Significance
MicroRNAs are important regulators of gene expression in unicellular and multicellular eukaryotes. They are generally embedded in stem–loops of precursor transcripts and are excised by the dsRNA-specific nuclease DICER with the assistance of dsRNA-binding proteins. In animals and plants, proteins harboring two or three dsRNA-binding domains (dsRBDs) are involved in microRNA (miRNA) biogenesis. In contrast, we found that the Dull slicer-16 (DUS16) protein, which contains a single dsRBD and also an ssRNA-binding domain, is involved in miRNA biogenesis in the unicellular green alga Chlamydomonas. This finding sheds light on a molecular mechanism of miRNA biogenesis in unicellular organisms that may be similar to that in a common ancestor of animals and plants.
Keywords: Chlamydomonas, microRNA biogenesis, dsRNA-binding protein, small RNA-seq, mutagenesis
Abstract
Canonical microRNAs (miRNAs) are embedded in duplexed stem–loops in long precursor transcripts and are excised by sequential cleavage by DICER nuclease(s). In this miRNA biogenesis pathway, dsRNA-binding proteins play important roles in animals and plants by assisting DICER. However, these RNA-binding proteins are poorly characterized in unicellular organisms. Here we report that a unique RNA-binding protein, Dull slicer-16 (DUS16), plays an essential role in processing of primary-miRNA (pri-miRNA) transcripts in the unicellular green alga Chlamydomonas reinhardtii. In animals and plants, dsRNA-binding proteins involved in miRNA biogenesis harbor two or three dsRNA-binding domains (dsRBDs), whereas DUS16 contains one dsRBD and also an ssRNA-binding domain (RRM). The null mutant of DUS16 showed a drastic reduction in most miRNA species. Production of these miRNAs was complemented by expression of full-length DUS16, but the expression of RRM- or dsRBD-truncated DUS16 did not restore miRNA production. Furthermore, DUS16 is predominantly localized to the nucleus and associated with nascent (unspliced form) pri-miRNAs and the DICER-LIKE 3 protein. These results suggest that DUS16 recognizes pri-miRNA transcripts cotranscriptionally and promotes their processing into mature miRNAs as a component of a microprocessor complex. We propose that DUS16 is an essential factor for miRNA production in Chlamydomonas and, because DUS16 is functionally similar to the dsRNA-binding proteins involved in miRNA biogenesis in animals and land plants, our report provides insight into this mechanism in unicellular eukaryotes.
MicroRNAs (miRNAs) generally function as posttranscriptional repressors by promoting degradation and/or translation inhibition of their target mRNAs. Further, miRNAs are key regulators of diverse biological processes, such as developmental timing, cell proliferation, and cell death, in animals and plants. Thus, miRNA abundance and activities must be tightly regulated at the levels of transcription, processing, and turnover of these small RNAs (1–3). The miRNA biogenesis pathway begins with transcription of primary-miRNAs (pri-miRNAs), generally synthesized by RNA polymerase II. After transcription, pri-miRNAs fold into hairpins and are processed by RNase III enzymes into precursor-miRNAs (pre-miRNAs), and then are processed further into duplexes consisting of guide (miRNA) and passenger (miRNA*) strands with 2-nt 3′ overhangs. Guide-strand miRNAs are preferentially incorporated into Argonaute (AGO) proteins and form an effector ribonucleoprotein complex termed the “RNA-induced silencing complex” (RISC). RISC induces endonucleolytic cleavage and/or translation repression of target mRNAs (2, 4, 5).
Several RNA-binding proteins play important roles in producing subsets of miRNAs by assisting the accurate and efficient processing of pri-miRNA and pre-miRNAs in animals and plants (6–8). In humans, pri-miRNAs are cotranscriptionally recognized by the microprocessor complex consisting of the RNase III enzyme Drosha and the dsRNA-binding protein DGCR8 and then are processed to liberate pre-miRNA hairpins from pri-miRNA in the nucleus (9–12). DGCR8 interacts directly at the junction between ssRNA and the dsRNA region of the pri-miRNA stem and directs dicing by Drosha 11 bp from the junction (10, 13). Following export of pre-miRNAs by Exportin-5 to the cytoplasm, another RNase III enzyme, Dicer, cleaves them into miRNA/miRNA* duplexes (14–16). Human Dicer also associates with two different dsRNA-binding proteins, TRBP and PACT (17, 18). These dsRNA-binding proteins are not required for dicing activity but function as key regulatory factors in defining the dicing site and facilitating RISC formation (19–21).
The biogenesis and functions of plant miRNAs have been documented mainly in Arabidopsis thaliana. Processing of pri-miRNAs is completed in two steps by the same RNase III enzyme, DICER LIKE 1 (DCL1). As in animals, DCL1 cleaves the base of pri-miRNAs to yield pre-miRNAs and then cleaves pre-miRNA again to release miRNA/miRNA* duplexes (22–24). Plant miRNA duplexes are generated in the nucleus and then are exported to the cytoplasm by HASTY, a homolog of Exportin-5, for RISC loading (25). DCL1 interacts with the dsRNA-binding protein HYPONASTIC LEAVES1 (HYL1/DRB1) and the ssRNA-binding protein SERRATE (SE) and forms a plant microprocessor complex in the nucleus (26–28). HYL1 assists in the efficient and precise cleavage of pri-miRNA by interaction with DCL1 (26). SE enhances the accuracy of DCL1-dependent pri-miRNA processing together with HYL1 (28). TOUGH (TGH), another ssRNA-binding protein, is also a component of the plant microprocessor. TGH contributes to the interaction between pri-miRNA and HYL1 and may regulate DCL1 cleavage efficiency and/or recruitment of pri-miRNAs (29). A. thaliana encodes four additional dsRNA-binding proteins, DRB2–5, which are closely related to HYL1. These DRBs also function in canonical and noncanonical miRNA biogenesis pathways (30, 31).
Although many studies have shed light on the mechanism(s) of RNA-mediated gene silencing, it remains poorly understood in the unicellular green alga Chlamydomonas reinhardtii (32–41), particularly with respect to miRNA biogenesis. Here we show that the Chlamydomonas RNA-binding protein Dull slicer-16 (DUS16), which has an ssRNA-binding domain (RRM) and a dsRNA-binding domain (dsRBD), associates with nascent pri-miRNA transcripts cotranscriptionally and assists in processing pri-miRNAs into mature miRNAs.
Results
DUS16 Is Involved in the Processing of pri-miRNAs.
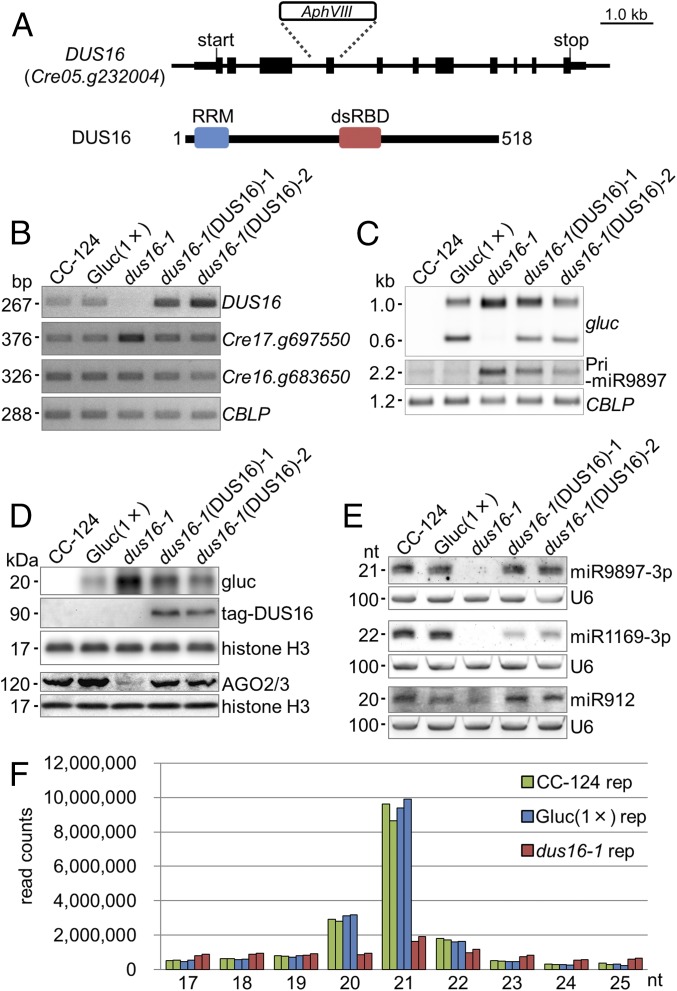
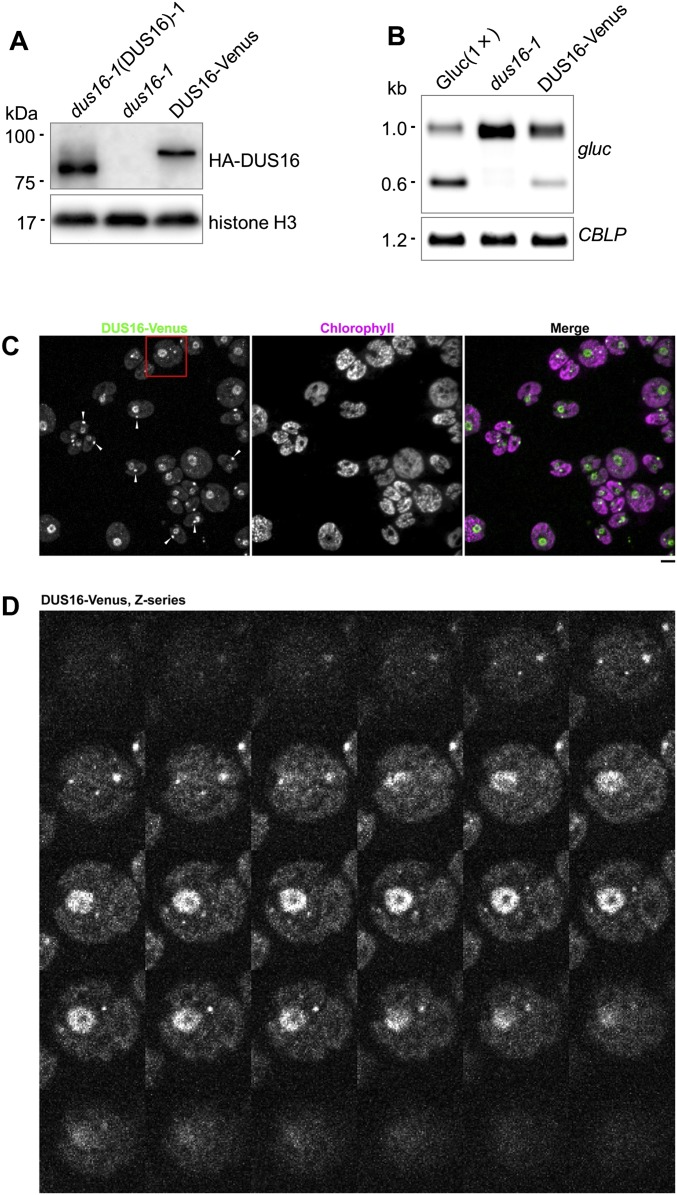
We have generated an insertional mutant library from the Chlamydomonas transgenic strain Gluc(1×), which expresses Gaussia luciferase (gluc) bearing a sequence perfectly complementary to miR9897-3p (38, 40). Measurement of gluc activity as an index of silencing allowed us to identify silencing-defective mutants in the library. One of the mutants, dus16-1, was defective in silencing by disruption of Cre05.g232004 (renamed “DUS16”) because of a single-tag insertion. It is noteworthy that DUS16 contains two different RNA-binding domains, an RRM and a dsRBD (Fig. 1 A and B and Fig. S1) (42–45). To verify the impairment of miR9897-3p–mediated silencing in dus16-1, we measured the abundance of the intact gluc transcript (∼1.0 kb) and the cleavage product of the gluc transcript (∼0.6 kb) (38). Gluc(1×) accumulated the sliced gluc transcript, but dus16-1 showed no detectable sliced gluc transcript (Fig. 1C). In agreement with the increase of intact gluc transcript, the gluc protein was increased significantly in dus16-1 (Fig. 1 C and D). By small RNA (sRNA) blotting, we confirmed that miR9897-3p and other miRNAs (miR1169-3p and miR912) were nearly absent in dus16-1 (Fig. 1E) (40). Moreover, the primary transcript of miR9897-3p (pri-miR9897), which is almost undetectable in the wild-type strain, was observed as an ∼2.2-kb RNA in dus16-1 (Fig. 1C). All these defects in dus16-1 were largely restored by the expression of tagged-DUS16, even though the fusion of the epitope tag might modestly affect the function of DUS16. (Fig. 1 B–E). These results clearly indicate that DUS16 is critically involved in the biogenesis of miR9897-3p, miR1169-3p, and miR912.
Fig. 1.
Characterization of the DUS16 mutant. CC-124, wild-type strain; Gluc(1×), CC-124 transgenic strain expressing Gaussia luciferase (gluc) bearing a sequence perfectly complementary to Chlamydomonas miR9897-3p; dus16-1, DUS16-defective mutant of Gluc(1×); dus16-1(DUS16)-1 and -2, strains expressing HA-FLAG-tagged DUS16 in a dus16-1 background. (A) Schematic diagram of the gene structure and transcript of DUS16 (Cre05.g232004). dsRBD, dsRNA-binding domain; RRM, ssRNA-binding domain. (B–E) Each panel is representative of three independent experiments. (B) RT-PCR analysis in the indicated strains. Cre17.g697550 is a target of miR B-mediated cleavage. Cre16.g683650 is a target of miR C-mediated translation repression. A housekeeping gene, CBLP, was used as a control. (C) Northern blotting of gluc mRNA and the pri-miR9897 transcript in the indicated strains. Detection of CBLP was performed to check for equivalent loading of the RNA samples. (D) Immunoblotting of gluc, tagged DUS16, and AGO2/3 proteins in the indicated strains. The tagged DUS16 protein was detected with a monoclonal antibody against the HA tag. Both AGO2 and AGO3 polypeptides are recognized by a polyclonal antibody. Detection of histone H3 was performed to confirm similar loading of the protein samples. (E) Northern blotting of Chlamydomonas mature miRNAs in the indicated strains. U6 small nuclear RNA was used as a control for equal loading of the RNA samples. (F) Small RNA-seq analysis of CC-124 (green), Gluc(1×) (blue), and dus16-1 (red). Biological duplicates of sRNA libraries were generated from the indicated strains. sRNA reads, 17–25 nt in length, were classified according to their size. Absolute read counts, without normalization, are indicated.
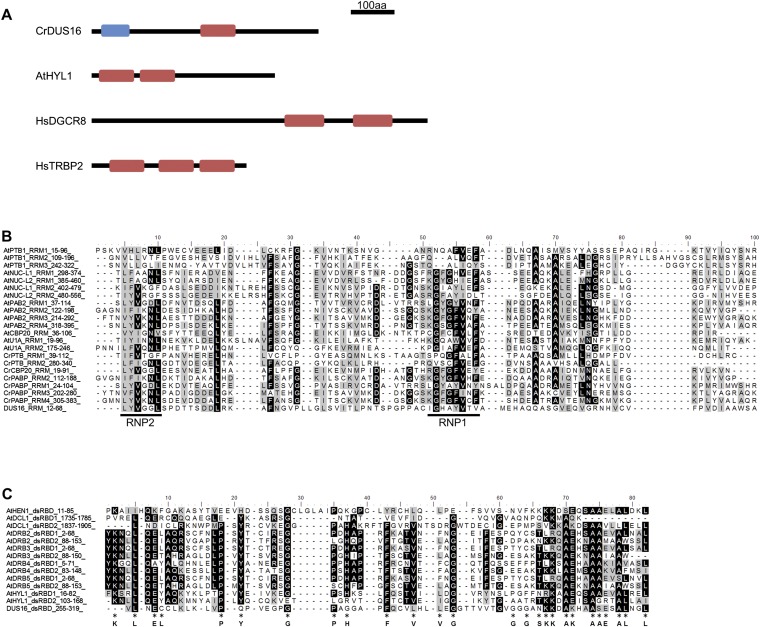
Fig. S1.
Domain structure of DUS16, HYL1, DGCR8, and TRBP2 and amino acid sequence alignment of the RRM and dsRBD from proteins from A. thaliana (At) and C. reinhardtii (Cr). Alignments were performed with ClustalX2. (A) Schematic domain structure of RNA-binding proteins involved in miRNA processing in C. reinhardtii (DUS16), A. thaliana (HYL1), and Homo sapiens (DGCR8 and TRBP2). Blue box, ssRNA recognition motif (RRM). Red box, dsRBD. (B) Alignment of RRMs. The conserved RNP1 and RNP2 sequences are indicated by black lines below the alignment (42, 43). (C) Alignment of dsRBDs. Amino acid residues conserved in animal polypeptides are indicated below the alignment (44, 45).
To investigate global changes in miRNA and sRNA levels, we performed sRNA-sequencing (sRNA-seq) analyses. The study revealed that the large majority of 20- and 21-nt sRNAs were absent in dus16-1 (Fig. 1F and Table S1). To determine the number of sRNAs generated from individual pre-miRNAs, we counted redundant sRNA reads mapped to individual pre-miRNA sequences. Although 50 stem–loop sequences corresponding to putative pre-miRNAs in Chlamydomonas have been registered in the miRBase database (www.mirbase.org/), few or no reads from Gluc(1×) were mapped to four of the 50 sequences. Accordingly, these four sequences were excluded from further analyses. In comparison with Gluc(1×), dus16-1 showed fewer than half the number of sRNA reads for 38 of the remaining 46 stem–loops; for eight stem–loops comparable or greater numbers of sRNA reads were observed (Fig. S2). These results suggest that DUS16 is involved in the production of most miRNA species. Marked decreases in miRNA (miR) B and miR C levels were also found in dus16-1 (Fig. S3). In agreement with this finding, the Cre17.g697550 transcript, a slicing target of miR B, was noticeably increased, whereas Cre16.g683650 mRNA, a translation repression target of miR C, was virtually unchanged in dus16-1 (Fig. 1B) (39, 40). Moreover, the AGO2/3 proteins were almost absent in dus16-1, probably because of their destabilization resulting from the global decrease in AGO2/3-associated sRNAs (Fig. 1D).
Table S1.
Analysis of sRNA libraries
| Filtering reads | CC-124 | Gluc(1×) | dus16-1 | |||
| Replicate no. 1 | Replicate no. 2 | Replicate no. 1 | Replicate no. 2 | Replicate no. 1 | Replicate no. 2 | |
| Total reads after adapter trimming | 23,826,133 | 21,811,027 | 21,999,293 | 22,796,748 | 18,196,871 | 18,946,433 |
| 17–25 nt with more than two reads for mapping (%) | 16,750,471 (70.3) | 15,450,769 (70.8) | 16,258,624 (73.9) | 16,989,609 (74.4) | 7,117,200 (39.1) | 8,001,591 (42.2) |
Percentages in parentheses are relative to the parent category.
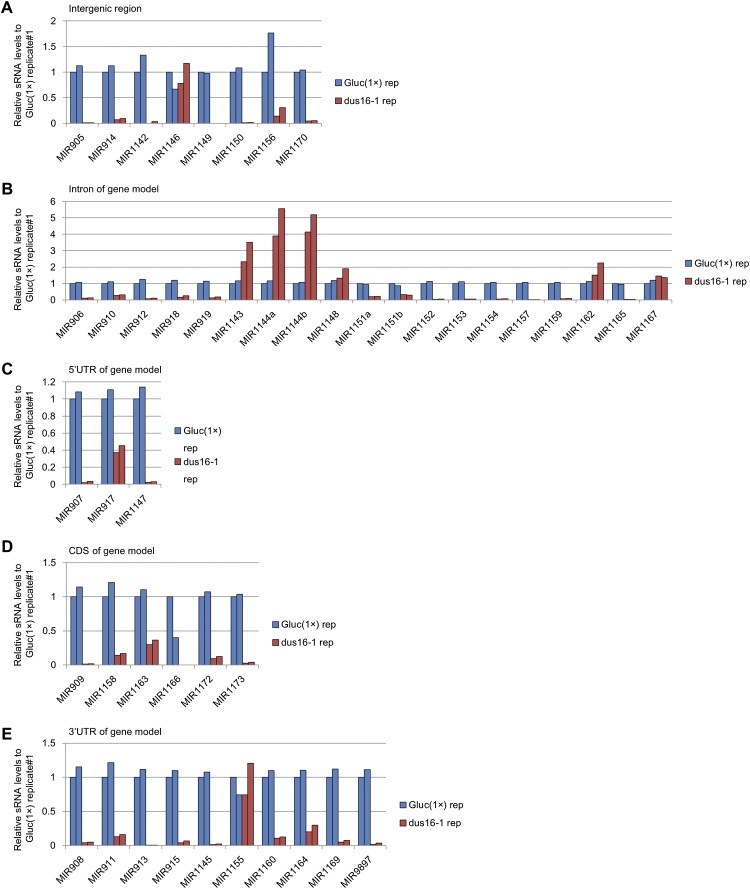
Fig. S2.
sRNA-seq analysis of Gluc(1×) and dus16-1. Biological duplicates of sRNA libraries were generated, and sRNA reads ranging from 17–25 nt were aligned to stem–loop sequences registered in miRBase. All sRNA reads that mapped to individual stem–loop sequences in each pre-miRNA were counted. The absolute read counts were normalized to that of Gluc(1×) replicate #1. Analyzed miRNA genes were grouped into five categories based on the location of stem–loops in the gene models. (A) Intergenic region. (B) Intron of the gene model. (C) The 5′ UTR of the gene model. (D) Coding sequence of the gene model. (E) The 3′ UTR of the gene model.
Fig. S3.
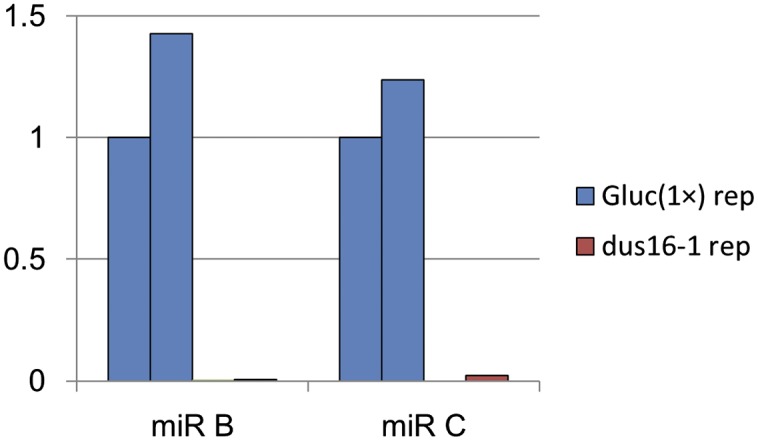
Comparison of the abundance of miR B and miR C in Gluc(1×) and dus16-1. Absolute read counts of mature miR B and miR C were normalized to that of Gluc(1×) replicate 1.
RRM and dsRBD Are Essential for DUS16 Function.
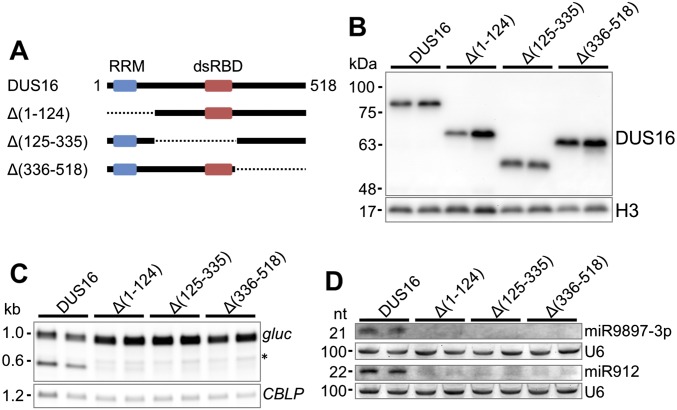
To evaluate the necessity of RRM and dsRBD for DUS16 function, we performed truncation analyses of DUS16. After removal of the RRM (Δ1–124), the dsRBD (Δ125–335), or the C-terminal region (Δ336–518) from the intact DUS16 expression cassette, individual truncated-DUS16 transgenes were transformed into the wild type and then were transferred to the dus16-1 background by a genetic cross (Fig. S4). As described above, production of miR9897-3p was clearly restored by the expression of intact, full-length DUS16 (Fig. S4). In contrast, no truncated DUS16 transgene could restore the production of miR9897-3p (Fig. S4). These results suggest that the RRM, the dsRBD, and also the C-terminal region are functionally and/or structurally important for DUS16 function in the processing of pri-miR9897.
Fig. S4.
Analysis of truncated versions of DUS16. (A) Schematic diagram of intact (full-length) and truncated forms of DUS16 protein. Black lines indicate existing polypeptide regions, and dotted lines indicate deleted polypeptide regions. DUS16, intact DUS16; Δ(1–124), DUS16 missing the RRM; Δ(125–335), DUS16 missing the dsRBD; Δ(336–518), DUS16 missing the C-terminal region. (B–D) Each panel is representative of three independent experiments. (B) Immunoblotting of each form of tagged DUS16 with an anti-HA antibody in the indicated strains. Pairs of transgenic strains were generated by the introduction of each tagged DUS16 form into the wild-type background, and the transgenes then were transferred to the dus16-1 background by genetic crossing. (C) Northern blotting of gluc mRNA in the indicated strains. The asterisk indicates naturally degraded gluc mRNA, which also is found in strains expressing gluc transgenes harboring no miRNA target. (D) Northern blotting of miR9897-3p and miR912 in the indicated strains.
DUS16 Is Localized Mainly to the Nucleus, and the Remaining DUS16 Forms Speckles in the Cytoplasm.
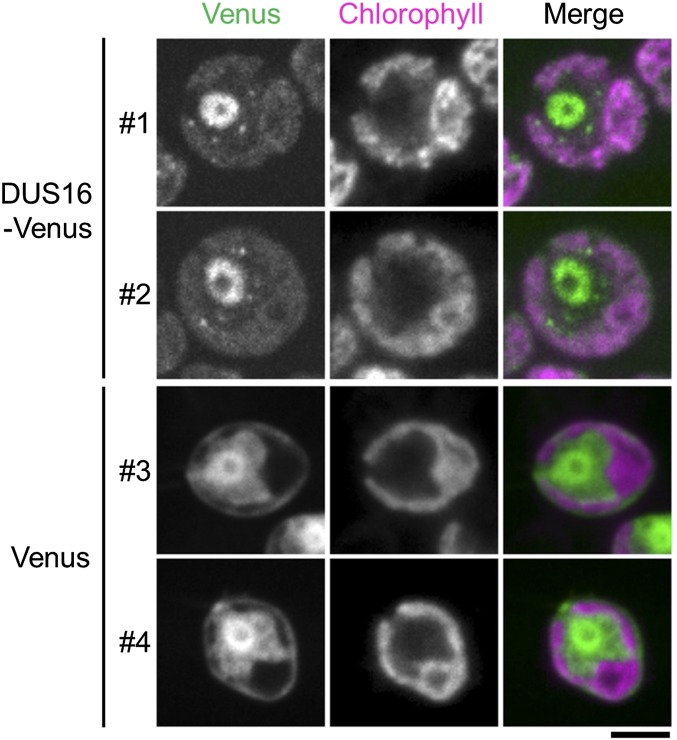
In animals and plants, initial processing of pri-miRNAs occurs exclusively in the nucleus. In agreement with this observation, HYL1, TGH, SE, and DCL1 are localized in specific subnuclear loci named “Dicer bodies” in A. thaliana (46–49). To observe the localization of DUS16 by confocal microscopy, we expressed Venus-fused DUS16 (DUS16-Venus) in dus16-1 and restored the production of miR9897-3p (Fig. S5 A and B). Intriguingly, most DUS16-Venus localized to the nucleus, but a fraction of the protein appeared to form speckles in the cytoplasm (Fig. 2 and Fig. S5). In A. thaliana, HYL1 functions in the precise cleavage of pri-miRNAs through interaction with DCL1 (26). Because the results described above suggest that DUS16 could be a substitute for HYL1 in Chlamydomonas, we compared the precision of dicing sites on stem–loop sequences in Gluc(1×) and dus16-1. By comparing the mapped sRNA reads in the two strains, we found no obvious increased inaccuracy of processing in dus16-1, even though the number of sRNA reads was quite small (Fig. S6). These results suggest that DUS16 localizes predominantly to the nucleus and contributes to the initial processing of pri-miRNA but does not assist strongly in adjusting dicing sites.
Fig. S5.
Complementation of miR9897-mediated silencing in dus16-1 by expression of DUS16-Venus and additional images of DUS16-Venus localization. (A) Immunoblotting of DUS16 tagged with an anti-HA antibody. Strain DUS16-Venus #1 expresses Venus-HA-FLAG-tagged DUS16 in a dus16-1 background. Each panel is representative of three independent experiments. (B) Northern blotting of gluc mRNA in the indicated strains. Each panel is representative of three independent experiments. (C) A field of dus16-1 cells expressing DUS16-Venus. Maximum-projection images of z-stacks covering the cell bodies are shown. The arrowheads indicate eyespot autofluorescence in some of the cells. (Scale bar, 5 µm.) (D) Montage of z-stacks of the area indicated by the red square in C, showing DUS16-Venus localization in the nucleus and as cytoplasmic punctae. The same cell also was used to generate a set of average-projection images shown in Fig. 2.
Fig. 2.
Subcellular localization of the DUS16-Venus fusion protein. Cells of transgenic Chlamydomonas expressing DUS16-Venus in a dus16-1 background (DUS16-Venus, strain #1 and #2) and wild-type cells expressing Venus (Venus, strain #3 and #4) were examined by confocal microscopy. Average-projection images of six z-stacks, each around the midplane, are shown. Note that, because of the low expression of the protein, a long exposure and high laser intensity were used for imaging DUS16-Venus; this imaging technique also increased chlorophyll and eyespot autofluorescence in the Venus channel (also see Fig. S5). (Scale bar, 5 µm.)
Fig. S6.
Mapping of sRNAs reads on the stem–loop sequences of selected miRNA genes. sRNA reads of Gluc(1×) replicate no. 1 and dus16-1 replicate no. 1 ranging from 19–24 nt were mapped to the stem–loop sequences of MIR908, MIR910, MIR912, MIR1151a, MIR1151b, MIR1152, MIR1153, MIR1164, MIR1169, and MIR9897.
Loss of DUS16 Results in Accumulation of Exonic pri-miRNAs, but Levels of Intronic pri-miRNAs Are Unchanged.
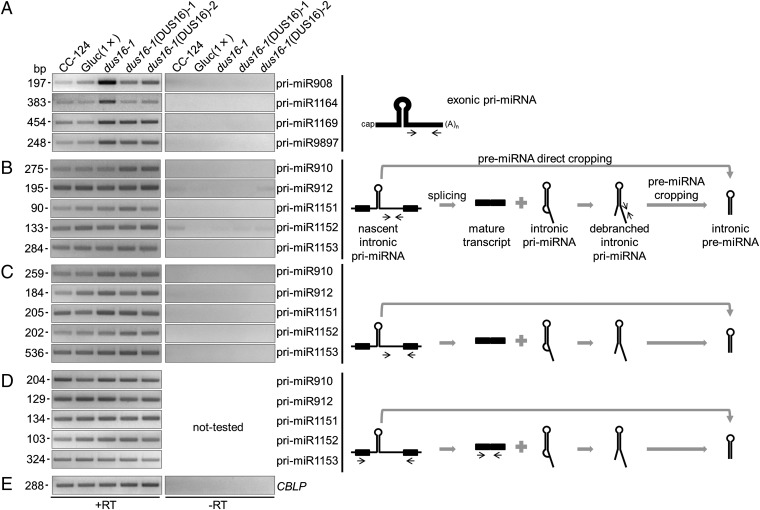
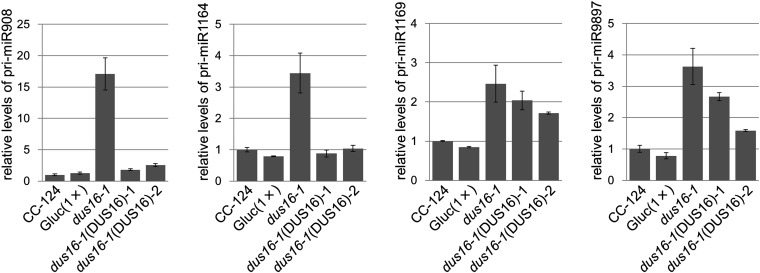
Mammalian miRNAs are embedded mainly (∼80%) into introns of protein-coding or noncoding genes, whereas plant miRNAs are encoded mostly in exonic regions of independent miRNA genes (50–52). In C. reinhardtii, 19 of the 46 stem–loop precursors discussed above are embedded into introns of gene models, whereas other 19 stem–loops are encoded in exons of gene models (three in 5′ UTRs, six in coding exons, and 10 in 3′ UTRs). The remaining eight stem–loops are found in intergenic regions (Fig. S2). In dus16-1, production of most sRNA species was impaired irrespective of the location of stem–loops in gene models (Fig. S2). We measured the accumulation levels of selected pri-miRNAs (pri-miR908, pri-miR1164, pri-miR1169, and pri-miR9897) that contain stem–loops in their 3′ UTRs. RT-PCR analyses revealed that these exonic pri-miRNAs were clearly increased in dus16-1, suggesting that they are processed by DCL protein(s) associated with DUS16 and that 5′- and 3′-cleavage fragments are likely destabilized and quickly degraded in the wild type (Fig. 3A and Fig. S7). We then measured the accumulation levels of other pri-miRNAs (pri-miR910, pri-miR912, pri-miR1151, pri-miR1152, and pri-miR1153) located in introns. In contrast to exonic pri-miRNAs, accumulation levels of these intronic pri-miRNAs appeared unchanged in dus16-1 (Fig. 3B). Nascent (unspliced form) intronic-miRNAs and the mature transcripts also appeared unchanged in dus16-1 (Fig. 3 C–E). These results suggest that splicing of intronic pri-miRNAs occurs efficiently and that the introns containing a stem–loop are degraded by the intron degradation pathway in dus16-1.
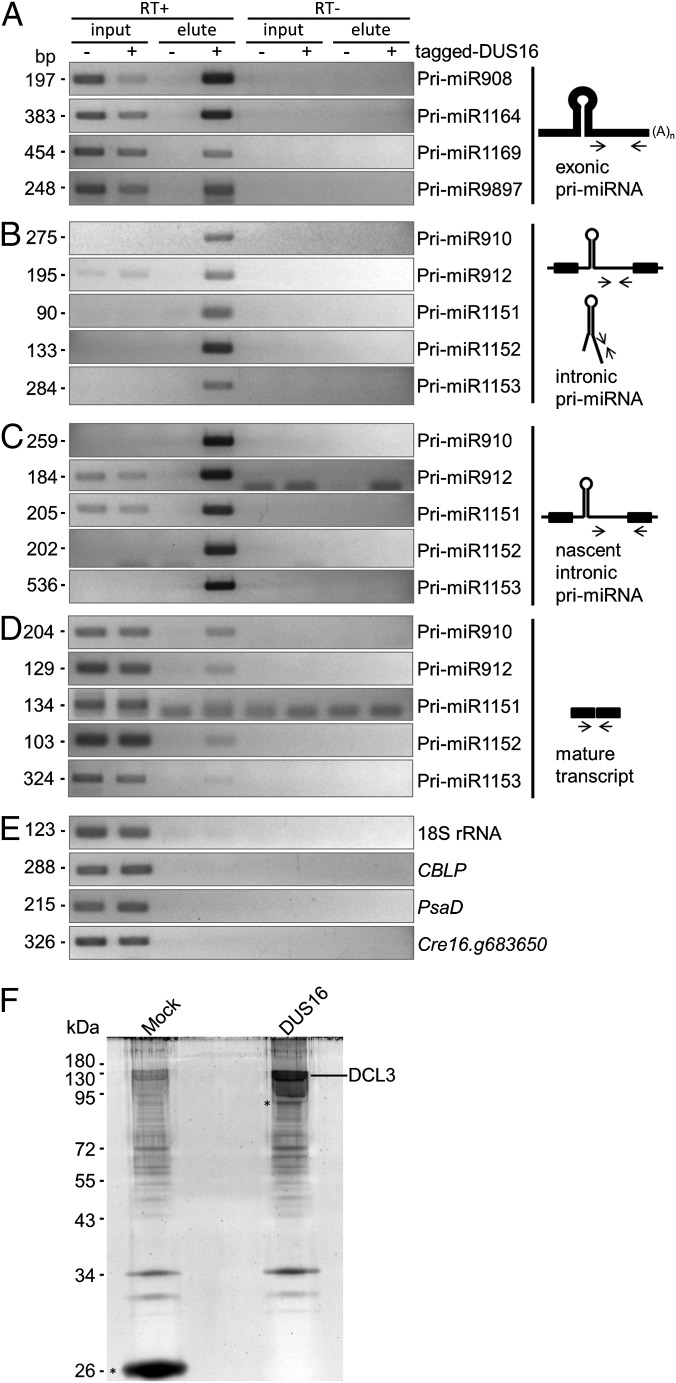
Fig. 3.
RT-PCR analysis of exonic and intronic pri-miRNAs. (A–E, Left) Each panel is representative of three independent experiments. (A) RT-PCR analysis of exonic pri-miRNAs in the indicated strains. The stem–loops are located in the 3′ UTRs, and primers were designed to amplify downstream of the stem–loops. (B) RT-PCR analysis of intronic pri-miRNAs in the indicated strains. The primers were designed to amplify downstream of the stem–loops. (C) RT-PCR analysis of nascent (unspliced form) intronic pri-miRNAs in the indicated strains. (D) RT-PCR analysis of mature transcripts resulting from proper splicing out of intronic pri-miRNAs. (E) RT-PCR analysis of mature CBLP mRNA as a control. (Right) Schematic diagrams of the secondary structure of exonic and intronic pri-miRNAs. Thick black lines in the diagrams indicate exons, and narrow black lines indicate introns. Narrow black arrows indicate the location and direction of primers for PCR amplification. Gray arrows and symbols indicate the direction of processing.
Fig. S7.
qRT-PCR analysis of exonic pri-miRNAs in the indicated strains. Levels of each exonic pri-miRNA were normalized to the levels of CBLP, after which the value corresponding to CC-124 was set to 1 and other values were adjusted accordingly. Values shown are the averages ± SD of three independent experiments.
DUS16 Associates with Nascent pri-miRNAs and the DCL3 Protein.
To obtain evidence that DUS16 associates with pri-miRNAs and promotes the production of miRNAs, we performed RNA immunoprecipitation (RIP) and subsequent RT-PCR (RIP RT-PCR). RNAs associated with tagged DUS16 were reverse-transcribed, and specific pri-miRNA regions were amplified by PCR with the primer sets used in Fig. 3. Interestingly, the exonic pri-miRNAs were recovered from the eluate of the DUS16-complemented strain, indicating that DUS16 associates with exonic pri-miRNAs (Fig. 4A). Furthermore, intronic pri-miRNAs (Fig. 4B) and nascent intronic pri-miRNAs (Fig. 4C) were recovered efficiently also. In contrast, DUS16 did not appear to interact efficiently with the mature transcripts (Fig. 4 D and E). Consistent with DUS16’s being part of a microprocessor complex, affinity purification of tagged DUS16 and subsequent mass spectrometry analysis revealed that the protein associates with DICER-LIKE 3 (DCL3), which is the predominant DICER function in Chlamydomonas (Fig. 4F) (41). These results suggest that DUS16 recognizes and associates with nascent pri-miRNAs cotranscriptionally and assists DCL3-mediated processing of pri-miRNAs.
Fig. 4.
RIP RT-PCR analysis and affinity purification of tagged-DUS16–associated transcripts and proteins. RIP with FLAG beads was performed with lysates from the dus16-1 (indicated as tagged-DUS16 −) and dus16-1(DUS16)-1 (indicated as tagged-DUS16 +) strains. Input RNA corresponded to 0.05% of the total input amount. Reactions were stopped after any of the PCRs using the same primer set reached the midlogarithmic phase of amplification. Each panel is representative of three independent experiments. Affinity purification of proteins associated with epitope-tagged DUS16 was also carried out with FLAG beads. Subsequent mass spectrometry analysis was performed with lysate of the tagged ble-expressing strain and dus16-1(DUS16)-1. (A) Exonic pri-miRNAs. (B) Intronic pri-miRNAs. (C) Nascent (unspliced form) intronic pri-miRNAs. (D) Mature transcript, the product of proper splicing out of intronic pri-miRNA. (E) Analysis of 18S rRNA, CBLP, PsaD, and Cre16.g683650 as negative controls. (F) Affinity purification of proteins associated with tagged DUS16 from the dus16-1(DUS16)-1 strain. Isolated tagged DUS16 and interacting proteins ware resolved by SDS/PAGE, stained with SYPRO Ruby, digested in the gel with trypsin, and identified by mass spectrometry. Mock purification with a tagged ble protein expressed in the CC-124 background served as a negative control. Tagged proteins are indicated by asterisks.
Discussion
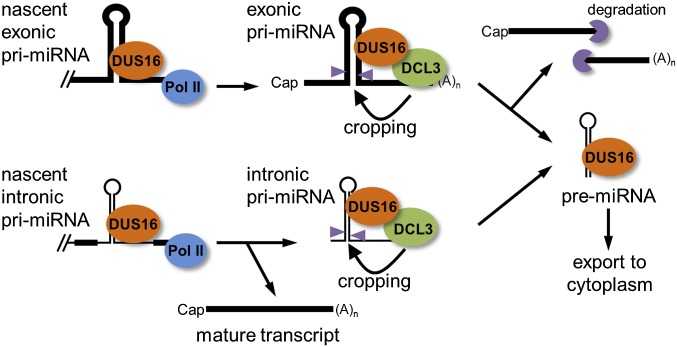
Based on our results, we propose that DUS16 is involved in processing pri-miRNAs (Fig. 5). In animals, stem–loop structures in pri-miRNAs are recognized cotranscriptionally by the microprocessor complex, which consists of two core components: the RNase III enzyme Drosha and DGCR8 (53–55). In A. thaliana, DCL1 interacts with HYL1 and SE to form the plant microprocessor complex, and this complex positively regulates the efficiency and accuracy of pri-miRNA processing in the nucleus (26–28). In C. reinhardtii, DUS16 is localized mostly in the nucleus, associates with DCL3 and nascent pri-miRNAs, and is required for the processing of pri-miRNAs into mature miRNAs. Therefore DUS16 is very likely a component of the Chlamydomonas nuclear microprocessor complex. Further, given that DUS16 also forms several speckles in the cytosol, this protein may accompany pre-miRNAs to the cytoplasm and may form distinct bodies with DCL3 for further processing of precursor miRNAs into mature miRNAs.
Fig. 5.
Proposed model for the role of DUS16 in miRNA biogenesis in Chlamydomonas. DUS16 associates, likely cotranscriptionally, with nascent pri-miRNAs. (Upper) In exonic pri-miRNA processing, DCL3-mediated cropping of a stem–loop generates pre-miRNA and cleavage fragments, which are quickly degraded in the wild type. Lack of processing results in exonic pri-miRNA accumulation in dus16-1. (Lower) In intronic pri-miRNA processing, because splicing appears to be unaffected by the DCL3-mediated processing of intronic pri- and/or pre-miRNA, the mature transcript is stably produced irrespective of the presence of functional DUS16. Generated pre-miRNAs may be exported to the cytoplasm and subjected to further processing.
In animals, spliceosome assembly of intronic pri-miRNAs promotes cropping of pre-miRNA hairpins from pri-miRNAs, but the splicing itself occurs more slowly than that of the adjacent introns (53, 55). In the A. thaliana HYL1 mutant background, some pri-miRNAs of unusual length, containing introns, accumulate (56). In Chlamydomonas, the efficiency of splicing of intronic pri-miRNAs was apparently unchanged in the dus16-1 background. In the analysis of the mature transcripts by RT-PCR, no additional longer amplicons that contained the intronic pri-miRNA were detected, indicating that the splicing efficiency of intronic pri-miRNAs was not strongly reduced. Thus, interactions between the DUS16 complex and the spliceosome in Chlamydomonas appear to be weak or nonexistent.
In the genome of C. reinhardtii, no multiple-dsRBD–containing gene, such as HYL1 or DGCR8, was found. Likewise, no DUS16 paralog was found. However, 8 of 46 examined pri-miRNAs were processed efficiently to mature miRNAs in dus16-1; indeed, some of these mature miRNAs were increased in the mutant. We hypothesize that Chlamydomonas likely harbors a DUS16-independent miRNA biogenesis pathway that is potentially activated in the dus16-1 background. Stem−loops in Chlamydomonas pri-miRNAs are greatly heterogeneous in length and structure, with variable positioning of the miRNA/miRNA* duplex (57). Given that the stem–loops of the eight pri-miRNAs appear to vary in secondary structure and size, structural signatures do not appear to be key determinants of the DUS16-independent miRNA biogenesis pathway. Production of sRNAs from miR1144a stem−loop and miR1144b stem−loop, which are embedded in the same gene (Cre04.g217937), was not inhibited in dus16-1, possibly implying that the processing pathway of pri-miRNAs is determined for each primary transcript.
A putative homolog of Chlamydomonas DUS16 was found only in the closely related alga Volvox carteri. Although many RNA-binding proteins have been well characterized as essential factors for the production of miRNAs in animals and plants, the composition of RNA-binding domains in DUS16 is unique among them (6–8, 49). Dead End 1 (Dnd1) does consist of a single RRM and a single dsRBD, but it counteracts the function of miRNAs by binding to transcripts at miRNA target sites in humans and zebrafish and is not involved in miRNA biogenesis (58). By functional analogy to other RNA-binding proteins, we propose that DUS16 plays a role mostly in pri-miRNA processing efficiency (but not accuracy) and functions as a partial substitute of plant HYL1. DUS16 may partly retain the character and function of ancestral RNA-binding proteins involved in miRNA/sRNA production. Further analysis of DUS16 may provide insight into the mechanism of miRNA biogenesis not only in the unicellular eukaryotes but also in the last common ancestor of eukaryotes in which an miRNA/sRNA-processing system evolved.
Materials and Methods
Culture Conditions and Genetic Crosses.
Unless otherwise noted, C. reinhardtii cells were grown photoheterotrophically in Tris/acetate/phosphate (TAP) medium (59). Genetic crosses were performed as described by Harris (60).
Plasmid Construction and Chlamydomonas Transformation.
Transformation of Chlamydomonas was performed as described previously (38, 61, 62). Mutant screening has been performed previously (40). The detailed construction procedures of the plasmids are described in SI Materials and Methods.
Fluorescence Microscopy.
Cells expressing Venus-tagged DUS16 were observed under a Leica DMI6000B microscope. The images were postprocessed using ImageJ version 1.50b (NIH) and Adobe Photoshop software. Detailed methods are provided in SI Materials and Methods.
DNA Analyses.
Isolation of genomic DNA, Southern blotting to determine the copy number of the insertion tag, and determination of the tag-insertion site were performed as described previously (59, 63, 64).
RNA Analyses.
Northern blotting and RT-PCR were performed as described previously (38, 65). The primers listed in Table S2 were used for RT-PCR and quantitative RT-PCR (qRT-PCR). Detailed methods are provided in SI Materials and Methods.
Table S2.
Primers used in this study
| Target | Forward (5′ to 3′) | Reverse (5′ to 3′) |
| DUS16 | CTGCGGTCTGAGATCCTTGGC | GCTCTGCAGCAGTCCGTTGA |
| Cre17.g697550 | CTCGAGAGGATCGCGGACAAC | TGAGGCACTGCACAACCTCATC |
| Cre16.g683650 | GGAGCCATTCGGCACATGGAG | CCTGCTTTACACCCCTGCTACTAC |
| CBLP | GAGTCCAACTACGGCTACGCC | CTCGCCAATGGTGTACTTGCAC |
| PsaD | AGAAGGAGCAGATCTTCGAGATGC | GTTCACCTTCTCGGGGTAGACG |
| 18S ribosomal RNA | AGCATGAGAGATGGCTACCACATC | CATTCCAATTACCAGACGCGAAGC |
| Pri-miR908 3′ UTR | GTGCCACACATTGGTCGAGTG | ACGTAACAGCGCTGAAGAGTACC |
| Pri-miR1164 3′ UTR | CTGTTTTGCCACCAGTGCTCAC | CCACCATGGACTGCCAGTAGC |
| Pri-miR1169 3′ UTR | CTGGACTTGCTGCCATTCTGTG | CAGTTTACACAAGTCACAACTGCTGC |
| Pri-miR9897 3′ UTR | AACCGAGAGTGGAAAGGGTCTTG | CCAATGCTCATTCGAACGCATGC |
| Mature pri-miR910 | CAAGCGCGAGCTGAATGACG | TCCAGCGATGCCTGCAGAG |
| Mature pri-miR912 | TGACGACGACACGGTCATGG | GCGTACTTGGCCATCTCCTCG |
| Mature pri-miR1151 | TGGCGGTGGCGGCTAC | AGTTGTTGTTGCCGCACTTGG |
| Mature pri-miR1152 | CCTCCTGGCTGTGGAGTTCG | GTTGCCGGCCATTCCCTTG |
| Mature pri-miR1153 | ACGCTGGCCGTGCTACAG | TCTTCCCGTTCTATCACCACGAAC |
| Nascent pri-miR910 | CGAACGGGCAAAGGAGTCAGG | TCCAGCGATGCCTGCAGAG |
| Nascent pri-miR912 | CCCTCATTGTCCCTGGCTACG | GCGTACTTGGCCATCTCCTCG |
| Nascent pri-miR1151 | TGGGCGAGAAACTCCATAGAGTC | AGTTGTTGTTGCCGCACTTGG |
| Nascent pri-miR1152 | ACCTTGCCTTCCTCAATGTCTCC | GTTGCCGGCCATTCCCTTG |
| Nascent pri-miR1153 | GCCTACACGCCTACGATGCAG | TCTTCCCGTTCTATCACCACGAAC |
| Pri-miR910 seventh intron | GGGACGGGTCTGTTCCGAG | CATAATGCAGGTGCGGTGATACG |
| Pri-miR912 sixth intron | CACGCTCGCACAAAACACACG | GCGGTGATGTTTGGGAGTCAC |
| Pri-miR1151 fourth intron | TGGGCGAGAAACTCCATAGAGTC | TGCCAACGTTGCAAGGCAAG |
| Pri-miR1152 10th intron | ACCTTGCCTTCCTCAATGTCTCC | GGGATCCCAGGCATTACCAGTC |
| Pri-miR1153 sixth intron | GCCTACACGCCTACGATGCAG | ACATCAGAAGCATGACAGGTGGAG |
Name of target genes are indicated in italics.
RIP.
RIP was performed as described previously (40). Detailed methods are provided in SI Materials and Methods.
sRNA-Seq.
sRNA-seq was performed as described previously (40). A summary of each sRNA library analyzed is presented in Table S1. Detailed methods are provided in SI Materials and Methods.
Immunoblotting.
Immunodetection was performed as described previously (38). For details of antibodies, see SI Materials and Methods.
Affinity Purification.
Affinity purification of tagged DUS16 and associated proteins was carried out in a similar way to RIP (66). Detailed methods are provided in SI Materials and Methods.
SI Materials and Methods
Plasmid Construction and Chlamydomonas Transformation.
Transformation of Chlamydomonas was performed as described previously (38, 61, 62). Mutant screening had been performed previously (40).
To generate the C-terminally tagged DUS16 expression vector (pC-tag-DUS16) for the complementation of dus16-1, the first half of the DUS16 genomic sequence was amplified by PCR from CC-124 genomic DNA using primers g5142-C-XhoI-F (5′-GCCGGCTCGAGATGGAAATCGATTCGGAACCCG-3′) and DUS16-R3 (5′-CGCCACACACGTTAGTTCACC-3′) with KOD Fx Neo DNA polymerase (Toyobo). The amplified first half of the DUS16 fragment was inserted into a p3xHA-2xgp64-3xFLAG(C) vector (Chlamydomonas Resource Center, www.chlamycollection.org/) at the XhoI and KpnI sites. A fragment corresponding to the second half of DUS16 was amplified using primers DUS16-F2 (5′-CCTTCCAGTGTGTGCTGCATCT-3′) and g5142-C-KpnI-R (5′-CTACTGGTACCCCTGGGCATGAAGTCCGC-3′). To reconstitute full-length DUS16, the amplified second half of the DUS16 fragment was inserted into the above plasmid at the KpnI site. The plasmid was digested with DraI, subjected to gel purification, and used for the transformation of dus16-1. Transgenic strains were selected on TAP medium containing 100 µg/mL of spectinomycin (Sigma).
To generate the truncated forms of DUS16 expression vectors, the RRM, dsRBD, or C-terminal region of the DUS16 sequence was removed from C-tagged DUS16 plasmid by inverse PCR with a KOD -Plus- Mutagenesis Kit (Toyobo) and the following primers: DUS16-aa0-R (5′-CTCGAGCATCCTGCAAATGGAAACGGCG-3′) and DUS16-aa125-F (5′-GTGACCGCCGCGCAAGCAGAAG-3′) for the Δ1–124 plasmid; DUS16-aa124-R (5′-GGCCTCCATTGCGTCAATGGGCTGG-3′) and DUS16-aa336-F (5′-CAGGTGCAGTCCCACTTGGTGCTGC-3′) for the Δ125–335 plasmid; DUS16-aa335-R (5′-GCGGAGGGATGCGACGACGTCG-3′) and DUS16-aa518-F (5′-GGTACCCTTCTGCAGGAGGGCGACATG-3′) for the Δ336–518 plasmid.
Plasmid pMO498 was constructed by excising an 11,160-bp DNA fragment containing DUS16-3×HA-2×gp64-3×FLAG from pC-tagged DUS16 plasmid using AscI and EcoRV and inserting it between the AscI and HpaI sites in pMO449; this construct is deposited in the Chlamydomonas resource center. pMO449 and pMO498 were digested with EcoRV and used for transformation. Transformants were selected on TAP agar plates supplemented with 10 µg/mL paromomycin. Venus-positive transformants were selected by fluorescence microscopy.
Fluorescence Microscopy.
Cells expressing Venus-tagged DUS16 were grown in TAP liquid medium to 1–2 × 106 cells/mL at 24 °C under constant illumination at 75 μmol photons⋅m−2⋅s−1, concentrated by centrifugation, and then mounted on a thin pad of TAP + 1% low-melting agar (SeaPlaque; FMC Corporation). The agar pad was then placed at the bottom of a glass-bottomed culture dish. The cells were observed under a Leica DMI6000B microscope equipped with a Yokogawa CSU10 spinning-disk confocal scanner unit, a Leica HCX PL Apo 63×/1.4–0.6 oil CORR lambda blue objective, and an Andor iXon camera. For each image, 36–40 z-series with 0.23-µm intervals were acquired for Venus fluorescence (excitation 514 nm, emission 543/22 nm) and chlorophyll autofluorescence (excitation 561 nm, emission 590/20 nm) using SlideBook 6.0 software. The images were postprocessed using ImageJ version 1.50b (NIH) and Adobe Photoshop software.
RNA Analyses.
The cDNA pools were generated with a ReverTra Ace cDNA synthesis kit (Toyobo). qRT-PCR was performed with KOD SYBR qPCR Mix (Toyobo) and StepOne (Life Technologies). The primers listed in Table S2 were used for RT-PCR and qRT-PCR.
Northern blotting was performed as described previously (38). DNA probes were generated by PCR amplification from plasmids harboring the sequence of the target transcripts (obtained by RT-PCR from cDNA pools of wild-type CC-124 and subsequent cloning). The following primers were used: gcluc-F2 (5′-ATCTGCCTGTCCCACATCAAGTG-3′) and gcluc-F1 (5′-CGACGGAGAACAACGAGGACTTC-3′) for the gluc probe; Pri-miR9897 3′ UTR-F (5′-AACCGAGAGTGGAAAGGGTCTTG-3′) and Pri-miR9897 3′ UTR-R (5′-CCAATGCTCATTCGAACGCATGC-3′) for the Pri-miR9897 probe; and CBLP-F (5′-GAGTCCAACTACGGCTACGCC-3′), and CBLP-R (5′-CTCGCCAATGGTGTACTTGCAC-3′) for the CBLP probe.
For sRNA blotting, total RNA was separated on 17% acrylamide/7M urea gels, electroblotted onto a nylon membrane, and subjected to UV crosslinking. Digoxigenin (Dig)-labeled oligonucleotide probes containing locked nucleic acids (LNA) were prepared, hybridized, and detected as previously described (65). The following LNA oligonucleotides were used for the production of Dig-labeled probes (LNAs are underlined): miR9897-3p (5′-GCCGATAAGAAGGAGCCGTAA-3′), miR912 (5′-GCCTGGCTGGGATCAATCCA-3′), and miR1169-3p (5′-ATCCAGCAAGCAACATCCACA-3′). The U6 DNA probe was generated by PCR with U6 snRNA-F (5′-GTGCTTCGGCACAACTGTTAAAAT-3′) and U6 snRNA-R (5′-AAAATTTGGAACCATTTCTCGATTTATGC-3′).
RIP.
RIP was performed as described previously (40). Approximately 2.5 × 109 cells were collected, washed once with 20 mM Tris⋅HCl (pH 7.6), and washed again with cell wash buffer [20 mM Tris⋅HCl (pH 7.6), 10% glycerol, and 150 mM KCl]. The washed cells were suspended in 5 mL of lysis buffer [20 mM Tris⋅HCl (pH 7.6), 10% glycerol, and 150 mM KCl, 0.2% Triton-X100, 0.2 mM PMSF, and 5 μL of protease inhibitor mixture (Sigma)] and were disrupted by two passages through a gas-pressure device (Mini-Bom Cell; Kontes) at 10.5 MPa under nitrogen gas. After removal of cell debris by two centrifugation steps at 16,000 × g for 10 min at 4 °C, the supernatant was passed through a 45-μm filter to obtain the lysates for affinity purification. At this point, input RNA was isolated from part of the lysate. The remaining lysate and 50 μL of packed FLAG M2 beads (Sigma) were incubated with rotation for 120 min at 4 °C. The beads were washed five times with the lysis buffer and then were incubated with FLAG elution buffer [20 mM Tris HCl (pH 7.6), 250 ng/mL 3×FLAG peptide (Sigma), 0.05% Tween-20] for 30 min at 4 °C. RNA was isolated from these eluates and precipitated with glycogen as carrier.
sRNA-Seq.
sRNA-seq was performed as described previously (40). sRNA libraries were generated using NEBNext Small RNA Library Prep Set for Illumina (New England Biolabs) following the manufacturer’s protocol. Deep sequencing was performed with Hiseq 1500 system (Illumina). Adapter sequences were removed from the generated data, and reads 17–25 nt in length were filtered for further analysis. The sorted sRNA reads were aligned to precursor transcripts of miRNA sequences annotated in miRBase (www.mirbase.org/) using the CLC genomic workbench (CLC bio). The summary of each sRNA library analyzed is presented in Table S1. Because 20- and 21-nt sRNAs were severely reduced in number in dus16-1, normalization to 1 million reads ranging from 17–25 nt or of total reads results in overestimation of the sRNA amounts in dus16-1. Thus, absolute counts of sRNA reads were used for the analyses throughout this study.
Immunoblotting.
Immunodetection was performed as described previously (38). To detect Gaussia luciferase, anti-gluc rabbit polyclonal antibody (E8023S; New England Biolabs) was used. To detect HA-tagged-DUS16, anti-HA rat monoclonal antibody (3F10; Roche) was used. The rabbit polyclonal anti-AGO2/3 antibody was raised against the C-terminal peptide of AGO2/AGO3 (36). To detect histone H3, a modification-insensitive rabbit polyclonal antibody (ab1791; Abcam) was used.
Affinity Purification.
Affinity purification of tagged DUS16 and associated proteins was carried out in a similar way to the RIP. Purified proteins were resolved by SDS/PAGE, stained with SYPRO Ruby (Bio-Rad) and were digested in gel with trypsin; peptide fragments were identified by tandem mass spectrometry as described previously (66). A minimum threshold of two unique peptides was used for validation of hits.
Acknowledgments
We thank the Functional Genomics Facility, National Institute for Basic Biology (NIBB) Core Research Facilities for technical support and Heather Cartwright (Carnegie Institution, Department of Plant Biology, Advanced Imaging Facility) for assistance with imaging. This work was supported by NIBB Collaborative Research Program 15-103 (T.Y.), by a grant from the National Science Foundation (NSF) (to H.C.), and by NSF Early Concept Grant for Exploratory Research (EAGER) Award 1548533 (to J. R. Pringle).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Small RNA-seq raw data have been deposited in the DNA Data Bank of Japan (DDBJ) sequence read archive [accession nos. (CC-124 replicate #1, DRR045092; CC-124 replicate #2, DRR045093; Gluc1(×) replicate #1, DRR045094; Gluc1(×) replicate #2, DRR045095; dus16-1 replicate #1, DRR048495; and dus16-1 replicate #2, DRR048496)]. Plasmid pMO449 is available on the Chlamydomonas Resource Center, www.chlamycollection.org.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523230113/-/DCSupplemental.
References
- 1.Sun G. MicroRNAs and their diverse functions in plants. Plant Mol Biol. 2012;80(1):17–36. doi: 10.1007/s11103-011-9817-6. [DOI] [PubMed] [Google Scholar]
- 2.Meister G. Argonaute proteins: Functional insights and emerging roles. Nat Rev Genet. 2013;14(7):447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- 3.Rogers K, Chen X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell. 2013;25(7):2383–2399. doi: 10.1105/tpc.113.113159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallory A, Vaucheret H. Form, function, and regulation of ARGONAUTE proteins. Plant Cell. 2010;22(12):3879–3889. doi: 10.1105/tpc.110.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 6.Siomi H, Siomi MC. Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell. 2010;38(3):323–332. doi: 10.1016/j.molcel.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Xie M, Zhang S, Yu B. microRNA biogenesis, degradation and activity in plants. Cell Mol Life Sci. 2015;72(1):87–99. doi: 10.1007/s00018-014-1728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 10.Han J, et al. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18(24):3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 12.Gregory RI, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432(7014):235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 13.Han J, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125(5):887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO J. 2002;21(17):4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chendrimada TP, et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436(7051):740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, et al. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25(3):522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukunaga R, et al. Dicer partner proteins tune the length of mature miRNAs in flies and mammals. Cell. 2012;151(3):533–546. doi: 10.1016/j.cell.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HY, Zhou K, Smith AM, Noland CL, Doudna JA. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013;41(13):6568–6576. doi: 10.1093/nar/gkt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RC, et al. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol Cell. 2015;57(3):397–407. doi: 10.1016/j.molcel.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurihara Y, Watanabe Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci USA. 2004;101(34):12753–12758. doi: 10.1073/pnas.0403115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez F, Gasciolli V, Crété P, Vaucheret H. The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr Biol. 2004;14(4):346–351. doi: 10.1016/j.cub.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 24.Han MH, Goud S, Song L, Fedoroff N. The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc Natl Acad Sci USA. 2004;101(4):1093–1098. doi: 10.1073/pnas.0307969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS. Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA. 2005;102(10):3691–3696. doi: 10.1073/pnas.0405570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurihara Y, Takashi Y, Watanabe Y. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA. 2006;12(2):206–212. doi: 10.1261/rna.2146906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Liu Z, Lu F, Dong A, Huang H. SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 2006;47(6):841–850. doi: 10.1111/j.1365-313X.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 28.Dong Z, Han MH, Fedoroff N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc Natl Acad Sci USA. 2008;105(29):9970–9975. doi: 10.1073/pnas.0803356105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren G, et al. Regulation of miRNA abundance by RNA binding protein TOUGH in Arabidopsis. Proc Natl Acad Sci USA. 2012;109(31):12817–12821. doi: 10.1073/pnas.1204915109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eamens AL, Kim KW, Curtin SJ, Waterhouse PM. DRB2 is required for microRNA biogenesis in Arabidopsis thaliana. PLoS One. 2012;7(4):e35933. doi: 10.1371/journal.pone.0035933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eamens AL, Wook Kim K, Waterhouse PM. DRB2, DRB3 and DRB5 function in a non-canonical microRNA pathway in Arabidopsis thaliana. Plant Signal Behav. 2012;7(10):1224–1229. doi: 10.4161/psb.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim F, Rohr J, Jeong WJ, Hesson J, Cerutti H. Untemplated oligoadenylation promotes degradation of RISC-cleaved transcripts. Science. 2006;314(5807):1893. doi: 10.1126/science.1135268. [DOI] [PubMed] [Google Scholar]
- 33.Molnár A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature. 2007;447(7148):1126–1129. doi: 10.1038/nature05903. [DOI] [PubMed] [Google Scholar]
- 34.Zhao T, et al. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007;21(10):1190–1203. doi: 10.1101/gad.1543507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casas-Mollano JA, et al. Diversification of the core RNA interference machinery in Chlamydomonas reinhardtii and the role of DCL1 in transposon silencing. Genetics. 2008;179(1):69–81. doi: 10.1534/genetics.107.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim F, et al. Uridylation of mature miRNAs and siRNAs by the MUT68 nucleotidyltransferase promotes their degradation in Chlamydomonas. Proc Natl Acad Sci USA. 2010;107(8):3906–3911. doi: 10.1073/pnas.0912632107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma X, et al. Small interfering RNA-mediated translation repression alters ribosome sensitivity to inhibition by cycloheximide in Chlamydomonas reinhardtii. Plant Cell. 2013;25(3):985–998. doi: 10.1105/tpc.113.109256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamasaki T, et al. Complementarity to an miRNA seed region is sufficient to induce moderate repression of a target transcript in the unicellular green alga Chlamydomonas reinhardtii. Plant J. 2013;76(6):1045–1056. doi: 10.1111/tpj.12354. [DOI] [PubMed] [Google Scholar]
- 39.Voshall A, Kim EJ, Ma X, Moriyama EN, Cerutti H. Identification of AGO3-associated miRNAs and computational prediction of their targets in the green alga Chlamydomonas reinhardtii. Genetics. 2015;200(1):105–121. doi: 10.1534/genetics.115.174797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamasaki T, Kim EJ, Cerutti H, Ohama T. Argonaute3 is a key player in miRNA-mediated target cleavage and translational repression in Chlamydomonas. Plant J. 2016;85(2):258–268. doi: 10.1111/tpj.13107. [DOI] [PubMed] [Google Scholar]
- 41.Valli AA, et al. Most microRNAs in the single-cell alga Chlamydomonas reinhardtii are produced by Dicer-like 3-mediated cleavage of introns and untranslated regions of coding RNAs. Genome Res. 2016;26(4):519–529. doi: 10.1101/gr.199703.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maris C, Dominguez C, Allain FH. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005;272(9):2118–2131. doi: 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- 43.Cléry A, Blatter M, Allain FH. RNA recognition motifs: Boring? Not quite. Curr Opin Struct Biol. 2008;18(3):290–298. doi: 10.1016/j.sbi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Masliah G, Barraud P, Allain FH. RNA recognition by double-stranded RNA binding domains: A matter of shape and sequence. Cell Mol Life Sci. 2013;70(11):1875–1895. doi: 10.1007/s00018-012-1119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gleghorn ML, Maquat LE. ‘Black sheep’ that don’t leave the double-stranded RNA-binding domain fold. Trends Biochem Sci. 2014;39(7):328–340. doi: 10.1016/j.tibs.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang Y, Spector DL. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr Biol. 2007;17(9):818–823. doi: 10.1016/j.cub.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujioka Y, Utsumi M, Ohba Y, Watanabe Y. Location of a possible miRNA processing site in SmD3/SmB nuclear bodies in Arabidopsis. Plant Cell Physiol. 2007;48(9):1243–1253. doi: 10.1093/pcp/pcm099. [DOI] [PubMed] [Google Scholar]
- 48.Song L, Han MH, Lesicka J, Fedoroff N. Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. Proc Natl Acad Sci USA. 2007;104(13):5437–5442. doi: 10.1073/pnas.0701061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren G, Yu B. Critical roles of RNA-binding proteins in miRNA biogenesis in Arabidopsis. RNA Biol. 2012;9(12):1424–1428. doi: 10.4161/rna.22740. [DOI] [PubMed] [Google Scholar]
- 50.Bartel B, Bartel DP. MicroRNAs: At the root of plant development? Plant Physiol. 2003;132(2):709–717. doi: 10.1104/pp.103.023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie Z, et al. Expression of Arabidopsis MIRNA genes. Plant Physiol. 2005;138(4):2145–2154. doi: 10.1104/pp.105.062943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 53.Kim YK, Kim VN. Processing of intronic microRNAs. EMBO J. 2007;26(3):775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morlando M, et al. Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol. 2008;15(9):902–909. doi: 10.1038/nsmb.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kataoka N, Fujita M, Ohno M. Functional association of the Microprocessor complex with the spliceosome. Mol Cell Biol. 2009;29(12):3243–3254. doi: 10.1128/MCB.00360-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szarzynska B, et al. Gene structures and processing of Arabidopsis thaliana HYL1-dependent pri-miRNAs. Nucleic Acids Res. 2009;37(9):3083–3093. doi: 10.1093/nar/gkp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuperus JT, Fahlgren N, Carrington JC. Evolution and functional diversification of MIRNA genes. Plant Cell. 2011;23(2):431–442. doi: 10.1105/tpc.110.082784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kedde M, et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131(7):1273–1286. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 59.Yamasaki T, Ohama T. Involvement of Elongin C in the spread of repressive histone modifications. Plant J. 2011;65(1):51–61. doi: 10.1111/j.1365-313X.2010.04400.x. [DOI] [PubMed] [Google Scholar]
- 60.Harris EH. 1989. The Chlamydomonas Sourcebook. A Comprehensive Guide to Biology and Laboratory Use, (Academic, San Diego)
- 61.Shimogawara K, Fujiwara S, Grossman A, Usuda H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics. 1998;148(4):1821–1828. doi: 10.1093/genetics/148.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamano T, Iguchi H, Fukuzawa H. Rapid transformation of Chlamydomonas reinhardtii without cell-wall removal. J Biosci Bioeng. 2013;115(6):691–694. doi: 10.1016/j.jbiosc.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 63.González-Ballester D, de Montaigu A, Galván A, Fernández E. Restriction enzyme site-directed amplification PCR: A tool to identify regions flanking a marker DNA. Anal Biochem. 2005;340(2):330–335. doi: 10.1016/j.ab.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 64.Yamasaki T, Miyasaka H, Ohama T. Unstable RNAi effects through epigenetic silencing of an inverted repeat transgene in Chlamydomonas reinhardtii. Genetics. 2008;180(4):1927–1944. doi: 10.1534/genetics.108.092395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim SW, et al. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic Acids Res. 2010;38(7):e98. doi: 10.1093/nar/gkp1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Dijk K, et al. Monomethyl histone H3 lysine 4 as an epigenetic mark for silenced euchromatin in Chlamydomonas. Plant Cell. 2005;17(9):2439–2453. doi: 10.1105/tpc.105.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]