Abstract
Spinal cord epidural stimulation has resulted in the initiation of voluntary leg movements and improvement in postural, bladder, and sexual function. However, one of the limitations in reaching the full potential of epidural stimulation for therapeutic purposes in humans has been the identification of optimal stimulation configurations that can neuromodulate the spinal cord for stepping. In the present work, we investigated the mechanisms underlying the specificity of interaction between the rostral and caudal spinal cord circuitries in enabling locomotion in spinal rats (n = 10) by epidural spinal cord stimulation. By using unique spatiotemporal epidural stimulation parameters of the lumbar and sacral spinal cords, a robust stepping pattern in spinal rats was observed with only six training sessions and as early as 3 weeks post-injury. Electrophysiological evidence reveals that in addition to frequency of stimulation pulses at the stimulation sites, the relative timing between stimulation pulses applied at the lumbar (L2) and sacral (S1) segments of the spinal cord heavily impacted stepping performance. Best stepping was established at a higher stimulation frequency (40 Hz vs. 5, 10, 15, and 20Hz) and at specific relative time-intervals between the stimulation pulses (L2 pulse applied at 18–25 msec after the onset of the S1 pulse; S1 pulse applied 0–7 msec after the L2 pulse). Our data suggest that controlling pulse-to-pulse timing at multiple stimulation sources provides a novel strategy to optimize spinal stepping by fine-tuning the physiological state of the locomotor networks. These findings hold direct relevance to the clinician who will incorporate electrical stimulation strategies for optimizing control of locomotion after complete paralysis.
Key words: : electromyography, epidural stimulation, locomotion, locomotor networks, neuromodulation, rat, spinal cord injury
Introduction
Treatment using spinal cord epidural stimulation (i.e., electrically-enabled motor control [eEmc]), in combination with locomotor training, has resulted in the initiation of voluntary leg movements,1–3 and gains in postural control and bladder and sexual function in individuals with complete sensory and motor paralysis.2 One of the challenges in reaching the full potential of eEmc for therapeutic purposes in humans has been the identification of optimal stimulation configurations and parameters that can neuromodulate the spinal cord for standing and stepping. It seems that eEmc essentially can drive the sub-threshold locomotor network excitability of the lumbosacral segments to a state that can enable intentional motor, as well as more automatic movements, like stepping in humans1,2 and rats4,5 with complete paralysis. While increasing the overall excitation of the neuronal circuitry to facilitate motor output is critical,6 it appears feasible to fine-tune combinations of stimulation parameters that will result in more effective sensorimotor responses than have been observed thus far.2,7
Although the entire extent of the lumbar cord is suggested to possess rhythmogenic capacity, it is generally accepted that the rostral region has a greater potential in generating bursting rhythm pattern than the caudal region, whether in neonatal rats,8,9 adult rats,10 cats,11 mice,12,13 or humans.14 Additionally, the sacral neuronal networks exhibit unique features: these networks retain their distinct rhythmogenic capacity for motor output, such as patterned tail movements,15 and also transmit excitatory signals to the lumbar cord via long and short propriospinal interneurons.16 Importantly, afferent stimulation of the sacral region of the spinal cord in neonatal rats can dramatically enhance excitation of the neuronal circuitries in the lumbar cord via activation of this interneuronal linkage.17 It also is well known that afferent input from hip and ankle mechanoreceptors can serve as the main driver of neuronal activation in awake adult rats,18 as well as in humans.19 Collectively, these observations emphasize the potential significance of the interactions of this input between the lumbar and sacral neuronal circuitries in defining locomotor success.
The objective of the present work was to determine the interactive effects of stimulation frequency and pulse intervals delivered at different spinal cord sites in facilitating locomotion in spinal rats. We employed a spatiotemporally-independent monopolar (SIM) epidural stimulation at the lumbar (L2) and sacral (S1) regions of the spinal cord that electrically enables motor control (SIM-eEmc) to investigate if this strategy would influence the excitability of the lumbar spinal networks. We hypothesized that SIM-eEmc would synergistically influence the excitability of the lumbar spinal networks to evoke a much stronger stepping response in spinal rats than when stimulating networks in either the lumbar or sacral segments alone. Additionally, we hypothesized that the stimulation frequencies at the sacral cord and the relative timing of the stimulation pulses between the lumbar and sacral cords would play an important role in the functional output of the locomotor networks. Our data demonstrate that SIM-eEmc allows robust stepping patterns within only six training sessions in spinal rats. Additionally, stepping behavior is impacted greatly by the frequency of stimulation pulses and the relative timing at which the L2 and S1 pulses are delivered.
Methods
All experimental procedures were approved by the University of California Los Angeles Chancellor's Animal Research Committee and complied with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.20 The timeline for the experimental procedures is shown in Figure 1A. All surgical, stimulation, and behavioral procedures are used routinely in our laboratory.21,22
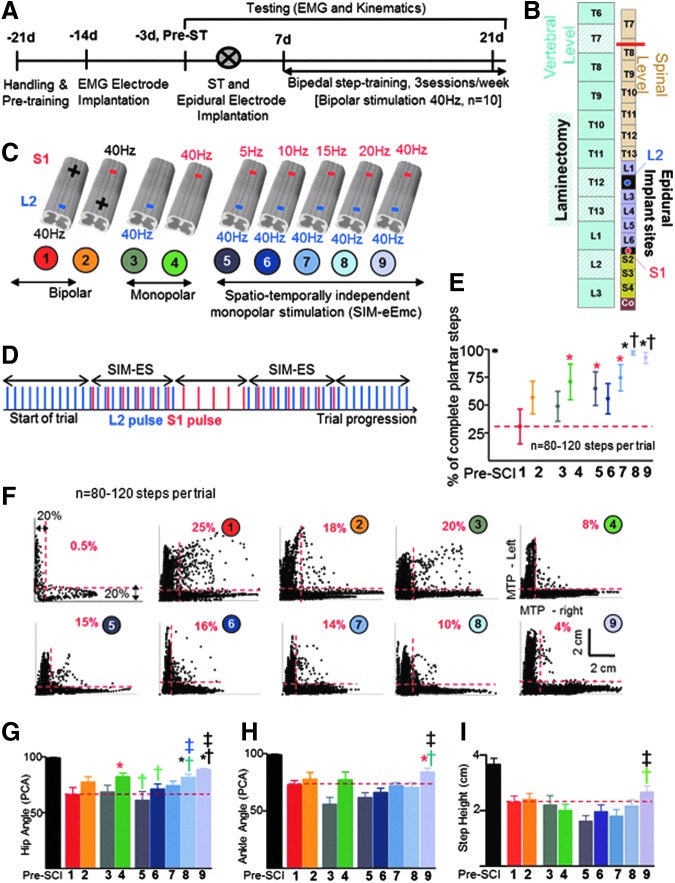
FIG. 1.
Timeline, experimental procedures, and kinematic results. (A, B) Rats (n = 10) underwent electromyographic (EMG) recording electrode implantation, implantation of spinal cord epidural stimulating electrodes (L2 and S1), and a complete spinal cord transection (ST at ∼T8). Rats were trained to step bipedally using a bipolar epidural stimulation regimen (40 Hz). (C) Cartoon of the spinal cord depicts nine combinations (trials) of epidural stimulation parameters administered randomly (1-2, bipolar stimulation; 3-4, monopolar stimulation; 5-9, spatiotemporally-independent monopolar stimulation to electrically-enabled motor control (SIM-eEmc) with L2 stimulation at 40 Hz and S1 stimulation at five different frequencies) during EMG and kinematics data collection. (D) A given SIM-eEmc trial progressed from initial stimulation at L2 at 40 Hz, switching “on” of S1 stimulation at frequencies depending upon the trial (SIM-eEmc), switching “off” L2 stimulation, switching “on” L2 (SIM-eEmc), and switching “off” S1 stimulation. Trials 8-9 generally resulted in a higher number of plantar toe contacts, reflective of good stepping (E). (F) The mean joint probability distributions of the y coordinates of the left and right metatarsophalangeal (MTP) joint markers during bipedal stepping before (pre-spinal cord injury) and post-ST for all rats are shown for all stimulation combinations. The dotted lines separate the data points that lie outside the 20% of data points for left and right y coordinates. Percentage indicates the percent of all data points outside the 20% margins for the L-shaped pattern. (G-I) By keeping the L2 epidural stimulation constant at 40 Hz, higher frequencies of stimulation at S1 (either S1-40 Hz alone or S1-20 Hz) generally result in greater consistency of hip (G) and ankle (H) angles and greater step heights (I), compared with the other stimulation conditions. The red dotted line in E, G, H and I indicates the mean values for bipolar L2-S1 stimulation (trial 1). Values are mean ± standard deviation for 8-12 steps/rat for all 10 rats. *, †: Significantly different from trials 1 and 2, and trials 3 and 4, respectively. *Significantly different from trial 1. †Significantly different from trial 4. †Significantly different from trial 3. ‡Significantly different from trials 5-8. ‡Significantly different from trials 5 and 6. The numbers and color code for each stimulation combination shown in (C) is maintained throughout the figures. Color image is available online at www.liebertpub.com/neu
Overall experimental design
Ten adult female Sprague-Dawley rats (200–250 g body weight) underwent electromyographic (EMG) and epidural stimulating electrode implantation procedures and complete spinal cord transection at spinal segment T8 as previously described.22 All surgeries were performed under aseptic conditions with the rats deeply anesthetized with isoflurane gas (1.0–2.5% via facemask as needed). Surgery was performed with the rats on a water-circulating heating pad maintained at 37°C to maintain body temperature. All incisions were closed in layers using 4.0 Dexon for the muscle and fascial layers and 4.0 Vicryl for the skin. After surgery, the rats were placed in an incubator until they fully recovered and antibiotics and analgesics were administered once or twice per day as needed for 3–4 days. Thereafter, the rats were housed in a room maintained at 26 ± 1°C and 40% humidity and on a 12:12 h light:dark cycle with access to food and water ad libitum. The cage floors were covered with CareFresh bedding. Pieces of fruit were given once daily. After the spinal cord transection surgery, the bladders of all rats were expressed manually three times daily for the first 2 weeks and two times thereafter throughout the study.
EMG implantation procedures
A small incision was made at the mid-line of the skull. The muscles and fascia were retracted laterally, small grooves were made in the skull with a scalpel, and the skull was dried thoroughly. Two Omnetics connectors with Teflon-coated stainless steel wires (AS632; Cooner Wire, Chatsworth, CA) were attached securely to the skull with screws and dental cement as previously described.22,23 A skin incision was made in the mid-dorsal region of the back and wires from the connector were routed subcutaneously. Four wires were coiled subcutaneously for later implantation as epidural stimulation electrodes on the spinal cord (see below). Skin and fascial incisions were made to bilaterally expose belly of the medial gastrocnemius (MG) and tibialis anterior (TA) muscles. Wires were routed subcutaneously from the back incision to each muscle site. Bipolar intramuscular EMG electrodes were formed and secured into the mid-belly of each muscle as described previously.23 The EMG wires were coiled near each implant site and in the mid-back region to provide stress relief. Stimulation through the connector implanted on the skull was used to verify the proper placement of the electrodes in each muscle. In addition, proper placement of the electrodes was verified post-mortem via dissection. Approximately 1 cm of Teflon coating was stripped at the distal end of an additional wire that served as a reference electrode. This wire was placed subcutaneously on the left side of the vertebral column at the level of the inferior scapular angle.
Spinal cord transection procedures
Spinal cord transection was performed as previously described.22 A dorsal mid-line skin incision was made from ∼T6 to T10 and the paravertebral muscles and fascia from ∼T7 to T9 were reflected laterally to expose the vertebrae. A partial laminectomy was performed via removal of the spinous processes and a portion of the lateral bodies of the T7 and T8 vertebrae, effectively exposing the spinal cord. The dura was picked up using fine forceps and micro-scissors were used to completely transect the spinal cord (including the entire extent of the dura) at ∼T8 spinal segment. Small cotton balls were used to separate the cut ends of the spinal cord and to clean the transection site. Two surgeons independently verified a complete transection by gently passing a fine glass probe through the transection site and then lifting the cut ends of the spinal cord. Gelfoam was inserted in the transection site to minimize bleeding and to separate (∼2–3 mm) the cut ends of the spinal cord.
Epidural stimulation electrode implant procedures
Epidural electrodes were implanted as described previously.22 Briefly, a partial laminectomy was performed over spinal cord segments L2 (between vertebral levels T12 and T13) and S1 (vertebral level L2; Fig. 1B). Two Teflon-coated stainless-steel wires were inserted through an opening made between the T10 and T11 vertebrae and one wire was passed epidurally to each partial laminectomy site. One additional wire that served as a reference electrode for use in monopolar stimulation at the L2 and S1 spinal segments was placed subcutaneously on the right side of the vertebral column at the level of the inferior scapular angle. A small region (∼1 mm notch) of the Teflon coating was removed from each wire to form the stimulating electrodes that were then secured to the dura at the mid-line of the spinal cord at each site with 9.0 silk sutures. The wires were coiled at the exit site from the vertebral column to provide stress relief. The two electrodes are at an approximate distance of 14 mm from each other.
Locomotor training procedures
The rats were trained in the morning for 3 days/week, 20 min/session for 2 weeks (six training sessions starting 7 days after the spinal cord transection surgery). In each training session, an upper body harness was used to position the rats over a treadmill belt and partially support their body weight during bipedal locomotion.22 Bipolar epidural electrical stimulation at L2 and S1 (40 Hz, 200 μs rectangular pulses) was delivered continuously during the training sessions as described previously,22 except that pharmacological agents were not administered in the present study. The treadmill belt speed was increased progressively from 6 to 13.5 cm/sec over the six training sessions.
Behavioral and EMG testing procedures
Kinematics and EMG data were collected from all rats (n = 10) prior to and at 25 days post-transection. Before surgery, testing was done without epidural stimulation, whereas after surgery testing was done with epidural stimulation. Epidural stimulation at L2 at 40–50 Hz initiates bilateral rhythmic hindlimb movements in spinal cord–injured rats.21,24,25 Low frequency stimulation (3–5 Hz) of the caudal lumbosacral cord facilitates postural limb reflexes in spinal rabbits and is accompanied by a predominant extensor activity.26 Therefore, we kept the frequency of stimulation at L2 constant at 40 Hz and varied the frequency of stimulation at S1 (5, 10, 15, 20, or 40 Hz) during stepping to determine any interactions between the rostral and caudal lumbosacral neuronal networks. Thus, each rat was tested with nine different epidural stimulation trials (10 min between trials) during the post-transection testing sessions (Fig. 1C): trial 1, bipolar stimulation from L2-S1; trial 2, bipolar stimulation from S1-L2; trial 3, monopolar stimulation at L2; trial 4, monopolar stimulation at S1; and trials 5 through 9, spatiotemporally-independent monopolar eEmc (SIM-eEmc) at L2 and S1 at five different frequencies of stimulation at S1. The order of the five SIM-eEmc trials was randomized for S1 stimulation frequency.
A stimulation frequency of 40 Hz with 200 μsec duration, monophasic rectangular pulses was used during monopolar and bipolar stimulation. For SIM-eEmc, the frequency at L2 was set at 40 Hz with 200 μsec duration, monophasic rectangular pulses, whereas the stimulation frequency at S1 was varied randomly (5, 10, 15, 20, or 40 Hz with 200 μsec duration, monophasic rectangular pulses). Additionally, each SIM-eEmc trial chronologically followed five stimulation sequences that were tested as follows: start L2 stimulation, start S1 stimulation at one of the five frequencies (SIM-eEmc), stop L2 stimulation, restart L2 stimulation (SIM-eEmc), and stop S1 stimulation (Fig. 1D). This order was followed to obtain two SIM-eEmc sequences from the same animal per trial. The frequency of S1 stimulation for each SIM-eEmc sequence within a SIM-eEmc trial was kept constant at one of the frequencies listed above. Each SIM-eEmc trial therefore consisted of at least two similar sequences; the tester subjectively determined the best stepping sequence from the video recordings.
The timing of one stimulation pulse relative to the other stimulation pulse was not controlled during the SIM-eEmc testing sessions. We identified the effects of the relative timing of the stimulation pulses on stepping ability during analysis of the data. The optimal stimulation intensity to enable stepping was determined by the rat's best stepping ability (determined subjectively by the tester) regardless of stimulation combination: this stimulation intensity (ranging from 1.0 to 3.5 V) then was used during the kinematics and EMG data collection for all trials. Note that we have previously shown that six weeks of training sessions are required for spinal rats to step optimally with monopolar or bipolar epidural stimulation.22,27 Additionally, spinal rats step best during the first few minutes of a training session (data not shown). Thus, any improvements in stepping performance in the SIM-eEmc trials (that were conducted after the monopolar/bipolar trials) is not attributed to facilitation from the preceding bipolar stimulation. All outcome measures from kinematics and electrophysiology data were objectively quantified after data collection using standard/customized software and were therefore least likely to be impacted by testers collecting or analyzing data. The investigators were therefore not required to stay blinded to the trials during data collection.
Kinematics data were collected using three-dimensional (3-D) video recordings as described previously.22,28 The SIMI motion capture system (SIMI Reality Motion Systems, UntersLHLeissheim, Germany) was used to obtain 3-D coordinates of limb markers attached bilaterally to bony landmarks of the hindlimbs.22 EMG data were collected from eight to 12 consistent consecutive step cycles bilaterally. The EMG signals were filtered (band passed, 30 Hz - 1 KHz) and amplified (×100) using an analog amplifier (differential AC amplifier; AM-systems Inc.). The signal then was digitized at a 10 KHz sampling rate and stored on a computer using a data acquisition card (NI-DAQ; National Instruments Inc.) using a custom-written program.
Data analysis
The body was modeled as an interconnected chain of rigid segments and joint trajectories were generated accordingly.22 Eight to 12 step cycles taken from when the rats were stepping consistently were analyzed for each stimulation parameter. Joint probability distribution (JPD) plots, a measure of coordination,28 were obtained from the vertical (y) positions of the left and right metatarsophalangeal markers. Percentage of steps that fell outside the 20% margin (of vertical positions for each limb) for an L-shaped pattern were quantified by normalizing the number of data points that fell outside this margin to the total number of data points.28 A range of kinematic gait parameters, including step height, percentage of plantar steps, and paw drag, were computed for each gait cycle.28 Spatial consistencies of the hindlimb trajectories from the x-y coordinates of the ankle and hip markers were measured as the amount of variance explained by the first principal component.
Additionally, the rats were objectively ranked for stepping ability using the following criteria: 1) ability to step rhythmically (unilaterally vs. bilaterally); 2) presence of plantar steps (vs. drags and toe curls); 3) ability to take consecutive steps (12 vs. <12 steps); and 4) x-y trajectory of limb motion (consistent vs. irregular) with adequate step height (low vs. high step height), compared with non-injured controls. Rats with ranks 1–3 were grouped as best steppers and those with ranks 4–6 were grouped as poor steppers. Grouping ranks across these categories allowed us to run a non-parametric test to determine the relationship between stepping ability and the time delay between stimulation pulses (see statistics below). Activation patterns (EMG amplitude with respect to time) for the TA and MG muscles were obtained by taking an average of 8–12 filtered, rectified, and normalized (to the step cycle) EMG bursts from each muscle. Mean peak EMG amplitudes and the time-to-peak amplitude were determined from the rectified linear EMG envelopes for the TA and MG.28 In addition, the rate at which the MG muscle rose to peak amplitude in a step cycle was calculated. The necessary and sufficient number of animals was determined using standard power calculations based on the mean ± standard deviation (SD) from our previous works.22,29,30 Significant differences in measurement outcomes were detected using a two-tailed significance level of p = 0.05, sample size of 8–12 steps in each animal to maintain a power of 0.90.
The electrophysiological characteristics of the MG and TA responses evoked by spatiotemporally-independent S1 and L2 monopolar epidural stimulation pulses (SIM-eEmc) were determined. Firstly, the evoked responses within a single EMG burst for both monopolar and SIM-eEmc combinations (S1 at 5, 10, 20, and 40 Hz) were analyzed. (Note that the 15Hz data set (S1 stimulation 15Hz, L2 40Hz) was not analyzed because the time-interval between L2 and S1 stimulation pulses in a L2-40Hz, S1-15Hz stimulation sequence is random within each muscle burst and can make averaging inaccurate). The start of each 25 msec evoked response (magenta or blue traces in Fig. 4C) within a burst was synchronized with the initiation of the pulse that evoked the response (blue or red dot for L2 and S1 pulses, respectively, in Fig. 4C). For all SIM-eEmc trials, the start time of each L2 pulse (blue dots, Fig. 4C, trials 5, 6, 8, and 9) was taken with reference to the S1 pulse at time “0” (red dot). The magenta trace within the SIM-eEmc bursts represents an “interaction-evoked response” (i.e., an evoked response influenced by both the S1 and L2 pulse). A blue trace represents an “isolated L2 response” (i.e., an L2 evoked response independent of the S1 pulse; Fig. 4C, 4D). Therefore, with an increasing stimulation frequency at S1 from trial 5 to trial 9, the number of isolated L2 responses (blue traces) decreases within each SIM-eEmc combination such that there are no isolated L2 responses for trial 9. Note that interaction responses were associated with either the L2 pulse following the S1 pulse (S1+L2, Fig. 4B), or the S1 pulse following the L2 pulse if the L2 pulse was delayed more than 18 msec after the S1 pulse (see below).
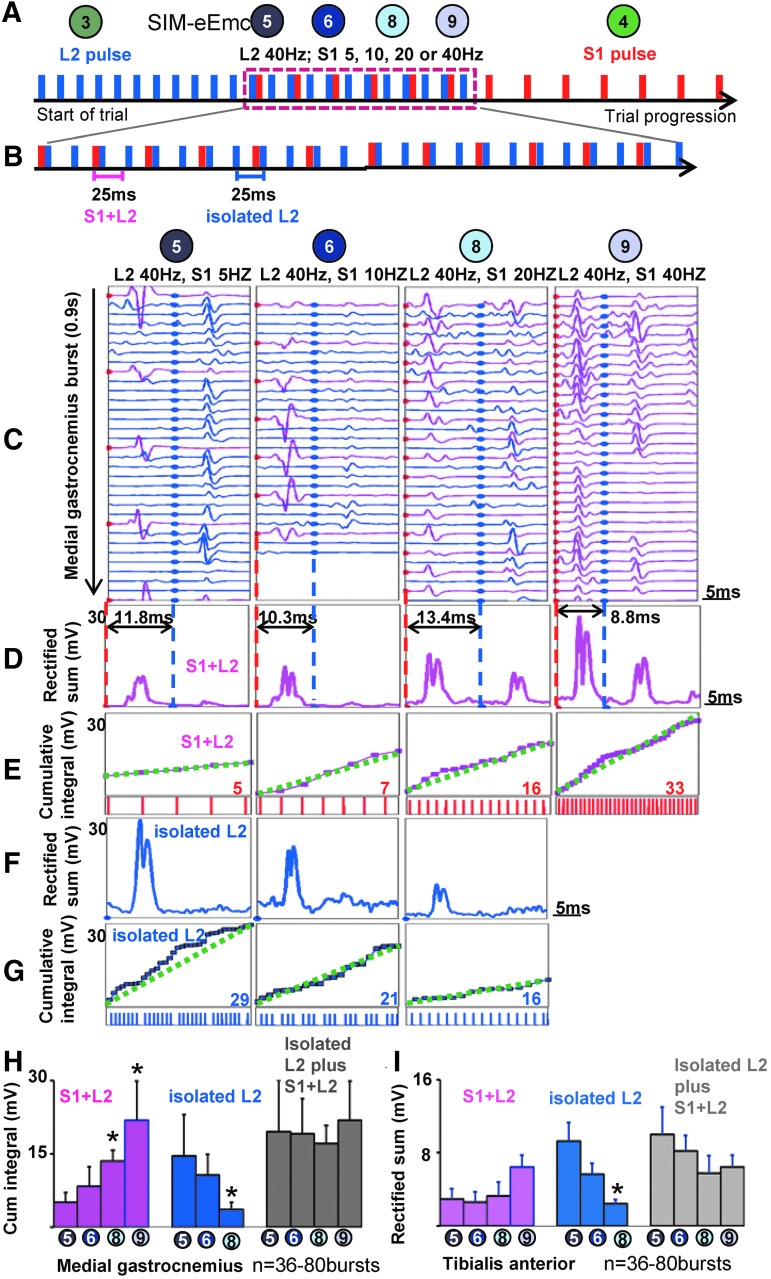
FIG. 4.
The locomotor networks for spinal stepping are stimulation frequency dependent. (A) Schematic of progression of a spatiotemporally-independent monopolar stimulation to electrically-enabled motor control (SIM-eEmc) trial. (B) The inter-stimulation pulse interval between L2 and S1 is such that the L2 pulse follows S1 (S1+L2) or precedes S1 (L2+S1; data not shown). (C) The modulation of evoked potentials generated for each stimulation pulse in an medial gastrocnemius (MG) electromyographic (EMG) burst in a S1+L2 trial at four different frequencies. For all SIM-eEmc trials, the S1 pulse is at time “0” and an intervening blue dot indicates the start time of each L2 pulse. (D) The sum of the rectified signal from all S1+L2 pulses within a single MG EMG burst increases with an increase in S1 stimulation frequency. (E) Given the greater number of S1 stimulation pulses, there was an increase in the pulse-by-pulse cumulative integrals of the interaction-evoked responses with increase in S1 stimulation frequency. Concurrently, the sum of the rectified EMG (F) and cumulative integral (G) of the isolated L2 pulse decreased with an increase in S1 stimulation frequency. The numbers in (E) and (G) in red and blue indicate the total number of S1 stimulation pulses and L2 isolated pulses, respectively, that are summated in each burst for each trial. The dotted green line represents a theoretical linear slope if the cumulative energies from consecutive pulse were linear; the magenta lines indicate the actual slope. Mean (± SD) cumulative integrals in the MG (H) and tibialis anterior (TA; I) from the “S1+L2” and “isolated” L2 evoked responses and the total cumulative integral for each burst. *Significantly different from trial 5. Color image is available online at www.liebertpub.com/neu
Secondly, two epidural stimulation parameters were studied (i.e., the effects of constant L2 and variable S1 stimulation frequency) and the relative timing between the L2 and S1 stimulation pulses on the characteristics of the resultant interaction-evoked responses within an EMG burst during stepping.
Frequency of stimulation
The cumulative integral was calculated from the EMG bursts for each SIM-eEmc trial by separately summing the integral from consecutive interaction-evoked responses and consecutive isolated L2 responses (Fig. 4). Thus, the integral values were separate for an isolated L2, S1+L2, and L2+S1 evoked response. Total cumulative integral values were obtained at every 25 msec within a burst, equivalent to a combination of isolated L2 and the S1+L2 or L2+S1 responses.
Latency of stimulation pulses (window analysis for relative timing between pulses)
Upon objectively ranking the rats for their stepping ability at a given S1 frequency in a SIM-eEmc trial, we were able to identify four windows between the L2 and S1 stimulation pulses that best determined the rat's ability to step (Fig. 5A, 5B). In a 25 msec evoked response within each muscle, windows were defined with reference to the S1 pulse (red dot) at “0” and a subsequent L2 pulse (blue dot) that was at a time-interval of: i) 0–2.9 msec (window 1, W1), ii) 3–10 msec (window 2, W2), iii) 10.1–18 msec (window 3, W3), and iv) 18.1–25 msec (window 4, W4) after the S1 stimulation pulse. Consequently, W1 was defined as the time window when the L2 stimulation pulse was between 0–2.9 msec from the S1 pulse, W2 when the L2 stimulation pulse was introduced at a time 3–10 msec from the S1 pulse, W3 when the L2 stimulation pulse was between 10.1–18 msec, and W4 when the L2 stimulation pulse was introduced at 18.1–25 msec from the S1 pulse. Note that at a S1 frequency of 40Hz, in window 4 (L2 follows 18–25 msec after S1) W4 is considered equivalent to L2 pulse preceding the S1 pulse. As such, based on the absence of a prominent polysynaptic response after 18 msec (more than 80% of all traces within a burst) in all of the monopolar combinations tested, the interaction responses for W4 was recognized as an L2+S1 interaction response (i.e., a L2 pulse that precedes the S1 pulse (instead of assigning it as a S1+L2 response).
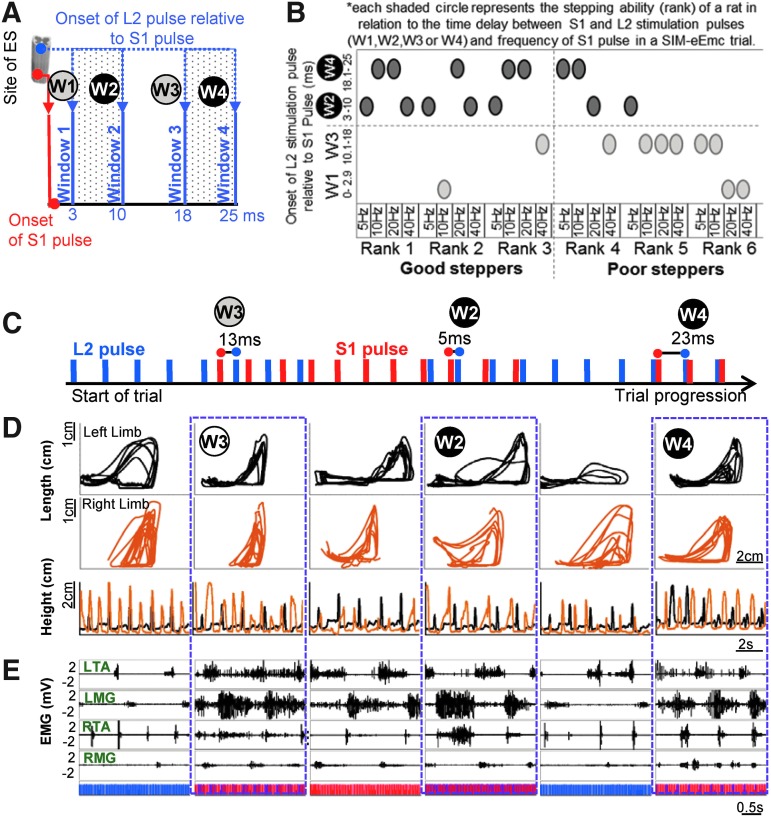
FIG. 5.
Two windows with relative time-intervals between the S1 and L2 stimulation pulses result in the best bipedal stepping. (A) Four windows with inter-stimulation time-intervals were defined with reference to the S1 pulse at “0” and a subsequent L2 pulse (blue dots) that was 1) 0–2.9 msec (window 1 [W1]), 2) 3–10 msec (window 2 [W2]), 3) 10.1–18 msec (window 3 [W3]), and 4) 18.1–25 msec (window 4 [W4]) after the S1 stimulation pulse. (B) The stepping ability (rank) of a rat in each spatiotemporally-independent monopolar stimulation to electrically-enabled motor control (SIM-eEmc) trial is represented relative to the time delay between stimulation pulses (four time windows W1, W2, W3 or W4) and S1 stimulation frequency (5, 10, 20, or 40 Hz). Each shaded circle therefore represents the stepping ability at a given frequency in a specific time window. The majority of the good steppers (rank 1-3), irrespective of stimulation frequency, fell in the time windows when L2 was at 3-10 (W2) or 18–25 msec (W4) after the onset of the S1 pulse. Rank 1 corresponds to the best stepping. (C) Progression of a SIM-eEmc sequence that consisted of multiple SIM-eEmc trials, each having the same frequency (L2 40 Hz; S1 40 Hz) but different pulse intervals (onset of L2 stimulation pulse at 13ms, 5ms, and 23 msec after the S1 pulse) in the same animal. (D) Limb kinematics and (E) corresponding electromyographic (EMG) signals indicate more a robust stepping pattern (bilateral coordinated steps and trajectory pattern) for the SIM-eEmc segments in W4 and W2 than W3. Color image is available online at www.liebertpub.com/neu
Lastly, for each monopolar evoked response and interaction evoked response, the early response was estimated in the window of 1–3 msec, the middle response in the window of 4–6 msec, and late response in the window of >7 msec, based on previously published data.31,32 Subsequently, the amplitudes for each evoked response or the interaction-evoked response were calculated from six out of 10 rats that were randomly picked using a coin toss (evoked response from four to six rats is found to be a sufficient number for analysis.29,32 The amplitudes of the interaction evoked responses were reported as the percent difference between the amplitudes of the resultant evoked response and amplitudes of evoked responses evoked by L2 alone or S1 alone pulses for that frequency. This was crucial to account for the variation on the frequency of S1 stimulation. All latencies were calculated with respect to the S1 pulse for W1 and W2, to the S1 or L2 pulse separately for W3, and to the L2 pulse for W4.
Statistical analysis
An estimate of the variation between trials was first obtained using standard deviations. Normality of distribution was assessed by the Shapiro-Wilk test. Overall significant differences between the stimulation trials were determined using repeated measures one- or two-way analysis of variance measures. Mauchly's Test of Sphericity was conducted to measure the homogeneity of variances for the repeated measures. Bonferroni post hoc tests were used to identify significant differences between stimulation trials. All data are reported as mean ± SD values. Differences between groups were considered statistically significant at p < 0.05.
To answer the question if the relationship between stepping ability and delay in latency was more than expected by chance, we ran a statistical Fisher's exact test for categorical data.33 Animals were grouped into two categorical variables: a) stepping ability [good versus poor steppers (ranks 1–3 = good steppers; ranks 4–6 = poor steppers) that formed the rows of a contingency table)] and b) delay between S1 and L2 stimulation pulses during stepping [(W2+W4 vs. W1+W3) that formed the columns of a contingency table]. A two-tailed p value was computed from the Fisher's test at α = 0.05. All statistical analyses were performed using MATLAB (Mathworks), STATISTICA (Statsoft Inc.), or GraphPad Prism 6 (GraphPad, Inc.).
Results
Compared with bipolar (L2-S1 [trial 1] and S1-L2 [trial 2]) and monopolar (L2-ref [trial 3] and S1-ref [trial 4]) stimulation, the SIM-eEmc combinations enabled superior kinematics patterns (Fig. 1 and Fig. 2; Supplementary Video 1; see online supplementary material at www.liebertpub.com/neu). In a representative rat, Figure 2 shows that during the same testing session, most optimal electromyography and stepping kinematic patterns are observed at the highest frequency of stimulation (L2 at 40 Hz, S1 at 40 Hz [trial 9]). Average data from all animals reveal that the stepping quality (characterized by plantar [Fig. 1E], continuous, alternating, coordinated, and rhythmic steps [Fig. 1F]) and hip joint angular kinematics (Fig. 1G) were closer to pre-injury measures for SIM-eEmc trials at the highest frequencies (L2 at 40 Hz, S1 at 20 Hz [trial 8] or 40 Hz [trial 9]) than with most other stimulation conditions. Compared with trials 5-7, trial 8 showed greater number of plantar steps (Fig. 1E) and hip angle consistency (Fig. 1G).
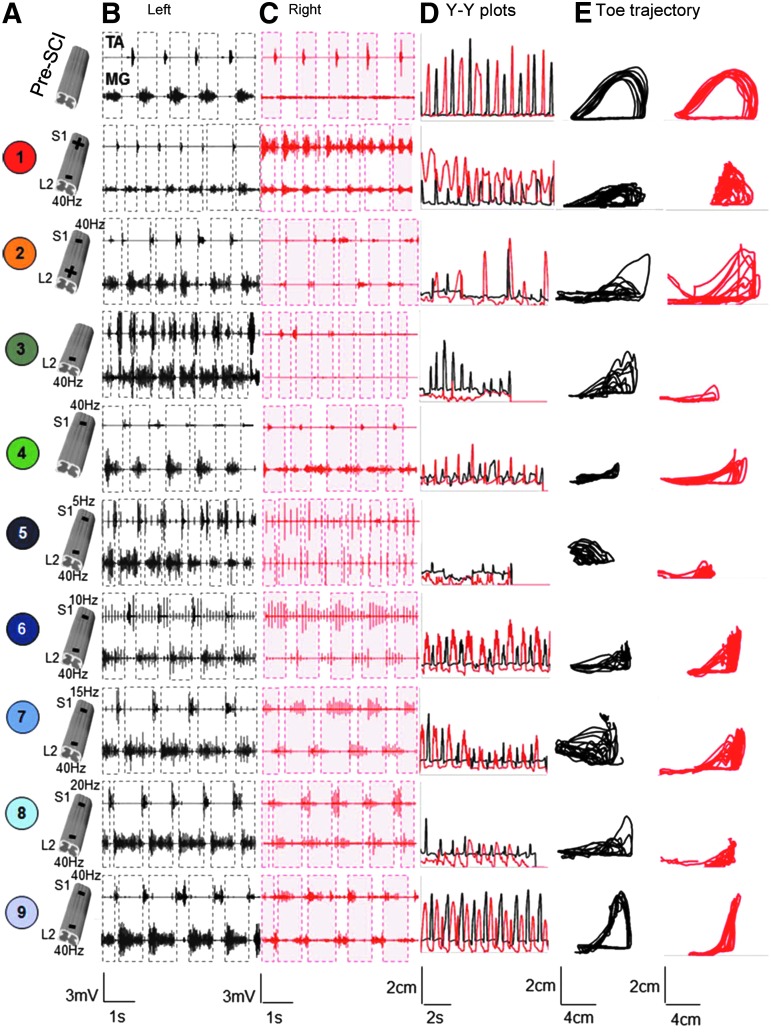
FIG. 2.
Electromyographic (EMG) and kinematics data from a representative rat during bipedal stepping using the nine combinations of epidural stimulation parameters described in Figure 1. (A) Cartoon of the spinal cord depicts pre-spinal cord injury (SCI), bipolar (trials 1 and 2), monopolar (trials 3 and 4) and spatiotemporally-independent monopolar stimulation to electrically-enabled motor control (SIM-eEmc) at five frequencies of stimulation at S1 (trials 5, 6, 7, 8, and 9). (B) and (C) Raw EMG signals from the left (B) and right (C) tibialis anterior (TA) and medial gastrocnemius (MG) muscles during a 3-sec period of bipedal stepping on a treadmill before (Pre-SCI, no stimulation) and 21 days post–spinal cord transection (ST; with all nine combinations of stimulation). (D) Y-Y plots of the toe marker for 8-12 consecutive and consistent steps on the treadmill. (E) Toe marker trajectories for the same steps as in (D). All traces/signals from the left and right hindlimbs are in black and red, respectively. Note that although all stimulation combinations enabled some stepping at 21 days post-ST, the most robust and coordinated stepping (i.e., most similar to pre-ST) was observed in trial 9 (SIM-eEmc: L2 at 40 Hz and S1 at 40 Hz). Color image is available online at www.liebertpub.com/neu
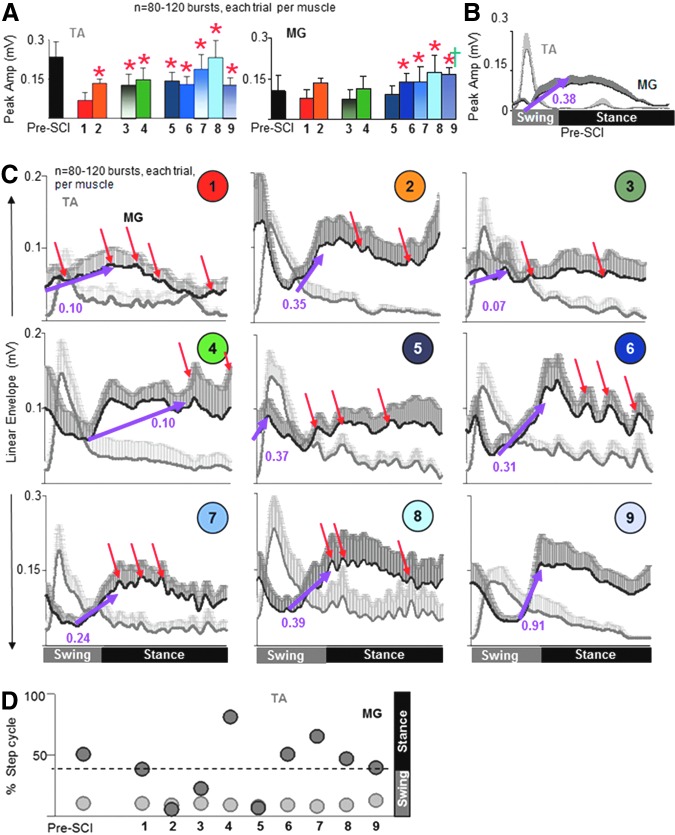
The quality of stepping was verified by the patterns of modulation of the EMG in the TA versus MG muscles. The peak TA EMG amplitude was lower in trial 1 than in all other trials (Fig. 3A) and the peak MG EMG amplitude was higher in trials 6-9 than in trial 1, consistent with the fewest number of plantar steps (Fig. 1E) and the most foot drags (data not shown) seen in trial 1. The time for the TA EMG to reach peak amplitude during a step was consistent among all trials, including pre-injury (Fig. 3C, 3D). In contrast, the time for the MG EMG to reach peak amplitude was altered dramatically for trials 2-5, compared with pre-injury values (Fig. 3C, 3D). The rate at which the MG EMG reached peak amplitude was variable across trials (Fig. 3C, purple arrows). Trial 9 showed a rapid rate to reach peak MG EMG, approximately two times faster than pre-injury and between three to nine times faster than most monopolar and bipolar combinations, most likely indicating the rapid recruitment of motoneurons with high-frequency ES at S1. MG activation throughout a step cycle for trial 9 followed a relatively smoother pattern (similar to pre-injury) than that for all other trials. Lower frequencies of stimulation at S1 (trials 5-8) were accompanied with an irregular jerky pattern (Fig. 3C, red arrows) during stepping. Note that there was no difference in the stimulation intensities of the S1 pulse during the S1 monopolar and SIM-eEmc trials (data not shown). Additionally, the body weight support provided to facilitate hindlimb stepping on the treadmill was similar between all trials (69% ± 5% in trials 1-4 versus 67% ± 3% in trials 5-9).
FIG. 3.
Spatiotemporally-independent monopolar stimulation to electrically-enabled motor control (SIM-eEmc) at higher frequencies results in electromyographic (EMG) temporal patterns similar to pre–spinal cord injury (SCI). (A) Peak tibialis anterior (TA) and medial gastrocnemius (MG) amplitudes of the rectified EMG linear envelopes before (pre-SCI) and post–spinal cord transection (ST) for all rats during 8-12 steps. Superimposed averaged (+SEM) integrated EMG linear envelopes normalized to the step cycle for the TA and MG pre-SCI (B) and post-ST (C, 1-9) for each combination of stimulation (n = 10 rats, 8-12 steps). The purple arrow connects the time of onset with the time to reach peak amplitude for the MG burst, while the numbers indicate the slope at which the MG burst reaches peak amplitude. Most SIM-eEmc configurations and SI-L2 bipolar stimulation showed similar slopes to pre-SCI, whereas trials 1, 3, and 4 showed a slower rate to reach peak amplitude. The red arrows indicate an irregular pattern in the MG burst during the stance phase (note the sharp increases and decreases in the EMG amplitudes for trials 1-7). Despite well-coordinated kinematics of movement, inadequate plantar placements during monopolar S1 stimulation can be attributed to an altered time-to-peak MG EMG (∼80% into the step cycle) and the rate at which this peak was reached (trial 4). (D) Timing of peak TA (light gray circles) and MG (dark gray circles) EMG normalized to the step cycle for all animals. The distance between the light and dark gray circles reflects the amount of co-contraction (i.e., the smaller the distance, the greater the amount of co-contraction). *, †: Significantly different from trial 1 and trial 3, respectively. Color image is available online at www.liebertpub.com/neu
The patterns of generation of evoked potentials during a step cycle for trials 5, 6, 8 and 9 are shown in Fig. 4A-H. Stimulation pulse sequence (Fig. 4B) and 25 msec traces from an EMG burst signal (Fig. 4C, 4D) are shown when S1 is the lead pulse. The increase in S1 stimulation frequency across trials 5-9 resulted in an increased cumulative integral of the evoked responses (Fig. 4C-H). In contrast there is an incremental decrease in the evoked potentials linked to L2 stimulation alone (Fig. 4F, 4G). Note that combining the cumulative integrals of L2 isolated pulses and interaction-evoked responses (S1+L2) within a burst did not alter the net cumulative integrals at the different frequencies (Fig. 4H, 4I). Our finding that the quality of stepping is relatively poorer in trials 5, 6, 8 than in trial 9 (Fig. 1E-I), whereas the net level of activation is similar across these conditions (Fig. 4H, 4I), demonstrates that the quality of stepping is dependent on the timing of interaction between stimulation pulses. Note that when L2 was the lead pulse, a similar effect on cumulative integral was observed (data not shown).
The more effective stepping (ranks 1–3 vs. 4–6 in stepping ability), irrespective of S1 stimulation frequency, occurred when the L2 stimulation pulse was 3–10 msec (W2) and 18.1–25 msec (W4) after the onset of the S1 pulse (Fig. 5A, 5B; p = 0.036). Some rats stepped better (and were ranked higher) than others at both the higher and lower frequencies at S1. Note that rank 1 corresponds to best stepping. Changing the specific delays between the stimulation pulses at the same stimulation frequency in the same rat elicited the best stepping response in W2 and W4 (Fig. 5C-E). The more consistent right and left hindlimb foot trajectories (bilateral step heights and coordination; Fig. 5D) and more consistent and clear EMG bursting patterns (Fig. 5E) occurred in W2 and W4. Note that a better stepping response is seen with a 23 msec delay (W4) than a 5 msec delay (W2) between stimulation pulses (Supplementary Video 2). Note that we used a variety of measures to fine-tune the ranking of stepping ability and found that there was a strong correlation (r = −0.8, p < 0.001) between the rank assigned to each animal and the percent of coordinated steps determined from step kinematics (data not shown).
We next investigated the electrophysiological significance of the time-interval between stimulation pulses. In a representative animal, when the L2 pulse followed the S1 pulse after 0–2.9 msec (W1), the response (S1+L2) was partially inhibited, compared with an evoked response to an S1 pulse only, particularly in the extensor muscle (MG; Fig. 6B, W1). When the L2 pulse occurred 3–10 msec after the onset of the S1 pulse (W2), a polysynaptic response was observed (Fig. 6B, W2). When the L2 pulse occurred 10.1–18 msec after the onset of the S1 pulse (W3), the interaction-evoked response was occasionally partially inhibited (Fig. 6, W3). The most unique feature of W2 and W4 is that both a monosynaptic and polysynaptic response was generated for each pair of pulses, whereas W1 andW3 had only a monosynaptic response (Fig. 6C).
FIG. 6.
Mechanisms underlying the specificity of L2-S1 inter-stimulation intervals in enabling stepping. (A) Schematic of the progression of a spatiotemporally-independent monopolar stimulation to electrically-enabled motor control (SIM-eEmc) trial. Inset demonstrates that the L2 pulse (blue dot and arrows) appears within one of the four time windows (W1-W4) relative to the S1 pulse (red dot and arrows). (B) Bilateral raw medial gastrocnemius (MG) and tibialis anterior (TA) electromyographic (EMG) bursts in a representative animal along with averaged evoked responses from individual bursts (corresponding to black and tan rectangles) secondary to L2 only, S1 only, and four SIM-eEmc trials each with four distinct time windows (W1 to W4) and related step kinematics. W1 shows a partial inhibition of the interaction evoked response (gray arrows) and W2 is accompanied by the presence of a polysynaptic response (green arrows). L2 stimulation from 10.1–18.0 msec (W3) is either partially inhibited or does not show any obvious alteration in the response. L2 preceding the S1 pulse (W4) results in a shift of the latency of the response in one muscle (purple dotted line for the MG) relative to both the L2 and S1 monopolar evoked responses and is frequently accompanied by a polysynaptic response. (C) Mean (± standard deviation [SD]) latency periods of the monosynaptic and polysynaptic evoked responses for conditions similar to that described in (B). (D) Average (± SD) percentage differences in the amplitude of interaction-evoked responses in each window in comparison to the L2 or S1 only stimulation trials in all animals for MG and TA muscles. *Significant latency shift differences of first response in W4 from both S1 only and L2 only. †Significantly different from W1 and W3. ‡Significantly different from W3. *Significantly different from L2 monopolar stimulation. *Significantly different from S1 monopolar stimulation. Color image is available online at www.liebertpub.com/neu
When the L2 pulse occurred 18.1–25 msec after the onset of the S1 pulse (W4), a scenario considered to be synonymous to the L2 pulse preceding the S1 pulse, there was a shift in the evoked response latency for the extensors (Fig. 6B, dotted vertical purple lines) or flexor (TA) muscles in a given step cycle relative to the first L2 stimulation artifact. Mean data for the latency of the evoked responses for all conditions are shown in Figure 6C. Compared with L2 stimulation alone, there was a two- to four-fold elevation in the amplitude of the interaction-evoked responses in all windows (facilitating effect of the S1 pulse) for the MG. Compared with S1 stimulation alone, there was a partial, although significant, inhibition (0.25- to 0.7-fold decrease) in the amplitude of the interaction-evoked responses in W1 and W3, respectively (Fig. 6D). Interactive effects for the TA were similar but less dramatic. These results demonstrate a consistent interactive effect of evoked potentials generated when stimulating at both the lumbar and sacral spinal cord sites, simultaneously compared to stimulating only one region.
Discussion
By utilizing unique epidural stimulation configurations and parameters of a simple two-point electrode system, we demonstrate that in the absence of any pharmacological facilitation, six training sessions can enable a robust stepping pattern in spinal rats as early as 3 weeks post-complete spinal cord injury. Our key and important finding of the present study is that the site of stimulation, frequency of stimulation pulses and the relative timing of stimulation pulses between L2 and S1 stimulation pulses are critical determinants of spinal stepping enabled by epidural stimulation. L2 stimulation applied at specific times after S1 stimulation uniquely modulates neuronal activity to evoke a range of patterns of spinal stepping (i.e., from poor to well-coordinated stepping). Additionally, L2 stimulation applied before S1 consistently generates a robust stepping response, thereby rendering itself a better stimulation strategy to enable spinal stepping. These findings are parallel to a recent report that investigated the modulatory effects of the paired spinal cord stimulation delivered to the lumbar and sacral spinal segments with different delays on spinally evoked motor potentials in leg muscles of non-injured human volunteers.34
Robust spinal stepping with six sessions of epidural stimulation alone
Robust stepping in spinal awake rats using epidural stimulation is possible when supplemented with pharmacological agents and/or extended motor training regimens that last for 4–6 weeks.4,22,35,36 Although pharmacological interventions facilitate spinal stepping in spinal rats and cats, the evidence for these interventions enhancing stepping ability in humans with a complete spinal cord injury (SCI) has been marginal to date.37 With the objective to translate an epidural stimulation technology to the human with SCI,1,38,39 we focused on engaging locomotor and postural networks in the lumbosacral cord to optimize the capacity of epidural stimulation to modulate sensorimotor pathways for functional motor recovery. We approached this by controlling the epidural configurations and stimulation parameters with which the spinal cord was stimulated. While motor training is undoubtedly an essential and necessary element for modulating the physiological state of the spinal circuitry to enable functional recovery,38–42 only six training sessions were adequate to elicit bilateral rhythmic and consistent stepping in all rats tested. We discuss below results that demonstrate the crucial role of the site of stimulation, frequency of stimulation and relative timing between stimulation pulses in shaping stepping behavior.
Specificity of the site of epidural stimulation
Based on electrophysiological in vivo experiments25,43 and computational models,44,45 epidural stimulation mainly involves activation of the low threshold afferent fibers that enter the spinal dorsal horn. The projecting afferent input excites the motoneurons through monosynaptic and/or polysynaptic pathways. Interestingly, comparisons between eEmc at S1 and L2 alone and eEmc at S1 and L2 together (SIM-eEmc) indicates that the overall quality and coordination of stepping and the timing and rate at which the flexor-extensor muscles reach peak activity was much more effective with the stimulations at L2 and S1 together. This unique interactive and synergistic effect suggests multi-segmental convergence of descending and ascending influences on the neuronal circuitries during epidural stimulation applied at L2 and S1 spinal segments combined than with stimulation at either segments alone. The question, however, remains as to how does the modulation of the networks with eEmc at L2 differ qualitatively and quantitatively from that by eEmc at S1, and how do these two sources of neuromodulation interact?
Physiologically, it is likely that the combined eEmc at L2 and S1 involves an optimal level of rhythm generation and pattern formation from the rostral lumbar segments46 along with their enhanced amplification from activation of interneuronal populations in the sacral cord.17 Note that robust activation of a variety of ascending propriospinal neurons from the sacral cord that terminate in the lumbar cord is well demonstrated during afferent stimulation of the sacral cord.17 Consequently, we propose that the projecting afferents at S1 not only act locally at the sacral spinal segment level (monosynaptically or polysynaptically), but afferent stimulation at S1 synaptically conveys information to the rostral lumbar circuits via the sacral ascending propriospinal interneurons (that receive input directly or indirectly from the sacral afferents). As discussed in the following sections, the frequency of stimulation and the relative timing of the stimulation pulses applied at L2 and S1 play a critical role in facilitating this interactive response.
The neuronal circuitry for mammalian locomotion is frequency dependent
Epidural stimulation at L2 at 40–50 Hz initiates bilateral rhythmic hindlimb movements both in the rat and human.14,21,24,25 Stimulation of the caudal lumbosacral cord facilitates postural limb reflexes in spinal rabbits and predominantly accompanies an extensor activity.26 Therefore, we kept the frequency of stimulation at L2 constant at 40 Hz and varied the frequency of stimulation at S1 (five different frequencies) during stepping to study the interaction between rostral and caudal lumbosacral neuronal networks.
The less robust stepping observed with lower stimulation frequencies at S1 (5, 10, 15 Hz) accompanied prominent muscle twitches in both the flexor and extensor muscles throughout the step cycle and interfered with the reciprocal rhythmic stepping pattern. The non-specific facilitation of all hindlimb muscles by the S1 pulse during both the swing and stance phases of the steps is attributable to direct activation of the myelinated sacral motor axons running into the ventral roots via currents that flow dorsoventrally within the cerebrospinal fluid. Each stimulation pulse at S1 at lower frequencies generated an evoked response in the hindlimb muscle during stepping with a latency of about 1.5–3.0 msec (data not shown), similar to that observed during sacral cord epidural stimulation (5 Hz) in humans with an incomplete SCI,47 reflective of direct activation of motor axons, as demonstrated previously.31,32,44 Note that such direct activation of motor axons is not observed with L2 stimulation.31
The most robust stepping was observed with 40 Hz stimulation applied at S1, consistent with previous observations in spinal cord–injured rats and humans.43,48 Stimulation frequencies greater than 50 Hz in spinal rats interferes with their stepping ability, perhaps due to interference with responses evoked by consecutive stimuli at high frequencies.43 This is consistent with stimulation at 40 Hz at L2 and 20–40 Hz at S1 enabling robust stepping by interactions of excitatory and inhibitory lumbosacral interneuronal networks as opposed to a mere summation of independent potentials from single sites (S1 or L2) of the same network.
The timing of the stimulation pulses between L2 and S1 impacts stepping performance The more optimal stepping was observed when the L2 pulse occurred either at 3–10 msec after the S1 pulse or 0–7 msec prior to the S1 pulse.
When L2 pulse follows the S1 pulse
Early after an SCI, spinally-evoked responses are composed of an early direct response (latency of 1–3 msec) and a middle monosynaptic response (latency of 5–7 msec).32 Therefore, eEmc applied at L2 within 3–10 msec after the onset of the S1 pulse will occur before, during, or just after the middle monosynaptic component evoked at S1. Our data indicate that when the L2 pulse follows the S1 pulse within 3–10 msec, there is no facilitation or inhibition in the amplitude of the resultant interaction response (with respect to the S1 pulse) during stepping. Instead, the resultant interaction response is comprised of a middle evoked response, as well as additional late polysynaptic responses not closely synchronized to a stimulation pulse. We suppose that eEmc at S1 excites lumbar motoneurons via afferent fibers projecting to multiple types and numbers of interneurons (that form a continuously changing network) resulting in a range of randomly-occurring synaptic potentials onto the rostral locomotor neuronal circuitry.
Conceptually, we suggest that responses generated from L2 modulate the responses evoked by S1 stimulation and trigger multiple polysynaptic responses of varying delays. In a continuously changing synaptic environment, we propose that modulation by eEmc at L2 effectively generates feed-forward (preparatory) modulatory and decisive events based on the changing shape of massive sensory ensembles of continuous proprioceptive and cutaneous input during load-bearing stepping. This interpretation is consistent with the rostral lumbar networks playing a primary role in modulating motor output through their action on “controller” interneurons that, in turn, select the combination of interneurons that define the level of recruitment within a given motoneuronal pool and among multiple motor pools to generate mammalian locomotion.46,49,50
The occurrence of the L2 pulse between 0–2.9 msec after the S1 pulse results in partial or complete inhibition of the evoked response (Fig. 6B, W1). Behaviorally, eEmc at L2 in this time window results in severe interference with stepping consistency. We suggest that when the L2 pulse is initiated at 0 msec or close to 0 msec after the S1 pulse, this inhibition is most likely due to collisions of action potentials individually generated by eEmc at S1 and L2. Specifically, the action potentials generated by the afferent fibers of the S1 pulse may collide with, and partly abolish, the action potentials when stimulating the L2 dorsal root. Similar observations for the interplay of short time-intervals between stimulation pulses to evoke an inhibitory response on muscle output have been made during paired stimulation pulses in the cervical51 and lumbosacral spinal cords.31 Alternatively, when the L2 pulse is at a 0–2.9 msec pulse delay after the S1 pulse, the poor stepping could be simply because of the effective balance of excitatory and inhibitory interneurons being recruited. Such strong inhibition of spinally evoked responses was recently demonstrated in the leg muscles in healthy human volunteers when S1 spinal segment transcutaneous stimulation preceded L2 spinal segment stimulation by 5–50 msec.34 Experiments in our laboratory are currently under way to address mechanisms underlying these observations in the rodent.
An L2 pulse generated ∼10–18 msec after the onset of the S1 pulse (window 3) does not alter the interaction-evoked response and results in relatively poor stepping. Since a middle monosynaptic response occurs at 5–7 msec after eEmc at S1, an L2 pulse at 10–18 msec after the onset of the S1 pulse appears during or after the hyperpolarized phase of the middle response. As such, we suggest that stimulation at L2 with a delay of 10–18 msec is less likely to evoke a response. And for L2 to be effective, modulation must take place at the level of an active network indicated by the presence of evoked responses (similar to what we observe when L2 is at 3–10 msec).
When L2 pulse precedes the S1 pulse
In the absence of S1 stimulation, evoked responses from the L2 pulse alone are of significantly lower amplitudes to be effective in evoking a robust stepping pattern. When L2 stimulation pulse precedes the S1 pulse by 0–7 msec (W4), we detected the most rhythmic, timed and consistent stepping patterns. Electrophysiologically, the data reveal a consistent facilitatory effect on the resultant evoked response in both the flexors, as well as extensor muscles (in comparison to evoked response from L2 pulse only) along with a considerable delay in the latency of evoked responses. These findings are consistent to data reported in a paired stimulation paradigm in the human34 that shows that stimulation at the L2 spinal segment that precedes S1 pulse by 5–50 msec resulted in robust facilitation of the evoked muscle response from all muscles tested. We propose that the significant alterations in physiology and behavior must involve complex interactions of excitatory and inhibitory locomotor networks. Specifically, we hypothesize that the rostral lumbar cord is capable of evoking its individual stepping response and is crucial in initiating a stepping rhythm/pattern,46 but is dependent on the sacral cord for its activation.
Unlike the interference of stepping when L2 immediately (0–2.9 msec) follows S1, S1 stimulation does not interfere with stepping when L2 immediately (0–2.9 msec) precedes S1. This suggests that the S1 pulse essentially has a facilitatory influence on the rostral cord and does not alter the rhythm or pattern that is generated by a preceding stimulation at L2. S1 most likely excites the sacral propriospinal relay projections that ascend to the lumbar cord to enhance stepping performance. Our proposition is supported by experiments in the neonatal rat52,53 that demonstrate that although the rostral lumbar cord independently produces a rhythmic alternating left-right bursting pattern, this activation is maximized when the sacral cord also is stimulated. Based on lesion and fluorescent labeling experiments, Etlin and colleauges16,17 have shown that rostrally projecting short and long glutamatergic sacral propriospinal interneurons that relay into lumbar segments can be activated via afferent activation. These interneuronal networks in the sacral cord are excitatory and can enhance activity of the lumbar central pattern generators (CPGs).
The relatively longer processing times of evoked responses during the combined stimulation could reflect a) the time delay for excitatory sacral networks to potentiate evoked response by the rostral lumbar networks and/or b) involvement of a greater number of synaptic components of CPG circuits.
Though a physiological time-delay for the S1 pulse to activate rostral lumbar circuits might seem like a plausible explanation for the shift in latency, excitation by sacral networks alone is not sufficient to explain the robust stepping pattern because mere potentiation of evoked response does not necessarily lead to better stepping (see W1 and W3 in Fig. 6D). Since the pattern and timing of the mammalian locomotor activity is substantially dependent on the excitability of spinal inhibitory interneurons,54,55 we suggest that combined stimulation at specific relative timing engages a wider pool of active inhibitory and excitatory interneurons involved in generating rhythmicity.56 The delay in the evoked responses could occur by specifically engaging rhythmically active spinal inhibitory interneurons (GABAgeric) that receive afferent input during treadmill stepping (complemented by sacral excitation). Therefore, the combined interaction between L2 and S1 pulses is complex, such that sacral ascending propriospinal excitatory neuronal pathways17 increase the general excitability of excitatory networks in the lumbar cord; and discrete inhibitory interneuronal cell populations in the rostral cord54,55 produce a delay in the timing of muscle activation in a phase dependent manner.
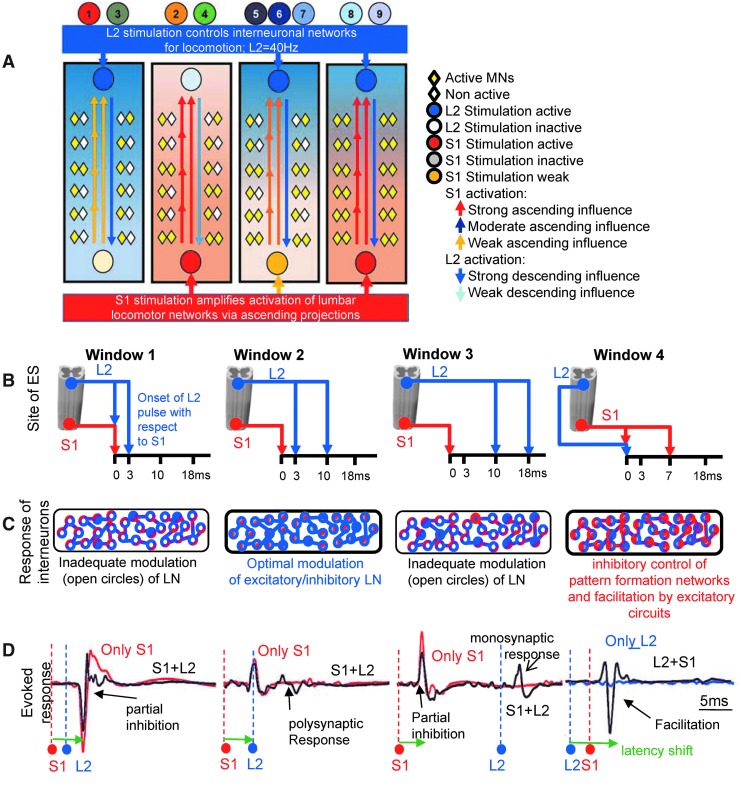
Collectively, we demonstrate here that none of the L2 and S1 stimulation pulses are as effective independently as they are when combined. When combined, eEmc at L2 and S1 involves an optimal level of rhythm generation and pattern formation from the rostral lumbar segments.46 These evoked responses show enhanced amplification from activation of interneuronal populations in the sacral cord17 (Fig. 7A, conceptual hypothesis). The relative timing of the stimulation pulses predict stepping quality such that eEmc at L2 modulates neuronal activity by engaging wider pools of excitatory and inhibitory interneuronal circuits; eEmc at S1 subsequently amplifies the excitability state of the spinal networks (Fig. 7B-D, conceptual hypothesis).
FIG. 7.
Conceptual hypothesis illustrating descending and ascending influences of sacral and lumbar spinal activation, respectively, during stepping enabled by electrically-enabled motor control (eEmc). (A) eEmc at L2 excites the locomotor networks (LNs) for motor output. Optimal facilitation of the interneuronal and motoneuronal pools depends upon the excitatory input from sacral spinal cord stimulation. Short interconnecting and long arrows indicate short and long ascending propriospinal interneurons, respectively. For L2 monopolar and L2-S1 trials, the ascending facilitation by S1 is minimal because the sacral cord is not stimulated. With S1 monopolar and S1-L2 trials, there is ascending excitation of the LNs from the sacral cord stimulation. Because of the lack of an optimal level of pattern generation directly by L2 stimulation, however, the motoneuronal pool excitation is not optimized for robust stepping. For trials 5-7, the rostral cord is activated for rhythm generation, but the S1 activation frequency is not adequate enough for effective stepping. For trials 8-9, the LNs that respond to L2 stimulation also are facilitated by S1 stimulation and the interaction between these networks is maximized for optimal stepping. (B) Spinal cord cartoon demonstrates that the relative timing of the stimulation pulses at L2 and S1 produce unique interactions in the LNs shown in (C). For Windows 1 and 3, the modulation of the LNs is not adequate for robust stepping. In contrast, the relative timing of the stimulation pulses in Windows 2 and 4 allows for appropriate modulation and facilitation. (D) Interaction-evoked responses (black) are superimposed over an evoked response from a single stimulation pulse (red or blue) to depict the interactions described in (C). Color image is available online at www.liebertpub.com/neu
Conclusion
We demonstrate here that the frequency and relative timing of the stimulation pulses induced at L2 and S1 is critical in shaping the physiological state of the locomotor networks. The complex interplay of rostral and caudal neuronal networks efficiently activates the locomotor circuitry as early as 3 weeks after a spinal transection in adult rats. A detailed understanding of the sensitivity of the frequency and timing of stimulation pulses can play an important role in electrical modulatory strategies for optimizing control of locomotion after complete paralysis. Clinicians can incorporate these stimulation strategies to optimize spinal epidural stimulation based therapies after central nervous system damage.
Supplementary Material
Acknowledgments
This work was supported by the Christopher and Dana Reeve Foundation, and the National Institute of Biomedical Imaging and Bioengineering R01EB007615. Y.G. was supported by a grant from the Russian Scientific Fund project No 14-45-00024. We thank Dr. Zaghloul Ahmed (City College of New York, Staten Island) for his comments on the manuscript draft and valuable feedback on interpretation of our electrophysiology data.
Author Disclosure Statement
V. Reggie Edgerton, Roland R. Roy, and Yury Gerasimenko hold shareholder interest in NeuroRecovery Technologies. Drs. Edgerton, Roy, and Gerasimenko also hold certain inventorship rights on intellectual property licensed by the Regents of the University of California to NeuroRecovery Technologies and its subsidiaries. For the remaining authors, there are no competing financial interests.
References
- 1.Angeli C.A., Edgerton V.R., Gerasimenko Y.P., and Harkema S.J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.G., and Edgerton V.R. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minassian K., Jilge B., Rattay F., Pinter M.M., Binder H., Gerstenbrand F., and Dimitrijevic M.R. (2004). Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42, 401–416 [DOI] [PubMed] [Google Scholar]
- 4.van den Brand R., Heutschi J., Barraud Q., DiGiovanna J., Bartholdi K., Huerlimann M., Friedli L., Vollenweider I., Moraud E.M., Duis S., Dominici N., Micera S., Musienko P., and Courtine G. (2012). Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 [DOI] [PubMed] [Google Scholar]
- 5.Gad P.N., Roy R.R., Zhong H., Lu D.C., Gerasimenko Y.P., and Edgerton V.R. (2014). Initiation of bladder voiding with epidural stimulation in paralyzed, step trained rats. PloS One 9, e108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musienko P., Heutschi J., Friedli L., van den Brand R., and Courtine G. (2012). Multi-system neurorehabilitative strategies to restore motor functions following severe spinal cord injury. Experimental Neurol. 235, 100–109 [DOI] [PubMed] [Google Scholar]
- 7.Tator C.H., Minassian K., and Mushahwar V.K. (2012). Spinal cord stimulation: therapeutic benefits and movement generation after spinal cord injury. Handb. Clin. Neurol. 109, 283–296 [DOI] [PubMed] [Google Scholar]
- 8.Cazalets J.R. and Bertrand S. (2000). Coupling between lumbar and sacral motor networks in the neonatal rat spinal cord. Eur. J. Neurosci. 12, 2993–3002 [DOI] [PubMed] [Google Scholar]
- 9.Cowley K.C. and Schmidt B.J. (1997). Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J. Neurophysiol. 77, 247–259 [DOI] [PubMed] [Google Scholar]
- 10.Magnuson D.S., Lovett R., Coffee C., Gray R., Han Y., Zhang Y.P., and Burke D.A. (2005). Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J. Neurotrauma 22, 529–543 [DOI] [PubMed] [Google Scholar]
- 11.Marcoux J. and Rossignol S. (2000). Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. J. Neurosci. 20, 8577–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie K.J. and Whelan P.J. (2005). Monoaminergic establishment of rostrocaudal gradients of rhythmicity in the neonatal mouse spinal cord. J. Neurophysiol. 94, 1554–1564 [DOI] [PubMed] [Google Scholar]
- 13.Hagglund M., Dougherty K.J., Borgius L., Itohara S., Iwasato T., and Kiehn O. (2013). Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc. National Acad. Sci. U. S. A. 110, 11589–11594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimitrijevic M.R., Gerasimenko Y., and Pinter M.M. (1998). Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 860, 360–376 [DOI] [PubMed] [Google Scholar]
- 15.Lev-Tov A. and Delvolve I. (2000). Pattern generation in non-limb moving segments of the mammalian spinal cord. Brain Res. Bull. 53, 671–675 [DOI] [PubMed] [Google Scholar]
- 16.Etlin A., Blivis D., Ben-Zwi M., and Lev-Tov A. (2010). Long and short multifunicular projections of sacral neurons are activated by sensory input to produce locomotor activity in the absence of supraspinal control. J. Neurosci. 30, 10324–10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etlin A., Finkel E., Mor Y., O'Donovan M.J., Anglister L., and Lev-Tov A. (2013). Characterization of sacral interneurons that mediate activation of locomotor pattern generators by sacrocaudal afferent input. J. Neurosci. 33, 734–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgerton V.R. and Roy R.R. (2009). Activity-dependent plasticity of spinal locomotion: implications for sensory processing. Exercise Sport Sci. Rev. 37, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietz V., Muller R., and Colombo G. (2002). Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain 125, 2626–2634 [DOI] [PubMed] [Google Scholar]
- 20.National Research Council (2011). Guide for the Care and Use of Laboratory Animals. National Academy Press: Washington, DC [Google Scholar]
- 21.Ichiyama R.M., Gerasimenko Y.P., Zhong H., Roy R.R. and Edgerton V.R. (2005). Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 383, 339–344 [DOI] [PubMed] [Google Scholar]
- 22.Shah P.K., Gerasimenko Y., Shyu A., Lavrov I., Zhong H., Roy R.R., and Edgerton V.R. (2012). Variability in step training enhances locomotor recovery after a spinal cord injury. Eur. J. Neurosci. 36, 2054–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy R.R., Hutchison D.L., Pierotti D.J., Hodgson J.A., and Edgerton V.R. (1991). EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J. Appl. Physiol. 70, 2522–2529 [DOI] [PubMed] [Google Scholar]
- 24.Gerasimenko Y., Roy R.R., and Edgerton V.R. (2008). Epidural stimulation: comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp. Neurol. 209, 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minassian K., Persy I., Rattay F., Pinter M.M., Kern H., and Dimitrijevic M.R. (2007). Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum. Mov. Sci. 26, 275–295 [DOI] [PubMed] [Google Scholar]
- 26.Musienko P.E., Zelenin P.V., Orlovsky G.N., and Deliagina T.G. (2010). Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J. Neurophysiol. 103, 1080–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichiyama R.M., Courtine G., Gerasimenko Y.P., Yang G.J., van den Brand R., Lavrov I.A., Zhong H., Roy R.R., and Edgerton V.R. (2008). Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J. Neurosci. 28, 7370–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah P.K., Garcia-Alias G., Choe J., Gad P., Gerasimenko Y., Tillakaratne N., Zhong H., Roy R.R., and Edgerton V.R. (2013). Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain 136, 3362–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gad P., Choe J., Nandra M.S., Zhong H., Roy R.R., Tai Y.C., and Edgerton V.R. (2013). Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats. J. Neuroeng. Rehabil. 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavrov I., Courtine G., Dy C.J., van den Brand R., Fong A.J., Gerasimenko Y., Zhong H., Roy R.R, and Edgerton V.R. (2008). Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J. Neurosci. 28, 7774–7780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerasimenko Y.P., Lavrov I.A., Courtine G., Ichiyama R.M., Dy C.J., Zhong H., Roy R.R., and Edgerton V.R. (2006). Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J. Neurosci. Methods 157, 253–263 [DOI] [PubMed] [Google Scholar]
- 32.Lavrov I., Gerasimenko Y.P., Ichiyama R.M., Courtine G., Zhong H., Roy R.R., and Edgerton V.R. (2006). Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J. Neurophysiol. 96, 1699–1710 [DOI] [PubMed] [Google Scholar]
- 33.Altman D.G. (1991). Practical Statistics for Medical Research. Chapman & Hall/CRC Texts in Statistical Science: London [Google Scholar]
- 34.Sayenko D.G., Atkinson D.A., Floyd T.C., Gorodnichev R.M., Moshonkina T.R., Harkema S.J., Edgerton V.R., and Gerasimenko Y.P. (2015). Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci. Lett. 609, 229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slawinska U., Majczynski H., Dai Y., and Jordan L.M. (2012). The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J. Physiol. 590, 1721–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenger N., Moraud E.M., Raspopovic S., Bonizzato M., DiGiovanna J., Musienko P., Morari M., Micera S., and Courtine G. (2014). Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 6, 255ra133. [DOI] [PubMed] [Google Scholar]
- 37.Domingo A., Al-Yahya A.A., Asiri Y., Eng J.J., and Lam T.; Spinal Cord Injury Rehabilitation Evidence Research Team. (2012). A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J. Neurotrauma 29, 865–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carhart M.R., He J., Herman R., D'Luzansky S., and Willis W.T. (2004). Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 32–42 [DOI] [PubMed] [Google Scholar]
- 39.Herman R., He J., D'Luzansky S., Willis W., and Dilli S. (2002). Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 40, 65–68 [DOI] [PubMed] [Google Scholar]
- 40.Edgerton V.R., Courtine G., Gerasimenko Y.P., Lavrov I., Ichiyama R.M., Fong A.J., Cai L.L., Otoshi C.K., Tillakaratne N.J., Burdick J.W., and Roy R.R. (2008). Training locomotor networks. Brain Res. Rev. 57, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harkema S.J. (2008). Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res. Rev. 57, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hubli M. and Dietz V. (2013). The physiological basis of neurorehabilitation–locomotor training after spinal cord injury. J. Neuroeng. Rehabil. 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavrov I., Dy C.J., Fong A.J., Gerasimenko Y., Courtine G., Zhong H., Roy R.R., and Edgerton V.R. (2008). Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J. Neurosci. 28, 6022–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capogrosso M., Wenger N., Raspopovic S., Musienko P., Beauparlant J., Bassi Luciani L., Courtine G., and Micera S. (2013). A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J. Neurosci. 33, 19326–19340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ladenbauer J., Minassian K., Hofstoetter U.S., Dimitrijevic M.R., and Rattay F. (2010). Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 18, 637–645 [DOI] [PubMed] [Google Scholar]
- 46.McCrea D.A. and Rybak I.A. (2008). Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 57, 134–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murg M., Binder H., and Dimitrijevic M.R. (2000). Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. muscle twitches - a functional method to define the site of stimulation. Spinal Cord 38, 394–402 [DOI] [PubMed] [Google Scholar]
- 48.Jilge B., Minassian K., Rattay F., and Dimitrijevic M.R. (2004). Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol. Cybern. 91, 359–376 [DOI] [PubMed] [Google Scholar]
- 49.Orlovsky G., Orlovskii G.N., and Grillner S. (1999). Neuronal Control of Locomotion: From Mollusc to Man. Oxford University Press: Oxford [Google Scholar]
- 50.Zelenin P.V., Deliagina T.G., Orlovsky G.N., Karayannidou A., Dasgupta N.M., Sirota M.G., and Beloozerova I.N. (2011). Contribution of different limb controllers to modulation of motor cortex neurons during locomotion. J. Neurosci. 31, 4636–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharpe A.N. and Jackson A. (2014). Upper-limb muscle responses to epidural, subdural and intraspinal stimulation of the cervical spinal cord. Journal of neural engineering 11, 016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strauss I. and Lev-Tov A. (2003). Neural pathways between sacrocaudal afferents and lumbar pattern generators in neonatal rats. J. Neurophysiol. 89, 773–784 [DOI] [PubMed] [Google Scholar]
- 53.Lev-Tov A., Etlin A., and Blivis D. (2010). Sensory-induced activation of pattern generators in the absence of supraspinal control. Ann. N. Y. Acad. Sci. 1198, 54–62 [DOI] [PubMed] [Google Scholar]
- 54.Wilson J.M., Blagovechtchenski E., and Brownstone R.M. (2010). Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion. J. Neurosci. 30, 1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talpalar A.E., Endo T., Low P., Borgius L., Hagglund M., Dougherty K.J., Ryge J., Hnasko T.S., and Kiehn O. (2011). Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 71, 1071–1084 [DOI] [PubMed] [Google Scholar]
- 56.McLean D.L., Masino M.A., Koh I.Y., Lindquist W.B., and Fetcho J.R. (2008). Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat. Neurosci. 11, 1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.