability in the participants' schedules related to school, illnesses, or family events. Hence, each participant was offered 40 opportunities to complete 30 rTMS sessions. Treatment was delivered with the Neuronetics Neurostar Therapy System (Neuronetics Inc., Malvern, PA). Motor threshold assessments were completed with a visualization of movement technique at baseline and after every 10 rTMS sessions (O'Reardon et al. 2007). Treatment intensity was 120% resting motor threshold with a 10 Hz frequency. Stimulus train durations were 4 seconds with an intertrain interval of 26 seconds. When necessary for participant comfort, the treatment intensity was titrated 10% daily starting at a minimum of 80% to the target 120% of resting motor threshold.
Clinical assessments
Depression severity was assessed at baseline, after 10 treatments, after 20 treatments, after 30 treatments (or at early withdrawal), and at a 6 month follow-up visit with the CDRS-R, Quick Inventory for Depressive Symptomatology Adolescent Seventeen Item Self Report (QIDS-A17-SR) (Bernstein et al. 2010), and the Clinical Global Impressions-Severity (CGI-S) and Improvement (CGI-I) scales (Guy 1976). Suicidality was monitored on a weekly basis during the rTMS treatment course with the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2008). Participants underwent neurocognitive testing at baseline, upon completion of active rTMS treatments, and at the 6 month follow-up visit. Neurocognitive assessments included the Children's Auditory Verbal Learning Test-Second Edition (Talley 1985) and the Delis–Kaplan Executive Function System (Delis et al. 2004). Tolerability and adverse events were assessed before and after each study visit. Symptoms were rated on a numerical scale and specific details were recorded on an Adverse Event Monitoring Form.
Statistical analysis
Clinical outcome measures were characterized as mean changes from baseline and standard deviation of the change. The ten participants were included in last observation carried forward analyses. Analyses compared change in scores between end-point in comparison with baseline, using the Wilcoxon signed rank test. Data analyses were performed with SAS (SAS Institute, version 9.2, NC). The mean group distances of DST to 5 cm and DST to Beam F3 locations were compared with a Wilcoxon signed rank test.
Results
Demographics
Six male and four female adolescent participants with a mean (SD) age of 15.9 (1.1) years of age (range 13.9–17.4 years of age) enrolled in the study. Demographics are described in Table 1. Three participants did not complete all 30 treatments.
Table 1.
Participant Demographics
| ID | Sex | Age at first treatment | Total number of prior med trials | Duration of current MDD episode (weeks) | Antidepressant during trial |
|---|---|---|---|---|---|
| 010 | M | 14.1 | 3 | 8 | Desvenlafaxine 50 mg daily |
| 011 | F | 16.1 | 5 | 52 | Lithium 1500 mg daily, Sertraline 100 mg daily |
| 012 | M | 15.9 | 3 | 32 | Sertraline 100 mg daily |
| 013 | M | 17.4 | 4 | 104 | Fluoxetine 80 mg daily |
| 014 | F | 16.3 | 3 | 260 | Fluoxetine 20 mg daily |
| 015 | F | 15.9 | 4 | 144 | Escitalopram 20 mg daily |
| 016 | M | 16.2 | 9 | 56 | Mirtazapine 45 mg daily |
| 017 | M | 16.5 | 5 | 240 | Desvenlafaxine 100 mg daily |
| 018 | M | 16.8 | 3 | 24 | Milnacipran 100 mg daily |
| 019 | F | 13.9 | 1 | 52 | Escitalopram 20 mg daily |
| Mean | 15.9 | 4.0 | 97.2 | ||
| SD | 1.1 | 2.1 | 89.7 |
MDD, major depressive disorder.
Clinical outcomes
Six of the ten participants responded to rTMS treatment. Total mean scores on the CDRS-R, QIDS-A17-SR, and CGI-S improved from baseline to treatment 20, 30, and at 6-month follow-up. The CDRS-R and CGI-S demonstrated improvement by treatment 10. The mean (SD) CDRS-R score at baseline was 62.9 (8.2), corresponding to a severely depressed range. The CDRS-R scores improved significantly at treatments 10 (mean = 54.6, SD = 7.4, p = 0.005), 20 (mean = 44.7, SD = 11.2, p = 0.001), and 30 (mean = 41.8, SD = 13.2, p = 0.002). Improvement maintained at the 6-month follow-up visits (39.9, SD 17.4, p = 0.03). The QIDS-A17-SR scores had a mean (SD) baseline rating of 16.0 (3.5) indicating a severe level of depression (15 is the cutoff for severe). Improvements with this measure became significant at treatments 20 (mean = 12.5, SD = 4.3, p = 0.02) and 30 (mean = 12.0, SD = 5.3, p = 0.045) and endured at 6-month follow-up (mean = 10.3, SD =6.7, p = 0.03). Clinician ratings of illness severity and improvement showed significant improvement as a group and individually. At baseline, the mean CGI Severity score was 5.4, indicating marked depression. Significant improvement was noted at treatment 10 (mean = 4.9, SD = 0.7, p = 0.02), treatment 20 (mean = 3.9, SD =1.3, p = 0.003), treatment 30 (mean = 3.4, SD = 1.5, p = 0.002) and 6 month follow-up (mean = 3.0, SD = 1.9, p = 0.002). Upon completion of treatment 30, 6 of 10 adolescents were rated as mildly ill (3), borderline mentally ill (2), or normal, not at all ill (1). At 6 month follow-up, improvement persisted, with 6 of 10 adolescents receiving depression severity ratings of mildly ill (3), borderline mentally ill (2), or normal, not at all ill (1). CGI Improvement scores were noted to be much improved (2) or very much improved (1) in 6 of 10 participants at treatment completion and at 6 month follow-up.
Safety findings
One participant did not tolerate the first session because of scalp discomfort. A second participant completed five sessions and was subsequently hospitalized for worsening depression. A third participant completed 17 sessions but became anxious about school expectations and developed multiple somatic symptoms. This patient chose not to continue rTMS treatment. It is of note that the study team and her parents thought her depressive symptoms had improved with rTMS.
The most common adverse event was transient scalp discomfort. Additional adverse events included headaches, dizziness, musculoskeletal discomfort, neck stiffness, eye twitching, and nausea. All of these events were characterized as mild and transient. There were no seizures or other significant treatment-related adverse events during the trial.
Neurocognitive testing did not reveal any significant decline in functioning. These findings have been reported previously (Wall et al. 2013). At baseline, 80% of the participants reported some degree of suicidal thinking the week prior to their first rTMS sessions. During the trial, two participants had worsening suicidal behaviors. One participant related that this was in the context of an argument with his mother regarding schoolwork and these symptoms quickly resolved. Another participant experienced self-injurious behavior during the 6 month follow-up period.
Comparison of targeting methods for coil placement
Plots of the euclidean distances between DST to 5 cm locations and DST to Beam F3 locations suggested variability between these techniques (Fig. 2). The mean (SD) distance between DST and 5 cm location was 3.93 (1.22) cm. The mean (SD) distance between DST and Beam F3 location was 2.45 (1.35 cm). The difference in mean distances reached statistical significance (p = 0.006).
Discussion
To our knowledge, this is the first open-label trial of MRI-guided, high-frequency rTMS applied to the DLPFC for adolescents with depression. This also was the first attempt to compare the 5 cm rule, Beam F3, and neuronavigation methods of targeting the DLPFC for high-frequency rTMS treatments in adolescents with severe MDD. The results of the present study provide additional data and experience with high-frequency rTMS in adolescents with treatment-recalcitrant depression. There are a number of important limitations to consider. First, the APB location and motor threshold measurements were based on observations of visual contractions rather than neurophysiological techniques with electromyography. An electromyography approach may have improved the specificity of the APB site localization. Some experts have raised important concerns regarding visualization methods for motor threshold, as this approach may also provide inaccurate information for dosing rTMS sessions (Rossi et al. 2009; Westin et al. 2014). However, the visualization of movement method for motor threshold testing is commonly used in clinical practice, and universal consensus is lacking (Pridmore et al. 1998; Rossi et al. 2009). Second, in terms of clinical outcomes, there are important threats to internal validity for consideration. Specifically, there was no control group, and the clinical ratings were not blinded. It is possible that symptom reduction was simply the result of the passage of time, involvement in a therapy, or interpersonal interaction with the treating psychiatrist or technician. Finally, given the small sample size and design of the study, caution is warranted to avoid over-interpreting the findings. However, a course of MRI-guided, high-frequency rTMS treatment was feasible, tolerable, and beneficial for the majority of participants. Further, retention, tolerability, and effectiveness results of this pilot study parallel our earlier work that employed the standard 5 cm rule method for localizing the rTMS stimulation site of the DLPFC (Wall et al. 2011). We note that titration methods for stimulus intensity dosing will likely improve the overall tolerability and retention of adolescent participants in future studies. Titrating from 80% to 120% of resting motor threshold over five treatment sessions with an increase by 10% as tolerated per session appeared beneficial and may be a reasonable strategy for future studies. In fact adult studies suggest that procedural scalp pain associated with rTMS improves over the course of treatment (Anderson et al. 2009).
Multiple techniques for MRI guided localization of the DLPFC have been examined previously (Glahn et al. 2005; Fitzgerald et al. 2006; Peleman et al. 2010; Rusjan et al. 2010). The methodology in the present study was supervised by a neuroradiologist and was relatively expedient as it utilized a pragmatic neuroimaging landmark. Ultimately, the accuracy of all MRI guided DLPFC localization techniques cannot be validated as Brodmann's areas are not imaged directly (Rajkowska and Goldman-Rakic 1995a,b). However, the technique we present corresponds with BA9 and 46 based on prior cytoarchitecture descriptions (Rajkowska and Goldman-Rakic 1995a,b). Although prior groups have described similar techniques, our methodology identifies the DBT as the interface of the superior and middle frontal gyri rather than center of the middle frontal gyrus (Peleman et al. 2010). This is noteworthy, as BA9 and BA46 are thought to include both superior and middle frontal gyri.
The optimal localization technique for rTMS treatment of MDD is an open question (Mir-Moghtadaei et al. 2015). Although practical, it has been widely suggested that the 5 cm method is inaccurate (Herwig et al. 2001; Ahdab et al. 2010; Bradfield et al. 2012). For example, in studies examining the precision of the 5 cm method in comparison with well-defined cortical landmarks for the DLPFC, the 5 cm method is inaccurate >50% of the time (Ahdab et al. 2010; Mir-Moghtadaei et al. 2015). This may have been a contributing factor in prior rTMS trials with suboptimal clinical outcomes (Fitzgerald et al. 2009; Mir-Moghtadaei et al. 2015). Although a 6 cm technique or the Beam F3 method may improve accuracy of localization, MRI-guided techniques are increasingly pondered as a touchstone approach for adult populations. However, the optimal MRI localization procedure has not been established. Given the limitations of the present study, it is unknown if MRI-guided rTMS treatment improves clinical outcomes in depressed adolescents. A definitive study would be a complex and expensive endeavor necessitating the comparison of outcomes with each stimulation site. Further, it is unlikely that MRI-guided rTMS treatment will be routinely employed in future clinical practice, as this could present unfortunate barriers for adolescents in need. Future practical approaches might ponder the utility of MRI-guided techniques for depressed adolescents failing an extended course of standard rTMS. The practical and economic feasibility of implementing MRI-guided localization broadly is of concern (Mir-Moghtadaei et al. 2015), and warrants further study.
Conclusions
This study and prior work suggest that high-frequency rTMS is a feasible, tolerable, and potentially effective modality for adolescents with treatment-resistant MDD. The fact that the majority of participants completed all 30 sessions suggests a perceived benefit of the treatment. The maintenance of clinical improvement at 6 month follow-up is also promising. Drawing definitive conclusions regarding clinical effectiveness is premature. Ideal future efforts will focus on randomized, sham-controlled trials with an adequate dose, duration, and blinding. Based on our early findings, rTMS dosing parameters (30 rTMS sessions of 10 Hz, 120% motor threshold, with 3000 pulses applied to the DLPFC) from prior pivotal adult studies appears to be a reasonable approach for further work. The present results suggest that 5 cm rule, Beam F3, and MRI-guided localization approaches in adolescents may yield different locations. However, the clinical significance of localization is unclear in terms of tolerability and clinical outcome. Given the potential family burden of daily rTMS treatments, future research efforts should also focus on baseline predictive biomarkers for patient selection and dosing strategies.
Clinical Significance
The present study suggests that 30 sessions of high-frequency rTMS applied to the dorsolateral prefrontal cortex is a promising intervention for adolescents with treatment-resistant depression. Standard localization techniques for rTMS treatment may identify divergent anatomical areas in adolescents, but the clinical impact of this is undetermined. In the future, collaborative, randomized, sham-controlled trials of rTMS for adolescent depression could advance understanding of rTMS in adolescents, and potentially address an unmet clinical need.
Disclosures
Drs. Wall and Sampson have received in-kind support for equipment and supplies from Neuronetics Inc. Dr. Croarkin has received grant support from the Brain and Behavior Research Foundation, the Mayo Clinic Foundation, NIMH (K23 MH100266), and Pfizer Inc. He has received in-kind support for equipment and supplies from Neuronetics Inc. Dr Frye has received grant support from AssureRx Health Inc, the Mayo Foundation, Myriad, the National Institute on Alcohol Abuse and Alcoholism (P20AA017830) in the National Institutes of Health at the US Department of Health and Human Services, NIMH (R01 MH079261), and Pfizer Inc. He has been a consultant to Janssen Global Services LLC, Mitsubishi Tanabe Pharma Corp, Myriad Genetics Inc, Sunovion Pharmaceuticals Inc, and Teva Pharmaceutical Industries Ltd. He has received continuing medical education/travel/presentation support from CME Outfitters LLC and Sunovion Pharmaceuticals Inc. Dr. Baruth, Dr. Port, Ms. Haugen, and Ms. Maroney-Smith have no financial relationships to disclose.
References
- Ahdab R, Ayache SS, Brugieres P, Goujon C, Lefaucheur JP: Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin 40:27–36, 2010 [DOI] [PubMed] [Google Scholar]
- Anderson BS, Kavanagh K, Borckardt JJ, Nahas ZH, Kose S, Lisanby SH, McDonald WM, Avery D, Sackeim HA, George MS: Decreasing procedural pain over time of left prefrontal rTMS for depression: Initial results from the open-label phase of a multi-site trial (OPT-TMS). Brain Stimul 2:88–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Beam W, Borckardt JJ, Reeves ST, George MS: An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul 2:50–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IH, Rush AJ, Trivedi MH, Hughes CW, Macleod L, Witte BP, Jain S, Mayes TL, Emslie GJ: Psychometric properties of the Quick Inventory of Depressive Symptomatology in adolescents. Int J Methods Psychiatr Res 19:185–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield NI, Reutens DC, Chen J, Wood AG: Stereotaxic localisation of the dorsolateral prefrontal cortex for transcranial magnetic stimulation is superior to the standard reference position. Aust N Z J Psychiatry 46:232–239, 2012 [DOI] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J: Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA 299:901–913, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Birmaher B: Treatment-resistant depression in adolescents: Recognition and management. Child Adolesc Psychiatr Clin N Am 15:1015–1034, 2006 [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Wall CA, McClintock SM, Kozel FA, Husain MM, Sampson SM: The emerging role for repetitive transcranial magnetic stimulation in optimizing the treatment of adolescent depression. J ECT 26:323–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agati D, Bloch Y, Levkovitz Y, Reti I: rTMS for adolescents: Safety and efficacy considerations. Psychiatry Res 177:280–285. 2010 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J: Reliability and validity of the Delis–Kaplan Executive Function System: An update. J Int Neuropsychol Soc 10:301–303, 2004 [DOI] [PubMed] [Google Scholar]
- Donaldson AE, Gordon MS, Melvin GA, Barton DA, Fitzgerald PB: Addressing the needs of adolescents with treatment resistant depressive disorders: A systematic review of rTMS. Brain Stimul 7:7–12, 2014 [DOI] [PubMed] [Google Scholar]
- Dunlop K, Gaprielian P, Blumberger D, Daskalakis ZJ, Kennedy SH, Giacobbe P, Downar J: MRI-guided dmPFC-rTMS as a treatment for treatment-resistant major depressive disorder. J Vis Exp 102:e53129, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, Brock DG, Bonneh–Barkay D, Cook IA, Lanocha K, Solvason HB, Demitrack MA: A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: Durability of benefit over a 1-year follow-up period. J Clin Psychiatry 75:1394–1401, 2014 [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, Bailey M, Been G, Kulkarni J, Daskalakis ZJ: A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology 34:1255–1262, 2009 [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ: An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res 148:33–45, 2006 [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE, 3rd, Schwartz T, Sackeim HA: Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch Gen Psychiatry 67:507–516, 2010 [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI: Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp 25:60–69, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Healthy, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976 [Google Scholar]
- Herwig U, Padberg F, Unger J, Spitzer M, Schonfeldt–Lecuona C: Transcranial magnetic stimulation in therapy studies: Examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry 50:58–61, 2001 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Krishnan C, Santos L, Peterson MD, Ehinger M: Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul 8:76–87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CT, Chen MH, Juan CH, Huang HH, Chen LF, Hsieh JC, Tu PC, Bai YM, Tsai SJ, Lee YC, Su TP: Efficacy of prefrontal theta-burst stimulation in refractory depression: A randomized sham-controlled study. Brain 137:2088–2098, 2014 [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 292:807–820, 2004 [DOI] [PubMed] [Google Scholar]
- March JS, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J: The Treatment for Adolescents With Depression Study (TADS): Long-term effectiveness and safety outcomes. Arch Gen Psychiatry 64:1132–1143, 2007 [DOI] [PubMed] [Google Scholar]
- Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J: Concordance Between BeamF3 and MRI-neuronavigated Target Sites for Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex. Brain Stimul 8:965–973, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA: Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry 62:1208–1216, 2007 [DOI] [PubMed] [Google Scholar]
- Peleman K, Van Schuerbeek P, Luypaert R, Stadnik T, De Raedt R, De Mey J, Bossuyt A, Baeken C: Using 3D-MRI to localize the dorsolateral prefrontal cortex in TMS research. World J Biol Psychiatry 11:425–430, 2010 [DOI] [PubMed] [Google Scholar]
- Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R: Effects of psychotropic drugs on seizure threshold. Drug Saf 25:91–110, 2002 [DOI] [PubMed] [Google Scholar]
- Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, Fisher P, Zelazny J, Burke A, Oquendo M, Mann J: Columbia-Suicide Severity Rating Scale (C-SSRS). New York: Columbia University; 2008 [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R: Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Psychiatry 23:191–197, 1984 [DOI] [PubMed] [Google Scholar]
- Pridmore S, Fernandes JA, Nahas Z, Liberatos C, George MS. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 14:25–27, 1998 [PubMed] [Google Scholar]
- Rajkowska G, Goldman–Rakic PS: Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex 5:307–322, 1995a [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman–Rakic PS: Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 5:323–337, 1995b [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascaual–Leone A, The Safety of TMS Consensus Group: Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, Daskalakis ZJ: Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp 31:1643–1652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA: The definition and meaning of treatment-resistant depression. J Clin Psychiatry 62 Suppl 16:10–17, 2001 [PubMed] [Google Scholar]
- Talley JL. Children's Auditory and Verbal Learning Test-2 Professional Manual. (CAVLT-2). Odessa, Florida: Psychological Assessments, Inc.; 1995 [Google Scholar]
- Walkup JT: Treatment of depressed adolescents. Am J Psychiatry 167:734–737, 2010 [DOI] [PubMed] [Google Scholar]
- Wall CA, Croarkin PE, McClintock SM, Murphy LL, Bandel LA, Sim LA, Sampson SM: Neurocognitive effects of repetitive transcranial magnetic stimulation in adolescents with major depressive disorder. Front Psychiatry 4:165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall CA, Croarkin PE, Sim LA, Husain MM, Janicak PG, Kozel FA, Emslie GJ, Dowd SM, Sampson SM: Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry 72:1263–1269, 2011 [DOI] [PubMed] [Google Scholar]
- Westin GG, Bassi BD, Lisanby SH, Luber B: Determination of motor threshold using visual observation oversestimates transcranial magnetic stimulation dosage: Safety implications. Clin Neurophysiol 125:142–147, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]