Significance
The anaphase-promoting complex/cyclosome (APC/C) is a large E3 ubiquitin ligase that controls progression through mitosis and entry into G1. Its capacity to recognize and ubiquitinate substrates is dependent on coactivator subunits that interact with substrate degrons and promote a conformational change of the APC/C to increase its affinity for the priming E2 UbcH10. We show that the WD40 domain of anaphase-promoting complex subunit 1 (Apc1) is required for communicating the conformational change initiated by the binding of coactivator to the catalytic module. In contrast to UbcH10, binding of the elongating E2 Ube2S and its APC/C-stimulated activity does not require the active state of the APC/C. The work raises the possibility that conformational changes of the Apc1 WD40 domain may play a role in regulating UbcH10 binding to the APC/C.
Keywords: APC/C, ubiquitination, cell cycle, UbcH10, Ube2S
Abstract
The anaphase-promoting complex/cyclosome (APC/C) is a large multimeric cullin–RING E3 ubiquitin ligase that orchestrates cell-cycle progression by targeting cell-cycle regulatory proteins for destruction via the ubiquitin proteasome system. The APC/C assembly comprises two scaffolding subcomplexes: the platform and the TPR lobe that together coordinate the juxtaposition of the catalytic and substrate-recognition modules. The platform comprises APC/C subunits Apc1, Apc4, Apc5, and Apc15. Although the role of Apc1 as an APC/C scaffolding subunit has been characterized, its specific functions in contributing toward APC/C catalytic activity are not fully understood. Here, we report the crystal structure of the N-terminal domain of human Apc1 (Apc1N) determined at 2.2-Å resolution and provide an atomic-resolution description of the architecture of its WD40 (WD40 repeat) domain (Apc1WD40). To understand how Apc1WD40 contributes to APC/C activity, a mutant form of the APC/C with Apc1WD40 deleted was generated and evaluated biochemically and structurally. We found that the deletion of Apc1WD40 abolished the UbcH10-dependent ubiquitination of APC/C substrates without impairing the Ube2S-dependent ubiquitin chain elongation activity. A cryo-EM structure of an APC/C–Cdh1 complex with Apc1WD40 deleted showed that the mutant APC/C is locked into an inactive conformation in which the UbcH10-binding site of the catalytic module is inaccessible. Additionally, an EM density for Apc15 is not visible. Our data show that Apc1WD40 is required to mediate the coactivator-induced conformational change of the APC/C that is responsible for stimulating APC/C catalytic activity by promoting UbcH10 binding. In contrast, Ube2S activity toward APC/C substrates is not dependent on the initiation-competent conformation of the APC/C.
The eukaryotic cell cycle is controlled by oscillations in the activities of key regulatory proteins through the interplay of reversible protein phosphorylation and irreversible ubiquitin-dependent proteolysis (1–3). By ubiquitinating essential cell-cycle proteins, the anaphase-promoting complex/cyclosome (APC/C) is the crucial RING E3 ubiquitin ligase that controls accurate sister chromatid segregation, cytokinesis, and the initiation of chromosome replication (4–9). The APC/C and a second cullin–RING E3 ligase, the Skp1–Cul1–F-box protein (SCF), coordinate cell-cycle regulation and are important players in cancer biogenesis (10).
APC/C activity is controlled by its association with one of two coactivator subunits (either Cdc20 or Cdh1) that function to specify substrate recognition and stimulate ubiquitin transfer reactions (11–14). The mitotic coactivator Cdc20 preferentially binds to hyperphosphorylated APC/C, whereas Cdh1 also binds to unphosphorylated APC/C. Coactivators enhance vertebrate APC/C catalytic activity by increasing its affinity for the priming E2 UbcH10 (also known as “Ube2C”) (12), whereas in budding yeast APC/C coactivators enhance E2 catalytic efficiency (13). The APC/C is a multisubunit E3 ligase composed of 15 different proteins (12). Five are tetratricopeptide repeat (TPR) proteins, four of which (Apc3, Apc6, Apc7, and Apc8) form structurally related homodimers. Apc12, Apc13, and Apc16 are TPR accessory subunits. Apc1 is the largest scaffolding subunit (molecular mass ∼200 kDa) (15). Its proteasome cyclosome (PC) domain shares the same PC repeat architecture as the Rpn1 and Rpn2 subunits of the 19S proteasome (16). The N-terminal region of Apc1 (Apc1N) is rich in β-strands and possesses a multitude of regulatory phosphorylation sites (17, 18). The APC/C catalytic module composed of Apc2 and Apc11 recruits canonical E2s (UbcH10 and UbcH5 in vertebrates, Ubc1 and Ubc4 in budding yeast) to catalyze substrate ubiquitination (19, 20). Apc2 is a cullin domain protein that interacts with the RING domain subunit Apc11. Apc10 and the coactivators are responsible for APC/C substrate recruitment. Apc15 is required for Cdc20 autoubiquitination in the context of the mitotic checkpoint complex (MCC), thereby regulating APC/C–MCC disassembly (21–23).
In human APC/C, UbcH10 and Ube2S synthesize polyubiquitin chains through a sequential mechanism. The association of UbcH10 with the RING domain of Apc11 (Apc11RING) and Apc2’s winged-helix B domain (Apc2WHB) initiates ubiquitin-chain formation (19, 20, 24–26). Ube2S, on the other hand, is responsible for ubiquitin-chain extension and specifically assembles Lys11-linked ubiquitin chains on substrates targeted by the APC/C (14, 25, 27–30). In vertebrates, the RING domain of Apc11 is repurposed to position the acceptor ubiquitin, conjugated to an APC/C substrate, for modification by Ube2S (14, 30, 31). UbcH10 and Ube2S together build branched ubiquitin chains on APC/C substrates, and these chains are recognized more efficiently by the proteasome (32). In budding yeast, APC/C modifies substrates with Lys48-linked ubiquitin chains. The E2 Ubc4 initiates the ubiquitin chain synthesis, whereas Ubc1 extends the Lys48-linked ubiquitin chains (33, 34).
Previous cryo-EM studies showed that Cdh1 association with the APC/C promotes substantial conformational changes in the catalytic module and the neighboring platform subunits Apc1, Apc4, and Apc5 (12). This change in conformation exposes the UbcH10-binding site of the catalytic module, enhancing UbcH10 association and thereby stimulating APC/C E3 ligase activity (12, 19). Here, we combined biochemical methods, X-ray crystallography, and single-particle EM to study the structure of Apc1N and to examine its functions in vitro. We demonstrate that Apc1N is essential for APC/C catalytic activity because it is required to mediate the coactivator-induced conformational changes necessary for UbcH10 to engage the APC/C catalytic module.
Results
Apc1N Comprises a WD40 β-Propeller Domain.
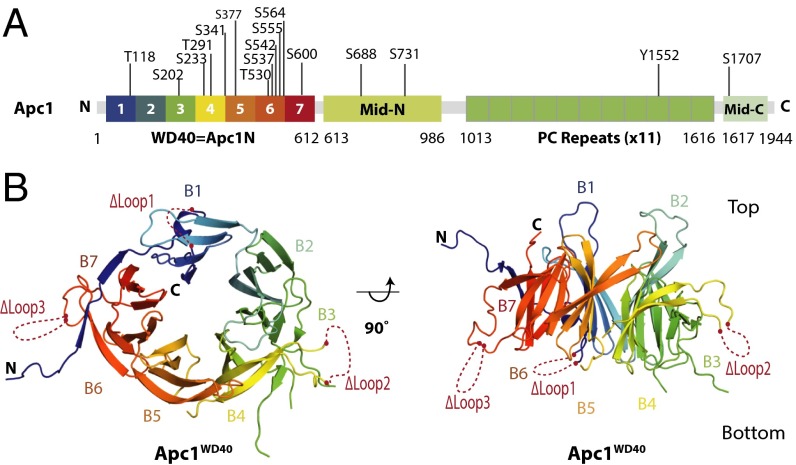
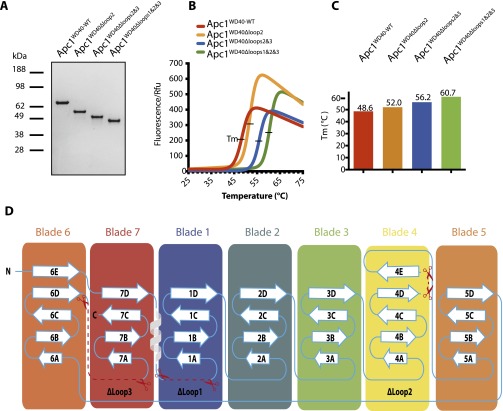
The domain architecture of full-length Apc1 is shown in Fig. 1A. Apc1N is followed by the middle domain (Mid-N), a PC domain, and a C-terminal domain (Mid-C) that coalesces with Mid-N to form Apc1Mid (19). Combining the secondary structure predictions of Apc1 with structural information from the APC/C atomic model determined using a 3.6-Å resolution cryo-EM map (19), we designed the following Apc1N constructs: Apc1NWT, Apc1NΔloop2, Apc1NΔloops2&3, and Apc1NΔloops1&2&3 (Fig. 1B). The resultant proteins were expressed using the insect cell/baculovirus system, purified (Fig. S1A), and screened for crystallization conditions. The stability of the four Apc1N constructs was examined using thermal shift assays. The combined deletion of loops 1, 2, and 3 (Apc1NΔloops1&2&3) greatly increased the thermal stability of the protein (Fig. S1 B and C) and allowed its crystallization.
Fig. 1.
Crystal structure of Apc1WD40. (A) Schematic of domain architecture of Apc1. The Apc1WD40 β-propeller structure determined in this study is rainbow color-coded. Phosphorylation sites as defined in ref. 19 are indicated. (B) Ribbons representation of the Apc1 N-terminal β propeller. Deleted loops are indicated by dashed red lines. Loop 1: residues 34–69; loop 2: residues 307–402; loop 3: residues 523–580.
Fig. S1.
Thermal shift assay of Apc1N constructs. (A) SDS/PAGE of Apc1N constructs: Apc1NWT, Apc1NΔloop2, Apc1NΔloops2&3, and Apc1NΔloops1&2&3. (B) Thermal shift assay analysis of each construct visualized in Prism. (C) The melting temperatures (Tm) of individual constructs are shown in vertical bars format with values at 48.6 °C, 52 °C, 56.2 °C, and 60.7 °C, respectively. (D) Schematic of secondary structure elements of Apc1WD40. Loops deleted in this study are indicated.
The Apc1NΔloops1&2&3 construct yielded protein crystals that diffracted to 2.2-Å resolution (Table S1). The crystal structure of Apc1NΔloops1&2&3 was determined using the cryo-EM–derived APC/C atomic model (19) as a search object in molecular replacement. The crystal structure of Apc1NΔloops1&2&3 was confirmed as a WD40 β-propeller domain, which consists of seven blades, each with either four or five β-strands (Fig. 1B and Fig. S1D). The Apc1 WD40 domain (Apc1WD40) is ∼70 Å in diameter across its top face and ∼50 Å in height (Fig. 1B). In blade 7, an N-terminal β-strand (β7D) joins strands β7A–C to close the propeller in a Velcro-like closure common to β-propeller domains (35). Loop 2, the longest disordered loop, was removed from the segment connecting β-strands β4D and β4E, and loops 1 and 3 were removed from the segments connecting β7D with β1A and β6D with β7A, respectively (Fig. 1B and Fig. S1D). There is only one helical region in Apc1WD40, located within the extended loop that emerges from strand β7D. The WD40 repeat domain is an ancient conserved architecture that functions in many cellular processes (36, 37). The similarities of Apc1WD40 to other WD40 domain proteins were assessed using the pairwise structural comparison server DALI (Table S2) (38).
Table S1.
Crystallographic data collection and refinement statistics
| Beam line | Diamond Light Source I02 |
| Wavelength, Å | 0.9795 |
| Resolution, Å | 40.13–2.15 (2.23–2.15) |
| Space group | C2221 |
| Unit cell, Å | 82.6 Å, 113.3 Å, 100.4 Å |
| Multiplicity | 4.8 (2.5) |
| Completeness, % | 97.8 (84.7) |
| Mean I/σ, I | 16.76 (2.01) |
| CC1/2 | 0.999 (0.891) |
| Rmerge | 0.059 (0.433) |
| Rwork | 0.179 (0.273) |
| Rfree | 0.227 (0.303) |
| No. of nonhydrogen atoms | 3152 |
| Protein residues | 378 |
| Rms bonds, Å | 0.009 |
| Rms angles, ° | 1.21 |
| Ramachandran favored, % | 98.4 |
| Ramachandran allowed, % | 1.6 |
| Ramachandran outliers, % | 0 |
| Clash score | 4.53 |
| Average B-factor | 56.7 |
| Rotamer outliers, % | 1.8 |
| Solvent, % | 56.13 |
Numbers in parentheses are for the outermost shells.
Table S2.
DALI analysis of the Apc1WD40 crystal structure
| No. | Protein match | PDB ID code | Z | Rmsd, Å | Aligned, no. | Res, no. | Identity, % |
| 1 | Nup120 | 4gq2-M | 16.7 | 4.0 | 281 | 904 | 6 |
| 2 | PTHB11 | 4yd8-B | 16.1 | 4.2 | 272 | 334 | 11 |
| 3 | BBS1 | 4v0n-B | 16.1 | 4.1 | 263 | 330 | 8 |
| 4 | Sni1 | 2oaj-A | 16.0 | 3.9 | 283 | 875 | 6 |
| 5 | Nup157 | 4mhc-A | 15.9 | 4.1 | 281 | 671 | 7 |
Res, residues.
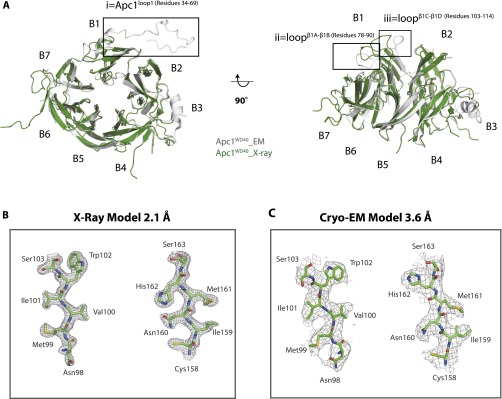
As expected, the Apc1WD40 crystal structure is in good agreement with the Apc1WD40 model from the cryo-EM structure of APC/C–Cdh1–Emi1 at 3.6 Å (19). The two structures aligned with an rmsd value of 1 Å (Fig. S2A). Because the crystal structure is at higher resolution than the cryo-EM structure, it better defines side-chain rotamers (Fig. S2 B and C), and Apc1WD40–water interactions also can be observed.
Fig. S2.
Structural analysis of the Apc1WD40 X-ray structure and comparison with the Apc1WD40 EM structure. (A) Superimposition of the Apc1WD40 crystal structure (green) with EM model (gray). The main differences are enlarged in squares i, ii, and iii. The extra residues built into the EM model are in box i. (B) The X-ray crystallization electron density map with the selected segments. (Left) Residues 98–103. (Right) Residues 158–163. (C) The cryo-EM density and EM model with the same selected segments.
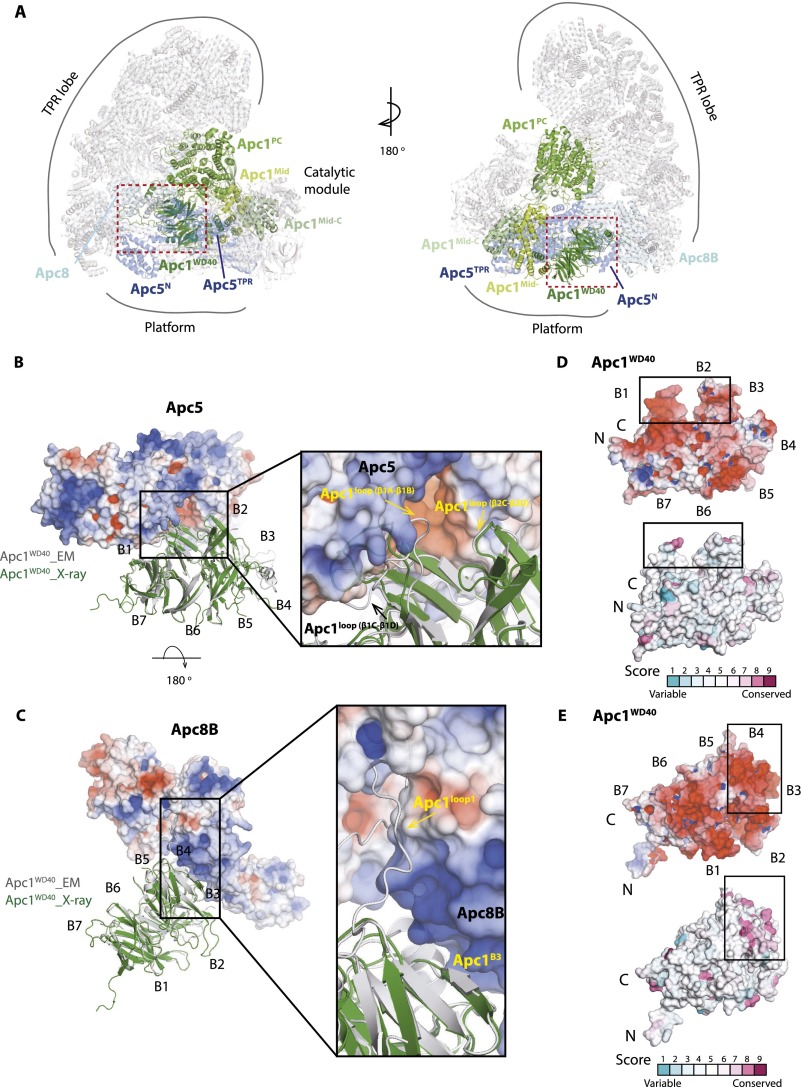
Apc1WD40 Interacts with Apc5 and Apc8B.
Within the context of the APC/C assembly, Apc1WD40 tucks into the helical groove of Apc5TPR (the TPR domain of Apc5) and forms an edge-on contact with the C-terminal TPR helix of Apc8B (one subunit of the homodimer Apc8) (Fig. S3) (12, 19). To gain structural insights into the interactions of Apc1WD40 with Apc5 and Apc8B, we mapped the sequence conservation and electrostatic potential of Apc1WD40 onto its molecular surface. The surface electrostatic analysis shows that Apc1WD40 is predominantly negatively charged, especially on surfaces that interact with complementary positively charged regions of Apc5 and Apc8B (Fig. S3), and, notably, these interacting regions are evolutionarily conserved (Fig. S3 D and E). The interface interactions between Apc1WD40 and Apc5 and Apc8B were analyzed using Protein Interfaces, Surfaces, and Assemblies (PISA) (Table S3). Conformational differences between the crystal and EM structures of Apc1WD40, confined mainly to surface loops, accommodate the interaction of Apc1WD40 with Apc5 and Apc8B (Fig. S3 B and C).
Fig. S3.
Apc1WD40 interacts with Apc5 and Apc8. (A) Structure of Apc1 within the context of the cryo-EM APC/C–Cdh1–Emi1 ternary structure (19). Apc1, Apc5, and Apc8B are highlighted. (B and C) Views showing the interface between Apc1WD40 and Apc5 (B) and Apc8B (C). The electrostatic potentials (color ramped from red to blue for negative to positive electrostatic potential) of Apc5 and Apc8B are shown, and Apc1WD40 is shown in a cartoon representation. The EM and X-ray structures of Apc1WD40 are in gray and green, respectively. Loops that differ in conformation in the two structures as a result of interactions with Apc5 and Apc8 are indicated. (D and E) Two views of the surface of Apc1WD40 showing surface electrostatic potential and structural conservation (colored from turquoise to maroon indicating variable to conserved).
Table S3.
PISA analysis of Apc1WD40 domain interactions with Apc5 and Apc8B
| Interaction | Protein A | Nres | Protein B | Nres | ΔG, kcal/mol |
| 1 | Apc1WD40 | 45 | Apc5 | 48 | −13.5 |
| 2 | Apc1WD40 | 20 | Apc8B | 35 | −12.7 |
ΔG, interface solvation energy; Nres, number of interface residues.
Apc1WD40 Is Required for APC/C–UbcH10 Ubiquitination Activity.
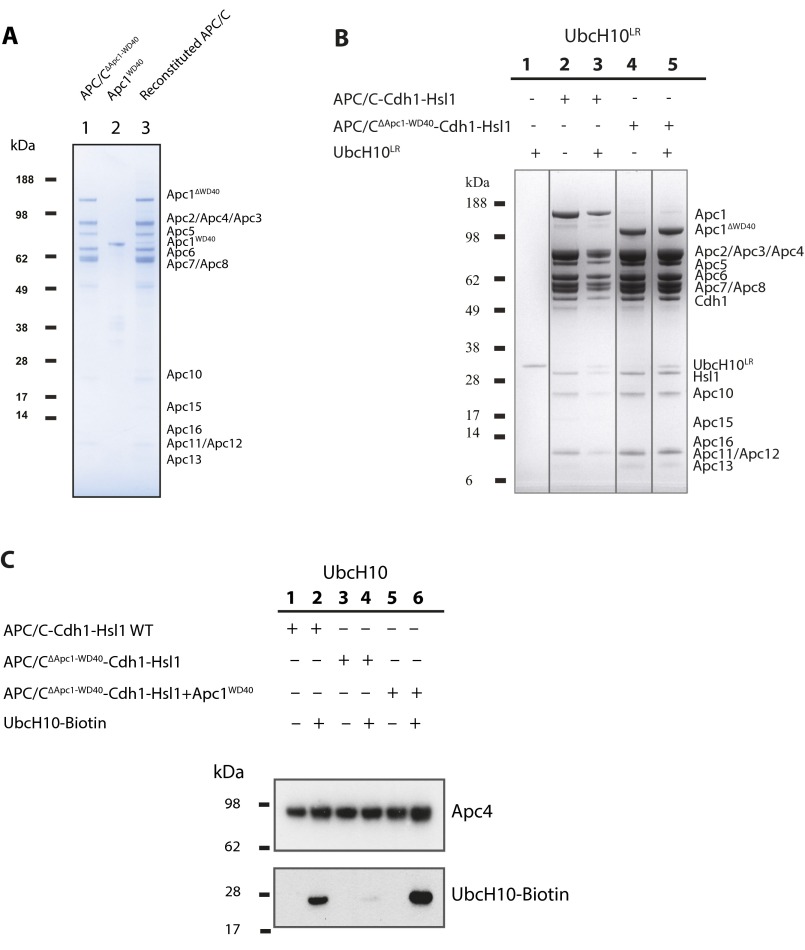
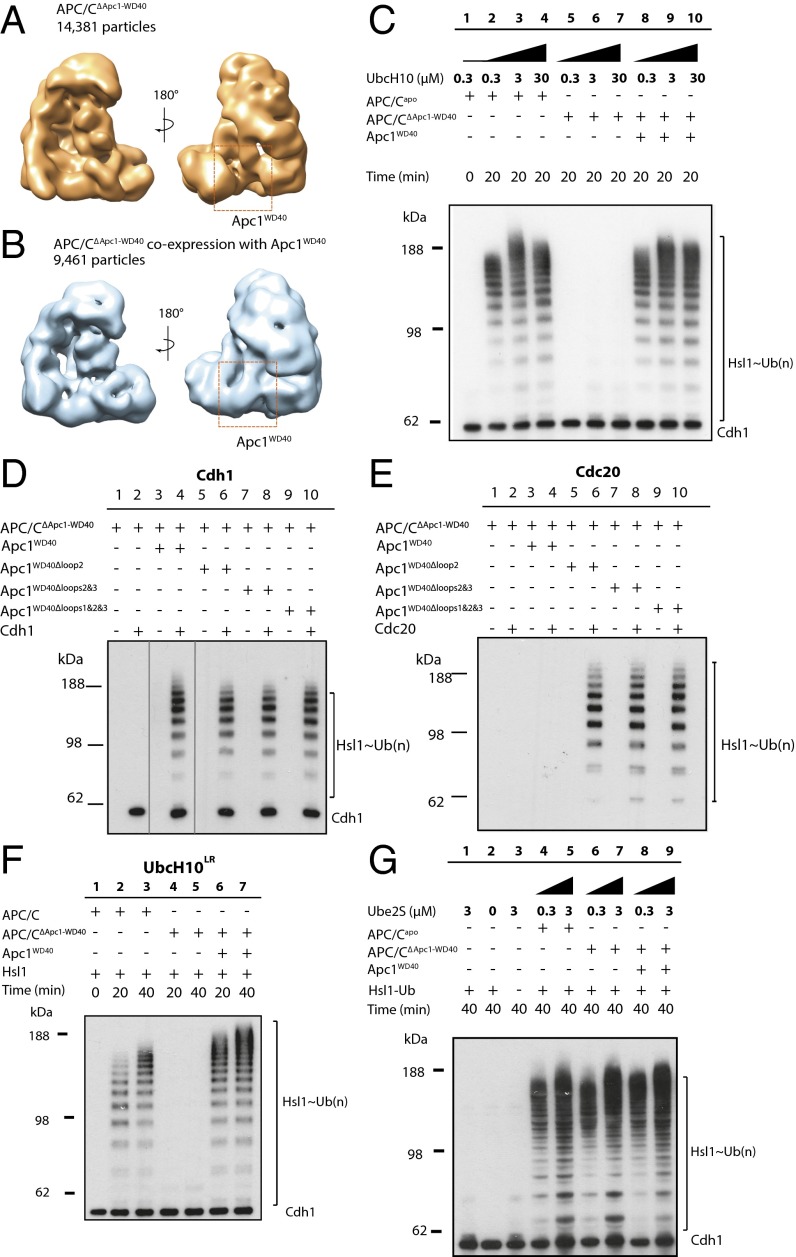
To understand better the function of Apc1WD40 for APC/C activity and its contribution to the overall conformation of the APC/C, we reconstituted recombinant mutant APC/C lacking Apc1WD40 (APC/CΔApc1−WD40) and tested its activity using in vitro ubiquitination assays. As judged by SDS/PAGE gels (Fig. S4A) and single-particle negative-stain EM (Fig. 2A), APC/CΔApc1−WD40 was assembled correctly. Strikingly however, in contrast to wild-type APC/C, the UbcH10-dependent APC/C ubiquitination activity was abolished, even at 30 μM UbcH10 [∼100-fold its Kd (12)] (compare lanes 2–4 with lanes 5–7 in Fig. 2C). When the purified Apc1WD40 was added back to the APC/CΔApc1−WD40, ubiquitination activity was restored (lanes 8–10 in Fig. 2C). In addition, the reconstituted APC/CΔApc1−WD40–Apc1WD40 complex is structurally equivalent to the wild-type PC/C complex as revealed by negative-stain EM (Fig. 2B), indicating that the mutant complex activity and structure could be fully recovered by the addition of Apc1WD40. Thus, Apc1WD40 is essential for APC/C–Cdh1–UbcH10 catalytic activity.
Fig. S4.
APC/C complexes and biochemical characterization of E2 interaction with APC/C complexes. (A) SDS PAGE gel of APC/CΔApc1−WD40 complexes. (B) SDS/PAGE analysis of UbcH10LR interactions with the APC/CΔApc1−WD40–Cdh1–Hsl1 complex (lane 5). APC/C subunits and the coeluted UbcH10LR are indicated on the right. Lanes 1-5 correspond to lanes 1, 4, 3, 5, and 7 of SDS PAGE gel (unwanted lanes 2 and 6 were removed). Lines were added to Fig. S4B to separate cropped lanes. (C) Western blot analysis of UbcH10 interactions with the ternary mutant APC/CΔApc1−WD40–Cdh1–Hsl1 complex (lane 4). The upper blot was performed with anti-Apc4 antibody to monitor the quantity of APC/C complexes used in this study, and the lower blot was performed with streptavidin to monitor the coeluted UbcH10 from the size-exclusion chromatography column.
Fig. 2.
APC/CΔApc1−WD40–Cdh1 is inactive toward UbcH10 but active toward Ube2S. (A and B) Negative-stain EM reconstructions of APC/CΔApc1−WD40 (A) and APC/CΔApc1−WD40 with coexpressed Apc1WD40 (B). (C) E3 ligase activity of the wild-type APC/C and APC/CΔApc1−WD40 with Cdh1, Hsl1, and UbcH10 (lanes 1 to 4). In contrast to wild-type APC/C, APC/CΔApc1−WD40–UbcH10 did not ubiquitinate Hsl1 (lanes 5 to 7). Adding back Apc1WD40 to APC/CΔApc1−WD40 restored activity (lanes 8 to 10). (D) Ubiquitination assays of the Cdh1-mediated APC/CΔApc1−WD40-loops activity. Lanes 1 to 10 correspond to lanes 1, 2, 11, 12, and 5-10 of the original Western blot. This is consistent with the order of lanes in Fig. 2E. Lines were added surrounding spliced lanes 3 and 4. As in C, APC/CΔApc1−WD40–Cdh1 showed no activity compared with apo APC/CΔApc1−WD40. The APC/C–Cdh1 ubiquitination activity toward Hsl1 was restored by adding back Apc1WD40, Apc1WD40–Δloop2, Apc1WD40–Δloops2&3, and Apc1WD40–Δloops1&2&3. (E) Ubiquitination activity of APC/CΔApc1−WD40 with Cdc20 and loop deletions of Apc1WD40. APC/CΔApc1−WD40–Cdc20 was not active and could not be activated with wild-type Apc1WD40. The addition of Apc1WD40–Δloop2, Apc1WD40–Δloops2&3, or Apc1WD40–Δloops1&2&3 to APC/CΔApc1 allowed APC/CΔApc1 to be activated by Cdc20. (F) Ubiquitination activity of APC/C–UbcH10LR toward Hsl1. In contrast to wild-type APC/C (lanes 2 and 3), APC/CΔApc1−WD40–UbcH10LR did not ubiquitinate Hsl1 (lanes 4 and 5). The addition of Apc1WD40 restored the ubiquitination activity (lanes 6 and 7). (G) Ubiquitination assay of APC/CΔApc1−WD40 with Cdh1, Hsl1–K1–Ub, and Ube2S. APC/CΔApc1−WD40–Cdh1 showed higher activity than the wild-type APC/C–Cdh1. The reconstituted APC/CΔApc1−WD40–Cdh1–Apc1WD40 complex showed activity similar to that of wild-type APC/C.
An Apc1WD40 Loop Regulates APC/CCdc20 Activity.
As discussed above, three disordered loops were deleted from Apc1WD40 to aid successful protein crystallization. Numerous mitotic phosphorylation sites are located within these loops, implicating a potential role in regulating Cdc20 interactions with the APC/C. This idea has been confirmed recently by structural and biochemical studies (39, 40). We therefore addressed the requirement of these Apc1WD40 loops for APC/C activity. To obtain versions of APC/C lacking one or more Apc1WD40 loops, APC/CΔApc1−WD40 was reconstituted with individual Apc1WD40 loop-deletion constructs. The activity of the resultant reconstituted APC/C was then tested. These tests showed that the catalytic activity of APC/CΔApc1−WD40–Cdh1 is restored with any of the Apc1WD40 loop-deletion constructs (lanes 4, 6, 8, and 10 in Fig. 2D). Therefore the three loops of Apc1WD40 are not required for APC/C–Cdh1 catalytic activity. Strikingly, when we used Cdc20 as the coactivator, although APC/CΔApc1−WD40 was not activated using wild-type Apc1WD40 (lane 4 in Fig. 2E), the loop 2 deletion construct of Apc1WD40 generated active APC/CCdc20 (lane 6 in Fig. 2E). Additional deletions of loops 1 and 3 did not further activate APC/CCdc20 (lanes 8 and 10 in Fig. 2E). This result, indicating that loop 2 (residues 307–402) of Apc1WD40 inhibits Cdc20 activity, is in agreement with the identification of an autoinhibitory segment within this loop that blocks the binding site of the coactivator C-box on Apc8B (39, 40). Phosphorylation of loop 2 (referred to as the “300s loop” in ref. 39) by mitotic kinases displaces the autoinhibitory segment, relieving the steric blockade of the C-box binding site, thereby permitting APC/C–Cdc20 interactions (39). Thus, deletion of loop 2 of Apc1WD40 (also termed the “300s loop”) enables APC/CCdc20 activation, mimicking the effects of mitotic APC/C phosphorylation. This finding is in agreement with recent studies (39, 40).
Apc1WD40 Is Required for the APC/C–Cdh1 Complex to Bind UbcH10.
The loss of APC/C catalytic activity in the absence of Apc1WD40 could result from the loss of either UbcH10 or substrate/coactivator interactions with the mutant APC/C. The latter possibility was excluded because an APC/CΔApc1−WD40–Cdh1–Hsl1 complex was isolated using size-exclusion chromatography (lane 4 in Fig. S4B). To test the association of UbcH10 with both the APC/C and APC/CΔApc1−WD40, size-exclusion chromatography was performed. An excess of biotinylated UbcH10, prepared as described (12), was incubated with either wild-type ternary APC/C–Cdh1–Hsl1 or mutant ternary APC/CΔApc1−WD40–Cdh1–Hsl1 complexes. Although wild-type ternary APC/C binds UbcH10 (lane 2 in Fig. S4C), virtually no UbcH10 coeluted with APC/CΔApc1−WD40–Cdh1–Hsl1 (lane 4 in Fig. S4C). However, UbcH10 associated with the reconstituted APC/CΔApc1−WD40–Cdh1–Hsl1–Apc1WD40 complex, as it did with wild-type APC/C (lane 6 in Fig. S4C). Thus, Apc1WD40 is required for UbcH10 to associate optimally with the APC/C–coactivator complex, and the loss of UbcH10-dependent catalytic activity of the APC/CΔApc1−WD40 mutant results (at least in part) from the loss of UbcH10 binding.
UbcH10LR [a fusion of the LR motif of Ube2S (residues 154–222) to the C terminus of UbcH10] has a higher affinity for APC/C than does wild-type UbcH10 because the LR motif of UbcH10LR engages the LR motif-binding site at the interface of Apc2 and Apc4 (12, 19). Using size-exclusion chromatography, we could detect binding of UbcH10LR to both APC/C–Cdh1–Hsl1 and APC/CΔApc1−WD40–Cdh1–Hsl1 (lanes 3 and 5 in Fig. S4B). However, despite the binding of UbcH10LR to APC/CΔApc1−WD40, the mutant APC/CΔApc1−WD40–Cdh1 complex was still unable to ubiquitinate Hsl1 (compare lanes 2 and 3 with lanes 4 and 5 in Fig. 2F). These data are consistent with the idea that in the APC/CΔApc1−WD40–Cdh1 complex the recognition site for the Ubc domain of UbcH10 on the APC/C’s catalytic module, necessary to stimulate UbcH10 catalytic activity (19, 20), is not accessible.
Apc1WD40 Is Not Required for APC/C–Ube2S Ubiquitination Activity.
Although the APC/CΔApc1−WD40–Cdh1–UbcH10 E2–E3 pair is deficient in substrate ubiquitination, it remained possible that APC/CΔApc1−WD40–Cdh1 might still promote Ube2S-dependent extension of ubiquitin moieties conjugated to APC/C substrates. To address this possibility, we tested whether APC/CΔApc1−WD40–Cdh1 could catalyze Ube2S-mediated elongation of an APC/C substrate primed with ubiquitin. We used a modified Hsl1 substrate in which all Lys residues except that in the KEN box were replaced with arginines and a ubiquitin moiety was fused to Hsl1’s C terminus (Hsl1–K1–Ub). Interestingly, both wild-type APC/C–Cdh1 (lanes 2 and 3 in Fig. 2G) and the mutant APC/CΔApc1−WD40–Cdh1 (lanes 4 and 5 in Fig. 2G) ubiquitinated Hsl1–K1–Ub. Surprisingly, APC/CΔApc1−WD40 had a slightly higher activity than wild-type APC/C. In conclusion, our results reveal that without Apc1WD40 the APC/CΔApc1−WD40–Cdh1–Hsl1 complex is impaired in substrate ubiquitination because its ability to bind UbcH10 in a catalytically productive mode is disrupted.
The APC/CΔApc1−WD40–Cdh1–Hsl1 Complex Is Locked in the Inactive Conformation.
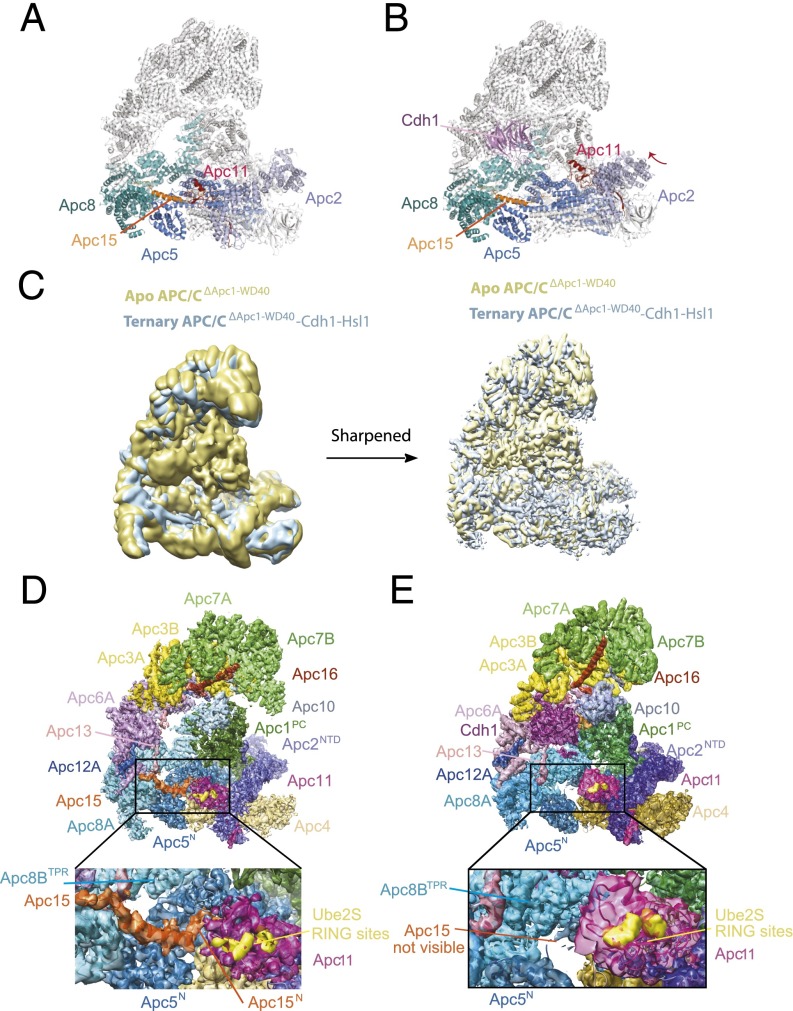
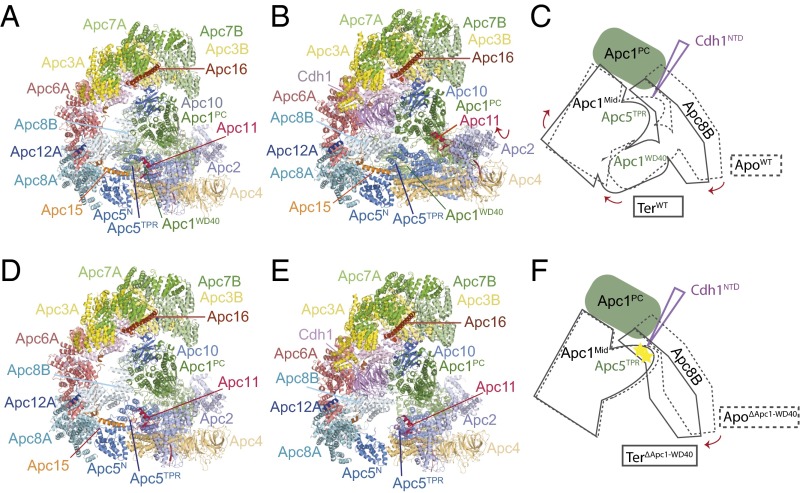
Previous cryo-EM reconstructions of human APC/C revealed that coactivator induces a conformational change of the platform subcomplex and the associated Apc2–Apc11 catalytic module. Additionally, the entire TPR subcomplex rotates relative to Apc1PC. In the presence of coactivator, the catalytic module is shifted to an upward position, away from Apc4 and Apc5 of the platform, thereby exposing the UbcH10-binding sites on Apc11RING and Apc2WHB (Fig. 3 A and B) (12, 19, 20). In these structures the small subunit Apc15, which is required for Cdc20 autoubiquitination (21–23), adopts an extended conformation anchored to Apc5 by its N terminus and bridging Apc5 and Apc8A through its adjacent N-terminal helix (Apc15NTH) (Fig. 3 A and B).
Fig. 3.
Cdh1 cannot induce the active conformation of APC/CΔApc1−WD40. (A and B) Ribbon representation of wild-type apo APC/C (A) and ternary APC/C–Cdh1–Hsl1 complex (B). These two structures show the coactivator-induced conformational change of the catalytic module of Apc2–Apc11. (C) Superimposition of the 6-Å resolution cryo-EM maps of apo APC/CΔApc1−WD40 (yellow) and the ternary APC/CΔApc1−WD40–Cdh1–Hsl1 complex (light blue). (D) View of the apo APC/CΔApc1−WD40 molecular envelope with the EM maps color-coded according to subunit assignments. (E) View of the ternary APC/CΔApc1−WD40–Cdh1–Hsl1 EM map. The EM density for Apc15 seen in D is not visible.
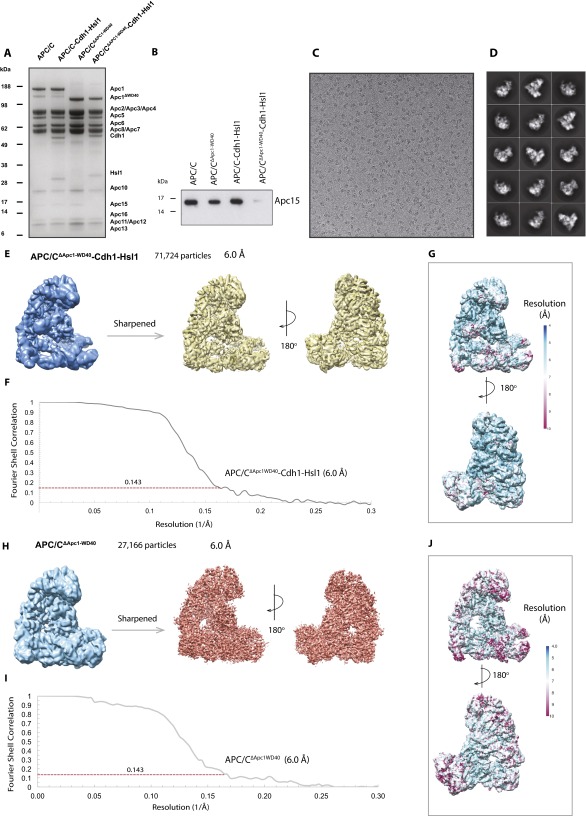
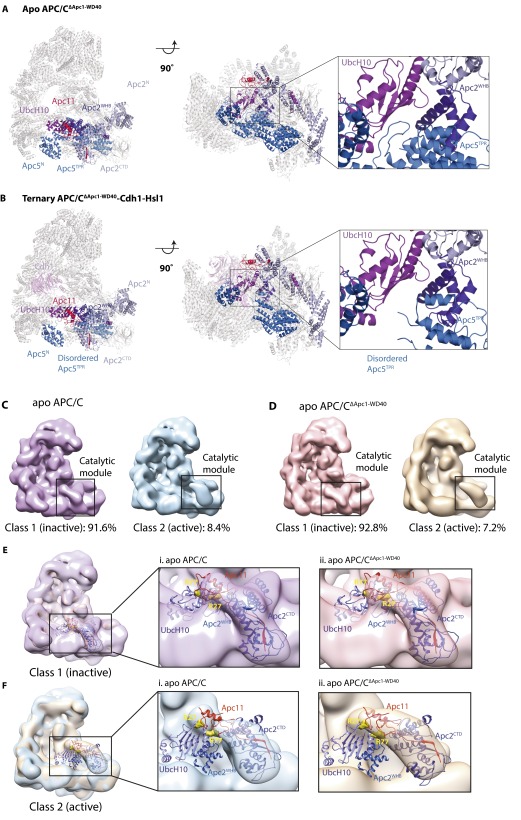
To obtain structural insights into the inability of APC/CΔApc1−WD40 to ubiquitinate substrates when paired with UbcH10, we determined the cryo-EM structures of both apo APC/CΔApc1−WD40 (Fig. 3 C and D) and a ternary APC/CΔApc1−WD40–Cdh1–Hsl1 complex (Fig. 3 C and E) at 6.0-Å resolution (Fig. S5 C–J). An atomic model of apo APC/CΔApc1−WD40 (Fig. 4D) was built by docking models of apo APC/C (39) into the cryo-EM reconstruction. APC/CΔApc1−WD40–Cdh1–Hsl1 (Fig. 4E) was built using apo APC/C (39) and APC/C–Cdh1–Hsl1–UbcH10 (19) coordinates. Except for the absence of Apc1WD40, the apo states of wild-type APC/C and mutant APC/CΔApc1−WD40 are essentially identical (Fig. 4 A and D).
Fig. S5.
APC/CΔApc1−WD40 and APC/CΔApc1−WD40–Cdh1–Hsl1 complexes for cryo-EM studies. (A) SDS/PAGE gel of APC/C complexes used for this study: wild-type apo APC/C (lane 1), ternary APC/C–Cdh1–Hsl1 (lane 2), mutant apo APC/CΔApc1−WD40 (lane 3), and ternary APC/CΔApc1−WD40–Cdh1–Hsl1 (lane 4). The molecular weight marker is indicated on the left, and the APC/C subunit corresponding to each band is indicated on the right. (B) Western blot analysis of Apc15 in APC/C complexes as shown in A. Blot was performed with an anti-Apc15 antibody. (C) A typical cryo-EM micrograph of APC/CΔApc1−WD40–Cdh1–Hsl1 particles. (D) Gallery of 2D averages of APC/CΔApc1−WD40–Cdh1–Hsl1 showing different orientation views. (E) Reconstruction map of APC/CΔApc1−WD40–Cdh1–Hsl1. (F) FSC plots for APC/CΔApc1−WD40–Cdh1–Hsl1. (G) Local resolution map of APC/CΔApc1−WD40–Cdh1–Hsl1 showing resolution range. (H) Reconstruction map of APC/CΔApc1−WD40. (I) FSC plots for APC/CΔApc1−WD40. (J) Local resolution map of APC/CΔApc1−WD40 showing resolution range.
Fig. 4.
Comparison of apo APC/CΔApc1−WD40 and ternary APC/CΔApc1−WD40–Cdh1–Hsl1 complexes with wild-type APC/C. (A) Apo APC/C. (B) Ternary APC/C–Cdh1–Hsl1. (C) Schematic of the conformational change on conversion from the apo to the ternary state. The N-terminal domain of Cdh1 (Cdh1NTD) acts as a wedge to separate Apc1PC from Apc8B. Apc8B is pushed down on Apc5TPR and Apc1WD40, shifting Apc4 and causing the platform subcomplex to rotate. (D) Apo APC/CΔApc1−WD40. (E) Ternary APC/CΔApc1−WD40–Cdh1–Hsl1. (F) Schematic of the conformational change on conversion from the apo to the ternary state. Apc8B pushes down on Apc5TPR; however, because of the absence of Apc1WD40, the conformational changes are not transmitted to Apc4; the platform remains unchanged, and the N-terminal domain of Apc5TPR becomes distorted, destabilizing its contacts with the N terminus of Apc15, which dissociates.
Strikingly, 3D classification of the APC/CΔApc1−WD40–Cdh1–Hsl1 cryo-EM dataset showed that even when associated with coactivator, APC/C particles are locked in the inactive state with the catalytic module occupying the downward position (Figs. 3 C and E and 4E). This APC/C conformation resembles apo APC/C (Fig. 4 A and D) and is associated with low affinity for UbcH10 and low ubiquitination activity (12). The smaller coactivator-induced rotation of the entire TPR subcomplex relative to Apc1PC is retained in APC/CΔApc1−WD40. In the downward conformation, UbcH10 is unable to engage the catalytic module for two reasons. First, docking UbcH10 onto Apc11RING, as observed in the APC/C–Cdh1–substrate–UbcH10 complex (19, 20), shows that UbcH10 would clash with Apc5TPR. Second, in this conformation Apc2WHB, which is required for high-affinity UbcH10 interactions and for stimulating ubiquitin transfer from UbcH10–Ub conjugates (20), would clash with Apc5TPR (see Fig. S7). In contrast to apo APC/CΔApc1−WD40 and the ternary APC/C–Cdh1–Hsl1 complex, EM densities corresponding to Apc15 and the N-terminal TPR helix of Apc5TPR are not visible in the APC/CΔApc1−WD40–Cdh1–Hsl1 complex (Fig. 3 D and E), indicating their structural disorder. Size-exclusion chromatography showed that Apc15 dissociated from the APC/CΔApc1−WD40–Cdh1–Hsl1 complex (Fig. S5 A and B).
Fig. S7.
UbcH10 and Apc2WHB clash with Apc5 in the inactive conformation. (A and B) Docking of UbcH10 and Apc2WHB onto Apc11RING in the inactive conformation in apo APC/C CΔApc1−WD40 (A) and ternary APC/CΔApc1−WD40–Cdh1–Hsl1 complex (B) shows that UbcH10 and Apc2WHB clash with Apc5. (C and D) 3D classification of wild-type apo APC/C (39) (C) and apo APC/CΔApc1−WD40 (this study) (D). For both wild-type and mutant apo APC/C, the majority of particles (∼92%) represent an inactive state. A small class (∼8% of the total) shows the catalytic module in the upward position. (E) Modeling of Apc2CTD–Apc11RING–UbcH10 coordinates (19) shows that in the inactive state the catalytic module (Apc2CTD–Apc11RING) is positioned close to Apc5, blocking the binding of UbcH10 and the ordering of Apc2WHB. (F) In the class with the catalytic module in the upward position, docking of Apc2CTD–Apc11RING–UbcH10 shows that Apc2WHB and UbcH10 can be accommodated with clashes with Apc5.
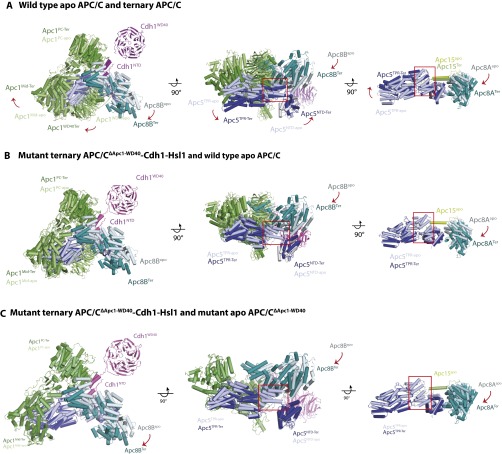
The disordering of Apc15 and the N-terminal TPR helix of Apc5TPR is a consequence of the Cdh1-induced conformational change of the APC/C that is disrupted in the APC/CΔApc1−WD40 mutant by the loss of Apc1WD40. In apo APC/C (wild type and APC/CΔApc1−WD40 mutant), Apc8B is well ordered, and its C-terminal TPR motifs interact with the outer α-helices of Apc1PC (Fig. 4 C and F and Fig. S6) (12, 19). On associating with the APC/C, the N-terminal domain of Cdh1 (Cdh1NTD) interacts both with Apc8B (C-box interaction) and with a site on Apc1PC that overlaps with the Apc1PC–Apc8B interface (Fig. S6) (12, 19). This latter interaction disrupts Apc8B–Apc1PC contacts, resulting in a downward shift of Apc8B’s C-terminal TPR motifs. The downward movement of Apc8B pushes simultaneously on the N-terminal TPR helix of Apc5TPR and on Apc1WD40. In turn, movement of Apc1WD40 causes a shift of the C-terminal TPR helix of Apc5TPR, resulting in a concerted motion of the whole Apc5TPR domain. The overall effect is that the platform subcomplex rotates, lifting it and the associated catalytic module upward at the front of the molecule into the catalytically active conformation (Fig. 4 B and C) (12, 19). In APC/CΔApc1−WD40–Cdh1–Hsl1, however, because of the loss of Apc1WD40, the downward movement of Apc8B causes a displacement of only the N-terminal TPR helix of Apc5TPR, whereas the C-terminal TPR helix of Apc5TPR remains in the inactive conformation. Motion of the C-terminal TPR helix of Apc5TPR is likely to be the main driving force for rotation of the platform. Thus, with Apc8B pushing down on the N-terminal TPR helix of Apc5TPR, without motion of Apc4 and the C-terminal TPR helix of Apc5TPR, the N-terminal TPR helix of Apc5TPR clashes with Apc8B, distorting the TPR helical geometry; this distortion is likely further accentuated by the noncoordinated motion of the C-terminal TPR helix of Apc5TPR (Fig. S6). Thus the loss of Apc1WD40 destabilizes and disorders the N-terminal TPR helix of Apc5TPR and disrupts interactions with the N-terminal extended segment of Apc15 that is responsible for anchoring Apc15 to Apc5 (Fig. 3D).
Fig. S6.
Comparison of conformational changes induced by Cdh1 in wild-type APC/C and mutant APC/CΔApc1−WD40. (A) Superimposition of wild-type ternary APC/C–Cdh1–Hsl1 and wild-type apo APC/C showing Cdh1, Apc1, Apc5, and Ac8B. (B) Superimposition of wild-type ternary APC/C–Cdh1–Hsl1 and ternary APC/CΔApc1−WD40–Cdh1–Hsl1. (C) Superimposition of ternary APC/CΔApc1−WD40–Cdh1–Hsl1 and apo APC/CΔApc1−WD40.
Interestingly, the conformation of APC/CΔApc1−WD40–Cdh1–Hsl1 resembles the hybrid class observed in the EM datasets of wild-type ternary APC/C–coactivator–substrate complexes (∼10% of APC/C particles) (12, 19, 39). In the hybrid state, the catalytic module adopts the inactive conformation, and EM densities for Apc1WD40 and Apc15 are absent. We assume that the hybrid state results from an N-terminal truncation of a minor portion of Apc1 during expression and purification of the recombinant APC/C (Figs. S4B and S5A).
Discussion
In this study we have used information from a cryo-EM model to determine the crystal structure of Apc1WD40 at higher resolution, highlighting the complementarity of X-ray crystallography and cryo-EM. Our results reveal that, in the absence of Apc1WD40, the APC/CΔApc1−WD40–Cdh1–Hsl1 complex is locked in the inactive conformation with the Apc2CTD–Apc11RING catalytic module positioned in the downward conformation. Thus, Apc1WD40 functions to stabilize the active conformation of the APC/C but is not required to stabilize the inactive conformation.
The inability of APC/CΔApc1−WD40 to adopt the active conformation results in a loss of catalytic activity of the APC/CΔApc1−WD40–Cdh1–UbcH10 E3–E2 pair because UbcH10 is unable to engage APC/C’s catalytic module. However, this inactive conformation did not affect the ability of Ube2S to assemble a polyubiquitin chain on Hsl1–K1–Ub. This finding indicates that, although coactivators regulate the catalytic activity of the APC/C toward the priming E2 (UbcH10) through a conformational change that renders the UbcH10-binding site on the catalytic module accessible, the intrinsic catalytic activity of the APC/C–Ube2S pair is independent of coactivator. Unlike UbcH10, the affinity of Ube2S for the APC/C is not dependent on coactivator (12). Ube2S interacts with the APC/C through its C-terminal LR tail at the interface of Apc2–Apc4 (19) and interacts with Apc2 through its Ubc catalytic domain (31). This LR tail-binding site does not change conformation on interconversion between active and inactive states (12, 39). Although independent of the catalytic module for APC/C binding, the catalytic activity of Ube2S requires the RING domain of Apc11 that is repurposed to engage the acceptor ubiquitin of the APC/C substrate for covalent linkage with the donor ubiquitin of the Ube2S–ubiquitin conjugate (14, 30). The acceptor ubiquitin-binding site on Apc11 is accessible in the inactive APC/C conformation (Fig. 3 D and E) as is consistent with our findings that Ube2S extends ubiquitin on the Hsl1–K1–Ub substrate in the context of the APC/CΔApc1−WD40–Cdh1–Hsl1–Ub complex. This study shows that, similar to the affinity of Ube2S for the APC/C (19), the catalytic activity of Ube2S in complex with the APC/C is not stimulated by the coactivator-induced conformational changes within the APC/C.
The affinity of apo APC/C for UbcH10 is four- to eightfold lower than for the ternary APC/C–Cdh1–substrate complex (12). However, the present study indicates that the inactive conformation adopted by apo APC/C would be incapable of engaging UbcH10. We suggest that the APC/C interconverts between an inhibited conformation that is unable to bind UbcH10 and an active conformation that binds UbcH10 even in the absence of coactivator. This interconversion would explain the capacity of apo APC/C to bind UbcH10 with low affinity, the small but detectable binding of UbcH10 to APC/CΔApc1−WD40–Cdh1–Hsl1 (Fig. S4C), and the low ubiquitination activity of APC/CΔApc1−WD40 (Fig. 2D). Analysis of 3D classes of apo wild-type APC/C and mutant APC/CΔApc1−WD40 EM datasets indicates a small population of molecules (roughly 8%) in which the catalytic module (Apc2CTD–Apc11) adopts an upward conformation because of rotation about the Apc2CTD–Apc2NTD interface, with the platform remaining in the inactive conformation (Fig. S7 C and D). This upward conformation of the catalytic module would allow binding of UbcH10 to Apc11RING and Apc2WHB (Fig. S7 E and F).
It is interesting to consider the possibility that controlling the association of Apc1WD40 with its binding pocket in the APC/C could provide a potential regulatory mechanism. Thus, proteins that bind to Apc1WD40 and compete for its association with its docking site on the APC/C would displace Apc1WD40, thereby inactivating the APC/C. The APC/CΔApc1−WD40 mutant has unexpectedly provided a system for exploring the different ubiquitination mechanisms of human APC/C with its two cognate E2s UbcH10 and Ube2S.
Materials and Methods
X-ray data were collected at the Diamond Light Source beamline I02 using a Pilatus 6M-F silicon pixel detector and were processed using XDS (41) and scaled using Aimless (42) in the CCP4i software package (43, 44). Cryo-EM data were collected using a 300-kV FEI Polara electron microscope and were processed using the RELION (Regularized Likelihood Optimization) software package (45). Detailed procedures for the protein preparation, ubiquitination assays, crystallization, and EM data processing are provided in SI Materials and Methods.
SI Materials and Methods
Cloning and Expression of the Recombinant Human Apc1N and APC/C Complexes.
Human Apc1N constructs (including wild type and loop-deletion mutants) were fused with the C-terminal tobacco etch virus (TEV)-cleavable double-StrepII (WSHPQFEK GGGSGGGSGGGSWSHPQFEK) tag present in the pF2 vector by means of uracil-specific excision reagent (USER) cloning (46). Primers for cloning Apc1N loop-deletion constructs were designed to exclude one or more of the following regions: loop 1 (residues 34–69), loop 2 (residues 307–402), and/or loop 3 (residues 523–580). Individual constructs were expressed in Sf9 cells.
All genes for recombinant human APC/C complexes were cloned into a modified MultiBac system as described previously (19, 46, 47). Apc4 was fused to a C-terminal double-StrepII tag for purification. A baculovirus expression system was applied for the protein expression. A recombinant baculovirus containing Apc1ΔApc1−WD4 and Apc11 was generated for this study. To coexpress the mutant APC/CΔApc1−WD40 complex in High Five cells, two other viruses (named “virus 2” and “virus 3” in this study) were used as described previously (12). Virus 2 contained Apc3, Apc6, Apc7, Apc12, and Apc16, and virus 3 contained Apc2, Apc4, Apc5, Apc8, Apc10, Apc13, and Apc15. Similarly, for APC/CΔApc1−WD40 –Cdh1–Hsl1 generation, one virus (named “virus 4”) containing Cdh1, Hsl1, Apc2, Apc4, Apc5, Apc8, Apc10, Apc13, and Apc15 was mixed with virus 1 and 2 for coexpression in High Five cells. To reconstitute the APC/CΔApc1−WD40 –Apc1WD40 complex, virus containing the Apc1N gene was coinfected with viruses 1, 2, and 3 in High Five cells.
All purification procedures were performed at 4 °C. Apc1N constructs were purified using a similar approach. Cell pellets were thawed and resuspended in lysis buffer [50 mM Hepes (pH 8.3), 200 mM NaCl, 10% (vol/vol) glycerol, 0.5 mM Tris(2-carboxyethyl)phosphine (TCEP), 1 mM EDTA, 0.1 mM PMSF, and Complete EDTA-free protease inhibitors (Roche)]. After sonication (10 min, 3-s pulse), the lysate was centrifuged at 50,000 × g for 1 h to remove the insoluble cell debris. The soluble supernatant was bound to a 5-mL Strep-Tactin Superflow Cartridge (Qiagen) with a flow rate of 1 mL/min. The column was washed with Strep binding/wash buffer [50 mM Hepes (pH 8.0), 200 mM NaCl, 10% (vol/vol) glycerol, 0.5 mM TCEP, 1 mM EDTA], and the protein was eluted with wash buffer supplemented with 2.5 mM Desthiobiotin (Sigma). TEV protease was used to cleave off the double-StrepII tag. Then the protein/TEV mixture was applied to the Ni-NTA column to remove TEV and noncleaved protein. The flowthrough from the Ni-NTA column was concentrated and applied to a gel-filtration column (Superdex75, 26/60; GE Healthcare). Purification of recombinant mutant APC/CΔApc1−WD40 and APC/CΔApc1−WD40–Ch1–Hsl1 complexes was carried out as described previously for wild-type human APC/C complexes (12, 19, 47).
Thermal Shift Assay.
A thermal shift assay was used to determine the thermal stability of Apc1N constructs in a series of buffer conditions. The hydrophobic fluorescent dye SYPRO orange was used in this assay. The stability curve is obtained by measuring the fluorescence signal of SYPRO orange at each point; its midpoint value is the melting point. More stable proteins will have higher a melting temperature and are more favorable for protein crystallization. Twenty microliters of 20 µM protein were mixed with 2.5 µL of SYPRO orange dye (50×) with an additional 2.5 µL of screen buffer stock (10×). The mixture then was transferred to a 96-well plate and spun to settle the protein/dye mixture to the bottom. The plate was sealed with heat-stable clear tape. The program was set for a melting curve on a real-time PCR machine (Bio-Rad) with 0.5 °C/min steps from 10–95 °C.
Crystallization and Data Collection and Processing.
To crystallize human recombinant Apc1N, we freshly prepared the insect cell-expressed protein and concentrated the protein to 5 mg/mL in buffer containing 20 mM Hepes (pH 8.0), 150 mM NaCl, and 0.5 mM TCEP. The protein was centrifuged at 45,000 × g for 30 min before mixing with crystallization screens. Initial crystals were obtained by mixing equal volumes of protein and the reservoir buffer by vapor diffusion (sitting-drop method) in a buffer containing 0.1 M citric acid (pH 5.0) and 2 M NaCl at 4 °C. To improve crystal size, larger hanging drops were used at 4 °C. Crystals grew to their full size in 2 wk. The protein crystals were soaked in cryoprotectant [the reservoir solution complemented with 25% (vol/vol) ethylene glycol] and then were cryo-cooled by plunging into liquid nitrogen.
The X-ray diffraction data were collected at Synchrotron Beamline I02 using a PILATUS detector at the Diamond Light Source. The data-collection wavelength was 0.9795 Å with an oscillation of 0.15° and an exposure time of 0.2 s with 100% transmission. Diffraction images were indexed and integrated by XDS (41) and scaled using Aimless (42) in the CCP4i software package (43, 44). The data-collection statistics of the best crystals are listed in Table S1.
The crystal structure of Apc1WD40 was determined using the cryo-EM–derived coordinates (19) using Phenix.Phaser (48). The molecule copy number used to search was based on the Matthews coefficient calculated using the program Matthews in the CCP4 software package (43, 44). Interactive manual model building and refinement cycles were run to improve the phases and determine the final structure. Model building of the X-ray crystal structure of Apc1 and the cryo-EM structure of APC/CΔApc1−WD40–Cdh1–Hsl1 was performed using COOT (49). Refinement was performed using the program Phenix.Refine (50) and Refmac (51) from the CCP4 suite software package. The progression of the refinement was monitored by R factors, which represent the fit of the model to the diffraction data (52). To check for model bias, simulated omit maps were calculated systematically at various stages of model building and refinement. The final refined structure was validated with the program MolProbity (53).
Structure Analysis.
The electrostatic surface potentials of Apc1WD40, Apc5, and Apc8 were calculated using the APBS plugin in PyMOL with default parameters (54). Structural conservation determined using ConSurf (55) was mapped onto the surface of Apc1WD40. Protein sequences were retrieved from UniProt (56), and the sequence alignment was performed in ClustalW2 (57). The similarities of the Apc1WD40 domain with other WD40 domain proteins were analyzed using DALI (Table S2) (38). The interface analysis of Apc1WD40 and Apc5 and Apc8 was calculated by the PISA service at the European Bioinformatics Institute (Table S3) (58). The structural conservation shown in Fig. 2 was performed using ConSurf-based Homo sapiens, Mus musculus, Schizosaccharomyces pombe, Saccharomyces cerevisiae, Arabidopsis thaliana, Dictyostelium discoideum, and Emericella discophora sequences.
EM.
Negative-stained and cryo-EM grids were prepared using methods similar to those described previously (12, 19). Protein was diluted in size-exclusion chromatography equilibration buffer to 40 μg/mL and 100 μg/mL for negative-staining EM and cryo-EM, respectively. Negative-staining EM micrographs were recorded by a 2 k × 2 k CCD camera on a 120-kV FEI Spirit electron microscope at a nominal magnification of 30,000×, yielding a pixel size of 3.5 Å.
Cryo-EM grids were loaded into an FEI Tecnai Polara electron microscope at an accelerating voltage of 300 kV and a Falcon III direct detector. Images were captured automatically using EPU software (FEI) at a nominal magnification of 78,000× (yielding a pixel size of 1.36 Å) with a defocus range of −2.0 to −4.0 μm. The exposure time for each micrograph was 1.1 s at a dose rate of 27 electrons/Å2/s generating 34 frames for each movie stack.
Image Processing.
Image processing for negative-stain and cryo-EM data was performed with RELION (45). The micrographs were manually screened with e2display.py in EMAN2 (59) to exclude micrographs with wrong defocus or a large area of ice contamination. The cryo-EM particles were automatically picked with APC/C–Cdh1–Hsl1 2D average images (12) as a reference using the batchboxer program in EMAN (60). Contrast transfer function (CTF) parameters were calculated with CTFFIND3 (61). The coordinates of particles and the CTF parameters from CTFFIND3 log files were imported to program RELION (45). The particles were extracted and inspected by discarding particles at the high end of the average Z-scores that were high-contrast false positives (e.g., ice contamination, carbon edges, protein aggregates, or pieces of junk) (62). From the 2D classification, particles in classes with poor structural features were discarded. The remaining particles were used for 3D classification using the 3D reconstruction map of APC/C–Cdh1–Hsl1 (12) as a reference. From this step, the conformational heterogeneity of the complex was identified, and the particles from the uncompleted complex reconstruction classes were excluded before the final 3D autorefinement and reconstruction in RELION using the same reference. The initial angular sampling rate, set at 7.5°, decreases iteratively during refinement according to the accuracy assessed by RELION (62, 63). The remaining particles were refined and corrected for beam-induced particle motion using particle polishing in RELION (64, 65). Polished particles were used for another round of 3D refinement. The reconstruction maps were sharpened using postprocessing in RELION, and the resolutions were estimated based on the gold-standard Fourier shell correlation (FSC) calculations using the criterion FSC value of 0.143 (63). The APC/CΔApc1−WD40–Cdh1–Hsl1 reconstruction map was refined from the 3D classification of the classes containing Cdh1 density to 6-Å resolution, whereas the apo state APC/CΔApc1−WD40 particles were extracted from the classes of the 3D classification of the APC/CΔApc1−WD40–Cdh1–Hsl1 complex.
Model Building of the Mutant APC/C Complex.
Initial fitting of coordinates was performed with Chimera (66). The atomic models of wild-type apo APC/C [Protein Data Bank (PDB) ID code: 5G05] (39) and APC/C–Cdh1–Hsl1–UbcH10 [Electron Microscopy Data Bank (EM-DB) ID code: 2926] (19) were docked into the cryo-EM reconstruction maps of APC/CΔApc1−WD40 and APC/CΔApc1−WD40–Cdh1–Hsl1. Further rigid body fitting of both structures was performed in COOT (49). The coordinates of Apc5TPR1–4 were deleted because of the absence of visible density in the EM map of APC/CΔApc1−WD40–Cdh1–Hsl1. The Cdh1 loop region containing residues 163–170, not seen in previous EM structures, was built using a polyalanine chain.
Ubiquitination Assays of APC/C Complexes.
The APC/C ubiquitination assay was adapted from refs. 12 and 19. Each ubiquitination reaction contained ∼80 nM of recombinant human APC/C, 150 nM of human ubiquitin-activating enzyme E1 (UBA1), 300 nM E2 (UbcH10), 2 µM substrate (Hsl1667–872), 70 µM human ubiquitin with an N-terminal His6-tag, and 40 μM recombinant human Cdh1 or Cdc20 (39) in a 10-µL reaction volume with reaction buffer [40 mM Hepes (pH 8.0), 10 mM MgCl2, 0.6 mM DTT], 5 mM ATP, and 0.25 mg/mL BSA. Ten microliters of the reaction mixtures were incubated at room temperature (22 °C) and terminated at various time points by adding 6 µL of SDS/PAGE loading buffer and heating at 100 °C for 2 min. Each reaction mixture was analyzed by SDS/PAGE (4–12% gradient gels) followed by Western blotting with an anti-His antibody against His6–ubiquitin.
Acknowledgments
We thank members of the D.B. group for discussion and reagents; C. Savva, S. Chen, and G. McMullan for help with EM data collection; X. Bai and K. Zhang for help with EM data analysis; J. Grimmet and T. Darling for computing; A. Boland, M. Yu, and G. Murshudov for advice and help with X-ray data collection, processing, and analysis; the staff at Diamond Light Source for help with data collection; and C. Alfieri for advice and comments on the manuscript. Recombinant Cdc20 was a generous gift of S. Zhang. This work was supported by a grant from the Cancer Research-UK Programme Grant C576/A14109 (to D.B.) and the Medcal Research Council Laboratory of Molecular Biology (MRC_UP_1201/6). Q.L. is the recipient of an Institute of Cancer Research Studentship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Protein coordinates of Apc1WD40 have been deposited with the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (RCSB PDB ID code 5LGG). EM maps have been deposited with the Electron Microscopy Data Bank (EM-DB) [accession codes EMD-4047 (ternary APC/CΔApc1−WD40–Cdh1–Hsl1 complex) and EMD-4048 (apo APC/CΔApc1−WD40)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607147113/-/DCSupplemental.
References
- 1.Morgan DO. 2006. The Cell Cycle: Principles of Control, Primers in Biology (New Science Press, London)
- 2.Hunt T, Nasmyth K, Novák B. The cell cycle. Philos Trans R Soc Lond B Biol Sci. 2011;366(1584):3494–3497. doi: 10.1098/rstb.2011.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wieser S, Pines J. The biochemistry of mitosis. Cold Spring Harb Perspect Biol. 2015;7(3):a015776. doi: 10.1101/cshperspect.a015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters JM. The anaphase promoting complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7(9):644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 5.Pines J. Cubism and the cell cycle: The many faces of the APC/C. Nat Rev Mol Cell Biol. 2011;12(7):427–438. doi: 10.1038/nrm3132. [DOI] [PubMed] [Google Scholar]
- 6.Barford D. Structure, function and mechanism of the anaphase promoting complex (APC/C) Q Rev Biophys. 2011;44(2):153–190. doi: 10.1017/S0033583510000259. [DOI] [PubMed] [Google Scholar]
- 7.Meyer HJ, Rape M. Processive ubiquitin chain formation by the anaphase-promoting complex. Semin Cell Dev Biol. 2011;22(6):544–550. doi: 10.1016/j.semcdb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Primorac I, Musacchio A. Panta rhei: The APC/C at steady state. J Cell Biol. 2013;201(2):177–189. doi: 10.1083/jcb.201301130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivakumar S, Gorbsky GJ. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol. 2015;16(2):82–94. doi: 10.1038/nrm3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochis OV, Fetica B, Vlad C, Achimas-Cadariu P, Irimie A. The importance of ubiquitin E3 ligases, SCF and APC/C, in human cancers. Clujul Med. 2015;88(1):9–14. doi: 10.15386/cjmed-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008;32(4):576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Zhang Z, Yang J, McLaughlin SH, Barford D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature. 2014;513(7518):388–393. doi: 10.1038/nature13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Voorhis VA, Morgan DO. Activation of the APC/C ubiquitin ligase by enhanced E2 efficiency. Curr Biol. 2014;24(13):1556–1562. doi: 10.1016/j.cub.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly A, Wickliffe KE, Song L, Fedrigo I, Rape M. Ubiquitin chain elongation requires E3-dependent tracking of the emerging conjugate. Mol Cell. 2014;56(2):232–245. doi: 10.1016/j.molcel.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Jörgensen PM, et al. Characterisation of the human APC1, the largest subunit of the anaphase-promoting complex. Gene. 2001;262(1-2):51–59. doi: 10.1016/s0378-1119(00)00511-4. [DOI] [PubMed] [Google Scholar]
- 16.He J, et al. The structure of the 26S proteasome subunit Rpn2 reveals its PC repeat domain as a closed toroid of two concentric α-helical rings. Structure. 2012;20(3):513–521. doi: 10.1016/j.str.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Steen JA, et al. Different phosphorylation states of the anaphase promoting complex in response to antimitotic drugs: A quantitative proteomic analysis. Proc Natl Acad Sci USA. 2008;105(16):6069–6074. doi: 10.1073/pnas.0709807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft C, et al. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22(24):6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang L, Zhang Z, Yang J, McLaughlin SH, Barford D. Atomic structure of the APC/C and its mechanism of protein ubiquitination. Nature. 2015;522(7557):450–454. doi: 10.1038/nature14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown NG, et al. RING E3 mechanism for ubiquitin ligation to a disordered substrate visualized for human anaphase-promoting complex. Proc Natl Acad Sci USA. 2015;112(17):5272–9. doi: 10.1073/pnas.1504161112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansfeld J, Collin P, Collins MO, Choudhary JS, Pines J. APC15 drives the turnover of MCC-CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat Cell Biol. 2011;13(10):1234–1243. doi: 10.1038/ncb2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster SA, Morgan DO. The APC/C subunit Mnd2/Apc15 promotes Cdc20 autoubiquitination and spindle assembly checkpoint inactivation. Mol Cell. 2012;47(6):921–932. doi: 10.1016/j.molcel.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uzunova K, et al. APC15 mediates CDC20 autoubiquitylation by APC/C(MCC) and disassembly of the mitotic checkpoint complex. Nat Struct Mol Biol. 2012;19(11):1116–1123. doi: 10.1038/nsmb.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, King RW, Peters JM, Kirschner MW. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr Biol. 1996;6(4):455–466. doi: 10.1016/s0960-9822(02)00513-4. [DOI] [PubMed] [Google Scholar]
- 25.Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133(4):653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson A, et al. Regulation of ubiquitin chain initiation to control the timing of substrate degradation. Mol Cell. 2011;42(6):744–757. doi: 10.1016/j.molcel.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnett MJ, et al. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat Cell Biol. 2009;11(11):1363–1369. doi: 10.1038/ncb1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson A, et al. Identification of a physiological E2 module for the human anaphase-promoting complex. Proc Natl Acad Sci USA. 2009;106(43):18213–18218. doi: 10.1073/pnas.0907887106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T, et al. UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci USA. 2010;107(4):1355–1360. doi: 10.1073/pnas.0912802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown NG, et al. Mechanism of polyubiquitination by human anaphase-promoting complex: RING repurposing for ubiquitin chain assembly. Mol Cell. 2014;56(2):246–260. doi: 10.1016/j.molcel.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown NG, et al. Dual RING E3 Architectures Regulate Multiubiquitination and Ubiquitin Chain Elongation by APC/C. Cell. 2016;165(6):1440–1453. doi: 10.1016/j.cell.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer HJ, Rape M. Enhanced protein degradation by branched ubiquitin chains. Cell. 2014;157(4):910–921. doi: 10.1016/j.cell.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130(1):127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigo-Brenni MC, Foster SA, Morgan DO. Catalysis of lysine 48-specific ubiquitin chain assembly by residues in E2 and ubiquitin. Mol Cell. 2010;39(4):548–559. doi: 10.1016/j.molcel.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri I, Söding J, Lupas AN. Evolution of the beta-propeller fold. Proteins. 2008;71(2):795–803. doi: 10.1002/prot.21764. [DOI] [PubMed] [Google Scholar]
- 36.Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: A common architecture for diverse functions. Trends Biochem Sci. 1999;24(5):181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, Min J. Structure and function of WD40 domain proteins. Protein Cell. 2011;2(3):202–214. doi: 10.1007/s13238-011-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holm L, Rosenstrom P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010;38(Web Server issue):W545–549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, et al. Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature. 2016;533(7602):260–264. doi: 10.1038/nature17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao R, et al. Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc Natl Acad Sci USA. 2016;113(19):E2570–E2578. doi: 10.1073/pnas.1604929113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans PR. An introduction to data reduction: Space-group determination, scaling and intensity statistics. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):282–292. doi: 10.1107/S090744491003982X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowtan K, Emsley P, Wilson KS. From crystal to structure with CCP4. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):233–234. doi: 10.1107/S0907444911007578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheres SH. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180(3):519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Yang J, Barford D. Recombinant expression and reconstitution of multiprotein complexes by the USER cloning method in the insect cell-baculovirus expression system. Methods. 2016;95:13–25. doi: 10.1016/j.ymeth.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, et al. Recombinant expression, reconstitution and structure of human anaphase-promoting complex (APC/C) Biochem J. 2013;449(2):365–371. doi: 10.1042/BJ20121374. [DOI] [PubMed] [Google Scholar]
- 48.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 52.Brünger AT, Kuriyan J, Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- 53.Chen VB, et al. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 1):12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98(18):10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Celniker G, et al. ConSurf: Using evolutionary data to raise testable hypotheses about protein function. Isr J Chem. 2013;53(3-4):199–206. [Google Scholar]
- 56.UniProt Consortium UniProt: A hub for protein information. Nucleic Acids Res. 2015;43(Database issue):D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 58.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Tang G, et al. EMAN2: An extensible image processing suite for electron microscopy. J Struct Biol. 2007;157(1):38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128(1):82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 61.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142(3):334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 62.Scheres SH. Semi-automated selection of cryo-EM particles in RELION-1.3. J Struct Biol. 2015;189(2):114–122. doi: 10.1016/j.jsb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheres SH, Chen S. Prevention of overfitting in cryo-EM structure determination. Nat Methods. 2012;9(9):853–854. doi: 10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai XC, Fernandez IS, McMullan G, Scheres SH. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. eLife. 2013;2:e00461. doi: 10.7554/eLife.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheres SH. Beam-induced motion correction for sub-megadalton cryo-EM particles. eLife. 2014;3:e03665. doi: 10.7554/eLife.03665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Z, et al. UCSF Chimera, MODELLER, and IMP: An integrated modeling system. J Struct Biol. 2012;179(3):269–278. doi: 10.1016/j.jsb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]