Abstract
In this paper, we offer a perspective on complementarity, acknowledging that it is not possible for human perception and cognition to grasp reality with unambiguous concepts or theories. Therefore, multiple concepts and perspectives are valid when they are not exaggerated beyond reasonable limits and do not claim exclusive validity. We recommend a humble stance enabling respectful dialogue between different perspectives in medical science and practice.
Key Points
No single perspective in clinical or scientific medicine can exhaustively explain medical phenomena.
Scientific attitude is characterised by a willingness to look for objections against what we prefer as truths.
Complementarity or unifying contradictions are concepts that allow for humility and pluralism in clinical and scientific medicine.
Keywords: Medical ethics, research ethics, philosophy of science
Introduction
Medical history may appear as a battleground where, through the centuries, one-eyed Cyclopes have fought furiously to defend particular versions of oversimplified medical theories. An example from fairly recent history is the nineteenth century tension between the contagionists (disease caused by contagions), the miasmatics (disease caused by poverty), and the constitutionalists (disease caused by hereditary factors). There is no doubt that science made progress through such wars, but in retrospect we understand that the wars were partly futile because theories were only temporarily valid, and were succeeded by new and more comprehensive theories that reconciled old contradictions. The scientific revolution and the modern scientific paradigm were fundamental for medical practice, and gained dominance in healthcare and in the academy. At the same time, unease has surfaced as we understand that human suffering and healing cannot be understood and resolved by biomedical or epidemiological knowledge alone.[1]
During the past four decades, a phenomenological shift has occurred in medicine and particularly in general practice. This shift emphasises the clinical and scientific relevance of personal experience, meaning, and the functional ability of people in the context of lived life. This perspective has gained increasing influence with ethical and theoretical claims from biopsychosocial medicine,[2] patient-centred clinical methods,[3] and other approaches for the recognition of patients as people.[4] The change is not only ethically founded, but is strongly supported by epidemiological and biomolecular research, revealing that human biology and human experience and biography are interdependent.[5]
Contemporary science wars
In spite of this shift in medical theory and attempts to reconcile the tensions between a biomedical and a biographical perspective, new conflicts arise. Patients and patient organisations may play a prominent role in contemporary conflicts. We increasingly observe this as we are confronted by the third wave of morbidity, with clusters of subjective ailments in modern societies. Attempts to make strong claims about the nature and management of these health complaints easily fuel conflicts. In Norway and Denmark inflamed debates in medical journals and in the press have revealed that the positions are not easily reconciled. Patients and patient advocacy organisations emphasise their rights to acknowledged diagnostic labels and corresponding welfare status. For example, members of the Norwegian Myalgic Encephalopathy Association oppose research findings that show benefits from graded training and cognitive therapy, claiming that their personal experiences invalidate such research findings.[6]
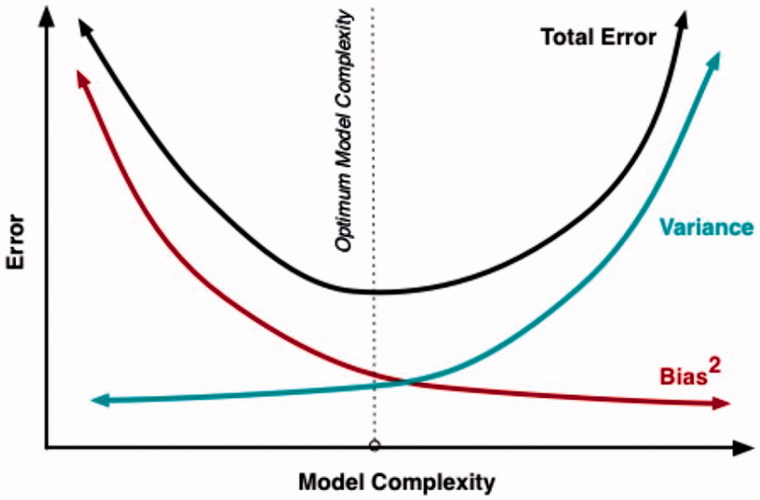
These conflicts are also fuelled by the introduction of personalised medicine or P4 medicine, which stands for predictive, personalised, preventive, and participatory. This initiative claims that it is a unifying effort answering the challenges of the digital revolution around managing Big Data. Such data will create deep insights into disease mechanisms and create metrics for assessing wellness, according to the proponents of the theory.[7] The assumption behind P4 theory is that voluminous data from sensors and personal analytic devices, and information from a multitude of other sources, will yield enough data to outweigh the effects from random variation and will safeguard reasonable protection against type I errors (erroneous confirmation) and type II errors (erroneous rejection). We acknowledge this intention as important in science. However, warnings are raised that beyond a certain limit the noise from random variation and the risk of overemphasising irrelevant associations will increase. Figure 1 illustrates this. The optimal interval is a middle ground, as the figure illustrates.[8]
Figure 1.
The relation between increasing complexity (amount of data) and the total number of errors in a scientific model. The figure illustrates a U-shaped relation resulting from diminishing bias (increased validity of the scientific model) and an increasing variation (reduced reliability of the model). A trade-off with an optimal interval is defined in the figure.[8]
A more fundamental critique against P4 medicine comes from researchers and medical theorists at the Norwegian University of Science and Technology, who represent a humanistic and phenomenological approach in medicine. They claim that subjective experience is of such fundamental consequence to human physiology that it represents a merger of human biography and biology.[5] For medicine to become person-centred, they advise us rather to understand the patient as an intentional and moral human being in a personal context. According to their view, P4 medicine does not meet the standards of humanistic medicine.[9]
A complementary approach
We acknowledge this as a relevant critique. However, we want to emphasise that subjective and objective medical knowledge are equally important for good clinical practice. We maintain that medicine is best off when we accept the tension between objective evidence and subjective experience as a source of learning and development in clinical and scientific dialogues.[10] Contradictions in medicine may appear as antagonisms and exclusive truths. An alternative perspective is to see contradictions as mutually uniting or, in other words, as complementary contradictions.
The concept of complementarity was introduced by the Danish physicist Niels Bohr in order to account for apparently contradictory phenomena in quantum mechanics. He showed that such phenomena arise when one maintains the view of objectivity as “God's Eye View” (or a “view from nowhere”), and can only be solved when it is acknowledged that the observer cannot be eliminated from the description of nature. For example, experiments have shown that electrons appear to be both particles and waves. According to classical physics this is simply impossible, and therefore it represents an apparent contradiction in quantum mechanics. However, when the observational conditions are taken into consideration, we may say that under some conditions electrons behave as if they are particles, and under other conditions they behave as if they are waves. According to Bohr, it is imperative to keep in mind that when we attribute one or another property to a physical entity, we have to specify under which conditions those properties can be observed.[11]
Bohr generalised this idea and applied it to other fields, for example to biology and psychology.[11] We may, for example, study an organism as a physical entity, but then we have to impose observational conditions on the organism that will necessarily suppress the aspects that characterise life. Bohr even maintained that if we try to investigate the ultimate secrets of life by physical means, we will destroy life. To function as an organism, the organism will always have enough freedom to hide its ultimate secrets from us (cited in [11], p. 97). If we adopt the idea of complementarity, we acknowledge that objectifying reductionism will fail. The same applies to an exclusively phenomenological approach. The two represent complementary aspects of the same endeavour. Both are relevant for medical practice and cannot be unified in one perspective. Bohr's legacy is thus an appeal to intellectual humility and pluralism.[12]
Evidence-based medicine (EBM) allowing for uncertainty
When EBM was introduced it challenged authoritarian traditions and expert based medicine by introducing principles for critically appraising the quality of research evidence in scientific papers.[13,14] Increasingly, EBM has been accused of establishing quantitative research evidence at the top of a knowledge hierarchy, and of informing clinical work unambiguously through guidelines used as instruments for governance of healthcare staff and institutions. Some of this critique is obviously relevant and justified.[15]
However, it seems self-contradictory to discard EBM, and replace it with phenomenology. The first person perspective also entails the human need for learning from other people's experience, i.e., asking for external evidence. We consider EBM as a tool for improving the quality of external evidence. When we want to examine whether a treatment is effective, a randomised clinical trial (RCT), or an estimate of the mean effects from two or more RCTs (meta-analysis), is the most favourable research design. The basic assumption in this kind of research is that some aspects of our bodies are universal, and that illness and disease that afflict us may be placed in a valid category, and thereby diagnosed in a fairly invariant and consistent manner. Such categories and treatment effects are less valid when diagnoses, treatments, and outcomes are complex and relational. Therefore, interpretation of RCTs and meta-analyses should be performed with caution.[15] Finally, RCTs that are conducted in an explanatory design ask whether an intervention works under ideal or selected conditions, while RCTs that are conducted in a pragmatic design ask whether an intervention works under real-life conditions and whether it works in terms that matter to the patient. It is for obvious reasons that the latter design is closest to the truth of how treatment effects are in a real life setting.
We cannot assume that diagnostic categories and treatment effects will ever reach absolute certainty. Rather, EBM gives us a tool to understand that research and diagnostics are always prone to type I and type II errors. With EBM we are guided to compare beneficial effects with unwanted side effects. It enables us to evaluate diagnostic accuracy and estimate the risks of under- and over-diagnosis depending upon the prevalence of diseases. We are also able to assess the uncertainty in prediction, and thereby understand that science is prone to bias and conflicts between external and internal validity.
In the clinical encounter, EBM is applied in order to examine three different perspectives: (1) the experiences, values, and preferences of the patients; (2) the context for the encounter and the doctor's experiences; and (3) the best available external evidence. When the former two perspectives are suppressed and external evidence is applied without reference to the context, personal circumstances, or professional appraisal, it is no longer evidence based but algorithm driven. Neither should external evidence be confused with epidemiological knowledge. When the questions are why? and how?, qualitative and phenomenological studies will constitute the best available evidence.[13] Used with critical consideration EBM is not a context-denying perspective that makes subjective experiences irrelevant.
Scientific pluralism in practice
We aim for a medical professionalism where biomedical and technical knowledge are complemented by the personal and subjective perspectives, and where these aspects of professionalism are integrated in both undergraduate and postgraduate medical training.[16] Our ambition is that when educating doctors we should enable them to assess health problems from more than one perspective of knowledge, and to make balanced decisions where technical facts and personal judgement mutually inform each other. In order to achieve this, we need educational change and a professional language that allows us to consider a patient or a health problem from different perspectives. We act in contexts that are complex, where simple and mono-causal explanations are rare, and where health and disease are unpredictable and open processes.[17,18]
Research never informs the public unambiguously and without mediation.[19] Uncertainty and indeterminacy are inherent in any research [17] and research findings must be interpreted and critically appraised.[20] Therefore, sociologists and philosophers of science maintain that research should not only be methodologically robust but also socially robust.[20] Socially robust research is characterised by researchers who are engaged in open dialogue about the consequences of their findings.
In these dialogues researchers should be willing to scrutinise in what ways their perspectives and preconceptions (upstream factors) decide which truths are possible to discover and which blind spots their methodological restrictions entail. These reflections should also examine the intended and unintended consequences of conveying the results and the conclusions from research papers (downstream factors). Systematic methods for such critical dialogue have been developed, e.g., at the Centre for the Study of the Sciences and the Humanities at the University of Bergen.[21] The purpose is not to humiliate the researcher or refer science to an indifferent place in a cacophony of random statements. On the contrary, using self-criticism the researchers should promote their integrity not only as experts of opinion, but also as dialogue partners in society.[22] A true scientific attitude is characterised by a willingness to look for objections against what we prefer as truths, and to invite others to promote such objections.
Acknowledgements
Edvin Schei and Ragnar Fjelland contributed with text, and together with Reidun S Kjome advised us on earlier drafts of this paper.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Cassell EJ. The nature of suffering and the goals of medicine. Oxford, New York: Oxford University Press; 2004. [Google Scholar]
- 2.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. [DOI] [PubMed] [Google Scholar]
- 3.McWhinney IR. Philosophical and scientific foundations of family medicine In: McWhinney IR, editor. A textbook of family medicine. New York, Oxford: Oxford University Press; 1989. p. 43–71. [Google Scholar]
- 4.Cassell EJ. Diagnosing suffering: a perspective. Ann Intern Med. 1999;131:531–534. [DOI] [PubMed] [Google Scholar]
- 5.Getz L, Kirkengen AL, Ulvestad E. The human biology-saturated with experience . Tidsskr Nor Laegeforen. 2011;131:683–687. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkum T, Wang CE, Waterloo K.. Pasienterfaringer med ulike tiltak for kronisk utmattelsessyndrom [Patients' experience with treatment of chronic fatigue syndrome] English summary. Tidsskr nor Legeforen. 2009;129:1214–1216. [DOI] [PubMed] [Google Scholar]
- 7.Golubnitschaja O, Costigliola V.. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortmann-Roe S. Understanding the bias-variance tradeoff. [Internet]; [cited 2015 Dec 8]. Available from: http://scott.fortmann-roe.com/docs/BiasVariance.html (accessed 8 Dec 2015). [Google Scholar]
- 9.Vogt H, Ulvestad E, Eriksen TE, et al. . Getting personal: can systems medicine integrate scientific and humanistic conceptions of the patient? J Eval Clin Pract. 2014;6:942–952. [DOI] [PubMed] [Google Scholar]
- 10.Heath I. “Uncertain clarity”: contradiction, meaning and hope. Br J Gen Pract. 1999;49:651–657. [Google Scholar]
- 11.Fjelland R. Niels Bohr on physics, biology and psychology. Teorie Modelli. 2001;VI:87–101. [Google Scholar]
- 12.Fjelland R. The “Copenhagen interpretation” of quantum mechanics and phenomenology In: Babich BE, editor. Hermeneutic philosophy of science, Van Goghs eyes, and God. New York: Kluwer Academic Publishers; 2002. [Google Scholar]
- 13.Sackett DL, Rosenberg WM, Gray JA, et al. . Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sackett DL, Haynes RB, Tugwell P.. Clinical epidemiology. A basic science for clinical medicine. Boston/Toronto: Little, Brown and Company; 1985. p. 3–45. [Google Scholar]
- 15.Skaftnesmo T. Evidensbasering. Det nye sannhetsmaskineriet [Evidence basing. The new machinery of truths]. Oslo: Paradigmeskifte Forlag AS; 2013. [Google Scholar]
- 16.Schei E. Lytt. Legerolle og kommunikasjon [Listen. Physician role and communication]. Bergen: Fagbokforlaget; 2014. [Google Scholar]
- 17.Funtowicz S, Strand R.. Change and commitment: beyond risk and responsibility. J Risk Res. 2011;14:995–1003. [Google Scholar]
- 18.Rortveit G, Strand R.. Risiko, usikkerhet og uvitenhet i medisinen [Risk, uncertainty and ignorance in medicine] English summary. Tidsskrift Den Norske Laegeforening. 2001;121:1382–1386. [PubMed] [Google Scholar]
- 19.Rustad LM. Kunnskap som delvise forbindelser [Knowledge as partial connections] In: Asdal K, Berg AJ, Brenna B, Moser I, Rustad LM, editors. Betatt av viten. Bruksanvisninger til Donna Haraway. Oslo: Spartacus Forlag; 1998. [Google Scholar]
- 20.Nowotny H, Scott G, Gibbons M.. Re-thinking science. Knowledge and the public in an age of uncertainty. Oxford: Blackwell Publishers; 2001. [Google Scholar]
- 21.Forssen A, Meland E, Hetlevik I, et al. . Rethinking scientific responsibility. J Med Ethics. 2011;37:299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cilliers P. Complexity, deconstruction and relativism. Theory Cult Soc. 2005;22:255–267. [Google Scholar]