Abstract
Bladder-related events, including neurogenic detrusor overactivity, are the leading cause of autonomic dysreflexia in spinal cord injured individuals. Self-reported autonomic dysreflexia is reduced following onabotulinumtoxinA treatment for neurogenic detrusor overactivity; however, none of these trials have assessed autonomic dysreflexia events using the clinical cutoff of an increase in systolic blood pressure ≥20 mm Hg. This study used a prospective, open-labelled design from 2013 to 2014 to quantitatively assess the efficacy of one cycle 200 U intradetrusor-injected onabotulinumtoxinA (20 sites) on reducing the severity and frequency of bladder-related autonomic dysreflexia events and improving quality of life. Twelve men and five women with chronic, traumatic spinal cord injuries at or above the sixth thoracic level, and concomitant autonomic dysreflexia and neurogenic detrusor overactivity, underwent blood pressure monitoring during urodynamics and over a 24 h period using ambulatory blood pressure monitoring pre- and 1 month post-treatment. Post-onabotulinumtoxinA, autonomic dysreflexia severity was reduced during urodynamics (systolic blood pressure increase: 42 ± 23 mm Hg vs. 20 ± 10 mm Hg, p < 0.001) and during bladder-related events across the 24 h period (systolic blood pressure increase: 49 ± 2 mm Hg vs. 26 ± 22 mm Hg, p = 0.004). Frequency of 24 h bladder-related autonomic dysreflexia events was also decreased post-onabotulinumtoxinA (4 ± 2 events vs. 1 ± 1 events, p < 0.001). Autonomic dysreflexia and incontinence quality of life indices were also improved post-onabotulinumtoxinA (p < 0.05). Intradetrusor injections of onabotulinumtoxinA for the management of neurogenic detrusor overactivity in individuals with high level spinal cord injuries decreased the severity and frequency of bladder-related episodes of autonomic dysreflexia, and improved bladder function and quality of life.
Key words: : ambulatory blood pressure monitoring, blood pressure, Botox, cardiovascular, neurogenic bladder
Introduction
Autonomic dysreflexia (AD) is a medical emergency that occurs in up to 90% of individuals with a spinal cord injury (SCI) at or above the sixth thoracic (T6) spinal segment. It is clinically defined as an elevation in systolic blood pressure (SBP) ≥20 mm Hg from baseline in response to noxious or innocuous stimuli below injury level.1 The urinary bladder is the cause of 85% of all AD episodes, because of bladder distension or involuntary detrusor contractions known as neurogenic detrusor overactivity (NDO).2,3 NDO management following SCI typically involves routine urodynamic studies (UDS)4 and the use of anticholinergic agents.5 When anticholinergics are ineffective, onabotulinumtoxinA (Botox, Allergan, Inc.) has provided a safe and effective alternative treatment option.6 Two human studies have documented reductions in self-reported AD symptoms following intradetrusor Botox injections7,8 whereas improved mean arterial blood pressure (BP) has been reported by one animal study.9 In order to determine the efficacy of Botox in reducing the severity of bladder-related AD, a proper evaluation of SBP responses using the clinically established AD criterion is necessary. Therefore, the objective of this study was to quantitatively assess the efficacy of 200 U intradetrusor-injected Botox for NDO on reducing the frequency and severity of AD during bladder-related events including UDS, the gold-standard assessment of bladder function, and prior to each clean intermittent catheterization (CIC) during a 24 h period. The primary outcome was the severity of AD during UDS. The secondary outcome of AD frequency and severity during a 24 h period was assessed using 24 h ambulatory BP monitoring (ABPM). Tertiary outcomes included AD and bladder-related quality of life (QoL), whereas quaternary outcomes included bladder function indices assessed during UDS. We hypothesized that our intervention would reduce the frequency and severity of AD during bladder-related events, and that these reductions would lead to an improvement in QoL.
Methods
Study participants
Inclusion criteria were as follows: males and females, 18–65 years of age, with chronic (>1 year post-injury), traumatic SCI at or above T6, confirmed AD during UDS, confirmed NDO, capable of CIC, and resistant to anticholinergic medications. Exclusion criteria included documented traumatic brain injury, previous use of Botox for the bladder, previous genitourinary disease or surgery, multiple injury levels, acute urinary tract infection (UTI) (culture-proven diagnosis), and history of cardiovascular disease.
Study design
This prospective, open-label, pre/post comparison study was conducted from April 2013 to August 2014 at the Blusson Spinal Cord Centre, in Vancouver, British Columbia, Canada. Approval was received by the Clinical Research Ethics Board at the University of British Columbia, and the Vancouver Coastal Health Research Institute Ethics Board, conforming to the Declaration of Helsinki, and was registered (#NCT02298660) at ClinicalTrials.gov. All participants provided written informed consent. The neurological level of injury and completeness of injury were classified according to the International Standards for Neurological Classification of SCI.10 Baseline (pre) testing consisted of a UDS assessment to confirm the presence of AD, 24 h ABPM, and QoL questionnaires. Two weeks following baseline evaluations, participants received Botox injections. One month following injections (post), participants repeated the same measurements as the baseline test. Participants were asked to refrain from exercise, coffee, alcohol, and anticholinergic medications ≥12 h prior to testing. To minimize the effects of the bowel (i.e., impaction and defecation) on AD,11,12 the UDS and 24 h ABPM assessments were scheduled at least 2 days prior to each participant's scheduled bowel program.
UDS assessment
Prior to UDS, a urinary dipstick test confirmed the absence of infection. UDS occurred between 0800 and 1200 h in a temperature-controlled room (21°C) according to established standards.13,14 Cystometry was performed by a double-lumen catheter (6 Fr, Laborie, Canada) with continuous filling of sterile water (37°C) at a fixed rate of 30 mL/min. Abdominal pressure was measured with an intrarectal balloon catheter (10 Fr, Laborie, Canada). Pelvic floor electromyography (EMG) (Aquarius TT, Laborie Model 94-R03-BT, Montreal, Quebec, Canada) was recorded using surface EMG. Filling was stopped if any of the following conditions were observed: 1) reported sensation of fullness, 2) spontaneous urine leakage, 3) intravesical pressure ≥40 cm H2O, 4) infused volume reached 500 mL, or 5) sustained SBP ≥180 mm Hg or intolerable AD symptoms.
Heart rate (HR) and BP were recorded every minute on the right arm during UDS using an automated sphygmomanometer (DinamapV100; GE Medical Systems, Fairfield, CT), using a medium (23–33 cm) or large (31–40 cm) adult-sized cuff. Three supine measurements were taken at the beginning of UDS and averaged to determine baseline supine SBP. After this, hemodynamic measurements corresponding to each of the following time points were documented: 1) first urge to perform CIC, 2) at maximum volume infused, and 3) the overall maximum SBP reached during UDS. Signs and self-reported symptoms of AD were documented. The change in SBP (ΔSBP) and HR (ΔHR) from baseline at each time point was calculated. At the end of the assessment, a CIC was performed to void the bladder. If AD persisted, it was managed according to established guidelines15,16 including sitting the participant upright to induce orthostatic BP response, loosening any restrictive clothing, confirming the bladder had been fully voided, and checking for any other possible sources of stimuli. If these steps did not result in the normalization of BP, the administration of an antihypertensive agent was considered if SBP remained ≥150 mm Hg. In the present study, no participants required the use of an antihypertensive agent to manage their AD following the UDS assessment.
Twenty-four hour ABPM assessment
To document the incidence of AD during bladder-related events across a 24 h period, ABPM was used (Meditech Card [X] plore; Meditech Ltd., Budapest, Hungary). A medium (24–32 cm) or large (32–42 cm) adult-sized cuff was used. With participants seated in their own wheelchairs, three discrete seated BP and HR measurements were taken on the nondominant arm and averaged to determine the baseline seated SBP and HR values with which the 24 h ABPM values would be compared. Automatic recordings were taken every 15 min from 0700 to 2300 h (daytime period), and every hour from 2300 to 0700 h. Manual BP measurements were also documented in an activity log before and after each CIC and suspected AD episode. The number of bladder-related events (i.e., CIC) was documented, and the highest SBP prior to each CIC was subtracted from the baseline seated SBP to determine if AD was occurring (ΔSBP ≥20 mm Hg). The corresponding HR during these events was also subtracted from baseline HR to determine the change (ΔHR). The average daytime and nighttime BP values were also determined.
Questionnaires
The AD Health-Related Quality of Life Questionnaire (AD QoL)12 and the Incontinence Quality of Life Questionnaire (I-QoL)17 were administered to assess the impact of AD and NDO on perceptions of health.
Botox injections
Two weeks following UDS, one cycle of Botox was injected according to the established clinical protocol for NDO.7 To avoid provocation of AD, a local anesthetic was utilized with instillation of 50 mL of 2% lidocaine into the bladder mucosa. In brief, 200 U of Botox diluted in 20 mL 0.9% saline solution was injected into 20 sites of the detrusor muscle (10 U/site), trigone sparing. Oral antibiotics were prescribed 5 days prior to treatment. Anticholinergic medications were progressively decreased 1 week post-treatment. Adverse events were documented 1 week (by phone call) and 1 month (in person) post-Botox.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (Version 19.0; IBM Corporation, Armonk, NY). All data were assessed for normal distribution using Shapiro–Wilk tests, and analyzed by paired t test and Wilcoxon signed rank test for normal and non-normal distributions, respectively. All statistical analyses were considered significant at p < 0.05.
Results
Twenty-two individuals were enrolled into the study; however, only 17 completed the study to entirety. Two subjects decided against Botox, whereas three were lost at follow-up because of relocation. Participant characteristics are provided in Table 1. There were no adverse events during the Botox injections. Post-Botox injections, the following adverse events were reported: culture-proven UTIs requiring antibiotic therapy in three participants (two cervical, one thoracic), and a headache of unidentified origin (not related to AD) lasting 1 week in two (cervical) participants.
Table 1.
Participant Characteristics
| ID | Lesion level | AIS | Age (yr) | Sex | Height (cm) | Mass (kg) | TPI (yr) | Injury cause | History of anticholinergic use |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C4 | A | 51 | F | 167 | 55 | 8 | MVA | Oxybutynin 5 mg BID |
| 2 | C4 | A | 37 | F | 165 | 64 | 14 | MVA | Oxybutynin 5 mg TID |
| 3 | C5 | C | 43 | M | 175 | 84 | 27 | MVA | Oxybutynin 5 mg BID |
| 4 | C5 | C | 62 | M | 180 | 98 | 4 | Fall | Oxybutynin 5 mg BID |
| 5 | C6 | A | 44 | M | 183 | 63 | 19 | Sport | Tolterodine tartrate 12 mg OD |
| 6 | C6 | B | 60 | M | 178 | 70 | 34 | MVA | Oxybutynin 5 mg BID |
| 7 | C6 | C | 43 | M | 183 | 81 | 24 | Sport | Fesoterodine fumarate 4 mg OD |
| 8 | C7 | A | 42 | F | 178 | 53 | 18 | MVA | Oxybutynin 5 mg BID |
| 9 | C7 | B | 40 | M | 172 | 66 | 17 | Fall | Solifenacin succinate 10 mg OD |
| 10 | C7 | B | 28 | M | 178 | 68 | 8 | MVA | Oxybutynin 5 mg TID |
| 11 | C8 | B | 46 | M | 165 | 45 | 40 | MVA | Oxybutynin 2.5mg BID |
| 12 | T3 | B | 38 | M | 182 | 95 | 21 | Sport | Oxybutynin 10 mg BID |
| 13 | T4 | A | 36 | F | 178 | 63 | 23 | Sport | Tolterodine tartrate 2 mg BID |
| 14 | T5 | A | 31 | M | 178 | 60 | 10 | Sport | Oxybutynin 5 mg BID |
| 15 | T5 | A | 46 | F | 165 | 73 | 29 | Fall | Fesoterodine fumarate 8 mg OD |
| 16 | T5 | A | 62 | M | 183 | 91 | 42 | MVA | Tolterodine tartrate LA 4 mg OD |
| 17 | T5 | A | 44 | M | 157 | 70 | 18 | Fall | Fesoterodine fumarate 8 mg OD |
| Mean ± SD | 11 C 6 T |
9 A 5 B 3 C |
44 ± 10 | 5 F 12 M |
175 ± 8 | 71 ± 15 | 21 ± 11 | 4 Fall 8 MVA 5 Sport |
3 Tolterodine tartrate 10 Oxybutynin 3 Fesoterodine fumarate 1 Solifenacin succinate |
AIS, American Spinal Injury Association Impairment Scale; BID, twice daily; C, cervical; MVA, motor vehicle accident; OD, once daily; T, thoracic; TID, three times daily; TPI, time post-injury.
Hemodynamic outcome measures during UDS
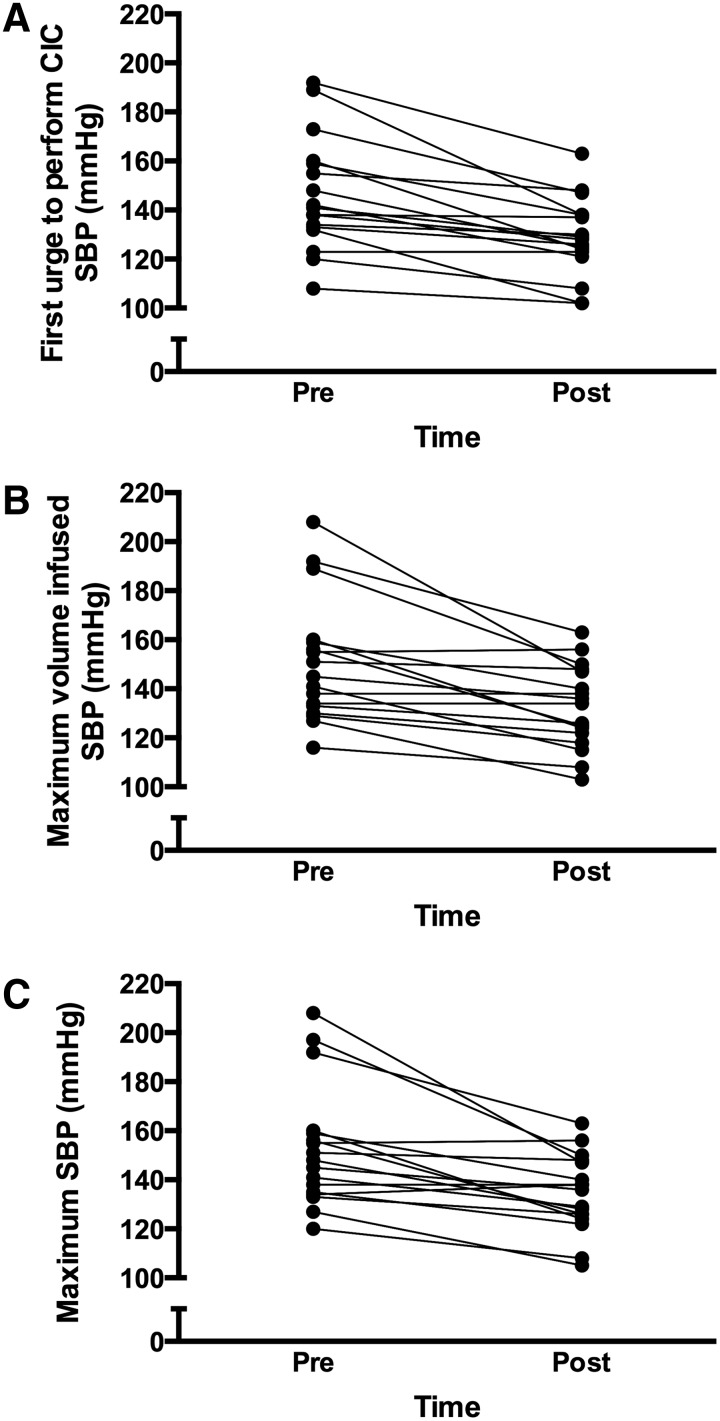
SBP and HR values during UDS are presented in Table 2, and individual SBP responses are illustrated in Figure 1. SBP and HR at baseline were unchanged between pre- and post-Botox assessments. However, SBP at the first urge to perform CIC, at the maximum volume infused, and the overall maximum SBP during UDS, were all decreased 1 month post-Botox. The change in SBP from baseline (ΔSBP) was used to quantify the severity of AD. All participants presented with AD pre-Botox (ΔSBP ≥20 mm Hg), whereas post-Botox it was eliminated in 10 (59%). AD severity was attenuated in the remaining seven. The majority of participants also experienced AD during the first urge to perform CIC and maximum infusion, and the severity of AD during these time points was significantly reduced post-Botox. Pre-Botox UDS, 15 participants (88%) reported at least one symptom of AD including goosebumps, chills/tingles, flushing, or headache. Post-Botox, self-reported symptoms decreased (p = 0.034), with only 9 (53%) reporting symptoms. The ΔHR was only reduced post-Botox at maximum infusion and maximum SBP. Of the 17 participants, 14 experienced the typical reduction in HR during AD whereas the other 3 participants experienced an increase in HR.
Table 2.
AD Severity and Incidence during UDS
| Variable | Pre-Botox | Post-Botox | p value |
|---|---|---|---|
| Supine baseline | |||
| SBP, mm Hg | 112 ± 17 | 114 ± 14 | 0.601 |
| HR, bpm | 71 ± 16 | 70 ± 10 | 0.768 |
| First urge to perform CIC | |||
| SBP, mmHg | 146 ± 23 | 129 ± 16 | <0.001 |
| ΔSBP, mmHg | 34 ± 20 | 15 ± 11 | 0.001 |
| ΔHR, bpm | −8 ± 11 | −6 ± 10 | 0.209 |
| AD incidence, no (%) | 14 (82) | 5 (29) | ----- |
| Maximum volume infusion | |||
| SBP, mm Hg | 151 ± 25 | 133 ± 17 | <0.001 |
| ΔSBP, mm Hg | 40 ± 24 | 18 ± 12 | <0.001 |
| ΔHR, bpm | −17 ± 12 | −9 ± 14 | 0.047 |
| AD incidence, no (%) | 16 (94) | 7 (41) | ----- |
| Maximum SBP | |||
| SBP, mm Hg | 153 ± 25 | 134 ± 16 | 0.001 |
| ΔSBP, mm Hg | 42 ± 23 | 20 ± 10 | <0.001 |
| ΔHR, bpm | −16 ± 13 | −8 ± 14 | 0.049 |
| AD incidence, no (%) | 17 (100) | 7 (41) | ----- |
Data are mean ± SD.
AD, autonomic dysreflexia; CIC, clean intermittent catheterization; HR, heart rate; SBP, systolic blood pressure; UDS, urodynamic studies.
FIG. 1.
Individual systolic blood pressure (SBP) responses during urodynamic studies (UDS) pre- and post-Botox. Panel A: SBP at the participant's first urge to perform a clean intermittent catheterization (CIC). Panel B: SBP at maximum bladder infusion. Panel C: Maximum SBP reached during UDS.
Twenty-four hour ABPM outcome measures
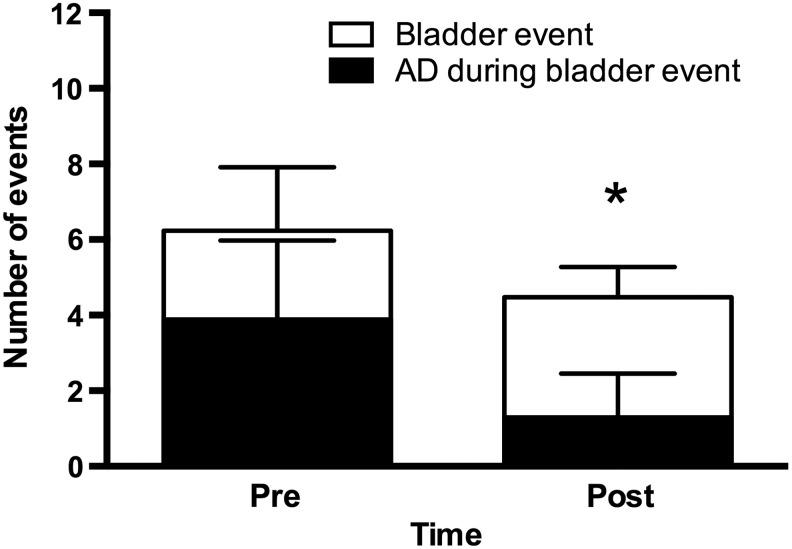
The severity of AD during bladder-related events was significantly reduced following Botox treatment, as evidenced by a reduction in both the maximum SBP and ΔSBP during bladder-related events (Table 3). The frequency of bladder-related events is presented in Figure 2. Pre-Botox, participants had 6 ± 2 bladder events in a 24 h period (i.e., performed a CIC), with AD occurring 67% of the time (4 ± 2 times). Although there was a significant reduction in the number of bladder events post-Botox to 4 ± 1 (p < 0.001), there was also a significant reduction in the frequency of AD during these events to 25% (1 ± 1 times, p < 0.001).
Table 3.
Summary of 24 h ABPM Outcomes
| Variable | Pre-botox | Post-botox | p value |
|---|---|---|---|
| Seated baseline | |||
| SBP, mm Hg | 108 ± 14 | 113 ± 14 | 0.064 |
| HR, bpm | 77 ± 13 | 76 ± 12 | 0.771 |
| Bladder-related events | |||
| Maximum SBP, mm Hg | 157 ± 21 | 139 ± 21 | 0.006 |
| ΔSBP, mm Hg | 49 ± 22 | 26 ± 22 | 0.004 |
| ΔHR, bpm | −11 ± 15 | −10 ± 14 | 0.880 |
| Daytime values | |||
| SBP, mm Hg | 108 ± 11 | 109 ± 12 | 0.532 |
| DBP, mm Hg | 64 ± 7 | 64 ± 8 | 0.708 |
| HR, bpm | 73 ± 10 | 75 ± 10 | 0.423 |
| Nighttime values | |||
| SBP, mm Hg | 98 ± 9 | 99 ± 7 | 0.245 |
| DBP, mm Hg | 54 ± 8 | 55 ± 6 | 0.571 |
| HR, bpm | 61 ± 11 | 65 ± 11 | 0.125 |
| Nocturnal dip, % | |||
| C4 – T5 | −8 ± 13 | −6 ± 11 | 0.325 |
| C4 – C8 | −2 ± 13 | −2 ± 12 | 0.806 |
| T3 – T5 | −17 ± 3 | −14 ± 4 | 0.129 |
Data are mean ± SD.
ABPM, ambulatory blood pressure monitoring; AD, autonomic dysreflexia; C, cervical; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure; T, thoracic.
FIG. 2.
Overview of the incidence of autonomic dysreflexia (AD) during bladder-related events during a 24 h period pre- and post-Botox. Open bars represent the number of bladder events (i.e., require participant to perform clean intermittent catheterization [CIC]). Black bars represent the number of these events which elicited AD (i.e., Δsystolic blood pressure [SBP] ≥20 mm Hg). Data are presented as mean ± SD. *p < 0.001 vs. pre-Botox for both number of bladder events, and AD during bladder event.
Questionnaire and bladder function outcome measures
All subsections for the AD HR-QoL and I-QoL were improved post-Botox treatment (Table 4). The total scores for AD HR-QoL and I-QoL were decreased and increased post-Botox, respectively, indicating an overall improvement in QoL. Overall, all indices of bladder function were improved post-Botox (Table 5).
Table 4.
Questionnaire Data
| Variable | Pre-botox | Post-botox | p value |
|---|---|---|---|
| AD HR-QoL – Total Score | 124 ± 30 | 86 ± 26 | <0.001 |
| Daily Basis AD | 21 ± 5 | 14 ± 4 | 0.001 |
| Bladder-Related AD | 19 ± 4 | 13 ± 3 | 0.001 |
| Daily Basis AD Severity | 15 ± 4 | 12 ± 3 | 0.001 |
| Bladder-Related AD Severity | 17 ± 3 | 12 ± 4 | 0.002 |
| AD Interference in Daily Life | 13 ± 2 | 10 ± 2 | 0.001 |
| Severity of Interference in Daily Life | 23 ± 10 | 14 ± 6 | 0.001 |
| AD Severity in Past 2 Weeks | 12 ± 4 | 8 ± 6 | 0.027 |
| AD Frequency in Past 2 Weeks | 4 ± 1 | 1 ± 2 | 0.001 |
| I-QoL – Total Score | 78 ± 19 | 93 ± 19 | 0.001 |
| Avoidance Limiting Behavior | 28 ± 6 | 34 ± 5 | 0.001 |
| Psychosocial Impact | 34 ± 9 | 40 ± 9 | 0.001 |
| Social Embarrassment | 16 ± 6 | 20 ± 6 | 0.001 |
Data are means ± SD.
AD, autonomic dysreflexia; HR-QoL, AD Health-Related Quality of Life Questionnaire; I-QoL, Incontinence Quality of Life Questionnaire.
Table 5.
Bladder Function Parameters during UDS
| Variable | Pre-botox | Post-botox | p value |
|---|---|---|---|
| Volume at first contraction, mL | 262 ± 146 | 391 ± 232 | 0.019 |
| Compliance, cm H2O−1 | 15 ± 17 | 42 ± 42 | <0.001 |
| Maximum detrusor pressure, cm H2O−1 | 38 ± 12 | 17 ± 9 | <0.001 |
| Contractions before leak, no | 3 ± 5 | 0 ± 0 | 0.012 |
| Volume before leak/maximum volume, mL | 380 ± 214 | 520 ± 139 | 0.010 |
Data are mean ± SD.
UDS, urodynamic studies.
Discussion
This is the first human clinical trial to assess the efficacy of Botox on reducing bladder-related AD events in individuals with cervical and high thoracic SCI using quantitative hemodynamic assessments and clinically relevant AD criteria. The main finding from this investigation was a reduction in AD severity and frequency during bladder-related events. These findings are likely attributed to our observation of improved bladder function, and resulted in an improvement in QoL.
The incidence of AD during bladder-related events was assessed using UDS, the gold-standard assessment of bladder function, as well as during daily living using 24 h ABPM. Following the Botox treatment, 59% of our sample no longer experienced AD during the UDS assessment (i.e., AD was eliminated), whereas the remaining 41% experienced a reduction in AD severity (i.e., AD was attenuated). Guidelines for the treatment of AD recommend pharmacological management at SBP ≥150 mm Hg.15 As illustrated in Figure 1C, 47% of our sample was above this cutoff pre-Botox, whereas only 18% had maximum SBP ≥150 mm Hg post-Botox. Therefore, whereas AD may not have been eliminated in all participants, the severity of the SBP increase was attenuated and in most individuals was reduced below a clinically relevant cutoff value. To date, no human studies have used BP assessments to quantify changes in AD following a Botox intervention for NDO. Previous investigations using 200 U intradetrusor-injected Botox reported the elimination of self-reported AD in 6%8 and 16%7 of their samples, and attenuated AD symptoms in 37%.7 In our investigation, AD determined using self-reported symptoms was eliminated in 40% of our sample (i.e., eight participants no longer reported symptoms during the UDS assessment post-Botox). Interestingly, only 88% of our sample reported AD symptoms during the pre-Botox UDS assessment, whereas 100% of the sample had AD according to the objective SBP criterion. This observation demonstrates that asymptomatic or “silent” AD does occur,18 and that subjective, self-reported measures of AD may not accurately reflect the hemodynamic responses. Therefore, although our objective and subjective assessments of AD demonstrate a greater efficacy of Botox for the treatment AD than previously reported, future investigations should consider using the objective measurement of SBP in their assessments of AD.
Previous studies have utilized 24 h ABPM to assess AD frequency and severity in SCI.12,19 We used this technique to assess how Botox treatment affected the incidence of bladder-related AD events during activities of daily living. This assessment complements our in-hospital UDS assessment, and demonstrates the translation of our findings into the real day-to-day life of individuals with SCI. Similar to the UDS assessment; we observed a reduction in the severity of AD indicated by reduction in both maximum SBP and ΔSBP. Additionally, we observed an overall reduction in the frequency of AD during bladder-related events, despite the reduction in the number of CIC performed within the 24 h period. The reduction in the number of CIC can be attributed to an increased bladder capacity, as evidenced by the post-Botox increase in maximum bladder volume assessed during UDS. In general, overall bladder function was improved post-Botox, which is similar to previous reports employing Botox for the treatment of NDO in SCI.7,20–22 The subsequent reduction in the frequency of AD prior to CIC is likely attributed to the mechanistic actions of Botox. Through temporary paralysis of the detrusor muscle, Botox inhibits involuntary detrusor contractions by preventing the transmission of noxious/innocuous stimuli from entering the dorsal column, thereby deactivating the initiation of the spinal-mediated reflex responsible for AD.7,23 Botox has also been found to contain anti-nerve growth factor properties that block the hyperexcitable afferent C fiber pathways responsible for the involuntary contractions.9 Therefore, in addition to improving bladder function and reducing NDO, our results suggest that Botox has the added benefit of reducing AD frequency and severity during bladder-related events. Botox also has also been shown to have an inhibitory effect on afferent axons positive for calcitonin-gene-related peptide and substance P,24 which are widely distributed throughout the bladder wall and are known to be involved in the development of AD.25,26 This may explain our observation that some individuals experienced less pronounced symptoms and/or asymptomatic AD during the post-Botox UDS.
The restoration of normal bladder function and eliminating episodes of AD are among the highest health priorities of individuals living with a high level of SCI.27 Therefore, it is not surprising that the reduction in AD and improvement in bladder function post-Botox resulted in an improved QoL. Based on the I-QoL, 53% were continent pre-Botox, whereas post-Botox this increased to 88%. Our study supports previous reports demonstrating improvements in QoL are attributed to clinical improvements in overall bladder function parameters and a reduction in urinary incontinence.7,20–22,28 A total of 13 participants (76%) underwent voluntary re-treatment. Of the remaining four participants, three declined re-treatment because of the side effects they experienced including culture-proven UTIs in two and headaches in the other. All three of these participants were positive responders to Botox, indicated by their reductions in bladder-related AD and improvements in bladder function and QoL. The remaining participant was a nonresponder to Botox, and declined retreatment because of no noticeable improvement in bladder-related AD, bladder function, or QoL. Although the long-term consequences of repeated AD events caused by bladder-related events is presently unknown, we recently demonstrated that a single bout of AD can be life threatening and even fatal.29 Therefore, in addition to improving QoL, the attenuation of daily bladder-related AD bouts with Botox also has the potential to improve longevity.
Presently, there are no safety data regarding the use of Botox for NDO in individuals with tetraplegia. Cruz and coworkers reported an incidence of UTIs in 52.6% of their paraplegic population following 200 U Botox.20 In the present study, culture-proven UTIs were reported by 2 of our 11 participants with tetraplegia (18%), and 1 of our 6 participants with paraplegia (17%). Headaches were reported by two participants with tetraplegia (18%). Although the trial by Cruz and coworkers did not report headache as a side effect, Herschorn and coworkers30 reported headaches in 21% of their sample with SCI and multiple sclerosis following Botox treatment for NDO. Therefore, our reported incidence of side effects is less than in previously published randomized control trials.
Limitations
From a clinical perspective, intravesical injections of Botox have an approximated average efficacy of 9 months.7 The duration of efficacy of Botox on reducing bladder-related AD frequency and severity beyond 1 month is not known. Other limitations included a small sample size, loss to follow-up, and lack of a control arm. Future research studies can confirm our preliminary findings through the conduction of a large-scale randomized controlled clinical design.
Conclusion
Episodes of AD are associated with potentially life-threatening events among individuals with SCI. Given that the bladder is responsible for up to 85% of AD events in individuals with high level SCI, strategies to mitigate this pathway are crucial. The present study demonstrated a reduction in the overall severity and frequency of bladder-related AD events 1 month following a Botox intervention for NDO. These findings may prove a viable treatment strategy for the successful alleviation of episodes of AD, and, possibly, amelioration of cardiovascular-related health risks associated with SCI. Furthermore, reductions in AD translated to an overall improvement in QoL. The observations from our study were likely attributed to the actions of Botox on temporary denervation of the detrusor muscle and consequential reduction in transmission of afferent sensory information from the urinary bladder to the spinal cord, known to trigger AD. Overall, findings from our study provide promising results, and highlight the necessity to further explore the clinical benefits of Botox for the treatment of AD in individuals living with SCI.
Acknowledgments
We thank the participants for their time and effort and acknowledge Teresa Lim and Colleen McLean for their assistance with the urodynamic assessments. This study was supported by a grant from the Rick Hansen Institute (201309). All Botox was supplied by in-kind donation from Allergan, Inc. Dr. Katharine D. Currie was supported by a fellowship from the Craig H. Neilsen Foundation (281863). All equipment used in this study was funded through a Canadian Foundation for Innovation grant to Dr. Andrei V. Krassioukov.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mathias C.J., andBannister R. (2002). Autonomic disturbances in spinal cord lesions. in: Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Bannister R., and Mathias C.J. (eds.). Oxford University Press: New York, pps. 839–881 [Google Scholar]
- 2.Kurnick N.B. (1956). Autonomic hyperreflexia and its control in patients with spinal cord lesions. Ann. Intern. Med. 44, 678–686 [DOI] [PubMed] [Google Scholar]
- 3.Guttmann L., and Whitteridge D. (1947). Effects of bladder distension on autonomic mechanisms after spinal cord injuries. Brain 70, 361–404 [DOI] [PubMed] [Google Scholar]
- 4.Abrams P., Agarwal M., Drake M., El-Masri W., Fulford S., Reid S., Singh G., and Tophill P. (2008). A proposed guideline for the urological management of patients with spinal cord injury. BJU Int. 101, 989–994 [DOI] [PubMed] [Google Scholar]
- 5.Fowler C.J. (2011). Systematic review of therapy for neurogenic detrusor overactivity. Can. Urol. Assoc. J. 5, S146–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangera A., Apostolidis A., Andersson K.E., Dasgupta P., Giannantoni A., Roehrborn C., Novara G., and Chapple C. (2014). An updated systematic review and statistical comparison of standardised mean outcomes for the use of botulinum toxin in the management of lower urinary tract disorders. Eur. Urol. 65, 981–990 [DOI] [PubMed] [Google Scholar]
- 7.Schurch B., Stohrer M., Kramer G., Schmid D.M., Gaul G., and Hauri D. (2000). Botulinum–A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J. Urol. 164, 692–697 [DOI] [PubMed] [Google Scholar]
- 8.Chen S.F., and Kuo H.C. (2012). Improvement in autonomic dysreflexia after detrusor onabotulinumtoxinA injections in patients with chronic spinal cord injuries. Tzu Chi Med. J. 24, 201–204 [Google Scholar]
- 9.Elkelini M.S., Bagli D.J., Fehlings M., and Hassouna M. (2012). Effects of intravesical onabotulinumtoxinA on bladder dysfunction and autonomic dysreflexia after spinal cord injury: role of nerve growth factor. BJU Int. 109, 402–407 [DOI] [PubMed] [Google Scholar]
- 10.Kirshblum S.C., Burns S.P., Biering–Sorensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A., Mulcahey M.J., Schmidt–Read M., and Waring W. (2011). International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faaborg P.M., Christensen P., Krassioukov A., Laurberg S., Frandsen E., and Krogh K. (2014). Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord 52, 494–498 [DOI] [PubMed] [Google Scholar]
- 12.Hubli M., and Krassioukov A.V. (2014). Ambulatory blood pressure monitoring in spinal cord injury: clinical practicability. J. Neurotrauma 31, 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaivas J.G., Awad S.A., Bissada N., Khanna O.P., Krane R.J., Wein A.J., and Yalla S. (1982). Urodynamic procedures: Recommendations of the Urodynamic Society. I. Procedures that should be available for routine urologic practice. Neurourol. Urodyn. 1, 51–55 [Google Scholar]
- 14.Biering–Sorensen F., Craggs M., Kennelly M., Schick E., and Wyndaele J.J. (2008). International lower urinary tract function basic spinal cord injury data set. Spinal Cord 46, 325–330 [DOI] [PubMed] [Google Scholar]
- 15.Krassioukov A., Warburton D.E., Teasell R., Eng J.J., and Spinal Cord Injury Rehabilitation Evidence Research Team (2009). A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch. Phys. Med. Rehabil. 90, 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consortium for Spinal Cord Medicine (2002). Acute management of autonomic dysreflexia: individuals with spinal cord injury presenting to health-care facilities. J. Spinal Cord Med. 25 Suppl 1, S67–88 [PubMed] [Google Scholar]
- 17.Schurch B., Denys P., Kozma C.M., Reese P.R., Slaton T., and Barron R. (2007). Reliability and validity of the Incontinence Quality of Life questionnaire in patients with neurogenic urinary incontinence. Arch. Phys. Med. Rehabil. 88, 646–652 [DOI] [PubMed] [Google Scholar]
- 18.Ekland M.B., Krassioukov A.V., McBride K.E., and Elliott S.L. (2008). Incidence of autonomic dysreflexia and silent autonomic dysreflexia in men with spinal cord injury undergoing sperm retrieval: implications for clinical practice. J. Spinal Cord Med. 31, 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curt A., Nitsche B., Rodic B., Schurch B., and Dietz V. (1997). Assessment of autonomic dysreflexia in patients with spinal cord injury. J. Neurol. Neurosurg. Psychiatry 62, 473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz F., Herschorn S., Aliotta P., Brin M., Thompson C., Lam W., Daniell G., Heesakkers J., and Haag–Molkenteller C. (2011). Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur. Urol. 60, 742–750 [DOI] [PubMed] [Google Scholar]
- 21.Chancellor M.B., Patel V., Leng W.W., Shenot P.J., Lam W., Globe D.R., Loeb A.L., and Chapple C.R. (2013). OnabotulinumtoxinA improves quality of life in patients with neurogenic detrusor overactivity. Neurology 81, 841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schurch B., de Seze M., Denys P., Chartier–Kastler E., Haab F., Everaert K., Plante P., Perrouin–Verbe B., Kumar C., Fraczek S., Brin M.F., and Botox Detrusor Hyperreflexia Study (2005). Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J. Urol. 174, 196–200 [DOI] [PubMed] [Google Scholar]
- 23.Apostolidis A., Popat R., Yiangou Y., Cockayne D., Ford A.P., Davis J.B., Dasgupta P., Fowler C.J., and Anand P. (2005). Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J. Urol. 174, 977–982 [DOI] [PubMed] [Google Scholar]
- 24.Durham P.L., and Cady R. (2011). Insights into the mechanism of onabotulinumtoxinA in chronic migraine. Headache 51, 1573–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Seze M., Wiart L., de Seze M.P., Soyeur L., Dosque J.P., Blajezewski S., Moore N., Brochet B., Mazaux J.M., Barat M., and Joseph P.A. (2004). Intravesical capsaicin versus resiniferatoxin for the treatment of detrusor hyperreflexia in spinal cord injured patients: a double-blind, randomized, controlled study. J. Urol. 171, 251–255 [DOI] [PubMed] [Google Scholar]
- 26.Rabchevsky A.G. (2006). Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog. Brain Res. 152, 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson K.D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
- 28.Schurch B., Denys P., Kozma C.M., Reese P.R., Slaton T., and Barron R.L. (2007). Botulinum toxin A improves the quality of life of patients with neurogenic urinary incontinence. Eur. Urol. 52, 850–858 [DOI] [PubMed] [Google Scholar]
- 29.Wan D., and Krassioukov A.V. (2014). Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J. Spinal Cord Med. 37, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herschorn S., Gajewski J., Ethans K., Corcos J., Carlson K., Bailly G., Bard R., Valiquette L., Baverstock R., Carr L., and Radomski S. (2011). Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: a randomized, double-blind trial. J. Urol. 185, 2229–2235 [DOI] [PubMed] [Google Scholar]