Significance
Leptin is an adipocytokine whose activity has been associated with the development and maintenance of proinflammatory immune responses. Here we show that genetic deficiency of leptin protected mice from systemic lupus erythematosus (SLE) and reduced production of autoantibodies and renal disease. In a spontaneous animal model of SLE, the administration of leptin promoted an acceleration of the disease, whereas leptin blockade protected from autoimmunity. We suggest that these effects of leptin on SLE could possibly be exploited therapeutically in the disease.
Keywords: systemic lupus erythematosus, leptin, autoimmunity, immune regulation, animal models
Abstract
Leptin is an adipocytokine that plays a key role in the modulation of immune responses and the development and maintenance of inflammation. Circulating levels of leptin are elevated in systemic lupus erythematosus (SLE) patients, but it is not clear whether this association can reflect a direct influence of leptin on the propathogenic events that lead to SLE. To investigate this possibility, we compared the extent of susceptibility to SLE and lupus manifestations between leptin-deficient (ob/ob) and H2-matched leptin-sufficient (wild-type, WT) mice that had been treated with the lupus-inducing agent pristane. Leptin deficiency protected ob/ob mice from the development of autoantibodies and renal disease and increased the frequency of immunoregulatory T cells (Tregs) compared with leptin-sufficient WT mice. The role of leptin in the development of SLE was confirmed in the New Zealand Black (NZB) × New Zealand White (NZW)F1 (NZB/W) mouse model of spontaneous SLE, where elevated leptin levels correlated with disease manifestations and the administration of leptin accelerated development of autoantibodies and renal disease. Conversely, leptin antagonism delayed disease progression and increased survival of severely nephritic NZB/W mice. At the cellular level, leptin promoted effector T-cell responses and facilitated the presentation of self-antigens to T cells, whereas it inhibited the activity of regulatory CD4 T cells. The understanding of the role of leptin in modulating autoimmune responses in SLE can open possibilities of leptin-targeted therapeutic intervention in the disease.
Although the causes of systemic lupus erythematosus (SLE) remain unclear, there is consensus that genetics and the environment, together with the activity of certain hormones, can play pivotal roles in the pathogenesis of the disease (1). Among the hormones that have been linked to the pathogenesis of SLE, certain sex hormones such as estradiol and prolactin have been shown to contribute significantly to the gender bias that characterizes the disease (2). In addition to these hormones, less characterized gender-related factors are believed to also take an important part in the female preponderance that is typically observed in SLE (3).
Leptin is a sexually dimorphic hormone/cytokine with strong proinflammatory activities that have been linked to autoimmune phenotypes (4). Although we and others have shown that leptin levels are elevated in SLE patients (5, 6), it is not known whether the abnormal levels of this adipocytokine in SLE could be secondary to the chronic inflammatory state of the disease and/or directly contribute to its development.
Here we show that the development of SLE manifestations, including autoantibody production and renal disease, required leptin both in experimentally induced SLE and in a spontaneous mouse model of the disease. We also report that leptin blockade protected lupus mice from disease progression and associated with the induction of immunoregulatory responses. Together, these results underscore a promoting role of leptin in SLE and hint at the possibility of leptin antagonism in the management of the aberrant inflammatory response in the disease.
Results
Leptin Promotes Pristane-Induced Lupus.
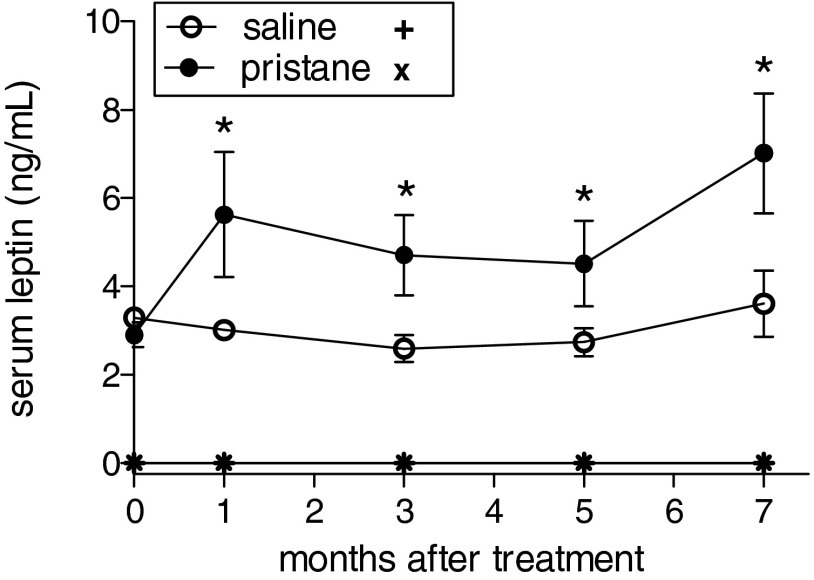
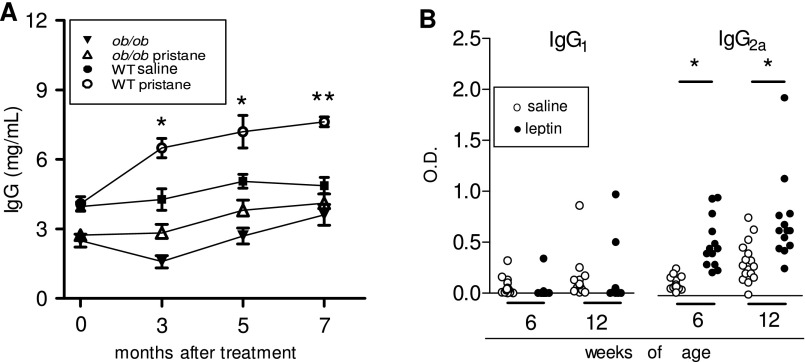
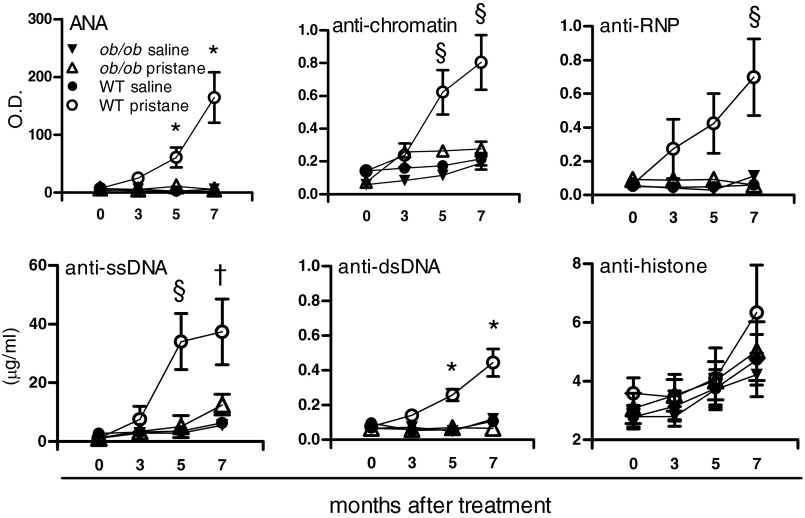
Pristane is a hydrocarbon oil that induces lupus-like disease in multiple strains of mice (7). We found increased levels of circulating leptin in wild-type (WT) C57BL/6 mice after treatment with pristane (Fig. S1), implying a promoting effect of pristane on the production of endogenous leptin. The measurement of autoantibodies (autoAb) showed an increase in total IgG in pristane-treated WT mice compared with mock-treated WT controls (Fig. S2A), similarly to what has been reported by others (8). Interestingly, the comparison between pristane-treated WT mice and syngeneic leptin-deficient ob/ob mice also treated with pristane revealed an increase in IgG levels in WT mice but no change in leptin-deficient ob/ob mice (Fig. S2A). Consistent with previous reports (7–9), WT mice started to develop multiple autoAb by 3 mo after pristane treatment, including antinuclear antibodies (ANA), anti-chromatin, anti-ribonucleoprotein (RNP), anti-ssDNA, and anti-dsDNA (all raising gradually to become significantly higher than in controls by 5 mo after treatment) (Fig. 1). Anti-histone autoAb only increased modestly and not significantly. Notably, leptin-deficient ob/ob mice did not develop any autoAb after pristane treatment (Fig. 1).
Fig. S1.
Plasma levels of leptin in leptin-deficient ob/ob mice (+, ×) and in WT mice (circles) after treatment with pristane. Mean ± SEM of six mice per group; *P < 0.02.
Fig. S2.
Dependence of (auto)Ab responses on leptin. (A) Total IgG in ob/ob mice (triangles) and in WT mice (circles) that had been treated or not with pristane (open or closed symbols, respectively). Mean ± SEM of six mice per group. In the comparison between pristane-treated and matched mock control animals, *P < 0.02; **P < 0.001. (B) Leptin facilitates Th1 responses in NZB/W mice. Anti-dsDNA IgG1 and IgG2a in NZB/W mice after treatment with leptin (closed circles) or saline (open circles) (see Materials and Methods for details). *P < 0.001.
Fig. 1.
Leptin deficiency protects from the development of lupus autoantibodies. Plasma from WT and leptin-deficient ob/ob mice was tested by ELISA at 3, 5, and 7 mo after pristane treatment for levels of different autoAb. Except for anti-histone autoAb, differences in autoAb levels were significantly higher in WT mice compared with ob/ob mice. Mean ± SEM from 8 to 12 animals per group. *P < 0.01; §P < 0.02; †P < 0.03.
Leptin Accelerates the Development of Renal Disease in Pristane-Induced Lupus.
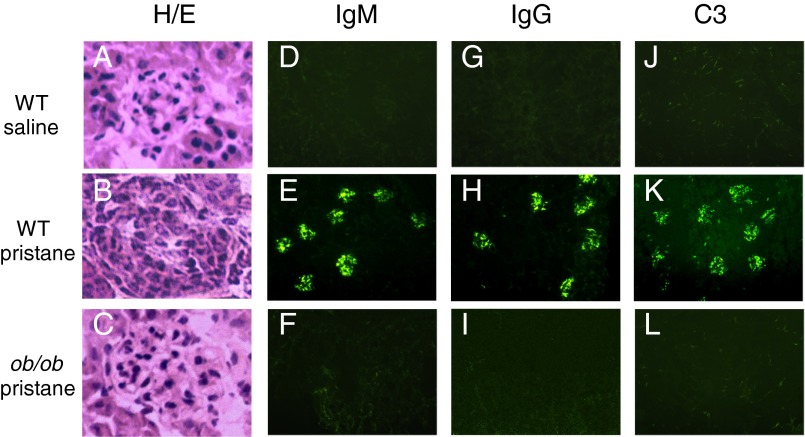
The effects of leptin deficiency on renal pathology were also examined. As shown in Fig. 2, pristane favored the development of immune complex-mediated proliferative glomerulonephritis in WT mice (Fig. 2 B, E, and H) but not in ob/ob mice (Fig. 2 C, F, and I). Whereas untreated WT mice had no IgM or IgG immune deposits (Fig. 2 D and G), as expected, pristane-treated WT mice had a significant glomerular deposition of IgM (Fig. 2E), IgG (Fig. 2H), and C3 (Fig. 2K) that was not seen in ob/ob mice also treated with pristane (Fig. 2 F, I, and L). Thus, leptin deficiency protected from pristane-induced lupus nephritis.
Fig. 2.
Leptin deficiency protects from pristane-induced lupus renal disease. Twenty-eight weeks after treatment with pristane or saline, kidneys from WT and ob/ob mice were explanted, and sections were stained with hematoxylin/eosin (H/E) (A–C) or by indirect immunofluorescence for anti-IgM (D–F), anti-IgG (G–I), or anti-complement factor C3 (J–L). Only pristane-treated WT mice developed diffuse proliferative glomerulonephritis (B), Ig (E and H), and complement deposition (K). Original magnification, 10×. Data were obtained from five to six fields from six mice per group.
Effects of Leptin on Immune Cells.
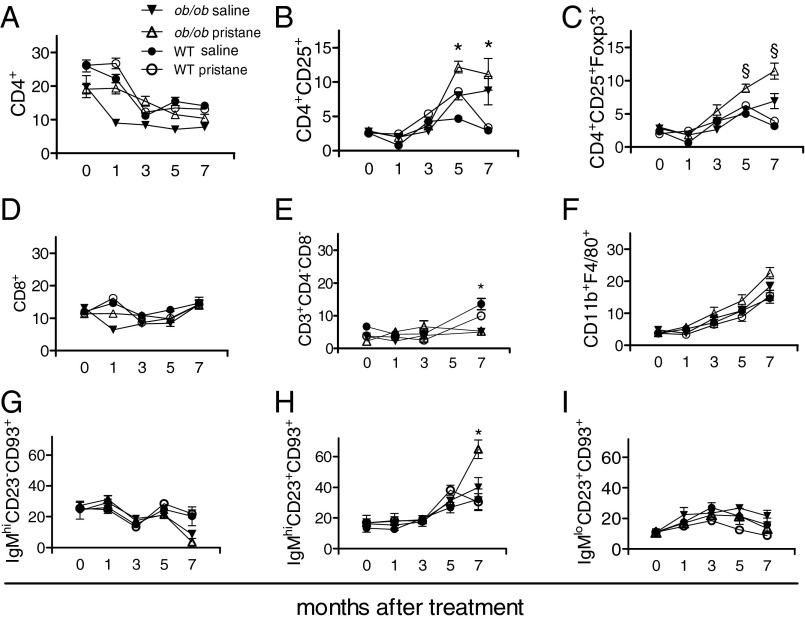
Dysregulated immune responses play a key role in the pathogenesis of SLE. To evaluate the effects of leptin on the immune response in SLE, we compared immune cell frequencies between leptin-sufficient WT mice and leptin-deficient ob/ob mice that had been treated with pristane. The frequency of CD4+ and CD8+ T cells did not change, except for a short-lived increase of the former around 1 mo after pristane treatment in the ob/ob mice (yet not maintained over time) (Fig. 3 A and D). Within the CD4 T-cell compartment, CD4+CD25+ T cells expanded in ob/ob mice by 5 mo after pristane treatment (Fig. 3B). These cells expressed Foxp3 (Fig. 3C) and suppressed the proliferation of syngeneic CD4+CD25− T cells (72.1 ± 14.6% at a 1:1 ratio of Tregs to CD4+CD25− T cells and 56.7 ± 8.9% at a 1:3 ratio, respectively; P < 0.03 for both). Also within the CD4+ T-cell compartment, the frequency of double-negative (DN) CD4−CD8− T cells [which are known to promote lupus (10−11)] was reduced in leptin-deficient ob/ob mice compared with WT mice (Fig. 3E). As for macrophages, pristane expectedly increased the frequency of CD11b+ cells but with little difference between WT and ob/ob mice in terms of activated phenotypes (Fig. 3F). For B cells, no significant differences were seen after pristane treatment between WT and ob/ob mice for transitional (B220+CD93+) and mature (B220+CD93−CD23+) B cells including T1 (IgMhi CD23−CD93+) and T3 (IgMloCD23+CD93+) B-cell subsets (12) (Fig. 3 G and I). There were also no differences between follicular (B220+CD21intCD23+) and marginal zone (B220+CD21hiCD23−) B cells (P not significant). The only significant difference among B-cell subsets related to T2 (IgMhiCD23+CD93+) cells, which increased in ob/ob mice around 7 mo after treatment with pristane more than in the other groups —where an increase over time was also observed (Fig. 3H).
Fig. 3.
Frequency of immune cell subsets in pristane-treated (open symbols) leptin-deficient ob/ob mice (triangles) and syngeneic leptin-sufficient wild-type (WT) mice (circles) compared with mock-treated controls (closed symbols). Flow cytometry for peripheral CD4 Tregs (A–C), CD8 (D), double-negative (DN) CD4−CD8− T cells (E), activated macrophages (F), and B cells (B220+) of the transitional (T)1 (G), T2 (H), and T3 (I) type. Mean ± SEM from 8 to 12 mice per group. *P < 0.01; §P < 0.005 in the comparison between pristane-treated ob/ob mice and pristane-treated WT mice.
Leptin Accelerates Spontaneous SLE in NZB/W Mice.
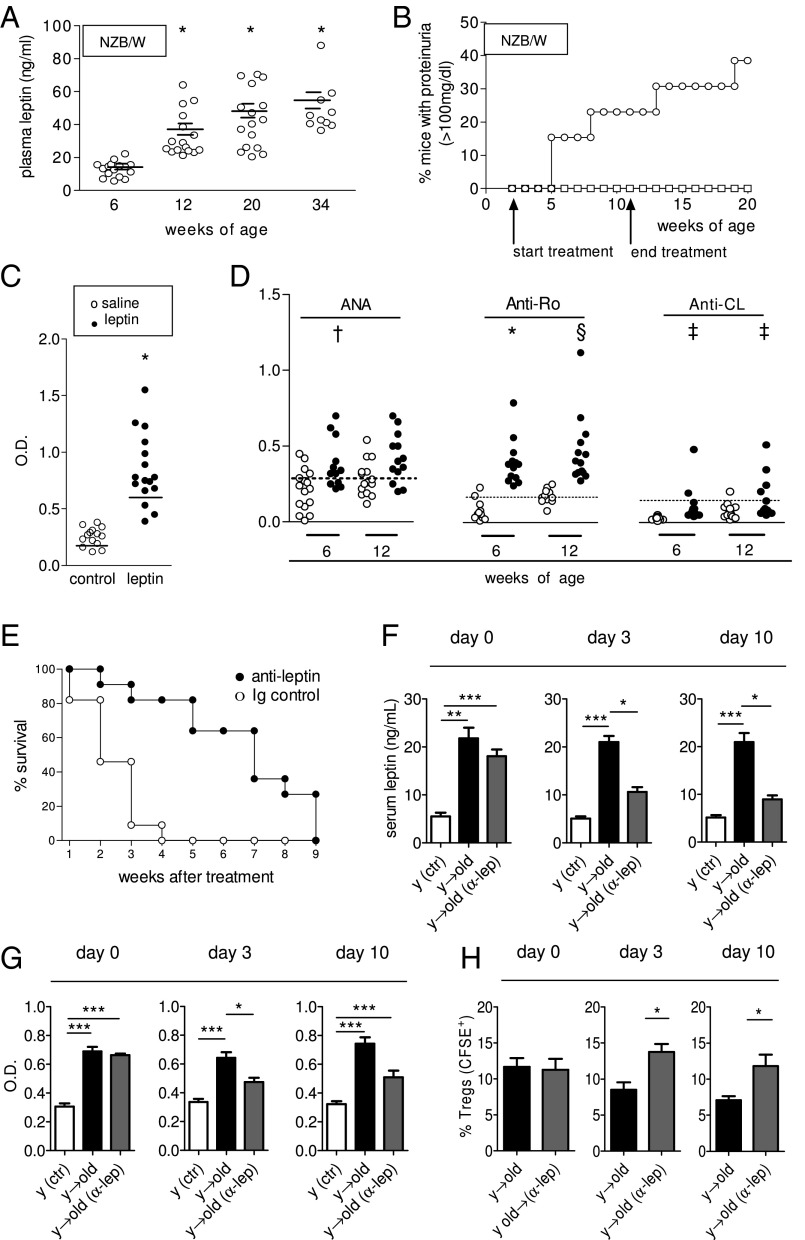
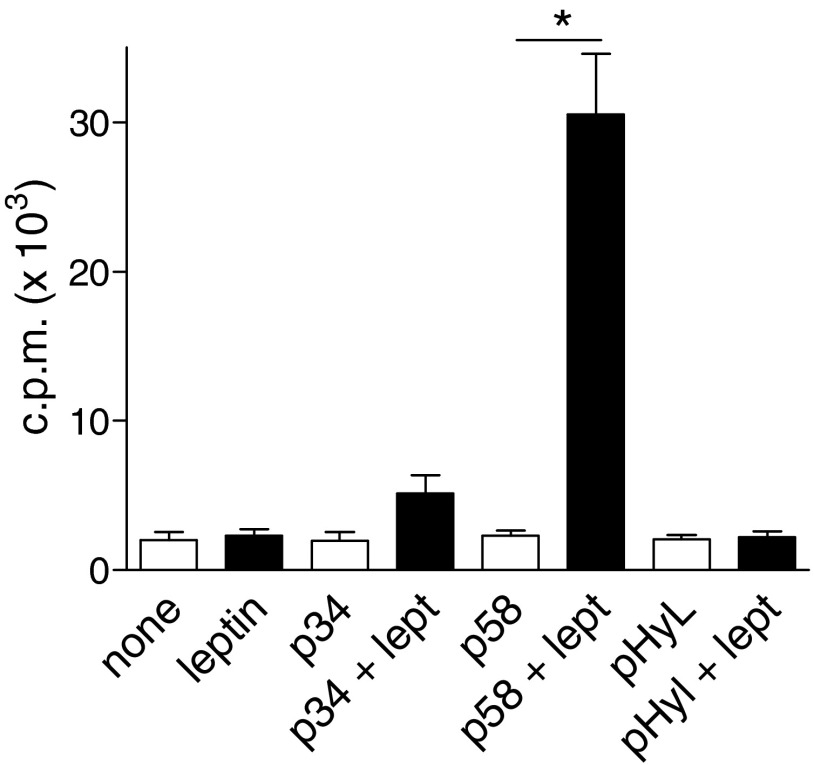
Because leptin promoted pristane-induced SLE, we wondered whether leptin had similar effects in a spontaneous model of the disease. In untreated lupus-prone New Zealand Black (NZB) × New Zealand White (NZW)F1 (NZB/W) mice, circulating leptin levels increased with age (Fig. 4A). Administration of leptin to young, premorbid NZB/W mice accelerated the development of lupus renal disease, as indicated by elevated proteinuria as early as 3 wk after treatment (Fig. 4B). The observed accelerated development of anti-dsDNA autoAb (Fig. 4C) was evocative of an underlying predominance of Th1 responses (increased IgG2a vs. IgG1) (Fig. S2B). Other autoAb, including ANA, anti-Ro, and anti-cardiolipin Ab, were also significantly increased in leptin-treated mice compared with controls (Fig. 4D). At the cellular level, leptin promoted T-cell autoreactivity in vitro to anti-DNA Ab-derived peptide [p58 is an anti–DNA-derived peptide that induces T-cell help for production of anti-DNA Ab in NZB/W mice (13, 14)] (Fig. S3).
Fig. 4.
Leptin promotes spontaneous SLE in NZB/W mice, and leptin blockade by anti-leptin Ab delays SLE progression and prolongs survival of NZB/W lupus mice. (A) Increased plasma leptin in aging female untreated lupus-prone NZB/W mice (n = 16) followed longitudinally from 6 to 34 wk of age. (B) Accelerated development of proteinuria (>100 mg/dL) in NZB/W mice after treatment with leptin (open circles, n = 14) vs. saline-treated controls (n = 16, open squares) using the protocol described in Materials and Methods. (C) ELISA for anti-dsDNA autoAb at 12 wk of age in mice treated as in B, with leptin (closed circles) or saline (open circles). (D) AutoAb in NZB/W mice treated as in B, with leptin (closed circles) and saline-treated controls (open circles) at different ages. The dashed line indicates the threshold of positivity. *P < 0.0001; §P < 0.003; †P < 0.01; ‡P < 0.05. (E) Severely nephritic NZB/W mice (proteinuria ≥300 mg/dL) were randomly divided into two groups (n = 11 each) and given either a single i.p. injection of 150 μg anti-leptin Ab (closed circles) or isotype-matched control Ab (open circles). Survival was monitored for the following 9 wk. P < 0.007. (F–H) Tregs expansion that occurs after leptin inhibition in vivo (30) associates with suppression of anti-dsDNA autoAb in diseased NZB/W lupus mice. Ex vivo-labeled (CFSE) Tregs were adoptively transferred into old NZB/W mice that had received a single i.p. injection of 150 μg anti-leptin Ab/mouse at 24 and 12 h before Tregs transfer. Arrows indicate the direction of transfer of mouse donor cells into host. Untreated young mice (y) were included as controls. Comparisons were made between old diseased NZB/W lupus mice receiving Tregs from young premorbid mice following leptin-blockade or mock treatment. Data are shown before (0) and at day 3 and 10 after Tregs transfer. Plasma leptin concentration (measured by ELISA) (F) and anti-DNA autoAb (G) before and after transfer of Tregs and expansion of Tregs (CFSE-gated) in vivo after leptin blockade (H). Mean ± SEM of cumulative data from two independent experiments (n = 8 per group). *P < 0.05; **P < 0.01; ***P < 0.005.
Fig. S3.
Leptin favors T-cell autoreactivity in NZB/W mice. In vitro T-cell proliferation to anti-DNA Ab-derived peptides p34 and p58 or to irrelevant control peptide pHyL, in the presence (closed bars) or not (open bars) of 100 ng/mL leptin. Purified CD3+ T cells from 30-wk-old NZB/W mice were cultured for 72 h with irradiated splenocytes in the conditions indicated on the x axis, and cell proliferation was assessed by labeling cultures with [3H]thymidine during the last 18 h. Mean ± SEM of two independent experiments in triplicate; *P < 0.01.
Leptin Inhibition Protects NZB/W Lupus Mice from Severe Nephritis and Induces Immunoregulatory Responses.
Because leptin accelerated the development of SLE both in experimentally induced SLE and in spontaneous SLE, we tested whether leptin inhibition could provide beneficial effects on ongoing lupus disease. NZB/W mice with severe lupus nephritis (proteinuria ≥300 mg/dL) were given a single injection of anti-leptin neutralizing Ab or matched control Ab and then monitored for survival. Marked protective effects were observed after leptin antagonism, with a significant extension of the lifespan in leptin-inhibited mice (Fig. 4E). To investigate these findings, we evaluated whether leptin inhibition affected Tregs frequency in lupus mice (because we had found that leptin constrained Tregs expansion, Fig. 3 B and C). Nephritic NZB/W lupus mice were treated with anti-leptin Ab and then adoptively transferred with purified syngeneic Tregs from premorbid mice. The decrease in leptin levels in leptin-neutralized mice (Fig. 4F) was associated with reduced levels of anti-dsDNA autoAb (Fig. 4G) and an increased frequency of Tregs in the transferred mice (Fig. 4H).
Leptin Inhibits the Conversion of CD4+CD25− T Cells into Tregs.
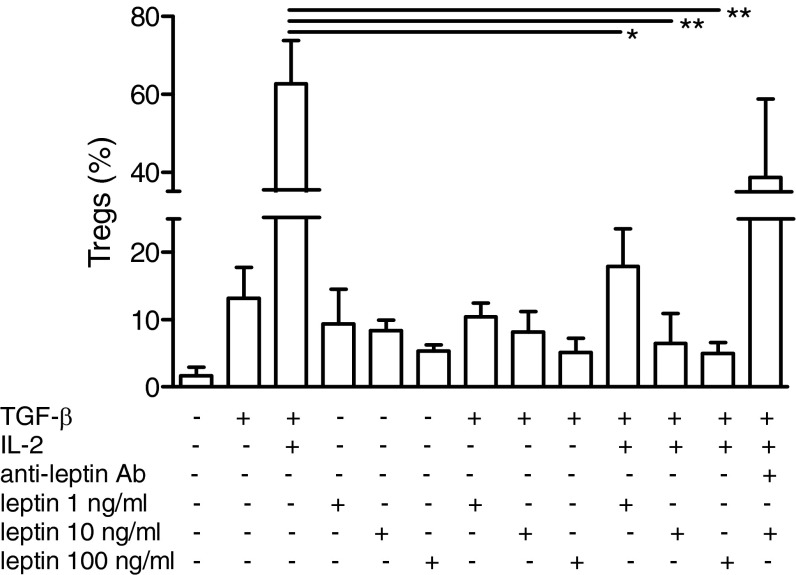
Tregs play a protective role in SLE (15), both when they are naturally derived or when induced in the periphery (16). With regard to the latter possibility, it has been shown that TGF-β and IL-2 can promote conversion of CD4+CD25− T cells into functional Tregs (17). After confirming an induction of Tregs by these cytokines (Fig. 5), as reported (17), we found that leptin inhibited the conversion of CD4+CD25− T cells into Tregs in a dose-dependent fashion, and this was reversed by leptin blockade (Fig. 5).
Fig. 5.
Leptin inhibits the conversion of CD4+CD25− T cells into Tregs. Magnetic-bead sorted CD4+CD25− T cells were incubated with 2 μg/mL anti-CD3/CD28 Ab for 3 d. TGF-β (2 ng/mL) and IL-2 (10 U/mL) were added to cultures, without or with leptin (0, 1, 10, and 100 ng/mL), and cells were analyzed by flow cytometry 3 d later. Specificity was indicated by reversal of leptin effects on the inhibition of conversion in the presence of 20 μg/mL anti-leptin receptor Ab. The graph shows percentages of cells coexpressing CD25 and Foxp3 among CD4-gated cells. Mean ± SEM of one of four independent experiments with similar results. *P < 0.05; **P < 0.005.
Discussion
Extensive clinical data and experimental results have demonstrated the involvement of different adipocytokines in the pathogenesis of autoimmune diseases (18). Here we report that leptin promoted experimentally induced and spontaneous SLE. The results also showed that leptin antagonism had beneficial effects in delaying the progression of severe SLE and that leptin constrained Tregs, whereas it promoted autoreactive immune responses. Together, these findings suggest a contribution of leptin to the gender bias that is observed in SLE [that had been previously associated with sexually dimorphic hormones such as estrogens and prolactin (2)]. This is of interest because circulating levels of leptin are typically 5- to 10-fold higher in females than in males (4).
Interestingly, the results reported in the current study showed that leptin influenced distinctively the development of different types of autoAb. Specifically, anti-histone autoAb developed modestly at late stages in leptin-sufficient mice but were absent in leptin-deficient mice (Fig. 1). Because anti-histone Ab are relatively insensitive to SLE disease activity (19), this could suggest that only the production of certain autoAb could be leptin-dependent, whereas the production of other autoAb might rely on leptin-independent pathways [in line with the concept that different autoAb are regulated in different ways in SLE (20)]. Specifically, anti-dsDNA autoAb—which are produced mainly during periods of disease activity and that associate with pathogenic disease manifestations, including lupus nephritis (21)—would be leptin-dependent because they did not develop in ob/ob mice (Fig. 1). This leptin dependence of anti-dsDNA and other autoAb (ANA, anti-chromatin, anti-RNP, anti-ssDNA) envisions possibilities of inhibition of certain autoAb in the disease through leptin manipulation, although this needs further investigation. Incidentally, the data also suggested that leptin’s functional effects on B cells might be more prominent than the quantitative effects on the same cells, given the finding of modest changes induced by leptin on the overall B cells’ frequencies (Fig. 3).
Another finding from this study was that leptin levels increased before the development of autoAb and clinical disease manifestations in mice that spontaneously developed SLE (Fig. 4). This may suggest that leptin could take part in the early proinflammatory events in SLE, e.g., by favoring an expansion of propathogenic cells including DN T cells (Fig. 3E) while inhibiting Tregs (Fig. 3 B and C). The results also showed an ability of leptin to inhibit conversion of CD4+CD25− T cells into Tregs (Fig. 5), implying that leptin could use multiple modalities to inhibit Tregs. This might be relevant for SLE, where an inhibition of Tregs conversion could contribute to the reduced Tregs function that characterizes the disease (22). In other words, by inhibiting Tregs conversion, leptin could help to reduce availability of these key suppressor cells in the disease.
Leptin could also influence the development and/or selection processes involved in the formation of a mature B-cell compartment (23), as suggested by the finding that leptin-deficient ob/ob mice displayed a larger increase in T2 B cells late in the disease (Fig. 3H)—an aspect that needs further investigation. Additionally, leptin favored autoimmune responses to self-antigen (Fig. S3). In this context, autoreactivity to the anti-DNA peptide p58 and not to p34 (that is from the same anti-DNA A6.1 Ab) could be explained with the immunodominance of the former, in line with past studies that found T-cell proliferative responses to p58 and not to p34 after immunization of NZB/W with the whole A6.1 anti-DNA Ab (due to the fact that p34 is a cryptic epitope that is only targeted at late disease stages) (13, 14).
Together, the data from the present study suggest a propathogenetic role of leptin that is not in conflict with our recent investigations on associations between selected leptin gene polymorphisms and human SLE (24). Weak associations with certain SNPs did not remain significant after correction for multiple testing, although associations with selected ethnicities and/or subgroups of SLE patients were identified (24). Those findings bring to mind the lack of genetic associations observed for SLE and gene polymorphisms of BLyS (25, 26), a proinflammatory cytokine that, like leptin, is elevated in SLE, promotes SLE in mice and in humans (27), and is targeted for therapy in certain SLE patients (28).
To summarize, leptin could promote unbalanced immune homeostasis in SLE by modulating both effector and regulatory immune responses toward a proinflammatory phenotype. Because leptin antagonism ameliorated disease and favored immune regulation, the possibility that leptin-based intervention in SLE might reduce proinflammatory responses in the disease should be investigated.
Materials and Methods
Mice.
Wild-type (WT) C57BL/6J (B6) mice, leptin-deficient (B6) ob/ob mice, and NZB/W lupus mice were purchased from The Jackson Laboratory and housed on a 12-h light/dark cycle, with food and water ad libitum. At 8 wk of age, mice received one i.p. injection of 500 μL pristane (Sigma-Aldrich) or saline (8). Blood was collected before pristane injection and after 1, 3, 5, and 7 mo. Plasma was tested for Ab, and peripheral blood mononuclear cells (PBMC) were used for flow cytometry. Urine samples were tested monthly for proteinuria using Albustix strips (Bayer). All mice were euthanized 7 mo after treatment and kidneys explanted for histopathology. Bioavailability and distribution of pristane was similar between WT and ob/ob mice, as indicated by the following experiments. Mice were given 3H-labeled radioactive pristane (Perkin–Elmer). Radioactivity monitored monthly in peripheral blood by liquid scintillation counting indicated comparable cpm in WT and ob/ob mice for up to 3 mo after [3H]pristane injection (P not significant), as well as comparable radioactive content per gram of tissue in liver, spleen, and kidneys (P not significant). Radioactivity did not influence results because mice given 3H-labeled pristane developed anti-dsDNA Ab at comparable levels to WT mice injected with nonradioactive pristane (P not significant). All studies were approved by the Institutional Animal Research Committees of the University of California, Los Angeles and Università degli Studi di Napoli Federico II.
In Vivo Treatment.
Starting at 2 wk of age, NZB/W mice were injected i.p. with recombinant mouse leptin (R&D Systems) dissolved in saline. Controls received equal amounts of saline only. Injections of 2 μg leptin/gram of body weight (or equal volume of saline) were repeated at 5-d intervals for the first four injections and then every 48 h until mice were 11 wk old. Sex-, age-, and strain-matched control mice received equal volume of saline i.p. following the same protocol. Mice were monitored for development of proteinuria on consecutive blind measurements with Albustix strips (Miles Inc.). For anti-leptin Ab treatment, NZB/W mice with proteinuria ≥300 mg/dL (on two consecutive measurements at least 1 d apart) were divided into two groups and given either a single i.p. injection of 150 μg anti-leptin mAb or control Ig (R&D Systems). For adoptive transfers, NZB/W Tregs from 8- to 12-wk-old NZB/W mice were sorted using the Mouse CD4+CD25+ Regulatory T Cell Isolation kit and an AutoMACS separator (Miltenyi Biotec). Cells were labeled with CFSE (eBioscience) before i.v. injection of 1 × 107 cells per transfer into >26-wk-old syngeneic mice that had proteinuria >100 mg/dL.
ELISA.
Leptin concentration was determined using commercial ELISA kits (R&D Systems). The minimum detection limit of the assay was 0.2 ng/mL; intra- and interassay coefficients of variation were <5%. Samples were tested in duplicate, and optical density (A450) was read using an MRX ELISA reader (Dynatech Laboratories). In some experiments, measurements were confirmed using two additional ELISA kits (RayBiotech and Alpco Diagnostic). Total IgG, anti-RNP, ANA, and anti-ssDNA Ab were measured using Alpha Diagnostic ELISA kits, according to the manufacturer’s instructions. Anti-dsDNA Ab titers were also quantified by ELISA, as previously described (22). Anti-chromatin Ab were detected in sera that had been diluted 1:500, using chicken chromatin (kind gift from Minoru Satoh, University of Florida, Gainesville, FL) as plate-coating antigen and using HRP-conjugated anti-mouse IgG (Promega) for detection. Plasma from nephritic NZB/W mice served as a positive control. All experiments were performed in blinded fashion.
Histopathology.
Kidney sections (4 μm) were stained with hematoxylin and eosin (H/E) according to standard procedures and evaluated in a blinded fashion. For indirect immunofluorescence (IIF) analyses, sections were fixed in cold acetone for 5 min, washed, and blocked with 3% (vol/vol) BSA for 1 h before staining with one of the following FITC-conjugated Ab: rabbit anti-mouse IgG, rabbit anti-mouse IgM, or goat anti-mouse C3 (all from Fisher Scientific). Six mice per group were scored on six to eight fields.
Flow Cytometry.
PBMC were stained for surface markers or control isotype after cell blockade of Fc-γ receptors (eBioscience). For surface staining, the following fluorochrome-labeled mAb from eBioscience were used: anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-B220, anti-CD23, anti-CD93, anti-IgM, anti-CD11b, and anti-F4/80. For intracellular staining with anti-Foxp3 (eBioscience), cells were fixed and permeabilized. Flow cytometry was acquired on a FACSCalibur (BD Biosciences) using Cell-Quest (BD Biosciences) software, and data were analyzed using Flowjo software (Tree Star).
T-Cell Proliferation.
Purified CD3+ T cells (5 × 105) (negatively selected using Miltenyi Biotec magnetic beads) were cultured with irradiated splenocytes and 20 μg anti–DNA-derived or irrelevant control peptide (13) for 3 d in leptin/serum-free HL-1 medium in the presence or absence of 10 ng/mL leptin. During the last 18 h of culture, [3H]thymidine was added and cpm counted following cell harvesting. Experiments were run on triplicates and repeated three times.
Peptides.
The synthetic peptides p34 and p58 were purchased from Chiron Mimotopes. Both derive from the VH region of the pathogenic anti-DNA Ab A6.1 (isolated from NZB/W mice) and induce spontaneous T-cell help for the production of anti-DNA Ab in unimmunized NZB/W mice (13, 14). pHyL is a control peptide that derives from a mAb against hen egg lysozyme (29). Similarly to p34 and p58, it binds to the NZB/W H-2 I-Ed but does not elicit T-cell help in unimmunized NZB/W mice (13, 14).
Suppression Assay.
After magnetic bead labeling with the CD25 MicroBead Kit (Miltenyi Biotec) and negative selection by AutoMACS (Miltenyi Biotec), CD4+CD25− T cells were incubated alone or with CD4+CD25+ T cells at ratios of 1:1 and 1:3 in round-bottom 96-well plates and stimulated with mouse anti-CD3/CD28 beads (Life Technologies) at a ratio of 0.1–0.5 beads per cell. After 3 d, cultures were analyzed for incorporation of [3H]thymidine and suppression of target cell proliferation determined by comparing percentages of inhibition of cell proliferation in cocultures of CD4+CD25+ T cells plus CD4+CD25− cells vs. CD4+CD25− cells alone.
In Vitro Conversion Assay.
Magnetic bead-sorted CD4+CD25− T cells were plated onto 96-well plates that had been precoated with 2 μg/mL anti-mouse CD3 and anti-CD28 Ab (eBioscience) for 3 d. Recombinant mouse IL-2 (10 U/mL), TGF-β (2 ng/mL), and leptin (1, 10, and 100 ng/mL) (all from R&D Systems) were added to cultures in leptin/serum-free HL-1 medium at 37 °C/5% CO2. After 3 d, cells were harvested for flow cytometry or in vitro suppression assays.
Statistics.
Statistical comparisons between two experimental groups were made with paired Student’s t test or Mann–Whitney U test using PRISM 4 software (GraphPad). Nonparametric testing between more than two groups was performed by one-way ANOVA. Significance was considered with P < 0.05.
Acknowledgments
This study was supported by the NIH Grants AR53239 and AI109677 (to A.L.C.). G.M. is supported by grants from the European Research Council (ERC) “menTORingTregs” 310496, the Italian Space Agency (ASI) 2014-033-R.O., and the European Foundation for the Study of Diabetes/Juvenile Diabetes Research Foundation (EFSD/JDRF)/Lilly Programme 2015.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607101113/-/DCSupplemental.
References
- 1.Kyttaris VC, Krishnan S, Tsokos GC. Systems biology in systemic lupus erythematosus: Integrating genes, biology and immune function. Autoimmunity. 2006;39(8):705–709. doi: 10.1080/08916930601061363. [DOI] [PubMed] [Google Scholar]
- 2.Jeganathan V, Peeva E, Diamond B. Hormonal milieu at time of B cell activation controls duration of autoantibody response. J Autoimmun. 2014;53:46–54. doi: 10.1016/j.jaut.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6(6):366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4(5):371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Gonzalez A, et al. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int. 2002;22(4):138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- 6.McMahon M, et al. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann Rheum Dis. 2011;70(9):1619–1624. doi: 10.1136/ard.2010.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han S, et al. 2015. Maintenance of autoantibody production in pristane-induced murine lupus. Arthritis Res Ther 30(17):384 (abstr)
- 9.Richards HB, et al. Interleukin 6 dependence of anti-DNA antibody production: Evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188(5):985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivakumar S, Tsokos GC, Datta SK. T cell receptor α/β expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143(1):103–112. [PubMed] [Google Scholar]
- 11.Crispín JC, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allman D, et al. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167(12):6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 13.Singh RR, et al. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J Exp Med. 1995;181(6):2017–2027. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh RR, Hahn BH, Tsao BP, Ebling FM. Evidence for multiple mechanisms of polyclonal T cell activation in murine lupus. J Clin Invest. 1998;102(10):1841–1849. doi: 10.1172/JCI3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17(5):421–425. doi: 10.1177/0961203308090028. [DOI] [PubMed] [Google Scholar]
- 16.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177(3):1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Sanctis JB, Zabaleta M, Bianco NE, Garmendia JV, Rivas L. Serum adipokine levels in patients with systemic lupus erythematosus. Autoimmunity. 2009;42(4):272–274. doi: 10.1080/08916930902828031. [DOI] [PubMed] [Google Scholar]
- 19.Wallace DJ, Lin HC, Shen GQ, Peter JB. Antibodies to histone (H2A-H2B)-DNA complexes in the absence of antibodies to double-stranded DNA or to (H2A-H2B) complexes are more sensitive and specific for scleroderma-related disorders than for lupus. Arthritis Rheum. 1994;37(12):1795–1797. doi: 10.1002/art.1780371213. [DOI] [PubMed] [Google Scholar]
- 20.Fields ML, Erikson J. The regulation of lupus-associated autoantibodies: immunoglobulin transgenic models. Curr Opin Immunol. 2003;15(6):709–717. doi: 10.1016/j.coi.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Quismorio FD, Torralba KD. Clinical applications of serologic tests, serum protein abnormalities, and other clinical laboratory tests in SLE. In: Wallace DJ, Hahn BH, editors. Dubois’ Lupus Erythematosus and Related Syndromes. 8th Ed. Saunders Elsevier; Philadelphia: pp. 526–540. [Google Scholar]
- 22.Hahn BH, Anderson M, Le E, La Cava A. Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58(8):2488–2497. doi: 10.1002/art.23609. [DOI] [PubMed] [Google Scholar]
- 23.Petro JB, et al. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277(50):48009–48019. doi: 10.1074/jbc.M200305200. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, et al. Genetic associations of leptin-related polymorphisms with systemic lupus erythematosus. Clin Immunol. 2015;161(2):157–162. doi: 10.1016/j.clim.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawasaki A, Tsuchiya N, Fukazawa T, Hashimoto H, Tokunaga K. Analysis on the association of human BLYS (BAFF, TNFSF13B) polymorphisms with systemic lupus erythematosus and rheumatoid arthritis. Genes Immun. 2002;3(7):424–429. doi: 10.1038/sj.gene.6363923. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki A, Tsuchiya N, Fukazawa T, Hashimoto H, Tokunaga K. Presence of four major haplotypes in human BCMA gene: lack of association with systemic lupus erythematosus and rheumatoid arthritis. Genes Immun. 2001;2(5):276–279. doi: 10.1038/sj.gene.6363770. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166(1):6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 28.Stohl W. BlySfulness does not equal blissfulness in systemic lupus erythematosus: a therapeutic role for BLyS antagonists. Curr Dir Autoimmun. 2005;8:289–304. doi: 10.1159/000082108. [DOI] [PubMed] [Google Scholar]
- 29.Hartman AB, Mallett CP, Sheriff S, Smith-Gill SJ. Unusual joining sites in the H and L chains of an anti-lysozyme antibody. J Immunol. 1988;141(3):932–936. [PubMed] [Google Scholar]
- 30.Liu Y, Yu Y, Matarese G, La Cava A. Cutting edge: Fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J Immunol. 2012;188(5):2070–2073. doi: 10.4049/jimmunol.1102835. [DOI] [PMC free article] [PubMed] [Google Scholar]