Abstract
Advanced glycation end-products (AGEs)-induced mesangial cell death is one of major causes of glomerulus dysfunction in diabetic nephropathy. Both endoplasmic reticulum (ER) stress and autophagy are adaptive responses in cells under environmental stress and participate in the renal diseases. The role of ER stress and autophagy in AGEs-induced mesangial cell death is still unclear. Here, we investigated the effect and mechanism of AGEs on glomerular mesangial cells. AGEs dose-dependently decreased mesangial cell viability and induced cell apoptosis. AGEs also induced ER stress signals in a time- and dose-dependent manner. Inhibition of ER stress with 4-phenylbutyric acid effectively inhibited the activation of eIF2α and CHOP signals and reversed AGEs-induced cell apoptosis. AGEs also activated LC-3 cleavage, increased Atg5 expression, and decreased p62 expression, which indicated the autophagy induction in mesangial cells. Inhibition of autophagy by Atg5 siRNAs transfection aggravated AGEs-induced mesangial cell apoptosis. Moreover, ER stress inhibition by 4-phenylbutyric acid significantly reversed AGEs-induced autophagy, but autophagy inhibition did not influence the AGEs-induced ER stress-related signals activation. These results suggest that AGEs induce mesangial cell apoptosis via an ER stress-triggered signaling pathway. Atg5-dependent autophagy plays a protective role. These findings may offer a new strategy against AGEs toxicity in the kidney.
Diabetes mellitus (DM) is one of the most common metabolic diseases in the world1. There are many DM-induced complications such as retinopathy, nephropathy, peripheral neuropathy, and microvascular injury, which accounts for high mortality rates in diabetic patients1,2,3. Advanced glycation end-products (AGEs) resulting from hyperglycemia are reactive derivatives formed by the Maillard reaction or during oxidation of lipids and nucleic acids. AGEs are known to be an important factor in diabetes-induced complications4,5. AGEs have been found to induce the pancreatic islet endothelial cell apoptosis and skeletal muscle atrophy2,4. Singh et al. also suggested that AGEs play an important role in diabetic nephropathy6. In particular, diabetic nephropathy is one of the DM-induced complications, which results in high mortality rates. 25–40% of diabetic patients develop diabetic nephropathy within 20–25 years after the onset of diabetes and over 20% of those diabetic nephropathy patients develop end-stage renal disease in the next 10–20 years1,7,8,9. The pathological changes of diabetic nephropathy include glomerular hyperfiltration, mesangial matrix expansion, and glomerular and tubular hypertrophy. There is a high chance that diabetic nephropathy progresses to glomerular sclerosis and renal fibrosis, which cause renal dysfunction10,11,12. Mesangial cells are known to play an important role in diabetic nephropathy progression. Mesangial cells are responsible for modulating glomerular filtration and phagocytosing apoptotic cells and maintaining the structure of glomerular capillary as well as mesangial matrix homeostasis in the kidney13. In diabetic nephropathy patients, mesangial matrix expansion, which could cause by AGEs or other diabetic nephropathy injury factors, is one of important mechanism for kidney dysfunction14,15. Furthermore, mesangial cell apoptosis induced by AGEs or high glucose was another important mechanism in diabetic nephropathy-induced albuminuria and renal dysfunction13,16,17. High glucose can induce oxidative stress and intrinsic proapoptotic pathway in mesangial cells18,19. AGEs also evoked apoptosis in both mouse and human mesangial cells20,21. Although the secretions of vascular endothelial growth factor and monocyte chemotactic protein 1 protein have been found to be associated with AGEs-induced mesangial cell apoptosis20, the mechanisms of mesangial cell apoptosis induced by AGEs are still unclear.
Endoplasmic reticulum (ER) stress and autophagy have known to be associated with many kinds of renal injuries. Protein synthesis, folding, and modification are major functions of ER in the cells. If ER functions are disturbed, unfolded proteins will accumulate in the cells and subsequently induce ER stress and an adaptive response - unfolded protein response (UPR)22,23. UPR can protect cells under mild or short-term stress, but induces apoptosis if cells receive server or unrecoverable damage22. There are three major signaling pathways in UPR, including PKR-like ER kinase (PERK)-Eukaryotic initiation factor 2α (eIF2α), activating transcription factor 6 (ATF6), and IRE-1. Three pathways can induce the expression of CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP), which plays an important role in ER stress-induced apoptosis24. Previous study showed that CHOP expression enhanced cell apoptosis in renal ischemia reperfusion injury25. Xu Y et al. found that ER stress regulated transforming growth factor (TGF)-β-induced mesangial cell injury26. However, the role of ER stress in AGEs-induced mesangial cell apoptosis remains to be clarified. On the other hand, autophagy is another adaptive response in the cell under stress condition in which the double-membrane vacuoles (autophagosomes) are formed and delivered the cell contents to lysosome for degradation27. Several studies have also demonstrated that autophagy can prevent stress-induced apoptosis in the kidney28,29,30, and ER stress plays an important role in autophagy regulation31,32. Therefore, we hypothesized that both ER stress and autophagy may be involved in the AGEs-induced mesangial cell apoptosis. In this study, we tried to clarify whether ER stress and autophagy are participated in AGEs-induced mesangial cell apoptosis and death.
Results
AGEs induced mesangial apoptosis in a dose dependent manner
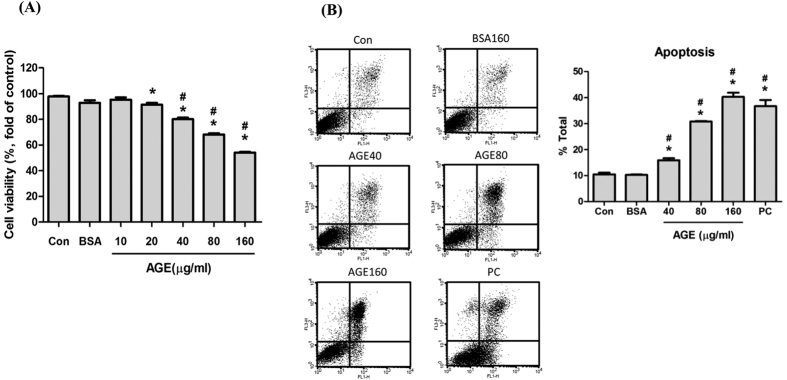
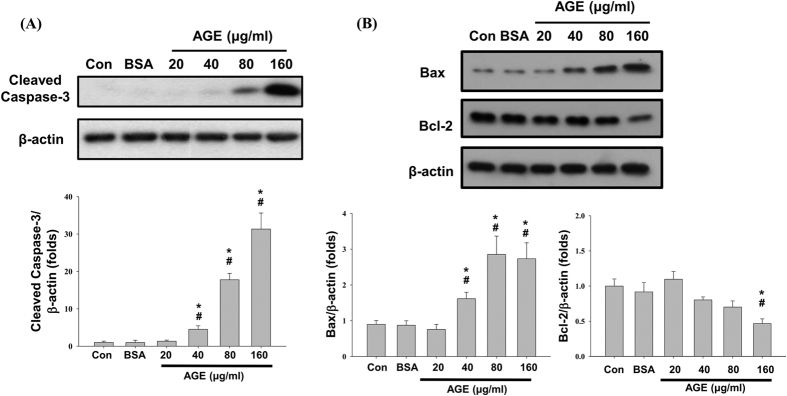
Mesangial cells were treated with various concentrations of AGEs (10, 20, 40, 80, and 160 μg/ml) and BSA (160 μg/ml) for 24 h to evaluate the cell viability. The cell viability significantly reduced in a dose-dependent manner after AGEs treatment as compared with normal control or BSA control (Fig. 1A). To understand whether apoptosis involved in AGEs-reduced cell viability, annexin V/PI staining was used. The results showed that AGEs (40–160 μg/ml) significantly induced apoptosis at in a dose-dependent manner (Fig. 1B). Furthermore, AGEs significantly increased the cleavage of caspase-3 protein (Fig. 2A). AGEs also significantly increased the Bax protein expression at the concentrations of 40–160 μg/ml, but decreased the Bcl-2 protein expression at the concentration of 160 μg/ml (Fig. 2B). These results show that AGEs induce mesangial cell apoptosis in a dose-dependent manner.
Figure 1. Effects of AGEs on mesangial cell viability and apoptosis induction.
Mesangial cells treated with AGEs (10, 20, 40, 80 and 160 μg/ml) for 24 h. BSA (160 μg/ml) was used as a negative control. The cell viability was evaluated by MTT assay (A). For apoptosis evaluation, cells were stained with Annexin V and PI after treated with AGEs. Cells were treated with 3% formaldehyde for 30 minutes as a positive control (PC). Percentages of apoptotic cells were determined by flow cytometry (B). Data are presented as mean ± SEM of three independent experiments performed in triplicates. *P < 0.05 as compared to control group. #P < 0.05 as compared to BSA-treated group.
Figure 2. Effects of AGEs on apoptosis-related protein expressions.
Cells were treated with AGEs (20–160 μg/ml) for 24 h. BSA (160 μg/ml) was used as a negative control. The cleavage of caspase-3 (A) and the protein expressions of Bax and Bcl-2 (B) were determined by Western blot. Data are presented as mean ± SEM of three independent experiments performed in duplicates. *P < 0.05 as compared to control group. #P < 0.05 as compared to BSA-treated group.
AGEs induced ER stress and autophagy in both dose and time-dependent manners
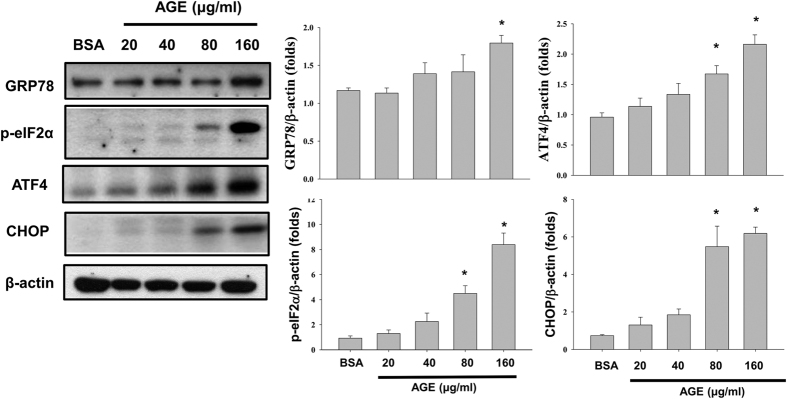
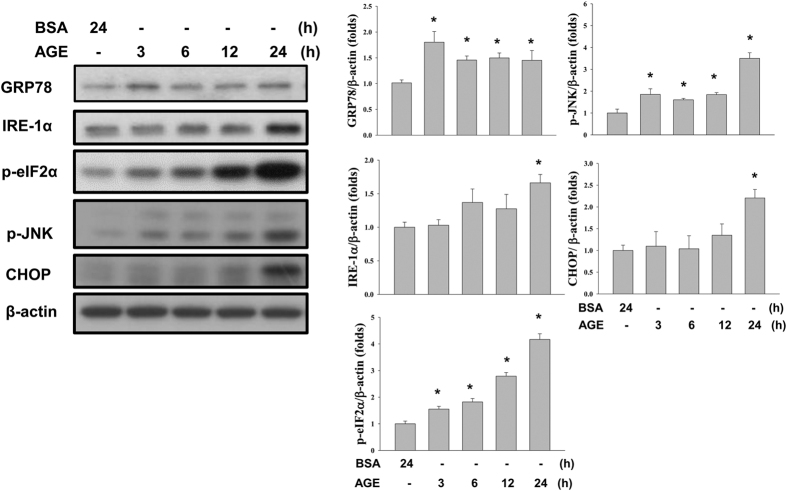
To understand whether ER stress involved in AGEs-induced mesangial cell apoptosis, ER stress-related proteins were detected by Western blots. Mesangial cells were treated with various concentrations of AGEs (20, 40, 80, and 160 μg/ml) and BSA (160 μg/ml) for 24 h. AGEs significantly upregulated the protein expressions of GRP78, ATF4, and CHOP and phosphorylation of eIF2α in a dose-dependent manner (Fig. 3). AGEs also increased the phosphorylations of eIF2α and c-Jun N-terminal kinase (JNK) and the protein expressions of GRP78, IRE-1α, and CHOP in a time dependent manner (Fig. 4). These results indicate that AGEs induced ER stress in mesangial cells, which may participate in AGEs-induced mesangial cell apoptosis.
Figure 3. Effects of AGEs on ER stress-related protein expressions.
Cells were treated with AGEs (20–160 μg/ml) for 24 h. BSA (160 μg/ml) was used as a negative control. The protein expressions of GRP78, phospho-eIF2α, ATF4, and CHOP were determined by Western blot. Data are presented as mean ± SEM of three independent experiments performed in duplicates. *P < 0.05 as compared to control group. #P < 0.05 as compared to BSA-treated group.
Figure 4. Effects of AGEs on ER stress-related proteins at various time points.
Cells were treated with AGEs (160 μg/ml) for 3, 6, 12, and 24 h. BSA (160 μg/ml) was used as a negative control. The protein expressions of GRP78, IRE-1α, phospho-eIF2α, phospho-JNK, and CHOP were determined by Western blot. Data are presented as mean ± SEM of three independent experiments performed in duplicates. *P < 0.05 as compared to control group. #P < 0.05 as compared to BSA-treated group.
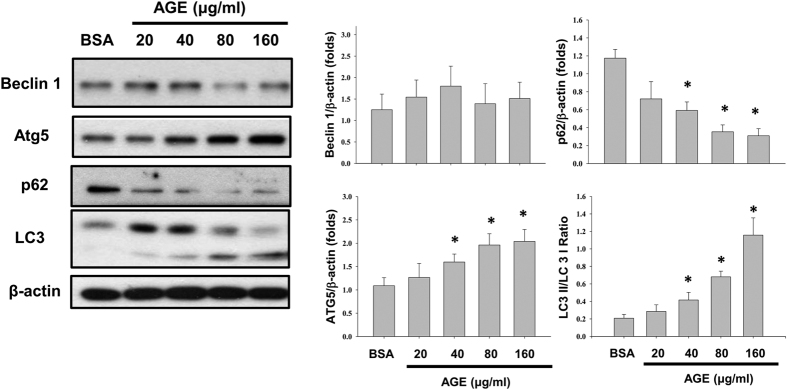
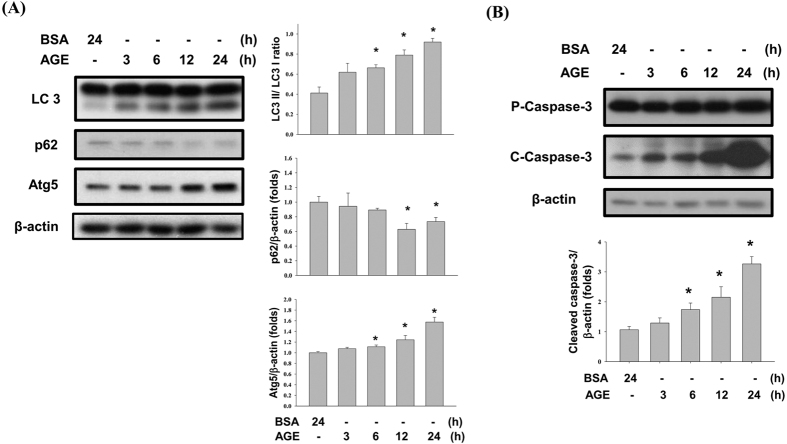
Autophagy is also an important mechanism involved in different renal injuries. AGEs significantly increased LC3 cleavage (LC3-II/LC3-I ratio) and autophagy-related gene 5 (Atg5) protein expression in mesangial cells in a dose- and time-dependent manner (Figs 5 and 6A). The protein expression of beclin1 was not affected by AGEs treatment (Fig. 5). The p62 protein, which is one of downstream signals of autophagy, was also decreased after AGEs treatment in a dose- and time-dependent manner (Figs 5 and 6A). Furthermore, the time course of caspase-3 activation was similar with the expression of ER stress and autophagy-related proteins after AGEs treatment (Fig. 6B). These results suggest that AGEs accumulation in mesangial cells induces autophagy activity.
Figure 5. Effects of AGEs on autophagy-related protein expressions.
Cells were treated with AGEs (20–160 μg/ml) for 24 h. BSA (160 μg/ml) was used as a negative control. The cleavage of LC3 and the protein expressions of Atg 5, beclin 1, and p62 were determined by Western blot. Data are presented as mean ± SEM of three independent experiments performed in duplicates. *P < 0.05 as compared to BSA-treated group.
Figure 6. Effects of AGEs on autophagy and apoptosis at various time points.
Cells were treated with AGEs (160 μg/ml) for 3, 6, 12, and 24 h. BSA (160 μg/ml) was used as a negative control. The protein expressions of LC3, p62, Atg5, and CHOP (A) and caspase-3 (B) were determined by Western blot. Data are presented as mean ± SEM of three independent experiments performed in duplicates. *P < 0.05 as compared to control group. #P < 0.05 as compared to BSA-treated group.
4-Phenylbutyric acid (4PBA) reversed AGEs-induced ER stress and apoptotic response in mesangial cells
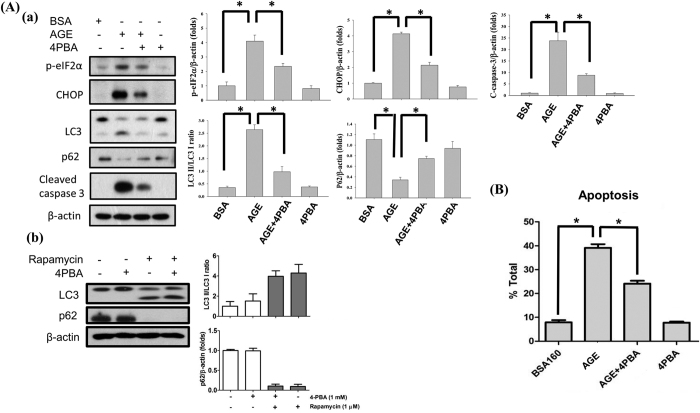
4PBA is a potent ER stress inhibitor, which can attenuate ER stress-induced cell injury33,34. We tried to use 4PBA to inhibit AGEs-induced ER stress and figure out the role of ER stress in AGEs-induced mesangial cell apoptosis. Mesangial cells were treated with AGEs (160 μg/ml) with or without 4PBA (1 mM) for 24 h. Co-treatment with 4PBA significantly reversed AGEs-induced CHOP protein expression and eIF2α phosphorylation and caspase-3 cleavage (Fig. 7Aa). Annexin V/PI staining also showed that 4PBA significantly reduced the percentage of AGEs-induced apoptotic mesangial cells (Fig. 7B). Moreover, co-treatment with 4PBA significantly reversed AGEs-induced LC3 cleavage (decrease of LC3-II/LC3-I ratio) and p62 protein degradation (Fig. 7Aa). 4PBA by itself did not affect the rapamycin-induced autophagy in mesangial cells (Fig. 7Ab). These results indicate that AGEs may induce caspase-3 activation and apoptosis and autophagy through an eIF2α/CHOP signaling pathway.
Figure 7. The role of ER stress in AGEs-induced mesangial cell apoptosis.
Cells were treated with AGEs (160 μg/ml) with or without ER stress inhibitor 4-phenylbutyric acid (4PBA, 1 mM) treatment for 24 h. The protein levels of phospho-eIF2α, CHOP, LC3, and cleaved caspase-3 were determined by Western blot (A-a). In some experiments, the effect of 4PBA on rapamycin-induced autophagy was tested (A-b). The percentages of apoptotic cells were determined by PI-Annexin V staining (B). Data are presented as mean ± SEM of three independent experiments performed in duplicates (A) or triplicates (B). *P < 0.05 as compared to BSA-treated group or AGEs-treated group.
Inhibition of Atg5 expression aggravated AGEs-induced injury in mesangial cells
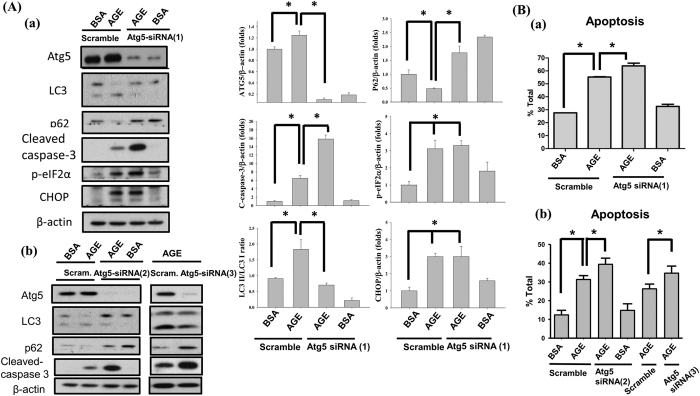
To clarify the role of autophagy in AGEs-induced mesangial cell apoptosis, cells were transfected with scramble or Atg5 siRNA for 6 h before AGEs treatment. Transfection with Atg5 siRNA (MSS247019) significantly reduced Atg5 protein expression and LC3 cleavage and p62 protein degradation (Fig. 8Aa). To confirm the effect of Atg5 knockdown, a single Atg5 siRNA (MSS247020) and a mixture of three Atg5 siRNAs (MSS247019, MSS247020, and MSS247021) were used. As shown in Fig. 8Ab, Atg5 siRNAs (MSS247020 and mixture) could also reduce Atg5 protein expression and LC3 cleavage and p62 protein degradation. These results indicate that Atg5 siRNA transfection inhibits AGEs-induced autophagy. Moreover, the AGEs-induced cleavage of caspase-3 was significantly enhanced by Atg5 siRNAs transfection (Fig. 8Aa,b). Similarly, transfection of Atg5 siRNAs significantly enhanced AGEs-induced cell apoptosis (Fig. 8Ba,b). These results may not due to the off-target effect of the Atg5 knockdown. However, transfection of Atg5 siRNA did not alter the protein expression of CHOP and the phosprylation of eIF2α in AGEs-treated mesangial cells (Fig. 8Aa). These results suggest that autophagy may play a protective role in AGEs-induced mesangial cell apoptosis. ER stress is capable of interfering with the function of autophagy in mesangial cells.
Figure 8. The role of autophagy in AGEs-induced mesangial cell apoptosis.
Mesangial cells were transfected with single Atg5 siRNA [(MSS247019; siRNA (1), (A-a)] or mixture of Atg5 siRNAs [(MSS247019, MSS247020, and MSS247021; siRNA (2), (A-b)] or single Atg5 siRNA [(MSS247020; siRNA (3), (B-a)] or scramble control before AGEs treatment. Cells were treated with BSA (160 μg/ml) or AGEs (160 μg/ml) for 24 h. The protein levels of Atg5, p62, LC3, phospho-eIF2α, CHOP, and cleaved caspase-3 were determined by Western blot (A). The percentages of apoptotic cells were determined by PI-Annexin V staining (B). Data are presented as mean ± SEM of three independent experiments performed in duplicates (A) or triplicates (B). *P < 0.05 as compared to BSA-treated group or AGEs-treated group.
Discussion
In this study, we found that AGEs induced renal glomerular mesangial cell apoptosis and death. We further found that both ER stress and autophagy were involved in AGEs-induced mesangial cells apoptosis. Inhibition of ER stress by 4PBA decreased eIF2α phosphorylation, CHOP protein expression, and cell apoptosis in AGEs-treated mesangial cells. Inhibition of autophagy by Atg5 siRNA transfection decreased Atg5 protein expression, LC3 cleavage, and p62 degradation, but aggravated cell apoptosis in AGEs-treated mesangial cells. Furthermore, ER stress inhibition could reduce the autophagy response, but autophagy inhibition did not affect ER stress activation. These results suggest that ER stress is the upstream regulator of autophagy in mesangial cells exposed to AGEs, and autophagy may play a protective role in AGEs-induced mesangial cell apoptosis.
The studies have shown that the serum levels of AGEs are significantly increased in the diabetic patients versus the control subjects: type 1 DM, 6.6 (5.1–9.9) vs. 5.5 (3.7–8.2) U/ml35; type 2 DM, 7.4 (4.4–10.9) vs. 4.2 (1.6–6.4) U/ml (1 AGE unit = 1 μg/mL AGE-BSA standard)36. The blood AGEs levels were also increased in the diabetic mouse models: control mice, 3–6 μg/ml; streptozotocin-induced diabetic mice, 65–75 μg/ml; high-fat, high-fructose-induced diabetic mice, 10–15 μg/ml37; db/db diabetic mice, 50–60 μg/ml38. AGEs have been reported to decrease cell viability and induce apoptosis in various cell types. Yamagishi et al. observed that AGEs (AGE-BSA) at 100 μg/ml reduced the viable cell numbers of retinal pericytes and induced apoptotic cell death in pericytes at 250 μg/ml39. Lan et al. also found that AGEs (AGE-BSA, 25–200 μg/ml) induced apoptosis in pancreatic islet endothelial cells2. Mahali et al. have demonstrated that AGEs [AGE-human serum albumin (HSA)] at 100 μg/ml induced apoptosis in some cancer cell lines40. Geoffroy et al. have shown that AGEs (AGE-BSA) at concentrations of <1 μM increase the rat mesangial cell proliferation, whereas AGEs at concentrations of >10 μM markedly inhibit the mesangial cell proliferation41. It has also been found that the concentrations of AGEs (AGE-BSA) at 10–50 μg/ml effectively reduced the mouse mesangial cell viability38. Yamabe et al. found that intracellular AGEs accumulation induced by AGE precursor (500 and 1000 μM glycolaldehyde) caused apoptosis and induced ER stress in chondrocytes42. In the present study, we found that 40–160 μg/ml AGEs (AGE-BSA) significantly reduced mesangial cell viability and induced mesangial cell apoptosis. Therefore, the concentrations of AGEs used in this study are reasonable and effectively induce mesangial cell injury.
The present study showed that AGEs induced mesangial cell apoptosis; however, some studies showed that AGEs induced cell proliferation and hypertrophy. Matrix accumulation induced by mesangial cell hypertrophy is already known also an important mechanism in diabetic nephropathy13,43. It is unclear that why there are two opposite responses in mesangial cells under hyperglycemia condition. Induction of inflammatory response may be one of important reasons that cause AGEs-induced mesangial cells apoptosis13. Meek et al. found that high level of AGEs induced strong inflammation response through the receptor for AGEs and subsequently induced apoptosis in mesangial cells and podocytes21. Furthermore, several studies have shown that ER stress possesses the ability to initiate the reactive oxygen species (ROS) cascades25,44,45. ROS is the most important mechanism for inflammatory response induction in cells46. In this study, we found that AGEs markedly induced ER stress and apoptosis in mesangial cells. It is possible that AGEs induce inflammatory response through ER stress-initiated ROS cascades and subsequently increase mesangial cells apoptosis. However, this hypothesis needs to be proved in the future.
Autophagy is a complicated response regulated by cellular nutrient and stress conditions. To adapt environment, autophagy by which plays a protective role or a harmful role depends on different situations. However, the mechanisms in which cells how to decide the role of autophagy were not totally understood. A previous study showed that AGEs induced autophagy through a RAGE/PI3K/AKT/mTOR signaling pathway in cardiomyocytes, which decreased the cell viability in a dose-dependent manner47. Autophagy induced by cadmium also impaired the viability of mesangial cells48. Atg5 is known to be a gene product required for autophagosome formation. Atg5 cleavage induced by death stimuli has been shown to trigger mitochondria-mediated apoptosis49. Leng et al. have found that Atg5, but not beclin1, plays a role in ursolic acid-induced autophagic cell death in cervical cancer cells50. Nevertheless, autophagy can play the protective roles in osteoblastic cells and podocytes under hyperglycemia circumstance51,52. Atg5-dependent autophagy has also been found to act the protective effect in paraquat and MPP+-induced apoptotic dopaminergic cell death53. In the present study, we found that AGEs activated autophagy by ER stress induction in mesangial cells. ER stress inhibition by 4PBA significantly reversed AGEs-induced autophagy, but autophagy inhibition did not influence the AGEs-induced ER stress-related signals. Inhibition of autophagy by Atg5 knockdown could significantly enhance the cytotoxic effect of AGEs on mesangial cells. These results suggest that Atg5-dependent autophagy plays a protective role in AGEs-induced mesangial cell injury.
In conclusion, this study provides evidence to suggest that AGEs induce mesangial cell apoptosis through ER stress induction. Moreover, Atg5-dependent autophagy activated by ER stress plays a protective role in AGEs-induced mesangial cell injury. These findings may offer a new strategy against AGEs toxicity in the kidney. These findings are provocative and deserve further animal study in the future.
Methods
Cell culture
Murine glomerular mesangial cell line (SV40 MES13) was purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan). Cells were cultured in 3:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium contained 5% fetal bovine serum, 14 mM HEPES, 100 U/ml penicillin, and 0.1 mg/ml streptomycin at 37 °C in 5% CO2. AGEs (AGE-BSA) were prepared as previously described4. The purity of AGEs was determined by liquid chromatography.
Cell viability assay
Cells were cultured in 24-well plates one day before AGEs treatment. For cell viability evaluation, cells were treated with 0, 10, 20, 40, 80, and 160 μg/ml AGEs for 24 h. BSA (160 μg/ml) treatment was as a negative control for AGEs-induced cytotoxicity. Medium was replaced with PBS contained 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO, USA) 24 h after AGEs treatment. The yellow tetrazolium is reduced by mitochondrial succinate dehydrogenase and purple formazan crystal is form in living cells. After 2 h incubation, MTT solution was replaced with dimethyl sulfoxide and then the plates were incubated at room temperature for 30 min with constant agitation to dissolve purple formazan crystal. The absorbance at 570 nm was detected by SpectraMax 190 spectrophotometer (Molecular Devices, Sunnyvale, CA), and the cell viability was calculated.
Annexin V and propidium iodide (PI) double staining
The numbers of AGEs-induced apoptotic cells were measured by FITC Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA). After cells treated with different concentrations of AGEs for 24 h, cells and media were collected and centrifuged at 1,000 rpm for 5 min at 4 °C. After washed by PBS twice, cells were resuspended in 100 μl 1x binding buffer contained 5 μl PI and 5 μl FITC-annexin V for 15 min. After that, 400 μl 1x binding buffer was replenished. Samples were analyzed by flow cytometry (BD Biosciences). In total 10,000 cells per sample, both early (PI negative and annexin V positive stained cells) and late (PI positive and annexin V positive stained cells) apoptotic cells were analyzed.
Immunoblotting analysis
After AGEs treatment, cells were washed with PBS twice and then lysed with RIPA buffer contained proteinase inhibitor (1 mM NaF, 1 mM phenylmethanesulfonylfluoride, 1 μg/ml aprotinin and 1 μg/ml leupeptin) on ice for 30 min. The cell lysates were collected and centrifuged at 13,000 rpm for 30 min at 4 °C. The supernatants were collected. The total proteins were denatured by sodium dodecyl sulfate (SDS) sampling buffer and boiled in water for 5 min. SDS polyacrylamide gel electrophoresis (6–15%) was used to separate the total protein samples. The proteins were then transferred from gel to polyvinyl difluoride membrane (Merck Millipore, Billerica, MA, USA). Membranes were blocked by Tris-buffered saline contained 0.1% Tween-20 (TBST) and 5% nonfat milk, and then immersed in sequence of TBST buffer contained primary antibodies for capspase-3, CHOP, phospho-eIF2α, LC3, Atg5, beclin1, and p62 (1:1000; Cell Signaling Technology, Danvers, MA), and secondary horseradish peroxidase antibody (Santa Cruz Biotechnology, San Diego, CA). Finally, signals were detected by Clarity ECL western blotting substrate (BIO-RAD).
Atg5 siRNA transfection
Small interfering RNA (siRNA) against Atg5 (Stealth Atg5-siRNA; MSS247019, MSS247020, and MSS247021) and negative control siRNA (Stealth RNAi Negative Control kit; 12935110) were purchased from Invitrogen (Carlsbad, CA, USA). Cells were seeded in 6-well plates one day before experiments. The growth medium was replaced with 800 μl OPTI-MEM medium (Invitrogen) before siRNA transfection. OPTI-MEM medium (200 μl) contained siLentFect Lipid (BIO-RAD, Philadelphia, PA) and 80 nM Atg5 siRNA or negative siRNA was added to each well. After 6 h of transfection, OPTI-MEM medium was replaced with 1 ml growth medium.
Statistics
Data are presented as mean ± SEM for at least three independent experiments. The whole procedure of cellular experiment was repeated at least three separate times with different cell samples. Each repetition was performed in multiple replicates. The significant difference from the respective controls for each experimental test condition was assessed by one-way analysis of variance (ANOVA) followed by post hoc analysis with Bonferroni’s test. A value of P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Chiang, C.-K. et al. Involvement of Endoplasmic Reticulum Stress, Autophagy, and Apoptosis in Advanced Glycation End Products-Induced Glomerular Mesangial Cell Injury. Sci. Rep. 6, 34167; doi: 10.1038/srep34167 (2016).
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology of Taiwan (MOST104-2320-B-002-021-MY3) and the National Taiwan University Hospital (NTUH105-S2953).
Footnotes
Author Contributions All authors approved the final version to be published. Study conception and design: S.-H.L., K.-H.H. and M.-L.S. Acquisition of data: C.-K.C., T.-F.L, K.-H.H. and C.-C.W. Analysis and interpretation of data: T.-F.L., K.-H.H., C.-C.W., S.-H.L., C.-K.C, K.-Y.H. and M.-L.S. Wrote the paper: S.-H.L. and C.-K.C.
References
- Yamagishi S., Nakamura K. & Imaizumi T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr. Diabetes Rev. 1, 93–106 (2005). [DOI] [PubMed] [Google Scholar]
- Lan K. C. et al. Advanced glycation end-products induce apoptosis in pancreatic islet endothelial cells via NF-κB-activated cyclooxygenase-2/prostaglandin E2 up-regulation. PLoS One 10, e0124418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Park K. H., Gul R., Jang K. Y. & Kim U. H. Role of kidney ADP-ribosyl cyclase in diabetic nephropathy. Am. J, Physiol. Renal Physiol . 296, F291–F297 (2009). [DOI] [PubMed] [Google Scholar]
- Chiu C. Y. et al. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J. Pathol. 238, 470–482 (2016). [DOI] [PubMed] [Google Scholar]
- Angoorani P., Ejtahed H. S., Mirmiran P., Mirzaei S. & Azizi F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int. J. Food Sci. Nutr. 67, 170–176 (2016). [DOI] [PubMed] [Google Scholar]
- Singh V. P., Bali A., Singh N. & Jaggi A. S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 18, 1–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Bo H., Villani V., Spencer P. J. & Fu P. The inhibitory effect of rapamycin on toll like receptor 4 and interleukin 17 in the early stage of rat diabetic nephropathy. Kidney Blood Press Res . 41, 55–69 (2016). [DOI] [PubMed] [Google Scholar]
- Yamagishi S. & Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid. Med. Cell Longev . 3, 101–108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann K. & Benz K. Structural renal changes in obesity and diabetes. Semin. Nephrol. 33, 23–33 (2013). [DOI] [PubMed] [Google Scholar]
- Qin G. et al. Overexpression of the foxo1 ameliorates mesangial cell dysfunction in male diabetic rats. Mol. Endocrinol. 29, 1080–1091 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. et al. Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr. Med. Chem. 22, 2858–2870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K. et al. Anti-inflammatory role of microrna-146a in the pathogenesis of diabetic nephropathy. J. Am. Soc. Nephrol . (In Press) 10.1681/ASN.2015010111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud H. E. Mesangial cell biology. Exp. Cell Res. 318, 979–985 (2012). [DOI] [PubMed] [Google Scholar]
- Steffes M. W., Osterby R., Chavers B. & Mauer S. M. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes 38, 1077–1081 (1989). [DOI] [PubMed] [Google Scholar]
- Anil Kumar P., Welsh G. I., Saleem M. A. & Menon R. K. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol . 5, 151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera T., Martin J., Riley S., Steadman R. & Phillips A. O. Glucose enhances mesangial cell apoptosis. Lab. Invest. 86, 566–577 (2006). [DOI] [PubMed] [Google Scholar]
- Pesce C. et al. Glomerular cell replication and cell loss through apoptosis in experimental diabetes mellitus. Nephron 90, 484–488 (2002). [DOI] [PubMed] [Google Scholar]
- Mishra R., Emancipator S. N., Kern T. & Simonson M. S. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int . 67, 82–93 (2005). [DOI] [PubMed] [Google Scholar]
- Menini S. et al. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 55, 1642–1650 (2006). [DOI] [PubMed] [Google Scholar]
- Yamagishi S. et al. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J. Biol. Chem. 277, 20309–20315 (2002). [DOI] [PubMed] [Google Scholar]
- Meek R. L. et al. Glomerular cell death and inflammation with high-protein diet and diabetes. Nephrol. Dial. Transplant. 28, 1711–1720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagi R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 10, 156–165 (2010). [DOI] [PubMed] [Google Scholar]
- Dickhout J. G. & Krepinsky J. C. Endoplasmic reticulum stress and renal disease. Antioxid. Redox. Signal. 11, 2341–2352 (2009). [DOI] [PubMed] [Google Scholar]
- Oyadomari S. & Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ . 11, 381–389 (2004). [DOI] [PubMed] [Google Scholar]
- Chen B. L. et al. CCAAT-Enhancer-Binding Protein Homologous Protein Deficiency Attenuates Oxidative Stress and Renal Ischemia-Reperfusion Injury. Antioxid. Redox. Signal. 23, 1233–1245 (2015). [DOI] [PubMed] [Google Scholar]
- Xu Y. et al. Role of the ER stress in prostaglandin E2/E-prostanoid 2 receptor involved TGF-beta1-induced mice mesangial cell injury. Mol. Cell. Biochem. 411, 43–55 (2016). [DOI] [PubMed] [Google Scholar]
- Kaushal G. P. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney int . 82, 1250–1253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. L. et al. Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. J. Nutr. Biochem. 25, 1226–1234 (2014). [DOI] [PubMed] [Google Scholar]
- Ko G. J., Bae S. Y., Hong Y. A., Pyo H. J. & Kwon Y. J. Radiocontrast-induced nephropathy is attenuated by autophagy through regulation of apoptosis and inflammation. Hum Exp Toxicol . (In Press) 10.1177/0960327115604198 (2015). [DOI] [PubMed] [Google Scholar]
- Cui J. et al. Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci. Rep. 5, 11256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakhar R., Paul S., Bhardwaj M. & Kang S. C. Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis. Cancer lett . 372, 89–100 (2016). [DOI] [PubMed] [Google Scholar]
- Chandrika B. B. et al. Endoplasmic Reticulum Stress-Induced Autophagy Provides Cytoprotection from Chemical Hypoxia and Oxidant Injury and Ameliorates Renal Ischemia-Reperfusion Injury. PLoS One 10, e0140025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. H. et al. Chemical chaperon 4-phenylbutyrate protects against the endoplasmic reticulum stress-mediated renal fibrosis in vivo and in vitro. Oncotarget 7, 22116–22127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung W. F. et al. 4-Phenylbutyric Acid (4-PBA) and Lithium Cooperatively Attenuate Cell Death during Oxygen-Glucose Deprivation (OGD) and Reoxygenation. Cell. Mol. Neurobiol. 35, 849–859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T. J. et al. The advanced glycation end product Nepsilon-(carboxymethyl)lysine is increased in serum from children and adolescents with type 1 diabetes. Diabetes Care 21, 1997–2002 (1998). [DOI] [PubMed] [Google Scholar]
- Kilhovd B. K., Berg T. J., Birkeland K. I., Thorsby P. & Hanssen K. F. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 22, 1543–1548 (1999). [DOI] [PubMed] [Google Scholar]
- Zhu W., Li W. & Silverstein R. L. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood 119, 6136–6144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S. S., Sheu M. L., Wu C. T., Chiang C. K. & Liu S. H. ATP synthase subunit-β down-regulation aggravates diabetic nephropathy. Sci. Rep. 5, 14561 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S. et al. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 290, 973–978 (2002). [DOI] [PubMed] [Google Scholar]
- Mahali S., Raviprakash N., Raghavendra P. B. & Manna S. K. Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: involvement of Ca2+ mediated by interleukin-8 protein. J. Biol. Chem. 286, 34903–34913 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy K., Wiernsperger N., Lagarde M. & El Bawab S. Bimodal effect of advanced glycation end products on mesangial cell proliferation is mediated by neutral ceramidase regulation and endogenous sphingolipids. J. Biol. Chem. 279, 34343–34352 (2004). [DOI] [PubMed] [Google Scholar]
- Yamabe S. et al. Intracellular accumulation of advanced glycation end products induces apoptosis via endoplasmic reticulum stress in chondrocytes. FEBS J . 280, 1617–1629 (2013). [DOI] [PubMed] [Google Scholar]
- Chen C. et al. Polydatin attenuates AGEs-induced upregulation of fibronectin and ICAM-1 in rat glomerular mesangial cells and db/db diabetic mice kidneys by inhibiting the activation of the SphK1-S1P signaling pathway. Mol. Cell. Endocrinol. 427, 45–56 (2016). [DOI] [PubMed] [Google Scholar]
- Zeeshan H. M., Lee G. H., Kim H. R. & Chae H. J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci . 17, 327 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Gou W. L. & Zhang R. FAM3A attenuates ER stress-induced mitochondrial dysfunction and apoptosis via CHOP-Wnt pathway. Neurochem. Int. 94, 82–89 (2016). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B., Vaziri N. D., Herrera-Acosta J. & Johnson R. J. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am. J. Physiol. Renal Physiol . 286, F606–F616 (2004). [DOI] [PubMed] [Google Scholar]
- Hou X. et al. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc. Diabetol. 13, 78 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. H., Shih Y. L., Ko W. C., Wei Y. H. & Shih C. M. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell. Mol. Life Sci. 65, 3640–3652 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. & Rubinsztein D. C. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ . 14, 1247–1250 (2007). [DOI] [PubMed] [Google Scholar]
- Lengm S. et al. Ursolic acid promotes cancer cell death by inducing Atg5-dependent autophagy. Int. J. Cancer 133, 2781–2790 (2013). [DOI] [PubMed] [Google Scholar]
- Yamahara K. et al. The role of autophagy in the pathogenesis of diabetic nephropathy. J. Diabetes Res. 2013, 193757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome A. et al. Autophagy impairment aggravates the inhibitory effects of high glucose on osteoblast viability and function. Biochem. J. 455, 329–337 (2013). [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia A. et al. Impairment of Atg5-dependent autophagic flux promotes paraquat- and MPP+-induced apoptosis but not rotenone or 6-hydroxydopamine toxicity. Toxicol. Sci. 136, 166–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]