Significance
Investigating enzyme function by genetic knockout is often complicated by indirect and compensatory changes, whereas supraphysiological levels of protein can compromise overexpression. These pitfalls have made it difficult to understand the functions of the enigmatic phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks); we are not even sure what lipid phosphorylation they catalyze in vivo. Here, we have used the unique genetic power of DT40 cells to genomically delete PI5P4Kα or remove the endogenous protein acutely (within 60 min). We used similar approaches to manipulate the endogenous catalytic activity of the enzyme. From this approach, we have gained unique and unexpected insights into the physiological role of PI5P4Kα and the ways in which it interacts with the Akt signaling pathway.
Keywords: phosphatidylinositol 5-phosphate 4-kinase; phosphatidylinositol 5-phosphate; phosphatidylinositol (4,5)-bisphosphate; Akt; mTOR
Abstract
Phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks) are enigmatic lipid kinases with physiological functions that are incompletely understood, not the least because genetic deletion and cell transfection have led to contradictory data. Here, we used the genetic tractability of DT40 cells to create cell lines in which endogenous PI5P4Kα was removed, either stably by genetic deletion or transiently (within 1 h) by tagging the endogenous protein genomically with the auxin degron. In both cases, removal impacted Akt phosphorylation, and by leaving one PI5P4Kα allele present but mutating it to be kinase-dead or have PI4P 5-kinase activity, we show that all of the effects on Akt phosphorylation were dependent on the ability of PI5P4Kα to synthesize phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2] rather than to remove PI5P. Although stable removal of PI5P4Kα resulted in a pronounced decrease in Akt phosphorylation at Thr308 and Ser473, in part because of reduced plasma membrane PIP3, its acute removal led to an increase in Akt phosphorylation only at Ser473. This process invokes activation primarily of mammalian target of rapamycin complex 2 (mTORC2), which was confirmed by increased phosphorylation of other mTORC2 substrates. These findings establish PI5P4Kα as a kinase that synthesizes a physiologically relevant pool of PI(4,5)P2 and as a regulator of mTORC2, and show a phenomenon similar to the “butterfly effect” described for phosphatidylinositol 3-kinase Iα [Hart JR, et al. (2015) Proc Natl Acad Sci USA 112(4):1131–1136], whereby through apparently the same underlying mechanism, the removal of a protein’s activity from a cell can have widely divergent effects depending on the time course of that removal.
The phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks) are an enigmatic family of three (PI5P4Kα, -β, and -γ) with cellular functions that remain poorly understood (1–3). In general, their principal activity is believed to be to remove and thus, regulate the levels of their substrate phosphatidylinositol 5-phosphate (PI5P). Phenotypes from knockout mice have highlighted that PI5P4Ks have important roles to play in physiology and pathology, including links between PI5P4Ks and the Akt signaling pathway, and other studies have pointed to roles in the generation of cancer (3). A number of key questions, however, remain unanswered. Knocking proteins out or down (by RNAi) is a long-term strategy that may lead to indirect changes, such as, for example, those highlighted in the recent study by Hart et al. (4) concerning the indirect long-term consequences of a point mutation in phosphatidylinositol 3-kinase–α (PI3Kα). Another issue still unresolved for PI5P4Ks is whether removal of PI5P is their only function or whether their ability to synthesize phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2] may also be important (5, 6).
We have recently used the high homologous recombination frequency of DT40 cells to tag endogenous PI5P4Ks and thus, bypass the interpretational problems associated with cell transfection, and this has led to the demonstration of heterodimerization of α and β and a nuclear localization of endogenous PI5P4Kβ (7–9). DT40s, being of avian origin, do not have a PI5P4Kγ gene, which facilitates the study of the functions and interrelationship of the α- and β-isoforms (8). Here, we have more fully exploited the genetic power of DT40s to gain unprecedented insight into the physiological functions of PI5P4Kα.
Results
Long-Term Loss of PI5P4Kα by Gene Disruption Results in Compromised Akt Phosphorylation.
Karyotype analysis of the DT40 cells with which we were working revealed trisomy on 2 (SI Appendix, Fig. S1), the chromosome on which PIP4K2A resides. For three main reasons, we chose to delete a 2.8-kb segment of the gene encompassing exons 8 and 9. First, with this short deletion, we hoped to avoid off-target effects, like those we saw in PIP4K2B−/− cells (SI Appendix, SI Results and Discussion and Figs. S2 and S3); second, these exons encode large portions of the PI5P and ATP binding sites of the kinase, and therefore, any N-terminal fragment is unlikely to possess any function relying on ATP or PI5P binding. Third, in cells with two of three alleles deleted, we could mutate the catalytic activity of the third. PIP4K2A−/−/− cells have a similar growth rate to controls (SI Appendix, Fig. S1) and unchanged PI5P4Kβ message (SI Appendix, Fig. S4). Analysis of the phenotype of PIP4K2A−/−/− cells revealed a significant decrease in Akt phosphorylation at both Thr308 and Ser473 (human numbering) both in synchronized exponential growth and on insulin stimulation (Fig. 1 A and B). Given this consistency between assays, we performed additional experiments using the former protocol only. As an independent test of the phenotype of PIP4K2A−/−/− cells, we knocked down PI5P4Kα by stable shRNA and found the same effect (SI Appendix, Fig. S5).
Fig. 1.
The effect of endogenous PIP4K2A mutations on Akt phosphorylation at Thr308 and Ser473. (A) Cells were synchronized at the same density in exponential growth before analysis. Complete PIP4K2A deletion results in reduced phospho-Akt at both Thr308 and Ser473. Deletion of two of three PIP4K2A alleles, while leaving the third unaffected aside from tagging with EmGFP, has no effect on Akt phosphorylation. This process acts as a control for the fourth and fifth lanes, where the undeleted allele is either rendered kinase-dead or mutated to encode a PI4P5K (SI Appendix, SI Materials and Methods) along with EmGFP tagging in both cases. A kinase-dead third allele does not support normal Akt phosphorylation in the way that a kinase-live one does, and it makes no difference to Akt phosphorylation whether the PI(4,5)P2 here is generated from PI5P or PI4P. Representative blot from three biological replicates. Note that these blots were captured electronically by a program that generates a negative image. To avoid any unnecessary image manipulation, the original negative image is presented. (B) The cell lines shown in A were serum starved and insulin stimulated (SI Appendix, SI Materials and Methods). The pattern of deficient Akt phosphorylation was found to be the same as when cells were in exponential growth. (C) Substrate specificity of wild-type PI5P4Kα and the A370E (substrate switch) mutant. Recombinant enzymes were assayed as described in SI Appendix, SI Materials and Methods using either PI5P or PI4P substrates. Note the logarithmic y axis. Wild-type PI5P4Kα has two orders of magnitude less activity toward PI4P than toward PI5P, whereas the A370E mutant prefers PI4P by almost three orders of magnitude. The activity of chicken PI5P4Kβ is included for comparison. The activity of chicken PI5P4Kα toward PI5P is about three orders of magnitude greater than that of chicken PI5P4Kβ, very similar to the human enzymes (3). Quantification is of four technical replicates.
To see the Akt phenotype, all three alleles of PIP4K2A had to be deleted. If we left one allele intact, the Akt phosphorylation levels were indistinguishable from those in wild-type cells. This process gave us the chance to explore the mechanism behind this phenotype. The remaining wild-type allele was mutated to encode a kinase-dead enzyme (D272K) (10). The allele was simultaneously tagged genomically at the C terminus with EmGFP, and as a control, we knocked in an EmGFP tag alone to the third allele. The data show that, whereas a remaining kinase-live third allele can support normal Akt phosphorylation, one that is kinase-dead cannot (Fig. 1). Note that these experiments are very tightly controlled, because PIP4K2A−/−/kinase-dead EmGFP cells and their controls (PIP4K2A−/−/EmGFP) differ by only one amino acid.
To explore whether the function of PI5P4Kα here is to remove PI5P, generate PI(4,5)P2, or both, we used the finding in the work by Kunz et al. (11) that a single mutation (A381E) in the activation loop of human PI5P4Kβ changes its specificity from a PI5P4K to a phosphatidylinositol 4-phosphate 5-kinase (PI4P5K). If PI5P removal is the main function of PI5P4Kα in this context, such a mutation introduced into the nondeleted PIP4K2A allele should see the Akt phenotype emerge; however, if PI(4,5)P2 synthesis is its function, because there is plenty of phosphatidylinositol 4-phosphate (PI4P) present in most cellular membranes (1), the mutant should support normal Akt phosphorylation. We tested directly using recombinant enzymes that the substrate specificity switch of human PI5P4Kβ (11) is valid in the chicken PI5P4Kα. The data (Fig. 1C) show that a marked (>98%) substrate specificity switch occurs (note the logarithmic y axis). Fig. 1 shows that, if the third PIP4K2A allele in PIP4K2A−/−/wt cells is mutated (A370E) to encode a PI4P5K, normal Akt phosphorylation is maintained. Our interpretation of these experiments is that PI5P4Kα synthesizes a pool(s) of PI(4,5)P2 required for sustained phosphorylation of Akt.
By generating PIP4K2B null cells, we excluded a similar role for PI5P4Kβ in the regulation of Akt phosphorylation. However, complete deletion of PIP4K2B did result in indirect down-regulation of PI5P4Kα (SI Appendix, Fig. S2). The consequent Akt phenotype was rescued only by overexpression of PI5P4Kα (not of PI5P4Kβ) and then, only if the PI5P4Kα was kinase-live, irrespective of whether it was mutated to a PI4P5K (SI Appendix, SI Results and Discussion and Figs. S2 and S3).
PI5P4Kα Is Necessary for Maintenance of Normal Plasma Membrane PIP3 Levels in DT40 Cells.
Given that PI5P4Kα is a lipid kinase, the simplest explanation of decreased Akt phosphorylation is that phosphatidylinositol (3,4,5)-trisphosphate (PIP3) synthesis may be compromised (see the introductory paragraphs). We, therefore, quantified PIP3 levels by MS (12) in the cell lines shown in Fig. 1. A decrease in the mass of PIP3 was evident that followed the pattern of loss of Akt phosphorylation (Fig. 2 and SI Appendix, Fig. S6), suggesting that at least part of this Akt phenotype is because of decreased PIP3 synthesis. We confirmed these findings by overexpression of the fluorescent 3-phosphoinositide reporter EmGFP-PH-Akt (13), which was recruited less to the plasma membrane in cells with deficient Akt phosphorylation (SI Appendix, Fig. S3C).
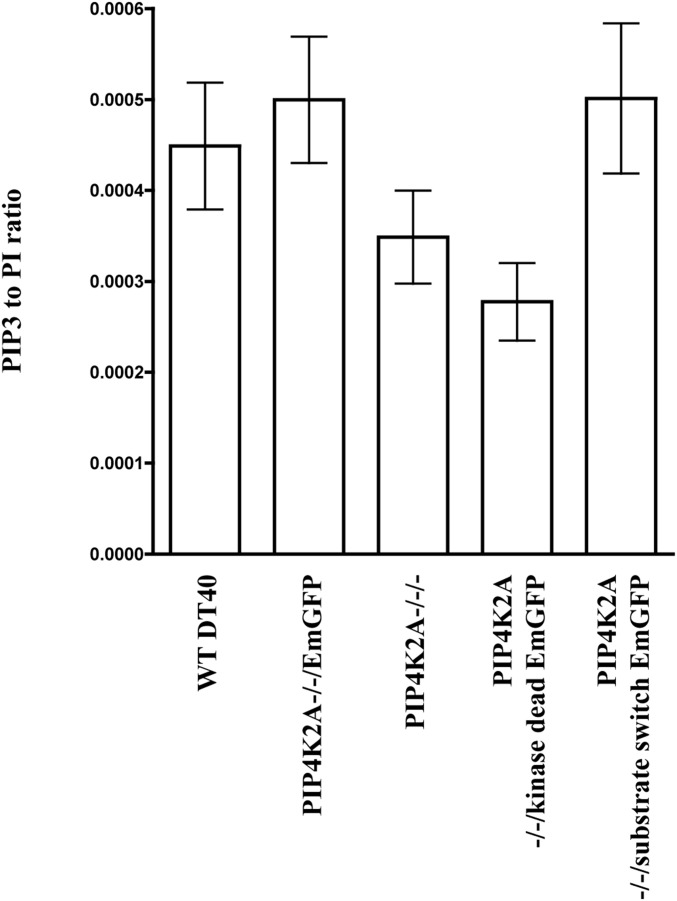
Fig. 2.
The effect of endogenous PIP4K2A mutations on PIP3 mass levels. Cells were synchronized at the same density in exponential growth before analysis by MS. PIP3 levels are expressed relative to phosphatidylinositol (PI) to correct for cell number. PIP3 and PI internal standards are present in the assay. Data were combined for 38:4, 38:3, and 36:2 PIP3 species. The chart displays means and 95% confidence intervals. Bonferroni’s multiple comparison tests are as follows: WT DT40 vs. PIP4K2A−/−/−: P = 0.065; PIP4K2A−/−/EmGFP vs. PIP4K2A−/−/kinase-dead EmGFP: P < 0.001; and PIP4K2A−/−/EmGFP vs. PIP4K2A−/−/substrate switch EmGFP: P = 0.97. Pooled data are from three replicates. The difference in PIP3 levels between WT and complete PIP4K2A knockout cells does not reach statistical significance, despite the central estimate of PIP3 levels in the latter cell lines being 25% lower than that in the former. This result may be because of an inadequate number of replicates, and therefore, we also pooled data for the cell lines exhibiting normal Akt phosphorylation and compared these with pooled data for the cell lines exhibiting abnormal Akt phosphorylation (SI Appendix, Fig. S6).
In light of these findings, we wondered if the PI5P4Kα-generated PI(4,5)P2 was acting as a substrate for class 1 PI3Ks to synthesize PIP3. Quantification of total PIP2 levels [which will be mostly PI(4,5)P2] in PIP4K2A−/−/− cells showed no change from the WT (SI Appendix, Fig. S6B), although this is not surprising given that the PI(4,5)P2 required for PIP3 synthesis is a very small fraction of total cellular PI(4,5)P2. However, in cells where all of the endogenous PI5P4Kα can be removed within 1 h by the addition of auxin (below), no change in PIP3 levels on acute removal of PI5P4Kα was detected, which would not be expected if PI5P4Kα-generated PI(4,5)P2 acted as an immediate precursor for PIP3. The simplest interpretation of these data suggests that the PI(4,5)P2 pool generated by PI5P4Kα regulates PIP3 synthesis indirectly over a time course longer than a few hours rather than by acting as its lipid precursor, but the latter possibility cannot be ruled out with our data.
Auxin-Inducible Degradation System for PI5P4Kα Reveals a Contrasting Phenotype of Akt Phosphorylation.
As just discussed, the simplest interpretation of the above data is that a pool of PI(4,5)P2 synthesized by PI5P4Kα is required for full PIP3 synthesis, but it is difficult to take this further in exploring what exactly is the primary defect using a knockout strategy. However, the power of DT40 genetics also gave us a unique opportunity to try a completely different approach but now looking at the effect of acute removal of the PI5P4Kα protein. To do this, we used the auxin degron system, which has been used in a number of cells by transfection of degron-tagged protein, including DT40s (14). Here, we tagged the PIP4K2A alleles directly with the auxin degron tag in a DT40 cell line that we had already stably transfected with the TIR1 protein (14) that is necessary for the auxin degron system to work. We also degron-tagged both alleles of PI5P4Kβ to make another cell line (PIP4K2Bdegron/degron), and this not only served as a control for any nonspecific effects of the manipulations but also, highlighted the specific role here of PI5P4Kα.
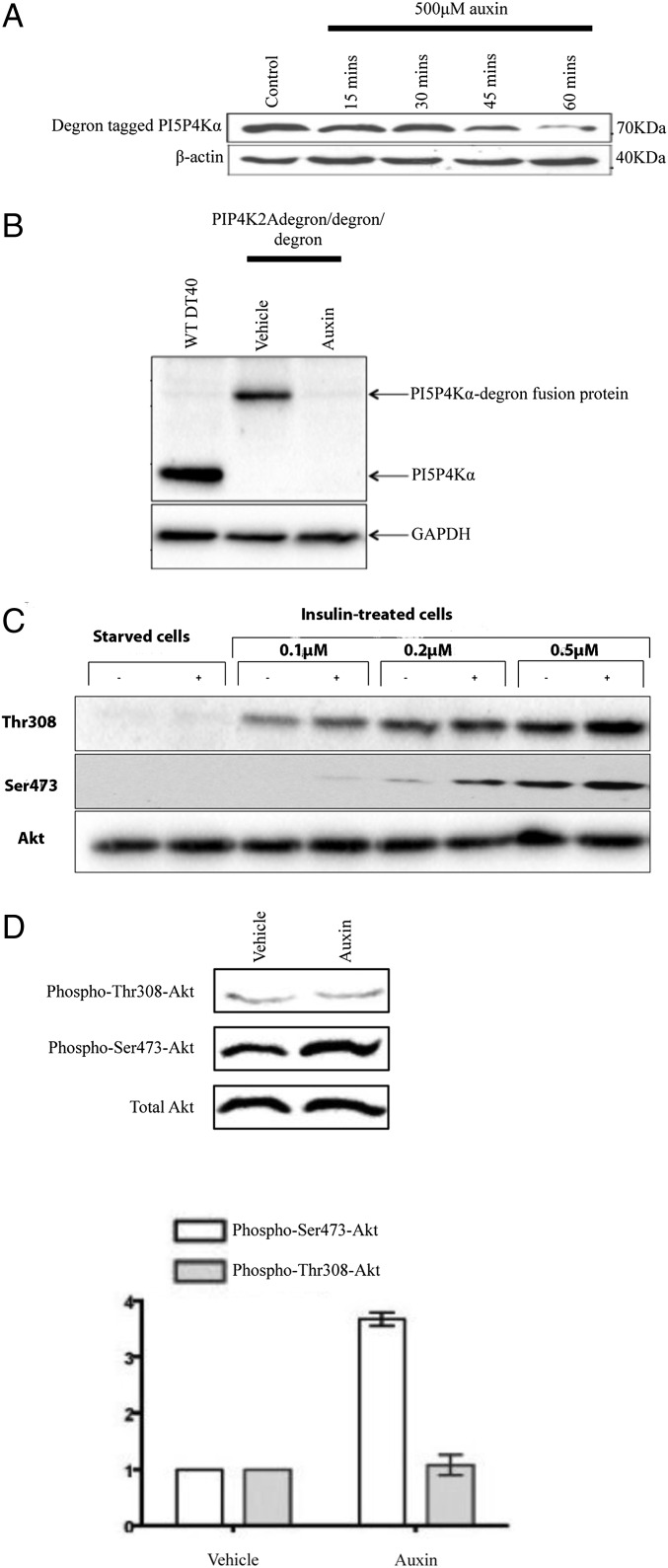
For PI5P4Kα, initially, we generated a cell line where all three alleles of the gene were degron-tagged (PIP4K2Adegron/degron/degron). By trying various strategies, we found that placing the degron tag at the C terminus of the protein followed by an (His)6-FLAG tag (as in ref. 8) worked best in that C-terminal tagging is easier than N-terminal tagging in this protocol, the tagged proteins could easily be quantified by immunoprecipitation and blotting, and the degron tag still worked effectively to target the protein for degradation. In both PIP4K2Adegron/degron/degron (Fig. 3) and PIP4K2Bdegron/degron (SI Appendix, Fig. S7) cells, addition of auxin led to the complete or near-complete removal of the tagged protein within 60 min, a process that is reversible (SI Appendix, Fig. S7). Blotting whole-cell lysates with an anti-PI5P4Kα antibody shows that no wild-type PI5P4Kα remains in PIP4K2Adegron/degron/degron cells (Fig. 3). The presence of the degron tag may reduce expression of PI5P4Kα slightly (Fig. 3), but we could detect no phenotypic difference in the cells.
Fig. 3.
Acute removal of PI5P4Kα with the auxin degron system. (A) Cells (PIP4K2Adegron/degron/degron) were treated with auxin as indicated, and any remaining PI5P4Kα degron fusion protein was immunoprecipitated against its FLAG tag and blotted against its poly-His tag. (B) Whole-cell lysates blotted with an anti-PI5P4Kα antibody. (C) PIP4K2Adegron/degron/degron cells were serum starved with or without 500 μM auxin for 60 min to remove PI5P4K. Insulin was added at the concentrations shown; after 10 min, the cells were lysed, and lysates were blotted sequentially (stripping in-between) for Akt phosphorylation at Thr308 and Ser473. (D) PIP4K2Adegron/degron/degron cells were synchronized in exponential growth and treated with either 500 μM auxin for 60 min to remove PI5P4Kα or vehicle. The effect on Akt phosphorylation at Thr308 and Ser473 sites was examined. Quantitation of four such blots by densitometry is shown in the graph. Bars show means and SEs.
Because we did not see the same changes in PIP3 mass levels on acute PI5P4Kα removal compared with PIP4K2A deletion (see above), we felt it important to reexplore the Akt signaling phenotype in these new cells. As with the knockout cell lines, we examined Akt phosphorylation under conditions of both insulin stimulation (Fig. 3C) and exponential growth in full medium (Figs. 3D and 4). After 1 h of serum starvation, cells not concurrently exposed to auxin exhibited insulin-induced Akt phosphorylation at both Thr308 and Ser473 as expected (15) in a dose-dependent fashion (Fig. 3C). In cells depleted of PI5P4Kα, the insulin-induced phosphorylation of Thr308 was unchanged or slightly increased, whereas the phosphorylation of Ser473 was significantly enhanced (Fig. 3C). We saw the same effect of increased Ser473 phosphorylation when cells were in exponential growth in full medium (Figs. 3D and 4A). This finding was confirmed to be a PI5P4Kα-specific effect, because acute removal of PI5P4Kβ from PIP4K2Bdegron/degron cells had no effect on Akt phosphorylation (SI Appendix, Fig. S8). We wondered whether the enhancement of Akt phosphorylation at Ser473 was stimulus-specific, and therefore, we treated our serum-starved PIP4K2Adegron/degron/degron B cells with the B-cell receptor agonist M4. Although as expected (16), M4 treatment resulted in increased phospho-Akt, this effect was not enhanced by removal of PI5P4Kα (SI Appendix, Fig. S9). There is, therefore, stimulus selectivity in the regulation of Akt Ser473 phosphorylation by PI5P4Kα.
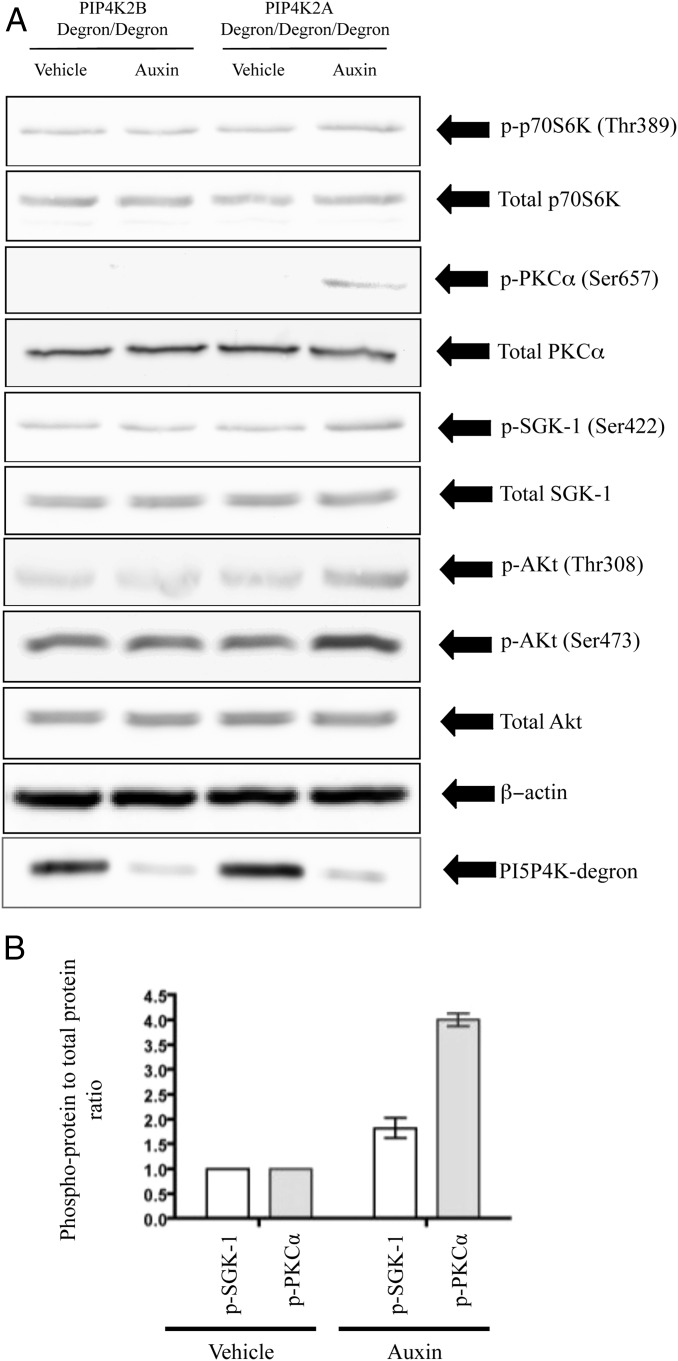
Fig. 4.
The effect of acute removal of PI5P4Kα or PI5P4Kβ on mTORC1 and mTORC2 phosphorylation targets. (A) Cells were synchronized at the same density in exponential growth before being treated for 60 min with 500 μM auxin and harvested for analysis. All blots use total cell-free extract, except those against the PI5P4K degron fusion protein, which are antipoly-His blots of anti-FLAG immunoprecipitates as in Fig. 3A. (B) Quantification of the phosphorylation status of the mTORC2 substrates SGK-1 and PKCα in response to acute PI5P4Kα removal by densitometry measurements from three such blots as in A. Results normalized to the phosphorylation status of these substrates in the presence of PI5P4Kα.
PI5P4Kα Is an Acute Negative Regulator of Mammalian Target of Rapamycin Complex 2.
The above data suggest that acute loss of PI5P4Kα results in enhanced Ser473 phosphorylation of Akt, with little or no effect on Thr308. Because the kinase primarily responsible for Ser473 phosphorylation is mammalian target of rapamycin complex 2 (mTORC2) (15), it seems most likely that PI5P4Kα is acting as a negative regulator of mTORC2 either directly or indirectly. To investigate this further, we examined the phosphorylation states of the mTORC2 targets SGK-1 and PKCα (15) and found phosphorylation of both to be increased on acute removal of PI5P4Kα but not of PI5P4Kβ (Fig. 4). In contrast and consistent with the lack of effect on Thr308 Akt phosphorylation (above), the phosphorylation state of the mTORC1 target p70S6K (15) is not affected by acute removal of PI5P4Kα (Fig. 4).
An alternative explanation for these findings is that PI5P4Kα removal inhibits the phosphatases responsible for dephosphorylating the three mTORC2 substrates. To address this idea we first confirmed that inhibition of TORC2 with the drug torin is successful in preventing phosphorylation of Akt at Ser473 in DT40 cells (SI Appendix, Fig. S11A). We then attempted to assay the kinetics of dephosphorylation of Ser473-Akt and Ser422-SGK1 in PIP4K2Adegron/degron/degron cells with or without auxin-induced removal of PI5P4Kα (SI Appendix, Fig. S11 B and C). Regardless of whether PI5P4Kα is depleted, complete dephosphorylation of the substrates examined occurs by the first time point at which we can reliably perform an assay (5 min). This finding rules out a fully quantitative experiment, but nevertheless, these findings coupled with the fact that different phosphatases are apparently responsible for removing the Ser473 phosphate of Akt and the Ser422 phosphate of SGK1 (17, 18) support PI5P4Kα being a negative regulator of mTORC2 as more likely than a positive regulator of two different phosphatases.
Acute Regulation of Akt by PI5P4Kα Depends on PI(4,5)P2 Generation.
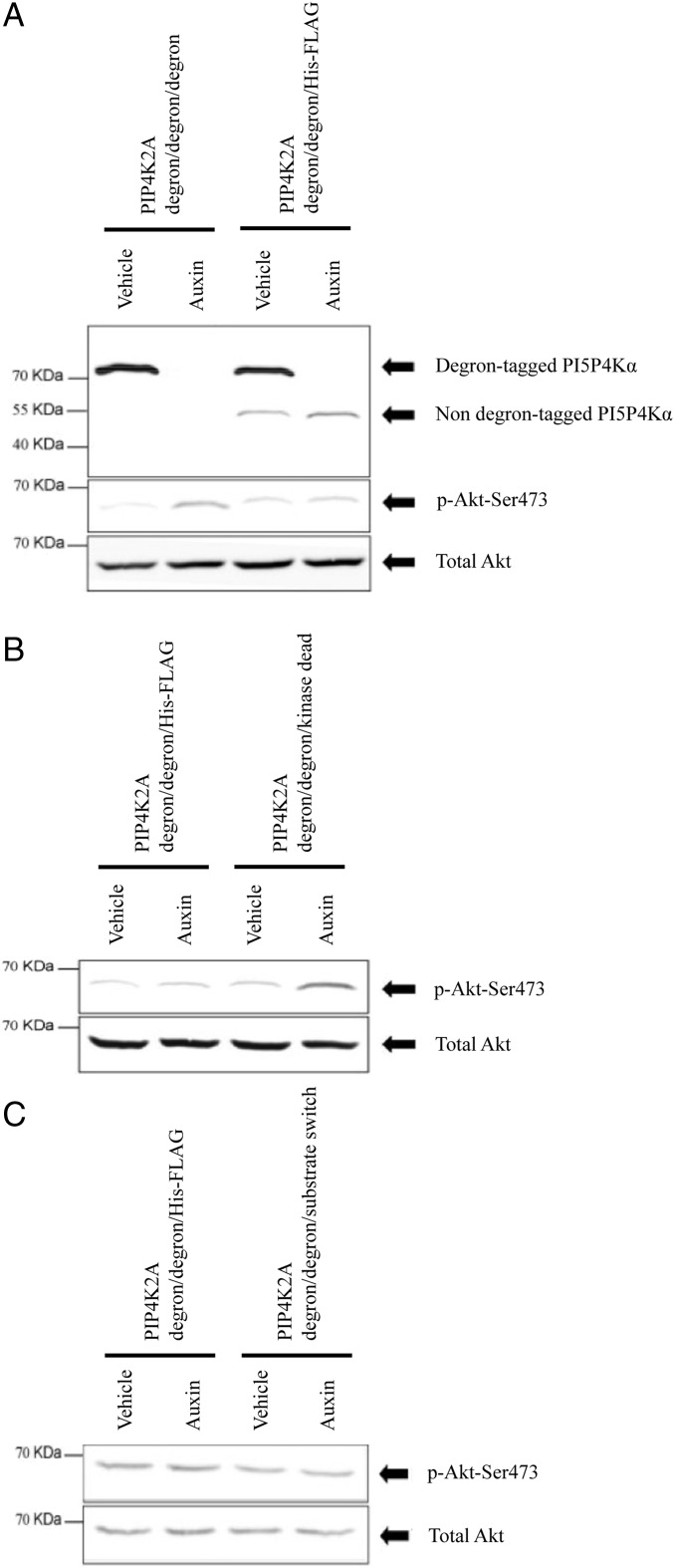
The unexpected finding that the Akt signaling phenotype of PI5P4Kα-deficient cells is completely different depending on the time course of PI5P4Kα removal begs the question of whether the mechanism by which PI5P4Kα acts is the same in both cases [that is, is kinase activity required in both cases, and if so, is PI5P removal or PI(4,5)P2 generation the primary effect?]. To address this question, we used a similar strategy to that described above for the stable knockout lines. Starting with PIP4K2Adegron/degron/wt cells, we either mutated the third allele to be kinase-dead or have PI4P5K activity while simultaneously tagging this allele at the C terminus with an (His)6-FLAG tag in both cases to generate PIP4K2Adegron/degron/kinase-dead and PIP4K2Adegron/degron/substrate switch cells, respectively. As a control, we generated a cell line where we simply knocked a C-terminal (His)6-FLAG tag into the third allele (PIP4K2Adegron/degron/His-FLAG cells). This strategy allows degron-tagged but otherwise wild-type PI5P4Kα to be acutely removed from the cell, while leaving behind either kinase-live, kinase-dead, or substrate switch PI5P4Kα. Note that, despite the likely homodimerization (8, 9) of PI5P4Kα monomers, the nondegron-tagged protein is not trafficked for degradation with its degron-tagged counterpart (Fig. 5), presumably because the kinetics of dimer dissociation are fast enough to prevent this.
Fig. 5.
The requirement for PI5P4Kα to synthesize a pool of PI(4,5)P2 to regulate mTORC2 signaling. Cells were synchronized at the same density in exponential growth before being treated for 60 min with 500 μM auxin and harvested for analysis. As shown previously, blots of Akt and phospho-Akt are on total cell lysates, whereas blots of PI5P4Kα are antipoly-His blots of anti-FLAG immunoprecipitates. (A) As previously shown, acute removal of PI5P4Kα from PIP4K2Adegron/degron/degron cells results in increased p-Ser473-Akt. In PIP4K2Adegron/degron/His-FLAG cells, removal of the protein product from the two degron-tagged alleles leaves the nondegron-tagged protein present, and this remaining protein is sufficient to support normal Akt phosphorylation. (B) When PIP4K2Adegron/degron/kinase-dead cells are treated with auxin so as to leave the cells with only kinase-dead PI5P4Kα, an increase in p-Ser473-Akt results. PI5P4Kα, therefore, has to be kinase-live to promote normal Akt phosphorylation. (C) By a similar strategy, treating PIP4K2Adegron/degron/substrate switch cells with auxin so that the cells are left with PI5P4Kα that has been mutated into a PI4P5K has no effect on Akt phosphorylation. Therefore, synthesis of PI(4,5)P2 rather than PI5P removal is the most likely function of PI5P4Kα here.
It should further be noted at this point that we were unable to achieve kinase-dead and substrate switch mutations by the same strategies as before: the single-triplet changes required were repeatedly edited out, presumably by homologous recombination between alleles. We reasoned that more substantial coding changes might not be edited out and found that, if the kinase-dead mutation was made by introducing a K366-368Q mutation into the activation loop the equivalent of the K377-379Q substitution that Kunz et al. (11) showed reduces the kinase activity of human PI5P4Kβ to less than 2% of wild-type, the mutation was retained. The substrate switch was generated by the A370E (11) mutation as above, but to avoid repair by homologous recombination, we had to introduce synonymous mutations into two neighboring triplets coding for K368 and A369.
Addition of auxin to the PIP4K2Adegron/degron/kinase-dead cells recapitulated the increase in Ser473 phosphorylation of Akt that was seen in PIP4K2Adegron/degron/degron cells (Fig. 5), implying that the kinase activity of PI5P4Kα is required for it to fulfill this aspect of its function. Therefore, the question again is whether PI5P removal or PI(4,5)P2 generation is relevant; note that Jude et al. (19) have reported that RNAi knockdown of PI5P4Kα leads to an increase in PI5P levels and mTOR activity. Adding auxin to PIP4K2Adegron/degron/substrate switch cells and therefore, leaving the cell with PI4P5K activity did not result in an increase in p-Ser473-Akt (Fig. 5), suggesting that generation of a PI(4,5)P2 pool by PI5P4Kα is crucial in this enzyme’s role as a negative regulator of mTORC2.
Discussion
A crucial result of our experiments is to establish that a functional pool (or pools) of PI(4,5)P2 is synthesized through a unique route: phosphorylation of PI5P rather than PI4P. For both the stable knockout and acute depletion of PI5P4Kα, we established clear phenotypes to provide “readouts” of enzyme function, and in both situations, it was clear that the role of PI5P4Kα was to synthesize PI(4,5)P2 rather than to deplete PI5P. Of course, in other settings, PI5P depletion by PI5P4Kα may well be of physiological importance. Furthermore, the PI5P4Kα-generated PI(4,5)P2 has a regulatory influence over mTORC2 and PI3K-Akt signaling.
It is striking, although perhaps not entirely unexpected, that very different phenotypes are identified on chronic and acute depletion of PI5P4Kα. Given that, in simple terms, increased Akt phosphorylation is seen with acute PI5P4Kα removal and reduced Akt phosphorylation is seen chronically, it is tempting to speculate that the long-term phenotype is an adaptive consequence of the initial pertubation. As discussed in the Introduction, a profound change in cell physiology stemming from a single-point mutation in type I PI3Kα has been shown by Hart et al. (4), which they called the “butterfly effect,” and a similar set of events may have taken place here.
In other studies, the impact of the PI5P4Ks on Akt signaling is not consistent between different organisms and different tissues using different techniques, and our demonstration of temporal variability may help to reconcile some of these data. For example, stable knockout of Drosophila dPIP4K (orthologous to the high-activity PIP4K2A in higher species) results in a dramatic attenuation of Akt phosphorylation (20), whereas acute depletion of PIP4K2A in human leukocytes by RNAi results in increased Akt phosphorylation (19). No Akt phenotype was found in PIP4K2A knockout mice (21), but as far as we are aware, cells of the hematopoietic lineage were not studied there; another study from the same group elegantly showed the tissue variability of gene knockouts, with PIP4K2B knockout mice having enhanced insulin-induced Akt phosphorylation in skeletal muscle and liver but not in white fat (22). Certainly, it is not unreasonable to suggest a particular role of PI5P4Kα in blood cells given that these are the only cells reported so far to have an excess of this enzyme over the other isoforms (23). Our data from exploring a simple, single-cell type system highlight how difficult it can be to interpret primary protein function from knockout phenotypes. More importantly, we suggest that the effects that we see in the degron-tagged cells, a strategy that gives the cells no chance to adapt around changes caused by the removal of protein, may be revealing a primary and previously unsuspected cellular function of PI5P4Kα related to mTORC2 regulation.
At this stage, it is unclear what mechanisms are leading to the different phenotypes, and clarification will take extensive additional work. This need is particularly so for the knockout phenotype because of its indirect nature. For the acute phenotype, it is interesting to note that the cellular location of endogenous PI5P4Kα in DT40s is largely cytoplasmic (SI Appendix, Fig. S10), with all of it being attached to membranes (8), raising the possibility that it is localized to the endoplasmic reticulum, the same location in which mTORC2 has been reported to function (15). PI(4,5)P2 is at very low levels in intracellular membranes (1), and therefore, any synthesized here by PI5P4Kα could have a disproportionate impact. Indeed, synthesizing a localized pool of PI(4,5)P2 by a different metabolic pathway from most of the cell’s (PI4P5K-generated) PI(4,5)P2 is an attractive concept for controlling localized functions of this highly multifunctional (1) lipid. Note that, if this is so, simplistically, the PI(4,5)P2 pool made by PI5P4Kα that we have invoked here must have a negative effect on mTORC2 activity. [Superficially, this might serve as the precursor for the PIP3 pool suggested recently by Liu et al. in mTORC2 regulation (24), although because this appears to be stimulatory to mTORC2, the relationship of the observations by Liu et al. with our observations is unclear.] There are two proteins reported as inhibiting mTORC2, DEPTOR (25) and XPLN (26), the latter being mTORC2-specific and thus, potentially, an endoplasmic reticulum protein, and both of these proteins have the theoretical potential to bind PI(4,5)P2 through their PDZ or PH domains, respectively; these considerations point to one way in which the negative influence of PI5P4Kα on mTORC2 activity might be mediated.
Materials and Methods
All methodology pertinent to this study, including cell line generation, signaling assays, and fluorescent and MS-based lipid assays, can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ashok Venkitaraman, Gerard Evan, and Tony Jackson for generous gifts of reagents. This work was supported in part by an A. J. Clark Studentship from the British Pharmacological Society (S.J.B.); Sidney Sussex College (A.D.); the Cambridge Overseas Trust (A.D.); the Säid Foundation (A.D.); and Medical Research Council Grant RG64071 (to J.H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522478113/-/DCSupplemental.
References
- 1.Balla T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93(3):1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viaud J, Boal F, Tronchère H, Gaits-Iacovoni F, Payrastre B. Phosphatidylinositol 5-phosphate: A nuclear stress lipid and a tuner of membranes and cytoskeleton dynamics. BioEssays. 2014;36(3):260–272. doi: 10.1002/bies.201300132. [DOI] [PubMed] [Google Scholar]
- 3.Bulley SJ, Clarke JH, Droubi A, Giudici ML, Irvine RF. Exploring phosphatidylinositol 5-phosphate 4-kinase function. Adv Biol Regul. 2015;57:193–202. doi: 10.1016/j.jbior.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart JR, et al. The butterfly effect in cancer: A single base mutation can remodel the cell. Proc Natl Acad Sci USA. 2015;112(4):1131–1136. doi: 10.1073/pnas.1424012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozenvayn N, Flaumenhaft R. Phosphatidylinositol 4,5-bisphosphate mediates Ca2+-induced platelet alpha-granule secretion: Evidence for type II phosphatidylinositol 5-phosphate 4-kinase function. J Biol Chem. 2001;276(25):22410–22419. doi: 10.1074/jbc.M008184200. [DOI] [PubMed] [Google Scholar]
- 6.Rozenvayn N, Flaumenhaft R. Protein kinase C mediates translocation of type II phosphatidylinositol 5-phosphate 4-kinase required for platelet alpha-granule secretion. J Biol Chem. 2003;278(10):8126–8134. doi: 10.1074/jbc.M206493200. [DOI] [PubMed] [Google Scholar]
- 7.Richardson JP, Wang M, Clarke JH, Patel KJ, Irvine RF. Genomic tagging of endogenous type IIbeta phosphatidylinositol 5-phosphate 4-kinase in DT40 cells reveals a nuclear localisation. Cell Signal. 2007;19(6):1309–1314. doi: 10.1016/j.cellsig.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, et al. Genomic tagging reveals a random association of endogenous PtdIns5P 4-kinases IIalpha and IIbeta and a partial nuclear localization of the IIalpha isoform. Biochem J. 2010;430(2):215–221. doi: 10.1042/BJ20100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bultsma Y, Keune WJ, Divecha N. PIP4Kbeta interacts with and modulates nuclear localization of the high-activity PtdIns5P-4-kinase isoform PIP4Kalpha. Biochem J. 2010;430(2):223–235. doi: 10.1042/BJ20100341. [DOI] [PubMed] [Google Scholar]
- 10.Hinchliffe KA, Giudici ML, Letcher AJ, Irvine RF. Type IIalpha phosphatidylinositol phosphate kinase associates with the plasma membrane via interaction with type I isoforms. Biochem J. 2002;363(Pt 3):563–570. doi: 10.1042/0264-6021:3630563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunz J, Fuelling A, Kolbe L, Anderson RA. Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a single amino acid residue. J Biol Chem. 2002;277(7):5611–5619. doi: 10.1074/jbc.M110775200. [DOI] [PubMed] [Google Scholar]
- 12.Clark J, et al. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat Methods. 2011;8(3):267–272. doi: 10.1038/nmeth.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25(4):491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods. 2009;6(12):917–922. doi: 10.1038/nmeth.1401. [DOI] [PubMed] [Google Scholar]
- 15.Gaubitz C, Prouteau M, Kusmider B, Loewith R. TORC2 structure and function. Trends Biochem Sci. 2016;41(6):532–545. doi: 10.1016/j.tibs.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, et al. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J Immunol. 1999;163(4):1894–1905. [PubMed] [Google Scholar]
- 17.Gao T, Furnari F, Newton AC. PHLPP: A phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Chao CC, Ma YL, Lee EHY. Protein kinase CK2 impairs spatial memory formation through differential cross talk with PI-3 kinase signaling: Activation of Akt and inactivation of SGK1. J Neurosci. 2007;27(23):6243–6248. doi: 10.1523/JNEUROSCI.1531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jude JG, et al. A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene. 2015;34(10):1253–1262. doi: 10.1038/onc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A, et al. Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc Natl Acad Sci USA. 2013;110(15):5963–5968. doi: 10.1073/pnas.1219333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emerling BM, et al. Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors. Cell. 2013;155(4):844–857. doi: 10.1016/j.cell.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamia KA, et al. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta-/- mice. Mol Cell Biol. 2004;24(11):5080–5087. doi: 10.1128/MCB.24.11.5080-5087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke JH, Emson PC, Irvine RF. Localization of phosphatidylinositol phosphate kinase IIgamma in kidney to a membrane trafficking compartment within specialized cells of the nephron. Am J Physiol Renal Physiol. 2008;295(5):F1422–F1430. doi: 10.1152/ajprenal.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, et al. PtdIns(3,4,5)P3-dependent activation of the mTORC2 kinase complex. Cancer Discov. 2015;5(11):1194–1209. doi: 10.1158/2159-8290.CD-15-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson TR, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna N, Fang Y, Yoon MS, Chen J. XPLN is an endogenous inhibitor of mTORC2. Proc Natl Acad Sci USA. 2013;110(40):15979–15984. doi: 10.1073/pnas.1310434110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.