Abstract
Spinal cord injury (SCI) is a condition with no available cure. The initial physical impact triggers a cascade of molecular and cellular events that generate a nonpermissive environment for cell survival and axonal regeneration. Spinal cord injured patients often arrive at the clinic hours after the initial insult. This indicates the need to study and develop treatments with a long therapeutic window of action and multiactive properties, which target the complex set of events that arise after the initial trauma. We provide evidence that tamoxifen (TAM), a drug approved by the Food and Drug Administration, exerts neuroprotective effects in an animal model when applied up-to 24 h after SCI. We hypothesized that continuous TAM administration will improve functional locomotor recovery by favoring myelin preservation and reducing secondary damage after SCI. Adult female Sprague-Dawley rats (∼230 g) received a moderate contusion to the thoracic (T9–T10) spinal cord, using the MASCIS impactor device. To determine the therapeutic window available for TAM treatment, rats were implanted with TAM pellets (15 mg) immediately or 24 h after SCI. Locomotor function (Basso, Beattie, Bresnahan open field test, grid walk, and beam crossing tests) was assessed weekly for 35 days post-injury. TAM-treated rats showed significant functional locomotor recovery and improved fine movements when treated immediately or 24 h after SCI. Further, TAM increased white matter preservation and reduced secondary damage caused by astrogliosis, axonal degeneration, and cell death after trauma. These results provide evidence for TAM as a potential therapeutic agent to treat SCI up to 24 h after the trauma.
Key words: : astrogliosis, neuroprotection, selective estrogen receptor modulator (SERM), spare tissue, therapeutic drug
Introduction
Spinal cord injury (SCI) research has been driven toward the development of therapeutic interventions to reverse the loss of sensory, autonomic, and motor function. Although some of these interventions have reached clinical trials, SCI continues to be a disease without a cure.1 Traumatic injury to the spinal cord triggers the activation of molecular and cellular events that result in cell death and the establishment of a repulsive environment for axonal outgrowth.2–4 As a consequence of the physical impact, secondary damage is related to an apoptotic wave, astrogliotic response, demyelination, and axonal degeneration, which contribute to the adverse behavioral outcomes.5,6 The sequence of events extends spatially (rostral and caudal from the lesion epicenter) and temporally (for months after the injury), which correlates with the physiological symptoms observed. Accordingly, any therapeutic drug aimed at improving locomotor recovery must be multiactive, being able to inhibit cell death and promote axonal regeneration.
Tamoxifen (TAM) is a selective estrogen receptor modulator (SERM) that has been shown to exert antiapoptotic, antioxidant, and neuroprotective actions in models of brain ischemia by middle cerebral artery occlusion (MCAo), brain injury, and SCI.7–18 Studies in our laboratory showed that TAM pre-treatment (7 days before SCI) favored locomotor recovery and increased white matter spared tissue, exerting a similar effect to estradiol (E2) administration without the detrimental effects of chronic E2 systemic exposure.15
Published studies provide strong evidence that TAM administration after trauma has beneficial effects in both acute and chronic models of SCI.12,14,17,18 Although advantageous effects were observed in these studies, all of them failed to evaluate a relevant therapeutic window of TAM infusion (over 2 h after SCI) that may provide some behavioral recovery. Because most patients are stabilized within the first 24 h after SCI, this study extended the window of TAM administration up to 24 h after the lesion.
At this moment, the only available therapeutic strategy to treat SCI is methylprednisolone, which is only administered up to 8 h after the injury.19 Its use, however, is still controversial.20 On the other hand, TAM is a compound approved by the Food and Drug Administration (FDA), used mainly for the treatment of breast cancer. Its beneficial effect has been widely studied in pathological conditions of the central nervous system, which support this drug as a useful therapy for SCI.7–18
The dynamic progression of the initial acute and chronic injury confirms the importance to investigate a pharmacological intervention, such as TAM, which tackles most of the events associated with the initial physical impact.21 The objective of this study was to evaluate the favorable therapeutic window to administer TAM after SCI and the effects of this drug in secondary damage.
We hypothesized that TAM administration immediately and 24 h after SCI will favor locomotor recovery by an increase in the amount of spared tissue. In a double-blinded study, female rats received placebo (PLB) or TAM pellets after a moderate contusion injury (t = 0 h and t = 24 h). Functional behavioral recovery was monitored weekly using the Basso, Beattie, Bresnahan (BBB) open field test, grid walking, and beam crossing tests. Terminal histological studies were performed to determine the level of spared tissue, number of neurons, reactive gliosis, and neurofilaments in control and TAM-treated animals. We provide the first evidence that TAM therapeutic window extends up to 24 h after SCI, supporting its beneficial use for the clinical setting.
Methods
Animal housing
Adult female Sprague-Dawley rats (∼230 g) were purchased from Hilltop Lab Animal (Scottdale, PA). Animals were housed in pairs during the acclimation period and were housed individually for post-operative care and throughout the rest of the experiments.22 Rats were maintained at a 12:12 h light-dark cycle and trained for behavioral studies after 1 week of acclimation. Water and rat food chow (Harlan Teklad) were provided ad libitum. The Institutional Animal Care and Use Committee of the University of Puerto Rico, Medical Sciences Campus, reviewed and approved all animal surgeries, procedures, and post-operational care. Laboratory personnel managed animals following the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Laminectomy, SCI, and drug administration
Surgical procedures were performed under sterile and aseptic conditions. A total of 126 rats underwent surgical procedures for the development of this study. A total of 80 rats were used for the behavioral and histological and immunofluorescence studies, and 15 rats were used for the Western blot experiments. A total of 31 rats were excluded from these studies because of errors during surgical procedures (damage of the spinal cord during laminectomy) or failure to exert a successful compression (height errors during compression with the impactor), which resulted in extensive movement of the hindlimbs at 2 days post-injury (DPI). The exclusion parameter of animals with BBB score ≥5 was established before the development of the experiments.
Female Sprague-Dawley rats were anesthetized with a cocktail of ketamine/xylazine/acepromazine, 87.5/4.2/0.8 mg/kg (Fort Dodge Animal Health, Fort Dodge, IO). All surgical procedures were performed as described previously.23,24 Vaginal smears were performed on at least five animals from each group to determine the estrus cycle stage of the animals. Cytology evaluation was performed blinded to treatment at the moment of surgery and gave us a representation of the cycle of the complete population of animals used for this study. Briefly, a sterile cotton swab was damped on sterile saline inserted in the vagina. The cotton swab was rotated gently until the uterine wall was reached. The sample was spread on frosted slides with a rotating motion and dried at room temperature for 5 min. The slides were stained with Dip Quick Stain (Jorgensen laboratories Inc., Loveland, CO #catJ-322) and evaluated by an experimenter blinded to treatment. No significant trend was observed between the rats that recovered better from SCI and the estrus cycle that the rats were staged at before SCI (data not shown).
Groups were randomly divided between SCI, SCI + treatment, and sham animals. Sham animals underwent laminectomy at the T9–T10 and were sutured immediately after surgery. Injured animals received a laminectomy at T9–T10 followed by a moderate contusion using the MACSIS impactor device. After laminectomy, the vertebral column was stabilized with clamps, and a 10 g rod was dropped from a 12.5 mm height over the exposed spinal cord and the compression maintained for 5 sec.
TAM pellets were designed for a continuous drug release of 0.71 mg/day for 21 days after SCI, similar to the amount used in our pre-treatment studies.15 Immediately or 24 h after SCI, a pellet of TAM (15 mg) or PLB (Innovative Research of America, Sarasota, FL, cat.# E-361) was placed subcutaneously in the mid-scapular region. The pellets were designed to release TAM immediately after implantation. Previous studies from our laboratory using UPLC-MS/MS have confirmed that TAM is consistently released in animals after implantation, and the levels decline with time (at 14, 21, and 28 days post-implantation).15 The release of TAM was monitored indirectly by weighing the animals weekly, because this drug provides an anorexic effect.25
Animals were sutured, and post-operative care included antibiotic injection (25 mg/kg cefazolin for 7 days), subcutaneous saline administration, eyedrops (to prevent excessive dryness to the cornea), and buprenorphine (Reckett & Colman Pharmaceuticals, Richmond, VA; 0.05 mg/kg) application twice a day for 3 days.24 Bladders were manually voided three times a day until micturition reflexes were fully reestablished (5–7 days). All animals were housed individually after laminectomy or SCI. Cereal and paper enrichment was provided to all animals (sham and injury) throughout the experiments.26
Parameters related to the compression injury performed with the MACSIS impactor were evaluated (Table 1). Compression injury was similar among groups, with no statistical difference observed, according to one-way analysis of variance (ANOVA) followed by a Tukey's multiple comparison post hoc test (F(3,51) = 0.4540; p = 0.7156). Analysis of the rod impact velocity revealed no significant difference among groups (F(3,50) = 1.124; p = 0.3484). The BBB test was performed at 2 DPI to establish the exclusion baseline of rats for this study. The animals that were able to perform extensive movement of the hindlimbs a this time point (BBB score ≥5) were excluded from all further tests and were not included in this article.
Table 1.
Impact Parameters Related to Compression Injury with the MASCIS Impactor
| Treatment groups | Compression (mm) | Impact velocity (m/sec) | BBB score (2 DPI) |
|---|---|---|---|
| Sham PLB | N/A | N/A | 21 |
| Sham TAM | N/A | N/A | 21 |
| SCI PLB | 2.24 ± 0.07 | 0.48 ± 0.00 | 0.55 ± 0.31 |
| t = 0 h | |||
| SCI TAM | 2.20 ± 0.07 | 0.47 ± 0.01 | 0.63 ± 0.29 |
| t = 0 h | |||
| SCI PLB | 2.23 ± 0.08 | 0.47 ± 0.00 | 0.30 ± 0.12 |
| t = 24 h | |||
| SCI TAM | 2.12 ± 0.06 | 0.47 ± 0.01 | 0.42 ± 0.15 |
| t = 24 h |
BBB, Basso, Beattie, Bresnahan; DPI, days post-injury; PLB, placebo; TAM, tamoxifen; SCI, spinal cord injury.
Evaluation of the impact parameters related to the compression injury with the NYU impactor showed no significant difference among groups. Compression injury was similar among groups; no statistical difference was found according to one-way analysis of variance, Tukey's multiple comparison test (F(3,51) = 0.4540, p = 0.7156). Analysis of the rod impact velocity revealed no significant difference among groups (F(3,50) = 1.124, p = 0.3484). Locomotor assessment at 2 DPI revealed no significant difference among groups, suggesting that the impact to the cord exerted a successful moderate compression (F(3,36) = 0.1948, p = 0.8992). Rats were not included in the behavioral, histological, and molecular data if the animals were capable of exerting extensive movement of two areas of the hindlimbs at 2 DPI (BBB score ≥5).
Behavioral experiments—recovery of locomotion, balance, and coordination
All animals were trained on the behavioral tests 1 week before SCI. Locomotor recovery was assessed weekly at 7, 14, 21, 28, and 35 DPI. The BBB open field test was used as a tool for evaluating locomotor behavior as reported previously.27 Briefly, rats walked individually on a plastic pool while two examiners, blinded to treatment, evaluated hindlimb locomotion. Each hindlimb was scored individually (between 0 and 21) based on joint movement, paw position, weight support, coordination, toe clearance, among other parameters. The average score of both limbs for each animal was reported. An initial evaluation was performed at 2 DPI to confirm that the injury was performed appropriately (Table 1).
The grid walk test was used to evaluate animal coordination when crossing a horizontal ladder (3 ft long, with bars separated by 1 or 2 in). Every week, the separators were alternated randomly to prevent habituation of the animal. Animals were evaluated on the number of errors (failure to correctly position the paw on the grid) and the number of correct paw positions. Because statistical analysis revealed no differences between left and right hindlimb scores of each group (data not shown), the number of errors and correct paw positions were averaged between left and right hindlimbs.
The narrow beam crossing test was used to evaluate recovery of equilibrium when challenged to cross a narrow surface. Rats crossed the square (2 × 2 cm) and round (2 cm diameter) beams at 36–44 cm (∼15–18 inches) from the ground and were scored according to their ability to cross from one side to the other.28 Animals were scored weekly for each beam as follows: half a bar, 0.5 point; entire beam without the use of hindlimbs 1.0 point; entire beam using one hindlimb, 1.5 points; or the entire beam using both hindlimbs; 2 points. The total score for both beams was combined, and the average was reported for each animal. Sham animals received a total score of 4 points throughout the experiment (data not shown).
Animal sacrifice and tissue collection
For histology and immunofluorescence studies, rats were anesthetized and sacrificed by intracardial perfusion with cold phosphate buffered saline (PBS) followed by fixation in 4% paraformaldehyde (PFA) at 35 DPI. The areas of laminectomy and lesion epicenter were identified, the vertebral column was cut open, spinal nerves were cut, and 1.5 cm of spinal cord was exposed and removed. The segment was obtained and presumed as a representation of the entire affected area (0.5 cm of the rostral, 0.5 cm epicenter, and 0.5 cm of the caudal area). The spinal cord was post-fixed in cold 4% PFA for 3 h. Tissue was washed in cold PBS and cryoprotected in 30% sucrose; tissue was stored at 4°C until further processing. To determine effect of TAM on tumorigenesis, the liver was extracted after fixation at 35 DPI.29 Also, the brain was dissected, and both organs were dried overnight and weighed 24 h after dissection.30
Luxol fast blue histology and densitometry analysis
Spinal cord tissue was embedded on freezing medium and transversely sectioned at 20 μm using Leica Cryostat. Transverse spinal cord sections were placed on gelatin coated slides and stored at −20°C until further processing. The amount of white matter spared tissue was determined by staining transverse tissue sections with Luxol fast blue as reported previously.15,24,31 To have a representation of the lesion epicenter, a total of three sections (∼200 μm apart) were selected for each animal evaluated in the Luxol fast blue experiments. Photomicrographs were taken using Motic (version 1.2 software).
Densitometry analysis was performed with MCID (Imaging Research, Inc. Ontario, Canada) as reported previously.15,24,31 Representative images (10x) were captured with Nikon E20L inverted microscope with DSRI2 CMOS camera. Briefly, to determine the amount of white matter spared tissue per area, the stained section of the spinal cord was delineated, selected, and the density per area (D × A) measured. Then, the area of the epicenter cavity was delineated, selected, and measured (D × A). The density measured on the epicenter cavity area was subtracted from the total density measured for the lesion epicenter, which represents the amount of white matter spared tissue on this area in density per unit area (density/area).
Immunofluorescence imaging and quantification
Cellular and structural markers, such as glial fibrillary acidic protein (GFAP) and neurofilament heavy chain (NF-H), were evaluated by immunofluorescence on 20 μm transverse sections, as proteins associated with secondary damage after SCI. Neuronal nuclei antibody (NeuN) was used to identify neuronal populations on the dorsal horn (DH) and ventral horn (VH) of the spinal cord. Slides were dried at 60°C for 30 min, and freezing media was removed with forceps.
All washes and incubations were performed at room temperature. First, cell membrane was permeabilized with 0.1% Triton X-100/PBS, fixed in 4% PFA, and blocked with 3% bovine serum albumin in PBS/0.01%NaN3 for 2 h. Slides were incubated for 1 h with the following primary antibodies: mouse anti-GFAP (1:2000 BD Biosciences, #cat 556327), mouse anti-NF-H (1:1000 Millipore, #cat CBL212), or mouse anti-NeuN (1:4000 Millipore, #cat MAB377). To assess the amount of nonspecific binding, every antibody was validated by the use of isotype controls at the same concentration as the primary antibody. Isotype controls were mouse IgG1 (CST, #cat G3A1) or mouse IgG2B (Millipore, #cat MABC006). Slides were washed with PBS and probed with Alexa Fluor® 488 donkey anti-mouse (1:500, Invitrogen).
Sections were visualized with an Axio Observer Z1 Zeiss Fluorescence microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY) and photographed with AxioCam MRm (Rev.3). Imaging parameters were established at the beginning of the experiment and kept constant throughout the experiment. Negative control slides from each experiment were used to calibrate the microscope before imaging. Because quantification of fluorescence may be limited by autofluorescence from the tissue or fluorescence from the fluorophore used, control slides were quantified to determine the amount of fluorescence emitted by these variables. Therefore, immunoreactivity of the primary antibody quantified was expressed as the amount of fluorescence emitted over the background fluorescence and autofluorescence.
To assess the effects of TAM on neuronal number as a consequence of spatial damage after SCI, a total of two sections rostral to the lesion epicenter were counted per animal (∼5 mm from the lesion epicenter, ∼500 μm apart). To reduce bias, two evaluators performed individual assessments of each anatomical area in the DH and VH. Evaluators identified neurons by positive immunoreactivity for NeuN on each area and counted the number of cells. Only cells that showed a complete soma on the DH and a defined nucleus in the VH were counted. Neuronal raw counts were normalized against sham animals to obtain the percent of cells that approximately survived after SCI.
The effect of TAM in GFAP or NF-H immunoreactivity after injury was determined from 8 bit images that were loaded into Image J. All measurements were limited to threshold and redirected to the image. GFAP and NF-H immunoreactivity were quantified using the Otsu method.32,33 Briefly, the Otsu method is a mathematical method that automatically defines the optimal threshold for an image with a gray histogram (8 bit) based on integration. The method uses a series of equations to define a value for the pixels with the highest intensity (immunoreactivity for GFAP or NF-H) and the pixels with the lowest intensities (background).
To assess the amount of GFAP at the lesion epicenter, images from the dorsal, ventral, and lateral white matter were captured and quantified with the Otsu method. The average of the total quantification for all the areas was reported. To assess the amount of neurofilaments at 35 DPI, we evaluated NF-H immunoreactivity in the lateral funiculus ∼2 mm caudal to the lesion epicenter. Images from the lateral funiculus (left and right side) were captured and quantified using the Otsu method.
Sham animals were quantified using the same method. The quantification of both sides of each sham animal was averaged and taken as 100%, which was compared to the injured animals. Injured spinal cords were quantified and the total amount (left and right) of NF-H was standardized against the sham animals. The fraction obtained represents the amount of NF-H spared as a consequence of both the moderate contusion to the cord and TAM effects neutralizing the detrimental environment as secondary damage develops after SCI.
Protein extraction and immunoblots
Animals were decapited at 35 DPI and three areas from the spinal cord were removed: lesion epicenter (0.5 cm), rostral (0.5 cm), and caudal (0.5 cm). The tissue was washed with cold PBS and immediately transferred to a 1.5 mL microcentrifuge tube containing 700 μL of the cold extraction buffer (CelLytic MT Lysis/Extraction Buffer [C3228], 1% phosphatase inhibitor cocktail 3, and SigmaFAST® Protease Inhibitor Cocktail Tablet, EDTA free [all from Sigma]).
Samples were homogenized using a pellet pestle and then placed in a shaker at 4°C for 45 min. The samples were centrifuged for 10 min at 4°C (20,000 g). The supernatant was collected for protein quantification, using the Bio-Rad protein assay (BioRad). For sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting, 12.5 μg of protein extracts were separated in 10% SDS polyacrylamide gels and transferred into nitrocellulose membranes using the Trans-Blot Turbo Transfer System (Bio-Rad Laboratories, Hercules, CA).
Membranes were blocked with blocking solution (Licor) and incubated overnight at 4°C with primary mouse monoclonal antibody against GFAP (1:1,000 BD Biosciences, #cat 556327) and rabbit anti-GAPDH (1:5,000 Sigma) as a loading control. To reduce variability, the GFAP antibody used for immunoblots was the same antibody used for the immunofluorescence experiments. Membranes were washed with PBS/0.1%Tween-20 and incubated with two secondary infrared antibodies: IRDye 800CW goat antirabbit and IR Dye 680RD goat antimouse (1:25,000). Membranes were washed with PBS, and blots were developed using the Odyssey CLx Quantitative Fluorescent Imaging Systems (Licor). Results were quantified with the Image Studio Lite software (Licor).
Statistical analysis
Results were presented as mean ± standard error of the mean (SEM). Differences between groups were considered significantly different if p < 0.05. Behavioral data were analyzed by repeated measures (RM) two-way ANOVA followed by the Bonferroni post hoc test. Densitometry analysis (Luxol histology) and immunofluorescence quantification experiments were analyzed by one-way ANOVA followed by the Tukey's multiple comparison test. Immunoblotting quantification and behavioral comparisons at 2 DPI between two groups (t = 0 and t = 24 h) were analyzed by two-tailed unpaired t test. All figures in this article were prepared for publication using FigureJ.34
Results
Tamoxifen administration, immediately and 24 h after SCI, produces locomotor recovery and improves coordination
Functional locomotor recovery was assessed with the BBB open field, grid walk, and the narrow beam crossing tests. Locomotor assessment at 2 DPI revealed no significant difference among groups (Table 1), suggesting that the impact to the cord exerted a successful moderate compression and TAM exerts no significant effect at the behavioral level at this time point (F(3,33) = 0.1680; p = 0.8992). In addition, independent analysis, using an unpaired two tailed t test, showed that the injured groups treated with TAM at t = 0 and t = 24 h exert similar locomotor function at 2 DPI, confirming that this drug did not improve behavioral recovery at this time point (t test SCI TAM 0 h vs. SCI TAM 24 h t = 0.4593; p = 0.6510). Rats were excluded from behavioral, histological, and molecular data if the animals were capable of exerting extensive movement of two areas of the hindlimbs at 2 DPI (BBB score ≥5).
Individual comparison of the SCI PLB-0 h with the SCI TAM-0 h showed significant differences at 7***, 14**, 21**, 28***, and 35** DPI according to RM two-way ANOVA treatment, F(1,39) = 13.29; p = 0.0008. Also, when we compare the SCI PLB 24 h with the SCI TAM 24 h, we observe a significant treatment effect at 28* and 35* DPI according to RM two-way ANOVA treatment, F(1,19) = 6.72; p = 0.0179. Because we found no significant difference between the SCI PLB-0 h and the SCI PLB-24 h groups (RM two-way ANOVA followed by Bonferroni post hoc treatment, F(1,27) = 2.145; p = 0.1546), we grouped the control animals together for power analysis.
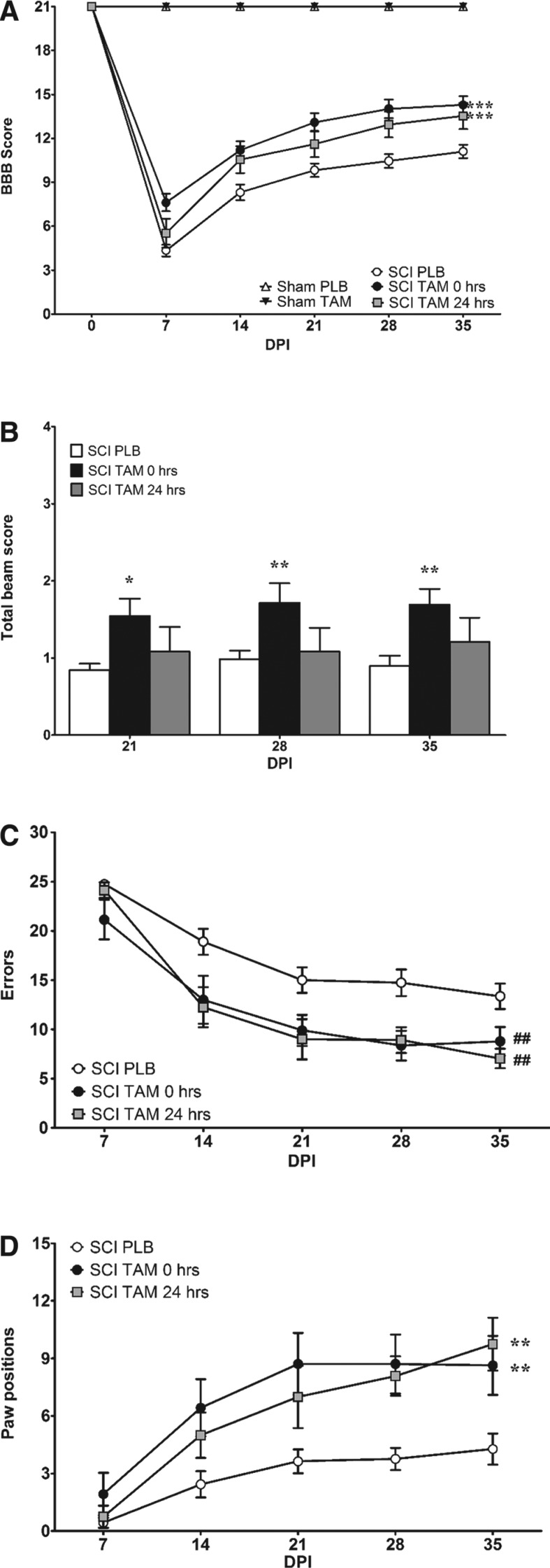
TAM treatment immediately (t = 0 h) after SCI improved BBB scores when compared with pooled PLB animals (t = 0 + 24 h), according to RM two-way ANOVA (treatment F(4,75) = 86.7; p < 0.0001). In addition, TAM beneficial effects after SCI depend on the time of the drug exposure and the recovery after SCI according to RM two-way ANOVA (interaction F(20,75) = 54.3; p < 0.0001) as evidenced in Fig. 1A. Hindlimb function was significantly better at 7***, 14***, 21***, 28***, and 35*** DPI (***p < 0.001) in animals treated with TAM immediately (t = 0 h) after SCI compared with injured animals treated with PLB (pooled placebo animals: t = 0 + 24 h). RM two-way ANOVA followed by a Bonferroni post hoc test revealed no significant difference between the SCI TAM at t = 0 h versus the SCI TAM t = 24 h groups (F(4,75) = 86.73; p > 0.05).
FIG. 1.
Tamoxifen (TAM) administration immediately and 24 h after spinal cord injury (SCI) improves locomotor recovery and coordination. (A) Sham animals obtained 21 points in the Basso, Beattie, Bresnahan (BBB) open field test at all test points evaluated (0–35 DPI). TAM treatment 0 and 24 h after SCI improved locomotor recovery after SCI (***p < 0.001 treatment, DPI, and interaction). (B) Rats treated with TAM (0 h) showed a significant improvement when crossing the narrow beam during chronic stages 21 (*p < 0.05), 28 DPI, and 35 DPI (**p < 0.01) (treatment, DPI, and interaction). (C,D) TAM improves coordination by reducing the number of errors (##p < 0.01; treatment) and increasing the number of correct paw positions in the grid walk test (**p < 0.01; treatment, DPI, and interaction). Data are the mean ± SEM (sham PLB n = 9, sham TAM n = 9, SCI PLB (rats from t = 0 + 24 h) n = 29, SCI TAM 0 h n = 21, and SCI TAM 24 h n = 12).
This result suggests that delayed treatment intervention has the same effect as administering TAM immediately after SCI. While the pool of PLB (t = 0 + 24 h) treated rats' locomotor capacity remained relatively constant after 21 DPI, animals treated with TAM reached a higher plateau with a difference of approximately three BBB scores (Fig. 1A). Mean scores at 7 DPI (7.6 vs. 4.3), 14 DPI (11.2 vs. 8.3), 21 DPI (13.1 vs. 9.9), 28 DPI (14.0 vs. 10.4), and 35 DPI (14.3 vs. 11.0) confirmed that TAM (t = 0 h) administration resulted in overall gross hindlimb improvement. Moreover, in the last 3 weeks of the BBB open field test, the animals treated with TAM immediately after SCI presented scores from stage III (consistent plantar stepping and coordination), which represent a significant improvement in locomotor function relative to PLB treated rats.
Similar results were obtained when TAM was administered 1 day after SCI. RM two-way ANOVA demonstrated that administration of TAM 24 h after the injury produced some functional locomotor recovery in the BBB open field test when compared with PLB treated animals pooled in one group (t = 0 + 24 h). Further, statistical analysis revealed that a significant locomotor recovery was observed at 14*, 28**, and 35** DPI (*p < 0.05, **p < 0.01), suggesting that administration of TAM 24 h after SCI delayed the recovery observed during the first week, but this had no effect on the overall outcome at 35 DPI. The effect was also time-dependent, because RM two-way ANOVA (DPI F(5,75) = 329.2; p < 0.0001) confirmed that the effect of this multiactive drug depends on the time of drug exposure and time after SCI (Fig. 1A).
Vestibular function and motor control was evaluated using the narrow beam crossing test. Rats treated with TAM immediately (t = 0 h) after SCI showed a significant improvement to cross the narrow beam during chronic stages of SCI (treatment F(2,59) = 4.5; p = 0.0150) as shown in Fig.1B. Statistical analysis demonstrated that the behavioral improvement depends on the effects of treatment and time (interaction F(8,59) = 2.0; p = 0.0418). The ability to cross the narrow beam was improved by immediate TAM (0 h) administration at 21*, 28**, and 35** DPI (*p < 0.05, **p < 0.01) relative to injured animals treated with PLB pellets (t = 0 + 24 h). This behavioral improvement was not observed in rats treated with TAM 24 h after the SCI.
The effect of TAM in hindlimb sensorimotor function and coordination of injured animals was determined using the grid walk test (Fig. 1C). Because a bilateral injury may result in an asymmetric lesion, we performed a statistical analysis to determine differences in the number of errors and paw positions exerted by each hindlimb (data not shown). The number of errors and correct paw positions were averaged for the left and right hindlimbs because no significant difference was found between the hindlimbs of each groups.
Injured rats treated with TAM immediately and 24 h after SCI made fewer errors (treatment F(2,48) = 6.7; p = 0.0028), relative to PLB treated animals. Immediate TAM administration (t = 0 h) resulted in a significant reduction in the number of errors at 14* and 28** DPI while a delayed therapy 24 h after SCI resulted in an improvement at 14**, 21*, 28*, and 35* DPI (*p < 0.05, **p < 0.01). This effect was independent of drug exposure and time after SCI (interaction F(8,48) = 1.9); p = 0.0559).
Importantly, injured rats with TAM administered immediately or 24 h after SCI resulted in a significant increase in the number of correct foot placements in the grid assay (Fig. 1D) as shown by RM two-way ANOVA (treatment F(2,48) = 6.8; p = 0.0025). TAM (t = 0 h) favored skilled locomotion coordination by increasing correct paw positions at 14*, 21**, 28**, and 35** DPI (*p < 0.05, **p < 0.01) while a delayed therapy 24 h after SCI resulted in an improvement at 28* and 35*** DPI (**p < 0.01, ***p < 0.001) when compared with PLB pooled animals (t = 0 + 24 h). Moreover, TAM beneficial effects in this assay were time dependent (interaction F (8,48) = 3.7; p = 0.0005).
TAM exerts neuroprotective effects enhancing white matter spared tissue when administered immediately and 24 h after SCI
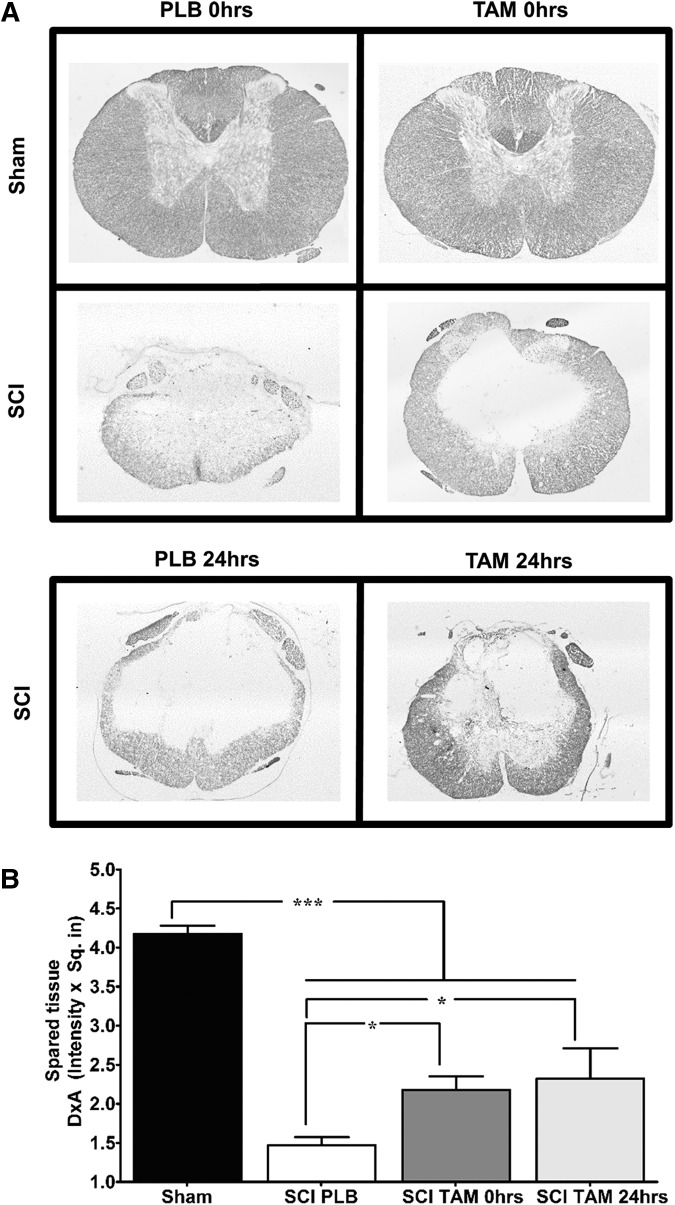
Luxol fast blue staining was used to evaluate the neuroprotective effects of TAM on white matter spared tissue at the lesion epicenter (Fig. 2A). Densitometry analysis revealed a significant reduction in white matter spared tissue in injured rats treated with PLB when compared with sham animals (Fig. 2B) according to one-way ANOVA (F(3,25) = 78.03; p < 0.0001). Treatment of injured rats with TAM immediately after SCI significantly increased white matter spare tissue relative to injured animals treated with PLB pellets (*p < 0.05) and, as a consequence, a reduced cavity size was observed (Fig. 2A). Moreover, if TAM was administered 24 h after SCI, the amount of white matter spared tissue was significantly higher in comparison with PLB treated animals (*p < 0.05).
FIG. 2.
Delayed tamoxifen (TAM) therapy 24 h after spinal cord injury (SCI) increases the amount of white matter spared tissue at the lesion epicenter. (A) Representative transverse spinal cord sections evaluated for Luxol fast blue staining at the lesion epicenter of sham and injured animals at 35 days post-injury (10x magnification). (B) Densitometry analysis of transverse sections at the lesion epicenter revealed that TAM treatment favors white matter preservation when administered immediately and 24 h after SCI. Three sections per animal were evaluated, one-way analysis of variance data are mean ± SEM. Sham (PLB+TAM) n = 13, SCI PLB (rats from t = 0 h + 24 h) n = 6, SCI TAM 0 h n = 7, SCI TAM 24 h n = 3 (*p < 0.05, **p < 0.01, ***p < 0.001).
TAM favors neuronal survival, increases the number of neurofilaments at the lateral funiculus, and reduces reactive gliosis when administered immediately and 24 h after SCI
The cellular mechanisms related to the locomotor recovery effects of TAM treatment (immediately or 24 h after SCI) in female rats were investigated using immunofluorescence and Western blot analysis. We focused on the neurons of the gray matter (NeuN immunostaining), reactive gliosis (GFAP immunoreactivity), and axonal cytoskeletal markers (neurofiliament marker). As a consequence of the primary and secondary events, the lesion epicenter turns into a hollow cavity, which extends rostrally and caudally, promoting neuronal death in both penumbras, worsening the outcome of recovery. For this reason, we evaluated the effects of TAM in neuronal number at the rostral level, because previous studies showed some beneficial effect of TAM in reducing reactive oxygen species (ROS) formation in this area.15
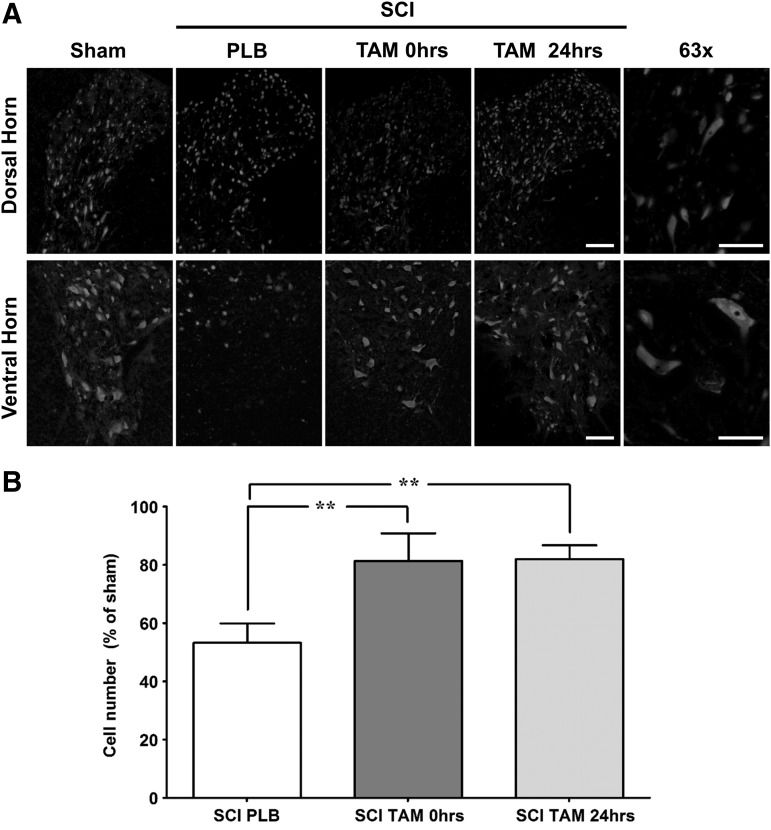
Representative photos illustrate the normal pattern of NeuN expression in the DH and VH of sham, injured rats treated with PLB, and injured animals treated with TAM immediately or 24 h after SCI (Fig. 3A). Bilateral VH and DH neurons were counted from two 20 μm sections ∼500 μm apart. Neuronal raw counts were averaged for the VH and DH of each animal. Average counts for the VH showed sham animals with 41.2 ± 1.3 (n = 9), SCI PLB 23.6 ± 3.9 (n = 4), SCI TAM 0 h 34.0 ± 3.6 (n = 3), and SCI TAM 24 h 28.6 ± 1.1 (n = 3) neurons. Average counts for the DH showed sham animals with 106.2 ± 5.8 (n = 9), SCI PLB 54.9 ± 11.4 (n = 4), SCI TAM 0 h 88.3 ± 13.1 (n = 3), and SCI TAM 24 hr 92.5 ± 6.2 (n = 3) neurons. TAM treatment increased the number of neurons rostral to the lesion epicenter as shown in Fig. 3B (one-way ANOVA F(3,15) = 25.5; p < 0.0001). The effect of TAM on cell number was observed if the drug was administered immediately or 24 h after the injury (**p < 0.01).
FIG. 3.
Tamoxifen (TAM) promotes neuronal survival in the dorsal and ventral horn at the rostral penumbra after spinal cord injury (SCI). (A) Representative images of NeunN immunoreactivity from the DH or VH in the rostral penumbra at 35 days post-injury (magnification = 20x, scale bar = 100 μm). High power magnification (63x) of dorsal and ventral horn neurons from sham animals (scale bar = 50 μm). (B) Neuronal cell count shows that SCI reduces the total number of neurons in the rostral area. TAM treatment at 0 and 24 h after SCI significantly increases the number of neurons from the DH and VH of injured animals. One-way analysis of variance Tukey's multiple comparison test (**p < 0.01), data are mean ± SEM, sham (PLB+TAM) n = 9, SCI PLB (rats from t = 0 h + 24 h) n = 4, SCI TAM 0 h n = 3, SCI TAM 24 h n = 3.
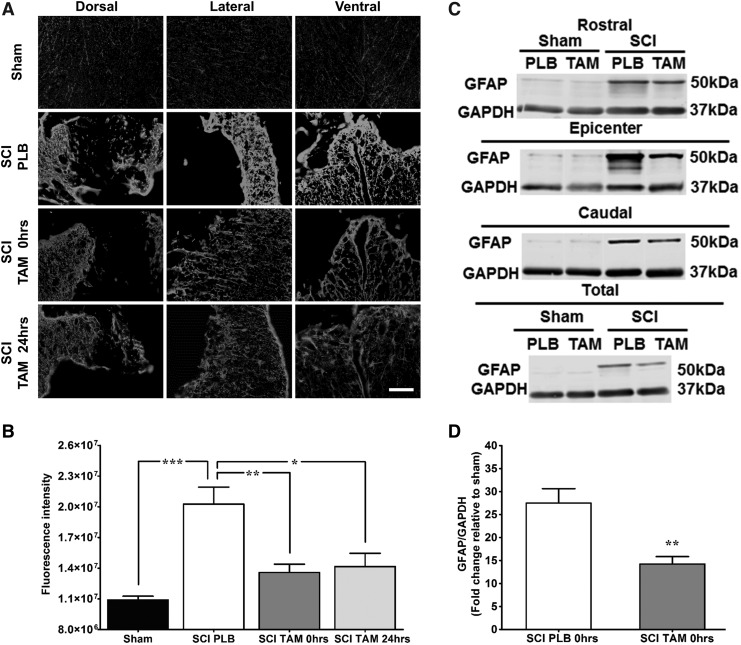
In addition, we observed that TAM treatment in injured animals reduced GFAP-mediated gliosis when compared with PLB animals (Fig. 4). Representative photos of GFAP immunoreactivity in dorsal, lateral, and ventral white matter revealed low immunoreactivity in sham animals and increased gliosis on SCI PLB animals (Fig. 4A). Injured animals presented an increase in GFAP immunoreactivity at 35 DPI, which decreased significantly when the animals were treated with TAM immediately or 24 h after SCI (Fig. 4B, one-way ANOVA F(3,22) = 18.0; p < 0.0001).
FIG. 4.
Immediate and delayed tamoxifen (TAM) administration after spinal cord injury (SCI) decreased secondary damage caused by astrogliosis. (A) Representative images of glial fibrillary acidic protein (GFAP) immunoreactivity at the lesion epicenter 35 DPI (magnification 20x, scale bar = 100 μm). (B) Relative fluorescence intensity quantification of GFAP immunoreactivity from the epicenter. Sections were divided into anatomical quadrants (dorsal, lateral, and ventral), and the total area was quantified and averaged for the analysis (scale bar = 100 μm). Animals with SCI showed significant increased GFAP immunoreactivity. TAM treatment at 0 and 24 h after SCI reduced secondary damage associated to reactive gliosis at the lesion epicenter according to one-way analysis of variance Tukey multiple comparison test (***p < 0.001, **p < 0.01, *p < 0.05). Data are mean ± SEM, sham (PLB+TAM) n = 11, SCI PLB (rats from t = 0 + 24 h) n = 7, SCI TAM 0 h n = 5, SCI TAM 24 h n = 3. (C) Representative immunoblots for GFAP expression at the rostral, epicenter, caudal levels. Equal amounts of proteins from each area were loaded (12.5 μg total), which represent that TAM treatment reduced astrogliosis extent of damage after SCI. (D) Semi-quantitative analysis revealed that TAM significantly reduced GFAP levels when administered immediately after SCI. Data are mean ± SEM standardized against sham and analyzed by unpaired t test (**p < 0.01). Sham PLB n = 2, sham TAM n = 3, SCI PLB (rats from t = 0 + 24 h) n = 4, SCI TAM n = 6.
Western blot analysis confirmed that TAM administration decreased GFAP expression after SCI, without changes in the housekeeping gene (GAPDH). Immunoblots of GFAP protein in regions rostral, caudal, and at the lesion epicenter from sham and injured animals, treated with PLB or TAM (0 h) were performed (Fig. 4C). The expression profile in all regions tested showed that the traumatic injury upregulates GFAP protein, relative to sham animals. Moreover, administration of TAM immediately after SCI reduced the level of this astrogliotic marker. Identical results were observed when protein extracts from all the regions were pooled and analyzed by Western blot. Densitometric analysis confirmed that TAM (0 h) reduced the expression of GFAP significantly (**p < 0.01) in animals with SCI, relative to injured animals treated with PLB (Fig. 4D, t = 4.1; p = 0.0033).
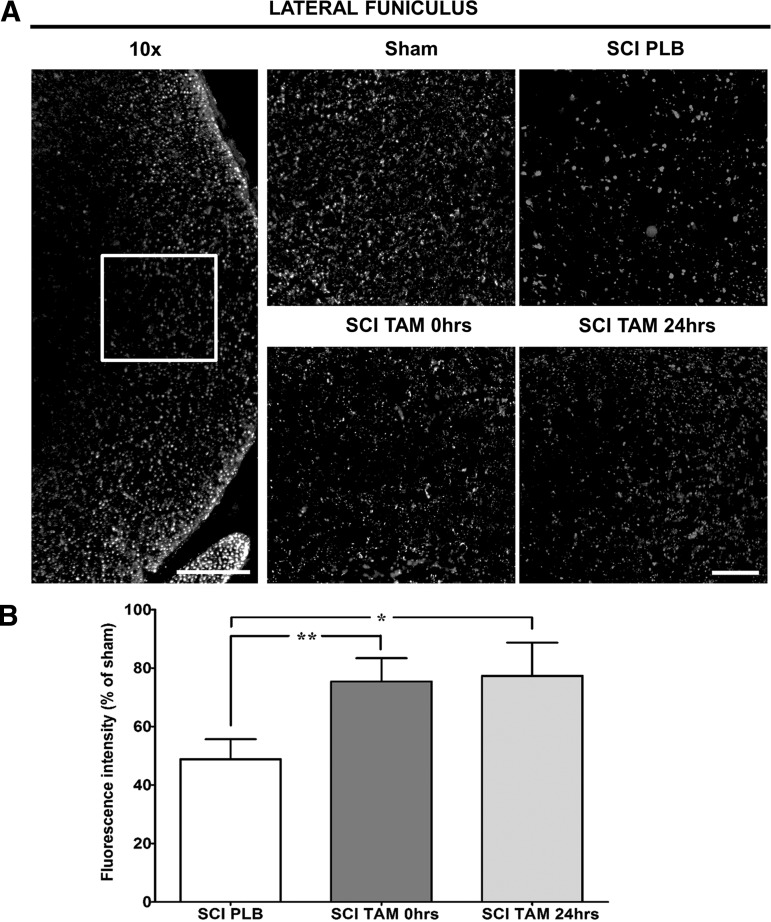
Another consequence of secondary injury is axonal degeneration both rostrally and caudally to the lesion epicenter. To evaluate the effect of TAM in the preservation of axonal structures after SCI, we evaluated NF-H immunoreactivity at the lateral funiculus of the spinal cord in caudal regions relative to the lesion epicenter (Fig. 5). The lateral funiculus was studied because some locomotor improvement was observed in skilled locomotion (grid walk test and narrow beam crossing), and this may be related to rubrospinal and reticulospinal fiber sparing in areas caudal to the epicenter. Increased immunoreactivity of the lateral funiculus that accompanied the behavioral effects provides valuable information as to a possible mechanism related to improved skilled locomotion.
FIG. 5.
Tamoxifen (TAM) favors axonal preservation at the lateral funiculus in the caudal spinal cord. (A) Representation of the lateral funiculus area photographed and quantified for the analysis (magnification = 10x, scale bar = 200 μm). Representative images of NF-H immunoreactivity, approximately 2 mm caudal to the lesion epicenter at 35 DPI (magnification = 40x, scale bar = 50 μm). (B) Relative fluorescence intensity quantification of NF-H immunoreactivity shows TAM treatment 0 and 24 h after SCI increased the amount of neurofilaments at the lateral funiculus after SCI. Isotype controls were kept for every experiment; IgG1 immunoreactivity was used to calibrate the microscope and quantified to determine the background level of fluorescence. Left and right lateral funiculus were analyzed, and the average of the total quantification was evaluated for statistical significance. One-way analysis of variance Tukey's multiple comparison test (**p < 0.01, *p < 0.05), data are mean ± SEM, sham (PLB+TAM) n = 10, SCI PLB (rats from t = 0 + 24 h) n = 6, SCI TAM 0 h n = 5, SCI TAM 24 h n = 3.
Low magnification picture (10x) depicts the region of interest within the lateral white matter and NF-H immunostaining in sham, SCI PLB, and TAM treated animals (Fig. 5A). Relative quantification shows that TAM administration immediately or 24 h after SCI increases NF-H immunoreactivity in the lateral funiculus at 35 DPI (Fig. 5B, One-way ANOVA F(3,20) = 19.1; p < 0.0001).
TAM appropriate release and safe use for the clinical setting
This study makes use of a continuous, systemic administration of a drug therapy, which is not targeted to a specific tissue. Because others have shown that TAM results in a decrease in food intake, adipose tissue, and body weight, we monitored changes in weight as an indication of appropriate drug release.25 TAM significantly reduces body weight at 21* and 35** DPI (Table 2; *p < 0.05, **p < 0.01) according to RM two-way ANOVA (F(1,40) = 4.5; p = 0.0399), confirming the continuous release of the drug during the period of this study.
Table 2.
Physiological effects of continuous tamoxifen treatment in the female rat
| Weight (g) | ||||
|---|---|---|---|---|
| Treatment groups | 0 DPI | 21 DPI | 35 DPI | Liver/brain weight ratio (35 DPI) |
| Sham PLB | 246 ± 5.6 | 264.5 ± 7.3 | 279.5 ± 8.1 | 7.87 ± 0.32 |
| n = 4 | n = 5 | |||
| Sham TAM | 250 ± 6.2 | 259.5 ± 5.7 | 264 ± 7.8 | 7.91 ± 0.18 |
| n = 4 | n = 4 | |||
| SCI PLB | 221.5 ± 2.0 | 222.3 ± 3.6 | 239.5 ± 4.5 | 7.21 ± 0.41 |
| t = 0 h | n = 10 | |||
| n = 20 | ||||
| SCI TAM | 221.6 ± 2.0 | 211.6 ± 3.3* | 226 ± 2.7** | 7.43 ± 0.67 |
| t = 0 h | n = 7 | |||
| n = 22 | ||||
Physiological effects of continuous tamoxifen (TAM) treatment in the rat with SCI. To assess TAM appropriate release, animal weight was monitored weekly. TAM significantly reduced the animal weight at 21, 28, and 35 days post-injury (DPI) according to RM two-way analysis of variance Bonferroni post-test (F(1,40) = 0.4540, treatment p = 0.0399, interaction p = 0.0015, DPI p < 0.001). Liver weight was normalized against the brain weight of each animal. Continous TAM administration for 21 days after SCI does not affect liver weight of treated rats when compared with placebo (PLB) (F(3,22) = 0.4434, p = 0.7243).
The liver is the major detoxification organ, and high drug levels may convey an increased workload to this organ, inducing hepatocarcinoma in rats and to a lower extent human livers.35,36 Therefore, to test for the possible toxic effect of continuous TAM infusion in the treated animals, the weight of the liver was examined in our samples at 35 DPI. Because body weights varied with time, we normalized the organ weight against the brain weight of each animal as recommended by the Society of Toxicology.30
One-way ANOVA was performed to compare brain weight between treatment groups, and no statistical difference was observed between sham and injury groups (F(3,25) = 0.6484 MS = 0.1485; p = 0.5923). Continous TAM (t = 0 h) for 21 days after SCI did not affect liver weight of treated rats when compared with PLB (F(3,22) = 0.4; p = 0.7243). Although this is not a complete set of outcome measures to assess the toxic effect of TAM in the studied animals, the gross structure of the major organ involved in the detoxification process was not affected by the dose used. The amount used of this FDA-approved drug was higher than the dose used to treat breast cancer in humans; still no noticeable changes were observed in the liver of the animals treated with TAM, and this could be related to the higher metabolism rate of these rodents.
Discussion
In this study, we demonstrated that TAM is a multiactive drug with a long therapeutic window (up to 24 h) that produces a permissive environment and locomotor recovery after SCI. Behavioral improvements observed with three different assays (BBB open field, grid walking, and beam crossing tests) confirmed the potential of this compound as a therapeutic drug for this condition. Among the long-term favorable results obtained at the cellular level are the increased spared tissue, increased number of neurons, decreased reactive gliosis, and decreased axonal degeneration. Moreover, this is the only chronic study available in female rats receiving TAM for a long period after SCI. The promising results observed with TAM after moderate SCI, without apparent toxic effects, provide strong evidence for its translation into the SCI clinical setting pending FDA approval for the treatment of this condition.
Previous studies showed that a single bolus of TAM (5 mg/kg), 30 min after a contusion SCI produced a significant locomotor recovery in male rats within the first 7 days of the study.12 Moreover, when continuous administration was used as a regimen of infusion 2 h after SCI, TAM (1 mg/day) favored locomotor recovery in the chronic phase of the injury (between 14 and 35 DPI) in male rats.14
Our results confirmed the behavioral observations obtained by these two groups, but expanded the information to the inclusion of female animals in our studies and extended the therapeutic window up to 24 h versus the previously reported up to 2 h after SCI.14 The acute behavioral locomotor recovery (7 DPI) observed in our studies is different from that obtained by Guptarak and colleagues,14 which observed a recovery at 14 DPI up to the end time point of 35 DPI. The discrepancy may be related to the sex of the animals used in each study and the lesion produced by the impactors used in each research project (NYU/MASCIS versus Infinite Horizon impactor).
In addition, we observed behavioral improvement in the beam crossing and grid walking tests suggesting that this drug may benefit those tracts associated with balance and sensorimotor functions. Although beneficial effects were observed in pre-treatment studies and in the acute and chronic studies,12,14,15 we still lack sufficient evidence regarding the molecular and cellular mechanisms activated by TAM that mediate the functional locomotor recovery.
Increased white matter spared tissue has been correlated well by others with locomotor improvement.37–41 This was confirmed in our studies with Luxol fast blue histology when TAM was administered immediately or 24 h after SCI, and either of these regimens significantly increased the amount of white matter spared tissue. The combination of acute and chronic neuroprotective effects by TAM could lead to increases in myelin preservation and, as a consequence, a reduction in cavity size.
Tamoxifen may stimulate neuroprotective mechanisms, which promote oligodendrocyte survival, remyelination of surviving tracts, or favor myelination of sprouted or regenerated tracts. Analyses performed in male rats demonstrated that TAM administration 2 h after SCI spares oligodendrocytes at the 10th thoracic segment (visualized by counting CC-1 labeled oligodendrocytes) and reduces the cavity size.14 Another possibility is that TAM may induce differentiation of progenitor cells into oligodendrocytes in the spinal cord, which may initiate a chronic remyelination process contributing to an increase in white matter after SCI.16,42,43 Both mechanisms were observed by Franco-Rodriguez and colleagues16 in brain injury, suggesting a similar mechanism may be activated by TAM after SCI.
The overall locomotor improvement suggests that TAM administered immediately or 1 day after SCI initiates neuroprotective mechanisms during acute and chronic stages. Among the expected effects of TAM are reduction of inflammatory cytokines, microglial activation, and reduction in astrogliosis, as has been shown by others in models of SCI12,14,17,18 and brain ischemia or trauma.8,13,16,44–48Administration of TAM, 30 min after spinal cord contusion, attenuates neuronal apoptosis within the first few days,12 possibly through the inactivation of caspase-3.18 The effect of the drug to reduce cell death is not only observed in SCI conditions; in a model of irradiation-induced brain injury, reversible focal cerebral ischemia, and MCAo, the investigators observed that TAM administration reduced neuronal cell death.13,45,47
Possible mechanisms that could be activated by TAM, which may result in a reduction of cell death, are a reduction in excitatory amino acid release, scavenger of ROS, and inhibition of peroxynitrite production.10,15,46 In our study, the number of NeuN positive cells augmented significantly at 35 DPI when the animals were treated with TAM immediately or 24 h after SCI. Although less neuron loss at the injured thoracic spinal cord segments likely did not contribute to locomotor recovery, other yet unknown sensorimotor functions may have been protected.38 Therefore, cellular mechanisms could be activated similar to those reported for brain and SCI that lead to increased neuronal number after traumatic injury to the central nervous system.10,12,15,18,46
TAM administration reduces the nonpermissive environment generated after SCI by decreasing the expression of repulsive genes like MAG, Nogo, and Ompg that block axonal outgrowth.12 In addition, this multiactive drug also decreased the level of reactive gliosis generated after SCI or brain trauma.13,14,47,48 In this study, we demonstrated that the levels of GFAP immunoreactive cells decreased significantly when TAM was administered immediately or 24 h after SCI. This result was confirmed by Western blot studies that demonstrated the effect of TAM to reduce the level of GFAP protein in regions rostral, caudal, and at the lesion epicenter. The observed effects seem to relate to an improvement in locomotor recovery, although others have shown that attenuating the astrogliotic response may worsen the outcome of SCI repair.49 Therefore, TAM could help to reduce the repulsive environment generated by the gliotic response and permit axonal outgrowth through the lesion epicenter.
In-vitro studies have shown that raloxifene, another SERM similar to TAM, favors neuronal survival and neurite outgrowth in hippocampal cells.50 The amount of NF-H observed in the lateral funiculus, caudal to the lesion epicenter, was significantly higher in animals treated with TAM immediately or 24 h after SCI. These results support the data obtained with the Luxol fast blue, where an increase in white matter spared tissue was observed together with a behavioral locomotor improvement.
The mechanisms that could explain the increase in neurofilament immunoreactivity after TAM administration are unknown, but possible explanations are a decrease in the inflammatory response, a decrease damage of the blood–brain barrier, and a reduction in edema formation. Studies from others have shown that TAM exerts antioxidant activity in a MCAo model by a receptor independent mechanism.10 This effect correlates well with our previous findings where estradiol reduces ROS formation by an estrogen receptor (ER)-independent mechanism, but the mechanisms by which TAM exerts chronic reduction in ROS were not assessed.15
It is possible that post-injury administration of TAM mediates neuroprotection both by ER-dependent (transcriptional or through proteins at the plasma membrane) and independent mechanisms (ROS scavenger), which could explain the robust effect observed after TAM administration in the acute and chronic stages. Therefore, this multiactive drug has several characteristics that make it an ideal treatment for SCI.11,21 Among those properties are (1) ability to cross the blood–brain barrier,51 (2) reduction in the inflammatory response,12,13,17,44 (3) decline in the levels of ROS,10,15,46 (4) reduce the level of apoptosis,12–14,18 (5) decrease the gliotic response,16,47,48,52 (6) reduction of blood–brain barrier permeability,12,17,44 (7) reduction of repulsive environment,12,14 and (8) myelin preservation.12,14,15 Other regenerative or survival mechanisms mediated by TAM are yet to be studied.
In this study, we observed a decrease in body weight in TAM treated rats that was used as indicative of appropriate drug delivery because of the anorexigenic effects of this drug.25 Although the appropriate release of TAM was observed, a significant reduction in body weight may be disadvantageous in this situation because of increased muscle, water, or fat loss in the injured animals. Because the significant weight loss did not impair the locomotor activity of these animals, the effect on body weight loss after SCI and TAM treatment is yet to be determined. In addition, TAM could be stored in adipose tissue, being released slowly into the plasma for a long period, and the long turnover of its active metabolites permits its activity for up to 2 weeks.36,53
Conclusions
This is a highly clinically relevant study that addressed the importance of evaluating the effects of TAM treatment after SCI. The current therapy for patients with SCI is methylprednisolone, and its use is questionable and controversial.20 Our results suggest that TAM, an FDA-approved drug with the capacity to cross the blood–brain barrier, exerts neuroprotection in female rats when administered up-to 24 h after SCI without considerable toxic effects. The robust effect on locomotion, cell survival, improved extracellular environment, and reduced gliosis provides evidence that TAM should be considered as a therapeutic agent to treat incomplete thoracic SCI.
Previous studies in our laboratory demonstrated that the pellet of 15 mg is the minimum amount of TAM necessary to provide beneficial consequences at the behavioral, anatomical, and cellular levels. The dose of TAM used in this study (0.71 mg/d) is lower than those used by others with neuroprotective effects after SCI (1 mg/d),12,14,15,17,18 but as mentioned by Guptarak and colleagues,14 still within the range used to manage other conditions.54 Because all studies with TAM after SCI were made in male rats, the novelties of these findings were the inclusion and analysis of this drug in female vertebrates, as well as the therapeutic window that produced some functional locomotor recovery after trauma using a low dose of TAM. Future experiments should target the therapeutic window of TAM in male rats, dose response to increase the potential therapeutic effect, as well as the effect of combinatorial strategies to potentiate the effect of this drug, and the molecular mechanism activated by this SERM.
Acknowledgments
This research project is in partial fulfillment of Jennifer M. Colón's doctoral thesis dissertation. The authors recognize the contributions and suggestions of Dr. Anne Etgen, for the development of this research project. Images were captured with a Nikon inverted microscope (grant P031S130068) of Dr. Jose Santiago and a Zeiss fluorescent microscope (grant 1P30NS069258-01) of Dr. Martine Behra with the assistance of Mr. Luis Colón. We give special thanks to Ms. Lyanne Garcia and Mr. Kenneth Garcia from the UPR-Carolina campus for their assistance during the behavioral and molecular experiments. We would like to recognize all personnel from the UPR-MSC Animal Resources for their assistance during surgical procedures and post-operative care. The project was supported by COBRE (P20-GM103642), MBRS-RISE Program (R25 GM061838), NIH-MARC (5T34GM007821-35) and RCMI (5G12MD007600) programs.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rabchevsky A.G., Patel S.P., and Springer J.E. (2011). Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol. Ther. 132, 15–29 [DOI] [PubMed] [Google Scholar]
- 2.McKerracher L., and Higuchi H. (2006). Targeting Rho to stimulate repair after spinal cord injury. J. Neurotrauma 23, 309–17 [DOI] [PubMed] [Google Scholar]
- 3.Fitch M.T., and Silver J. (2008). CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulsebosch C.E. (2002). Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol. Educ. 26, 238–255 [DOI] [PubMed] [Google Scholar]
- 5.Sofroniew M.V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson M.A., Ao Y., and Sofroniew M.V. (2014). Heterogeneity of reactive astrocytes. Neurosci. Lett. 565, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhandapani K.M., and Brann D.W. (2002). Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol. Reprod. 67, 1379–1385 [DOI] [PubMed] [Google Scholar]
- 8.Kimelberg H.K., Jin Y., Charniga C., and Feustel P.J. (2003). Neuroprotective activity of tamoxifen in permanent focal ischemia. J. Neurosurg. 99, 138–42 [DOI] [PubMed] [Google Scholar]
- 9.Mehta S.H., Dhandapani K.M., De Sevilla L.M., Webb C.R., Mahesh V.B., and Brann D.W. (2003). Tamoxifen, a selective estrogen receptor modulator, reduces ischemic damage caused by middle cerebral artery occlusion in the ovariectomized female rat. Neuroendocrinology 77, 44–50 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Milatovic D., Aschner M., Feustel P.J., and Kimelberg H.K. (2007). Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp. Neurol. 204, 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DonCarlos L.L., Azcoitia I., and Garcia-Segura L.M. (2009). Neuroprotective actions of selective estrogen receptor modulators. Psyconeuroendocrinology 34, Suppl 1, S113–S122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian D.S., Liu J.L., Xie M.J., Zhan Y., Qu W.S., Yu Z.Y., Tang Z.P., Pan D.J., and Wang W. (2009). Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J. Neurochem. 109, 1658–1667 [DOI] [PubMed] [Google Scholar]
- 13.Liu J.L., Tian D.S., Li Z.W., Qu W.S., Zhan Y., Xie M.J., Yu Z.Y., Wang W., and Wu G. (2010). Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 1316, 101–111 [DOI] [PubMed] [Google Scholar]
- 14.Guptarak J., Wiktorowicz J.E., Sadygov R.G., Zivadinovic D., Paulucci-Holthauzen A. a, Vergara L., and Nesic O. (2014). The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J. Neurotrauma 31, 268–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosquera L., Colón J.M., Santiago J.M., Torrado A.I., Meléndez M., Segarra A.C., Rodríguez-Orengo J.F., and Miranda J.D. (2014). Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: their antioxidant effect and role of estrogen receptor alpha. Brain Res. 1561, 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco Rodríguez N.E., Dueñas Jiménez J.M., De la Torre Valdovinos B., López Ruiz J.R., Hernández Hernández L., and Dueñas Jiménez S.H. (2013). Tamoxifen favoured the rat sensorial cortex regeneration after a penetrating brain injury. Brain Res. Bull. 98, 64–75 [DOI] [PubMed] [Google Scholar]
- 17.Ismailoğlu O., Oral B., Görgülü A., Sütçü R., and Demir N. (2010). Neuroprotective effects of tamoxifen on experimental spinal cord injury in rats. J. Clin. Neurosci. 17, 1306–1310 [DOI] [PubMed] [Google Scholar]
- 18.Wei H.Y., and Ma X. (2014). Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF-kB pathway after spinal cord injury in rats. Neurol. Sci. 35, 1763–1768 [DOI] [PubMed] [Google Scholar]
- 19.Baptiste D.C., and Fehlings M.G. (2006). Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma 23, 318–334 [DOI] [PubMed] [Google Scholar]
- 20.Bydon M., Lin J., Macki M., Gokaslan Z.L., and Bydon A. (2013). The current role of steroids in acute spinal cord injury. World Neurosurg. 82, 848–854 [DOI] [PubMed] [Google Scholar]
- 21.Salgado I.K., Torrado A.I., Santiago J.M., and Miranda J.D. (2015). Tamoxifen and Src kinase inhibitors as neuroprotective/neuroregenerative drugs after spinal cord injury. Neural Regen. Res. 10, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke D.A., Magnuson D.S., Nunn C.D., Fentress K.G., Wilson M.L., Shum-Siu A.H., Moore M.C., Turner L.E., King W.W., and Onifer S.M. (2007). Use of environmentally enriched housing for rats with spinal cord injury: the need for standardization. J. Am. Assoc. Lab. Anim. Sci. 46, 34–41 [PubMed] [Google Scholar]
- 23.Rosas O.R., Figueroa J.D., Torrado A.I., Rivera M., Santiago J.M., Konig-Toro F., and Miranda J.D. (2011). Expression and activation of ephexin is altered after spinal cord injury. Dev. Neurobiol. 71, 595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santiago M., Rosas O., Torrado A.I., Gonzalez M.M., Kalyan-Masih P.O., and Miranda J.D. (2009). Molecular, anatomical, physiological, and behavioral studies of rats treated with buprenorphine after spinal cord injury. J. Neurotrauma 29, 1783–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wade G.N., and Heller H.W. (1993). Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. Am. J. Physiol. 264, R1219–R1223 [DOI] [PubMed] [Google Scholar]
- 26.Ramsey J.B., Ramer L.M., Inskip J.A, Alan N., Ramer M.S., and Krassioukov A.V. (2010). Care of rats with complete high-thoracic spinal cord injury. J. Neurotrauma 27, 1709–1722 [DOI] [PubMed] [Google Scholar]
- 27.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 28.Merkler D., Metz G.A., Raineteau O., Dietz V., Schwab M.E., and Fouad K. (2001). Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J. Neurosci. 21, 3665–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauerbeck A.D., Laws J.L., Bandaru V.V., Popovich P.G., Haughey N.J., and McTigue D.M. (2015). Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma 32, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michael B., Yano B., Sellers R.S., Perry R., Morton D., Roome N., Johnson J.K., Schafer K., and Pitsch S. (2007). Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 35, 742–750 [DOI] [PubMed] [Google Scholar]
- 31.Figueroa J.D., Benton R.L., Velazquez I., Torrado A.I., Ortiz C.M., Hernandez C.M., Diaz J.J., Magnuson D.S., Whittemore S.R., and Miranda J.D. (2006). Inhibition of EphA7 up-regulation after spinal cord injury reduces apoptosis and promotes locomotor recovery. J. Neurosci. Res. 84, 1438–1451 [DOI] [PubMed] [Google Scholar]
- 32.Otsu N. (1979). A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cybern. 9, 62–66 [Google Scholar]
- 33.Liao P.S., Chen T.S., and Chung P.C. (2001). A fast algorithm for multilevel thresholding. J. Inf. Sci. Eng. 17, 713–727 [Google Scholar]
- 34.Mutterer J., and Zinck E. (2013). Quick-and-clean article figures with FigureJ. J. Microsc. 252, 89–91 [DOI] [PubMed] [Google Scholar]
- 35.Yang G., Nowsheen S., Aziz K., and Georgakilas A.G. (2013). Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol. Ther. 139, 392–404 [DOI] [PubMed] [Google Scholar]
- 36.Robinson S., Langa-Fahey S.M., Johnson D.A., and Jordan V.C. (1991). Metabolites, pharmacodynamics and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab. Dispos. 19, 36–43 [PubMed] [Google Scholar]
- 37.Basso D.M., Beattie M.S., and Bresnahan J.C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 [DOI] [PubMed] [Google Scholar]
- 38.Magnuson D.S., Trinder T.C., Zhang Y.P., Burke D., Morassutti D.J., and Shields C.B. (1999). Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 156, 191–204 [DOI] [PubMed] [Google Scholar]
- 39.Loy D.N., Magnuson D.S., Zhang Y.P., Onifer S.M., Mills M.D., Cao Q., Darnall J.B., Fajardo L.C., Burke D.A., and Whittemore S.R. (2002). Functional redundancy of ventral spinal locomotor pathways. J. Neurosci. 22, 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schucht P., Raineteau O., Schwab M.E., and Fouad K. (2002). Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176, 143–153 [DOI] [PubMed] [Google Scholar]
- 41.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E., Jr. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 42.Barnabé-Heider F., Göritz C., Sabelström H., Takebayashi H., Pfrieger F.W., Meletis K., and Frisén J. (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482 [DOI] [PubMed] [Google Scholar]
- 43.Tripathi R.B., Rivers L.E., Young K.M., Jamen F., and William D. (2011). PDGFRA / NG2 glia generate new oligodendrocytes but few astrocytes in a murine EAE model of demyelinating disease. J. Neurosci. Res. 30, 16383–16390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X., Ji C., Hu T., Wang Z., and Chen G. (2013). Tamoxifen as an effective neuroprotectant against early brain injury and learning deficits induced by subarachnoid hemorrhage: possible involvement of inflammatory signaling. J. Neuroinflammation 10, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimelberg H.K., Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW, Jr, and Tranmer BI. (2000). Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport 11, 2675–2679 [DOI] [PubMed] [Google Scholar]
- 46.Osuka K., Feustel P.J., Mongin A.A., Tranmer B.I., and Kimelberg H.K. (2001). Tamoxifen inhibits nitrotyrosine formation after reversible middle cerebral artery occlusion in the rat. J. Neurochem. 76, 1842–1850 [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Jin Y., Behr M.J., Feustel P.J., Morrison J.P., and Kimelberg H.K. (2005). Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp. Neurol. 196, 41–46 [DOI] [PubMed] [Google Scholar]
- 48.Barreto G., Santos-Galindo M., Diz-Chaves Y., Pernía O., Carrero P., Azcoitia I., and Garcia-Segura L.M. (2009). Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain: effects of aging and prolonged depletion of ovarian hormones. Endocrinology 150, 5010–5015 [DOI] [PubMed] [Google Scholar]
- 49.Faulkner J.R., Herrmann J.E., Woo M.J., Tansey K.E., Doan N.B., and Sofroniew M.V. (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Neill K., Chen S., and Diaz Brinton R. (2004). Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer's disease. Exp. Neurol. 188, 268–278 [DOI] [PubMed] [Google Scholar]
- 51.Biegon A., Brewster M., Degani H., Pop E., Somjen D., and Kaye A.M. (1996). A permanently charged tamoxifen derivative displays anticancer activity and improved tissue selectivity in rodents. Cancer Res. 56, 4328–4331 [PubMed] [Google Scholar]
- 52.Arevalo M.A., Diz-Chaves Y., Santos-Galindo M., Bellini M.J., and Garcia-Segura L.M. (2012). Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. J. Neuroendocrinol. 24, 183–190 [DOI] [PubMed] [Google Scholar]
- 53.Lien E.A., Solheim E., and Ueland P.M. (1991). Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 51, 4837–4844 [PubMed] [Google Scholar]
- 54.Robins H.I., Won M., Seiferheld W.F., Schultz C.J., Choucair A.K., Brachman D.G., Demas W.F., and Mehta M.P. (2006). Phase 2 trial of radiation plus high-dose tamoxifen for glioblastoma multiforme: RTOG protocol BR-0021. Neuro. Oncol. 8, 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]