Abstract
One approach to the development of an artificial graft material could rely on uniform coverage of a resorbable biomaterial with bone extracellular matrix (ECM). To achieve this on the surface of poly(propylene fumarate) (PPF) scaffolds, we selected a growth factor regime of basic fibroblast growth factor (FGF-2) (5 ng/mL), platelet-derived growth factor (PDGF-BB) (40 ng/mL), and epidermal growth factor (EGF) (20 ng/mL) to stimulate proliferation of bone marrow-derived human mesenchymal stem cells (BM-hMSCs). Bone morphogenetic protein (BMP) 4 (50 ng/mL), 6 (50 ng/mL), and 7 (27 ng/mL) in the presence of the following osteogenic substances: dexamethasone (10−7 M), β-glycerophosphate (10 mM), and ascorbic acid (50 μg/mL) were chosen to induce differentiation of BM-hMSCs into ECM-secreting osteoblasts. These growth factors were also studied at 10× concentration to determine dose effect. Proliferation was analyzed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, scanning electron microscopy (SEM), and toluidine blue staining, whereas differentiation was analyzed through alizarin red S staining and assay, alkaline phosphatase (ALP) staining and assay, and SEM. The proliferation study suggests that a combination of EGF, PDGF-BB, and FGF-2 growth factors at optimal concentration over a period of 1 week exhibits significantly (p = 0.001) higher number of cells (116,024 ± 5165) than these cytokines without EGF (91,706 ± 11,965). Increasing the dosage does not show any significant effect. The BM-hMSC differentiation study results show that ALP enzyme production and mineral deposition increase from day 14 to day 21 in all groups containing BMPs and osteogenic medium. However, mineralization is significantly higher in the BMP-7 group. Furthermore, the feasibility of translating the results from two dimensional thin films to three dimensional-printed PPF scaffolds was determined through uniform initial seeding and spreading of BM-hMSCs. Therefore, we have determined the optimum dose of growth factors for proliferation and differentiation of BM-hMSCs on the surface of PPF scaffolds, which can be used to produce ECM-coated implants for the treatment of bone defects.
Introduction
Critical size1 bone defects are an important health concern and there is a high demand worldwide for restorative bone materials. In the United States alone, annually more than 0.5 million patients receive bone defect repair surgery, with an associated cost of >$2.5 billion.2 Bone tissue engineering uses a regeneration-based method to address the limitations of current approaches, which depend largely on tissue transplantation along with bone cement/ceramic fillers, drugs, and artificial prosthesis/fixation devices. Some of the limitations associated with tissue transplantation are patient morbidity, infection, insufficient donor tissue, and high cost.2 Tissue engineering mainly involves three effectors, either as individual entities or in combination: biomaterials, cells, and cytokines.3
Biomaterials used for bone tissue engineering can be classified into four main categories: (1) polymers, (2) ceramic, or bioactive glass, (3) composites of polymer and ceramics, and (4) resorbable metals.4,5 Some of the polymers used for bone tissue engineering are hyaluronic acid, chitosan, collagen, poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(caprolactone) (PCL), poly(propylene fumarate) (PPF), and so on.6 Of these, PPF belongs to the category of photocrosslinkable polyesters. PPF can be three dimensionally (3D) printed under the guidance of ultra violet (UV) light.7 PPF also exhibits other favorable properties such as cell attachment, useful resorption kinetics, and biocompatibility.7–13
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have been established as one of the favorable progenitor cells for studying tissue regeneration because of their immunomodulatory potential.14–17 They present low to medium amounts of human leukocyte antigen classes I and II and, therefore, evade the immune system because they are not recognized as foreign cells.15 This unique property of BM-MSCs allows the use of allogeneic sources for human applications. Unlike BM-MSCs, other sources of allogeneic progenitor cells, such as adipose-derived MSCs, muscle-derived stem cells, induced pluripotent stem cells, and so on, have not been demonstrated to lack antigens after differentiation into bone.18 Therefore, until those data exist, if stem cells other than BM-MSCs are used, it may be wise to avoid an immune response by using only autologous stem cells.
Proliferation and differentiation of these progenitor cells on many biomaterial surfaces can be influenced by cytokines and other bioactive factors. Some of the molecules that have been shown to increase the proliferation of BM-MSCs include basic fibroblast growth factor (FGF-2), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF-BB).19–21 Growth factors that have been shown to drive BM-MSCs toward the bone lineage are bone morphogenetic protein (BMP)-4,22,23 BMP-2,24–27 BMP-6,28 BMP-7,29–31 etc. The BMPs can be used to bring about different stages of BM-MSC differentiation. Since all of these cytokines are very powerful molecules that can have pleiotropic effects, it may be wise to limit their use to in vitro preculturing of tissue-engineered implants outside the body, that is, in vitro, rather than administering them systemically, that is, in vivo. System administration has been associated with dangerous sequelae such as malignancy, severe pain, teratogenicity, and so on.32,33
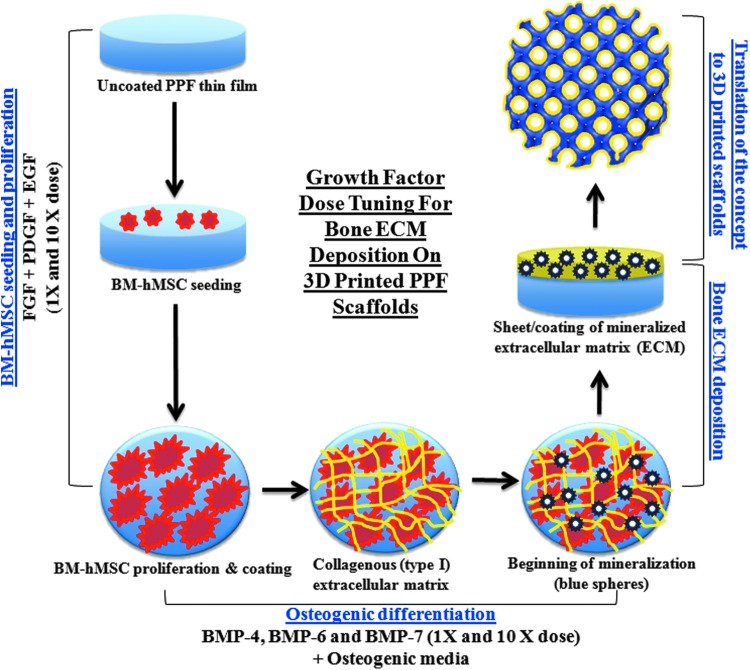
We hypothesize that an optimal combination of a suitable biomaterial scaffold, bone marrow-derived human mesenchymal stem cells (BM-hMSCs), and bioactive molecules including cytokines can be used to achieve a coating of bone extracellular matrix (ECM) that fully coats the scaffold surface. The cells will not be decellularized and they are expected to assist in bone formation upon implantation inside the body. The goal of this cellularized bone ECM-coated scaffold would be to serve as a tissue-engineered bone graft. Although this approach is consistent with many tissue engineering studies, to our knowledge no such device has been translated to the clinic. More specifically, we are unaware of any report of a strategy to prepare a tissue-engineered bone graft similar to the subject of this study. In this article, we present results of our study on this matter. Combinations of FGF-2, EGF, and PDGF and BMP-4, BMP-6, or BMP-7 plus osteogenic factors were used for the analysis of BM-hMSC proliferation and differentiation, respectively. A schematic representation of the process for ECM layer deposition is presented in Figure 1. Although our comprehensive optimization study was performed on PPF thin films, we also present the results of a study validating optimal seeding and proliferation of BM-hMSCs on three dimensional (3D)-printed PPF scaffolds.
FIG. 1.
Study schema: A schematic representation of BM-hMSC (red) proliferation, osteogenic differentiation and further deposition of a mineralized (blue dots) ECM layer (yellow) on the surface of poly(propylene fumarate) (PPF) (blue) thin films under the influence of cytokines and other bioactive molecules. Finally, our goal of a tissue-engineered bone graft requires translation of our method for the production of bone substitute material, what we refer to as a “tissue-engineered bone graft” requires translating our protocol for ECM layer deposition on resorbable PPF thin films to 3D-printed PPF scaffolds with a Schoen gyroid pore geometry. 3D, three dimensional; BM-hMSC, bone marrow-derived human mesenchymal stem cell; ECM, extracellular matrix. Color images available online at www.liebertpub.com/tec
Materials and Methods
Experimental design
Four experimental groups were studied to validate our hypothesis on the optimal medium for BM-hMSC proliferation on PPF thin films: (1) 1X dose of FGF-2, PDGF-BB, and EGF (E1X), (2) 10X dose of FGF-2, PDGF-BB, and EGF (E10X), 1X dose of FGF-2 and PDGF-BB (NE1X), and 10X dose of FGF-2 and PDGF-BB (NE10X) in hMSC Differentiation Basal Medium from RoosterBio, Inc. In these experimental group abbreviations “E” stands for groups containing EGF and “NE” stands for the group not containing EGF. The control medium (CM) group (without growth factors) in our cell proliferation study was hMSC Differentiation Basal Medium from RoosterBio, Inc.
Seven experimental groups were studied for BM-hMSC differentiation to an ECM-secreting osteoblast: OM (osteogenic medium containing 10−7 M dexamethasone, 10 mM β-glycerophosphate, and 50 μg/mL ascorbic acid), 1X and 10X dose of BMP-4 in OM (1X and 10X BMP-4), 1X and 10X dose of BMP-6 in OM (1X and 10X BMP-6), and 1X and 10X dose of BMP-7 in OM (1X and 10X BMP-7). The control group for this study was the same as proliferation study.
The respective doses for each growth factor (obtained from PeproTech, NJ) are mentioned in Table 1. The 10 times doses (10X) were chosen as a second group of study to confirm whether there was a significant effect while testing a wide difference in the doses.
Table 1.
The Growth Factor Doses Chosen for Optimization Study of Osteogenesis of Bone Marrow-Derived Human Mesenchymal Stem Cells on Poly(propylene fumarate) Surfaces
| Growth factor type | Dose (1X), ng/mL | Dose (10X), ng/mL | Reference |
|---|---|---|---|
| Proliferation regime | |||
| FGF-2 | 5 | 50 | 34 |
| PDGF-BB | 40 | 400 | 35 |
| EGF | 20 | 200 | 36 |
| Differentiation regime | |||
| BMP-4 | 50 | 500 | 23,28 |
| BMP-6 | 50 | 500 | 28 |
| BMP-7 | 27 | 270 | 12 |
BMP, bone morphogenetic protein; EGF, epidermal growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor.
PPF thin film and scaffold fabrication
Six thin films per group were used for quantitative analysis, whereas three thin films/scaffolds/group were used for qualitative analysis. PPF was synthesized in and provided by the laboratory of Dr. Matthew Becker, Department of Polymer Science, University of Akron (Akron, OH). Our storage resin of 3:1 PPF:DEF was diluted to 1:1 using DEF (Diethyl Fumarate; Sigma-Aldrich, MO). The following photoinitiators and dyes were mixed homogenously into the resin to create optimal material flow and photo-cross-linking: Irgacure 819/BAPO (BASF, NJ) (0.7% w/w%), oxybenzone/2-hydroxy-4-methoxybenzophenone (Sigma-Aldrich, MO) (0.4% w/w%), and Irgacure 784 (BASF, NJ) (0.3% w/w%). PPF thin films were created by placing 6–7 drops of resin between two glass microscope slides. The slides were then placed into a UV box for 30 min to promote enough cross-linking to allow samples to be cut into 1 × 1 cm squares. After cutting, samples were placed back into the UV box for 7.5 h to ensure complete cross-linking of the polymer. The thin films were used to conduct the proliferation and differentiation medium optimization studies.
After completion of those studies, 3D-printed porous cylindrical scaffolds with Schoen gyroid triply periodic minimal surface were fabricated with dimensions of 6 mm diameter and 5 mm height, using the same resin chemistry to validate the optimized medium regime on the 3D scaffolds. This pore geometry included a strut size of 187.5 μm, pore size of 625 μm, surface area of 342.27 mm2, and volume of 17.77 mm3 of PPF. A computer-aided design file of these features was created using SolidWorks software (Dassault Systèmes, Waltham, MA) and 3D printing was performed in an EnvisionTEC Perfactory® (Dearborn, MI) P3 3D printer.
To remove the uncured resin from the cross-linked polymer in the case of both thin films and scaffolds, washing was performed using 70% acetone (VWR, PA) and phosphate-buffered saline (PBS) (Life Technologies, NY). Before cell seeding, these thin films and scaffolds were incubated in fetal bovine serum (FBS) overnight.
Cell culture
BM-hMSCs (MSC-001; RoosterBio, Inc., Frederick, MD) were obtained from RoosterBio at passage No. 3 and were seeded at passage No. 5 using hMSC Differentiation Basal Medium (KT-004; RoosterBio, Inc., MD). PPF thin films were seeded at a density of 2.5 × 104 cells/cm2, whereas the 3D scaffolds were seeded with 3.6 × 105 cells/scaffold. The scaffold cell density was determined through previous mock proliferation studies on the same size scaffolds. It was ascertained that the cell density used in the thin films was not sufficient for proliferation to occur in the scaffolds. For cell seeding, briefly, the FBS was aspirated from the thin films and scaffolds followed by drop-wise pipetting of cell suspension into each well. In the case of scaffolds, the FBS aspiration was performed on all sides of the scaffolds while holding them with a sterile forceps to ensure complete aspiration of FBS. Cells were cultured on PPF thin films and scaffolds in ultra-low attachment well plates at 37°C in an atmosphere of >95% humidity and 5% carbon dioxide.
Bright field imaging
Five groups (n = 3) of cell densities, that is, 10,000, 25,000, 50,000, 100,000, and 200,000 cells/mL, were created by adding medium containing respective cell densities to the thin films. Cells were incubated for 4 h to allow cell attachment while preventing proliferation. After 4 h, bright field imaging was performed using a 12.8 MP digital camera on an Olympus CKX41 microscope (Olympus, Center Valley, PA).
MTT assay
Six thin films (N = 6) per group were cultured for each respective time point and thereafter collected for MTT (Sigma-Aldrich, MO) assay. In brief, once collected, they were washed in PBS and then incubated in 1 mL plain medium containing 5 mg/mL MTT dye. The incubation was carried out for 4 h in humidified (>95%) conditions in the presence of 5% CO2 at 37°C. After incubation, the well plate was removed from the incubator and MTT reagent was aspirated. One milliliter dimethyl sulfoxide (DMSO; VWR, PA) was added to each well for formazan crystal solubilization. The absorbance was read in absorbance units (A.U.) using a spectrophotometer at 490 nm wavelength.
SEM analysis
Three samples (N = 3) from each group were used for SEM analysis. Sample preparation was as follows: wash in PBS, fix in 1% glutaraldehyde for 30 min, treat with OsO4 for 30 min (to enhance image contrast). Next, graded ethanol dehydration (from 50% to 100%), drying in a critical point dryer (Pelco, CA), was conducted. Finally, these specimens were coated with gold plasma in a sputter coater (Cressington Scientific Instruments, Watford, United Kingdom). After gold coating, samples were viewed and imaged in a FEI Nova NanoSEM 400 scanning electron microscope (FEI, Hillsboro, OR) under the following settings: high voltage = 5 kV, spot size = 3, and working distance = 6–7 mm.
Toluidine blue assay
Samples for toluidine blue (Sigma-Aldrich, MO) staining (n = 3) were first washed in 1X PBS for 5 min then fixed in 3.7% formaldehyde (Sigma-Aldrich, MO) solution in PBS for 10 min at room temperature. Samples were washed again in PBS then incubated with 0.1% Triton X-100 (Sigma-Aldrich, MO) for 5 min in humidified conditions. Samples were then incubated in 1% bovine serum albumin (Life technologies, NY) for 30 min at room temperature followed by PBS wash. A solution of 1% toluidine blue and 1% sodium tetraborate (Sigma-Aldrich, MO) was made in distilled water (w/v%), mixed and added drop wise to the well plate, which was then placed into a 50°C oven until the stain began to dry. Samples were rinsed several times with distilled water followed by 95% and 100% ethanol. Finally, the samples were viewed microscopically.
ALP assay
One tablet of p-nitrophenol phosphate (Sigma-Aldrich, MO) (pNPP) and one tablet of Tris buffer were dissolved in distilled water (20 mL) to prepare ready-to-use pNPP substrate solution. The samples (n = 6) were washed thoroughly twice in PBS for 10 min. The pNPP substrate solution was added to each sample and incubated in the dark at room temperature for 30 min. After incubation, 200 μL from each well was aliquoted in a 96-well plate and the absorbance was read at 405 nm using a multiwell plate reader (Molecular Devices, CA).
ALP staining
The ALP staining samples (n = 3) were fixed in citrate-buffered acetone solution. Diluted diazonium salt solution was created by dissolving a Fast Violet B (Sigma-Aldrich, MO) capsule in distilled water. Alkaline dye solution was made by combining the dilute diazonium salt solution with Napthol AS-MX Phosphate alkaline solution (Sigma-Aldrich, MO) (4% v/v). The samples were incubated in alkaline dye solution at room temperature for 30 min in the dark. Samples were rinsed with deionized water for 2 min and then placed in Mayer's Hematoxylin Solution (Sigma-Aldrich, MO) for 10 min. Observations were made using a bright field microscope.
Alizarin Red S staining and assay
The medium from samples (n = 6) was removed and samples were gently washed three times in 1X PBS. Cells were fixed using cold 70% ethanol for 1 h and allowed to air dry. A 0.5% ammonium hydroxide (Sigma-Aldrich, MO) solution was prepared by diluting 30% stock solution in distilled water. Alizarin Red S (ARS) (Sigma-Aldrich, MO) staining solution (40 mM) was prepared by dissolving the dye in distilled water. The pH was adjusted to 4.1–4.3 using 0.5% ammonium hydroxide. The samples were incubated in ARS solution for 1 h at room temperature, followed by three distilled water rinses and imaging. A 10% cetylpyridinium chloride (Sigma-Aldrich, MO) solution (w/v) was made by dissolving in 0.1 M sodium phosphate buffer (pH 7.0). The ARS samples were destained in this solution and the absorbance readings (A.U.) were obtained at 562 nm.
Statistical analysis
All the statistical analyses were performed using univariate ANOVA and post hoc tests were conducted using Tukey HSD test. We used SPSS 16 software to conduct all the statistical analyses at 95% confidence interval level and p < 0.05 was considered significant.
Results
Determination of cell seeding density
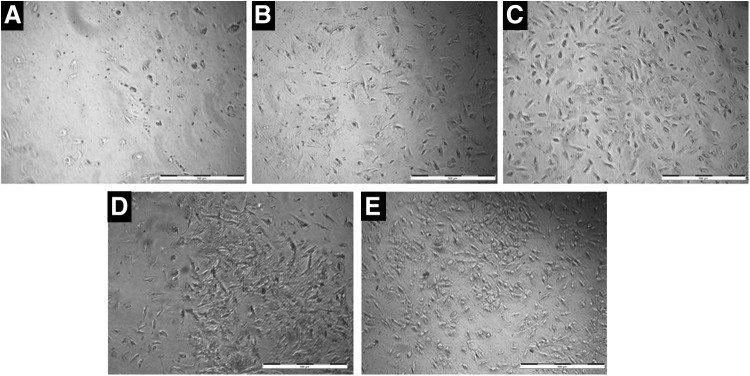
Optimal cell seeding density was determined by seeding cells at different densities onto PPF scaffolds and observing the cells under microscope for uniform coating and lower density. In Figure 2, it is clear that 10,000 cells/mL (Fig. 2A) did not produce a uniform, complete surface cell coating due to lower cell density; therefore, at this density more time is needed to proliferate and cover the thin film surface than with 25,000 cells/mL (Fig. 2B) and cell seeding densities of 50,000 cells/mL (Fig. 2C) or higher, that is, 100,000 (Fig. 2D) or 200,000 (Fig. 2E) cells/mL led to high cell densities that would limit further cell proliferation. It is preferable to observe the proliferation phase in the shortest duration of time; therefore, 25,000 cells/mL (Fig. 2B) was chosen for the proliferation study experiments.
FIG. 2.
Determination of optimum cell seeding density for the study of BM-hMSC proliferation on PPF polymer surface. The bright field microscope images of BM-hMSC-seeded PPF thin films are shown at cell densities of 10,000 (A), 25,000 (B), 50,000 (C), 100,000 (D), and, 200,000 cells/mL (E), respectively. The density of 25,000 cells/mL was chosen as initial cell density due to uniform and scattered distribution of cells (scale bar = 500 μm).
Analysis of cell proliferation
MTT assay
MTT assay was used to determine cell proliferation. A standard plot is prepared to correlate the absorbance values (absorbance units, A.U.) with the number of cells. Cell proliferation is represented in the form of number of cells/group/time point. All groups showed a significantly increasing proliferation trend from days 1 to 7 (Fig. 3A). At day 7, the number of cells for NE1X, NE10X, E1X, E10X, and, CM groups was 91,706 ± 11,965, 110,209 ± 4902, 116,024 ± 5165, 102,212 ± 16,282 and, 369,76 ± 4490, respectively. Among the groups at 1X dosage, E1X showed significantly higher proliferation than NE1X (p = 0.001) and CM (p = 0.000) groups. Similarly, NE1X had significantly higher proliferation than CM (p = 0.000). Therefore, the addition of growth factors supports cell proliferation at 1X and 10X dosages, but increasing the dosage to 10X concentrations did not significantly increase the outcome in comparison with the 1X dosages.
FIG. 3.
Study of the effect of including EGF on cell proliferation in addition to the presence of FGF-2 and PDGF growth factors. EGF was added at 1X (i.e., single dose, which is the dose that is optimal based on the literature or our previous experience) and 10X doses of EGF, FGF-2, and PDGF. As shown in (A), the number of cells determined by MTT assay shows a significantly (p = 0.001) higher cell proliferation with the addition of a 1X EGF-containing (E1X) dose than the group without EGF (NE1X) or the 10X doses. Whereas the CM depicts lowest cell proliferation. (B) represents the qualitative analysis of these results through toluidine blue staining. All the groups show an increasing trend of cell proliferation from days 1 to 7, similar to that of the MTT results (scale bar = 500 μm). CM, control medium; EGF, epidermal growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor. Color images available online at www.liebertpub.com/tec
Toluidine blue staining
Toluidine blue staining was performed for qualitative confirmation of the trends observed during MTT assay. As observed in Figure 3B, the cells (represented by blue color) show an increasing proliferation trend from days 1 to 7 in all the groups. As observed, the cells reached confluence by day 7. Proliferation was found to be relatively low for the CM group (which did not contain any growth factors) compared with rest of the groups (i.e., E1X, E10X, NE1X, and NE10X). Hence, the qualitative toluidine blue assay confirmed results seen from the quantitative MTT assay.
SEM analysis
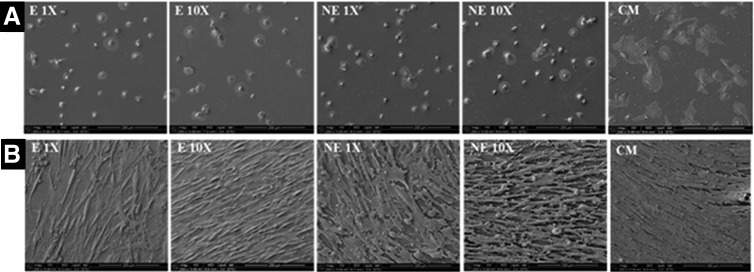
SEM imaging was performed for observation of hMSC cell distribution on the PPF surface. At day 0, all growth factor-containing groups showed uniform cell distribution on the surface of thin films (Fig. 4A), with cells showing a rounded morphology indicating initial attachment. By day 7 (Fig. 4B), all groups showed uniform cell coating, hence, we chose this as the time point for switching the medium for the analysis of hMSC cell differentiation towards the bone lineage. Whereas the CM group shows higher cell spreading at both days 0 and 7 time points.
FIG. 4.
Scanning electron microscope image analysis of BM-hMSC proliferation. As shown in (A), all the growth factor-containing groups (E1X, NE1X, E10X, and NE10X) show similar initial attachment at day 0 with mostly rounded morphology. Whereas these cells spread uniformly and coat the polymer surface by day 7 as demonstrated in (B). However, the CM group shows greater cell spreading at both time points (magnification of all images = 250X).
Analysis of hMSC differentiation to bone lineage
Alkaline phosphatase assay and staining
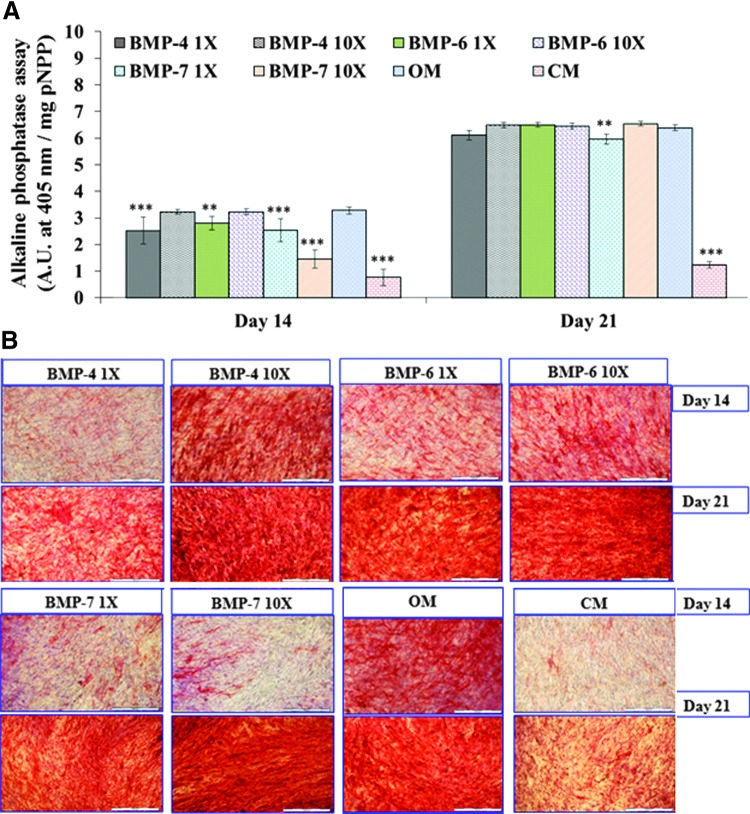
Proliferating osteoblasts are known to produce alkaline phosphatase (ALP) enzyme and, therefore, quantification of ALP levels can track the differentiation of hMSCs to osteoblasts. Absorbance values at 405 nm were used to quantify ALP enzyme production for each of the groups. All groups exhibited increasing differentiation from MSC to osteoblast from day 14 to day 21 (Fig. 5A). Days 0 to 7 were part of the proliferation study. The average values at day 14 for BMP-4 1X, BMP-4 10X, BMP-6 1X, BMP-6 10X, BMP-7 1X, BMP-7 10X, OM, and CM are 2.52 ± 0.51, 3.23 ± 0.10, 2.80 ± 0.25, 3.22 ± 0.11, 2.54 ± 0.43, 1.45 ± 0.34, 3.28 ± 0.14, and 0.76 ± 0.30 A.U., respectively. Readings for the same sequence of groups at day 21 were as follows: 6.11 ± 0.18, 6.49 ± 0.10, 6.50 ± 0.09, 6.45 ± 0.11, 5.96 ± 0.19, 6.54 ± 0.09, 6.39 ± 0.11, and 1.23 ± 0.11 A.U., respectively. It was found that at day 14, OM showed significantly higher values than 1X doses of BMP-4 (p = 0.000), BMP-6 (p = 0.010), and BMP-7 (p = 0.000), along with BMP-7 10 X (p = 0.000) and CM (p = 0.000). Whereas at day 21, OM showed significantly higher values than only BMP-7 1 X (p = 0.006) group and CM (p = 0.000). Dosage effect was observed at both time points and values were significantly (p < 0.05) higher at 10 X concentration for all the three growth factor types except BMP-6 at day 21 (p = 0.729).
FIG. 5.
Analysis of osteogenic differentiation through ALP enzyme assay and staining. During ALP enzyme assay, shown in (A), all the groups show increase in absorbance readings with increase in time from days 14 to 21. The OM group at the day 14 time point shows significantly (**p ≤ 0.01, ***p ≤ 0.001) higher values than all other groups except BMP-4 10X and BMP-6 10X. Whereas at day 21, all growth factor-containing groups show a rapid increase in ALP production. The OM and CM groups show statistically lower values than the other groups. (B) depicts the ALP staining results for the same group types and the diffused red staining indicates the presence of this enzyme. All groups showed results that are in line with those of the ALP assay during staining (scale bar = 500 μm). ALP, alkaline phosphatase; BMP, bone morphogenetic protein; OM, osteogenic medium. Color images available online at www.liebertpub.com/tec
ALP staining was performed for qualitative confirmation of the results obtained from the ALP assay. Diffuse red staining in and around the cells indicated the presence of ALP enzyme. As seen in Figure 5B, increasing ALP enzyme production can be correlated with increased staining from day 14 to day 21, this confirms the quantitative results.
ARS staining and assay
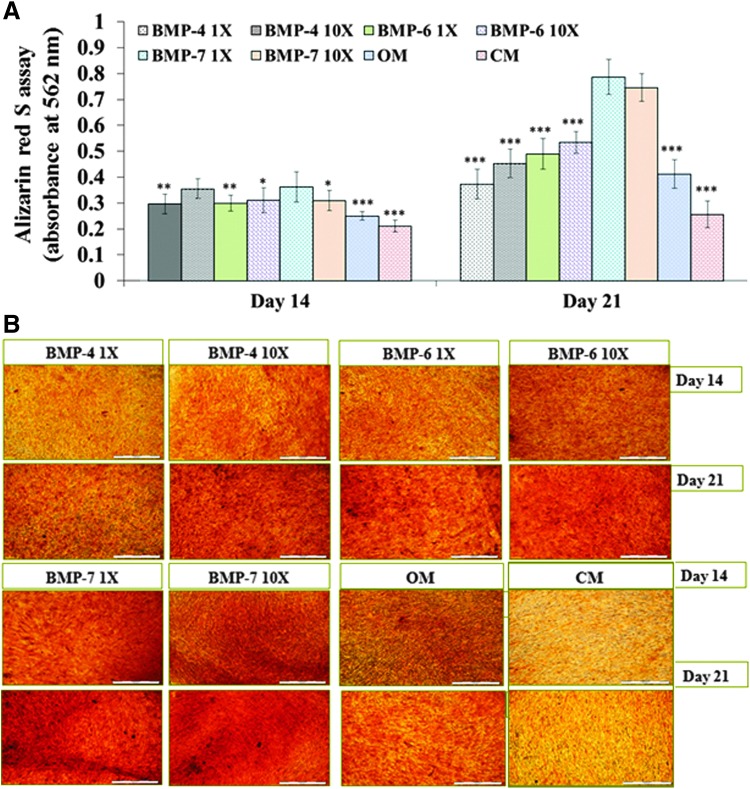
ARS stain is used to depict bone mineralization by orange-red staining of calcium-based mineral deposits/hydroxyapatite crystals, which are formed during the later phase of osteoblast maturation. As shown in Figure 6A, all groups displayed increasing bone mineralization through assay from days 14 to 21. The average values at day 14 for BMP-4 1X, BMP-4 10X, BMP-6 1X, BMP-6 10X, BMP-7 1X, BMP-7 10X, OM, and CM are 0.30 ± 0.04, 0.35 ± 0.04, 0.30 ± 0.03, 0.31 ± 0.05, 0.36 ± 0.06, 0.31 ± 0.04, 0.25 ± 0.02, and 0.21 ± 0.02 A.U., respectively. Whereas the readings for the same sequence of groups at day 21 were as follows: 0.37 ± 0.06, 0.45 ± 0.05, 0.49 ± 0.06, 0.53 ± 0.04, 0.79 ± 0.07, 0.75 ± 0.05, 0.41 ± 0.06, and 0.26 ± 0.05 A.U., respectively. The BMP-7 group, regardless of concentration, showed a significantly higher level of mineralization at day 21 than all the other groups (Fig. 6A, p < 0.000). However, results indicate that 1 X and 10 X BMP dosages do not differ.
FIG. 6.
Study of osteogenic mineralization through alizarin red S assay and staining. As shown in (A), the BMP-7 1X group shows significantly (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001) higher mineralization than all the other groups except BMP-4 10X group at day 14 time point. Whereas at day 21, BMP-7 (1X and 10X) groups display significantly higher mineralization than all other groups. As observed in (B), similar results were obtained during the staining studies for mineralization represented by orange-red staining at the mineralized areas (scale bar = 500 μm). The conclusion from this experiment was that the 1X dose of BMP-7 and OM is the best performing differentiation regime. Color images available online at www.liebertpub.com/tec
These results were qualitatively confirmed with ARS staining as shown in Figure 6B. Therefore, OM along with 1X BMP-7 would be sufficient and cost effective for inducing mineralization by osteoblastic cells formed during osteogenic differentiation of hMSCs on PPF polymer surfaces.
SEM analysis
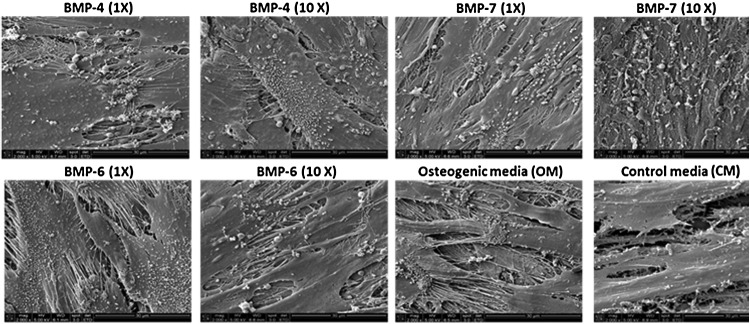
SEM imaging is performed at day 21 on samples from each group (Fig. 7) to observe bone mineralization. Imaging revealed crystal-like structures (whitish dots on cell layers), suggesting mineralization of the osteoblast. These crystals are closely associated with ECM threads, which further indicates the presence of hydroxyapatite-like crystals. These crystals are formed at nucleation sites on collagenous matrix during the maturation of osteoblasts. All except the CM group depicted the uniform distribution of many such crystals.
FIG. 7.
Scanning electron microscopy image analysis of mineralization. All the groups (except the control group, CM) show extensive mineralization through presence of hydroxyapatite-like crystals seen as white specks or granules associated with ECM. As expected, the CM group shows the least mineralization (magnification of all images = 2000X).
Validation of cell seeding and spreading on 3D-printed PPF scaffolds
Translation of the hMSC proliferation results obtained using two-dimensional (2D) thin film surfaces to 3D-printed scaffolds is of crucial importance to the preparation of scaffolds to repair bone defects. Therefore, to validate the use of our initial proliferation regime, we assessed 3D-printed porous PPF scaffolds through SEM imaging at days 0 and 7, post cell seeding (Fig. 8). As shown in Figure 8A and B, at day 0, the BM-hMSCs initially show a rounded morphology with observable indications of initial attachment. By day 7, the hMSCs were well attached to, and fully coating, the scaffold surface as observed in Figure 8C and D.
FIG. 8.
Translation of proliferation cytokine regime from two-dimensional PPF thin films to 3D-printed, porous PPF scaffolds. hMSCs were seeded and observed to attach, spread, and coat these 3D-printed, porous PPF scaffolds. At day 0, these cells mostly show a rounded morphology with uniform distribution at lower magnification (A). Some of these cells show the initiation of spreading upon observation at higher magnification (B). At the day 7 time point, all the cells show uniform spreading and coating at lower (C) and higher (D) magnifications. Magnification for images (A, B) = 250X, and that of images (C, D) = 500X.
Discussion
The formation of ECM on the surface of a resorbable scaffold is expected to allow positive recognition and incorporation of the scaffold by the host tissue. In the case of this study, our goal is to cover resorbable, 3D-printed, PPF scaffolds with bone ECM. We refer to these constructs as “bone tissue-engineered grafts.” Recent studies emphasize the importance and utility of ECM-covered 3D scaffolds, which mimic the microenvironment of natural bone tissue ECM. ECM-covered scaffolds perform better in in vivo conditions than bare scaffolds37 by allowing better penetration, proliferation, and differentiation of infiltrating bone progenitor cells and vasculature from the host tissue.
We expect that an important characteristic of a biomaterial to be used as a “bone tissue-engineered graft,” along with resorbability, is the geometry to provide guidance for infiltrating host tissue as well as subsequent bone vascularization and remodeling. A remodeling phase is necessary for development of mechanical and biological properties matching those of surrounding bone tissue (i.e., the production of cortical bone where needed). The presence of scaffold materials may interfere with the remodeling phase.38 Where needed, it is expected that remodeling will commence between 3 and 9 months after implantation. Therefore, we expect that among 3D-printable and resorbable biomaterials, PPF will provide a resorption rate more commensurate with this need than polylactides such as poly(lactic-co-glycolic acid) or PGA39 and slow resorbing polymers such as PCL (2–5 years).40 It is expected to fit into the required time frame for the resorption of bone tissue-engineered biomaterials with simultaneous remodeling of neo-bone.
Selection of the right cell source for the development of tissue-engineered bone grafts is also of utmost importance. BM-hMSCs, having the potential to evade immune system detection, can be used as an allogenic cell source for such applications.14,17 However, careful surveillance should be made so that cells do not differentiate to the point of becoming osteocytes, which are expected to present cell surface antigens. This is the reason why we have not included BMP-2 among the mineralization growth factors studied. Based on these concepts, we chose BM-hMSCs for studying the effect of growth factors on the proliferation and differentiation potential of progenitor cells on PPF scaffold surfaces.
In an earlier study, we observed the initial attachment of these cells after 48 h (in presence of FGF-2) on 3D-printed PPF scaffolds. These scaffolds had a different pore geometry, that we refer to as a “plate and post” geometry, and were also fabricated through continuous digital light processing, light-based, 3D printing.12 However, in this study, we provide comprehensive information on an optimized growth factor cocktail required for uniform stem cell coating (proliferation regime) and bone ECM formation (differentiation regime) on PPF thin films. The BM-hMSCs proliferated and covered the surface of these thin films within 7 days of culture in the presence of EGF, FGF, and PDGF growth factors. This study found that the addition of EGF reduced the time of BM-hMSC implant coating. The fact that single doses of these growth factors performed best reduces the cost of this regimen. These cell-covered PPF surfaces were further exposed to differentiation medium containing BMP-7, and mineralized ECM was formed within a period of 2 weeks after the proliferative phase. Validation of initial seeding and spreading of BM-hMSCs on 3D-printed, porous, PPF scaffold surfaces confirmed translation of our 2D PPF thin film proliferation regime results on these scaffolds. Currently, studies in our laboratory are being performed for validation of differentiation along with the study of translation of cylindrical pore geometry to 3D-printed, porous mandibular implant structures for a canine mandibular defect model. Finally, these 3D scaffolds are intended to be used for the treatment of human patients with craniomaxillofacial bone defects.
Conclusions
This study provides an optimized regimen of growth factors for the proliferation and differentiation of BM-hMSCs on the surface of PPF scaffolds. We found that to form a mineralized ECM layer on the PPF scaffold surface, EGF, FGF-2, and PDGF-BB perform as the best combination for proliferation, whereas for differentiation, BMP-7 works best along with OM. These results further suggest the suitability of PPF for the development of “bone tissue-engineered grafts” for the treatment of critical size or larger bone defects.
Acknowledgments
Partial support was provided by the Army, Navy, NIH, Air Force, VA, and Health Affairs to support the AFIRM II effort under Award No. W81XWH-14-2-0004. The U.S. Army Medical Research Acquisition Activity is the awarding and administering acquisition office for award No. W81XWH-14-2-0004. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Department of Defense. We thank Prof. Matthew Becker (Department of Polymer Science, University of Akron, Akron, OH) for providing the PPF resin used in this study. We thank Mr. Brian Kemmenoe for technical assistance with critical point drying and SEM image analysis at the Campus Microscopy Imaging Facility (Ohio State University, Columbus). We also thank Ms. Briana A. Swan for helping with cell culture experiments and Mr. Archie Tram for preparing the image of 3D-printed scaffolds shown in Figure 1.
Disclosure Statement
D.D. has pending and issued patents related to the preparation and use of PPF. He has ownership in three companies (Osteoplastics, 3DBioResins, and 3DServicePros, all of which are located in the the state of Ohio), and has received compensation from one company (Osteoplastics, LLC) that has interests in this technology.
References
- 1.Schmitz J.P., and Hollinger J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res 299, 1986 [PubMed] [Google Scholar]
- 2.Amini A.R., Laurencin C.T., and Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40, 363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mistry A.S., and Mikos A.G. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol 94, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Burg K.J., Porter S., and Kellam J.F. Biomaterial developments for bone tissue engineering. Biomaterials 21, 2347, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Gardin C., Ferroni L., Favero L., Stellini E., Stomaci D., Sivolella S., Bressan E., and Zavan B. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int J Mol Sci 13, 737, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bose S., Roy M., and Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30, 546, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean D., Topham N.S., Meneghetti S.C., Wolfe M.S., Jepsen K., He S., Chen J.E., Fisher J.P., Cooke M., Rimnac C., and Mikos A.G. Poly(propylene fumarate) and poly(DL-lactic-co-glycolic acid) as scaffold materials for solid and foam-coated composite tissue-engineered constructs for cranial reconstruction. Tissue Eng 9, 495, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Dean D., Jonathan W., Siblani A., Wang M.O., Kim K., Mikos A.G., and Fisher J.P. Continuous digital light processing (cDLP): Highly accurate additive manufacturing of tissue engineered bone scaffolds. Virtual Phys Prototyp 7, 13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean D., Mott E., Luo X., Busso M., Wang M.O., Vorwald C., Siblani A., and Fisher J.P. Multiple initiators and dyes for continuous digital light processing (cDLP) additive manufacture of resorbable bone tissue engineering scaffolds. Virtual Phys Prototyp 9, 3, 2014 [Google Scholar]
- 10.Fisher J.P., Dean D., and Mikos A.G. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials 23, 4333, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Kim K., Yeatts A., Dean D., and Fisher J.P. Stereolithographic bone scaffold design parameters: osteogenic differentiation and signal expression. Tissue Eng Part B Rev 16, 523, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace J., Wang M.O., Thompson P., Busso M., Belle V., Mammoser N., Kim K., Fisher J.P., Siblani A., Xu Y., Welter J.F., Lennon D.P., Sun J., Caplan A.I., and Dean D. Validating continuous digital light processing (cDLP) additive manufacturing accuracy and tissue engineering utility of a dye-initiator package. Biofabrication 6, 015003, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Wang M.O., Vorwald C.E., Dreher M.L., Mott E.J., Cheng M.H., Cinar A., Mehdizadeh H., Somo S., Dean D., Brey E.M., and Fisher J.P. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv Mater 27, 138, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol 217, 318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy M.B., Moncivais K., and Caplan A.I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45, e54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma S., Xie N., Li W., Yuan B., Shi Y., and Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ 21, 216, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdi R., Fiorina P., Adra C.N., Atkinson M., and Sayegh M.H. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 57, 1759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niemeyer P., Kornacker M., Mehlhorn A., Seckinger A., Vohrer J., Schmal H., Kasten P., Eckstein V., Südkamp N.P., and Krause U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Engineering 13, 111, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Eom Y.W., Oh J.E., Lee J.I., Baik S.K., Rhee K.J., Shin H.C., Kim Y.M., Ahn C.M., Kong J.H., Kim H.S., and Shim K.Y. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 445, 16, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Gharibi B., and Hughes F.J. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med 1, 771, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auletta J.J., Zale E.A., Welter J.F., and Solchaga L.A. Fibroblast growth factor-2 enhances expansion of human bone marrow-derived mesenchymal stromal cells without diminishing their immunosuppressive potential. Stem Cells Int 2011, 235176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo A., Tokuda H., Matsushima-Nishiwaki R., Kuroyanagi G., Yamamoto N., Mizutani J., Kozawa O., and Otsuka T. Rho-kinase limits BMP-4-stimulated osteocalcin synthesis in osteoblasts: regulation of the p38 MAP kinase pathway. Life Sci 96, 18, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Park K.H., Han D.I., Rhee Y.H., Jeong S.J., Kim S.H., and Park Y.G. Protein kinase C betaII and delta/theta play critical roles in bone morphogenic protein-4-stimulated osteoblastic differentiation of MC3T3-E1 cells. Biochem Biophys Res Commun 403, 7, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Burkus J.K., Heim S.E., Gornet M.F., and Zdeblick T.A. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech 16, 113, 2003 [DOI] [PubMed] [Google Scholar]
- 25.De Biase P., and Capanna R. Clinical applications of BMPs. Injury 36 Suppl 3, S43, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Park J.S., Yang H.N., Jeon S.Y., Woo D.G., Na K., and Park K.H. Osteogenic differentiation of human mesenchymal stem cells using RGD-modified BMP-2 coated microspheres. Biomaterials 31, 6239, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Honda Y., Ding X., Mussano F., Wiberg A., Ho C.M., and Nishimura I. Guiding the osteogenic fate of mouse and human mesenchymal stem cells through feedback system control. Sci Rep 3, 3420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sammons J., Ahmed N., El-Sheemy M., and Hassan H.T. The role of BMP-6, IL-6, and BMP-4 in mesenchymal stem cell-dependent bone development: effects on osteoblastic differentiation induced by parathyroid hormone and vitamin D(3). Stem Cells Dev 13, 273, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Shen B., Wei A., Whittaker S., Williams L.A., Tao H., Ma D.D., and Diwan A.D. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem 109, 406, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Eap S., Keller L., Schiavi J., Huck O., Jacomine L., Fioretti F., Gauthier C., Sebastian V., Schwinte P., and Benkirane-Jessel N. A living thick nanofibrous implant bifunctionalized with active growth factor and stem cells for bone regeneration. Int J Nanomedicine 10, 1061, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanakaris N.K., Calori G.M., Verdonk R., Burssens P., De Biase P., Capanna R., Vangosa L.B., Cherubino P., Baldo F., Ristiniemi J., Kontakis G., and Giannoudis P.V. Application of BMP-7 to tibial non-unions: a 3-year multicenter experience. Injury 39 Suppl 2, S83, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Benglis D., Wang M.Y., and Levi A.D. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery 62, ONS423, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Epstein N.E. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int 4, S343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn H.J., Lee W.J., Kwack K., and Kwon Y.D. FGF2 stimulates the proliferation of human mesenchymal stem cells through the transient activation of JNK signaling. FEBS Lett 583, 2922, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Jin Y., Zhang W., Liu Y., Zhang M., Xu L., Wu Q., Zhang X., Zhu Z., Huang Q., and Jiang X. rhPDGF-BB via ERK pathway osteogenesis and adipogenesis balancing in ADSCs for critical-sized calvarial defect repair. Tissue Eng Part A 20, 3303, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Fekete N., Rojewski M.T., Lotfi R., and Schrezenmeier H. Essential components for ex vivo proliferation of mesenchymal stromal cells. Tissue Eng Part C Methods 20, 129, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Pati F., Song T.H., Rijal G., Jang J., Kim S.W., and Cho D.W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 37, 230, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Zhou L., Shang H., Feng Z., Ding Y., Liu W., Li D., Zhao J., and Liu Y. Prototyped flexible grafting tray for reconstruction of mandibular defects. Br J Oral Maxillofac Surg 50, 435, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Cohen S.R., Holmes R.E., Meltzer H.S., Levy M.L., and Beckett M.Z. Craniofacial reconstruction with a fast resorbing polymer: a 6- to 12-month clinical follow-up review. Neurosurg Focus 16, E12, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Kasper F.K., Tanahashi K., Fisher J.P., and Mikos A.G. Synthesis of poly(propylene fumarate). Nat Protoc 4, 518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]