Significance
We show that vascular pericytes significantly contribute to cancer invasion and metastasis by the mechanism of the pericyte–fibroblast transition (PFT). This study proposes this concept and indicates the vascular pericyte’s role. Vascular pericytes were considered to remodel tumor vessels toward a mature phenotype. However, once dissociated from tumor vessels their functions within the tumor tissue are not known. In the present study, we show that pericytes, once detached from tumor microvasculatures, underwent differentiation to become stromal fibroblasts, which are known to contribute to tumor invasion and metastasis. Our results show that vascular pericytes are the important source of stromal fibroblasts and targeting PFT may offer a new treatment option in cancer metastasis.
Keywords: pericyte, PDGF, fibroblast, metastasis, mesenchymal cell
Abstract
Vascular pericytes, an important cellular component in the tumor microenvironment, are often associated with tumor vasculatures, and their functions in cancer invasion and metastasis are poorly understood. Here we show that PDGF-BB induces pericyte–fibroblast transition (PFT), which significantly contributes to tumor invasion and metastasis. Gain- and loss-of-function experiments demonstrate that PDGF-BB-PDGFRβ signaling promotes PFT both in vitro and in in vivo tumors. Genome-wide expression analysis indicates that PDGF-BB–activated pericytes acquire mesenchymal progenitor features. Pharmacological inhibition and genetic deletion of PDGFRβ ablate the PDGF-BB–induced PFT. Genetic tracing of pericytes with two independent mouse strains, TN-AP-CreERT2:R26R-tdTomato and NG2-CreERT2:R26R-tdTomato, shows that PFT cells gain stromal fibroblast and myofibroblast markers in tumors. Importantly, coimplantation of PFT cells with less-invasive tumor cells in mice markedly promotes tumor dissemination and invasion, leading to an increased number of circulating tumor cells and metastasis. Our findings reveal a mechanism of vascular pericytes in PDGF-BB–promoted cancer invasion and metastasis by inducing PFT, and thus targeting PFT may offer a new treatment option of cancer metastasis.
The tumor microenvironment possesses diverse cellular components including malignant cells, inflammatory cells, stromal fibroblasts, various progenitor cells, endothelial cells, and perivascular cells. These tumor-associated cellular components constantly change their identities and functions to cope with tumor growth and invasiveness (1). Tumor cells often produce various growth factors and cytokines as instrumental signals to manipulate host cells that in most cases facilitate tumor growth and metastasis. Although the role of endothelial, inflammatory, and mesenchymal cells in tumor growth and invasion have been extensively studied (2–10), functions of pericytes in the tumor microenvironment remain largely unknown.
Pericytes are mainly described as mural cells that are associated with vasculatures in various healthy and pathological tissues (11). Recruitment of pericytes to newly formed angiogenic vessels prevents excessive sprouting, stabilizes the nascent vasculature, prevents vascular leakiness, and remodels primitive vasculatures toward mature vasculatures (12, 13). It is believed that these vasculature-related pericyte functions could possibly impede tumor invasion and metastasis. Recent studies show that pericytes retain progenitor cell properties and can differentiate into other cells, including adipocytes, chondrocytes, osteoblasts, phagocytes, granulocytes, and skeletal muscle (14–18). Under pathological conditions, pericytes may differentiate into myofibroblasts, contributing to kidney fibrosis (19, 20). Unlike most host cells in tumors, pericytes exhibit unique features by expressing a number of surface markers and respond to certain growth factors. The PDGF-BB-PDGFRβ signaling pathway is best known for its modulation of pericyte coverage on microvessels, and endothelial cells are the primary source of PDGF-BB (11). Endothelial cell-derived PDGF-BB recruits pericytes onto angiogenic vessels through activation of PDGFRβ. Deletion of Pdgfb or Pdgfrß genes in mice resulted in vascular defect-related embryonic lethality due to lack of pericytes (21, 22), indicating the pivotal role of PDGF-BB-PDGFRβ signaling in pericyte biology. Various tumors also express a high level of PDGF-BB and the impact of tumor cell-derived PDGF-BB on pericytes is controversial. In contrast to endothelial cells, tumor cell-derived PDGF-BB may potentially attract pericytes to migrate from vessels through a chemoattractant gradient mechanism (23). The fate of vessel disassociated pericyte (PC) has not been clearly investigated. PDGF receptor signaling has been suggested to contribute to pericyte–myofibrolast transition in kidney fibrosis (19).
CAFs, especially myofibroblasts, are known to facilitate tumor invasion and metastasis (6). In certain tumor types such as pancreatic cancers, CAFs dominate tumor cellular components and constitute a major part of the tumor mass (24). Unresolved key issues include the origin of CAFs and the signaling pathways that control the CAF population in tumor tissues. Could it be possible that other host cells in tumors differentiate into CAFs under the influence of specific signaling systems? In the present study, we provide evidence to demonstrate that vascular pericytes are an important source of CAFs, which markedly promote cancer metastasis. The pericyte–CAF transition is modulated by PDGF-BB-PDGFRβ signaling through the mechanism of pericyte–fibroblast transition (PFT). Gain- and loss-of-function experiments using genetic and pharmacological approaches validate that PFT in tumors is the primary driving force for cancer invasion and metastasis. Genetic tracing in tumor-bearing mice demonstrates that a significant number of pericytes undergo PFT. Finally, we provide evidence that PDGF-BB expression levels and fibroblast components correlate with poor survival in patients with different cancer types. Thus, our findings are clinically relevant and shed mechanistic insights on pericyte-mediated cancer metastasis and suggest that targeting PFT may potentially offer a new therapeutic option for cancer treatment.
Results
PDGF-BB Expression and Stromal Fibroblast Components in Naturally Occurring Human Tumors.
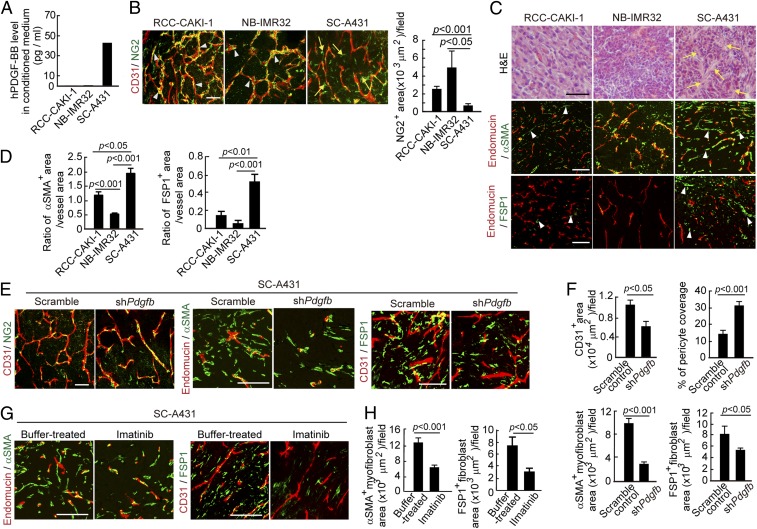
To study the role of PDGF-BB, the pluripotent member of the PDGF family, in supporting CAFs, we analyzed expression levels of PDGF-BB mRNA and protein in a dozen human tumor cell lines. We previously reported a high PDGF-BB expression level in SC-A431 squamous carcinoma tumor tissue and a low PDGF-BB expression level in NB-IMR32 neuroblastoma tumor tissue (23). We have now validated those in vivo findings with in vitro cell cultures. Naturally occurring SC-A431 squamous carcinoma cells expressed a high level of PDGF-BB (40 pg/mL) in a 48-h-conditioned medium (Fig. 1A). In contrast, a renal cell carcinoma (RCC-CAKI-1) and a neuroblastoma (NB-IMR32) have undetectable levels of PDGF-BB.
Fig. 1.
Knockdown of PDGF-BB expression and inhibition of PDGFR signaling decrease stromal fibroblasts. (A) Expression levels of PDGF-BB protein in RCC-CAKI-1, NB-IMR32, and SC-A431 tumor cells in conditioned medium. (B) Tumor microvessels and stromal components. CD31+ (red) endothelial cell and NG2+ pericyte signals (green) in various tumors. Arrowheads point to vessel-associated pericytes; arrows indicate vessel-disassociated pericytes. Quantification of NG2+ pericyte area (n = 7 random fields per group). (Scale bar, 50 μm.) (C, Top) H&E staining of tumor tissues. Arrows point to tumor stroma. (Scale bar, 100 μm.) (Middle) Endomucin+ tumor vessels (red) and αSMA+ cells (green). Arrowheads point to αSMA+ myofibroblasts. (Scale bar, 100 μm.) (Bottom) Endomucin+ tumor vessels (red) and FSP1+ stromal fibroblasts (green). Arrowheads point to stromal fibroblasts. n = 4 animals per group. (Scale bar, 100 μm.) (D) Quantification of the ratio of αSMA+ signals vs. microvascular areas (n = 12 random fields per group) and the ratio of FSP1+ signals vs. microvascular areas (n = 12 random fields per group) in various human tumors. (E) CD31+ (red) endothelial cell and NG2+ pericyte signals (green), endomucin+ tumor vessels (red) and αSMA+ cells (green), and CD31+ tumor vessels (red) and FSP1+ stromal fibroblasts (green) in scrambled-shRNA and Pdgfb-shRNA–transfected SC-A431tumors. (Scale bars: left panels, 50 μm; middle and right panels, 100 μm.) (F) Quantification of microvessel density (n = 7 random fields per group), pericyte coverage (n = 7 random fields per group), αSMA+ myofibroblast signals (n = 12 random fields per group), and FSP1+ signals (n = 12 random fields per group) in scrambled-shRNA and Pdgfb-shRNA–transfected SC-A431tumors. (G) Endomucin+ tumor vessels (red) and αSMA+ cells (green) and CD31+ tumor vessels (red) and FSP1+ stromal fibroblasts (green) in imatinib- and buffer-treated SC-A431tumors. (Scale bar, 100 μm.) (H) Quantification of αSMA+ myofibroblast signals (n = 12 fields per group) and FSP1+ signals (n = 12 random fields per group) in imatinib- and buffer-treated SC-A431tumors. Data are represented as mean ± SEM.
Immunohistochemical staining of tumor tissues showed that these tumors contained high densities of microvessels and decreased NG2+ pericyte area (Fig. 1B) (23). SC-A431 tumors possessed high content of fibroblast-specific protein 1 (FSP1)+ stromal fibroblast and α-smooth muscle actin (αSMA)+ myofibroblast components (Fig. 1 C and D). To exclude the possibility that αSMA+ cell populations were vascular smooth muscle cells (VSMCs), tumor tissues were costained with αSMA and endomucin (a pan-endothelial cell specific marker). Notably, the majority of αSMA+ cell populations did not show association with endomucin+ positive signals (Fig. 1C), suggesting that the majority of αSMA+ cells were myofibroblasts but not VSMCs. In contrast to SC-A431, both RCC-CAKI-1 and NB-IMR32 tumors have much fewer FSP1+ and αSMA+ fibroblast components. These findings suggest a possible link between PDGF-BB expression and high content of CAFs in naturally occurring human tumors.
Genetic Knockdown and Pharmacological Inhibition of the PDGF-BB-PDGFR Signaling Ablates CAFs in Human Tumors.
To investigate the relation between PDGF-BB expression and the stromal CAF composition in SC-A431 tumors, Pdgfb mRNA was knocked down using a specific shRNA. This approach effectively ablated the expression level of Pdgfb (Fig. S1A). Knockdown of Pdgfb significantly increased pericyte coverage in tumor microvessels (Fig. 1 E and F). Conversely, FSP1+ and αSMA+ stromal fibroblasts and myofibrolasts were markedly decreased in Pdgfb-shRNA–transfected compared with scrambled-shRNA–transfected SC-A431 tumors (Fig. 1 E and F).
Fig. S1.

Knockdown efficiency of shPdgfb in SC-A431 tumor cells. Pdgfb mRNA expression levels in scrambled- and Pdgfb-shRNA transfected SC-A431 tumor cells (n = 3 independent measurements/group). Data are represented as mean ± SEM.
To further validate these findings, SC-A431 tumors were treated with imatinib, a BCL-ABL tyrosine kinase inhibitor with a potential inhibitory effect on the PDGFR signaling (25–27). Imatinib inhibited SC-A431 tumor growth and the tumor growth of other cell lines as previously described (23, 28). Similar to the shRNA knockdown approach, imatinib treatment also restored pericyte coverage in tumor microvessels as previously described (23). Importantly, treatment with imatinib markedly reduced the stromal FSP1+ fibroblast and αSMA+ myofibroblast components in tumors (Fig. 1 G and H). These data demonstrate that the PDGF-BB-PDGFR signaling significantly contributes to increases of CAFs in naturally occurring human tumors.
Gain of Function of PDGF-BB in Mouse Tumors Enhances CAFs and Decreases Pericytes.
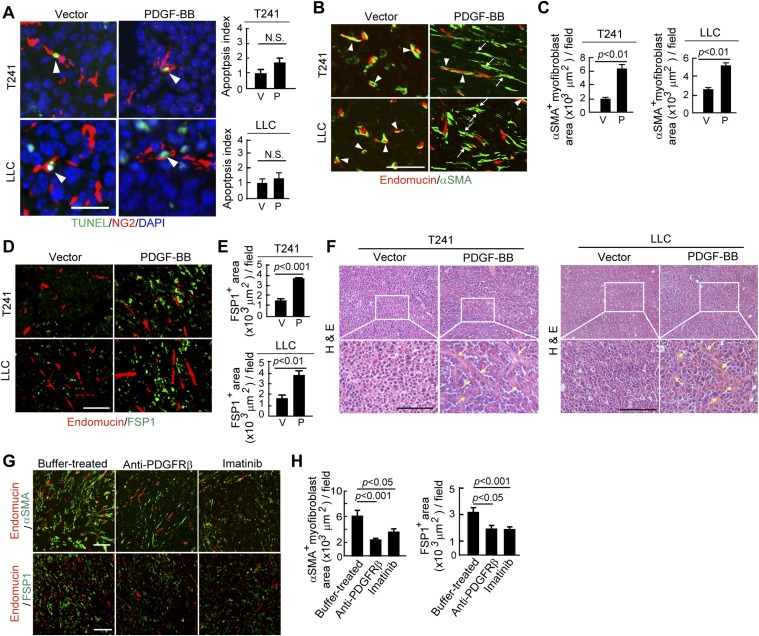
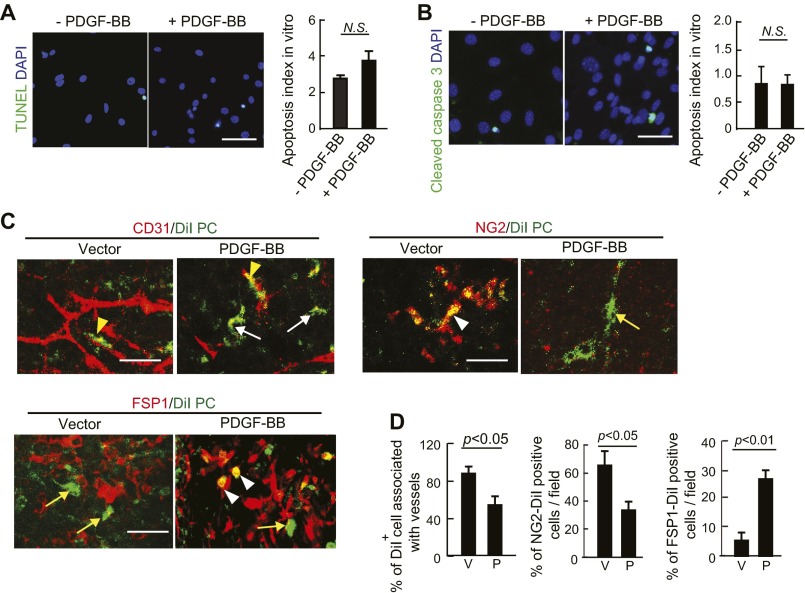
To further validate our findings in naturally occurring human tumors, we next performed a gain-of-function experiment by overexpressing PDGF-BB in mouse tumors that lacked detectable PDGF-BB as previously described (23). Similar to human SC-A431 tumors, overexpression of PDGF-BB in T241 fibrosarcoma and Lewis lung carcinoma (LLC) nearly completely ablated pericytes in tumors as previously described (23). Both vessel- and nonvessel-associated pericytes were depleted by overexpression of PDGF-BB (23). The decrease in pericytes in PDGF-BB–expressing T241 and LLC tumors was not due to pericyte apoptosis, because the pericyte apoptotic rates in PDGF-BB+ and PDGF-BB− tumors were similar (Fig. S2A). Expression of PDGF-BB in T241 and LLC tumors led to a marked increase of FSP1+ stromal fibroblasts and αSMA+ myofibrolasts, which were disassociated with tumor microvasculatures (Fig. S2 B–E). Consequently, total amounts of stromal components in these PDGF-BB–expressing tumors were markedly increased relative to PDGF-BB–negative tumors (Fig. S2F). These gain-of-function experiments provide independent evidence that PDGF-BB is primarily responsible for recruitment of CAFs and cancer-associated myofibroblasts (CAMFs).
Fig. S2.
Gain of PDGF-BB function in mouse tumors increases stromal fibroblasts and myofibroblasts and ablates pericytes. (A) TUNEL immunostaining of apoptotic cells in T241-vector and -PDGF-BB and LLC-vector and -PDGF-BB tumors. NG2+ and TUNEL+ apoptotic cells were quantified (n = 30 fields per group). P, PDGF-BB; V, vector. (Scale bar, 25 μm.) (B) Endomucin+ vessels (red) and αSMA+ signals (green) in T241-vector and -PDGF-BB and LLC-vector- and -PDGF-BB tumors. Yellow color indicates the overlapping signals (arrowheads). Arrows point to vessel-disassociated αSMA+ signals. (Scale bar, 100 μm.) (C) Quantification of αSMA+ myofibroblasts in T241-vector and -PDGF-BB and LLC-vector- and -PDGF-BB tumors (n = 12 fields per group). P, PDGF-BB; V, vector. (D) Endomucin+ vessels (red) and FSP1+ signals (green) in T241-vector and -PDGF-BB and LLC-vector- and -PDGF-BB tumors. (Scale bar, 100 μm.) (E) Quantification of FSP1+ fibroblasts in T241-vector and -PDGF-BB and LLC-vector- and -PDGF-BB tumors. (n = 12 fields per group). P, PDGF-BB; V, vector. (F) H&E staining of T241-vector and -PDGF-BB tumor tissues. Arrows indicate the tumor stromal components. (Scale bar, 100 μm.) (G) (Upper) Endomucin+ tumor vessels (red) and αSMA+ cells (green). (Lower) Endomucin+ tumor vessels (red) and FSP1+ stromal fibroblasts (green) in buffer-, anti-PDGFRβ-, and imatinib-treated T241-PDGF-BB tumors. (Scale bar, 100 μm.) (H) Quantification of αSMA+ myofibroblsts (n = 12 fields per group) and FSP1+ fibroblasts (n = 12 fields per group) in buffer-, anti-PDGFRβ-, and imatinib-treated T24-PDGF-BB tumors. Data are represented as mean ± SEM.
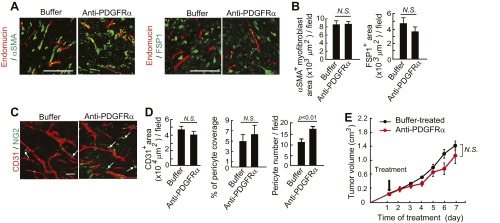
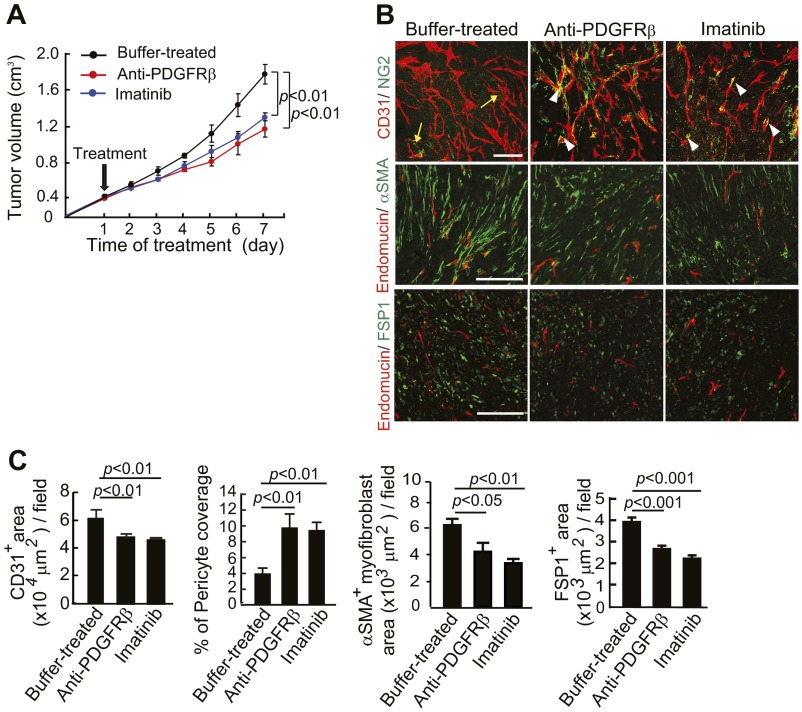
Additionally, PDGFRβ blockade treatment markedly decreased the populations of FSP1+ stromal fibroblasts and αSMA+ myofibrolasts in tumors (Fig. S2 G and H). In contrast to PDGFRβ blockade, anti-PDGFRα treatment did not significantly alter the FSP1+ and αSMA+ stromal fibroblast components in PDGF-BB–expressing tumors (Fig. S3 A and B), suggesting that PDGF-BB-PDGFRα signaling is not significantly involved in mediating the PDGF-BB–induced expansion of stromal fibroblasts. Similar to PDGFRβ blockade, treatment of T241-PDGF-BB tumors with imatinib resulted in nearly identical effects of pericyte restoration and loss of CAFs and CAMFs (Fig. S2 G and H). Also, treatment with an anti-PDGFRα neutralizing antibody increased NG2+ signals that were disassociated with blood vessels (Fig. S3 C and D). In addition to alterations of pericytes and stromal fibroblasts, treatment using imatinib, but not anti-PDGFRα, significantly inhibited tumor angiogenesis (20). Both anti-PDGFRβ and imatinib treatments significantly inhibited the growth rates of T241-PDGF-BB tumors as previously described (23). In contrast, treatment using anti-PDGFRα antibody did not alter the tumor growth rate significantly (Fig. S3E). To further validate our findings, we treated the established tumors (tumor size around 0.4 cm3) with PDGFRβ blockade and imatinib and obtained nearly identical results of fibroblast loss, reduction of stromal components, and inhibition of the tumor growth rate (Fig. S4). These gain-of-function results from mouse models validate our findings in human tumor models that the PDGFRβ-mediated signaling pathway is critical for the expansion of CAFs and CAMFs in tumors.
Fig. S3.
Anti-PDGFRα treatment of T241-PDGF-BB tumors. (A) Endomucin+ tumor vessels (red) and αSMA+ cells (green) and endomucin+ tumor vessels (red) and FSP1+ stromal fibroblasts (green) in buffer- or anti-PDGFRα–treated T241-PDGF-BB tumors. (Scale bar, 100 μm.) (B) Quantification of αSMA+ myofibroblsts (n = 12 fields per group) and FSP1+ fibroblasts (n = 12 fields per group) in buffer- or anti-PDGFRα–treated T241-PDGF-BB tumors. N.S., not significant. (C) CD31+ (red) vessels and NG2+ pericyte (green) signals in buffer- or anti-PDGFRα–treated T241-PDGF-BB tumors. Arrows point to vessel-disassociated pericytes. (Scale bar, 50 μm.) (D) Quantification of CD31+ microvessel density (n = 7 fields per group), pericyte coverage (n = 7 fields per group), and pericyte numbers (n = 7 fields per group). N.S., not significant. (E) Tumor growth curves in buffer- or anti-PDGFRα–treated T241-PDGF-BB tumor-bearing mice (n = 4–6 animals per group). Data are represented as mean ± SEM. N.S., not significant.
Fig. S4.
Tumor growth rates and changes of pericytes and stromal fibroblasts in established tumors that received various treatments. (A) Tumor growth curves in buffer-, anti-PDGFRβ-, and imatinib-treated T241-PDGF-BB tumor-bearing mice (n = 4–6 animals per group). (B) CD31+ (red) vessels and NG2+ pericyte (green) signals, endomucin+ tumor vessels (red) and αSMA+ cells (green), and endomucin+ tumor vessels (red) and FSP1+ stromal fibroblasts (green) in imatinib-, anti-PDGFRβ, and buffer-treated T241-PDGF-BB tumors. Arrows indicate the vessel-disassociated pericytes and arrowheads point to vessel-associated pericyes. (Scale bars: Top, 50 μm; Middle and Bottom, 100 μm.) (C) Quantification of CD31+ microvessel density (n = 7 fields per group), pericyte area (n = 7 fields per group), and coverage (n = 7 fields per group), αSMA+ myofibroblsts (n = 12 fields per group), and FSP1+ fibroblasts (n = 12 fields per group) in imatinib-, anti-PDGFRβ-, and buffer-treated T241-PDGF-BB tumors. Data are represented as mean ± SEM.
Genetic Deletion of PDGFRβ in Mice Attenuates PDGF-BB–Recruited Stromal Fibroblasts and Myofibrolasts.
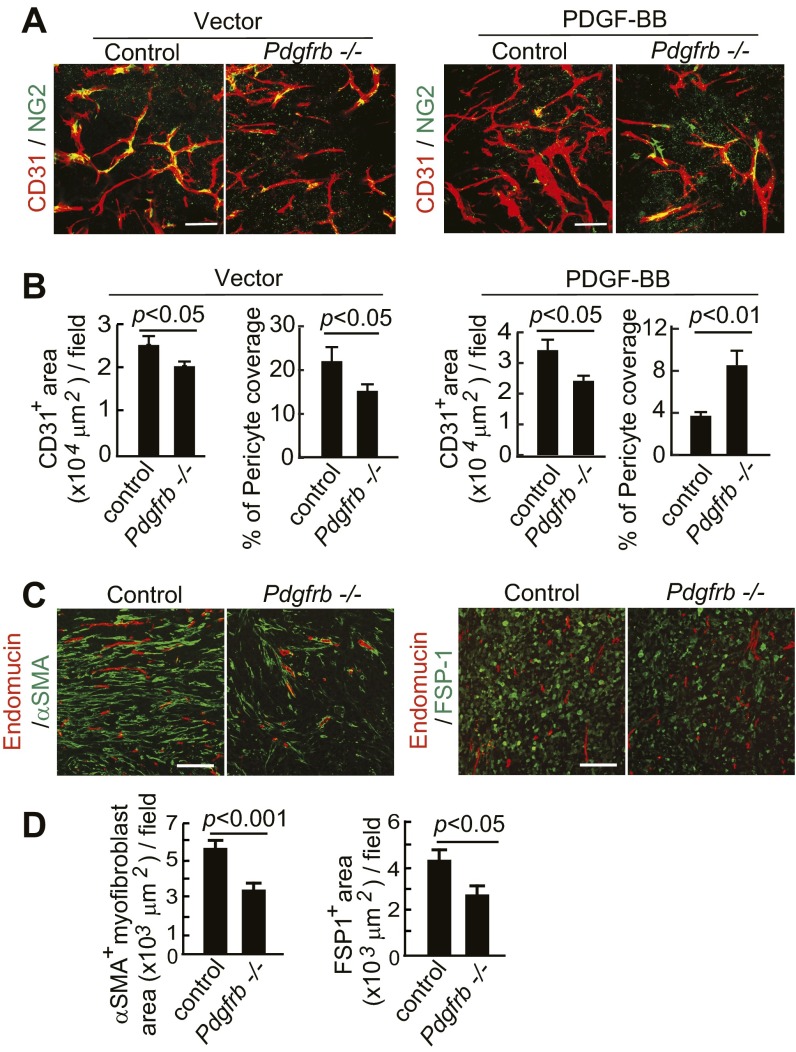
To further define the receptor signaling pathways that mediate PDGF-BB–recruited CAFs and CAMFs, we genetically deleted PDGFRβ in mice in a conditional knockout model (29, 30). Deletion of PDGFRβ significantly decreased vascular pericyte coverage in PDGF-BB–negative control vector tumors, supporting the vascular function of PDGF-BB in recruitment of pericytes to angiogenic vessels (Fig. S5 A and B). In contrast, implantation of PDGF-BB tumors in Pdgfrß−/− mice resulted in increased pericyte coverage on tumor vessels, as seen with the PDGFRβ blockade. It is possible that PDGFRα also played a role in recruitment of pericytes in this experimental setting. The CD31+ microvessel density in vector and PDGF-BB tumors was also decreased in Pdgfrß−/− mice (Fig. S5 A and B). Similar to pharmacological approaches, genetic deletion of Pdgfrß−/− significantly decreased FSP1+ CAF and αSMA+ CAMF components in tumors (Fig. S5 C and D). These results obtained from genetic defective models provide independent evidence to support the crucial role of PDGF-BB-PDGFRβ signaling in recruitment of CAFs and CAMFs.
Fig. S5.
Deletion of Pdgfrb in mice ablates PDGF-BB–stimulated stromal components. (A) CD31+ (red) vessels and NG2+ pericytes (green) in T241-vector and T241-PDGF-BB tumors grown in Pdgfrbflox/flox mice (control mice) and Pdgfrb−/− mice. (Scale bar, 50 μm.) (B) Quantification of CD31+ microvessel density and pericyte coverage in T241-vector and T241-PDGF-BB tumors grown in Pdgfrbflox/flox mice (control mice) and Pdgfrb−/− mice. (n = 7 fields per group). (C) Endomucin+ (red) vessels and αSMA+ signals (green) and endomucin+ (red) vessels and FSP1+ signals (green) in T241-PDGF-BB tumors grown in Pdgfrbflox/flox mice (control mice) and Pdgfrb−/− mice. (Scale bar, 100 μm.) (D) Quantification of αSMA+ myofibroblasts and FSP1+ fibroblasts in T241-PDGF-BB tumors grown in Pdgfrbflox/flox mice (control mice) and Pdgfrb−/− mice. (n = 12 fields per group). Data are represented as mean ± SEM.
PDGF-BB Promotes PFT of Isolated Mouse Pericytes.
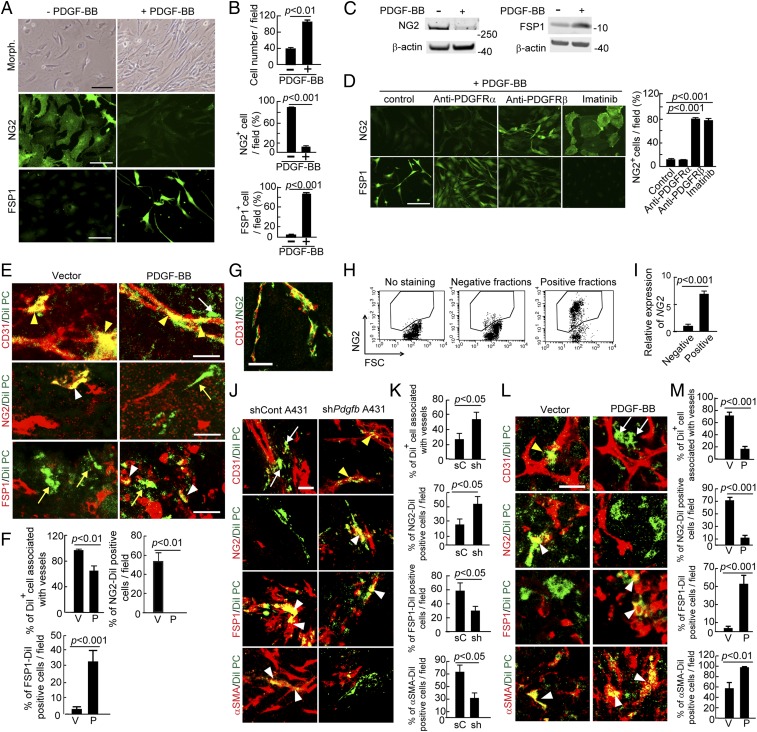
The loss of NG2+ pericytes and increase of CAFs and CAMFs in PDGF-BB–expressing tumors suggested that vascular pericytes might undergo differentiation into stromal fibroblasts. To investigate this possibility, we isolated primary pericytes and stimulated them with PDGF-BB in vitro for an extended period. First, we observed that PDGF-BB–stimulated pericytes changed their morphologies to an elongated and spindle-like cell shape that was morphologically similar to that of fibroblasts (Fig. 2 A and B), and PDGF-BB had no effect on pericyte apoptosis compared with vehicle-stimulated controls (Fig. S6 A and B). After 5-d stimulation with PDGF-BB, pericytes lost their NG2 expression (Fig. 2 A and B). Surprisingly, the PDGF-BB–stimulated pericytes gained FSP1 expression, a fibroblast-specific marker, indicating that pericytes underwent a PFT transition (Fig. 2 A and B). The loss of expression of NG2 and gain of expression of FSP1 were further validated by Western immunoblot with specific antibodies (Fig. 2C).
Fig. 2.
PDGF-BB induces PFT in vivo and in vitro. (A) Morphology, NG2 positivity, and FSP1 positivity in PDGF-BB–stimulated and nonstimulated primary pericytes. (Scale bar, 100 μm.) (B) Quantification of cell proliferation, NG2+ signals, and FSP1+ signals (n = 8 fields per group). The experiments were repeated three times. (C) Western blot analysis of NG2 and FSP1 proteins in PDGF-BB–stimulated and nonstimulated primary pericytes. β-actin detection indicates the standard loading in each lane. (D) NG2+ signals and FSP1+ signals in buffer-, anti-PDGFRα-, anti-PDGFRβ-, and imatinib-treated pericytes that were stimulated with PDGF-BB. Quantification of NG2+ cells in various treated groups (n = 8 fields per group). The experiments were repeated two times. (Scale bar, 100 μm.) (E) DiI-labeled pericytes (green) were implanted into T241-vector and T241-PDGF-BB tumors. Tumor vessels were stained with CD31 (red in Top), pericytes were stained with NG2 (red in Middle), and fibroblasts were stained with FSP1 (red in Bottom). Yellow arrowheads indicate vessel-associated DiI-labeled pericytes. White arrows indicate vessel-disassociated injected pericytes. The white arrowhead in the middle panel points to NG2 and DiI double signals and the yellow arrow in the middle panel indicates NG2− Dil+ cells. Yellow arrows in the lower panels indicate FSP1− Dil+ cells and white arrowheads in the lower panels point to FSP1+ Dil+ cells. (Scale bars, 25 μm.) (F) Quantification of CD31+ vessel-associated DiI+pericytes, NG2+DiI+ structures, and FSP1+DiI+ structures (n = 12 fields per group). Data are represented as mean ± SEM. P, PDGF-BB; V, vector. (G) Double immunohistochemical staining of human cancer tissue with CD31 (red) and NG2 (green). (Scale bar, 50 μm.) (H) FACS analysis of isolated NG2+ human pericytes from human tumors. (I) Quantification of NG2 positive signals of isolated human pericytes by quantitative PCR (triplicates per sample). (J) Tracing DiI-labeled human pericytes (green) implanted in scrambled shRNA- and shPdgfb-transfected human A431 tumors. White arrows indicate vessel-disassociated pericytes, yellow arrowheads point to vessel associated pericytes, and white arrowheads indicate double positive signals. (Scale bar, 25 μm.) (K) Quantification of CD31+vessel-associated DiI+pericytes, NG2+DiI+ structures, FSP1+DiI+ structures, and αSMA+DiI+ structures (n = 10 fields per group). Data are represented as mean ± SEM. sC, scrambled control tumor; sh, shPdgfb-transfected tumor. (L) Tracing DiI-labeled human pericytes (green) implanted in vector- and PDGF-BB-LLC tumors. The yellow arrowhead points to vasculature-associated pericytes, and white arrows indicate vasculature-disassociated pericytes. White arrowheads indicate double positive signals. (Scale bar, 25 μm.) (M) Quantification of CD31+vessel-associated DiI+pericytes, NG2+DiI+ structures, FSP1+DiI+ structures, and αSMA+DiI+ structures (n = 10 fields per group). Data are represented as mean ± SEM. P, PDGF-BB; V, vector.
Fig. S6.
DiI-labeled pericytes contribute to PFT in vivo tumors. (A) TUNEL apoptotic staining of PDGF-BB–stimulated and nonstimulated pericytes. Quantification of apoptotic index (n = 12 fields per group). (Scale bar, 100 μm.) (B) Cleaved caspase-3 apoptotic staining of PDGF-BB–stimulated and nonstimulated pericytes. Quantification of apoptotic index (n = 12 fields per group). (Scale bar, 100 μm.) (C) DiI-labeled pericytes (green) were implanted into LLC-vector and LLC-PDGF-BB tumors. Tumor vessels were stained with CD31 (red in upper left panels), pericytes were stained with NG2 (red in upper right panels), and fibroblasts were stained with FSP1 (red in lower panels). Yellow arrowheads in Upper Left panels indicate vessel-associated DiI-labeled pericytes. White arrows in the Upper Left panels indicate vessel-disassociated injected pericytes. The arrowhead in the Upper Right panels points to NG2 and DiI double-positive signals and the arrow in the Upper Right panels indicates NG2− Dil+ cells. Arrows in the Lower panels indicate FSP1− Dil+ cells and arrowheads in the Lower panels point to FSP1+ Dil+ cells. (Scale bars, 25 μm.) (D) Quantification of CD31+vessel-associated DiI+pericytes, NG2+DiI+ structures, and FSP1+DiI+ structures (n = 10 fields per group). Data are represented as mean ± SEM. P, PDGF-BB; V, vector.
We used anti-PDGFRα and anti-PDGFRβ specific neutralizing antibodies to delineate the receptor signaling responsible for PDGF-BB–induced PFT (23, 30). PDGFRβ neutralizing antibody blocked the PDGF-BB–induced PFT, whereas the anti-PDGFRα specific antibody had virtually no effect (Fig. 2D). Similarly, imatinib also effectively inhibited the PDGF-BB–induced PFT in this in vitro experiment setting. Both anti-PDGFRβ and imatinib prevented the loss of NG2 in pericytes (Fig. 2D). These data show that PDGF-BB is capable of inducing PFT in isolated pericytes.
To further study whether PDGF-BB was able to induce PFT in in vivo tumors, isolated pericytes were labeled with DiI dye and injected into PDGF-BB and vector tumors. Consistent with in vitro findings, NG2+ pericytes lost NG2 expression in PDGF-BB fibrosarcomas and gained FSP1 expression (Fig. 2 E and F). Similar results were also obtained from an independent PDGF-BB–expressing tumor, LLC-PDGF-BB (Fig. S6 C and D). Thus, PDGF-BB–induced PFT occurs in tumors in vivo.
Primary Pericytes Isolated from Human Tumors Undergo PFT in in Vivo Tumors.
To link our findings to clinical relevance, we isolated primary pericytes from fresh human tumors using NG2 as a specific marker (Fig. 2 G–I). The isolated human pericytes were labeled with DiI dye and injected into naturally occurring human squamous carcinomas implanted in immunodeficient SCID mice. Injection of human pericytes into control shRNA-A431 human squamous carcinoma resulted in disassociation of pericytes from tumor vasculatures and gain of FSP1 and αSMA expression (Fig. 2 J and K). However, knockdown of PDGF-BB by a shPdgfb markedly inhibited PFT and the remaining pericytes were associated with tumor vessels (Fig. 2 J and K). To further validate these findings in human tumors, DiI-labeled isolated human pericytes were also injected into mouse tumors. Similar to human tumors, PDGF-BB disassociated pericytes from tumor vasculatures and stimulated PFT by gaining FSP1 and αSMA expression and loss of NG2 expression (Fig. 2 L and M). Taken together, these findings show that pericytes isolated from human tumor tissues are capable of PFT in human and mouse tumors. Thus, it is highly plausible that PFT also occurs in human tumors.
Genome-Wide Profiling Defines Fibroblast-Like Signatures of PDGF-BB–Stimulated Pericytes.
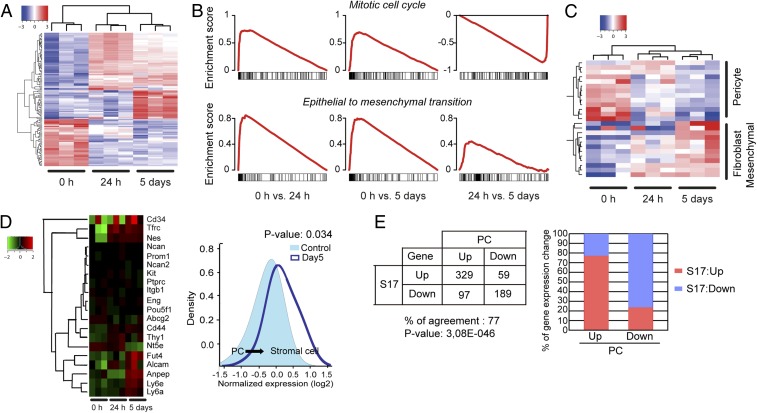
To further define fibroblast signatures in PDGF-BB–stimulated pericytes, we performed genome-wide gene expression profiling and revealed marked changes of clusters of genes in PDGF-BB–stimulated and nonstimulated pericytes (Fig. 3A). A multiclass rank product analysis identified differentially expressed genes. Gene set enrichment analysis showed that proliferation was strongly induced early at day 1 but was later decreased at day 5 after PDGF-BB stimulation (Fig. 3B). A gene set representing epithelial-to-mesenchymal transition was enriched among up-regulated genes both after day-1 and day-5 stimulation with PDGF-BB (Fig. 3B). A heat map of known marker genes for pericytes, fibroblasts, and mesenchymal cells showed that PDGF-BB treatment of primary pericytes induced differentiation toward a fibroblast fate (Fig. 3C). Further analysis of stromal or mesenchymal cell-related genes using the random variance model supported the PDGF-BB–stimulated PFT (Fig. 3D). Further, we used a known mesenchymal fibroblast cell (S17) as a positive control to profile the similarities of up- and down-related genes in PDGF-BB–stimulated pericytes. Strikingly, we found that 77% of up- and down-regulated genes were in agreement with the fibroblast signature (Fig. 3E). Taken together, the genome-wide gene expression analysis demonstrates that PDGF-BB–stimulated pericytes possess fibroblast features.
Fig. 3.
Gene-expression profiling by genome-wide array analysis. (A) Differentially expressed genes (FDR < 0.05) at different time points as identified using a multiclass rank product analysis. (B) Gene set enrichment analysis shows that proliferation is strongly induced early after stimulation of pericytes with PDFGF-BB but decreased at day 5 in comparison with 24-h stimulation. A gene set representing epithelial-to-mesenchymal transition is enriched among up-regulated genes both after 24-h and 5-d stimulation with PDFG-BB. (C) A heat map of known marker genes for pericytes, fibroblasts, mesenchymal cells, and epithelial-to-mesenchymal transition, showing that PDFGF-BB treatment of primary pericytes induced differentiation toward a fibroblast fate. (D) Heat map of stromal and mesenchymal markers, supporting the PDGF-BB–induced differentiation from pericytes toward stromal fibroblasts. (E) Gene profiling similarities between known stromal fibroblasts (S17 mesenchymal fibroblasts) and PDGF-BB–stimulated pericytes (day 5).
Genetic Tracing of PFT in Tumors with Two Independent Mouse Strains.
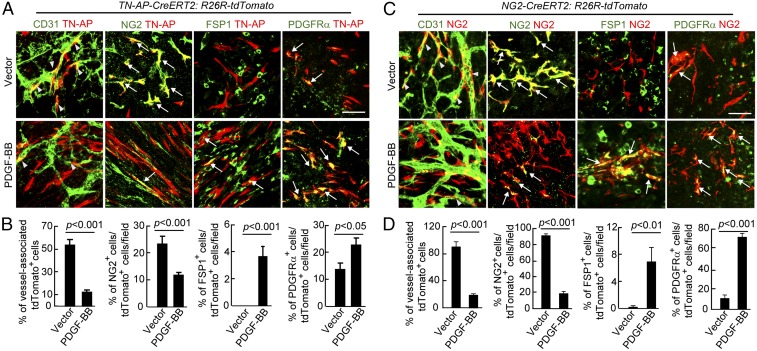
To provide definite evidence to further support the PDGF-BB–induced PFT, we generated two tumor models using genetically modified mouse strains that allowed us to trace pericytes in the tumor microenvironment. In the first model (TN-AP Cre ERT2:R26R-tdTomato), pericytes were genetically labeled with Tomato Red as previously described (15). Both T241 vector and PDGF-BB–expressing tumors were implanted in TN-AP Cre ERT2:R26R-tdTomato mice. Expectedly, Texas Red tomato-labeled pericytes were mainly associated with tumor vasculatures in the vector tumors and a substantial number of Texas Red Tomato-labeled pericytes expressed NG2 marker (Fig. 4 A and B). In contrast, most Texas Red tomato-labeled pericytes lost NG2 expression and their association with tumor vasculatures in PDGF-BB tumors. Instead, these Texas Red tomato-labeled cells exhibited elongated morphologies typical of fibroblasts in appearance. Moreover, these labeled pericytes gained expression of FSP1 and PDGFRα, two commonly expressed cell surface markers in stromal fibroblasts (Fig. 4 A and B). These findings support the notion that genetically labeled pericytes in the tumor microenvironment undergo PFT in response to PDGF-BB stimulation.
Fig. 4.
Genetic tracing of pericytes in contribution to PFT. (A) Genetic tracing of differentiation of alkaline phosphatase (AP)-marked pericytes into fibroblasts in T241-vector and T241-PDGF-BB tumors. Tamoxifen-induced AP-tdTomato-red (red) tumor tissues were contained with CD31, NG2, FSP1, and PDGFRα. Yellow arrows indicate overlapping positive signals in each panel. Arrowheads point to vessel-associated AP-tdTomato+ pericytes. (Scale bar, 50 μm.) (B) Quantification of vessel-associated AP-tdTomato+ cells, the total number of NG2+ AP-tdTomato+ structures, FSP1+ AP-tdTomato+ structures, and PDGFRα+ AP-tdTomato+ structures (n = 15 fields per group) in T241-vector and T241-PDGF-BB tumors. (C) Genetic tracing of differentiation of NG2-marked pericytes into fibroblasts in T241-vector and T241-PDGF-BB tumors. Tamoxifen-induced NG2-tdTomato-red (red) tumor tissues were contained with CD31, NG2, FSP1, and PDGFRα. Yellow arrows indicate overlapping positive signals in each panel. Arrowheads point to vessel-associated NG2-tdTomato+ pericytes. (Scale bar, 50 μm.) (D) Quantification of vessel-associated NG2-tdTomato+ cells, the total number of NG2+ NG2-tdTomato+ structures, FSP1+ NG2-tdTomato+ structures, and PDGFRα+ NG2-tdTomato+ structures (n = 15 fields per group) in T241-vector and T241-PDGF-BB tumors. Data are represented as mean ± SEM (n = 4 animals per group).
To further validate these findings, we chose to use an independent mouse tracing strain in which specific expression of Texas Red Tomato was controlled by the NG2 Cre recombinase (NG2 Cre ERT2:R26R-tdTomato) (31). Again, in the vector tumor, most NG2 Texas Red Tomato-labeled pericytes were associated with tumor vessels and they retained their NG2 expression (Fig. 4 C and D). Strikingly, almost all NG2 Texas Red Tomato-labeled pericytes lost their NG2 expression and association with tumor vasculatures in PDGF-BB tumors, supporting our findings in nongenetically manipulated WT mice. In contrast, NG2 Texas Red Tomato-labeled pericytes significantly gained expression of FSP1 and PDGFRα (Fig. 4 C and D). These findings support PDGF-BB–triggered PFT in the tumor microenvironment.
PDGF-BB–Primed Pericytes Stimulate Tumor Growth and Metastasis.
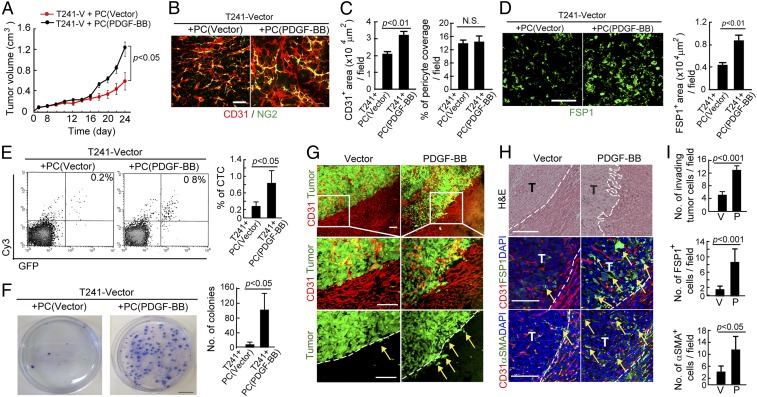
We next investigated functional impacts of PDGF-BB–stimulated pericytes in tumor growth and metastasis. Pericytes were stimulated with PDGF-BB–containing medium for 7 d and were subsequently coimplanted with EGFP-T241 tumor cells. Nonstimulated pericytes were used as controls. At day 24 after implantation, the PDGF-BB-pericytes+ tumor cell group grew significantly faster compared with the vehicle pericytes+ tumor cell group, indicating that PDGF-BB–stimulated pericytes play a significant role in stimulating tumor growth (Fig. 5A). Histological examination showed that tumors with PDGF-BB–stimulated pericytes possessed a higher density of microvessels relative to control tumors (Fig. 5 B and C). However, percentages of pericyte coverage in both PDGF-BB-pericyte and vehicle-pericyte+ tumors were identical (Fig. 5 B and C), suggesting that in vitro stimulation of pericytes with PDGF-BB did not affect their association with angiogenic vessels. Conversely, FSP1+ CAFs were markedly increased in PDGF-BB-pericyte tumors compared with control tumors (Fig. 5D), supporting the view that PDGF-BB–stimulated pericytes underwent PFT transition.
Fig. 5.
PDGF-BB–stimulated pericytes promote tumor growth, angiogenesis, stromal fibroblast expansion, and metastasis. (A) Growth rates of nonstimulated pericyte-T241-vector cell- and PDGF-BB–stimulated pericyte-T241-vector-coimplanted primary tumors (n = 8 animals per group). (B) Microvessel density (CD31) and pericyte coverage (NG2) of nonstimulated pericyte-T241-vector cell- and PDGF-BB–stimulated pericyte-T241-vector-coimplanted primary tumors. (Scale bar, 50 μm.) (C) Quantification of CD31+ vessel density and NG2+ vascular pericyte coverage of nonstimulated pericyte-T241-vector cell- and PDGF-BB–stimulated pericyte-T241-vector-coimplanted primary tumors (n = 7 fields per group). N.S., not significant. (D) Immunostaining and quantification of FSP1+ stromal fibroblasts of nonstimulated pericyte-T241-vector cell- and PDGF-BB–stimulated pericyte-T241-vector-coimplanted primary tumors (n = 7 fields per group). (Scale bar, 50 μm.) (E) FACS analysis of bloodstream CTCs of animals implanted with nonstimulated pericyte-T241-vector cell- and PDGF-BB–stimulated pericyte-T241-vector-coimplanted primary tumors. Right upper plots indicate CTCs and percent is shown on the plots. Quantification of percent of CTCs (n = 7 animals per group). (F) Tumor colony formation of peripheral blood from nonstimulated pericyte-T241-vector cell- and PDGF-BB–stimulated pericyte-T241-vector-coimplanted primary tumors (n = 7 animals per group). (Scale bar, 2 cm.) (G) Invasiveness of EGFP+ T241-vector and T241-PDGF-BB tumors in relation to intra- and peritumoral microvessels (CD31+). Dashed lines mark the tumor rims and arrows point to the invasive front. (Scale bar, 100 μm.) (H) FSP1+ and αSMA+ contents in relation to microvessels in the border regions of tumors. Dashed lines mark the tumor rims and arrows point to stromal fibroblasts or myofibroblasts. (Scale bar, 100 μm.) T, tumor. (I) Quantification of invasive tumor cells, numbers of FSP1+ stromal fibroblasts, and numbers of αSMA+ stromal myofibroblasts at the invasive fronts of tumors (n = 10 fields per group). Data are represented as mean ± SEM. P, PDGF-BB; V, vector.
It is known that increases of CAFs and angiogenic phenotype are correlated with invasiveness and metastatic phenotypes of solid tumors (6). To study the impact of PDGF-BB–stimulated pericytes in promoting cancer metastasis, we measured the number of circulating tumor cells (CTCs) in PDGF-BB-pericytes– and vehicle-pericytes–containing tumor-bearing mice. Interestingly, a significantly higher number of CTCs were found in PDGF-BB-pericytes–containing tumor-bearing mice compared with vehicle-pericytes–containing tumor-bearing mice (Fig. 5E). These data suggest that PDGF-BB–stimulated pericytes promote tumor cell intravasation in the primary sites. Consistent with increases of CTCs, culturing blood from PDGF-BB-pericytes–containing tumor-bearing mice resulted in a significantly increased number of tumor colonies compared with control group (Fig. 5F). Taken together, these results show that PDGF-BB–stimulated pericytes facilitate primary tumor growth and cancer metastasis through the mechanisms of tumor angiogenesis and intravasation.
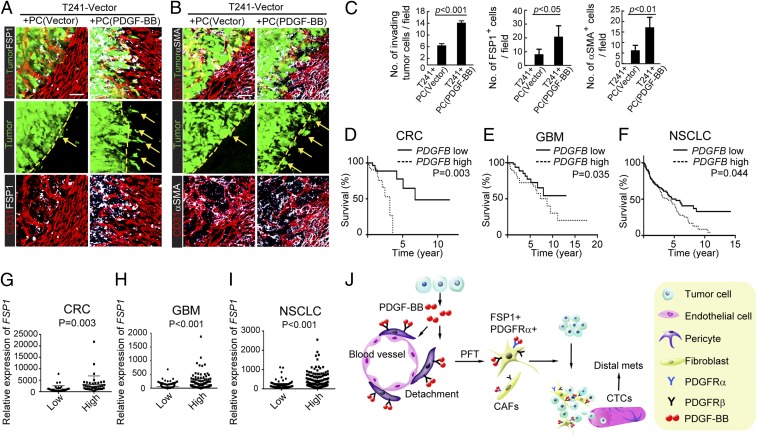
Liver implantation of T241-PDGF-BB-EGFP and T241-vector-EGFP tumors allowed us to visualize the invasive front of tumor masses. T241-PDGF-BB tumors showed irregular fronts with invasive tumor cells compared with control tumors (Fig. 5 G–I). Importantly, numbers of FSP1+ and αSMA+ CAFs in PDGF-BB tumors were markedly increased in the invasive front of PDGF-BB tumors compared with those in control tumors (Fig. 5 H and I), suggesting that these CAFs, likely to be originated from pericytes, are involved in tumor invasion and metastasis. To further delineate the role of pericytes in tumor invasion, we coimplanted EGFP-T241 tumors with PDGF-BB– or vehicle-stimulated pericytes. Interestingly, coimplantation of PDGF-BB–stimulated pericytes with tumor cells resulted in an invasive phenotype and invasive scattered tumor cells could be detected in the tumor rims (Fig. 6 A–C). Again, high densities of FSP1+ and αSMA+ CAFs were accrued at the invasive leading front in tumors with PDGF-BB–stimulated pericytes compared with tumors containing vehicle-stimulated pericytes (Fig. 6 A–C). These data provide an independent line of evidence that PDGF-stimulated pericytes promote tumor invasiveness in the invasive tumor front.
Fig. 6.
PDGF-BB–stimulated pericytes promote primary cancer invasiveness. (A) Invasiveness of nonstimulated pericyte- EGFP+ T241-vector cell- and PDGF-BB–stimulated pericyte- EGFP+ T241-vector-coimplanted primary tumors in relation to FSP1+ stromal fibroblasts (white) and CD31+ intra- and peritumoral microvessels (red). Dashed lines mark the tumor rims and arrows point to the invasive front. n = 8 animals per group. (Scale bar, 50 μm.) (B) Invasiveness of nonstimulated pericyte- EGFP+ T241-vector cell- and PDGF-BB–stimulated pericyte- EGFP+ T241-vector-coimplanted primary tumors in relation to αSMA+ (white) stromal myofibroblasts and CD31+ intra- and peritumoral microvessels (red). Dashed lines mark the tumor rims and arrows point to the invasive front. n = 8 animals per group. (Scale bar, 50 μm.) (C) Quantification of invasive tumor cells, numbers of FSP1+ stromal fibroblasts, and numbers of αSMA+ stromal myofibroblasts at the invasive fronts of nonstimulated pericyte-T241-vector cell- and PDGF-BB–stimulated pericyte-T241-vector-coimplanted primary tumors (n = 10 fields per group). (D) Kaplan–Meier survival of PDGFB-high (n = 36) and PDGFB-low (n = 36) COADREAD patients. (E) Kaplan–Meier survival of PDGFB-high (n = 107) and PDGFB-low (n = 105) LGG patients. (F) Kaplan–Meier survival of PDGFB-high (n = 199) and PDGFB-low (n = 192) LUSC patients. (G) Correlation of FSP1 expression in PDGFB-high (n = 36) and PDGFB-low (n = 36) COADREAD patients. (H) Correlation of FSP1 expression in PDGF-BB-high (n = 107) and PDGF-BB-low (n = 105) LGG patients. (I) Correlation of FSP1 expression in PDGFB-high (n = 199) and PDGFB-low (n = 192) LUSC patients. (J) Diagram of tumor-derived PDGF-BB in promoting tumor invasion and metastasis. Tumor cell-derived PDGF-BB builds up a high gradient that attracts pericytes to move away from tumor microvessels. Once pericytes detach, they differentiate into stromal fibroblast (PFT) in the presence of PDGF-BB. Increases of stromal fibroblasts and myofibroblasts in tumors significantly promote tumor cell invasion and intravasation into the circulation, leading to an increase of CTCs and distal metastasis. Data are represented as mean ± SEM.
Reverse Correlation of High PDGFB and FSP1 Expression with Survival in Colorectal, Glioblastoma Multiforme, and Lung Cancer Patients.
To relate our preclinical findings to clinical relevance, we performed metaanalyses of cancer patients to correlate PDGFB and FSP1 expression levels to survival. Because cancer invasion and metastasis are the most common causes of cancer-related death, survival prognosis often reflects the invasive and metastatic situation of cancer patients. We chose three common cancers, including lung squamous cell carcinoma (LUSC), colorectal adenocarcinoma (COADREAD), and lower-grade glioma (LGG), for further analysis. In this clinical study, datasets of 391 LUSC, 72 COADREAD, and 212 LGG patients were included in our study. Numbers of PDGFB-low patients include 192 LUSC, 36 COADREAD, and 105 LGG, and PDGFB-high patients include 199 LUSC, 36 COADREAD, and 107 LGG. Markedly, PDGFB-high groups in all three cancer types showed significantly shortened survivals compared with their corresponding PDGFB-low groups (Fig. 6 D–F).
We next correlated PDGFB-high and -low groups with the FSP1 expression levels in these tumors. Strikingly, FSP1+ fibrotic contents were significantly higher in PDGFB-high groups compared with PDGFB-low groups of these cancer patients (Fig. 6 G–I). The results from three cohort analyses provide clinical evidence of reverse correlation between PDGFB and FSP1 expression with survival. Based on these clinical findings, it is reasonable to speculate that PDGFB and FSP1 could potentially serve as prognostic markers to predict survivals of LUSC, COADREAD, and LGG patients.
Discussion
CAFs are one of the major host cellular components in many solid tumors, and infiltration of this stromal component has been correlated with invasiveness and poor prognosis of cancer disease (5, 6). In this study, we address an important issue related to the origin of CAFs in the tumor microenvironment. We have revealed a mechanism by which perivascular cells serve as a reservoir for stromal fibroblasts and the PFT is controlled by tumor-derived factors. Our study provides another example of how tumor cells manipulate the host-cell interaction for their growth and invasion. Although genetic mutations in malignant cells are important for tumor growth and invasion, the microenvironment composed of host cells is a crucial element that determines the invasive phenotype of tumor cells. To metastasize to distal organs, malignant cells at the primary site have to interact with host cells, including inflammatory cells, fibroblasts, and vascular cells, to intravasate into the circulation (32, 33). Intravasation, one of the initial steps of the metastatic cascade, is a complex process that requires several cell types to interact and transmigrate through the vascular endothelium in a cooperative manner. Several key issues related to tumor cell invasion and the underlying molecular mechanism remain unclear. How is this multicellular process modulated in the tumor microenvironment? What are the origins of CAFs? What are the functions of perivascular cells in cancer metastasis? How are these host cells positioned at the invasive fronts? What are the signaling molecules and pathways that control tumor invasion?
Whereas most host-cell types in the tumor microenvironment, including inflammatory cells, endothelial cells, and stromal fibroblasts, are well-characterized, the biological functions of pericytes in association with cancer metastasis remain largely unknown. Would high numbers of pericytes be advantageous for tumor growth and metastasis? If so, would associated or diassociated pericytes facilitate tumor growth and metastasis? In tumors, pericytes are widely believed to play a role in the recruitment of pericytes onto angiogenic vessels, leading to vascular remodeling toward a maturation phenotype (12, 16). PDGF-BB is one of the key factors involved in pericyte migration and association with angiogenic vessels. Disruption of pericyte coverage in tumor angiogenic vessels would likely increase the tortuosity and disorganization of tumor vessels, resulting in an accelerated tumor growth rate (34). In support of this view, ablation of pericytes by anti-PDGF agents has been reported to increase vascular tortuosity and tumor growth, suggesting that vascular recruitment of pericytes by PDGF-BB plays a negative role in tumor angiogenesis and growth (35, 36). Paradoxically, inhibition of PDGF-BB–mediated pericyte association to tumor vessels has also been reported to be a valid target for cancer therapy, particularly when targeted in combination with other angiogenic factors (35, 37–39). For example, combining anti-VEGF and anti-PDGF drugs provides an additive therapeutic effect (40). One explanation of the combination approach is that anti-PDGF drugs increase exposure of vascular endothelial cells to anti-VEGF agents by ablating perivascular cells. Despite these claims of vasculature-related functions, the action of pericytes per se on modulation of the tumor microenvironment, tumor growth, and metastasis are poorly understood. In particular, molecular players controlling pericyte differentiation in tumors remain unidentified.
We are beginning to understand the complex role of pericytes in modulation of cancer metastasis. Our recent work and work published by others shows that loss of pericytes makes the tumor vessels more susceptible for cancer cell intravasation and eventually metastasis (23, 41). Apart from pericytes and malignant cancer cells, other cell types such as tumor-associated macrophages and cancer-associated fibroblasts are also involved in the intravasation cascade (6, 33). Loss of pericytes from tumor vessels may either permit tumor cell intravasation and PFT or hijack tumor cells for intravasation, and perhaps even the formation of the initial metastatic niches in distal tissues and organs. Indeed, it has been described that tumors can carry their own fibroblasts as “soil” for them to “seed” and grow in distal organs (42). Therefore, pericytes play dynamic roles in cancer invasion and metastasis.
In this study, we show that PDGF-BB plays dual roles in modulation of pericyte functions. First, tumor cell-derived PDGF-BB ablates pericytes from tumor microvessels. The possible mechanism underlying pericyte ablation is that the high PDGF-BB gradient from the tumor cell source attracts pericytes to move toward tumor cells rather than to endothelial cells (23). Second, once pericytes disassociate from tumor vasculatures, they undergo PFT under persistent PDGF-BB stimulation in the tumor microenvironment (Fig. 6J). These findings may imply that (i) pericytes have an intrinsic property that displays high potential capacity and plasticity of differentiating into other cell types, (ii) endothelial cells in the vessel wall might prevent differentiation of pericytes into other cell types and maintain pericyte stemness features, and (iii) under the influence of a specific signaling pathway pericytes may commit to differentiation toward a specific cell type. In our mouse and human tumor experimental models we demonstrate that the PDGF-BB-PDGFRβ signaling system drives pericyte differentiation toward fibroblasts. It is unclear whether the PDGF-BB-PDGFRβ signaling pathway either alone or in combination with other signaling pathways induces pericyte differentiation toward other lineages.
One of the surprising findings of our present work is that PDGF-BB–expressing tumors almost completely lack NG2+ pericyte expression. Assuming that tumor cell-derived PDGF-BB ablates pericytes from tumor vessels, these NG2+ pericytes would have remained as vessel-disassociated NG2+ cells, which would still be detected. The loss of NG2+ cells is neither due to down-regulation of this cell surface marker by PDGF-BB stimulation nor to increased cell death. Two genetic tracing mouse strains provide compelling and convincing evidence supporting PFT. Although it is known that PDGF-BB tumors contain high amounts of stromal components (30), their origins remain unclear. There are three possible mechanisms by which PDGF-BB contributes to the tumor stromal cell component: (i) induction of proliferation of existing fibroblasts for expansion, (ii) recruitment of fibroblasts from neighbor or distal tissues because PDGF-BB is a potent factor for cell migration, and (iii) differentiation of stem cells and other cell types into fibroblasts. Here, we demonstrate pericyte differentiation as a mechanism of increasing fibroblast components in PDGF-BB tumors. Our data could also be applicable in PDGF-BB negative tumors because PDGFRβ could be activated by alternative mechanisms. For example, other members in the PDGF family, including PDGF-DD and PDGF-CC, could also activate PDGFRβ (43–46). Moreover, genetic mutations of PDGFRβ could cause ligand-independent activation of this receptor (47). Also, high expression of PDGFRβ in pericytes and stromal cells might cause autophosphorylation of PDGFRβ, leading to the formation of receptor dimers, oligomers, and aggregates (48–50). These interesting possibilities warrant future validation.
Preclinical and clinical evidence shows that the tumor stroma is strongly correlated with an invasive and metastatic phenotype of most solid tumors (51–53). In a mouse experimental metastatic model in our previous study we showed that PDGF-BB–producing tumor cells readily form clusters and are colonized in distal tissues and organs such as the lung (23). These findings are consistent with results by others demonstrating that stromal fibroblasts promote metastatic tumor growth via stimulation of tumor angiogenesis (37). Moreover, in the tumor environment, angiogenic vessels would facilitate pericyte infiltrations, which would subsequently undergo PFT by PDGF-BB. CAFs are crucial for the formation of tumor niches, and they often serve as feeder cells that support malignant cell adhesion, expansion, and invasion. Thus, tumor-derived PDGF-BB contributes to the metastatic cascade by facilitating multiple steps including stimulation of dissemination, recolonization, and regrowth. Our findings from animal tumor models are highly relevant to clinical settings in which human tumors produce high levels of PDGF-BB. Similar to genetically engineered mouse tumors, we provide evidence that human PDGF-BB–producing tumors in their intrinsic status exhibit pericyte ablation and disassociation from tumor vessels. Conversely, we demonstrate substantial expansion of the stromal compartment in PDGF-BB–positive tumors compared with PDGF-BB–negative tumors. Thus, these findings recapitulate the clinical situation in cancer patients. A rational speculation of our work is that PDGF-BB might serve as an important biomarker for predicting tumor invasion, metastasis, and drug resistance.
Our clinical data support the fact that high PDGFB expression in human cancers is reversely correlated with survival prognosis. In three cohort analyses of LUSC, COADREAD and LGG we show that high PDGFB levels serve as an independent prognostic marker for poor survival. Moreover, high PDGFB expression is positively correlated to the high content of stromal fibroblasts in tumors. Although tracing the fate of pericytes in human patients remains as a challenging issue because it cannot be performed, it is highly plausible that PDGF-BB is the driving force for expansion of CAFs in these cancer patients as well. In support of this notion, we demonstrate that pericytes isolated from human tumors are capable of undergoing PFT in human tumors. Because cancer metastasis is a common reason for cancer death, the reduced survival time of PDGFB-high and FSP1-high patients is likely due to cancer invasion and metastasis.
Taken together, our data provide evidence of cell-type switching in the tumor microenvironment and define functions of pericytes in cancer invasion and metastasis. Targeting the PDGF-BB-PDGFRβ–induced PFT would be an important therapeutic approach for the treatment of cancer and metastasis.
Materials and Methods
Animals.
Male and female C57/B6 mice at age 4 to 6 wk were used for xenograft tumor studies. Male or female SCID mice, Pdgfrβ−/− mice, TN-AP Cre ERT2:R26R-tdTomato mice, and NG2 Cre ERT2:R26R-tdTomato mice at age 4 to 8 wk were used as knockout and transgenic mouse strains. All mouse studies were approved by the Northern Stockholm Experimental Animal Ethical Committee. See SI Materials and Methods for more details.
Human Samples.
Human tissues materials were obtained from the Karolinska Hospital and the procedure of human sample handling and informed consent were followed according to the regulation approved by the Karolinska Biobank Review Board (permission no. BbK1228). Accordingly, all patient materials were anonymized before being transferred to research laboratories. Isolated cells from fresh human samples were injected into mice immediately without cultivation.
Cell Lines and Culture.
T241, LLC, A431, and CAKI-1 tumor cell lines were cultured in DMEM. The IMR32 tumor cell line was maintained in RPMI1640 medium. Mouse primary PCs were maintained in DMEM. See SI Materials and Methods for details and also for details on Pdgfb-shRNA knockdown, whole-mount staining, immunohistochemical staining, isolation of primary pericytes from fresh human tumor tissues, measurement of CTC, ELISA, quantitative real-time and RT-PCR, immunoblotting, and correlation of PDGFB expression in human cancer patients.
Affymetrix Gene-Array Analysis.
Data have been deposited in the Gene Expression Omnibus with accession nos. GSE85955 and GSE33717 (30). See SI Materials and Methods for details.
Statistical Analysis.
Statistical analyses of results except gene array analysis were performed using the standard two-tailed Student t test, and P < 0.05 was considered statistically significant.
SI Materials and Methods
Whole-Mount Staining.
Whole-mount immunohistochemical staining of tissue samples was performed according to our previously published methods (54–58). Briefly, tumor tissues were fixed with 4% (wt/vol) paraformaldehyde (PFA) overnight and cut into small pieces. Tissues were digested with 20 mM proteinase K in 10 mM Tris buffer (pH 7.5) for 5 min, followed by incubation with 100% (vol/vol) methanol for 30 min. Tissues were washed with PBS and incubated at 4 °C overnight in PBS containing 3% (wt/vol) skim milk and 0.3% Triton X-100, followed by incubation with various combinations of a rat anti-mouse CD31 monoclonal antibody (553370, 1:200; BD-Pharmingen), a rabbit anti-NG2 antibody (AB5320, 1:200; Millipore), a rabbit anti-mouse FSP1 polyclonal antibody (07-2274, 1:200; Millipore), and a rat anti-mouse PDGFRα antibody (14-1401-81, 1:200; eBioscience). After rinsing, endothelial cells and pericytes were detected using fluorescein-labeled secondary antibodies: an Alexa Fluor 555-labeled goat anti-rat (A21434, 1:200; Invitrogen) antibody, a Cy5-labeled goat anti-rabbit (AP132S , 1:200; Millipore) antibody, and a Cy5-labeled goat anti-rat antibody (AP183S , 1:200; Millipore). After washing, stained tissue samples were mounted using Vectashield mounting medium (Vector Laboratories) and images were captured a by confocal microscopy (Nikon C1 confocal microscope; Nikon Corporation).
Cell Culture.
Stable expression of PDGF-BB in murine T241 and LLC tumor cell lines was previously described (56, 59). IMR32 neuroblastoma and A431 squamous carcinoma cell lines were kindly provided by Keiko Funa, Gothenburg University, Sweden. The CAKI-1 renal cell carcinoma cell line was kindly provided by Xuri Li, National Eye Institute, Bethesda, MD. Except for IMR32 cells grown and maintained in RPMI medium (SH30027.01; HyClone) with 10% (vol/vol) FBS, other tumor cells were grown and maintained in DMEM supplemented with 10% (vol/vol) FBS (SH30160.03; HyClone) and 100 μg/mL streptomycin (SV30010; HyClone). Mouse primary PCs were isolated and maintained as previously described (23). All cell lines are negative for mycoplasma (LT07-318; Lonsa).
Pdgfb-shRNA Knockdown in Human A431 Cells.
Plasmids containing an shRNA specific for the human Pdgfb gene and a lentiviral vector-based expression packaging kit were purchased from GeneCopoeia. The transfection procedure was performed according to the manufacturer’s protocol. To produce Pdgfb-shRNA containing viral particles, Pdgfb-shRNA containing plasmid and the viral packaging vectors were cotransfected into the log-phase growing HEK293T monolayer cells. Viral particles carrying Pdgfb-shRNA were harvested from the conditioned medium and were subsequently used to infect A431 cells, which were selected with puromycin resistance. The knockdown efficiency was validated using a quantitative PCR method (qPCR). The human PDGFB primers used for qPCR were as follows: 5′-end forward primer: 5′-CGAATGGTCACCCGAGTTTG-3′; and 3′-end reverse primer: 5′-GAGATGCTGAGTGACCACTC-3′.
Animals.
Male or female C57/B6 mice at age 4 to 6 wk were used for xenograft tumor studies. Age- and sex-matched mice were randomly allocated to each group. The investigators were blinded to group allocation. Approximately 1–2 × 106 of mouse tumor cells were subcutaneously injected into the back along the middorsal line of each C57/B6 mouse. For human tumors, 3–5 × 106 cells were subcutaneously injected into each of 4- to 8-wk-old SCID mice. Tumor volumes were measured and calculated according to the standard formula (volume = length × width2 × 0.5241). For treatment, mice were randomly allocated to treatment groups. A rat anti-mouse PDGFRα neutralizing antibody (40 mg/kg; ImClone Pharmaceuticals) and a rat anti-mouse PDGFRβ neutralizing antibody (40 mg/kg; ImClone Pharmaceuticals) were injected intraperitoneally twice per week (n = 4–6 mice per group). Imatinib (LC laboratories) was injected intraperitoneally at a dose of 50 mg/kg per mouse daily (n = 4–8 animals per group). In some experiments, treatments were started at tumor size 0.3–0.4 cm3. Animals were killed by inhaling a lethal dose of CO2. In some experimental settings, 0.2 × 106 DiI-labeled pericytes were injected into each of the established T241 and LLC tumors. For the tumor invasion assay, 3 × 106 T241-vector or -PDGF-BB tumor cells were injected into the liver of each mouse. All mouse studies were approved by the Northern Stockholm Experimental Animal Ethical Committee.
Knockout and Transgenic Mouse Strains.
The mouse Pdgfrb gene was conditionally depleted by crossing the Pdgfrbflox/flox mice with the Cre–estrogen receptor transgenic mice (University of Toyama) by oral administration of tamoxifen (Sigma-Aldrich; 9 mg/40 g of body weight for 5 consecutive days) at the age of 4 wk. Pdgfrbflox/flox mice were treated identically and used as controls. TN-AP-CreERT2 mice were generated as previously described (15). The R26R-tdTomato reporter mice expressing the cytoplasmic tdTomato were crossed with the TN-AP-CreERT2 mice. Mice at age 6 d were subcutaneously injected with 0.25 mg tamoxifen for 3 consecutive days. At the age of 5 wk, mice were used for tumor implantations. The NG2-CreERT2 mice (Jackson laboratory) were crossed with R26R-tdTomato mice. Adult animals were treated for 5 consecutive days with 2 mg/20 g of tamoxifen by oral administration.
ELISA.
Human tumor cells were grown in a 75-cm2 dish supplement with DMEM or RPMI containing 10% (vol/vol) FBS. Conditioned media were harvested at 48 h after incubation and 100 μL conditioned media from each cell line were used for ELISA to detect human PDGF-BB protein according to the instructions from the manufacturer’s protocol (R&D Systems Inc.).
Quantitative Real-Time and RT-PCR.
Total RNAs extracted from primary human pericytes were used for quantitative real-time PCR (qPCR) analysis. Briefly, 100 ng total RNA from each sample was reversely transcribed using a RevertAid H minus First Strand cDNA Synthesis Kit (Fermentas). Reverse transcription was performed at 42 °C for 60 min, followed by 70 °C for 5 min to inactivate the enzyme activity. Samples were stored at –20 °C and subjected to qPCR using an ABI Prism 7500 System (Applied Biosystems). Each qPCR sample was performed in duplicate and 20 μL reaction contained SYBR Green (Applied Biosystems), 150 nM forward and reverse primers, and 1 μL of cDNA. The qPCR protocol was executed for 40 cycles and each cycle consisted of denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min. The primer pairs specific for various genes used in our experiments included the following: human GAPDH forward: 5′-CATTTCCTGGTATGAAACGA-3′; human GAPDH reverse: 5′-GTCTACATGGCAACTGTGAG-3′; human NG2 forward: 5′-CACGGCTCTGACCGACATAG-3′; and human NG2 reverse: 5′-CCCAGCCCTCTACGACAGT-3′.
Measurement of CTCs Using FACS.
Measurement of CTCs was performed according to our previously published methods (23). Briefly, tumor-bearing mice were killed by inhalation of a lethal dose of CO2 when tumor size reached around 1.2 cm3 in each group. Blood was collected by intracardiac puncture using a heparinized syringe. About 250 μL of whole blood were lysed in 2.5 mL of red blood cell lysis buffer (eBioscience) followed by adding 10 mL PBS. Samples were centrifuged at 400 × g for 5 min and cell pellets were incubated in 4% (wt/vol) PFA for 15 min at room temperature followed by a permeabilization step using 0.15% Triton X-100 in PBS. Fixed and permeabilized cells were centrifuged at 400 × g for 5 min after adding 10 mL of PBS, followed by incubation with 2% BSA in PBS to block nonspecific bindings. Cell pellets were rinsed with PBS and incubated with a rabbit anti-EGFP antibody (A11122, 1:50; Invitrogen) for 45 min followed by goat anti-rabbit Cy3 (1:200; Invitrogen) incubation for 30 min on ice. Cell pellets were washed in PBS and replaced with 1% PFA in PBS. Samples were analyzed by flow cytometry using FACScan and CellQuest software (BD Bioscience). Healthy mouse blood and EGFP-T241 tumor cell samples alone were used for positive and negative cell detection. Forward and side scatter gates were set to delete debris and 10,000 events per sample were analyzed using CellQuest software.
Isolation of Primary Pericytes from Fresh Human Tumor Tissues.
Fresh human tumor tissues were cut into small pieces by scissors, followed by incubation with Type I and II collagenase (each 1.5 mg/mL; Sigma) at 37 °C for 40–60 min. Filtered single-cell suspensions were collected by centrifugation at 100 × g for 10 min. Pellets were incubated with a rabbit anti-NG2 antibody (Millipore) for 45 min on ice followed by a Cy5-labeled goat anti-rabbit antibody (Millipore) incubation for 15 min on ice. Washed cells were further incubated with anti-Cy5 magnetic beads (Miltenyi Biotec) and beads’ positive fractions were collected using magnetic columns. Positive cell fractions were labeled with DiI dye (42364; Sigma) and injected into established tumor tissues or injected with tumor cells into mice immediately after isolation without cultivation. Purity of positive fractions was confirmed using FACSort and CellQuest software (BD Bioscience). Human tissues materials were obtained from the Karolinska Hospital, and the procedure of human sample handling and informed consent were followed according to the regulation approved by Karolinska Biobank (Permission no. BbK1228). Accordingly, all patient materials were anonymized before transferring to research laboratories.
Immunohistochemical Staining.
Immunomhistochemical staining was performed according to our previously published methods (58, 60). Paraffin-embedded tumor issue sections or frozen sections in 5-μm thickness were stained with a rat anti-endomucin antibody (14-5851-85, 1:400; eBioscience), a mouse anti-αSMA antibody (M0851, 1:200; DAKO), or a rabbit anti-FSP1 antibody antibody (07-2274, 1:300; Millipore). The secondary antibodies were an Alexa Fluor 555-labeled goat anti-rat (1:400; Molecular Probes) antibody, an Alexa Fluor 488-labeled donkey anti-mouse (1:400; Molecular Probes) antibody, and an Alexa Fluor 488-labeled donkey anti-rabbit (1:400; Molecular Probes) antibody. Cryostat tissue sections in 5-μm thickness were incubated with specific antibodies against NG2 (1:400), followed by staining with secondary antibodies of an Alexa Fluor 555-labeled goat anti-rat antibody. Tumor tissues were stained with H&E. Mouse primary PCs were seeded on the cover glasses and stimulated with PDGF-BB. PFA fixed cells were stained with a rabbit anti-cleaved caspase 3 antibody (1:500; Cell Signaling), followed by an Alexa Fluor 488-labeled goat anti-rabbit secondary antibody (1:400; Molecular Probes). TUNEL staining was performed according the manufacture’s instruction (In Situ Cell Death Detection Kit; Roche). Cells were mounted using Vectashield containing DAPI. Positive signals were captured using a fluorescence microscope equipped with a camera (DS-QilMC; Nikon).
Stimulation of Pericyte in Vitro and Immunostaining.
Primary PCs were treated with 250 ng/mL PDGF-BB (220-BB-050; R&D Systems, Inc.) for the indicated time points. In some experiments, PCs were incubated with a rat anti-mouse PDGFRα neutralizing antibody (60 ng/mL), a rat anti-mouse PDGFRβ neutralizing antibody (60 ng/mL), or imatinib (1 mg/mL) before PDGF-BB stimulation. Cells were fixed with 4% (wt/vol) PFA for 20 min and PCs were incubated with or without PBS containing 0.15% Triton X-100 (Sigma) for 5 min, followed by incubation with a blocking buffer made of 2% (wt/vol) skim milk and PBS. After 1-h incubation, cells were stained overnight with a rabbit polyclonal anti-NG2 (1:400) antibody, a rabbit anti-mouse FSP1 antibody (1:400), and a rabbit anti-cleaved caspase-3 (9661L; Cell Signaling). Positive signals were detected using an Alexa Fluor 488-conjugated donkey anti-rabbit antibody (A21206, 1:400; Life) and a Cy3-conjugated goat anti-rabbit antibody (AP187C, 1:400; Millipore). For detection of cellular apoptosis, TUNEL staining was performed according the manufacturer’s instructions. Stained cells were mounted using Vectashield containing DAPI and images were taken using fluorescence microscopy (Nikon) at random fields.
Immunoblotting.
Total cellular proteins from total cell lysates along with a protein ladder (SM1811; Fermentas) were subjected to SDS/PAGE (NP0301; Invitrogen), followed by wet transferring onto methanol-activated polyvinylidene fluoride membranes (LC2002; Invitrogen). Membranes were blocked at 4 °C for 30 min with 5% (wt/vol) BSA (A8806; Sigma) before incubation overnight with an anti-mouse NG2 antibody, an anti-mouse FSP1 antibody, or an anti-mouse β-actin (3700S , 1:1,000; Cell Signaling) monoclonal antibody. Membranes were incubated for 45 min with a labeled and species-matching secondary antibody (IRDye 680 or 800, 1:1,000; LI-COR Biosciences). Positive signals were visualized using infrared fluorescence (ODYSSEY CLx; LI-COR Biosciences).
Affymetrix Gene-Array Analysis.
RNA samples from PDGF-BB–stimulated and nonstimulated pericytes were prepared using the RNAeasy kit (Qiagen). Three replicates for each sample were hybridized using Affymetrix 1.0 ST Gene arrays. Custom CDF definitions (ENTREZGENE) for data extraction (61) used to improve precision and accuracy and data normalization were performed using robust multichip averaging (RMA) in R (https://www.r-project.org) by applying the “rma” function within the “affy” package (v1.32.1) and using default settings. Differentially expressed genes between each of the treated conditions (days 1 or 5) and the untreated control (day 0) condition were identified using either the multiclass rank product analysis or the random variance model two-tailed t test (62). The resulting P values were adjusted for multiple testing using the Benjamini–Hochberg false-discovery rate (FDR) method. Genes with an FDR < 0.05 were considered differentially expressed. PFT-related genes were normalized per individual genes (by centering the expression values across all replicates and conditions for each gene to 0 [log2 scale]) and subjected to hierarchical clustering. Densities of normalized expression values for the PFT-related genes at the control and day-5 time points were plotted and the Kolmogorov–Smirnov test was used to test the null hypothesis (i.e., that the distributions are the same). The binomial test was used to test whether more congruent regulation was observed compared with chance. The percentages showing congruent and noncongruent regulation were also plotted for genes that were up- or down-regulated after day 5 of PDGF-BB treatment. Pericyte gene-array data and S17 stromal cell data have been deposited in the Gene Expression Omnibus with accession numbers GSE85955 and GSE33717 (30).
Correlation of PDGFB Expression in Human Cancer Patients.
Survival data of 72 COADREAD patients, 212 brain LGG, and 391 LUSC patients from The Cancer Genome Atlas are analyzed for PDGFB-high (above median or top 25/10%) and PDGFB-low (below median or lowest 25/10%) groups. The statistical difference was analyzed using the Kaplan–Meier survival method followed by log-rank test. Gene expression of S100A4 is further analyzed between PDGFB-low and PDGFB-high group using the Mann–Whitney U test.
Acknowledgments
We thank Funeng Jiang, Xiaojuan Yang, Yin Zhang, and Hideki Iwamoto for technical assistance; and Dr. Funa Keiko (Gothenburg University) and Dr. Xuri Li (Zhongshan Ophthalmology Center, Sun Yat-sen University) for providing tumor cell lines. Y.C.’s laboratory is supported through research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, a Karolinska Institute distinguished professor award, the Torsten Soderbergs Foundation, the Tore Nilsons Foundation, the Ruth and Richard Julin Foundation, the Ogonfonden Foundation, the Martin Rinds Foundation, the Maud and Birger Gustavssons Foundation, the Lars Hiertas Minne Foundation, the Alex and Eva Wallströms Foundation, the Robert Lundbergs Memorial Foundation, the Swedish Diabetes Foundation, the Swedish Children Cancer Foundation, European Research Council Advanced Grant ANGIOFAT (Project 250021), the Knut Alice Wallenberg Foundation, and an advanced grant from NOVO Nordisk Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE85955 and GSE33717).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608384113/-/DCSupplemental.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, et al. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3(114):114rv3. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller MM, Fusenig NE. Friends or foes—Bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 8.Lim SD, et al. Expression of the neural stem cell markers NG2 and L1 in human angiomyolipoma: Are angiomyolipomas neoplasms of stem cells? Mol Med. 2007;13(3-4):160–165. doi: 10.2119/2006-00070.Lim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindarajan B, et al. Cooperative benefit for the combination of rapamycin and imatinib in tuberous sclerosis complex neoplasia. Vasc Cell. 2012;4(1):11. doi: 10.1186/2045-824X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai VB, et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:6656. doi: 10.1038/ncomms7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF. Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet. 1998;18(4):385–388. doi: 10.1038/ng0498-385. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18(4):478–489. doi: 10.1016/j.cmet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Dellavalle A, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 16.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32(4):687–698. [PubMed] [Google Scholar]
- 17.Mills SJ, Cowin AJ, Kaur P. Pericytes, mesenchymal stem cells and the wound healing process. Cells. 2013;2(3):621–634. doi: 10.3390/cells2030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian X, et al. Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science. 2014;345(6192):90–94. doi: 10.1126/science.1251487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YT, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80(11):1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys BD, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betsholtz C. Role of platelet-derived growth factors in mouse development. Int J Dev Biol. 1995;39(5):817–825. [PubMed] [Google Scholar]
- 22.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 23.Hosaka K, et al. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun. 2013;4:2129. doi: 10.1038/ncomms3129. [DOI] [PubMed] [Google Scholar]
- 24.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 25.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: A novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20(6):1692–1703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 26.Savage DG, Antman KH. Imatinib mesylate—A new oral targeted therapy. N Engl J Med. 2002;346(9):683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 27.Shah NP, Sawyers CL. Recent success with the tyrosine kinase inhibitor STI-571—Lessons for targeted therapy of cancer. Curr Opin Investig Drugs. 2001;2(3):422–423. [PubMed] [Google Scholar]
- 28.Sawyers CL. Disabling Abl-perspectives on Abl kinase regulation and cancer therapeutics. Cancer Cell. 2002;1(1):13–15. doi: 10.1016/s1535-6108(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, et al. Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing. J Biol Chem. 2005;280(10):9375–9389. doi: 10.1074/jbc.M413081200. [DOI] [PubMed] [Google Scholar]
- 30.Xue Y, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2011;18(1):100–110. doi: 10.1038/nm.2575. [DOI] [PubMed] [Google Scholar]
- 31.Kramann R, Humphreys BD. Kidney pericytes: Roles in regeneration and fibrosis. Semin Nephrol. 2014;34(4):374–383. doi: 10.1016/j.semnephrol.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 34.McCarty MF, et al. Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest. 2007;117(8):2114–2122. doi: 10.1172/JCI31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuhashi M, et al. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004;64(8):2725–2733. doi: 10.1158/0008-5472.can-03-1489. [DOI] [PubMed] [Google Scholar]
- 36.Pietras K, Sjöblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3(5):439–443. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 37.Crawford Y, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5(1):e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhnert F, et al. Soluble receptor-mediated selective inhibition of VEGFR and PDGFRbeta signaling during physiologic and tumor angiogenesis. Proc Natl Acad Sci USA. 2008;105(29):10185–10190. doi: 10.1073/pnas.0803194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xian X, et al. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116(3):642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duda DG, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. 2010;107(50):21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergsten E, et al. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3(5):512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- 44.Cao R, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16(12):1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- 45.LaRochelle WJ, et al. PDGF-D, a new protease-activated growth factor. Nat Cell Biol. 2001;3(5):517–521. doi: 10.1038/35074593. [DOI] [PubMed] [Google Scholar]
- 46.Li X, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2(5):302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 47.Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 48.Drummond-Barbosa DA, Vaillancourt RR, Kazlauskas A, DiMaio D. Ligand-independent activation of the platelet-derived growth factor beta receptor: Requirements for bovine papillomavirus E5-induced mitogenic signaling. Mol Cell Biol. 1995;15(5):2570–2581. doi: 10.1128/mcb.15.5.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrlich A, et al. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc Natl Acad Sci USA. 1998;95(15):8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai CC, Henningson C, DiMaio D. Bovine papillomavirus E5 protein induces oligomerization and trans-phosphorylation of the platelet-derived growth factor beta receptor. Proc Natl Acad Sci USA. 1998;95(26):15241–15246. doi: 10.1073/pnas.95.26.15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 52.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 53.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Dong M, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013;18(1):118–129. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hedlund EM, et al. Tumor cell-derived placental growth factor sensitizes antiangiogenic and antitumor effects of anti-VEGF drugs. Proc Natl Acad Sci USA. 2013;110(2):654–659. doi: 10.1073/pnas.1209310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nissen LJ, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117(10):2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X, et al. Vascular endothelial growth factor-dependent spatiotemporal dual roles of placental growth factor in modulation of angiogenesis and tumor growth. Proc Natl Acad Sci USA. 2013;110(34):13932–13937. doi: 10.1073/pnas.1309629110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci USA. 2013;110(29):12018–12023. doi: 10.1073/pnas.1301331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao R, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6(4):333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 60.Cao R, et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci USA. 2012;109(39):15894–15899. doi: 10.1073/pnas.1208324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai M, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33(20):e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright GW, Simon RM. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics. 2003;19(18):2448–2455. doi: 10.1093/bioinformatics/btg345. [DOI] [PubMed] [Google Scholar]