Significance
Endocytic receptors regulate the internalization of extracellular antigens and are often targeted to induce a potent immune response (e.g., tumor vaccinations), albeit with limited success. Here, we describe a role of an endocytic receptor on the regulation of T-cell functionality. We demonstrate that the expression of the mannose receptor on antigen-presenting cells (APCs) impaired full activation of cytotoxic T cells by direct interaction with CD45 on the T-cell surface, resulting in CD45 inhibition, T-cell reprogramming, and the induction of T-cell tolerance. These findings demonstrate that the immune-regulatory properties of endocytic receptors expressed on APCs have an important impact on the potency of T-cell activation.
Keywords: endocytosis receptors, T-cell activation, tolerance, CD45
Abstract
The mannose receptor (MR) is an endocytic receptor involved in serum homeostasis and antigen presentation. Here, we identify the MR as a direct regulator of CD8+ T-cell activity. We demonstrate that MR expression on dendritic cells (DCs) impaired T-cell cytotoxicity in vitro and in vivo. This regulatory effect of the MR was mediated by a direct interaction with CD45 on the T cell, inhibiting its phosphatase activity, which resulted in up-regulation of cytotoxic T-lymphocyte–associated Protein 4 (CTLA-4) and the induction of T-cell tolerance. Inhibition of CD45 prevented expression of B-cell lymphoma 6 (Bcl-6), a transcriptional inhibitor that directly bound the CTLA-4 promoter and regulated its activity. These data demonstrate that endocytic receptors expressed on DCs contribute to the regulation of T-cell functionality.
The mannose receptor (MR) is an endocytic receptor belonging to the C-type lectin family and is expressed by distinct populations of dendritic cells (DCs), macrophages, and endothelial cells (1). It consists of an N-terminal cysteine-rich domain (CR), a fibronectin type II (FN II) domain, eight C-type lectin-like domains (CTLDs), a transmembrane region, and a short intracellular region. The CTLDs of the MR can bind to glycoconjugates terminated in mannose, fucose, or glucose. Despite the presence of eight CTLDs, only CTLD4 is responsible for carbohydrate binding. Additionally, the MR can bind to sulfated carbohydrates via its CR and to collagen via its FN II domain (1).
In a previous study, we demonstrated that antigens internalized by the MR are processed specifically for cross-presentation (2). The correlation between the MR and cross-presentation offered the possibility that antigens targeted toward the MR could induce potent cytotoxic T-cell responses. Because the induction of a strong cytotoxic T-cell response against tumor-specific antigens is a crucial process in many tumor vaccination strategies, antigen targeting toward the MR seemed to be a promising approach. However, in in vivo tumor models, antigen targeting toward the MR did not result in a strong cytotoxic T-cell response but, instead, led to the induction of antigen-specific T-cell tolerance (3, 4). Therefore, we analyzed whether the MR has a regulatory effect on the induction of immune responses by means distinct from antigen uptake and presentation, and we investigated a direct influence of the MR on T-cell activation. We could demonstrate that the presence of the MR on the antigen-presenting cell (APC) directly impairs the cytotoxic activity of T cells in vitro and in vivo. We showed that this effect was due to a direct interaction of the MR with CD45 on the T-cell surface. This interaction inhibited CD45 activity and resulted in the up-regulation of the inhibitory molecule cytotoxic T-lymphocyte–associated Protein 4 (CTLA-4), which was responsible for the impaired cytotoxic activity of the T cells.
Results
The Presence of the MR on DCs Reduces Cytotoxic Activity of CD8+ T Cells.
To investigate whether the MR influences T-cell activation by means distinct from antigen presentation, we made use of Désiré T-cell receptor (DesTCR) transgenic CD8+ T cells. Because these T cells recognize endogenous proteins (5), the presentation of such antigens is independent of MR-mediated endocytosis.
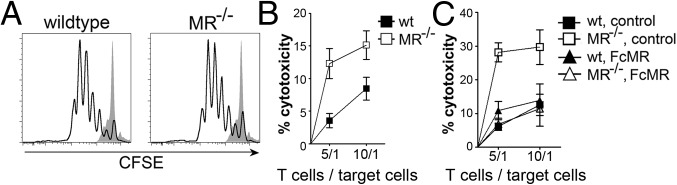
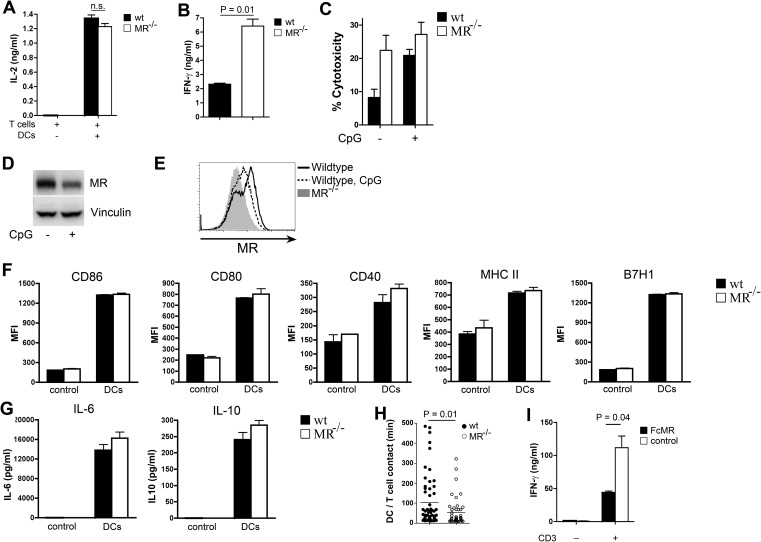
First, we cultured carboxyfluorescein succinimidyl ester (CFSE)-labeled DesTCR T cells on wild-type or MR-deficient DCs and monitored T-cell proliferation. DesTCR T cells activated by MR-deficient DCs proliferated to the same extent as T cells activated by wild-type DCs (Fig. 1A), indicating that the presence of the MR on the DC does not influence T-cell proliferation. Consistent with these observations, no differences in IL-2 secretion were observed (Fig. S1A). To analyze whether the presence of the MR influences effector functions of these T cells, we performed an in vitro cytotoxicity assay. To this end, DesTCR T cells were activated by either wild-type or MR-deficient DCs and cocultured with a mixture of differentially labeled target and control cells. Specific elimination of the target cells was monitored by flow cytometry. Interestingly, T cells activated by MR-deficient DCs displayed a markedly increased cytotoxic activity compared with T cells activated by wild-type DCs (Fig. 1B), indicating that the presence of the MR influences the cytotoxic activity of CD8+ T cells. Consistent with these findings, restimulation of T cells initially activated by MR-deficient DCs with a CD3-specific antibody resulted in an increased secretion of IFN-γ compared with T cells activated by wild-type DCs (Fig. S1B). Additionally, MR-induced T-cell tolerance was diminished after strong activation of the DC with CpG (Fig. S1C), which results in down-regulation of the MR (Fig. S1 D and E), again demonstrating an inhibitory effect of the MR on T-cell activation.
Fig. 1.
MR expression on DCs reduces cytotoxicity of activated T cells. (A) Proliferation of CFSE-labeled DesTCR T cells cocultured with wild-type or MR-deficient (MR−/−) BM-DCs. (B) Cytotoxic activity of DesTCR T cells activated by wild-type (wt) or MR−/− BM-DCs. (C) Cytotoxic activity of DesTCR T cells activated by wt or MR−/− BM-DCs in the presence of 10 μg/mL FcMR or isotype control. Line graphs depict statistical analysis (mean ± SEM) of replicates from at least three independent experiments.
Fig. S1.
Expression of the MR on APCs prevents T cells from being activated. (A) DesTCR T cells were cocultured with wild-type (wt) or MR-deficient (MR−/−) BM-DCs. Secretion of IL-2 in the supernatant was determined after 24 h by ELISA. n.s., not significant. (B) DesTCR T cells were cocultured with wt or MR−/− BM-DCs. After 3 d, T cells were collected and restimulated with a αCD3 antibody. Secretion of IFN-γ in the supernatant was determined after another 18 h by ELISA. (C) Cytotoxic activity of DesTCR T cells activated by BM-DCs that were untreated or pretreated with 6 μg/mL CpG for 24 h. MR expression is shown in wt DCs after pretreatment with 6 μg/mL CpG for 24 h monitored by Western blot (D) or flow cytometry (E). (F) The wt or MR−/− BM-DCs were cultured with DesTCR T cells for 18 h, and the expression of CD86, CD80, CD40, MHC II, and the coinhibitory molecule B7H1 was determined by flow cytometry. Isotype control staining was used as a control. MFI, mean fluorescence intensity. (G) The wt or MR−/− BM-DCs were cocultured with DesTCR T cells. After 18 h, IL-6 and IL-10 concentrations in the supernatant were determined by ELISA. T-cell samples without DCs were used as controls. (H) DesTCR T cells were cultured with wt or MR−/− BM-DCs. The interaction time between T cells and DCs was monitored by time-lapse microscopy for at least 40 randomly chosen cells. (I) DesTCR T cells were cocultured with wild-type or MR-deficient BM-DCs in the presence of 10 μg/mL FcMR or isotype control proteins. After 3 d, T cells were restimulated with αCD3 and secretion of IFN-γ was determined by ELISA.
Regulation of Cytotoxic CD8+ T-Cell Activity by the MR Is Mediated by Direct Interaction with Proteins on the T-Cell Surface.
The influence of the MR on T-cell activation could be due to two different mechanisms. First, MR engagement could result in signaling events within the DC, reducing their immunogenic properties. However, no differences in expression of costimulatory molecules or secretion of cytokines were observed between wild-type and MR-deficient DCs (Fig. S1 F and G). Alternatively to regulating immunogenic properties of the DC, the MR could influence T-cell activity directly by interacting with proteins on the T-cell surface. To investigate this hypothesis, we first monitored the interaction time between DesTCR T cells and wild-type or MR-deficient DCs by time-lapse microscopy. A prolonged interaction of T cells with MR-bearing DCs could be observed (Fig. S1H), pointing out the possibility that the MR indeed might contribute to the interaction with the T-cell. To answer the question unequivocally as to whether the influence of the MR on T-cell activation is due to a direct interaction with proteins on the T-cell surface, we generated soluble MR proteins (encompassing the CR region, the FN II domain, and CTLD1-2) fused to the Fc region of human IgG1 [hIgG1 (FcMR)] (6) and added these proteins to the cytotoxicity assays. If the regulatory effect of the MR was due to effects on the MR-expressing DC, addition of these soluble proteins should not influence the cytotoxic activity of T cells. If, however, the regulatory effect of the MR on the cytotoxic activity of CD8+ T cells was due to a direct interaction with proteins on the T-cell surface, addition of FcMR to the cytotoxicity assay should show a similar regulatory effect. Clearly, the cytotoxic activity of T cells activated by MR-deficient DCs in the presence of FcMR was reduced to a level comparable to T cells activated by wild-type DCs (Fig. 1C). This finding demonstrated that MR-induced impairment of cytotoxic activity was indeed due to a direct interaction of the MR with proteins on the T-cell surface and not due to signaling processes within the DC. Consistently, T cells activated in the presence of FcMR displayed clearly reduced secretion of IFN-γ after restimulation with α-CD3 (Fig. S1I).
Reduced Cytotoxic Activity Is Caused by Binding of the MR to CD45, Reducing Its Phosphatase Activity.
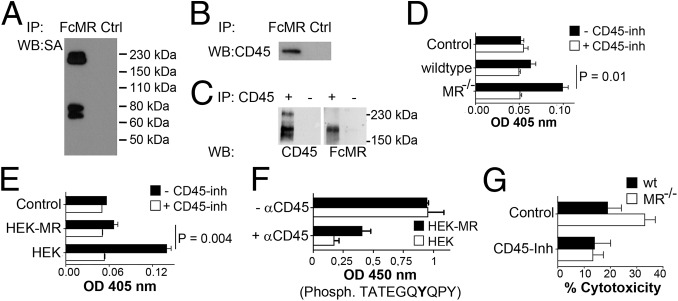
To identify possible interaction partners of the MR on the T cell, we added soluble FcMR to cell lysates of surface-biotinylated DesTCR T cells. Afterward, we immunoprecipitated FcMR and analyzed all interacting membrane proteins by Western blot. By this means, we could detect a clear MR interaction partner between 150 and 230 kDa (Fig. 2A). Because it has been demonstrated before that the CR of the MR bears affinity for the phosphatase CD45 (7), whose molecular mass is within the observed range and is expressed on the surface of CD8+ T cells, we verified whether the observed interaction partner resembles CD45. To this end, we performed coimmunoprecipitation experiments with the FcMR protein. Subsequent Western blot analysis revealed a clear interaction between CD45 and the MR (Fig. 2B). Additionally, we precipitated CD45 from DesTCR T cells. Subsequent Western blot analysis after staining with FcMR further confirmed the interaction of the MR with CD45 (Fig. 2C).
Fig. 2.
Interaction of the MR with CD45 impairs its phosphatase activity and is responsible for reduced cytotoxic activity. (A) FcMR or isotype controls (Ctrl) were added to the lysate of surface biotinylated DesTCR T cells and immunoprecipitated (IP). Eluates were analyzed by Western blot (WB) using HRP-conjugated streptavidin (SA). (B) Identical to A using a CD45-specific antibody. (C) Lysates of wild-type splenocytes were incubated with or without a CD45-specific antibody and immunoprecipitated. Eluates were analyzed by Western blot using FcMR or a CD45-specific antibody. (D) CD45 from lysates of DesTCR T cells that were incubated with wild-type or MR−/− BM-DCs was immunoprecipitated, and 2 mM 4-nitrophenyl phosphate was added in the presence or absence of 1 μM CD45 inhibitor (Inh) N-(9,10-dioxo-9,10-dihydro-phenanthren-2-yl)-2,2-dimethyl-propionamide. Dephosphorylation of 4-NPP was monitored by colorimetry at 405 nm. Samples without αCD45 were used as controls. (E) Identical to D using HEK293T (HEK) or MR-expressing HEK293T (HEK-MR) cells. (F) Dephosphorylation of TATEGQ-pY-QPQ by immunoprecipitated CD45 after incubation with HEK or HEK-MR cells. Phosph., phosphorylated. (G) Cytotoxic activity of DesTCR T cells activated by wt or MR−/− BM-DCs in the presence or absence of 40 nM SF1670. Bar graphs depict statistical analysis (mean ± SEM) of replicates from three independent experiments. OD, optical density.
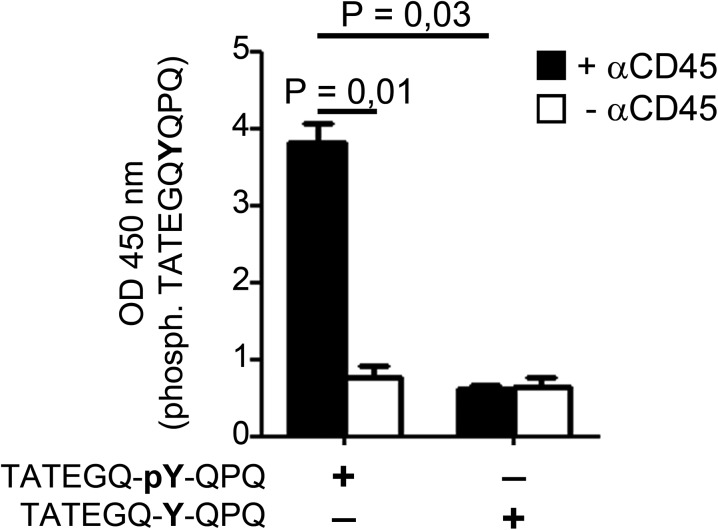
Next, we investigated whether the interaction of the MR with CD45 affected its phosphatase activity. To this end, we generated cell lysates from DesTCR T cells after incubation with wild-type or MR-deficient DCs. Subsequently, we precipitated CD45 from these lysates and added 4-nitrophenyl phosphate, whose dephosphorylation can be quantified by colorimetry. Indeed, the phosphatase activity of CD45 was reduced when T cells were cocultured with MR-bearing DCs (Fig. 2D). Similar results were obtained when T cells were cultivated with HEK293T cells that were stably transfected with the MR (8) (Fig. 2E), pointing out an inhibitory effect of the MR on CD45 activity. Finally, to demonstrate that observed dephosphorylation indeed was mediated by CD45, we monitored the influence of the MR on dephosphorylation of a synthetic peptide of Lck containing pY505, a specific substrate of CD45 (Fig. S2). Similar to the observations described above, the presence of the MR clearly prevented Y505 dephosphorylation (Fig. 2F), proving that the MR indeed inhibits the phosphatase activity of CD45.
Fig. S2.
Assay to determine phosphatase activity of CD45. CD45 from lysates of T cells was immunoprecipitated and incubated with biotinylated TATEGQ-pY-QPQ [epitope of Lck containing phosphorylated (phosph.) Y505] or TATEGQ-Y-QPQ (epitope of Lck containing unphosphorylated Y505). Subsequently, peptides were isolated via affinity chromatography and phosphorylation was determined after staining with the phosphospecific 4G10 antibody by colorimetry.
To investigate whether the reduced CD45 phosphatase activity after MR binding was responsible for the impaired cytotoxic activity of activated T cells, we added a CD45 inhibitor (9) to the cytotoxicity assays. Importantly, after inhibition of CD45, no differences in cytotoxic activity between T cells primed by wild-type or MR-deficient DCs could be detected (Fig. 2G), demonstrating that reduced CD45 activity is crucial for the impairment of cytotoxic activity of T cells activated in the presence of the MR.
Transcriptional Changes in T Cells Stimulated in the Presence of the MR.
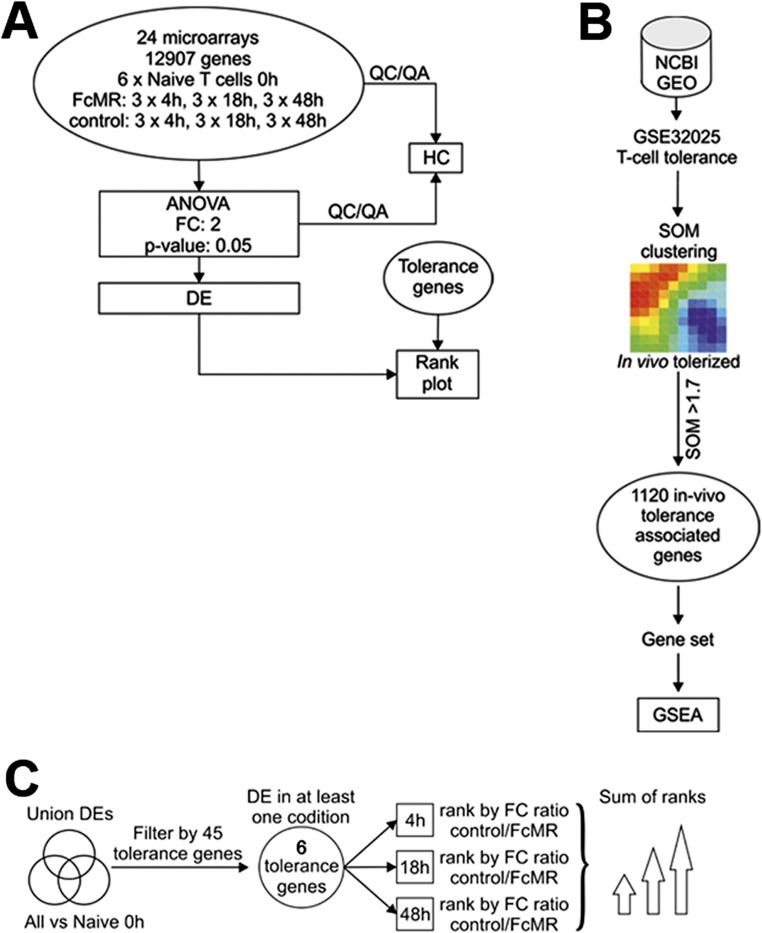
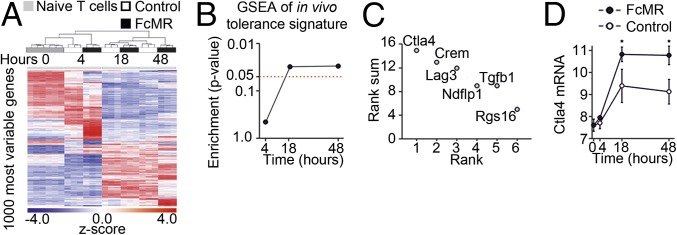
Next, we aimed at identifying genes involved in MR-mediated T-cell impairment and determined genome-wide gene expression changes in DesTCR T cells incubated with MR-deficient DCs in the presence of FcMR or isotype controls at an early, intermediate, and late time points (t = 4 h, 18 h, and 48 h, respectively) (Fig. S3A). Hierarchical clustering of the 1,000 most variable genes revealed a clear separation between control and FcMR-treated T cells (Fig. 3A). Applying an ANOVA model to define differentially expressed (DE) genes clearly revealed that the number of DE genes in FcMR-treated cells at early time points was higher than in control cultures and at later time points (Dataset S1), indicating that loss of T-cell effector function in these cells might be associated with an active reprogramming as has been observed for T-cell tolerance or anergy. To relate the mRNA signatures associated with MR-induced T-cell impairment to previously described tolerance signatures (10), we used a previous dataset (GSE32025) to generate an in vivo tolerance-associated gene set in a data-driven fashion applying significance of microarray clustering (Fig. S3B). The tolerance-related gene set contained 1,120 transcripts and was used as input for gene set enrichment analysis (GSEA) in our own dataset. GSEA revealed a significant enrichment for the tolerance-related gene set when comparing FcMR-treated with control-treated naive T cells at 18 and 48 h (Fig. 3B), suggesting that MR-mediated T-cell impairment shares mechanisms operative in T-cell tolerance. Next, we applied a combined approach to identify and prioritize candidate effector molecules responsible for MR-mediated T-cell impairment as defined by differential expression at least at one time point (first criterion), being part of the tolerance gene set (second criterion), and being part of a set of genes (n = 45) that were previously associated with T-cell anergy or tolerance (third criterion) (11–20). For genes within the intersection of these three gene groups, a sum rank was determined based on most significant differential expression (Fig. S3C). This approach revealed only six genes, with CTLA-4 being the most highly ranked gene (Fig. 3C). Visualizing differential gene induction, a clear increase in CTLA-4 transcription in FcMR-treated cells was revealed (Fig. 3D). Our analysis, together with previous observations concerning ligation of CD45 with specific antibodies, which was associated with up-regulation of CLTA-4 and subsequent induction of tolerance against allografts (21), suggested CTLA-4 to be the most promising candidate for mediating MR-induced T-cell tolerance.
Fig. S3.
Schemes of transcriptome analysis. (A) Scheme describing the work flow for Fig. 3. (B) Scheme of tolerance signature generation of an in vivo tolerance signature generated by microarray clustering using a T-cell tolerance dataset (GSE21025) and utilization of these genes for GSEA. (C) Scheme to derive candidate genes associated with FcMR-associated T-cell impairment. The intersection of three groups of genes was generated using the tolerance gene set in B (n = 1,120), 45 tolerance or anergy-associated genes, and DE genes [FDR-corrected P value <0.05, fold change (FC) of ±2] between FcMR-stimulated and control T cells for at least one time point.
Fig. 3.
Transcriptome analysis of T cells activated in the presence of the MR. (A) Hierarchical clustering visualizing z-transformed log2 expression values for the 1,000 most variably expressed genes (FDR-corrected P value <0.05) in the complete dataset (naive T-cells, t = 0 h), control, and FcMR-treated T cells at t = 4 h, 18 h, or 48 h. (B) GSEA was performed comparing FcMR-treated T cells against the controls for each time point separately. The enrichment P value was plotted in a line plot. (C) Rank plot of the sum of ranks as determined in Fig. S2C. (D) Line/scatter plot of log2 expression of CTLA-4. All graphs depict statistical analysis (mean ± SEM) of three biological replicates.
Reduced CD45 Activity by the MR Leads to Up-Regulation of CTLA-4.
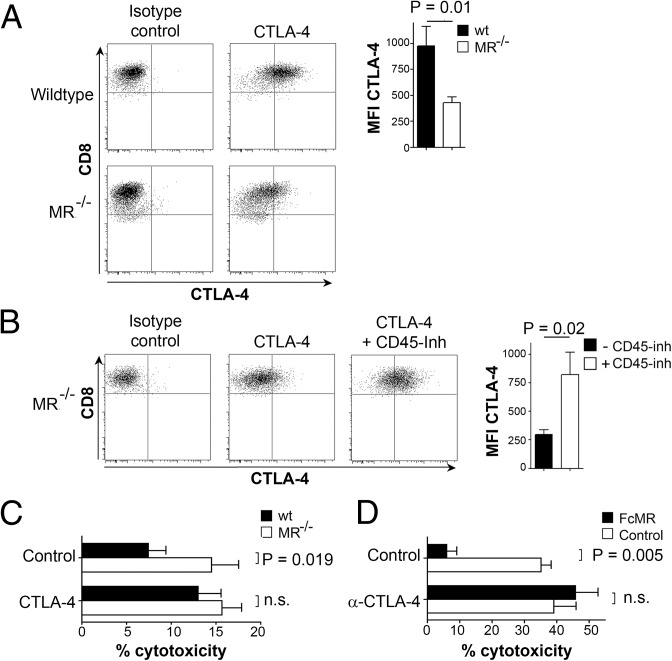
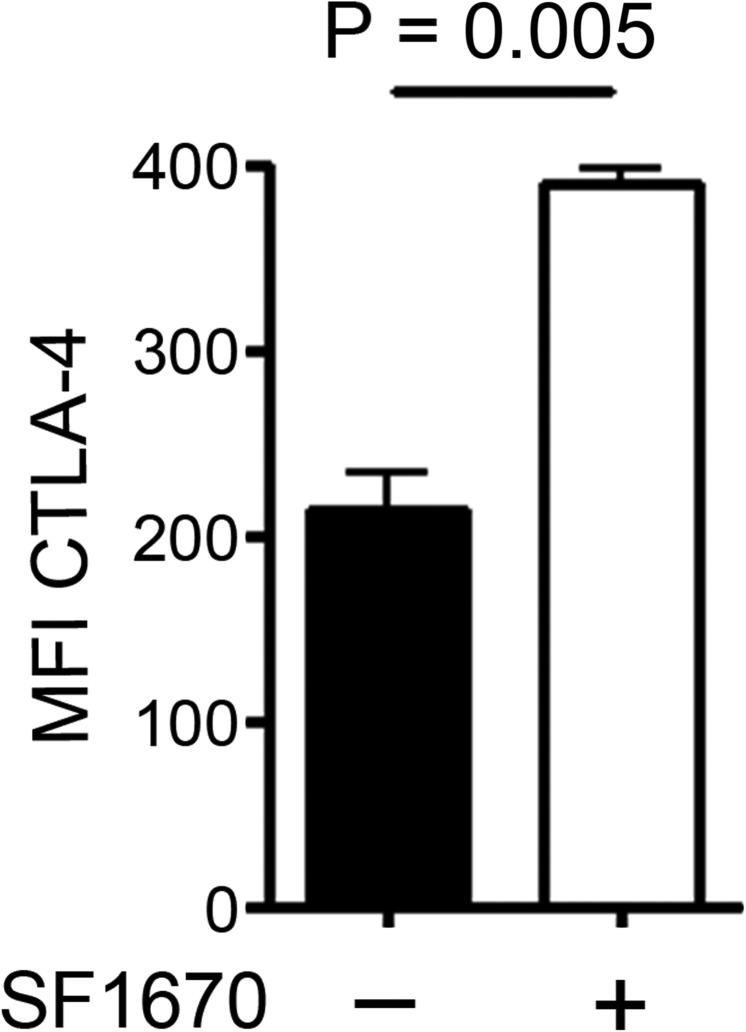
To investigate whether CTLA-4 indeed plays a role in MR-mediated T-cell tolerance, we first confirmed CTLA-4 up-regulation after MR binding on protein level. T cells activated by MR-bearing, but not by MR-deficient, DCs showed strong up-regulation of CTLA-4 (Fig. 4A), confirming our observations from the transcriptome analysis. To investigate whether this up-regulation of CTLA-4 in the presence of the MR was due to impaired CD45 activity, DesTCR T cells were activated in the absence of the MR but in the presence of two different CD45 inhibitors. Flow cytometric analysis revealed that inhibition of CD45 indeed resulted in a clear up-regulation of CTLA-4 (Fig. 4B and Fig. S4), pointing out a central role of CD45 activity in the regulation of CTLA-4.
Fig. 4.
Impaired CD45 activity after MR interaction results in up-regulation of CTLA-4 on T cells. (A) DesTCR T cells were incubated with wild-type or MR−/− BM-DCs, and CTLA-4 expression on CD8+ cells was determined. (B) CTLA-4 expression on DesTCR T cells activated by MR−/− BM-DCs in the presence or absence of 50 μM CD45 inhibitor methyl-3,4-dephostatin. (C) The wt or MR−/− BM-DCs were cocultured with DesTCR T cells in the presence or absence of 1 μg/mL anti–CTLA-4 blocking antibody. Specific killing of the target cells was monitored as above. (D) Identical to C using MR−/− BM-DCs in the presence of either FcMR or isotype controls. Bar graphs depict statistical analysis (mean ± SEM) of replicates from three independent experiments. MFI, mean fluorescence intensity; n.s., not significant.
Fig. S4.
Up-regulation of CTLA-4 after addition of a CD45 inhibitor. CTLA-4 expression on DesTCR T cells activated by MR−/− BM-DCs in the presence or absence of 1 μM CD45 inhibitor N-(9,10-dioxo-9,10-dihydro-phenanthren-2-yl)-2,2-dimethyl-propionamide (SF1670).
Subsequently, we analyzed whether up-regulation of CTLA-4 on T cells activated by MR-bearing DCs was actually responsible for the observed impaired cytotoxicity (Fig. 1 B and C). To this end, we analyzed the cytotoxic activity of T cells activated in the presence of a CTLA-4–specific blocking antibody (αCTLA-4). Differences in cytotoxic activity between T cells activated by wild-type or MR-deficient DCs were overcome in the presence of αCTLA-4 (Fig. 4C), confirming a decisive role of CTLA-4 in MR-mediated inhibition of T-cell cytotoxicity. Similar effects of αCTLA-4 were observed after addition of FcMR to cocultures of MR-deficient DCs with T cells (Fig. 4D), further confirming that reduced cytotoxic activity of T cells in the presence of the MR indeed was due to the up-regulation of CTLA-4.
Up-Regulation of CTLA-4 in the Presence of the MR Was Mediated by Impeded Expression of B-Cell Lymphoma 6, Which Directly Binds to the CTLA-4 Promoter and Regulates Its Transcription.
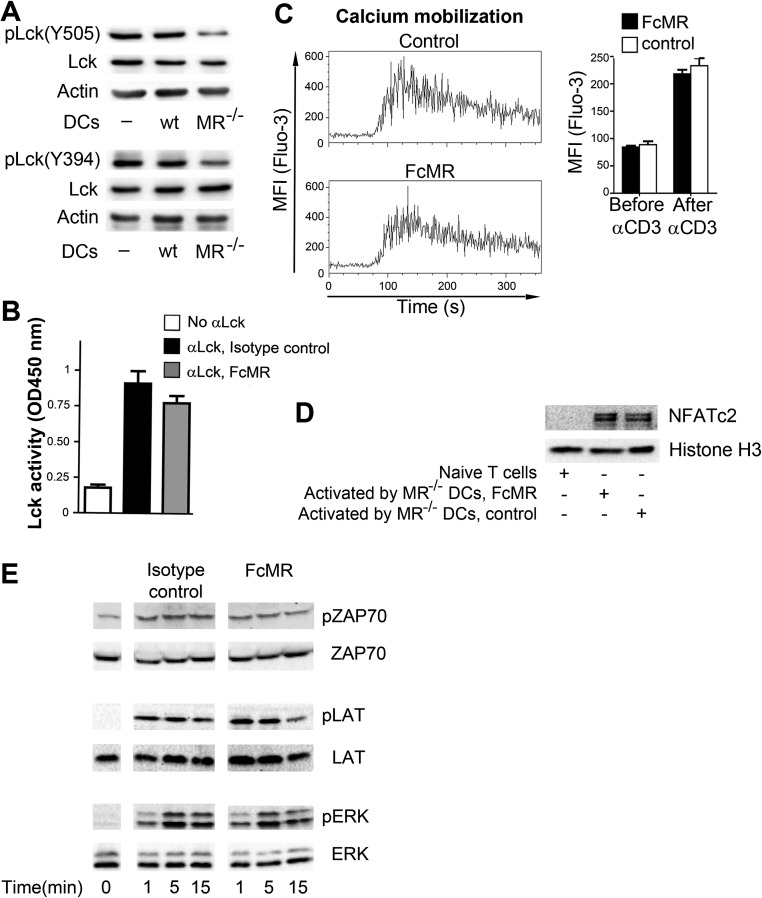
Next, we investigated how MR-mediated inhibition of CD45 influenced CTLA-4 expression. One of the most prominent substrates of CD45 is Lck, a kinase whose activation results in increased intracellular calcium mobilization. This increased intracellular calcium mobilization, in turn, induces the nuclear recruitment of nuclear factor of activated T cells (NFAT), a transcription factor (TF) family of which NFATc2 directly regulates CTLA-4 expression. Because NFATc2 translocation has been demonstrated to be altered in CD45-deficient cells (22), we analyzed whether CTLA-4 up-regulation after MR-induced inhibition of CD45 was due to a similar mechanism. Therefore, we first investigated the phosphorylation status of the activating tyrosine residue 394 (Y394) and the inhibitory tyrosine 505 (Y505) of Lck. Consistent with MR-mediated inhibition of CD45 phosphatase activity, dephosphorylation of both Y394 and Y505 was impaired in the presence of the MR (Fig. S5A). However, inefficient dephosphorylation of both the stimulatory and inhibitory tyrosine residues did not have a clear effect on overall Lck activity (Fig. S5B). Likewise, intracellular calcium mobilization (Fig. S5C) and NFATc2 translocation into the nucleus (Fig. S5D) remained unaltered, pointing out that the differences in CTLA-4 expression on T cells activated in the presence of the MR might not be due to altered T-cell receptor signaling. Likewise, no differences in phosphorylation of ZAP70, LAT, and ERK were detected in the presence of FcMR (Fig. S5E).
Fig. S5.
Up-regulation of CTLA-4 is not mediated by alterations in T-cell signaling. (A) Phosphorylation of Lck in DesTCR T cells activated by wt or MR−/− BM-DCs. (B) Lck activity in DesTCR T cells activated by wild-type or MR-deficient BM-DCs. (C) Calcium flux in T cells stimulated with α-CD3 after 90 s in the presence of FcMR. (D) NFATc2 expression in nuclear extracts from DesTCR T cells activated by wild-type or MR−/− BM-DCs. (E) DesTCR T cells were stimulated with αCD3 in the presence of 10 μg/mL FcMR or isotype control proteins for the indicated time points. Phosphorylation of ZAP, LAT, and ERK was determined by Western blot. Bar graphs depict statistical analysis (mean ± SEM) of replicates from three independent experiments.
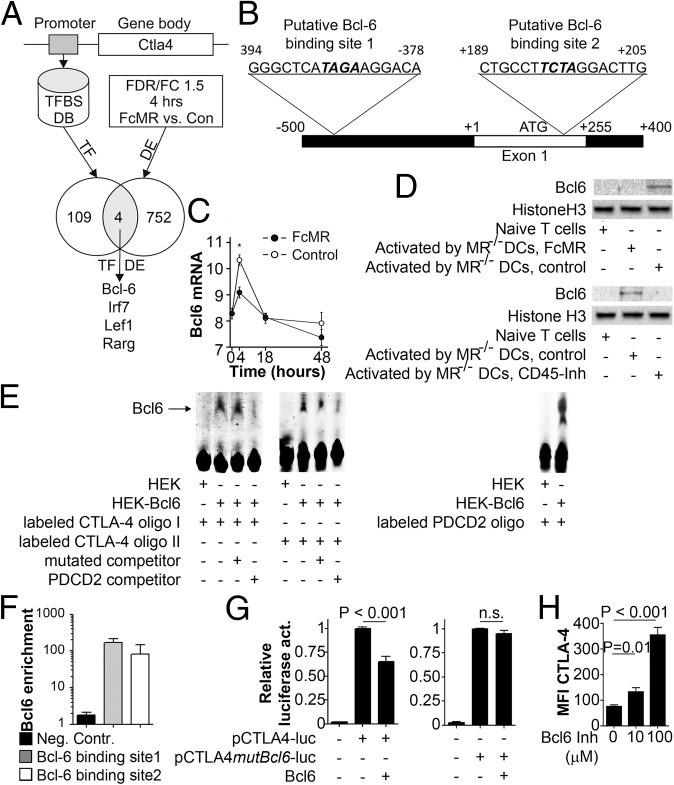
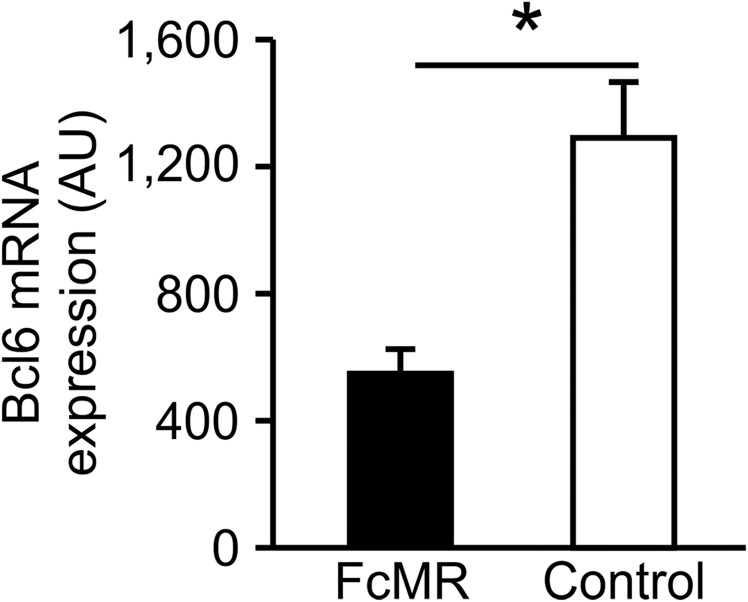
To identify potential regulators of CTLA-4 expression, we performed TF binding prediction analysis at the CTLA-4 promoter using algorithms provided by Genomatix and identified 113 potential candidate TFs (Fig. 5A). Only four of these TFs showed significant changes [false discovery rate (FDR) P value < 0.05 and fold change of 1.5] in expression at the 4-h time point (Fig. 5A). By this means, we identified B-cell lymphoma 6 (Bcl-6), a transcription inhibitor known to be involved in the differentiation of T-follicular helper cells (23), for example, as a candidate, with two putative binding sites within the CTLA-4 promoter (Fig. 5B) being up-regulated shortly after T-cell activation in the absence, but not in the presence, of the MR (Fig. 5C and Fig. S6). To confirm the presence of Bcl-6 in activated T cells on the protein level, we isolated nuclear extracts from T cells and confirmed Bcl-6 expression in T cells activated only in the absence of the MR (Fig. 5D). Bcl-6 expression was not only impeded after addition of soluble MR but also after addition of a CD45 inhibitor (Fig. 5D), indicating that lack of Bcl-6 in the presence of the MR was indeed due to inhibition of CD45. To investigate whether Bcl-6 was indeed able to bind to the identified sequences within the CTLA-4 promoter (Fig. 5B), we performed EMSA experiments. Fig. 5E demonstrates clear binding of Bcl-6 to both identified motifs. Such binding was inhibited by adding a 50-fold excess of a well-known Bcl-6 recognition side from the programmed cell death 2 (PDCD2) gene (24), but not by an excess of mutated recognition sequences from the CTLA-4 promoter, further confirming specificity of Bcl-6 binding. Next, we aimed to identify whether Bcl-6 was also recruited to the CTLA-4 promotor in activated T cells. Chromatin immunoprecipitation experiments clearly demonstrated the binding of Bcl-6 to both binding sites within the CTLA-4 promoter (Fig. 5F). To investigate whether Bcl-6 binding affected transcription of CTLA-4, we performed luciferase reporter assays. Expression of Bcl-6 clearly repressed transcription from the CTLA-4 promoter (Fig. 5G), which was overcome by mutating its Bcl-6 recognition site, demonstrating that Bcl-6 binding to the CTLA-4 promoter prevented its transcription. Next, we verified whether increased expression of CTLA-4 in T cells activated in the presence of the MR was indeed due to lack of Bcl-6 binding to the CTLA-4 promoter. Therefore, we activated T cells in the presence of a Bcl-6 inhibitor (25). Indeed, T cells activated in the absence of Bcl-6 activity showed a markedly increased expression of CTLA-4 (Fig. 5H), proving that Bcl-6 prevents expression of CTLA-4 in T cells bearing CD45 activity.
Fig. 5.
Activation of CD45 induces the expression of the transcriptional inhibitor Bcl-6, which regulates expression of CTLA-4. (A) Scheme of TF prediction. Con, control; TFBS DB, transcription factor binding site database. (B) Putative Bcl-6 binding sites within the CTLA-4 promoter. (C) Bcl-6 expression (log2) over time. The graph depicts statistical analysis of three biological replicates. (D) Bcl-6 expression in T cells activated by MR-deficient DCs in the presence of 10 μg/mL FcMR or 50 μM methyl-3,4-dephostatin. (E) EMSA using nuclear extracts from HEK293T cells transfected with Bcl-6 incubated with oligos encompassing both Bcl-6 binding sites (CTLA-4 oligos I and II) with or without 50 μM competitor (binding side from PDCD2 promoter) or mutated CTLA-4 oligos. (F) Chromatin immunoprecipitation analysis using a Bcl-6 antibody and primers for the Bcl-6 binding sites within the CTLA-4 promoter or for the INADL promoter [negative control (Neg. Contr.)]. (G) Luciferase activity of the CTLA-4 promoter (−400 to +1) with or without the mutated Bcl-6 binding site. (H) CTLA-4 expression on T cells activated in the presence of the Bcl-6 inhibitor 2-[(5Z)-5-(5-bromo-2-oxo-1H-indol-3-ylidene)-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]butanedioic acid. All bar graphs depict statistical analysis (mean ± SEM) of replicates from at least three independent experiments.
Fig. S6.
Influence of the MR on expression levels of Bcl-6. Absolute expression of Bcl6 in T cells that were activated by MR-deficient BM-DCs in the presence of 10 μg/mL FcMR or isotype control protein after 4 h. *P < 0.05. AU, arbitrary units.
Increased Expression of CTLA-4 and Reduced Cytotoxicity Induced by the MR in Vivo.
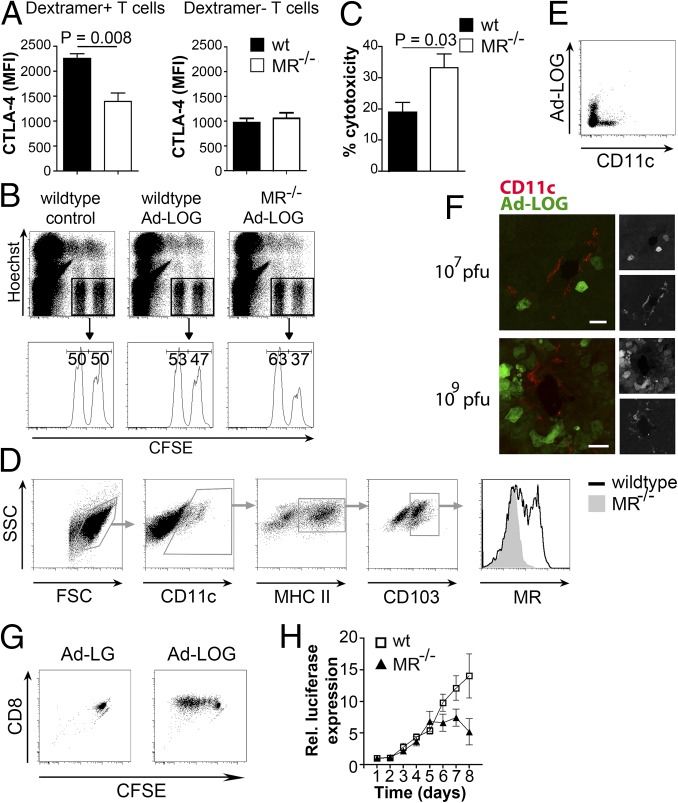
Next, we investigated whether the MR also influences up-regulation of CTLA-4 and the cytotoxic activity of T cells in vivo. Therefore, we transduced wild-type or MR-deficient DCs with luciferase-ovalbumin (OVA)-GFP–expressing adenoviruses (Ad-LOGs) and injected these cells into wild-type recipient mice. After 6 d, we determined CTLA-4 expression on endogenous OVA-specific splenic CD8+ T cells. Despite equal transduction levels of wild-type and MR-deficient DCs (Fig. S7A), we monitored an increased CTLA-4 expression only on antigen-specific T cells after priming with wild-type compared with MR-deficient DCs (Fig. 6A), confirming our in vitro observations.
Fig. S7.
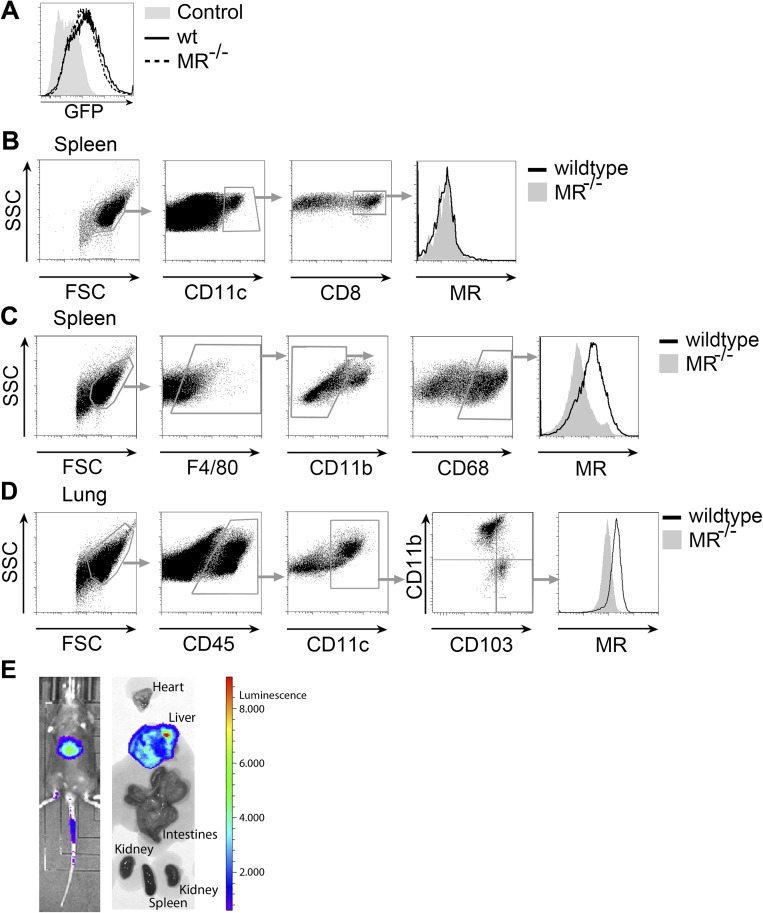
In vivo expression of the MR and distribution of Ad-LOGs in vivo. (A) The wt or MR−/− BM-DCs were transduced with Ad-LOGs. GFP expression was monitored by flow cytometry. Untransduced cells served as a control. (B) MR expression in CD8+ DCs from the spleen. (C) MR expression in F4/80+CD11b−CD68+ RPM of the spleen. (D) MR expression in DCs isolated from the lung. (E) Luciferase activity in mice or isolated organs from mice infected with 107 pfu Ad-LOGs.
Fig. 6.
Presence of the MR on DCs impairs cytotoxicity of activated T cells in vivo. (A) The wt or MR−/− BM-DCs were transduced with Ad-LOGs and injected into wild-type mice. After 6 d, CTLA-4 expression was monitored on SIINFEKL/Kb dextramer+ CD8+ and SIINFEKL/Kb dextramer− splenic CD8+ T cells. The graphs depict statistical analysis (mean ± SEM) of four biological replicates. (B) Wild-type or MR−/− BM-DCs were transduced with Ad-LOGs and injected into wild-type mice. After 5 d, mice were challenged with target (CFSEhigh) and nontarget (CFSElow) cells. After 4 h, their ratio was determined by flow cytometry. Untransduced DCs were used as controls. (C) Combined statistical analysis (mean ± SEM) of B from two independent experiments with n = 7. (D) MR expression in DCs from the liver of wild-type or MR−/− mice. (E) GFP expression in DCs from the liver of wild-type mice infected with 107 pfu of Ad-LOGs. (F) GFP expression in the liver from wild-type mice infected with 107 or 109 pfu of Ad-LOGs. (Scale bars: 30 μm.) (G) Proliferation of CFSE-labeled OT-I cells after incubation with sorted CD11c+ cells from the liver of mice infected with Ad-LOGs or control virus (Ad-LG). (H) The wt or MR−/− mice were injected i.v. with 107 pfu of Ad-LOGs. Total luciferase activity was determined after i.p. injection of 50 mM luciferin. The graph shows combined statistical analysis (mean ± SEM) from two independent experiments with n = 11. Rel., relative.
Additionally, we determined whether these T cells displayed a reduced cytotoxic activity in vivo. Therefore, we treated mice with Ad-LOG–transduced DCs, and 5 d later, we injected differentially labeled target and nontarget cells. Indeed, an increased cytotoxic T-cell response was observed after priming by MR-deficient compared with wild-type DCs (Fig. 6 B and C), demonstrating that the presence of the MR on the DC also results in an impaired cytotoxicity in vivo.
In a previous study, we reported the isolation of MR-expressing CD11c+ splenocytes that were able to cross-present MR-internalized antigens (26). In contrast, work from other groups pointed out that CD8+ DCs, which are the physiologically relevant cross-presenting cells of the spleen under homeostatic conditions, did not express the MR (27). Here, we now demonstrate that the initially identified MR-expressing cells represent F4/80+CD11b−CD68+ red pulp macrophages (RPMs) (28) (Fig. S7 B and C), confirming previous observations by others (29, 30). However, because the role of RPMs in cross-priming is unclear, the physiological relevance of MR expression in these cells in terms of cross-presentation remains elusive.
MR-mediated endocytosis has previously been shown to be important in cross-presentation by monocyte-derived DCs (27). Additionally, expression data from the Immunological Genome Project (www.immgen.org) suggested MR expression in liver and lung CD103+ DCs, which are potent cross-presenters and considered to be the tissue-resident equivalent of CD8+ DCs from the spleen. In accordance with the expression data, we could verify a clear MR expression in CD103+ DCs from both liver (Fig. 6D) and lung (Fig. S7D). To investigate whether the expression of the MR on these cells influenced in vivo T-cell activation in a more physiological context, we directly infected wild-type or MR-deficient mice with Ad-LOGs, which resulted in a strong infection of the liver (Fig. S7E). Although liver DCs were not directly infected by Ad-LOGs (Fig. 6 E and F), they were able to activate OVA-specific CD8+ T cells (Fig. 6G), indicating that they cross-presented viral antigens. Monitoring viral load over time showed that, from day 5 after infection onward, viral clearance was much more effective in MR-deficient mice compared with wild-type mice (Fig. 6H), demonstrating that the presence of the MR determines the strength of an antiviral T-cell response in the liver and substantiating a regulatory role of the MR on CD8+ T-cell activation in vivo.
Discussion
In this study, we demonstrated that MR-bearing DCs reduce the cytotoxic capacity and IFN-γ induction of activated CD8+ T cells. This effect was due to a direct interaction of the MR with CD45, as has also been demonstrated for the macrophage galactose-type C-type lectin (MGL) (31). Interaction of the MR with CD45 impaired its activity and resulted in the up-regulation of the inhibitory receptor CTLA-4, which was responsible for induction of T-cell tolerance. These data imply that endocytic receptors not only determine the intracellular fate, and hence the presentation of internalized antigens within the APCs, but can also directly regulate the functionality of primed T cells.
A correlation of the MR with the induction of T-cell tolerance is supported by various previous observations. First, antigen targeting toward the MR via antigen mannosylation has been demonstrated to induce antigen-specific T-cell tolerance (3). Such T-cell irresponsiveness has been shown to inhibit the development of experimental autoimmune encephalomyelitis after injection of mannosylated myelin peptide (4). Second, MR engagement on monocyte-derived DCs induces an immune-suppressive phenotype (32, 33). Third, substantial evidence points out that MR expression is directly correlated with the immunogenicity of the cellular environment. It has been demonstrated that antiinflammatory agents up-regulate (34, 35) and immune-stimulatory agents down-regulate (36, 37) MR expression. Fourth, several tolerance-inducing cells, including alternative activated macrophages, tolerogenic DC subtypes, and endothelial cells in the liver, express high levels of MR (38, 39). Because these cells have a strong inhibitory effect on the activation of T cells, a direct interaction of the MR with CD45 on T cells might contribute to the immunosuppressive features of these cells.
Expression of CTLA-4 by MR-mediated inhibition of CD45 activity was not due to differences in T-cell signaling, because no differences in Lck activity or NFATc2 translocation could be observed. Instead, MR-mediated inhibition of CD45 prevented Bcl-6 expression. We could demonstrate that Bcl-6 binds and inhibits transcription from the CTLA-4 promoter, and, therefore, if T cells are activated in the presence of the MR, lack of Bcl-6 allows expression of CTLA-4. Bcl-6 is a key regulator in Th-cell differentiation and in the regulation of CD4+ and CD8+ T-cell memory (40). Although it has been demonstrated before that Bcl-6 is transiently expressed in activated T cells and continuously up-regulated in CD8+ effector T cells (41), its role in these cells is less understood. Here, we could demonstrate that Bcl-6 prevents CTLA-4 from being expressed in these cells.
Our findings describing the induction of T-cell tolerance mediated by MR-induced up-regulation of CTLA-4 might open new perspectives for therapeutic approaches aimed at dampening the immune system. It has been demonstrated before that CTLA-4 up-regulation results in the promotion of allograft survival in transplantation studies (42). Because soluble MR fragments such as the ones used in this study induce up-regulation of CTLA-4, injection with such fragments could be a tempting approach to reduce immune responses in vivo. Future studies will have to reveal whether such a strategy might be a way to induce immune tolerance, for example, in transplantation studies or autoimmune diseases.
Materials and Methods
All animal experiments have been approved by the local authorities [Landesamt für Natur, Umwelt und Verbraucherschutz North Rhine-Westphalia (LANUV NRW)]. Detailed procedures are available in SI Materials and Methods.
In Vitro Cytotoxicity Assay.
DesTCR T cells were cocultured with DCs in the presence of 10 μg/mL FcMR or control protein. After 3 d, T cells were purified by density gradient centrifugation and incubated with 2,000 target and 2,000 control cells. For use as target cells, murine lymphoma Rauscher virus-induced T-cell lymphoma cells (RMA) cells were stained with 1 μM CFSE. As control cells, RMA-S cells, which do not express surface MHC I (43), were labeled with 0.1 μM CFSE. After 4 h, specific kill was determined.
In Vivo Cytotoxicity Assay and Expression of CTLA-4.
DCs were transduced with Ad-LOGs and injected i.v. into wild-type mice (1 × 106 DCs per mouse). After 6 d, splenocytes were stained with SIINFEKL/Kb dextramers and αCTLA-4. To determine T-cell cytotoxicity, we injected 5 × 106 target cells and 5 × 106 control cells 5 d after injection of DCs. As control cells, C57BL/6J splenocytes were labeled with 0.1 μM CFSE. As target cells, C57BL/6J splenocytes were pulsed with SIINFEKL peptide (2 μM) and labeled with 1 μM CFSE. After another 4 h, spleens were collected and the cytotoxicity was determined by flow cytometry.
For direct infection experiments, mice were injected i.v. with Ad-LOG (107 pfu/0.2 mL) per mouse. Bioluminescence was measured with an IVIS 200 (Caliper LifeSciences) 5 min after i.p. injection of luciferin (50 mM) in 200 μL of PBS. Data analysis was performed with Living Image 2.50.1 software.
RNA Isolation and Microarray Analysis.
DesTCR T cells were incubated with MR-deficient DCs in the presence of 10 μg/mL FcMR (details are provided in SI Materials and Methods) or isotype control. Afterward, at least 5 × 104 CD8+ T cells were isolated by cell sorting and lysed in TRIZOL, and total RNA was extracted. The quality of the RNA was assessed by spectroscopy at 260 nm and 280 nm and by visualization on an agarose gel. Before array-based gene expression profiling, total RNA was further purified using the MinElute Reaction Cleanup Kit (Qiagen). Biotin-labeled cRNA was generated using the TargetAmp Nano-g Biotin-aRNA Labeling Kit (Epicentre). Biotin-labeled cRNA (1.5 μg) was hybridized to MouseWG-6 v2.0 Beadchips (Illumina) and scanned on an Illumina HiScanSQ instrument.
The microarray data have been deposited in the Gene Expression Omnibus under accession number GSE45805 (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45805).
Statistical Analysis.
For microarray analysis, statistical significance of biological replicates was determined by ANOVA modeling. P values <0.05 were defined as significant, and were corrected for multiple testing using the FDR. Statistical analysis was performed by the Partek Genomics Suite.
For all other experiments, statistical significance was calculated using replicates from independent experiments. P values were calculated by Student’s t test (Excel). All graphs depict mean value ± SEM.
SI Materials and Methods
Antibodies, Reagents, and Mice.
αCTLA-4 (UC10-4B9), αCD3 (145-2C11), and αCD8 (53-6.7) were from Ebiosciences; αLck (2752), αpLck (Tyr505), and αBcl-6 (D65C10) were from Cell Signaling; αNFATc2 (4G6-G5) and αpLck (Tyr394) were from Santa Cruz Biotechnology; and H-2 Kb/SIINFEKL dextramers were from Immudex. αCD45 was purified from YBM42.2.2 cells. CTLA-4–IgG fusion proteins were from BD Pharmingen.
All mice were bred under specific pathogen-free conditions and used in accordance with local animal experimentation guidelines. For all experiments, mice between 8 and 16 wk of age were used.
Generation of Bone Marrow-Derived DCs.
Bone marrow-derived DCs (BM-DCs) were flushed from the bone marrow of femur and tibia of mice and cultured for 7 d in medium containing the supernatant of a GM-CSF–producing cell line.
Generation and Purification of FcMR.
FcMR proteins (encompassing the CR region, the FN II domain, and CTLD1-2 fused to the Fc region of hIgG1) were generated as described previously (6). Before their use in experiments, FcMR constructs were incubated with an anti-human IgG1 antibody.
Proliferation of DesTCR T Cells.
DesTCR T cells were incubated for 15 min with 1 μM CFSE and cocultured with wild-type or MR-deficient DCs. After 3 d, T-cell proliferation was analyzed by monitoring the CFSE dilution profile by flow cytometry.
Analysis of DC/T-Cell Interaction Time by Time-Lapse Microscopy.
For analysis of DC/T-cell interaction time, CD8+ DesTCR T cells were isolated by magnetic cell separation (MACS) and labeled with 0.1 μM CFSE. Subsequently, 160,000 T cells were incubated with 40,000 wild-type or MR-deficient DCs in plastic channel slides (μ-slide I; Ibidi) coated with 5.0 μg/mL ICAM-1/FC (R&D Systems) following incubation with 12.0 μg/mL goat anti-human IgG antibody (Dianova). Subsequently, time-lapse series were monitored with an inverted Olympus Fluoview 1000 confocal microscope equipped with an automated motorized xyz stage (Märzhäuser) and a climate chamber [Evotec; 37 °C and 5% (vol/vol) CO2 with humidity]. DC/T-cell interactions were recorded over a period of 12 h by capture of green fluorescence and differential interference contrast images every 2 min with a 0.75 Plan S Apo 20× objective (Olympus). The duration of DC/T-cell interactions was calculated with Fluoview software version 3.0 (Olympus).
Monitoring Intracellular Ca Flux.
DesTCR T cells were incubated with the fluorescent calcium probe Fluo-3 (Life Technologies) for 30 min. Fluorescence was measured by flow cytometry for 90 s before and 270 s after addition of a α-CD3 antibody.
Analysis of Lck Activity.
Lck from T-cell lysates was precipitated using protein A/G Sepharose beads. Lck activity was determined using the Tyrosine Kinase Assay Kit (Millipore). Briefly, precipitated Lck was incubated with biotinylated Poly(Glu4Tyr) peptides. After 1 h, peptides were isolated and precipitated using streptavidin-coated beads. Colorimetric analysis at 450 nm was performed after addition of a phosphospecific antibody (4G10; Millipore).
Monitoring Bcl-6 and NFAT Expression in Nuclear Extracts.
Cells were harvested and lysed in 10 mM Hepes (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.7% Nonidet P-40, and 1 mM DTT in the presence of protease inhibitor. After centrifugation, the residual pellet was resuspended in 20 mM Hepes (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT. Resulting nuclear extracts were analyzed by Western blot.
T-Cell Surface Biotinylation and Coimmunoprecipitation Experiments.
DesTCR T cells were incubated with 0.5 mg/mL biotin for 30 min and washed extensively. Afterward, cells were lysed and 10 μg/mL FcMR was added for 1 h on ice. Subsequently, FcMR was immunoprecipitated using protein A/G-based affinity chromatography, and samples were loaded on an SDS/PAGE gel for analysis by Western blot using streptavidin or a CD45-specific antibody. Alternatively, a CD45-specific antibody was added to the lysates of DesTCR T cells and precipitated by protein A/G-based affinity chromatography for subsequent Western blot analysis using FcMR.
Analysis of CTLA-4 Expression.
DesTCR T cells were cocultured with wild-type or MR-deficient BM-DCs. After 3 d, a fluorochrome-labeled CTLA-4–specific antibody or isotype control was added. After another 4 h, CTLA-4 expression was determined by flow cytometry.
CD45 Phosphatase Assay.
CD45 was precipitated from cell lysates and incubated with either 2 mM 4-nitrophenyl phosphate (4-NPP) or 0.25 μg of phosphorylated or nonphosphorylated biotin-conjugated TATEGQYQPQ for 18 h at 37 °C in the presence or absence of 1 μM CD45-specific inhibitor N-(9,10-dioxo-9,10-dihydro-phenanthren-2-yl)-2,2-dimethyl-propionamide. Phosphatase activity was quantified by colorimetry at 405 nm (4-NPP) or at 450 nm (TATEGQYQPQ) after affinity chromatography using streptavidin-agarose, staining with the phosphospecific antibody 4G10, an HRP-conjugated secondary antibody, and addition of TMB One (Kementec).
EMSA Experiments.
HEK293T cells were transfected with an expression vector encoding murine Bcl-6. Nuclear extracts were incubated for 30 min with 1 μM double-stranded 5-carboxyfluorescein–labeled oligos in the presence or absence of 50 μM unlabeled competitor or mutated oligos and analyzed on a 5% (wt/vol) polyacrylamide gel. The following oligos were used: CTLA-4 oligo I (actgaggggctcatagaaggacacaatttt), CTLA-4 oligo II (actgcagctgccttctaggacttggccttt), mutated CTLA-4 oligo I (actgagggggattctcggctacacaatttt), mutated CTLA-4 oligo II (actgcagctggtcagctcatgttggccttt), and PDCD2 competitor (ggacctacccttctaggaaaaaaccatcc).
Luciferase Activity Assays.
Expression from the CTLA-4 promoter was analyzed using the Dual-Luciferase Reporter Assay (Promega). Wild-type CTLA-4 promoter (−400 to +1) and the CTLA-4 promoter with a mutated Bcl-6 recognition site (GGGCTCAGATCAGGACA instead of GGGCTCATAGAAGGACA, underlined Bcl-6 recognition site) were cloned in front of the firefly luciferase reporter gene. Reporter plasmids were transfected into HEK293T cells, together with an expression plasmid for Bcl-6 (or empty plasmid in control samples) and a Renilla luciferase expression plasmid. Activity of the CTLA-4 promoter was depicted as the ratio between firefly and Renilla luciferase activity.
Chromatin Immunoprecipitation.
DesTCR T cells were activated by αCD3. Nuclear extracts were isolated, and chromatin immunoprecipitation was performed as described (44) using anti-Bcl-6 (sc858; Santa Cruz Biotechnology) and AATAAACCCTTTTGTGTACTGAG and CTCTTACTTAGCGTCCCATG (Bcl-6 binding region 1), AGGACCTCAGCACATTTG and GTCTCCACTCACCTTCAG (Bcl-6 binding region 2), or GTGGGAGGGTCCATATACTG and ACACATACACACAGCATATACTC (INADL promoter, negative control). The enrichment of target regions in the immunoprecipitation products relative to input chromatin was calculated by the change in the cycling threshold (2−ΔΔCT method). Immunoprecipitation with control IgG (12-370; Millipore) was used to normalize for nonspecific background. Results are depicted relative to input, normalized to samples with an isotype control.
Isolation of DCs from Liver, Lung, or Spleen.
Organs were treated with 0.2 mg/mL collagenase and 1,000 U/mL DNase I for 1 h, homogenized, prepurified by Percoll density gradient centrifugation, and stained with DC-specific antibodies.
For activation of OT-I cells by liver DCs, CD11c+ cells were sorted using a FACSAria (Becton Dickinson).
Bioinformatic Analysis.
Raw intensity microarray data were processed using GenomeStudio V2011.1 (Illumina). Subsequent analyses were performed using Partek Genomics Suite V6.6 (PGS). Nonnormalized data were imported from GenomeStudio using the default PGS report builder. After quantile normalization in PGS, all transcripts not expressed in at least one condition over the background of 7,248 log2 expression were removed from the dataset. Variable transcripts and significantly DE transcripts were calculated using a two-way ANOVA model. Variable genes were defined by an FDR-corrected P value <0.05, and significantly DE transcripts were defined by an FDR-corrected P value <0.05 and a fold change of ±2. Variable genes were analyzed by hierarchical clustering using PGS default settings visualizing condition-specific similar or differential gene expression. To generate a tolerance signature that could be used in the GSEA, we performed self-organizing map (SOM) clustering on a dataset (GSE32025) that comprises microarray data of naive, memory, rescued, tolerant, and in vivo tolerized T cells. Genes were grouped in gene sets associated with one of the five conditions showing a SOM coefficient higher than 1.7. This analysis resulted in an in vivo tolerized T-cell signature of 1,120 transcripts. GSEA was performed on the in vivo tolerized T-cell signature in 1,000 permutations by using PGS. P values of GSEA were plotted by a line plot comparing FcMR-treated T cells against controls over time. To determine whether genes associated with tolerance showed differential transcriptional regulation, we performed a literature search for T-cell anergy and tolerance-associated genes (11–20). Only those genes were used for the further analysis if they were DE (FDR-corrected P value <0.05, fold change of ±2) at least at one time point (6). A rank plot was calculated of the sum of ranks for each time point (t = 4 h, 18 h, 48 h) ranking the ratio of FcMR-stimulated and control T cells for anergy and/or tolerance genes. The highest positive ratio of a gene was rank 6, and the smallest ratio was rank 1. To determine putative binding of TFs to the CTLA-4 locus, we performed a promoter binding prediction using Genomatix (www.genomatix.de).
Supplementary Material
Acknowledgments
We thank Luisa Martinez-Pomares for plasmids encoding FcMR and Maren Schell for technical support. This work was supported by the Collaborative Research Center 704 (Project A24) and Grant INST 217/575-1 (to J.L.S.) from the German Research Foundation. S.B., I.F., W.K., P.A.K., M.B., and J.L.S. are members of the Deutsche Forschungsgemeinschaft Excellence Cluster ImmunoSensation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE45805).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605885113/-/DCSupplemental.
References
- 1.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92(6):1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 2.Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316(5824):612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 3.Luca ME, et al. Mannosylated PLP(139-151) induces peptide-specific tolerance to experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;160(1-2):178–187. doi: 10.1016/j.jneuroim.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Kel J, et al. Soluble mannosylated myelin peptide inhibits the encephalitogenicity of autoreactive T cells during experimental autoimmune encephalomyelitis. Am J Pathol. 2007;170(1):272–280. doi: 10.2353/ajpath.2007.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guimezanes A, et al. Identification of endogenous peptides recognized by in vivo or in vitro generated alloreactive cytotoxic T lymphocytes: Distinct characteristics correlated with CD8 dependence. Eur J Immunol. 2001;31(2):421–432. doi: 10.1002/1521-4141(200102)31:2<421::aid-immu421>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Pomares L, et al. Carbohydrate-independent recognition of collagens by the macrophage mannose receptor. Eur J Immunol. 2006;36(5):1074–1082. doi: 10.1002/eji.200535685. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Pomares L, et al. Cell-specific glycoforms of sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the mannose receptor. J Biol Chem. 1999;274(49):35211–35218. doi: 10.1074/jbc.274.49.35211. [DOI] [PubMed] [Google Scholar]
- 8.Zehner M, et al. Intraendosomal flow cytometry: A novel approach to analyze the protein composition of antigen-loaded endosomes. Eur J Immunol. 2012;42(8):2187–2190. doi: 10.1002/eji.201142089. [DOI] [PubMed] [Google Scholar]
- 9.Urbanek RA, et al. Potent reversible inhibitors of the protein tyrosine phosphatase CD45. J Med Chem. 2001;44(11):1777–1793. doi: 10.1021/jm000447i. [DOI] [PubMed] [Google Scholar]
- 10.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335(6069):723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battke F, Symons S, Nieselt K. Mayday--integrative analytics for expression data. BMC Bioinformatics. 2010;11:121. doi: 10.1186/1471-2105-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7(8):599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 14.Hochweller K, Anderton SM. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur J Immunol. 2005;35(4):1086–1096. doi: 10.1002/eji.200425891. [DOI] [PubMed] [Google Scholar]
- 15.Parish IA, et al. The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood. 2009;113(19):4575–4585. doi: 10.1182/blood-2008-10-185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safford M, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6(5):472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 18.Vang T, et al. Protein tyrosine phosphatases in autoimmunity. Annu Rev Immunol. 2008;26:29–55. doi: 10.1146/annurev.immunol.26.021607.090418. [DOI] [PubMed] [Google Scholar]
- 19.Williams O, Brady HJ. The role of molecules that mediate apoptosis in T-cell selection. Trends Immunol. 2001;22(2):107–111. doi: 10.1016/s1471-4906(00)01797-x. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9(1):50–55. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fecteau S, et al. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat Immunol. 2001;2(1):58–63. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 22.Barbeau B, Robichaud GA, Fortin JF, Tremblay MJ. Negative regulation of the NFAT1 factor by CD45: Implication in HIV-1 long terminal repeat activation. J Immunol. 2001;167(5):2700–2713. doi: 10.4049/jimmunol.167.5.2700. [DOI] [PubMed] [Google Scholar]
- 23.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron BW, et al. Repression of the PDCD2 gene by BCL6 and the implications for the pathogenesis of human B and T cell lymphomas. Proc Natl Acad Sci USA. 2007;104(18):7449–7454. doi: 10.1073/pnas.0701770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerchietti LC, et al. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell. 2010;17(4):400–411. doi: 10.1016/j.ccr.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgdorf S, et al. Steady-state cross-presentation of OVA is mannose receptor-dependent but inhibitable by collagen fragments. Proc Natl Acad Sci USA. 2010;107(13):E48–E49, author reply E50–E51. doi: 10.1073/pnas.1000598107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci USA. 2009;106(48):20377–20381. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohyama M, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457(7227):318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linehan SA, Martínez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189(12):1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franken L, et al. Splenic red pulp macrophages are intrinsically superparamagnetic and contaminate magnetic cell isolates. Sci Rep. 2015;5:12940. doi: 10.1038/srep12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Vliet SJ, Gringhuis SI, Geijtenbeek TBH, van Kooyk Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 2006;7(11):1200–1208. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 32.Chieppa M, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171(9):4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 33.Allavena P, et al. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010;2010:547179. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longoni D, Piemonti L, Bernasconi S, Mantovani A, Allavena P. Interleukin-10 increases mannose receptor expression and endocytic activity in monocyte-derived dendritic cells. Int J Clin Lab Res. 1998;28(3):162–169. doi: 10.1007/s005990050037. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber S, et al. Prostaglandin E specifically upregulates the expression of the mannose-receptor on mouse bone marrow-derived macrophages. Cell Regul. 1990;1(5):403–413. doi: 10.1091/mbc.1.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepherd VL, Abdolrasulnia R, Garrett M, Cowan HB. Down-regulation of mannose receptor activity in macrophages after treatment with lipopolysaccharide and phorbol esters. J Immunol. 1990;145(5):1530–1536. [PubMed] [Google Scholar]
- 37.Harris N, Super M, Rits M, Chang G, Ezekowitz RA. Characterization of the murine macrophage mannose receptor: Demonstration that the downregulation of receptor expression mediated by interferon-gamma occurs at the level of transcription. Blood. 1992;80(9):2363–2373. [PubMed] [Google Scholar]
- 38.Limmer A, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35(10):2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 39.van Vliet SJ, van Liempt E, Geijtenbeek TBH, van Kooyk Y. Differential regulation of C-type lectin expression on tolerogenic dendritic cell subsets. Immunobiology. 2006;211(6-8):577–585. doi: 10.1016/j.imbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11(2):114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida K, et al. Bcl6 controls granzyme B expression in effector CD8+ T cells. Eur J Immunol. 2006;36(12):3146–3156. doi: 10.1002/eji.200636165. [DOI] [PubMed] [Google Scholar]
- 42.Ariyan C, et al. Cutting edge: Transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003;171(11):5673–5677. doi: 10.4049/jimmunol.171.11.5673. [DOI] [PubMed] [Google Scholar]
- 43.Townsend A, et al. A mutant cell in which association of class I heavy and light chains is induced by viral peptides. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):299–308. doi: 10.1101/sqb.1989.054.01.038. [DOI] [PubMed] [Google Scholar]
- 44.Beyer M, et al. Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat Immunol. 2011;12(9):898–907. doi: 10.1038/ni.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.