Significance
Protein synthesis initiates in eukaryotes when the 40S ribosomal subunit, loaded with initiator tRNA, attaches to the 5′ end of the mRNA, scans the 5′ UTR, and selects the AUG start codon. Ribosome attachment and scanning are impeded by structures in the 5′ UTR that can be resolved by RNA helicases Ded1 and eukaryotic translation initiation factor 4A (eIF4A), with cofactors eIF4B and eIF4G. We show that eIF4B can stimulate translation independently of eIF4A and that eIF4B, eIF4A, and Ded1 are preferentially required for translating long mRNAs, burdened with 5′ UTR structures, that inefficiently form the closed-loop intermediate with the mRNA ends joined by eIF4G. In contrast, eIF4G appears to be most crucial for closed-loop assembly on short, highly translated, and unstructured mRNAs.
Keywords: eIF4B, eIF4A, eIF4G, Ded1, translation
Abstract
DEAD-box RNA helicases eukaryotic translation initiation factor 4A (eIF4A) and Ded1 promote translation by resolving mRNA secondary structures that impede preinitiation complex (PIC) attachment to mRNA or scanning. Eukaryotic translation initiation factor 4B (eIF4B) is a cofactor for eIF4A but also might function independently of eIF4A. Ribosome profiling of mutants lacking eIF4B or with impaired eIF4A or Ded1 activity revealed that eliminating eIF4B reduces the relative translational efficiencies of many more genes than does inactivation of eIF4A, despite comparable reductions in bulk translation, and few genes display unusually strong requirements for both factors. However, either eliminating eIF4B or inactivating eIF4A preferentially impacts mRNAs with longer, more structured 5′ untranslated regions (UTRs). These findings reveal an eIF4A-independent role for eIF4B in addition to its function as eIF4A cofactor in promoting PIC attachment or scanning on structured mRNAs. eIF4B, eIF4A, and Ded1 mutations also preferentially impair translation of longer mRNAs in a fashion mitigated by the ability to form closed-loop messenger ribonucleoprotein particles (mRNPs) via eIF4F–poly(A)-binding protein 1 (Pab1) association, suggesting cooperation between closed-loop assembly and eIF4B/helicase functions. Remarkably, depleting eukaryotic translation initiation factor 4G (eIF4G), the scaffold subunit of eukaryotic translation initiation factor 4F (eIF4F), preferentially impacts short mRNAs with strong closed-loop potential and unstructured 5′ UTRs, exactly the opposite features associated with hyperdependence on the eIF4B/helicases. We propose that short, highly efficient mRNAs preferentially depend on the stimulatory effects of eIF4G-dependent closed-loop assembly.
The translation initiation codon in most eukaryotic mRNAs is identified by the scanning mechanism, which commences with binding of initiator Met-tRNAi to the small (40S) ribosomal subunit in a ternary complex (TC) with eukaryotic translation initiation factor (eIF) 2 and GTP. The resulting 43S preinitiation complex (PIC) attaches to the mRNA 5′ end in a manner facilitated by eIF4F, comprised of cap-binding protein eIF4E, scaffolding protein eIF4G, and DEAD-box RNA helicase eIF4A. The helicase activity of eIF4A is thought to facilitate PIC attachment by resolving secondary structures in cap-proximal mRNA nucleotides. Interactions between eIF4G and eIF3 (in mammals) and eIF5 and eIF1 (in budding yeast) stabilize PIC association with the eIF4F–messenger ribonucleoprotein particles (mRNPs) (reviewed in refs. 1 and 2). Eukaryotic translation initiation factor 4B (eIF4B) also promotes PIC attachment to mRNA, and mammalian eIF4B stimulates the ATPase and RNA helicase activities of eIF4A (1, 3, 4), increases coupling of ATP hydrolysis to duplex unwinding by eIF4A (5), and increases the processivity of eIF4A helicase function (6).
Although yeast eIF4B lacks the C-terminal RNA-binding domain involved in stimulating eIF4A helicase activity by mammalian eIF4B (7–9), recent results suggests that yeast eIF4B and eIF4G equally stimulate ATP hydrolysis and RNA unwinding by eIF4A in the manner expected for increased coupling of ATP hydrolysis to unwinding (10), and they appear to accelerate the transition between the open and closed states of eIF4A (11). In a reconstituted yeast translation system, eIF4B cooperates with eIF4E⋅eIF4G, eIF4A, and eIF3 to promote the rapid assembly of 48S PICs positioned at the start codons of native capped mRNAs (12), enhancing the functional affinity of eIF4A for the PIC (13). Yeast eIF4B binding to the 40S subunit is crucial for its functions in vivo and in vitro, and eIF4B binds to the head domain of the 40S subunit and alters the mRNA entry channel (13), suggesting that eIF4B promotes an open conformation of the 40S subunit conducive to mRNA recruitment or scanning. It is unknown whether eIF4B remodeling of the 40S mRNA entry channel occurs independently of its role as an eIF4A cofactor.
Results from a mammalian reconstituted system indicated that eIF4A can facilitate PIC attachment and scanning through a stem–loop (SL) structure of moderate stability distal from the cap (14), whereas helicases DHX29 and yeast DEAD-box protein Ded1 (an ortholog of mammalian DDX3X) were required to resolve SLs of higher stability (15, 16). Consistently, in yeast cells, a ded1 mutation had a stronger effect than an eIF4A mutation (tif1-A79V in a strain lacking TIF2) on translation of a reporter harboring a long 5′ UTR (17), and a ded1 mutation impaired scanning through a cap-distal SL (18). By ribosome footprint profiling of eIF4A and Ded1 mutants, we previously identified a much larger cohort of mRNAs with a greater-than-average requirement for Ded1 vs. eIF4A in achieving WT translational efficiencies (TEs) and found that Ded1-hyperdependent mRNAs tend to have unusually long or structured 5′ UTRs. That only a small number of mRNAs were found to be hyperdependent on eIF4A, despite a strong reduction in bulk translation in the eIF4A mutant, implied that the absolute TEs of most mRNAs were reduced comparably by inactivation of eIF4A. Nevertheless, moderate reductions in relative TE conferred by the eIF4A mutation were associated with increased propensity for 5′ UTR structure. These results, combined with the differential effects of Ded1 and eIF4A mutations on reporter mRNAs with SLs at different 5′ UTR locations, suggested that Ded1 is crucial for scanning through structured 5′ UTRs, whereas eIF4A performs an essential function common to virtually all mRNAs, e.g., enhancing PIC attachment, and that in the presence of Ded1 eIF4A is either ineffective or dispensable in resolving highly stable structures (19).
To shed light on the functional overlap between eIF4A and eIF4B in yeast cells, we have conducted ribosome profiling of a tif3∆ mutant (lacking eIF4B) under the same growth conditions used previously to profile tif1 and ded1 mutants. If eIF4B acts primarily as a cofactor for eIF4A, then mRNAs whose relative TEs are reduced by impairing eIF4A should be similarly affected by eliminating eIF4B, and few mRNAs should be affected exclusively in cells lacking eIF4B. However, if eIF4B can function independently of eIF4A, e.g., to remodel the 40S mRNA entry channel, then we should identify mRNAs exhibiting a heightened requirement for eIF4B but not for eIF4A. Our results indicate that eIF4B cooperates with eIF4A to stimulate the translation of many mRNAs with a heightened propensity for 5′ UTR secondary structure, as is consistent with eIF4B functioning as an eIF4A cofactor. However, we also identified mRNAs with an enhanced requirement for eIF4B but not for eIF4A and observed distinct effects of eliminating eIF4B and inactivating eIF4A on the expression of reporters with 5′ UTR SL insertions, suggesting that eIF4B also can stimulate translation independently of eIF4A. Interestingly, mRNAs that are hyperdependent on eIF4B, eIF4A, or Ded1 tend to be longer and less prone to forming the closed-loop intermediate mediated by eIF4G interactions with eIF4E bound to the cap and poly(A)-binding protein (Pab1) bound to the poly(A) tail of mRNAs. Closed-loop formation is thought to enhance eIF4F binding to mRNA and to facilitate its functions in PIC recruitment and eIF4A activation and also might enable terminating ribosomes to reinitiate translation efficiently on the same mRNAs (20). Surprisingly, mRNAs found to be hyperdependent on eIF4G have the opposite characteristics, being relatively short, prone to closed-loop formation, and devoid of 5′ UTR structure. We conclude that eIF4G has a critical function in vivo beyond stimulating eIF4A helicase activity; we believe this function involves enhancing closed-loop assembly and the advantages it provides for rapid initiation.
Results
Ribosome Profiling of a tif3Δ Mutant Reveals a Cohort of eIF4B-Hyperdependent Genes.
We previously conducted ribosome footprint profiling of a yeast mutant lacking both chromosomal genes encoding eIF4A (TIF1/TIF2) and harboring the temperature-sensitive (Ts−) allele tif1-A79V as the only source of eIF4A (tif1-ts) and an isogenic strain containing the Ts− allele ded1-952 (ded1-ts). The mutants complemented by WT TIF1 or DED1 alleles were examined in parallel as controls. The strains were cultured at 30 °C followed by a shift to 37 °C for 1 h for the tif1-ts mutant and its WT control or for 2 h for the ded1-ts and WT strains, evoking marked reductions in bulk polysome assembly in the mutant strains (19). To analyze cells lacking eIF4B under similar conditions, a tif3Δ mutant and isogenic TIF3 strain were cultured at 30 °C and shifted to 37 °C for 1 h. Bulk polysomes in tif3Δ cells were reduced to ∼23% of the WT level, a slightly smaller reduction than seen for the tif1-ts strain (17% of WT) and somewhat greater than observed in the ded1-ts mutant (36% of WT) (SI Appendix, Fig. S1 A–C). [Henceforth, results obtained from this experiment are designated as “tif3Δ (37)”.] Because bulk polysomes are diminished in the mutant cells, absolute TEs of most mRNAs will be reduced compared with WT, but these reductions are dampened by normalization to total ribosome footprint reads, and TE changes are determined relative to all other mRNAs. Those genes exhibiting reductions in relative TE in tif3Δ vs. WT cells display a greater-than-average dependence on eIF4B, whereas genes exhibiting increased relative TE in the mutant show a lower-than-average dependence on eIF4B and might even be repressed by eIF4B.
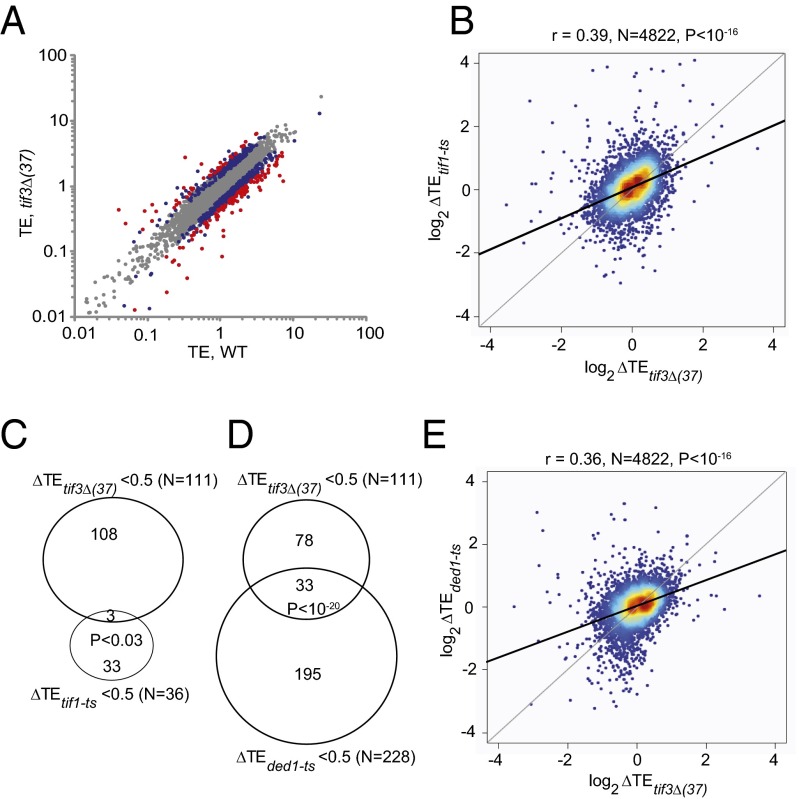
Both ribosome footprinting and RNA-seq results were highly reproducible in biological replicates (Pearson’s r ∼0.99) (SI Appendix, Fig. S2 A–D). Comparing ribosome footprint densities in tif3Δ and WT cells revealed a set of ∼530 genes with substantially altered translation in the mutant in both replicates [SI Appendix, Fig. S2E; red dots indicate a greater than twofold deviation from identity at a false-discovery rate (FDR) <0.01], whereas RNA sequencing (RNA-seq) data identified only about one-half that number with comparable changes in mRNA abundance (SI Appendix, Fig. S2F). By dividing the ribosome footprint reads by the total RNA reads across the coding sequence (CDS), we identified 111 genes whose relative TE (henceforth “relative TE” is referred to simply as “TE”) is twofold or more higher in WT than in tif3Δ cells [ΔTEtif3Δ(37) = TEtif3Δ(37)/TEWT <0.5; FDR <0.01], indicating a heightened dependence on eIF4B; whereas 48 genes displayed less-than-average dependence on eIF4B and exhibited twofold or higher TE in the mutant [ΔTEtif3Δ(37) = TEtif3Δ(37)/TEWT >2.0; FDR <0.01] (Fig. 1A, red dots). We refer to the genes with ΔTEtif3Δ(37) <0.5 as being “hyperdependent on eIF4B.”
Fig. 1.
Correlation between genome-wide changes in TE conferred by the elimination of eIF4B or inactivation of eIF4A or Ded1 at 37 °C. (A) Scatterplot of TEs in tif3Δ vs. WT. Genes exhibiting twofold or greater changes in tif3Δ cells at FDR <0.01 or >1.4-fold changes at FDR <0.05 are highlighted in red and blue, respectively. (B) Density plot of log2∆TE values for 4,822 expressed genes for tif1-ts vs. tif3Δ strains at 37 °C, using tif1-ts data from ref. 19, excluding genes with −6 > log2∆TE >6. The solid line is the determined regression line; the dotted line is the theoretical regression line for identical changes in ∆TE values. Individual genes are shown by blue filled circles; the coloring indicates increased density of genes (red is maximum). (C) Overlap between eIF4B- and eIF4A-hyperdependent genes exhibiting twofold or greater reductions in TE at 37 °C in tif3Δ and tif1-ts strains. (D) Overlap between eIF4B- and Ded1-hyperdependent genes exhibiting twofold or greater reductions in TE at 37 °C in tif3Δ and ded1-ts strains. (E) Density plot of log2∆TE values for 4,822 expressed genes for ded1-ts vs. tif3Δ strains at 37 °C, using ded1-ts data from ref. 19, generated as in B.
Cooperation of eIF4B with eIF4A and Ded1 in Promoting Efficient Translation in Vivo.
In our previous study (19), using the criteria defined above, only 36 genes were found to be hyperdependent on eIF4A. Despite the small number of eIF4A-hyperdependent genes, the changes in TE conferred by tif1-ts and tif3Δ for all genes exhibit a significant positive correlation (Fig. 1B), consistent with functional cooperation between eIF4A and its cofactor eIF4B at a fraction of genes. However, only three genes show an unusually strong dependence on both eIF4A and eIF4B (Fig. 1C). That most eIF4A-hyperdependent genes are not equally dependent on eIF4B could be explained by proposing that eIF4A functions independently of eIF4B at these genes, e.g., by relying exclusively on eIF4G cofactor function. Alternatively, the eIF4A activity in cells lacking eIF4B, although reduced from WT levels, could be sufficient to prevent a strong reduction in TE for most of the 33 eIF4A-hyperdependent genes in tif3Δ cells. On the other hand, our finding that only 3 of the 111 eIF4B-hyperdependent genes are also hyperdependent on eIF4A (Fig. 1C) suggests that eIF4B acts beyond its role as eIF4A cofactor, because hyperdependence on eIF4B should dictate hyperdependence on eIF4A if eIF4B’s only function is enhancing eIF4A activity. An eIF4A-independent function for eIF4B is also consistent with the slope of the trend-line of the ΔTEtif1-ts vs. ΔTEtif3Δ(37) scatterplot being <1 (Fig. 1B), indicating that tif3Δ has a relatively greater effect than tif1-ts on TEs genome-wide despite similar reductions in bulk translation initiation in the two mutants (SI Appendix, Fig. S1 A and C).
Comparing changes in TE conferred by ded1-ts and tif3Δ for all genes also revealed a significant correlation (r = 0.36, P < 10−16) (Fig. 1E) comparable to that observed in the tif1-ts/tif3Δ comparison (r = 0.39) (Fig. 1B). Interestingly, a more substantial overlap of genes hyperdependent on both Ded1 and eIF4B was observed, representing 30% of the eIF4B-hyperdependent genes (Fig. 1D). Including the results of our previous comparison of genome-wide TE changes in ded1-ts vs. tif1-ts cells (19) reveals essentially the same degree of correlation for all three pairwise comparisons (Fig. 2A), whether all genes are compared or only those for which a statistically significant change in TE relative to WT (at FDR <0.05) was observed in any of the three mutants (Fig. 2A, values in parenthesis). These findings suggest similar degrees of functional cooperation of eIF4B with eIF4A and Ded1.
Fig. 2.
Common and distinct changes in TE conferred by tif3Δ, ded1-ts, and tif1-ts mutations at 37 °C. (A) Correlation between TE changes conferred by tif3Δ, ded1-ts, or tif1-ts for all expressed genes (n = 4,822) or for genes with statistically significant TE changes in any of the three mutants (FDR <0.05; n = 1,487). The r values for the latter subset of genes are given in parentheses. P values for all correlations are <10−16. Published ∆TE values for tif1-ts and ded1-ts mutants were used from ref. 19. (B) Hierarchical clustering analysis of ∆TE values conferred by tif1-ts, tif3Δ, and ded1-ts at 37 °C for 1,487 genes with statistically significant TE changes in any of the three mutants (FDR <0.05), presented as a heat-map using the R heatmap.2 function from the R gplots library and the default hclust hierarchical clustering algorithm. Only one gene (YNL042W-B) with >64-fold increase in TE in tif1-ts cells was excluded because including it would diminish the color differences among the remaining genes analyzed in the heat-map. Published ∆TE values for tif1-ts and ded1-ts mutants were used from ref. 19.
The conclusions reached above are reinforced by hierarchical clustering of TE changes for the 1,487 genes exhibiting significant differences from WT in any of the three mutants (Fig. 2B). Thus, a substantial proportion of genes showing reduced TE in tif1-ts cells also display diminished TE in the tif3Δ mutant (Fig. 2B, red bars in left and center columns), as is consistent with the role of eIF4B as eIF4A cofactor. However, numerous genes, including some of the most highly eIF4B-dependent genes, exhibit reduced TE in tif3Δ cells (red) but no change or even increased TE (white or blue) in tif1-ts cells (Fig. 2B). The corresponding ded1-ts heat map supports the conclusion that many genes whose TE is altered in ded1-ts cells are similarly altered in tif1-ts or tif3Δ cells but also reveals many genes with a heightened requirement for Ded1 but not for eIF4A or eIF4B (Fig. 2B, compare the right column with the left or middle column). Thus, although functional cooperation is widespread, many genes exhibit a heightened requirement for only one or two of the three factors.
The 5′ UTRs of eIF4B-Dependent mRNAs Are Atypically Long with Heightened Propensity for Structure.
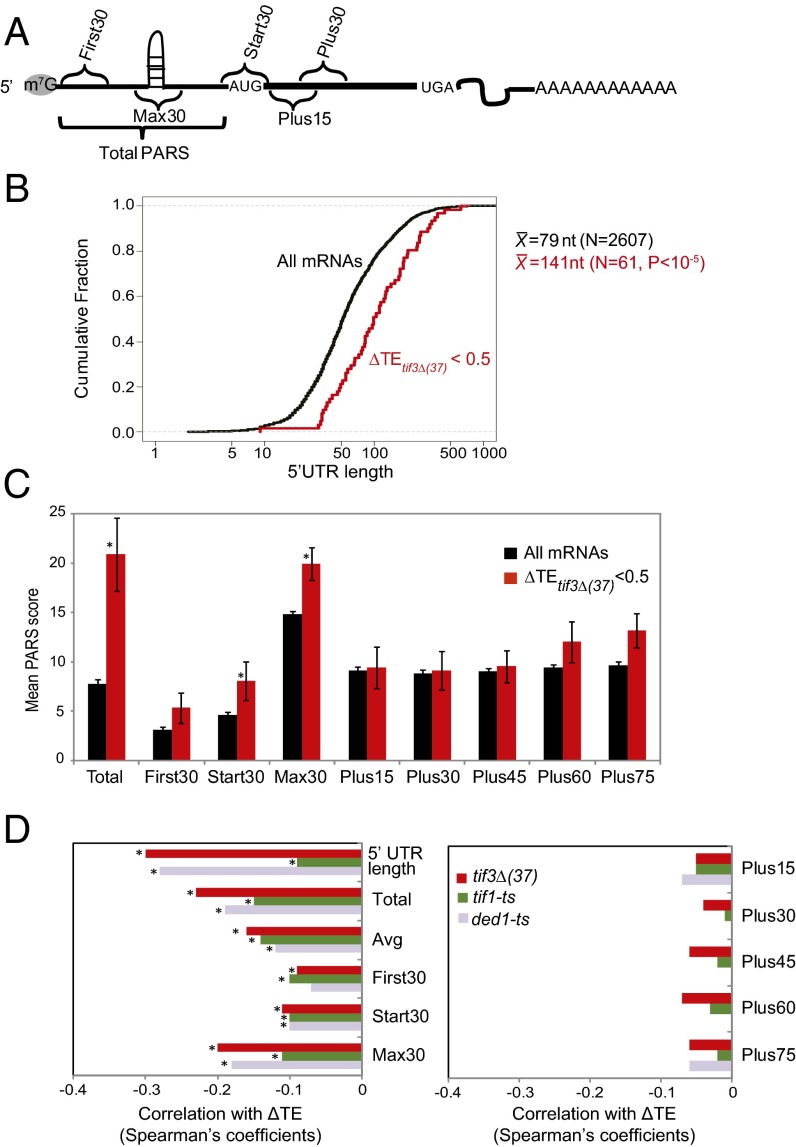
We analyzed the 5′ UTR features of eIF4B-hyperdependent genes using a compilation of 5′ UTR lengths and propensities for secondary structure (21), where each nucleotide in 3,000 different yeast transcripts was assigned a parallel analysis of RNA structure (PARS) score, based on susceptibility to digestion with single- or double-strand–specific nucleases in mRNA reannealed in vitro; higher scores denote greater residency in the double-stranded conformation. For each transcript we calculated several cumulative PARS scores (Fig. 3A), including the sum of scores for all 5′ UTR nucleotides (Total PARS); for the first 30 nucleotides (First30 PARS); for 30 nucleotides surrounding the start codon (Start30 PARS; for genes with a 5′ UTR ≥15 nt); and the highest cumulative score in any 30-nt window across the 5′ UTR (Max30 PARS). We also calculated the average score over the 5′ UTR (Avg PARS) and sums of scores for nucleotides downstream of the AUG, including intervals +1 to +30 (Plus15), +16 to +45 (Plus30), +31 to +60 (Plus45), +46 to +75 (Plus60), and +61 to +90 (Plus75).
Fig. 3.
The 5′ UTRs of eIF4B-dependent genes exhibit longer-than-average length and PARS scores. (A) Schematic showing 5′ UTR and CDS intervals assigned for calculating cumulative PARS scores for each gene. (B) Cumulative distributions of 5′ UTR lengths for all 2,607 genes (black line) or for eIF4B-hyperdependent genes (red line), curated by ref. 21, with the P value indicating a statistically significant difference in mean length (X) for the 61 eIF4B-hyperdependent genes vs. all genes, as determined by the Student’s t test. (C) Average PARS scores calculated for the indicated gene set for each 5′ UTR or CDS interval described in A, with P values from Student’s t tests indicated (*P < 0.05). (D) Spearman coefficients from correlations between ∆TE values conferred by the indicated mutations and 5′ UTR lengths or the indicated cumulative PARS scores for all 2,607 curated genes (*P < 10−16). Results for tif1-ts and ded1-ts mutants are reproduced from ref. 19 for comparison.
Interestingly, the 61 eIF4B-hyperdependent genes for which 5′ UTR PARS scores were available exhibit a mean 5′ UTR length of 140 nt, substantially greater than the mean length of 79 nt for all 2,607 compiled genes examined in our study (P < 0.0001) (Fig. 3B). These genes also exhibit significantly higher mean Total and Max30 PARS scores (P < 0.01) and higher Avg PARS scores (0.06 ± 0.01 vs. 0.18 ± 0.04, P < 0.03) for their 5′ UTRs but have typical PARS scores for all CDS intervals (Fig. 3C). Because there are too few eIF4B-hypodependent genes with available PARS data, we adjusted the threshold to ∆TEtif3Δ(37) >1.4 and compared these 161 genes with a corresponding group of 255 genes with greater-than-average eIF4B dependence [∆TEtif3Δ(37) <0.7]. Similar to our findings for the eIF4B-hyperdependent genes (Fig. 3C), the 255 genes with ∆TEtif3Δ(37) <0.7 show greater-than-average 5′ UTR lengths and PARS scores for all 5′ UTR features except the First30 PARS score (Table 1). Remarkably, the 161 genes least dependent on eIF4B display below-average 5′ UTR lengths and PARS scores for all 5′ UTR features (Table 1), strengthening the association between eIF4B dependence and propensity for 5′ UTR structure. However, neither group of genes shows an atypical propensity for secondary structure in the CDS (Table 1, Plus PARS features).
Table 1.
Comparison of 5′ UTR lengths and PARS features for genes exhibiting significant changes in TE in tif3Δ mutant vs. WT cells at FDR <0.05 at 37 °C
| 5′ UTR feature | All mRNAs, n = 2,607 | ∆TEtif3Δ(37) <0.7, n = 255 | P value | ∆TEtif3Δ(37) >1.4, n = 161 | P value |
| Total length | 79.0 ± 1.6 | 119.3 ± 6.61 | <0.0001 | 60.81 ± 6.57 | <0.006 |
| Total PARS | 7.75 ± 0.49 | 18.55 ± 2.03 | <0.0001 | −2.79 ± 1.68 | <0.0001 |
| Average PARS | 0.058 ± 0.008 | 0.147 ± 0.019 | <0.002 | −0.135 ± 0.049 | <0.0001 |
| First30 PARS | 3.12 ± 0.26 | 4.16 ± 0.744 | 0.24 | −1.37 ± 1.48 | <0.0001 |
| Start30 PARS | 4.59 ± 0.31 | 7.08 ± 0.923 | <0.02 | −0.037 ± 1.58 | <0.0005 |
| Max30 PARS | 14.8 ± 0.31 | 19.31 ± 0.917 | <0.0001 | 8.5 ± 1.50 | <0.0001 |
| Plus15 PARS | 9.15 ± 0.31 | 9.61 ± 0.944 | 0.66 | 7.44 ± 1.45 | 0.19 |
| Plus30 PARS | 8.84 ± 0.33 | 10.15 ± 0.936 | 0.23 | 8.57 ± 1.53 | 0.84 |
| Plus45 PARS | 9.01 ± 0.33 | 10.62 ± 1.002 | 0.14 | 8.99 ± 1.48 | 0.99 |
| Plus60 PARS | 9.38 ± 0.32 | 11.29 ± 1.14 | 0.08 | 8.86 ± 1.4 | 0.70 |
| Plus75 PARS | 9.65 ± 0.33 | 12.06 ± 1.03 | <0.03 | 8.00 ± 1.25 | 0.22 |
The mean (± SEM) 5′ UTR length or indicated PARS feature was calculated for all 2,607 genes or for 255 of the 443 genes having ∆TEtif3Δ(37) <0.7 that were compiled in the PARS database and for 161 of the 270 genes with ∆TEtif3Δ(37) >1.4 at FDR <0.05 that were compiled in the PARS database.
Supporting these last conclusions, analysis of all 2,607 genes in our study with available PARS data revealed significant negative correlations between ΔTEtif3Δ(37) and each 5′ UTR feature, with the strongest associations for length, Total PARS, and Max30 PARS scores of 5′ UTRs (Fig. 3D, Left, red bars), whereas no significant correlations were observed for any CDS features (Fig. 3D, Right, red bars). Interestingly, changes in TE in ded1-ts cells observed previously (19) also show negative correlations with all 5′ UTR features similar in magnitude to those identified here for tif3Δ(37) cells (Fig. 3D, Left, gray bars). Relatively weaker, albeit significant, negative correlations were found for these same features and the ΔTEtif1-ts values measured for the tif1-ts mutant (Fig. 3D, Left, green bars). Thus, greater-than-average 5′ UTR length and propensity for secondary structure are important determinants of heightened dependence on eIF4B, eIF4A, and Ded1.
We extended our comparative analysis of Ded1 and eIF4B dependence with additional ribosome-profiling experiments on the tif3Δ mutant shifted from 30 °C to 15 °C for 10 min, designated “tif3Δ (15),” the regime used previously to inactivate a cold-sensitive ded1-cs mutant (19). Polysome depletion in the tif3Δ mutant (12% of WT) was somewhat greater than seen in ded1-cs (23% of WT) under these conditions (SI Appendix, Fig. S1 D and E). Comparing highly reproducible replicate ribosome footprint and RNA-seq data for tif3Δ and identically cultured WT cells (r values >0.97) revealed a cohort of 156 genes hyperdependent on eIF4B under these conditions (SI Appendix, Fig. S3A). Again, we observed significant genome-wide negative correlations between ΔTEtif3Δ(15) and 5′ UTR length and PARS features (SI Appendix, Fig. S3B) and between TE changes in tif3Δ vs. ded1-cs cells shifted to 15 °C (SI Appendix, Fig. S3C), and there is extensive overlap between genes hyperdependent on eIF4B or Ded1 under these conditions (SI Appendix, Fig. S3D). Thus, eIF4B and Ded1 cooperate at both reduced and elevated growth temperatures to enhance translation of a cohort of genes with a tendency to contain long, structured 5′ UTRs.
In an effort to identify genes for which sequences conferring eIF4B hyperdependence are confined to the 5′ UTR, we analyzed the expression of reporter genes constructed previously (19) containing the promoters and 5′ UTRs of candidate genes fused to luciferase coding sequences (LUC) (SI Appendix, Fig. S4A). Eight reporters were analyzed that displayed appreciable reductions in TE in the tif3Δ mutant at 37 °C, which were driven primarily by reductions in ribosome density with minimal changes in mRNA expression (SI Appendix, Fig. S4B). LUC expression was reduced in tif3Δ vs. WT cells cultured at 37 °C for all eight reporters, and the magnitude of these reductions correlated with reductions in ribosome density measured by ribosome profiling in the tif3Δ(37) experiment (SI Appendix, Fig. S4 B and C). Thus, the 5′ UTRs of these genes contribute to the reductions in TE conferred by elimination of eIF4B in vivo.
eIF4B Is Critically Required for PIC Attachment to Reporter mRNA Burdened with a Cap-Proximal SL.
To provide independent support for our conclusion that the 5′ UTRs of eIF4B hyperdependent genes tend to be atypically long and prone to secondary structure, we examined the effects of tif3Δ on LUC reporters containing SL insertions at sites 5 nt (cap-proximal) or 44 nt (cap-distal) from the 5′ end of a 70-nt 5′ UTR comprised primarily of (CAA)n repeats and thus considered to be relatively unstructured. Because 43S PICs protect ∼45 nt of mRNA (22), we reasoned that the cap-proximal SLs should inhibit PIC attachment, whereas the cap-distal SL insertions should inhibit scanning without impeding 43S attachment.
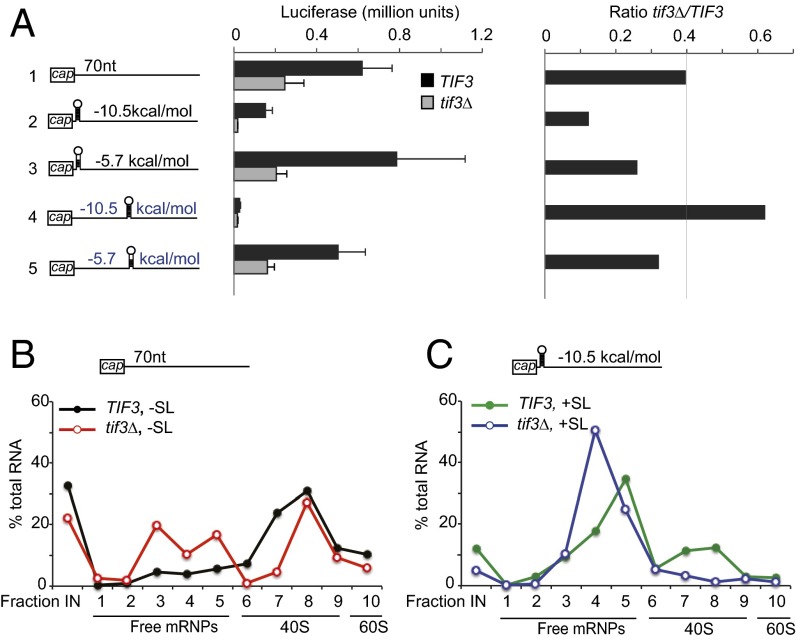
Expression of the reporter lacking an SL insertion (−SL) was reduced by ∼60% in tif3Δ cells (Fig. 4A, Left, row 1, TIF3 vs. tif3Δ), as is consistent with the reduction in bulk translation initiation in this mutant (SI Appendix, Fig. S1A). The presence of SL insertions of moderate predicted stability (−5.7 kcal/mol) at either cap-proximal or cap-distal locations had little or no impact on LUC expression both in WT and tif3Δ cells (Fig. 4A, Left, rows 3 and 5 vs. row 1) and thus did not substantially alter the tif3Δ/TIF3 expression ratio from that observed for the −SL construct (Fig. 4A, Right, rows 3 and 5 vs. row 1). The more stable SL insertions (−10.5 kcal/mol) at cap-proximal or cap-distal locations reduced reporter expression in WT cells relative to that of the −SL reporter, with the cap-distal SL exerting a stronger effect (Fig. 4A, Left, rows 2 and 4 vs. row 1, TIF3). Interestingly, the cap-proximal insertion was substantially more inhibitory in tif3Δ cells, reducing the tif3Δ/TIF3 expression ratio by a factor of approximately threefold compared with the −SL reporter (Fig. 4A, Right, row 2 vs. rows 1 and 3). That eliminating eIF4B exacerbates the inhibitory effect of the stable cap-proximal SL implicates eIF4B in promoting PIC attachment at structured 5′ ends. By contrast, tif3Δ did not further reduce the expression of the reporter harboring the more inhibitory cap-distal SL (Fig. 4A, row 4). Because we showed previously that a highly stable cap-distal SL introduces a strong block to ribosomal scanning that cannot be overcome efficiently in WT cells (19), we suggest that the defect in PIC attachment conferred by the absence of eIF4B in tif3Δ cells is no longer rate-limiting for initiation in the presence of the cap-distal SL.
Fig. 4.
A cap-proximal SL increases eIF4B dependence for the expression of reporter mRNA at the step of 43S PIC attachment. (A, Left) Schema of LUC reporters indicating position and predicted stabilities of SLs inserted into a 70-nt unstructured 5′ UTR, either 5 nt or 44 nt from the cap. LUC expression was determined as in SI Appendix, Fig. S4 for six independent transformants of WT (FJZ046) and tif3Δ (FJZ052) strains for each reporter following approximately three cell doublings in SC-Ura at 23 °C. Mean (± SEM) luciferase expression in WT and tif3Δ cells (Center) and ratios of expression in tif3Δ vs. WT cells (Right) are plotted. (B) WT and tif3Δ transformants harboring reporter plasmids derived from those described in A but with a truncated LUC CDS (78 nt), containing (+SL) or lacking (−SL) the cap-proximal −10.5 kcal/mol SL, were cultured as in A, and cells were cross-linked with 2% HCHO before harvesting. WCEs were resolved by sedimentation through sucrose gradients, and fractions were collected with continuous scanning at 254 nm to locate free 40S and 60S subunits. RNA was extracted, and the abundance of reporter mRNA was quantified by quantitative RT-PCR, conducting three technical replicates for each fraction, and is reported as the percentage of total RNA in fractions 1–10. Highly similar results were obtained in an independent experiment.
To demonstrate that the reduced LUC expression of the strong cap-proximal SL in tif3Δ cells (Fig. 4A, rows 1 and 2) involves diminished PIC attachment, we measured the association of reporter mRNAs containing (+SL) or lacking (−SL) this SL with native 48S PICs and free mRNPs resolved by sedimentation through sucrose density gradients. These reporters were derived from the LUC constructs by truncation of the LUC CDS to 78 nt, which should accommodate only one translating ribosome (23), reducing the mRNA length to allow separation of 48S PICs from free mRNPs. Cells were treated with formaldehyde to prevent dissociation of PICs during sedimentation (24). In WT cells, the SL reduces but does not abolish the association of reporter mRNA with 48S PICs (compare TIF3, +SL in Fig. 4C vs. TIF3, −SL in Fig. 4B), as is consistent with the reduced luciferase expression of the corresponding +SL LUC reporter in WT (Fig. 4A, rows 1 and 2, TIF3). In tif3Δ cells, the +SL mRNA was present almost exclusively in the free mRNP fractions (Fig. 4C, tif3Δ, +SL), whereas an appreciable pool of 48S-associated reporter lacking the SL is retained in tif3Δ cells (Fig. 4B, tif3Δ, −SL). These last results are consistent with the greater reduction in luciferase expression observed for the +SL than for the −SL LUC reporter in the tif3Δ mutant (Fig. 4A, rows 1 and 2, tif3Δ). The findings shown in Fig. 4 B and C indicate that a stable cap-proximal SL increases the requirement for eIF4B to achieve robust PIC attachment and translation initiation.
eIF4B Promotes Efficient Translation of Long mRNAs Underenriched for eIF4E and Pab1 Association.
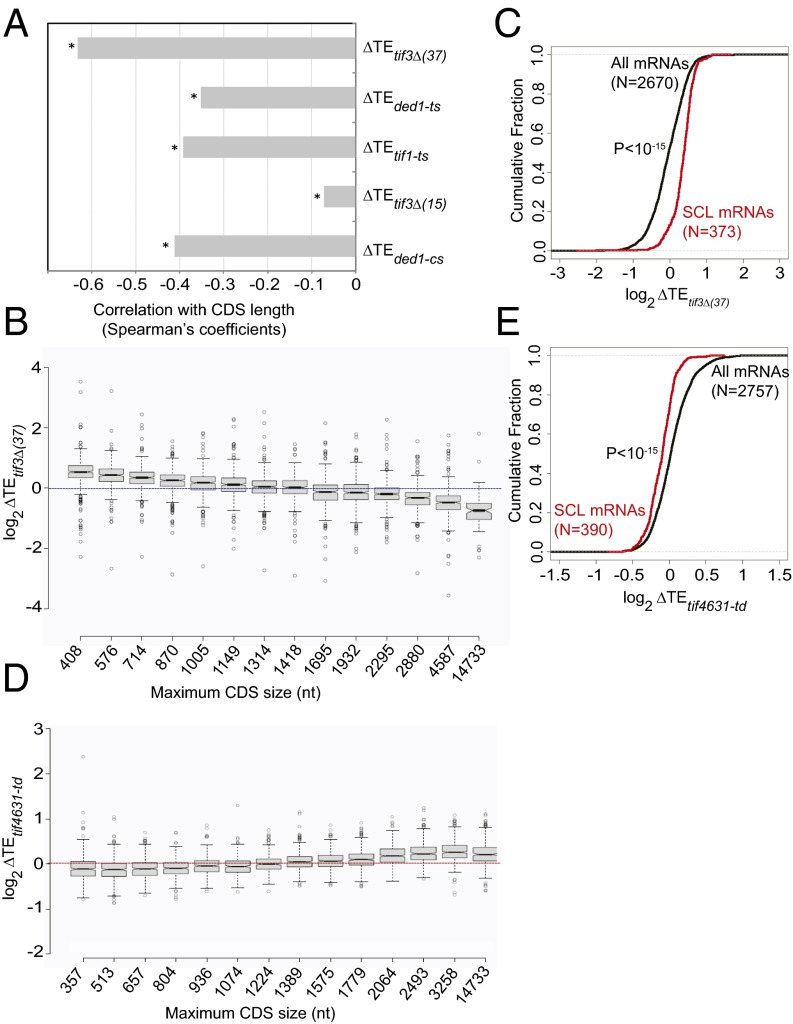
In budding yeast, translation initiation is less efficient on longer mRNAs than on shorter ones (23, 25). Interestingly, we discovered that eIF4B hyperdependence is also linked to mRNA length, because changes in TE conferred by tif3Δ at 37 °C [ΔTEtif3Δ(37)] exhibit a pronounced negative correlation with CDS lengths (Fig. 5A), and a significant but smaller negative correlation also was observed for tif3Δ cells at 15 °C [Fig. 5A, ΔTEtif3Δ(15)]. Negative correlations also were observed for the tif1-ts, ded1-ts, and ded1-cs mutants (Fig. 5A). [Note that transcript length is highly correlated with CDS length in yeast (r = 0.98) (26).] Dividing all genes into 14 bins based on CDS length (Fig. 5B) reveals that reductions in TE occur in tif3Δ cells at 37 °C [ΔTEtif3Δ(37) <1] only for genes with CDS lengths longer than ∼1,500 nt, whereas genes with CDS shorter than ∼1,100 nt exhibit increased TEs in tif3Δ cells (Fig. 5B). Similar findings were made for the ded1-ts and tif1-ts mutants (SI Appendix, Fig. S5A), confirming that longer mRNAs generally display heightened dependence on eIF4A, eIF4B, and Ded1.
Fig. 5.
mRNAs with heightened eIF4B dependence tend to be longer and underenriched in closed-loop–forming initiation factors. (A) Spearman coefficients from correlations between ∆TE values conferred by the indicated mutations vs. CDS lengths for ∼5,000 expressed genes (*P < 10−16). Published ∆TE values for tif1-ts and ded1 mutants were used from ref. 19. (B) Notched box-plots of ∆TE values in the tif3Δ mutant vs. WT at 37 °C for bins of 400 genes of increasing CDS length (except for the last bin, which had 112 genes). Each box depicts the interquartile range containing 50% of the data, intersected by the median; the notch indicates a 95% confidence interval around the median. (C) Cumulative distributions of ∆TE values conferred by tif3Δ at 37 °C for all 2,670 mRNAs or for the 373 SCL mRNAs, characterized for occupancies of eIF4F-, Pab1-, and eIF4E-binding proteins as shown in ref. 28. The P value from a Kolmogorov–Smirnov test of statistical significance of the difference between the two distributions is shown. (D) Notched box-plot plots of ∆TE values in the tif4631-td mutant vs. WT cells constructed as in B using published data from ref. 29. (E) The same analysis as C except using published ∆TE values conferred by eIF4G depletion in the tif4631-td mutant (29).
A possible mechanism underlying the inverse relationship between mRNA length and initiation rate is that longer mRNAs can form more RNA structures involving 5′ UTR base-pairing with CDS sequences that in sum impede PIC attachment or ribosomal scanning (25). Indeed, we discovered genome-wide positive correlations between CDS length and the length of 5′ UTRs [correlation coefficient (ρ) = 0.26; P = e−16], Total PARS scores (ρ = 0.18; P = e−16), and average PARS scores (ρ = 0.12; P = e−16) but not with CDS PARS features. That shorter mRNAs tend to have shorter, less structurogenic 5′ UTRs could help explain their reduced requirement for eIF4B, eIF4A, and Ded1.
To determine whether CDS length also contributes to eIF4B dependence beyond its participation in 5′ UTR structures, we conducted local polynomial regression (LOESS) of ΔTEtif3Δ(37) vs. CDS length, 5′ UTR length, or Total PARS scores and examined correlations between the residuals of each regression and the other two variables (SI Appendix, Fig. S5B). After controlling for CDS length, both 5′ UTR length and Total PARS scores still make significant contributions to the variance of ΔTEtif3Δ(37), although the correlation coefficients are reduced compared with those shown in Fig. 5A for correlations of total ΔTEtif3Δ(37) vs. 5′ UTR or Total PARS scores (compare columns 1 and 2 in SI Appendix, Fig. S5B). Similarly, after controlling for 5′ UTR length, both CDS length and Total PARS scores still contribute significantly (SI Appendix, Fig. S5B, column 3), and after controlling for Total PARS scores, both CDS length and 5′ UTR length make significant contributions to the variance of ΔTEtif3Δ(37) (SI Appendix, Fig. S5B, column 4). This analysis indicates that contributions of 5′ UTR length and RNA structure to variations in ΔTEtif3Δ(37) are at least partly independent of CDS length and that 5′ UTR length per se is an important determinant independent of its contribution to total 5′ UTR structure. Furthermore, CDS length is the strongest determinant of ΔTEtif3Δ(37), independent of its possible involvement in RNA structures formed with 5′ UTR sequences.
Given the strong, independent contribution of long transcript length to eIF4B dependence (SI Appendix, Fig. S5B), we considered the alternative hypothesis that hyperdependence of long mRNAs on eIF4B, eIF4A, and Ded1 reflects their relative inability to form the closed-loop intermediate (27), stabilized by mutual interaction of eIF4G with Pab1 bound to the poly(A) tail and eIF4E bound to the cap. To address this possibility, we interrogated a published dataset describing the genome-wide association of mRNAs with eIF4E, eIF4G1, eIF4G2, Pab1, and the inhibitory eIF4E-binding proteins Caf20 and Eap1, determined by sequencing mRNAs immunoprecipitated with these proteins (RIP-seq) (28). A subset of 395 mRNAs was found to be enriched for eIF4E, eIF4G1, eIF4G2, and Pab1 and depleted for Caf20 and Eap1 and thus was considered to have the greatest potential for closed-loop formation. Remarkably, we found that this “strong closed-loop” (SCL) set of mRNAs exhibits relatively small reductions, or even increases, in TE in the tif3Δ mutant at 37 °C (Fig. 5C), and similar results were found for tif1-ts, ded1-ts, and ded1-cs mutants at their nonpermissive temperatures. Thus, mRNAs considered to be optimized for closed-loop formation tend to be hypodependent on eIF4B, eIF4A, and Ded1.
As might be expected, the SCL mRNAs constitute one of the most highly translated subsets of all yeast mRNAs; however, another subset of highly translated mRNAs is enriched only for Pab1 (28). The latter group (designated “group I”) does not exhibit the increase in TE in tif3Δ cells at 37 °C displayed by the SCL group of mRNAs (designated “group III”) and instead displays a response more typical of all other yeast genes (SI Appendix, Fig. S6A). Thus, robust translation in WT cells does not necessarily dictate hypodependence on eIF4B, which appears to be a specific property of the SCL mRNAs.
As recently reported (26), and as is consistent with previous in vitro analysis of closed-loop assembly by mRNAs of different lengths (27), the mRNAs in the SCL group have a substantially shorter-than-average median CDS length (SI Appendix, Fig. S6B). This observation raises the question of whether shorter length or higher closed-loop potential is the key determinant of the hypodependence of SCL mRNAs on eIF4B, eIF4A, and Ded1. To address this question, we divided the SCL mRNAs into three sets (A, B, and C) based on CDS length and compared their TE changes in tif3Δ cells with length-matched sets of non-SCL mRNAs (SI Appendix, Fig. S6 D–F). Similar to our findings for all SCL mRNAs, in set C also, which contained the longest mRNAs in the SCL group, the tif3Δ mutation conferred a significantly smaller reduction in TE in the SCL than in the “all mRNA” cohort (SI Appendix, Fig. S6 F vs. C). However, this difference was not observed for SCL mRNA sets A and B, which contain the shortest and intermediate CDS lengths, respectively (SI Appendix, Fig. S6 compare D and E vs. C). Thus, a short CDS (or some other feature enriched in shorter mRNAs) is sufficient to confer reduced dependence on eIF4B, and high closed-loop potential does not confer additional independence of eIF4B for shorter mRNAs. For longer mRNAs, by contrast, strong closed-loop potential mitigates the impact of longer mRNA lengths and prevents the marked decline in TE in tif3Δ cells typically observed for longer mRNAs (SI Appendix, Fig. S6F). Thus, it appears that eIF4B function and closed-loop assembly make overlapping contributions to translation of longer mRNAs.
eIF4G-Hyperdependent mRNAs Tend to Be Short, Prone to Closed-Loop Formation, and Devoid of 5′ UTR Structure.
Previously, we examined the genome-wide effects of depleting eIF4G1 in the tif4631-td mutant, which lacks the functionally equivalent isoform eIF4G2, by analyzing changes in mRNA association with large polysomes relative to total mRNA abundance (29). Interestingly, we found that mRNAs with heightened dependence on eIF4G1 tend to have relatively short CDS (Fig. 5D), in contrast to the relationship between ΔTE and CDS length noted above for eIF4B, eIF4A, and Ded1 mutants (Fig. 5B and SI Appendix, Fig. S5A). In addition, we discovered here that TE changes on depletion of eIF4G1 are positively correlated with 5′ UTR length and PARS features (SI Appendix, Fig. S7A), indicating that the eIF4G1-hyperdependent genes have relatively short, unstructured 5′ UTRs, as opposed to the relatively long, structured 5′ UTRs of genes hyperdependent on eIF4B, eIF4A, and Ded1 (Fig. 3D). Consistent with their relatively short CDS lengths and short unstructured 5′ UTRs, the eIF4G1-hyperdependent genes tend to be translated very efficiently in WT cells (29), as indicated by the strong inverse correlation between changes in TE evoked by eIF4G1 depletion and TE values in WT cells (SI Appendix, Fig. S7B). We suggested previously (29) that this trend might be explained by proposing that the closed-loop formation, achieved more readily by shorter mRNAs (27), is a key determinant of the relatively higher TEs of shorter mRNAs in WT cells and that this functional advantage is lost on depletion of eIF4G1. Consistent with this model, as reported recently (26) and illustrated in Fig. 5E, genes belonging to the SCL group with the greatest closed-loop potential (28) are atypically sensitive to depletion of eIF4G—the opposite of our findings that SCL genes are hypodependent on eIF4B, eIF4A, and Ded1 (Fig. 5C). Thus, promoting the closed-loop intermediate for SCL genes and achieving the high-level TEs characterizing these genes in WT cells appears to be the most critical function of eIF4G in determining TEs in vivo. By contrast, it seems that eIF4B, eIF4A, and Ded1 are not crucial for closed-loop assembly and are more critically required for translation of longer mRNAs with a diminished capacity for closed-loop formation and containing longer, more structured 5′ UTRs.
Discussion
We used a combination of ribosome profiling and reporter analysis to probe in vivo functions of eIF4B in the yeast translatome. Because yeast eIF4B was shown to enhance eIF4A helicase activity (10) and stimulate 48S PIC assembly by eIF4A in vitro (13), the relative dependence of mRNAs on eIF4B in vivo could be expected to mirror their dependence on eIF4A. However, because eIF4B interacts directly with the 40S subunit near the mRNA entry channel, and 40S-binding is associated with robust eIF4B function (13), we considered that eIF4B could also act independently of eIF4A to promote translation of particular mRNAs. Our results support the latter possibility but also provide evidence consistent with eIF4B’s known role as cofactor for eIF4A.
By ribosome profiling we identified a cohort of >100 mRNAs that are hyperdependent on eIF4B for robust translation, exhibiting TE reductions of more than twofold in tif3Δ vs. WT cells, and whose 5′ UTRs are significantly longer and more structurogenic than the average 5′ UTR. Considering TE changes of any magnitude genome-wide also reveals that 5′ UTR length and propensity for structure are inversely correlated with TE changes conferred by tif3Δ. Having reached the same conclusion from ribosome profiling of tif1-ts cells with impaired eIF4A function (19), we believe our current results on tif3Δ are consistent with eIF4B acting as a cofactor for eIF4A to resolve 5′ UTR structures that impede 43S PIC attachment or scanning. This notion is further supported by the significant correlation between genome-wide changes in TE in tif3Δ and tif1-ts cells. It is noteworthy, however, that the magnitude of TE changes is greater in cells lacking eIF4B than in cells lacking eIF4A, even though these mutants display similar reductions in bulk translation under our profiling conditions. Likewise, only 36 genes were found to be hyperdependent on eIF4A, and only three belong to the group of 111 genes hyperdependent on eIF4B. We reached a similar conclusion by comparing changes in ribosome occupancies rather than TE, with only 61 genes exhibiting twofold or greater reductions in the tif1-ts mutant; of these 61 genes, only 30 belong to the larger group of 246 genes with comparable reductions in ribosome occupancy in tif3Δ(37) cells at 37 °C. Thus, even if all changes in ribosome occupancy result from altered translation rates (regardless of changes in mRNA abundance), there would still be many fewer genes strongly affected by tif1-ts vs. tif3Δ at 37 °C, as we concluded from analyzing TE changes. The finding that unusually strong dependence on both eIF4A and eIF4B is not widespread is consistent with the model that eIF4B can function in parallel with eIF4A, e.g., by remodeling the 40S mRNA entry channel, in addition to enhancing eIF4A helicase activity. Presumably both modes of eIF4B function are important for mRNAs with structurogenic 5′ UTRs. Our findings are consistent with previous studies indicating that certain mRNAs with structured 5′ UTRs exhibit an unusually strong dependence on mammalian eIF4B (4, 30).
By ribosome profiling of ded1 mutants we previously identified a large cohort of Ded1-hyperdependent genes whose 5′ UTRs also tend to be unusually long and structurogenic (19). Surprisingly, the correlations between genome-wide TE changes in ded1 vs. tif3Δ mutants are similar in magnitude to those observed for tif1-ts vs. tif3Δ cells, and the overlap between Ded1- and eIF4B-hyperdependent genes, ∼40–70% for various ded1 mutations, is greater than the 3% overlap between genes hyperdependent on eIF4B and eIF4A. Thus, extensive functional cooperation occurs between Ded1 and eIF4B that is comparable to, or exceeds, that between eIF4A and eIF4B. Perhaps eIF4B performs an unsuspected cofactor function for Ded1; alternatively, many mRNAs could require independent contributions of eIF4B and Ded1 to enhance PIC attachment or scanning through structured 5′ UTRs, as we suggested previously to explain the functional overlap between eIF4A and Ded1 (19).
Our analysis of LUC reporters with unstructured 5′ UTRs harboring SL insertions revealed that a strong cap-proximal SL confers a nearly complete dependence on eIF4B at the stage of PIC recruitment to the mRNA. Previously, we found that a cap-proximal SL moderately increased the requirement for Ded1 but did not increase the dependence on eIF4A for efficient translation compared with that seen for a reporter with unstructured 5′ UTR (19). We speculated that eIF4A is either ineffective in resolving stable SL structures or dispensable for this reaction in cells containing Ded1. To explain the relatively stronger effect of eliminating eIF4B on a LUC reporter with a stable cap-proximal SL, it could be proposed that eIF4B supports the overlapping functions of Ded1 and eIF4A in resolving such structures or that eIF4B acts independently of the helicases to overcome the inhibitory effect of a stable SL on PIC attachment.
An inverse correlation exists between transcript length and TE in WT yeast (25), which might reflect an increased probability that 5′ UTRs of longer mRNAs engage in secondary structures involving the extended CDS of these mRNAs. Indeed, we found that shorter mRNAs tend to contain short, unstructured 5′ UTRs and they harbor nonstructurogenic sequences surrounding their start codons (31). The inefficient translation of longer mRNAs also could reflect their decreased ability to form the closed-loop intermediate stabilized by eIF4G interactions with eIF4E and Pab1 (27). Interestingly, we found that longer mRNAs tend to be unusually dependent on eIF4B, eIF4A, and Ded1. Although mRNA length, 5′ UTR length, and 5′ UTR structure all contribute independently to eIF4B hyperdependence, mRNA length is the strongest determinant by far. We also observed that mRNAs with the strongest potential for closed-loop assembly (SCL/group III) (28) tend to be hypodependent on eIF4B/eIF4A/Ded1. The SCL group has shorter-than-average transcript lengths (26), however, and we deduced that belonging to the SCL group imparts a translational advantage in cells lacking eIF4B only for longer genes with CDS lengths >700 nt. Thus, eIF4B and the helicases are particularly important for translation of mRNAs with both longer length and reduced capacity for closed-loop formation, whereas shorter mRNAs are relatively independent of eIF4B/eIF4A/Ded1 regardless of their closed-loop potential. The tendency toward short, unstructured 5′ UTRs also contributes to the relative independence of short mRNAs on eIF4B and the helicases.
Interestingly, interrogating data from our previous study (32) revealed that yeast eIF4G is most critically required for mRNAs that are relatively short, have a high probability for closed-loop assembly (26), have relatively short and unstructured 5′ UTRs, and exhibit greater-than-average TEs in WT cells. Similarly, depletion of eIF4G was found to diminish translation of short vs. long genes preferentially in Caenorhabditis elegans, a condition that is exploited via down-regulation of eIF4G expression in starved animals to up-regulate long, stress-response genes (33). The remarkable fact that depleting eIF4G preferentially impairs the translation of the categories of mRNAs least impacted by inactivating eIF4B, eIF4A or Ded1 implies that eIF4G carries out a function beyond stimulating the unwinding of 5′ UTR structures by eIF4A or Ded1 and that this additional function is required for the robust translation of short mRNAs with strong closed-loop potential.
An obvious possibility for this additional function of eIF4G is promoting closed-loop assembly, because forming this intermediate is favored for eIF4G-hyperdependent mRNAs by their relatively short transcript lengths (27) and high eIF4F and Pab1 occupancies (28). Closed-loop assembly stabilizes eIF4F binding to mRNA (32, 34), which should enhance eIF4G functions in recruiting the 43S PIC, through its direct interactions with eIFs 1 and 5 (35), and in recruiting or activating eIF4A and Ded1 (36). The diminished capacity of many long mRNAs to form the closed-loop and thereby stabilize eIF4G binding should render them particularly sensitive to mutations in eIF4A, Ded1, or eIF4B that reduce helicase activity or eIF4B function in PIC recruitment or scanning, thus helping to explain the tendency of long mRNAs to be hyperdependent on the helicases/eIF4B. Closed-loop assembly also might facilitate reinitiation by ribosomes recycled at the stop codon of the mRNA and delivered to the 5′ end of the same transcript (37, 38); this function could be particularly important for achieving the high-level translation of small mRNAs with strong closed-loop potential. By eliminating the multiple advantages of closed-loop assembly in PIC recruitment and reinitiation, depletion of eIF4G should disproportionately impair translation of the short, highly efficient mRNAs optimized for closed-loop assembly and have relatively lesser effects on the longer, more structured mRNAs that form the closed loop poorly at WT levels of eIF4G. The resulting strong reduction in translation of the former highly efficient mRNAs should allow the longer, more structured mRNAs to compete better for limiting PICs, offsetting the diminished PIC recruitment evinced by eIF4G depletion and attendant diminished eIF4A and Ded1 activity for these less efficient mRNAs. If, as seems plausible, the helicases/eIF4B are not critically required for closed-loop assembly, then helicase/eIF4B inactivation should produce the observed small reductions in the translation of highly efficient short mRNAs, which are also depleted of 5′ UTR structures. Their relative TEs frequently increase in the helicase/eIF4B mutants because of reduced competition from the longer, more structured mRNAs that have greater dependence on helicase/eIF4B activities.
In conclusion, although eIF4G is a cofactor for eIF4A and also appears to regulate Ded1 (36), its most critical role in yeast appears to be stabilizing closed-loop assembly. As a result, depleting eIF4G most strongly impairs the translation of short, unstructured mRNAs with the highest closed-loop potential, whereas eliminating eIF4B or inactivating eIF4A and Ded1 most strongly impairs mRNAs with exactly the opposite features.
Methods
Construction of Yeast Strains and Plasmids.
Yeast strains FJZ046 (MATa his3Δ1 leu2Δ0 lys2Δ0 met15Δ0 ura3Δ0 TIF3) and FJZ052 (MATa his3Δ1 leu2Δ0 lys2Δ0 met15Δ0 ura3Δ0 tif3Δ::hisG) were described previously (13). Plasmids used are listed in SI Appendix, Table S1, and generated as described in the SI Appendix.
Yeast Biochemical Methods.
Methods for analysis of bulk polysome profiles, assays of LUC reporters in whole cell extracts (WCEs) (19), and association of reporter mRNA with native 40S subunits (13) were described previously; details are provided in the SI Appendix.
Ribosome Footprint Profiling and RNA-Seq.
Ribosome profiling was conducted essentially as described (39, 40), as detailed in the SI Appendix, on isogenic tif3Δ (FJZ052) and WT (FJZ046) cells cultured in synthetic complete (SC) medium and treated with 100 ug/mL cycloheximide for 2 min before harvesting. Statistical analysis of differences in ribosome footprint, RNA-seq read counts, or TE values between WT and mutant samples was conducted using DESeq (41) (tabulated in Dataset S1). Genes with fewer than 128 total mRNA reads in the four samples combined (two replicates of both WT and mutant strains) were excluded from the calculation of TE values.
Supplementary Material
Acknowledgments
We thank members of the A.G.H. laboratory and the Dever and Lorsch groups for many helpful suggestions; Wendy Gilbert and colleagues for critical insights about the closed-loop potential of short eIF4G-dependent genes; Sumit Sen, Razvan Chereji, and Tingfen Yan for help with bioinformatics; and Philip McQueen and Peter Munson for advice on statistical analysis. N.T.I. was supported by Searle Scholars Program Grant 11-SSP-229 and National Institute of Environment Health Sciences Grant R21 ES22575-01. This work was supported in part by the Intramural Research Program of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Data deposition: Sequencing data from this study have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo/ (accession no. GSE81966).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612398113/-/DCSupplemental.
References
- 1.Pestova TV, Lorsch JR, Hellen CUT. The mechanism of translation initiation in eukaryotes. In: Mathews M, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2007. pp. 87–128. [Google Scholar]
- 2.Hinnebusch AG, Lorsch JR. The mechanism of eukaryotic translation initiation: New insights and challenges. Cold Spring Harb Perspect Biol. 2012;4(10):a011544. doi: 10.1101/cshperspect.a011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altmann M, et al. A Saccharomyces cerevisiae homologue of mammalian translation initiation factor 4B contributes to RNA helicase activity. EMBO J. 1993;12(10):3997–4003. doi: 10.1002/j.1460-2075.1993.tb06077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahbazian D, et al. Control of cell survival and proliferation by mammalian eukaryotic initiation factor 4B. Mol Cell Biol. 2010;30(6):1478–1485. doi: 10.1128/MCB.01218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Özeş AR, Feoktistova K, Avanzino BC, Fraser CS. Duplex unwinding and ATPase activities of the DEAD-box helicase eIF4A are coupled by eIF4G and eIF4B. J Mol Biol. 2011;412(4):674–687. doi: 10.1016/j.jmb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-García C, Frieda KL, Feoktistova K, Fraser CS, Block SM. RNA biochemistry. Factor-dependent processivity in human eIF4A DEAD-box helicase. Science. 2015;348(6242):1486–1488. doi: 10.1126/science.aaa5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Méthot N, Pause A, Hershey JW, Sonenberg N. The translation initiation factor eIF-4B contains an RNA-binding region that is distinct and independent from its ribonucleoprotein consensus sequence. Mol Cell Biol. 1994;14(4):2307–2316. doi: 10.1128/mcb.14.4.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naranda T, Strong WB, Menaya J, Fabbri BJ, Hershey JW. Two structural domains of initiation factor eIF-4B are involved in binding to RNA. J Biol Chem. 1994;269(20):14465–14472. [PubMed] [Google Scholar]
- 9.Rozovsky N, Butterworth AC, Moore MJ. Interactions between eIF4AI and its accessory factors eIF4B and eIF4H. RNA. 2008;14(10):2136–2148. doi: 10.1261/rna.1049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreou AZ, Klostermeier D. eIF4B and eIF4G jointly stimulate eIF4A ATPase and unwinding activities by modulation of the eIF4A conformational cycle. J Mol Biol. 2014;426(1):51–61. doi: 10.1016/j.jmb.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Harms U, Andreou AZ, Gubaev A, Klostermeier D. eIF4B, eIF4G and RNA regulate eIF4A activity in translation initiation by modulating the eIF4A conformational cycle. Nucleic Acids Res. 2014;42(12):7911–7922. doi: 10.1093/nar/gku440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell SF, et al. The 5′-7-methylguanosine cap on eukaryotic mRNAs serves both to stimulate canonical translation initiation and to block an alternative pathway. Mol Cell. 2010;39(6):950–962. doi: 10.1016/j.molcel.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker SE, et al. Yeast eIF4B binds to the head of the 40S ribosomal subunit and promotes mRNA recruitment through its N-terminal and internal repeat domains. RNA. 2013;19(2):191–207. doi: 10.1261/rna.035881.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pestova TV, Hellen CUT, Shatsky IN. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol Cell Biol. 1996;16(12):6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisareva VP, Pisarev AV, Komar AA, Hellen CU, Pestova TV. Translation initiation on mammalian mRNAs with structured 5'UTRs requires DExH-box protein DHX29. Cell. 2008;135(7):1237–1250. doi: 10.1016/j.cell.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abaeva IS, Marintchev A, Pisareva VP, Hellen CU, Pestova TV. Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs during ribosomal scanning. EMBO J. 2011;30(1):115–129. doi: 10.1038/emboj.2010.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthelot K, Muldoon M, Rajkowitsch L, Hughes J, McCarthy JE. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol Microbiol. 2004;51(4):987–1001. doi: 10.1046/j.1365-2958.2003.03898.x. [DOI] [PubMed] [Google Scholar]
- 18.Chiu WL, et al. The C-terminal region of eukaryotic translation initiation factor 3a (eIF3a) promotes mRNA recruitment, scanning, and, together with eIF3j and the eIF3b RNA recognition motif, selection of AUG start codons. Mol Cell Biol. 2010;30(18):4415–4434. doi: 10.1128/MCB.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen ND, Zhou F, Ingolia NT, Hinnebusch AG. Genome-wide analysis of translational efficiency reveals distinct but overlapping functions of yeast DEAD-box RNA helicases Ded1 and eIF4A. Genome Res. 2015;25(8):1196–1205. doi: 10.1101/gr.191601.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachs A. Physical and functional interactions between the mRNA cap structure and the poly(A) tail. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2000. pp. 447–465. [Google Scholar]
- 21.Kertesz M, et al. Genome-wide measurement of RNA secondary structure in yeast. Nature. 2010;467(7311):103–107. doi: 10.1038/nature09322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak M. Nucleotide sequences of 5′-terminal ribosome-protected initiation regions from two reovirus messages. Nature. 1977;269(5627):391–394. doi: 10.1038/269390a0. [DOI] [PubMed] [Google Scholar]
- 23.Arava Y, et al. Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2003;100(7):3889–3894. doi: 10.1073/pnas.0635171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen KH, Valásek L. In vivo deletion analysis of the architecture of a multiprotein complex of translation initiation factors. Methods Enzymol. 2007;431:15–32. doi: 10.1016/S0076-6879(07)31002-1. [DOI] [PubMed] [Google Scholar]
- 25.Arava Y, Boas FE, Brown PO, Herschlag D. Dissecting eukaryotic translation and its control by ribosome density mapping. Nucleic Acids Res. 2005;33(8):2421–2432. doi: 10.1093/nar/gki331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson MK, Rojas-Duran MF, Gangaramani P, Gilbert WV. The ribosomal protein Asc1/RACK1 is required for efficient translation of short mRNAs. eLife. 2016;5:5. doi: 10.7554/eLife.11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amrani N, Ghosh S, Mangus DA, Jacobson A. Translation factors promote the formation of two states of the closed-loop mRNP. Nature. 2008;453(7199):1276–1280. doi: 10.1038/nature06974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello J, et al. Global mRNA selection mechanisms for translation initiation. Genome Biol. 2015;16:10. doi: 10.1186/s13059-014-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park EH, Zhang F, Warringer J, Sunnerhagen P, Hinnebusch AG. Depletion of eIF4G from yeast cells narrows the range of translational efficiencies genome-wide. BMC Genomics. 2011;12(1):68. doi: 10.1186/1471-2164-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dmitriev SE, Terenin IM, Dunaevsky YE, Merrick WC, Shatsky IN. Assembly of 48S translation initiation complexes from purified components with mRNAs that have some base pairing within their 5′ untranslated regions. Mol Cell Biol. 2003;23(24):8925–8933. doi: 10.1128/MCB.23.24.8925-8933.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y, Shah P, Plotkin JB. Weak 5′-mRNA secondary structures in short eukaryotic genes. Genome Biol Evol. 2012;4(10):1046–1053. doi: 10.1093/gbe/evs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park EH, et al. Multiple elements in the eIF4G1 N-terminus promote assembly of eIF4G1•PABP mRNPs in vivo. EMBO J. 2011;30(2):302–316. doi: 10.1038/emboj.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers AN, et al. Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in C. elegans. Cell Metab. 2011;14(1):55–66. doi: 10.1016/j.cmet.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanagiya A, et al. Requirement of RNA binding of mammalian eukaryotic translation initiation factor 4GI (eIF4GI) for efficient interaction of eIF4E with the mRNA cap. Mol Cell Biol. 2009;29(6):1661–1669. doi: 10.1128/MCB.01187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He H, et al. The yeast eukaryotic initiation factor 4G (eIF4G) HEAT domain interacts with eIF1 and eIF5 and is involved in stringent AUG selection. Mol Cell Biol. 2003;23(15):5431–5445. doi: 10.1128/MCB.23.15.5431-5445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilliker A, Gao Z, Jankowsky E, Parker R. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol Cell. 2011;43(6):962–972. doi: 10.1016/j.molcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchida N, Hoshino S, Imataka H, Sonenberg N, Katada T. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent\ translation. J Biol Chem. 2002;277(52):50286–50292. doi: 10.1074/jbc.M203029200. [DOI] [PubMed] [Google Scholar]
- 38.Kopeina GS, et al. Step-wise formation of eukaryotic double-row polyribosomes and circular translation of polysomal mRNA. Nucleic Acids Res. 2008;36(8):2476–2488. doi: 10.1093/nar/gkm1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingolia NT. Genome-wide translational profiling by ribosome footprinting. Methods Enzymol. 2010;470:119–142. doi: 10.1016/S0076-6879(10)70006-9. [DOI] [PubMed] [Google Scholar]
- 40.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324(5924):218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.