Significance
Africa hosts contrasting communities of mammal browsers and is, thus, the ideal background for testing their effect on plant communities and evolution. In this study at the continental scale, we reveal which mammal browsers are most closely associated with spiny communities of trees. We then show a remarkable convergence between the evolutionary histories of these browsers (the bovids) and spiny plants. Over the last 16 My, plants from unrelated lineages developed spines 55 times. These convergent patterns of evolution suggest that the arrival and diversification of bovids in Africa changed the rules for persisting in woody communities. Contrary to our current understanding, our data suggest that browsers predate fire by millions of years as agents driving the origin of savannas.
Keywords: Africa, Bovidae, coevolution, mammalian herbivory, savanna
Abstract
Savannas first began to spread across Africa during the Miocene. A major hypothesis for explaining this vegetation change is the increase in C4 grasses, promoting fire. We investigated whether mammals could also have contributed to savanna expansion by using spinescence as a marker of mammal herbivory. Looking at the present distribution of 1,852 tree species, we established that spinescence is mainly associated with two functional types of mammals: large browsers and medium-sized mixed feeders. Using a dated phylogeny for the same tree species, we found that spinescence evolved at least 55 times. The diversification of spiny plants occurred long after the evolution of Afrotherian proboscideans and hyracoids. However, it is remarkably congruent with diversification of bovids, the lineage including the antelope that predominantly browse these plants today. Our findings suggest that herbivore-adapted savannas evolved several million years before fire-maintained savannas and probably, in different environmental conditions. Spiny savannas with abundant mammal herbivores occur in drier climates and on nutrient-rich soils, whereas fire-maintained savannas occur in wetter climates on nutrient-poor soils.
The origin and spread of savannas have been topics of intensive research, but many questions remain. The C4 grasses that dominate savannas emerged in the late Oligocene (∼30 Ma), but savannas only began to emerge as one of the world’s major biomes in the late Miocene more than 20 My later (1). What changed to roll back the forests, allowing the rapid spread of grasslands? Ehleringer et al. (2) first linked the rise of savannas to a drop in atmospheric CO2, which would favor C4 grasses over their C3 grass predecessors. Low CO2 can also reduce woody cover by increasing the risk of recruitment failure in woody plants whether from drought, fire, or browsing (3). However, the timing of the onset of low CO2 is much earlier than the spread of savannas; therefore, although low CO2 may have contributed to savanna expansion, it cannot explain the long time lag between C4 origins and savanna spread. Climate change is the usual explanation for changing vegetation over time. Increased aridity in the late Miocene has been shown to cause the retreat of forests in North America and Eurasia, allowing grasslands to spread in their place (4, 5). However, large areas of extant savannas occur in climates that are wet enough to support forests and other closed woody types (6–8). Fires are frequent in high-rainfall savannas and have been considered the major agents accounting for open ecosystems in climates that can support forests. Fossil charcoal, mostly from marine cores, shows a surge in fire activity from the late Miocene correlated with the spread of savannas (9, 10). Phylogenetic studies have shown the emergence of fire-adapted woody plants from the late Miocene through to the Pleistocene in both Brazil and Africa, consistent with fossil evidence for increasing fire activity from this time (11, 12).
An alternative hypothesis, that mammal herbivory creates open ecosystems, was first proposed by Owen-Smith (13). He argued that increased forest cover from the last glacial to interglacial conditions was partly the result of extinction of the Pleistocene fauna (13). Many experimental and observational studies have shown that mammals have the capacity to create open ecosystems by reducing tree biomass whether in the tropics or temperate and boreal regions (14, 15). Although Owen-Smith (13) emphasized megaherbivores (animals >1,000 kg) as primarily responsible for open habitats, mesobrowsers (4–450 kg), such as deer, antelope, and caprids, are very effective at preventing woody plants from escaping the “browse trap” and growing into larger size classes. They have also been implicated as agents maintaining open ecosystems and preventing forest development. Here, we explore the potential role of mesobrowsers in opening up ancient ecosystems and promoting the spread of African savannas. Africa is particularly suitable for such a study, because it retains a largely intact megafauna, although now greatly reduced in abundance and area.
Fossil tests of the importance of herbivory in opening up ancient forests are difficult because of the lack of sites with suitable data on both plant and animal fossils. This lacuna in the fossil record is explained partly because contrasting conditions favor fossil development in plants vs. mammals (16). For example, where plant fossils are well-preserved in acidic deposits, animal bones are not. Recently, paleoecologists have begun using the spores of a coprophilous fungus, Sporormiella, as a proxy for high herbivore activity. Fungal spores can be counted along with pollen and charcoal to determine changes in herbivory, fire, and vegetation (17). Such studies have suggested changes from open ecosystems with high Sporormiella counts to closed woody vegetation or in some cases, a switch to flammable vegetation when the dung spores decline (18, 19). Thus, there is growing paleoecological evidence that mammal herbivory helped maintain open ecosystems in the past and that large mammal extinction triggered major vegetation change. However, these studies have focused on the Late Pleistocene and Holocene, and the earlier origins of open grassy formations have been less explored (20).
Here, we report on a phylogenetic approach for exploring the importance of mammal browsers in opening up African vegetation and promoting the spread of savannas from the late Miocene. Although several phylogenetic analyses have explored plant defenses against insect herbivory, we report here an analysis of the evolution of tree defenses against vertebrate herbivores. We used stem (not leaf) spines on woody plants as markers of high mammal herbivory (Fig. S1). Spines are considered a defense specific to mammal herbivores (21). We did not include leaf spines or species with soft organs, stinging hairs, or spines shorter than 5-mm long, because their defensive function against vertebrate herbivory remains unclear. Spines are a peculiar defense in that the foliage of spiny trees is often highly palatable and favored by browsers. Spines function by reducing bite size of the browsing animal, thereby reducing food intake and driving the animal to move away to seek more rewarding targets (22). In this paper, we first establish how the present day distribution of spiny species relates to the abundance of different herbivore functional types, abiotic environmental factors (precipitation, soil fertility, fire, and temperature), and major vegetation types. We used a recent classification of African herbivores that groups together animals according to their functional traits (body mass, diet, gut type, social behavior, and water dependence) (23). We then explore coevolutionary dynamics by comparing the accumulation of spiny plant lineages in African savanna with the diversification of bovids (antelope and their relatives). Although bovids and mesobrowsers are not strictly equivalent, most mesobrowsers in Sub-Saharan Africa are bovids. The bovid lineage includes the browsers most related to spiny plant distribution today. A close match would imply a causal link between the diversification of bovids and spiny plants. If spines emerged much earlier than bovid radiations, then it is possible that these plants were preadapted to bovid herbivory, and it is unlikely that mammal herbivory was a major factor in their spread. Finally, we compared the timing of the increase in spiny plant lineages and the diversification of their mammal browsers (bovids) with phylogenetic and fossil evidence for the emergence of savannas.
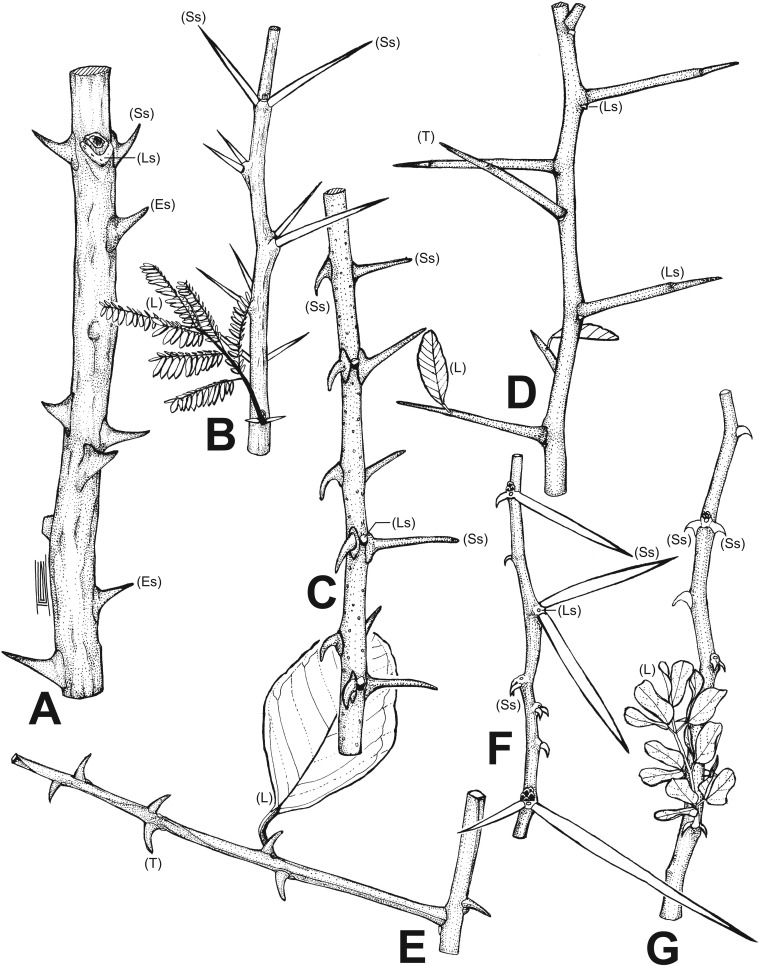
Fig. S1.
Types of spines. (A) Prickles: Zanthoxylum davyi. (B) Straight stipular spines: Vachellia robusta. (C) Straight stipular spines and stipular hooks: Ziziphus mucronata. (D) Straight thorns: Gymnosporia harveyana. (E) Hook thorns: Scutia myrtina. (F) Straight stipular spines and stipular hooks: Vachellia tortilis. (G) Stipular hooks: Senegalia nigrescens. Es, epidermic spine; L, leaf; Ls, leaf scar; Ss, stipular spine; T, thorn (i.e., branch with a sharp tip).
Results
Contemporary Environmental Correlates.
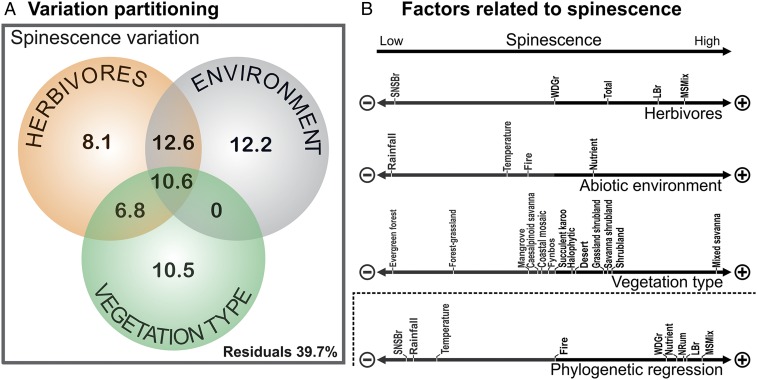
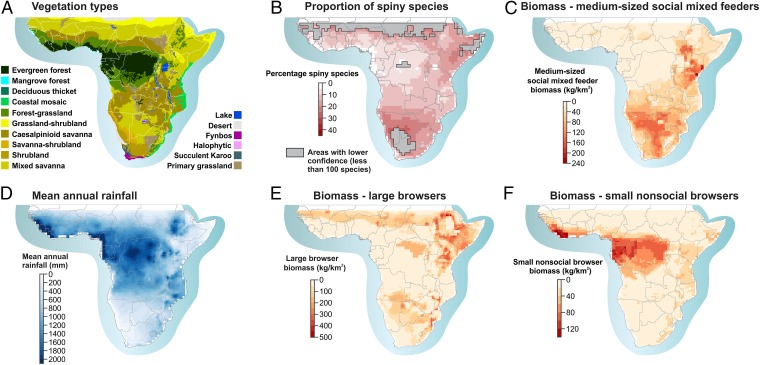
Our spatial model explained over 60% of the distribution of spiny communities in Africa (Fig. 1A and Table S1). Spinescence of vegetation was significantly related to the suite of herbivores present (adjusted R2 = 0.38), abiotic factors (0.35), and the distribution of biomes (0.27). Spiny communities are favored in open environments, such as mixed savannas (all scores are reported in Table S2), shrubland, savanna–shrubland, and grassland–shrubland, and poorly represented in evergreen forest and forest/grassland mosaics (Figs. 1B and 2). The abiotic environments related to spinescence include low mean annual precipitation (Fig. 1B), high-nutrient soils, colder temperature, and low fire frequencies. Importantly, the unique contribution of herbivores (0.081) is significant even after removing the variation coexplained with abiotic factors or biome distribution. The relationship between spiny plant and herbivore communities is strong and positive for medium-sized mixed feeders (consuming grass and trees) and large browsers (Fig. 1B). In contrast, spinescence is strongly negatively associated with small nonsocial browsers, which are largely restricted to rainforest (Fig. 2). The weaker relationship with the total biomass of herbivores emphasizes the usefulness of the functional grouping of herbivores to identify groups best associated with spinescence.
Fig. 1.
Environmental factors related to spinescence of African tree species. (A) Variation partitioning of the proportion of spiny species explained by the biomass of different herbivore functional types, environmental variables, and vegetation type. (B) Relationships between environmental factors and proportion of spiny species. Black arrows indicate a positive relationship; gray arrows indicate a negative relationship. The position on the arrow indicates the strength of the relationship. Names of functional types of mammals follow the work in ref. 23. LBr, large browser; MSMix, medium-sized social mixed feeders; NRum, nonruminant; SNSBr, small nonsocial browser; Total, total biomass of mammalian herbivores; WDBr, water-dependent grazer.
Table S1.
Details of the variation partitioning: Partition table
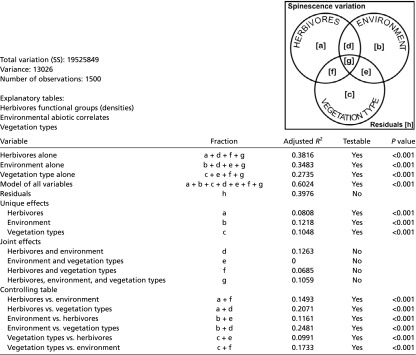 |
Total variation (sum of squares): 19,525,849. Variance: 13,026. Number of observations: 1,500. Explanatory tables: herbivores functional groups (densities), environmental abiotic correlates, and vegetation types.
Table S2.
Scores of selected variables on the first axis of CCA
| Vegetation types | Scores |
| Species | |
| Spiny | +0.31 |
| Non spiny | −0.05 |
| Variables selected | |
| Evergreen forest | −0.68 |
| Forest grassland | −0.42 |
| Mangrove forest | −0.11 |
| Caesalpinioid savanna | −0.05 |
| Coastal mosaic | −0.05 |
| Fynbos | −0.01 |
| Succulent Karoo | +0.02 |
| Halophytic | +0.09 |
| Desert | +0.12 |
| Grassland shrubland | +0.23 |
| Savanna shrubland | +0.25 |
| Shrubland | +0.27 |
| Mixed savanna | +0.72 |
| Variables not selected | |
| Primary grassland | — |
| Deciduous thicket | — |
| Abiotic factors | |
| Species | |
| Spiny | +0.30 |
| Non spiny | −0.05 |
| Variables selected | |
| MAP | −0.87 |
| MAT | −0.25 |
| Burn % | −0.14 |
| Nutrient | +0.22 |
| Herbivores | |
| Species | |
| Spiny | +0.33 |
| Non spiny | −0.05 |
| Variables selected | |
| SNSBr | −0.83 |
| WDGr | +0.01 |
| Total | +0.29 |
| LBr | +0.55 |
| MSMix | +0.68 |
| Variables not selected | |
| Nrum | — |
Stepwise selection by permutation was applied on variables from each dataset separately using a stopping criterion of 0.05 (68). Number of sites: 1,500. Number of species: 1,800. Burn %, percentage burnt annually; LBr, large browser in biomass per surface area; MAP, mean annual precipitation; MAT, mean annual temperature; MSMix, medium-sized social mixed feeders in biomass per surface area; NRum, nonruminants in biomass per surface area; nutrient, −(soil nutrient availability index); SNSBr, small nonsocial browser in biomass per surface area; total, total biomass of mammalian herbivores in biomass per surface area; WDBr, water-dependant grazer in biomass per surface area.
Fig. 2.
Maps of spiny species distribution and environmental correlates. (A) Vegetation types. (B) Proportion of spiny species: values for areas in gray are not reported because fewer than 100 tree species have been reconstructed in each degree pixel; interpretation of proportions should then be subject to caution. (C) Total biomass of medium-sized social mixed feeders. (D) Mean annual rainfall. (E) Total biomass of large browsers. (F) Total biomass of small nonsocial browsers.
Phylogenetic regression on the principal components of the combined explanatory dataset identified similar important variables (Fig. 1B). The best model included only the first principal component (Table S3), which was positively related to spinescence (P = 0.022) (Table S4). Variable correlations with the first principal component (Table S2) suggest that spinescence is associated with dry, somewhat cooler environments with high soil nutrient status (all scores are reported in Table S4). Medium-sized mixed feeders, large browsers, nonruminants, and to some extent, water-dependent grazers are positively related to spinescence, whereas small nonsocial browsers have a strong negative correlation with spinescence (Fig. 1B and Table S4). Fire prevalence seems to have virtually no association with spinescence.
Table S3.
Parameter estimates for phylogenetic regression models
| Rank and parameter | Estimate | SE | z Value | P value | α | AIC | ΔAIC | Combined Mantel correlations |
| 1 | ||||||||
| Intercept | −1.316 | 0.559 | −2.355 | 0.019* | 0.011 | 528.23 | 0 | 0.038 |
| PC1 | 0.048 | 0.021 | 2.283 | 0.022* | ||||
| 2 | ||||||||
| Intercept | −1.478 | 0.541 | −2.734 | 0.006** | 0.012 | 528.80 | 0.58 | 0.0375 |
| PC1 | 0.057 | 0.024 | 2.369 | 0.018* | ||||
| PC3 | −0.011 | 0.042 | −0.255 | 0.799 | ||||
| 3 | ||||||||
| Intercept | −1.318 | 0.557 | −2.366 | 0.018* | 0.011 | 530.20 | 1.97 | 0.0379 |
| PC1 | 0.048 | 0.021 | 2.243 | 0.025* | ||||
| PC2 | −0.001 | 0.027 | −0.038 | 0.969 | ||||
| 4 | ||||||||
| Intercept | −1.469 | 0.526 | −2.792 | 0.005** | 0.013 | 530.82 | 2.59 | 0.0245 |
| PC1 | 0.053 | 0.024 | 2.218 | 0.027* | ||||
| PC2 | −0.003 | 0.030 | −0.098 | 0.922 | ||||
| PC3 | −0.011 | 0.041 | −0.262 | 0.793 |
The four models, including PC1, all had broadly similar support based on the AIC. However, PC1 is the only significant predictor variable (*P < 0.05; **P < 0.01) in this set of models. The cumulative Mantel correlation shows the spatial autocorrelation in model residuals. Combined Mantel correlations (Pearson) are of all significant (positive and negative) distance classes tested within the Mantel correlograms (with a Holm correction for multiple testing). PC, axis in the principal component analysis.
Table S4.
Variable weightings on principal components axes
| Variable | PC1 (54%) | PC2 (22%) | PC3 (11%) |
| Mean annual rainfall | −0.838 | 0.411 | 0.281 |
| Mean annual temperature | −0.691 | 0.599 | −0.162 |
| Soil nutrients | 0.725 | −0.153 | 0.040 |
| Burn percentage | 0.006 | 0.746 | −0.644 |
| Small nonsocial browsers | −0.879 | 0.075 | 0.445 |
| Medium-sized social mixed feeders | 0.881 | −0.228 | −0.126 |
| Large browsers | 0.785 | 0.364 | 0.315 |
| Water-dependent grazers | 0.665 | 0.658 | 0.238 |
| Nonruminants | 0.768 | 0.532 | 0.254 |
The relationship between each variable and principal component axis is bound by the range from −1 to 1. The percentage of total variance explained by each axis is shown in parentheses. PC, axis in the principal component analysis.
Phylogenetic Analyses.
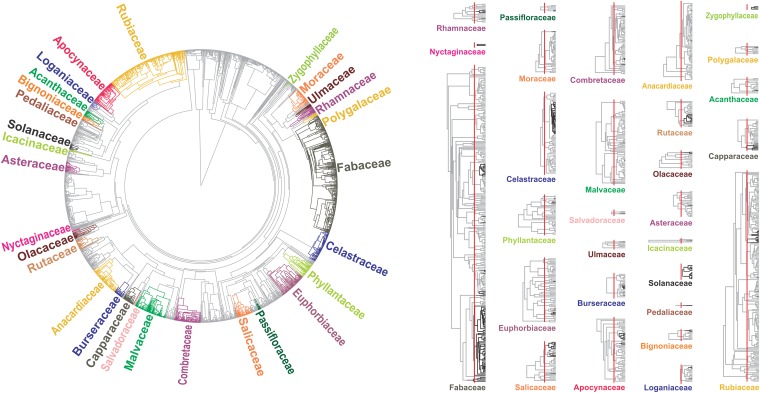
The distribution of spinescence in African trees is phylogenetically dispersed (Fig. 3). We recorded 213 spiny species (from a sample of 1,852 tree species) distributed in 29 families, indicating at least 55 independent evolutionary origins across the angiosperm tree of life (Dataset S1). Spiny clades have relatively recent origins, with the great majority arising within the last 17 Ma (Fig. 3) but with some older origins, frequently associated with clades that likely diversified elsewhere (Table S5). The mid-Miocene radiation of spiny plants indicates that early Cenozoic African herbivores, notably proboscideans (including elephants) and hyracoids, did not select for this structural defense.
Fig. 3.
(Left) Phylogeny of southern African woody flora and position of spiny species. Flora were reconstructed based on DNA barcodes using a maximum likelihood approach after transforming branch lengths to millions of years ago by enforcing a relaxed molecular clock and multiple calibrations. Colored branches highlight families containing at least one spiny species. The subtrees for these families are presented (Right), with the position of spiny species indicated in black (shades of gray indicate the probability of the ancestral lineages being spiny). The red bar indicates 17 Ma on all subtrees.
Table S5.
Dating and biogeography of spiny clades in our analyses and the literature
| Clade no. | Genus (era) | Family | Age of clade, My (Refs.) | Age in this analysis, My | Sampled species (total in the clade) (Refs.) | Biogeography | Comments |
| 1 | Balanites | Zygophyllaceae | 15 (68) | 10.32 | 3 (9) | Seven species across Africa and two species in Burma and India (69); groups with two other African genera (70), likely sister to Sisyndite (70) | Not clear if sister group (Sisyndite) is also spiny, herbarium images suggest not |
| 2 | Maclura | Moraceae | 46 (71) | 20.78 | 1 (12) | South America origin, single species in Africa | Clade of spiny species, spines evolved outside Africa |
| 3 | Chaetachme | Ulmaceae | — | — | 1 (1) (72) | Native to eastern and western Africa | |
| 4 | Ziziphus | Rhamnaceae | 33.6 (73) | 29.71 | 5 (110) | African Ziziphus monophyletic clade; Richardson et al. (74) suggest Laurasian origin for Ziziphus, with later migration into South America and Africa | Ziziphus nonmonophyletic, composed of two clades OW and NW Ziziphus (75); total no. of species and age for OW species only |
| 5 | Scutia | Rhamnaceae | 34 (69) | 10.05 | 1 (1) | Rhamnus, Frangula, and Scutia widespread (74), biogeography unclear | Genus paraphyletic, one species in Africa (76) |
| 6 | Nylandtia (= Muraltia) | Polygalaceae | 21.0 ± 3.5 (77) | 20.11 | 1 (117) | South Africa, mainly the Cape, except for one species, Muraltia xanaganii, which is found in disjunct alpine habitats in eastern Africa northward to southern Tanzania | |
| 7 | Erythrina | Fabaceae | 7.4 (78) | 20.08 | 11 (38) | 38 Species across Africa and Madagascar, 12 species in Asia and Australia, and ∼70 species in Central America (72) | Total species no. for African species only, but monophyly of African clade unclear (79); phylogenetic placement of these taxa inconsistent with recent literature |
| 8 and 9 | Dalbergia | Fabaceae | 9.6 ± 2.6 (80) | 17.79 | 7 (250) | Pantropical distribution with centers of diversity in Central and South America, Africa, Madagascar, and Asia; African clades paraphyletic (81) | |
| 10 | Dichrostachys | Fabaceae | 10.45 (82) | 16.22 | 2 (13) | 11 East Africa/Madagascar, 1 pantropical, 1 Australia (72) | |
| 11 | Fabaceae | Faidherbia | 24.2 (83) | 10.11 | 1 (1) | Africa to east Mediterranean | Monotypic spiny genus, sister to genus Zapoteca, which is not spiny (84) |
| 12 | Acacia | Fabaceae | — | — | — | ||
| 13 | Umtiza | Fabaceae | 54.0 ± 3.4 (85) | 38.38 | 1 (1) | A monotypic genus endemic to South Africa | |
| 14 | Vachellia: -Senegalia | Fabaceae | 30.5 ± 3.1 (85) | 16.96 | 59 (138) | African Acacias represented in two genera, Senegalia and Vachellia, distributed in Africa (86) | |
| 15 | Mezoneuron: -Guilandina, -Haematoxylon, -Pterolobium | Fabaceae | 54.5 ± 0.4 (85) | 58.99 | 4 (66) | Pterolobium: Africa and Asia; Mezoneuron: Asia; Haematoxylon: Central America, South America, and Africa; Guilandina: pantropical (87) | Reported age is Casalpinioid crown node (85) |
| 16 | Gymnosporia: -Putterlickia, -Gloveria | Celastraceae | 12.83 (69) | 10.28 | 27 (59) | African clade monophyletic (88, 89) | Total species no. for monophyletic African clade |
| 17 | Phyllanthus | Phyllanthaceae | 2.05 (69) | 1.75 | 6 (800) (90) | Phylanthus part of a large clade mostly endemic to Madagascar but Phylanthus reticulatus from Sri Lanka (91) | Total no. of species for the whole genus |
| 18 | Phyllanthus | Phyllanthaceae | 5.87 (69) | 5.37 | 6 (800) (90) | Total no. of species for the whole genus | |
| 19 | Erythrococca | Euphorbiaceae | 6.57 (69) | 8.55 | 3 (41) | The clade is distributed in eastern and southern Africa from Kenya to Namibia (92) | |
| 20 | Euphorbia | Euphorbiaceae | 11 (93) | 8.28 | 7 (250) (93) | African clade (93) | |
| 21 | Euphorbia | Euphorbiaceae | 10 (93) | 16.85 | 7 (250) (93) | African clade (93) | |
| 22 | Adenia | Passifloraceae | 8.73 (94) | 12.81 | 3 (40) | African clade (94) | |
| 23 | Dovyalis | Salicaceae | — | — | 6 (14) | 15 Species, 14 in Africa and 9 southern African species | Total species no. for African species only |
| 24 | Scolopia | Salicaceae | — | — | 3 (5) | Genus includes 40 species of trees and shrubs occurring in Africa, Comores, Mascarenes, Madagascar, Malaysia, and Australia (95), 4 species from southern African | Total species no. for South African species only |
| 25 | Flacourtia | Salicaceae | — | — | 1 (2) | A paleotropical genus with about 10 species, 2 in Africa, 1 of them in East Africa and extending to tropical Asia and Malesia (96) | |
| 26 and 27 | Terminalia | Combretaceae | 17 (97) | 18.91 | 10 (28) | African Terminalia clade (97) | Total species no. for African species only (98) |
| 28 | Hibiscus | Malvaceae | 6 (99) | 21.65 | 4 (100) | Originated in Africa and likely dispersed to Madagascar, then India, and southeastern Asia (100) | |
| 29 | Azima | Salvadoraceae | — | — | 1 (2) | Widespread distribution | |
| 30 | Cadaba aphylla | Capparaceae | 17.65 (69) | 10.91 | 2 (30) | African clade (101) | |
| 31 | Capparis | Capparaceae | 23 (102) | 26.92 | 5 (110) | African clade (102, 103) | Total species no. for OW clade only (104); age from European fossil (105) |
| 32 and 34 | Commiphora | Burseraceae | 10.26 (69) | 7.13 | 20 (190) | Commiphora seems to have dispersed and radiated within continental Africa during the Middle Eocene (106) | Spiny Commiphora nested within nonspiny clade |
| 35 and 36 | Searsia | Anacardiaceae | 35 (111) | Predominantly southern Africa but extending to Arabian Peninsula (107) | Crown age for Searsia 11.4 My (108) | ||
| 37 | Zanthoxylum | Rutaceae | 11.78 (69) | 12.75 | 7 (250) (109) | Pantropical (109) | |
| 38 | Ximenia | Olacaceae | 23.7 (69) | 37.86 | 2 (8) | Tropical | |
| 39 | Pisonia and Phaeoptilum | Nyctanginaceae | 32.7 (69) | 31.53 | Pisonia: 1 (40); Phaeoptilum: 1 (1) | Pisonia: tropical America; Phaeoptilum: southwest Africa | Pisonia is paraphyletic, with Phaeoptilum and other genera derived within |
| 40 | Didelta | Asteraceae | — | 1 (2) | Southwest Africa (110) | ||
| 41 | Cassinopsis | Icacinaceae | 2 (11) | Southern Africa and Madagascar (111) | |||
| 42 | Solanum: -Lycium | Solanaceae | 20.98 ± 2.28 (112) | 15.11 | Solanum: 5 (1,325); Lycium: 6 (80) (112) | Solanum: subcosmopolitan; Lycium: warm tropical (112) | |
| 43 | Sesamothamnus | Pedaliceae | — | — | 1 (6) | Africa, drylands southwest and northeast (72) | |
| 44 | Rhigozum | Bignoniaceae | — | — | 2 (7) | Northeast and South Africa (71) | |
| 45 | Barleria | Acanthaceae | 8.55 (69) | 8.3 | 2 (300) | Tropical (72) | |
| 46 | Strychnos | Loganiaceae | — | — | 2 (4) (113) | African clade of four species (113) | Genus age: 12.72 My (114) |
| 47 | Strychnos | Loganiaceae | — | — | 1 (8) | Clade mostly from Asia (114) | Genus age: 12.72 My (114) |
| 48 | Carissa | Apocynaceae | 9.5 (69) | 11.99 | 6 (35) | African and Asian/Australian (115) | |
| 49 | Pachypodium | Apocynaceae | 1.1 (69) | 14.49 | 2 (5) | Two clades (total of five species) comprising all African species thought monophylletic (116,117) | |
| 50 | Catunaregam | Rubiaceae | 2.26 (69) | 9.78 | 3 (15) | African clade (118) | |
| 51 | Hyperacanthus | Rubiaceae | 1.74 (69) | 16.02 | 2 (5) | African clade (118) | |
| 52 | Canthium | Rubiaceae | 1.96 (69) | 4.72 | 6 (45) | African clade (118, 119) | |
| 53 | Pyrostria | Rubiaceae | — | — | 2 (80) | Madagascan origins with dispersal to Africa (119) | |
| 54 | Vangueria | Rubiaceae | 1.21 (69) | 5.54 | 11 (61) | African clade (119) | |
| 55 | Psydrax | Rubiaceae | 21.75 (120) | 9.32 | 3 (81) | Historical biogeography unclear (119, 120) |
Reported ages are from the literature for clades most closely matching clades in our analysis. In some cases, there is not an exact match between clades, because taxonomic sampling differs between studies, and we only include African lineages, but they provide a reasonable approximation for contrasting estimates of divergence times. NW, New World; OW, Old World.
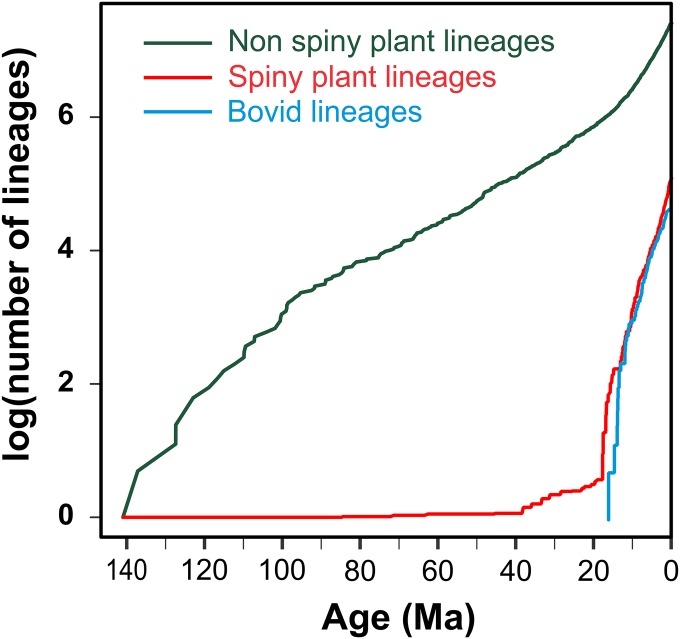
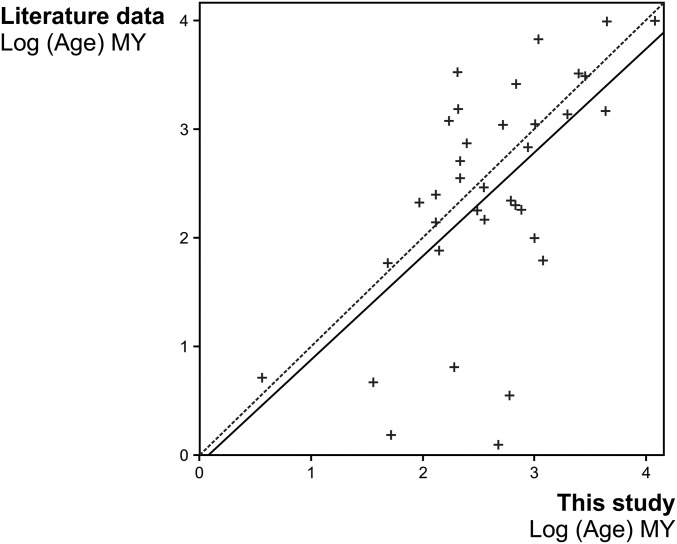
Accumulation of lineages of spiny trees and bovids (data from ref. 24) (both bovid and tree phylogenies have multiple calibrations by fossils) is remarkably congruent in time (Fig. 4). The origin and timing in acceleration of bovid lineage diversification match closely to the increase in spiny plant lineages with present day descendants in African savanna. The suggestion of some apparently older dates for the origin of spines, perhaps as far back as 40 Ma, reflects uncertainty in character states deeper in time and the fact that some spiny taxa are nested within non-African clades (i.e., we are missing their close relatives) (Fig. S2 and Table S5). Our sampling of plant lineages only encompasses woody tree species with southern African distributions but is relatively complete for spiny taxa, and we have reasonable confidence that we are not missing species-rich spiny lineages with earlier origins.
Fig. 4.
Lineage accumulation through time of bovids and spiny and nonspiny southern African woody species. Information for the bovid lineages is sourced from ref. 24. Plant lineage richness for spiny and nonspiny taxa was estimated as the sum of the number of lineages multiplied by their probability for the respective character state.
Fig. S2.
Comparison of ages estimated in this study and previously published studies. Table S5 shows data sources. The dotted line represents no difference between estimations of ages in this study and the literature (slope = 1; intercept = 0); the solid line is the regression line of the linear model (slope = 0.95; intercept = −0.08). The intercept is not significantly different from zero (P value = 0.89). The ages estimated in our analyses do not differ significantly (paired t test; mean of the differences = −0.34 My; P value = 0.84) from ages estimated in the literature.
Discussion
Are Spines a Good Proxy for Mammal Presence in Contemporary Savannas?
We analyzed three linked explanations for the present day distribution of spinescent species in Africa: (i) growing conditions in which spines are more effective, (ii) carbon costs, and (iii) mammal herbivore pressure. In our analyses, spiny communities are associated with more arid and nutrient-rich savannas. These communities are the “eutrophic” savannas of Africa (25). These environments support high mammal biomass (23, 25) and high browser diversity (26). Spinescent species are rare in forest and negatively associated with its mammalian fauna. In contrast, open savannas have a high proportion of spiny species, suggesting that the costs of structural defenses (27) may be incompatible with low light levels found in forests. Our results constitute evidence at large spatial scales that the abundance of mammals is the factor best related to the distribution of spiny communities. Our results are not explained by covarying abiotic environmental factors or vegetation types and remain significant, even after controlling for these factors. Additionally, phylogenetic analyses show that herbivory has selected for the evolution of structural defenses in multiple woody plant lineages.
Using the functional grouping of mammal herbivores (23), we found that “medium-sized social mixed feeders” are the functional type most closely associated with spinescence. This group of species generally grazes more during the wet season and browses more in the dry season. Mixed feeding has several consequences. First, high-quality new grass growth can support large herds of mixed feeders during the wet season, which then amplifies their selective effect on trees when these herds switch to browsing during the dry season; second, mixed feeder impacts on plant populations should be stronger where dry seasons are longer, as suggested by our results that show spinescence to be associated with more arid savannas.
Bovids and Spiny Trees: A Coevolutionary Relationship?
Patterns of convergent evolution provide a test of adaptation between herbivores and plant defenses (28). We show that spinescence evolved independently multiple times in the phylogeny of African trees and many different families and was achieved by three different developmental pathways: stipular spines, epidermic prickles, and thorns. Character optimization on the dated phylogeny indicates that nearly all of the modern spiny species evolved since the early Miocene.
Before the Miocene, spinescence evolution could have been limited by unfavorable physical environmental conditions or their ineffectiveness against modes of feeding of early Cenozoic African mammals. The rarity of spines in modern forests could imply that a more forested environment in the Paleogene may have inhibited spine development. However, this argument is contradicted by the presence in Africa of thicket from the early Eocene (29). Thicket is a low, dense woody vegetation with intermediate light level between forests and savannas that supports a high diversity of spiny trees (29). The early appearance of thicket and the much later appearance of spiny plants in our analysis, thus, suggest that the evolution of spinescence is linked to the arrival of bovids in Africa during the Neogene.
Structural defenses vary with mode of feeding: for example, defenses against large bird browsers are quite different from those of “mammal” browsers (30). However, there are few studies of variation in defense structures in response to different modes of mammal feeding. Africa was an island continent from the beginning of the Cenozoic with an endemic African fauna, the Afrotheria, including the extinct rhinoceros-like embrithopods, Hyracoidea, and Proboscidea (of which hyraxes and elephants, respectively, are the sole extant examples) (16). Hyracoids were diverse, ranging in size from small rabbits to rhinoceros, and the ecological analogs of perissodactyls and artiodactyls on other continents (16). Apparently, none of these lineages selected for spines as a plant defense. Modern elephants, the last of the proboscids, have been documented as destroying stands of spiny tree species (31). A land bridge developed between Africa and Eurasia in the late Oligocene/Miocene, and Eurasian mammals (rhinoceros and suids) entered Africa for the first time. By mid-Miocene (16 Ma), there was a second invasion of Eurasian elements, including horned bovids and antlered giraffoids, with another wave of Eurasian immigrants in the late Miocene (16).
The diversification of bovids (24) from mid-Miocene closely matches the rapid accumulation of spiny plant lineages in African savanna (Fig. 4), suggesting that bovids presented a novel mode of feeding that spread in Africa after the Miocene and selected for the evolution of spinescence. Interactions between trees and mammals might have resulted in niche diversification for both groups. This suggestion is consistent with contemporary patterns of diversity identified for Acacia species (sensu lato; i.e., Vachellia and Senegalia) and mammalian herbivores (26). Several processes could be involved in niche diversification: (i) segregation of niches in height: herbivores have distinct feeding strategies (bite size and tolerated fiber content) depending on their body size (32), and the vertical deployment of spines on plant species has been shown to match the body size of herbivores present (33); (ii) niche specialization: mammals that browse more have narrower muzzles, longer tongues, and prehensile lips, allowing them to handle thorny plants better than grazers (22); and (iii) segregation of niches in time for trees: browser impacts vary with season, differentially affecting evergreen and deciduous tree species (34, 35).
The second functional type of herbivores related to spinescence (“large browsers”) in modern Africa includes giraffes and okapi, two members of the Giraffidae family. Fossil giraffids were formerly more diverse and are known since the middle Miocene in Africa (16). Although not as abundant or diverse as bovids, giraffids might also have played a role in selecting for spinescence.
Bovids and the Spread of Savannas.
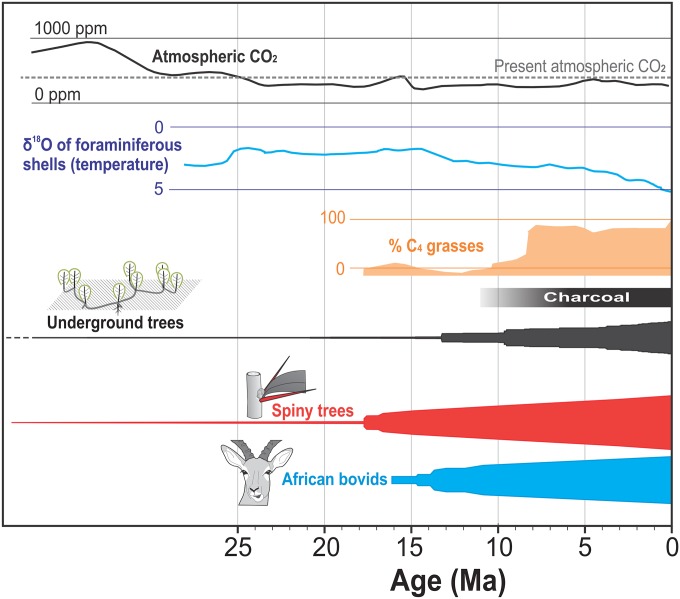
The timing of increase in spinescence, which we have shown to associate with savannas, provides a proxy for dating the spread of mammal-dominated savannas independent of isotopic evidence (36, 37). The rise of savanna has been attributed to increased aridity promoting grasses over trees (4) or a drop in CO2 concentrations promoting the spread of C4 grasses (Fig. 5). Although these two factors define the climatic envelope suitable for grasses, they do not account for the exclusion of forests that could be supported along the precipitation gradient in Africa (38, 39). Simulations illustrate well that aridity and low CO2 are not enough to explain the spread of savannas; an additional force opening up forests is needed (40). Fire is one likely causative agent (9, 41). Here, we have shown that herbivory pressure might provide another pathway. Our results indicate that the influx of bovids triggered savanna formation from the mid-Miocene, long before its explosive spread because of fire in the late Miocene. The precise dating of this ecological transition is difficult: molecular dating could overestimate divergence ages, and the use of fossils most often underestimates them (42). This problem is especially true in the African context, where the fossil record for trees and mammals is patchy and restricted to few localities that poorly describe the full ecological spectrum of the continent (4, 16, 43). However, we can compare relative dating using phylogenetic analysis of spines as a marker of mammal-dominated savannas and geoxylic suffrutices as markers of fire-dominated savannas (12). The same woody plant phylogeny shows an accumulation of spiny plant lineages in Africa several million years earlier than the appearance of geoxyles, suggesting that mammal-dominated savannas predate fire-dominated savannas by millions of years (Fig. 5). Moreover, savannas maintained by fire and herbivory seem to be favored in contrasting environmental settings: fire-dominated (dystrophic) savannas on seasonally humid and nutrient-poor environments vs. herbivore-dominated (eutrophic) savannas on arid and nutrient-rich environments (7, 23, 25). If these two types of savannas occurred elsewhere in the world, then Pleistocene mammal extinctions may only have had limited effects on releasing fire as an agent and then, only in eutrophic, semiarid savannas.
Fig. 5.
Potential factors responsible for the rise of savannas. Percentages of C4 grasses reconstructed from δ13C of tooth enamel of mammalian herbivores (49), atmospheric CO2 measured from Antarctic ice cores (50), temperature inferred from 18O levels in foraminiferous shells in marine sediments (51), and charcoal abundance from marine sediments (9). For underground trees (12), spiny trees, and African bovids (24), line widths are proportional to the log of lineage numbers.
The structure of the first savannas is intriguing: were they similar to modern savannas but with a C3 grass layer (1, 4), or were they carved out of ancient thickets as browse pressure increased? How do the distinct histories of browsing on other continents relate to the evolution of structural defenses? Answers to these questions require a better understanding of the interactions between photosynthetic constraints and efficacy of defense and of how structural defenses vary with different modes of feeding.
Materials and Methods
Taxon Sampling and Plant Distribution Data.
We sampled a total of 1,852 (of ∼2,200) woody plant species in southern Africa from 127 families and 651 genera, including 213 spiny taxa. Species names were extracted from the African plants database (www.ville-ge.ch/cjb/) and cross-checked against The Plant List (www.theplantlist.org/). Species were defined as spinescent if they had hard sharp-pointed structures developed from modified epidermis (prickles), modified stipules (stipular spines), or lignification of the apex of a stem (thorns) (Fig. S1).
Locality records for each species were extracted from the African plants database (www.ville-ge.ch/cjb/) and supplemented with records from the Naturalis Biodiversity Center (www.naturalis.nl/nl/) and the Global Biodiversity Information Facility (www.gbif.org). All records were thoroughly checked, and those with points falling in the sea, inverted latitude/longitude, or duplicate records were removed. Genera with fewer than 10 records were also excluded from the analysis. Species distribution models were constructed using MaxEnt (details are in SI Materials and Methods).
Environmental Variables.
Environmental variables were derived from WorldClim (44) (mean annual rainfall and temperature), the Food and Agriculture Organization of the United Nations (www.fao.org/home/en/; soil nutrient status “SQ1”), and the Global Fire Emissions Database (45) (annual mean burn percentage for 1997–2009) and downscaled to a 1° × 1° grid resolution. Vegetation classifications were based on major vegetation types and mosaics for Africa by White (46). The original 80-level vegetation mapping units scheme by White (46) was simplified to 15 vegetation types based on the relative predominance of plant growth forms and functional groups (Figs. 1 and 2).
Large mammal herbivore functional types and biomass surfaces were obtained from the work in ref. 23. Herbivore functional types were based on hierarchical cluster analysis of five species-level traits (body mass, diet, gut type, herd size, and water dependence) for 92 African herbivore species. Biomass surfaces were created from spatially explicit species-level historical biomass reconstructions (∼1,000 y ago) produced from models of protected area census data in relation to rainfall, soil, and vegetation.
Phylogenetic Reconstruction and Dating.
Phylogenetic reconstruction and dating for trees follow the work in ref. 12 (details are in SI Materials and Methods). Phylogenetic reconstruction for bovids is from the work in ref. 24, and it is a dated phylogeny based on the full mitochondrial genome and calibrated with 16 fossils.
Statistical Analyses.
We first investigated environmental factors that correlate with the spatial distribution of spinescence to (i) identify the group of herbivores most associated spatially with the presence of spiny vegetation, (ii) reveal the environmental factors favoring spinescence, and (iii) explore the interaction between abiotic environment, spiny species, and herbivory.
Variables from the three datasets—herbivore densities, abiotic factors, and vegetation types—were standardized and analyzed using canonical correspondence analyses (CCAs). Stepwise selection in CCAs (permutation under reduced model) (47) was applied to each dataset separately using a stopping criterion of 0.05. We then used variation partitioning to estimate the unique and joint effects of herbivores, abiotic environment, and vegetation type on the proportion of spiny species. CCAs and partial CCAs were used with three partitions, and the significance of testable fractions was evaluated using permutation tests (9,999 permutations; α < 0.05) (47). For each of these analyses, adjusted R2 values provided unbiased estimates of the variation explained by the fractions (48). Venn diagrams were used to illustrate the results of variation partitioning.
Phylogenetic logistic regression was used to assess the extent to which spines are associated with particular environments and forms of herbivory pressure across plant lineages. Species-specific climate and herbivory scores were obtained by averaging each of four environmental variables (mean annual rainfall, mean annual temperature, soil nutrient status, and percentage burned area) and the biomass of five herbivore functional types (small nonsocial browsers, medium-sized social mixed feeders, large browsers, water-dependent grazers, and nonruminants) across their distribution range. Because of the strong correlations among variables, principal components analysis was performed to obtain orthogonal axes for inclusion in the subsequent regression analysis. Spinescence, coded as spiny = 1 and nonspiny = 0, was then modeled using logistic regression with the first three principal components (87.5% of total variation) (Table S1) fitted as explanatory variables and the nonindependence among residuals informed by the phylogenetic tree (phyloglm using maximum penalized likelihood estimate; R Statistical Software). Model selection was based on Akaike information criterion scores, with only significant predictor variables described. Variable loadings on principle components (Table S2) were used to gauge environmental and herbivory effects on spinescence. All analyses were conducted in the following R packages: FactomineR, vegan, packfor, and phylolm.
Spatial autocorrelation in the residuals accounted for only a very small part of the total variation in both our spatial analyses (Mantel correlation = 0.0015) and phylogenetic logistic regressions (cumulated Mantel correlation always smaller than 0.04) (Table S3); we, therefore, did not fit an explicit description of spatial structure into the models.
SI Materials and Methods
Species Distribution Models.
Species distribution models were constructed using MaxEnt (52) enforcing eigenvector-based spatial filtering in SAM (53) to impose dispersal constraints in the spatial models (54). Filters were resampled to a resolution of 5 min, and we included the first 14 spatial filters as predictor variables along with 19 bioclimatic variables from the WorldClim (43) following the work in ref. 54. We used the equal training sensitivity and specificity threshold (55) to create presence–absence maps using the presence-only species data and 10,000 background points. Model performance was evaluated using the area under the receiver operator curve (56). Distribution maps were aggregated at a resolution of 1° × 1°.
Phylogenetic Reconstruction and Dating.
An initial maximum likelihood analysis was conducted on a combined dataset of DNA bacording regions (rbcLa: 552 bp; matK: 942 bp) (57) using RAxML-HPC2 v.7.2.6 (58) on the CIPRES cluster (www.phylo.org/sub_sections/portal/) and enforcing topological constraints assuming the APG III backbone from Phylomatic v.3 (59). First, the RAxML starting tree was adjusted, so that branch lengths satisfied all fossil prior constraints using PATHd8 v.1.0 (60). Second, we assumed an uncorrelated log-normal model for rate variation among branches and the generalized time-reversible + I + Γ model of sequence evolution for each partition based on the Akaike information criterion (AIC) evaluated using Modeltest v.2.3 (61). The phylogeny was rooted using representatives of Acrogymnospermae (62, 63), and branch lengths were calibrated in millions of years using a Bayesian Markov chain Monte Carlo (MCMC) approach implemented in BEAST v.1.4.8 (64). We used 20 fossil calibration points (65) as minimum age constraints on the stem node of each group, except for the crown Eudicots, which were set at 124 My, with a log-normal distribution (66). We performed four independent MCMC runs, each for 100 million generations and sampling every 1,000 generations. We assessed the MCMC log files for convergence using the effective sample size (ESS) statistics in Tracer v.1.5 (64). ESS values were all >100, indicating that the posterior estimates were not unduly influenced by autocorrelation. We combined the resulting tree files from the four runs in LogCombiner v.1.7.5 (64), downsampling 1 in 20,000 trees and discarding the first 25% trees as burn in. The maximum clade consensus tree, with means and 95% highest posterior density intervals, was generated with TreeAnnotator v.1.7.5 (64). Voucher specimen information and GenBank accession numbers are listed on the BOLD DataSystem (www.boldsystems.org) and in Table S5. We inferred probability of the ancestral lineages being spiny using stochastic character mapping (66, 67) assuming a symmetrical transition rate matrix. The reconstructed estimates were only used for illustration (Fig. 3). We explored temporal patterns of lineage formation using lineage through time plots for the following groups: all woody species, spinescent species, and African browsers.
Supplementary Material
Acknowledgments
We thank Sally Archibald for providing data about fire frequencies and Norman Owen-Smith for comments. We thank the Government of Canada through Genome Canada and the Ontario Genomics Institute (2008-OGI-ICI-03), the International Development Research Centre (Canada), the University of Johannesburg Analytical Facility (South Africa), the South African National Research Foundation, and the Royal Society (United Kingdom) for financial support and various local and international authorities who granted plant collection permits. T.C.-D., G.P.H., and W.J.B. thank the Mellon Foundation, the Claude Leon Foundation, and the National Research Foundation for financial support.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper are available in Dataset S1 and at www.boldsystems.org/, www.ncbi.nlm.nih.gov/genbank/, www.ville-ge.ch/cjb/, www.naturalis.nl/nl/, www.gbif.org/, worldclim.org/, climate.geog.udel.edu/∼climate/, www.fao.org/home/en/, www.globalfiredata.org/, and modis.gsfc.nasa.gov/.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607493113/-/DCSupplemental.
References
- 1.Edwards EJ, et al. C4 Grasses Consortium The origins of C4 grasslands: Integrating evolutionary and ecosystem science. Science. 2010;328(5978):587–591. doi: 10.1126/science.1177216. [DOI] [PubMed] [Google Scholar]
- 2.Ehleringer JR, Cerling TE, Helliker BR. C4 photosynthesis, atmospheric CO2, and climate. Oecologia. 1997;112(3):285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- 3.Bond WJ, Midgley GF. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Philos Trans R Soc Lond B Biol Sci. 2012;367(1588):601–612. doi: 10.1098/rstb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strömberg CA. Decoupled taxonomic radiation and ecological expansion of open-habitat grasses in the Cenozoic of North America. Proc Natl Acad Sci USA. 2005;102(34):11980–11984. doi: 10.1073/pnas.0505700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang ZH, Ding ZL. A palynological insight into the Miocene aridification in the Eurasian interior. Palaeoworld. 2013;22(3):77–85. [Google Scholar]
- 6.Hirota M, Holmgren M, Van Nes EH, Scheffer M. Global resilience of tropical forest and savanna to critical transitions. Science. 2011;334(6053):232–235. doi: 10.1126/science.1210657. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann CE, Archibald SA, Hoffmann WA, Bond WJ. Deciphering the distribution of the savanna biome. New Phytol. 2011;191(1):197–209. doi: 10.1111/j.1469-8137.2011.03689.x. [DOI] [PubMed] [Google Scholar]
- 8.Staver AC, Archibald S, Levin SA. The global extent and determinants of savanna and forest as alternative biome states. Science. 2011;334(6053):230–232. doi: 10.1126/science.1210465. [DOI] [PubMed] [Google Scholar]
- 9.Keeley JE, Rundel PW. Fire and the Miocene expansion of C4 grasslands. Ecol Lett. 2005;8(7):683–690. [Google Scholar]
- 10.Hoetzel S, Dupont L, Schefuß E, Rommerskirchen F, Wefer G. The role of fire in Miocene to Pliocene C4 grassland and ecosystem evolution. Nat Geosci. 2013;6(12):1027–1030. [Google Scholar]
- 11.Simon MF, et al. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc Natl Acad Sci USA. 2009;106(48):20359–20364. doi: 10.1073/pnas.0903410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurin O, et al. Savanna fire and the origins of the ‘underground forests’ of Africa. New Phytol. 2014;204(1):201–214. doi: 10.1111/nph.12936. [DOI] [PubMed] [Google Scholar]
- 13.Owen-Smith N. Pleistocene extinctions: The pivotal role of megaherbivores. Paleobiology. 1987;13(3):351–362. [Google Scholar]
- 14.Beschta RL, Ripple WJ. Recovering riparian plant communities with wolves in northern Yellowstone, USA. Restor Ecol. 2010;18(3):380–389. [Google Scholar]
- 15.Bakker ES, et al. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc Natl Acad Sci USA. 2016;113(4):847–855. doi: 10.1073/pnas.1502545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werdelin L, Sanders WJ. Cenozoic Mammals of Africa. Univ of California Press; Berkeley, CA: 2010. [Google Scholar]
- 17.Burney DA, Robinson GS, Burney LP. Sporormiella and the late Holocene extinctions in Madagascar. Proc Natl Acad Sci USA. 2003;100(19):10800–10805. doi: 10.1073/pnas.1534700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 2009;326(5956):1100–1103. doi: 10.1126/science.1179504. [DOI] [PubMed] [Google Scholar]
- 19.Rule S, et al. The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science. 2012;335(6075):1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 20.Bouchenak-Khelladi Y, et al. The origins and diversification of C4 grasses and savanna‐adapted ungulates. Glob Chang Biol. 2009;15(10):2397–2417. [Google Scholar]
- 21.Grubb PJ. A positive distrust in simplicity—lessons from plant defences and from competition among plants and among animals. J Ecol. 1992;80(4):585–610. [Google Scholar]
- 22.Shipley LA. The influence of bite size on foraging at larger spatial and temporal scales by mammalian herbivores. Oikos. 2007;116(12):1964–1974. [Google Scholar]
- 23.Hempson GP, Archibald S, Bond WJ. A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Science. 2015;350(6264):1056–1061. doi: 10.1126/science.aac7978. [DOI] [PubMed] [Google Scholar]
- 24.Bibi F. A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics. BMC Evol Biol. 2013;13(1):166. doi: 10.1186/1471-2148-13-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholes RJ. The influence of soil fertility on the ecology of southern African dry savannas. J Biogeogr. 1990;17(4/5):415–419. [Google Scholar]
- 26.Greve M, et al. Continental-scale variability in browser diversity is a major driver of diversity patterns in acacias across Africa. J Ecol. 2012;100(5):1093–1104. [Google Scholar]
- 27.Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst. 2007;4(8):157–178. [Google Scholar]
- 28.Agrawal AA. Current trends in the evolutionary ecology of plant defence. Funct Ecol. 2011;25(2):420–432. [Google Scholar]
- 29.Cowling RM, Procheş Ş, Vlok JH, van Staden J. On the origin of southern African subtropical thicket vegetation. S Afr J Bot. 2005;71(1):1–23. [Google Scholar]
- 30.Bond WJ, Silander JA. Springs and wire plants: Anachronistic defences against Madagascar’s extinct elephant birds. Proc Biol Sci. 2007;274(1621):1985–1992. doi: 10.1098/rspb.2007.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor TG, Page BR. Simplification of the composition, diversity and structure of woody vegetation in a semi-arid African savanna reserve following the re-introduction of elephants. Biol Conserv. 2014;180:122–133. [Google Scholar]
- 32.Wilson SL, Kerley GI. Bite diameter selection by thicket browsers: The effect of body size and plant morphology on forage intake and quality. For Ecol Manage. 2003;181(1):51–65. [Google Scholar]
- 33.Burns KC. Are there general patterns in plant defence against megaherbivores? Biol J Linn Soc Lond. 2014;111(1):38–48. [Google Scholar]
- 34.Bryant JP, Reichardt PB, Clausen TP. Chemically mediated interactions between woody plants and browsing mammals. J Range Manage. 1992;45(1):18–24. [Google Scholar]
- 35.Massei G, Hartley SE, Bacon PJ. Chemical and morphological variation of Mediterranean woody evergreen species: Do plants respond to ungulate browsing? J Veg Sci. 2000;11(1):1–8. [Google Scholar]
- 36.Ségalen L, Lee-Thorp JA, Cerling T. Timing of C4 grass expansion across sub-Saharan Africa. J Hum Evol. 2007;53(5):549–559. doi: 10.1016/j.jhevol.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 38.Sankaran M, et al. Determinants of woody cover in African savannas. Nature. 2005;438(7069):846–849. doi: 10.1038/nature04070. [DOI] [PubMed] [Google Scholar]
- 39.Bond WJ. What limits trees in C4 grasslands and savannas? Annu Rev Ecol Evol Syst. 2008;39(1):641–659. [Google Scholar]
- 40.Scheiter S, et al. Fire and fire-adapted vegetation promoted C4 expansion in the late Miocene. New Phytol. 2012;195(3):653–666. doi: 10.1111/j.1469-8137.2012.04202.x. [DOI] [PubMed] [Google Scholar]
- 41.Beerling DJ, Osborne CP. The origin of the savanna biome. Glob Chang Biol. 2006;12(11):2023–2031. [Google Scholar]
- 42.Marshall CR. The fossil record and estimating divergence times between lineages: Maximum divergence times and the importance of reliable phylogenies. J Mol Evol. 1990;30(5):400–408. doi: 10.1007/BF02101112. [DOI] [PubMed] [Google Scholar]
- 43.Bibi F, et al. The fossil record and evolution of Bovidae: State of the field. Palaeontol Electronica. 2009;12(3):1–11. [Google Scholar]
- 44.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965–1978. [Google Scholar]
- 45.van der Werf GR, et al. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997-2009) Atmos Chem Phys. 2010;10(23):11707–11735. [Google Scholar]
- 46.White F. Vegetation of Africa—A Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa. UNESCO; Paris: 1983. [Google Scholar]
- 47.Borcard D, Gillet F, Legendre P. Numerical Ecology with R. Springer; New York: 2011. [Google Scholar]
- 48.Peres-Neto PR, Legendre P, Dray S, Borcard D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology. 2006;87(10):2614–2625. doi: 10.1890/0012-9658(2006)87[2614:vposdm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Cerling TE, Harris JM, Ambrose SH, Leakey MG, Solounias N. Dietary and environmental reconstruction with stable isotope analyses of herbivore tooth enamel from the Miocene locality of Fort Ternan, Kenya. J Hum Evol. 1997;33(6):635–650. doi: 10.1006/jhev.1997.0151. [DOI] [PubMed] [Google Scholar]
- 50.Beerling DJ, Royer DL. Convergent cenozoic CO2 history. Nat Geosci. 2011;4(7):418–420. [Google Scholar]
- 51.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 52.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190(3):231–259. [Google Scholar]
- 53.Rangel TF, Diniz‐Filho JA, Bini LM. SAM: A comprehensive application for spatial analysis in macroecology. Ecography (Cop.) 2010;33(1):46–50. [Google Scholar]
- 54.Blach‐Overgaard A, Svenning JC, Dransfield J, Greve M, Balslev H. Determinants of palm species distributions across Africa: The relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography (Cop.) 2010;33(2):380–391. [Google Scholar]
- 55.Liu C, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distributions. Ecography (Cop.) 2005;28(3):385–393. [Google Scholar]
- 56.Bewick V, Cheek L, Ball J. Statistics review 13: Receiver operating characteristic curves. Crit Care. 2004;8(6):508–512. doi: 10.1186/cc3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CBOL Plant Working Group A DNA barcode for land plants. Proc Natl Acad Sci USA. 2009;106(31):12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 2008;57(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 59.Webb CO, Donoghue MJ. Phylomatic: Tree assembly for applied phylogenetics. Mol Ecol Notes. 2005;5(1):181–183. [Google Scholar]
- 60.Britton T, Anderson CL, Jacquet D, Lundqvist S, Bremer K. Estimating divergence times in large phylogenetic trees. Syst Biol. 2007;56(5):741–752. doi: 10.1080/10635150701613783. [DOI] [PubMed] [Google Scholar]
- 61.Nylander JAA. Modeltest v2. Uppsala Univ; Uppsala, Sweden: 2004. [Google Scholar]
- 62.Cantino PD, et al. Towards a phylogenetic nomenclature of Tracheophyta. Taxon. 2007;56(3):822–846. [Google Scholar]
- 63.Soltis DE, et al. Angiosperm phylogeny: 17 genes, 640 taxa. Am J Bot. 2011;98(4):704–730. doi: 10.3732/ajb.1000404. [DOI] [PubMed] [Google Scholar]
- 64.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7(1):214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bell CD, Soltis DE, Soltis PS. The age and diversification of the angiosperms re-revisited. Am J Bot. 2010;97(8):1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- 66.Bollback JP. SIMMAP: Stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics. 2006;7(1):88. doi: 10.1186/1471-2105-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Revell LJ. phytools: An R package for phylogenetic comparative biology (and other things) Methods Ecol Evol. 2012;3(2):217–223. [Google Scholar]
- 68.Zanne AE, et al. Three keys to the radiation of angiosperms into freezing environments. Nature. 2014;506(7486):89–92. doi: 10.1038/nature12872. [DOI] [PubMed] [Google Scholar]
- 69.Sands MJS. In: Flora of Tropical East Africa: Balanitaceae. Beentje HJ, editor. A. A. Balkema; Rotterdam: 2003. [Google Scholar]
- 70.Sheahan MC, Chase MW. Phylogenetic relationships within Zygophyllaceae based on DNA sequences of three plastid regions, with special emphasis on Zygophylloideae. Syst Bot. 2000;25(2):371–384. [Google Scholar]
- 71.Sarraf P. 2014 Unraveling the Evolutionary History of a Cosmopolitan Plant Genus: Phylogeny and Biogeography of Maclura (Moraceae). Available at www.thenurj.com/phylogeny-and-biogeography-of-maclura/. Accessed August 17, 2016.
- 72.Mabberley DJ. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications and Uses. 3rd Ed Cambridge Univ Press; Cambridge, UK: 2008. [Google Scholar]
- 73.Onstein RE, Carter RJ, Xing Y, Richardson JE, Linder HP. Do Mediterranean-type ecosystems have a common history?--insights from the Buckthorn family (Rhamnaceae) Evolution. 2015;69(3):756–771. doi: 10.1111/evo.12605. [DOI] [PubMed] [Google Scholar]
- 74.Richardson JE, Chatrou LW, Mols JB, Erkens RH, Pirie MD. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philos Trans R Soc Lond B Biol Sci. 2004;359(1450):1495–1508. doi: 10.1098/rstb.2004.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Islam MB, Simmons MP. A thorny dilemma: Testing alternative intrageneric classifications within Ziziphus (Rhamnaceae) Syst Bot. 2006;31(4):826–842. [Google Scholar]
- 76.Hauenschild F, et al. Analysis of the cosmopolitan buckthorn genera Frangula and Rhamnus sl supports the description of a new genus, Ventia. Taxon. 2016;65(1):65–78. [Google Scholar]
- 77.Forest F, Nänni I, Chase MW, Crane PR, Hawkins JA. Diversification of a large genus in a continental biodiversity hotspot: Temporal and spatial origin of Muraltia (Polygalaceae) in the Cape of South Africa. Mol Phylogenet Evol. 2007;43(1):60–74. doi: 10.1016/j.ympev.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 78.Li H, et al. Diversification of the phaseoloid legumes: Effects of climate change, range expansion and habit shift. Front Plant Sci. 2013;4:386. doi: 10.3389/fpls.2013.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruneau A. Phylogenetic and biogeographical patterns in Erythrina (Leguminosae: Phaseoleae) as inferred from morphological and chloroplast DNA characters. Syst Bot. 1996;21(4):587–604. [Google Scholar]
- 80.Pennington RT, Richardson JE, Lavin M. Insights into the historical construction of species-rich biomes from dated plant phylogenies, neutral ecological theory and phylogenetic community structure. New Phytol. 2006;172(4):605–616. doi: 10.1111/j.1469-8137.2006.01902.x. [DOI] [PubMed] [Google Scholar]
- 81.Vatanparasta M, et al. First molecular phylogeny of the pantropical genus Dalbergia: Implications for infrageneric circumscription and biogeography. S Afr J Bot. 2013;89:143–149. [Google Scholar]
- 82.Bouchenak-Khelladi Y, Maurin O, Hurter J, van der Bank M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): An emphasis on African acacias. Mol Phylogenet Evol. 2010;57(2):495–508. doi: 10.1016/j.ympev.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 83.Bouchenak-Khelladi Y, Maurin O, Hurter J, van der Bank M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): An emphasis on African acacias. Mol Phylogenet Evol. 2010;57(2):495–508. doi: 10.1016/j.ympev.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 84.Hernandez HM. Zapoteca: A new genus of neotropical Mimosoideae. Ann Mo Bot Gard. 1986;73(4):755–763. [Google Scholar]
- 85.Lavin M, Herendeen PS, Wojciechowski MF. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol. 2005;54(4):575–594. doi: 10.1080/10635150590947131. [DOI] [PubMed] [Google Scholar]
- 86.Kyalangalilwa B, et al. Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations Vachellia and Senegalia. Bot J Linn Soc. 2013;172(4):500–523. [Google Scholar]
- 87.Gagnon E, Lewis GP, Sotuyo JS, Hughes CE, Bruneau A. A molecular phylogeny of Caesalpinia sensu lato: Increased sampling reveals new insights and more genera than expected. S Afr J Bot. 2013;89:111–127. [Google Scholar]
- 88.Simmons MP, et al. Phylogeny of the Celastraceae inferred from phytochrome B gene sequence and morphology. Am J Bot. 2001;88(2):313–325. [PubMed] [Google Scholar]
- 89.McKenna MJ, et al. Delimitation of the segregate genera of Maytenus s. l. (Celastraceae) based on morphological and molecular characters. Syst Bot. 2011;36(4):922–932. [Google Scholar]
- 90.Samuel R, et al. Molecular phylogenetics of Phyllanthaceae: Evidence from plastid MATK and nuclear PHYC sequences. Am J Bot. 2005;92(1):132–141. doi: 10.3732/ajb.92.1.132. [DOI] [PubMed] [Google Scholar]
- 91.Kathriarachchi H, Hoffmann P, Samuel R, Wurdack KJ, Chase MW. Molecular phylogenetics of Phyllanthaceae inferred from five genes (plastid atpB, matK, 3'ndhF, rbcL, and nuclear PHYC) Mol Phylogenet Evol. 2005;36(1):112–134. doi: 10.1016/j.ympev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Wurdack KJ, Hoffmann P, Chase MW. Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using plastid RBCL and TRNL-F DNA sequences. Am J Bot. 2005;92(8):1397–1420. doi: 10.3732/ajb.92.8.1397. [DOI] [PubMed] [Google Scholar]
- 93.Bruyns PV, Klak C, Hanáček P. Age and diversity in Old World succulent species of Euphorbia (Euphorbiaceae) Taxon. 2011;60(6):1717–1733. [Google Scholar]
- 94.Hearn DJ. Adenia (Passifloraceae) and its adaptive radiation: Phylogeny and growth form diversification. Syst Bot. 2006;31(4):805–821. [Google Scholar]
- 95.Meyer NL. Plants of Southern Africa: An Annotated Checklist. National Botanical Institute; Pretoria, South Africa: 2003. [Google Scholar]
- 96.Sleumer H. Flora of Tropical East Africa. Rijksherbarium; Leiden, The Netherlands: 1975. [Google Scholar]
- 97.Gere J, Yessoufou K, Daru BH, Maurin O. African continent a likely origin of family Combretaceae (Myrtales). A biogeographical view. Annu Res Rev Biol. 2015;8(5):1–20. [Google Scholar]
- 98.Griffith ME. A revision of the African species of Terminalia. J Linn Soc Bot. 1959;55(364):818–907. [Google Scholar]
- 99.Koopman MM, Baum DA. Phylogeny and biogeography of tribe Hibisceae (Malvaceae) on Madagascar. Syst Bot. 2008;33(2):364–374. [Google Scholar]
- 100.Wilson FD. The genome biogeography of Hibiscus L. section Furcaria DC. Genet Resour Crop Evol. 1994;41(1):13–25. [Google Scholar]
- 101.Hall JC. Systematics of Capparaceae and Cleomaceae: An evaluation of the generic delimitations of Capparis and Cleome using plastid DNA sequence data. Botany. 2008;86(7):682–696. [Google Scholar]
- 102.Inocencio C, Rivera D, Concepción Obón M, Alcaraz F, Barreña JA. A systematic revision of Capparis (Capparaceae) Ann Mo Bot Gard. 2006;93(1):122–149. [Google Scholar]
- 103.Hall JC, Sytsma KJ, Iltis HH. Phylogeny of Capparaceae and Brassicaceae based on chloroplast sequence data. Am J Bot. 2002;89(11):1826–1842. doi: 10.3732/ajb.89.11.1826. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez RR, Greuter W. A study of differentiation patterns in Capparis sect. Breyniastrum in Cuba, with a nomenclatural and taxonomic survey of Cuban Capparis (Capparaceae) Willdenowia. 2004;34(1):259–276. [Google Scholar]
- 105.Selmeier A. Capparidoxylon holleisii nov. spec., a silicified Capparis (Capparaceae) wood with insect coprolites from the Neogene of southern Germany. Zitteliana. 2005;45:199–209. [Google Scholar]
- 106.Weeks A, Daly DC, Simpson BB. The phylogenetic history and biogeography of the frankincense and myrrh family (Burseraceae) based on nuclear and chloroplast sequence data. Mol Phylogenet Evol. 2005;35(1):85–101. doi: 10.1016/j.ympev.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 107.Moffett RO. Name changes in the Old World Rhus and recognition of Searsia (Anacardiaceae) Bothalia. 2007;37(2):165–175. [Google Scholar]
- 108.Weeks A, et al. To move or to evolve: Contrasting patterns of intercontinental connectivity and climatic niche evolution in “Terebinthaceae” (Anacardiaceae and Burseraceae) Front Genet. 2014;5:409. doi: 10.3389/fgene.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Groppo M, Pirani JR, Salatino ML, Blanco SR, Kallunki JA. Phylogeny of Rutaceae based on twononcoding regions from cpDNA. Am J Bot. 2008;95(8):985–1005. doi: 10.3732/ajb.2007313. [DOI] [PubMed] [Google Scholar]
- 110.Funk VA, Chan R, Keeley SC. Insights into the evolution of the tribe Arctoteae (Compositae: subfamily Cichorioideae ss) using trnL-F, ndhF, and ITS. Taxon. 2004;53(3):637–655. [Google Scholar]
- 111.Stull GW, Duno de Stefano R, Soltis DE, Soltis PS. Resolving basal lamiid phylogeny and the circumscription of Icacinaceae with a plastome-scale data set. Am J Bot. 2015;102(11):1794–1813. doi: 10.3732/ajb.1500298. [DOI] [PubMed] [Google Scholar]
- 112.Särkinen T, Bohs L, Olmstead RG, Knapp S. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): A dated 1000-tip tree. BMC Evol Biol. 2013;13(1):214. doi: 10.1186/1471-2148-13-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frasier C. 2008. Evolution and systematics of the angiosperm order gentianales with an in-depth focus on loganiaceae and its species-rich and toxic genus Strychnos. PhD thesis (Rutgers, The State University of New Jersey, New Brunswick, NJ)
- 114.Adebowale A, Nicholas A, Lamb J, Naidoo Y. Divergence times estimates and historical biogeography of southern African Strychnos L. (Loganiaceae) S Afr J Bot. 2015;98:205 (abstr). [Google Scholar]
- 115.Stodart DW, Barker NP. The species level phylogenetic relationships of the genus Carissa L. (Apocynaceae) S Afr J Bot. 2015;98:202 (abstr). [Google Scholar]
- 116.Burge D. Diversification of Pachypodium. Cactus Succul J. 2013;85(6):250–258. [Google Scholar]
- 117.Burge DO, Mugford K, Hastings AP, Agrawal AA. Phylogeny of the plant genus Pachypodium (Apocynaceae) PeerJ. 2013;1:e70. doi: 10.7717/peerj.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robbrecht E. Generic distribution patterns in subsaharan African Rubiaceae (Angiospermae) J Biogeogr. 1996;23(3):311–328. [Google Scholar]
- 119.Wikström N, Avino M, Razafimandimbison SG, Bremer B. Historical biogeography of the coffee family (Rubiaceae, Gentianales) in Madagascar: Case studies from the tribes Knoxieae, Naucleeae, Paederieae and Vanguerieae. J Biogeogr. 2010;37(6):1094–1113. [Google Scholar]
- 120.Wikström N, Kainulainen K, Razafimandimbison SG, Smedmark JE, Bremer B. A revised time tree of the asterids: Establishing a temporal framework for evolutionary studies of the coffee family (rubiaceae) PLoS One. 2015;10(5):e0126690. doi: 10.1371/journal.pone.0126690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.