Abstract
Objective
The study objective was to measure the rates of inclusion of populations at risk of advanced melanoma in a pilot targeted screening project involving general practitioners.
Design
This cross-sectional database study compared the inclusion rates of patients who signed inclusion in a targeted screening project with those of patients who did not, during a period in which both groups of patients consulted investigators.
Setting
Data were extracted from the national healthcare insurance records in western France from 11 April to 30 October 2011.
Patients
Patients, older than 18, considered for the data extraction had consulted one of the 78 participating GPs during the study period, and were affiliated with the national healthcare insurance.
Main outcome measures
Inclusion in the screening was the main outcome measure. Patients at risk of advanced melanoma were characterized by male gender, age over 50, low income, rural residence, farmer, and presence of chronic disease.
Results
A total of 57,279 patients consulted GPs during the inclusion period and 2711 (4.73%) were included in the targeted screening. Populations at risk of advanced melanoma were less included: men (OR = 0.67; 95%CI [0.61–0.73]; p < 0.001), older than 50 (OR = 0.67; 95%CI [0.60–0.74]; p < 0.001), low income (OR = 0.65; 95%CI [0.55–0.77]; p < 0.001), farmer (OR = 0.23; 95%CI [0.17–0.30]; p < 0.001) and presence of a chronic disease (OR = 0.87; 95%CI [0.77–0.98]; p < 0.028).
Conclusion
This study demonstrated inequalities in the inclusion of patients in a melanoma screening. Patients at risk of advanced cancer were screened less often. Further studies should focus on GPs ability to identify and screen these patients.
Key Points
Advanced melanoma is more frequently diagnosed in men, older patients and socioeconomically disadvantaged populations, which leads to survival inequalities.
• Despite the involvement of general practitioners, the implementation of targeted melanoma screening did not avoid inclusion inequalities.
• Men, older patients, patients suffering from chronic diseases, and low-income patients were less likely to benefit from screening.
• The display of a conventional or an alarmist poster in the waiting room did not statistically reduce these inclusion inequalities.
Keywords: Early detection of cancer, France, general practice, melanoma, population characteristics, primary healthcare, screening
Introduction
Melanoma screening is a challenge for both clinicians and researchers.[1] On the one hand, the impact of screening the general population on mortality remains debated,[1] and on the other hand, patient survival is correlated with the disease stage and with the Breslow index at the time of diagnosis: specifically, the five-year survival rate of patients with melanoma in the localised stage is 98.3% compared with 16% for patients with metastatic disease.[2] Significant funds are spent by policymakers to enhance melanoma awareness and ensure that patients are self-directed to seek consultations. One solution might be to increase the involvement of primary care providers in the screening of patients at elevated risk of melanoma, regardless of whether the patients would have sought a clinical skin examination on their own.
Various researchers claim that reducing the number of patients with advanced lesions at the time of the diagnosis, rather than multiplying the diagnosis of very thin lesions, is a priority.[3,4] Based on the findings reported by Lyratzopoulos, melanoma exhibits the highest inequalities when diagnosed at advanced stages compared with the rest of the cancers.[5] Researchers have previously reported that inequalities impact survival rates in disadvantaged populations [6,7] and have demonstrated that men, older patients, and socioeconomically disadvantaged populations are at risk of advanced melanoma.[3,5,8,9] A solution to this problem might be the development and validation of tailored and effective awareness interventions to reduce inequalities at the screening step.[1,5,10–12]
Our team developed a targeted melanoma screening approach using a validated selection tool grounded in primary care, based on general practitioner (GP) involvement, and focused on patients at risk of melanoma.[13–15] Previous publications have reported the potential benefits of this type of targeted screening based on the following findings: a high melanoma incidence in the selected patients (25-fold greater than that in the general population), diagnosis of melanomas <1 mm in thickness, and a positive impact on patient awareness and prevention behaviours.[13,14] However, it is unclear whether more education and counselling would decrease the observed inequalities. A few authors have suggested that more education might increase inequalities because these efforts may have a greater influence on more-educated patients.[16]
A major issue is the ability of GPs to screen patients at risk of advanced melanoma (men, older patients, and socioeconomically disadvantaged populations) and who would not consult a physician on their own initiative. On the one hand, it is well known that the inclusion of patients in trials and research programs is difficult because the attention of GPs cannot always focus on the study subject and varies between GPs,[17,18] and on the other hand, in our pilot screening, we expected that participation would not rely on patient motivation and that populations at risk of advanced melanoma would be included at an equal or higher rate than other populations.
The following database study focused on inclusions in a pilot targeted melanoma screening program involving GPs. The main objective was to evaluate whether the rates of inclusion of populations at risk of advanced melanoma were lower than, equal to or higher than those of other populations.
Method
Design and setting
This cross-sectional comparison database study was part of a wider targeted melanoma screening project entitled “COhort of PAtient at elevated RIsk of MElanoma” (COPARIME, http://clinicaltrials.gov NCT01610531).[14] The reported data were collected in western France from 11 April to 30 October 2011. The characteristics of the patients who consulted at the GP offices during the inclusion period were extracted from the national healthcare insurance system administrative records.
A pilot targeted screening strategy
The COPARIME screening strategy was based on three steps: (1) all patients who consulted GPs were asked to complete a SAMScore questionnaire that was available in the waiting room, regardless of their reasons for seeking a consultation; (2) the skin of all patients at elevated risk of melanoma was examined by the GPs; and (3) all patients requiring further examination were referred to the dermatologist based on the physician's opinion according to his/her usual practice.
Patient eligibility in the targeted screening program
The eligibility of patients in the screening was based on the Self-Assessment of Melanoma risk score.[13–15] GPs were provided printed SAMScore questionnaires (Figure 1) that listed seven risk factors associated with melanoma (e.g., phototype, freckling tendency, number of moles, residence in a country with strong sunshine, severe sunburn during infancy, personal history of melanoma, and family history of melanoma).
Figure 1.
Questionnaire used for the Self-Assessment of the Melanoma Risk Score (Quereux, 2012).
GP awareness of melanoma screening
Before starting the study, all of the GPs (1) had to view an e-learning module on melanoma screening to update their knowledge and skin examination practices, (2) participated in a 2-h meeting that presented the study, and (3) personally received the same documents necessary for study participation, a poster to be displayed in the waiting room, information leaflets on melanoma produced by the French National Cancer Institute, and printed questionnaires listing seven risk factors for melanoma. A total of 78 GPs volunteered to participate.
Posters randomly displayed in GP waiting rooms
Two different posters were randomly provided to GPs (Figure 2). The size of both posters was 110 cm ×150 cm. The alarmist poster showed a large picture of a patient’s back with a melanoma, a picture in a medallion of the body of a patient with metastases, and the following sentences “More than 1700 French citizens die of melanoma every year. However, when melanoma is diagnosed early, this cancer can be cured without complications. Diagnosis is necessary! Participate in the COPARIME study!” The conventional poster showed the smiling faces of four patients and the following sentences: “Naevus or melanoma, how to make the difference? Learn how to perform a skin examination and when to consult a doctor.”
Figure 2.
Conventional versus alarmist posters randomly displayed in the GP waiting rooms.
Scoring and inclusion during consultations
Between 11 April and 30 October 2011, when consulting with patients who were seen for other reasons, the GPs were asked to systematically identify patients at risk of melanoma. The GPs were asked to access a SAMScore risk calculator on a server (http://www.dmg-nantes.fr/coparime/english) during the consultation and to enter each patient’s responses to seven questions. The calculator integrated the risk factors using the SAMScore algorithm and expressed the risk in a dichotomous format, reporting either an elevated risk or a non-elevated for melanoma.
Patients who were eligible for the targeted screening were required to (1) have an increased risk of melanoma, (2) be at least 18 years of age, (3) have an adequate knowledge of French, and (4) have no personal history of melanoma (as a dermatologist follow-up was recommended). The GPs provided oral and written information on the pilot targeted screening program and collected written informed consent from their patients. Thereafter, the patient was formally included in the screening program.
Data extraction from the national healthcare insurance records
Patients included in the database study
The following criteria were applied for data extraction: the patients (1) had consulted one of the 78 GPs who participated in the melanoma targeted screening program during the enrolment period, (2) were at least 18 years of age, and (3) were affiliated with the national healthcare insurance at the beginning of the study (1 April 2011).
Data collection and variables extracted from the database study
All data relative to the GP consultations during the study period were extracted from the national healthcare insurance administrative records. Based on a literature review [8,9,11,12,19–22], the following factors were selected to identify populations at elevated risk of advanced melanoma: marital status, gender, age >50, low socioeconomic status, location of residence, and comorbidities. Because we could not access the corresponding information in the national insurance system database, marital status was not collected.
The following patient characteristics were extracted from the healthcare national database: gender, age, socioeconomic status (implying a specific reimbursement facility), location of residence (i.e., rural, semi-rural, or urban), and presence of a chronic disease (implying that they benefited from a “disorder of long duration” reimbursement facility). Low socioeconomic status was defined by an annual income lower than 8593€ for an individual or lower than 12,889€ for a couple.
Statistical analysis
We analysed the data with R 3.1.1. We performed two-tailed analyses and set the significance threshold to 0.05. A generalized linear mixed model was used. The GPs were considered a random effect, whereas other variables tested were considered fixed cofactors. No stratification was planned. The results were analysed with an adjustment for GPs.
Ethical approval
Ethical approval was provided by the ethics committee of the University Hospital of Tours (no. 2011-R2-BRD 10/11-N).
Results
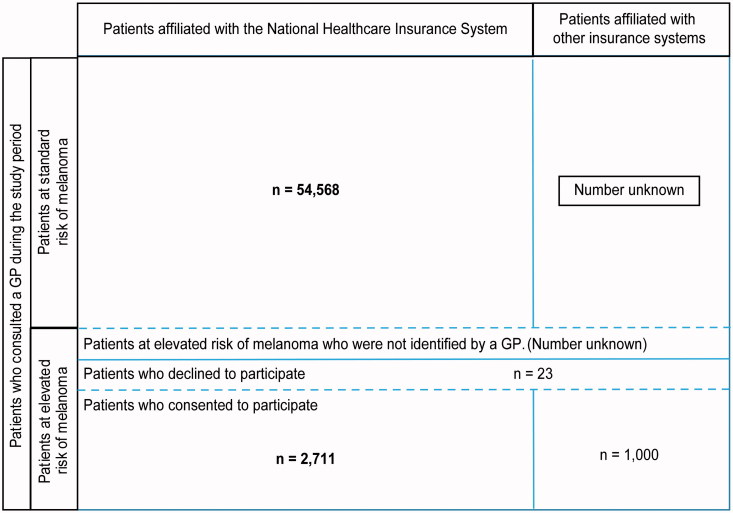
Figure 3 reports the patients included in the database study after data extraction from the national healthcare insurance records. The characteristics of 57,279 patients were analysed: 2711 were included in the targeted screening project, and 54,568 consulted GPs without being included. A total of 23 had been identified to be at high risk of melanoma by the GPs but refused to be included.
Figure 3.
Patients included in the database study after extraction from the national healthcare insurance records.
The characteristics of the patients who consulted with the GPs during the inclusion period are reported in Table 1. Males comprised 37.7% of all patients. The mean age was 47 years old. A low socioeconomic status was identified in 9.63% of all patients. Approximately 19.0% of all patients suffered from chronic disease. Patients who lived in rural geographic areas comprised 27.9% of the study population. Table 1 also reports the characteristics of the study patients, depending on whether they consulted GPs who received the conventional or the alarmist poster.
Table 1.
Characteristics of patients who consulted with GPs during the study period (France, 2011).
| Total N = 57,279 n, % |
Conventional poster group N = 26,765 n, % |
Alarmist poster group N = 30,514 n, % |
|
|---|---|---|---|
| Male gender | 21,586, 37.69 | 9986, 37.31 | 11,600, 38.02 |
| Agea | 46.95, 18.20 | 46.30, 17.90 | 47.50, 18.40 |
| Age group | |||
| 18–25 | 7538, 13.16 | 3629, 13.56 | 3909, 12.81 |
| 26–50 | 26,405, 46.10 | 12,583, 47.01 | 13,822, 45.30 |
| 51–75 | 18,606, 32.48 | 8606, 32.15 | 10,000, 32.77 |
| 76–100 | 4730, 8.26 | 1947, 7.27 | 2783, 9.12 |
| Low socioeconomic status | 5515, 9.63 | 2554, 9.54 | 2961, 9.70 |
| Presence of a chronic disease | 10,894, 19.02 | 4971, 18.57 | 5923, 19.41 |
| Farmer insurance system | 3249, 5.67 | 1528, 5.71 | 1721, 5.64 |
| Residential area | |||
| Rural | 15,979, 27.90 | 8377, 31.30 | 7602, 24.91 |
| Urban | 34,652, 60.50 | 15,323, 57.25 | 19,329, 63.34 |
| NAb | 6648, 11.61 | 3065, 11.45 | 3583, 11.74 |
Mean, SD.
Not available.
Table 2 reports the patient characteristics associated with non-inclusion in the targeted screening: male gender (OR = 0.67; 95%CI [0.61–0.73]; p < 0.001), over 50 years of age (OR = 0.67; 95%CI [0.60–0.74]; p < 0.001), low socioeconomic status (OR = 0.65; 95%CI [0.55–0.77]; p < 0.001), farmer insurance system (OR = 0.23; 95%CI [0.17–0.30]; p < 0.001), and presence of a chronic disease (OR = 0.87; 95%CI [0.77–0.98]; p < 0.028).
Table 2.
Comparison of characteristics of the included and non-included patients in a targeted melanoma screening (France, 2011) (logistic regression).
| Included patientsa N = 2711 n, % |
Non-included patientsb N = 54,568 n, % |
OR [95%CI] | p | |
|---|---|---|---|---|
| Male gender | 806; 29.73 | 20,780; 38.08 | 0.67 [0.61–0.73] | <0.001 |
| Age >50 years | 878; 32.39 | 22,458; 41.16 | 0.67 [0.60–0.74] | <0.001 |
| Low socioeconomic status | 183; 6.75 | 5332; 9.77 | 0.65 [0.55–0.77] | <0.001 |
| Presence of a chronic disease | 403; 14.87 | 10,491; 19.23 | 0.87 [0.77–0.98] | 0.028 |
| Farmer insurance system | 100; 3.69 | 3149; 5.77 | 0.23 [0.17–0.30] | <0.001 |
| Rural residential area | 929; 34.27 | 15,050; 27.58 | 1.02 [0.91–1.14] | 0.76 |
Patients who signed informed consent to participate to the melanoma targeted screening project.
Patients who did not sign informed consent to participate to the melanoma targeted screening project.
Table 3 provides a comparison of the characteristics of the included patients and the non-included patients with respect to which poster was displayed in the waiting rooms they visited. Among the 45 GPs who received the alarmist posters, 20 (44.4%) did not follow the protocol and did not actually display the poster in their waiting room. Therefore, the results provided were obtained from a per-protocol subgroup analysis of the 25 GPs who actually displayed the poster. None of the population characteristics were associated with the display of a particular poster (Table 3).
Table 3.
Characteristics of included and non-included patients in a targeted melanoma screening programme according to the poster displayed in the waiting room (France, 2011) per protocol analysis.
| Conventional poster | Alarmist poster | ||||
|---|---|---|---|---|---|
| Included patients N = 1066 n, % |
Non-included patients N = 20,056 n, % |
Included patients N = 956 n, % |
Non-included patients N = 16,732 n, % |
pb | |
| Male gender | 343; 32.18 | 7385; 36.82 | 251; 26.26 | 6487; 38.77 | 0.88 |
| Agea | 44.50; 15.97 | 46.15; 17.95 | 44.78; 14.76 | 48.45; 18.68 | – |
| Age group | |||||
| <25 | 112; 10.51 | 2840; 14.16 | 75; 7.85 | 2114; 12.63 | 0.74 |
| 26–50 | 617; 57.88 | 9369; 46.71 | 571; 59.73 | 7166; 42.83 | 0.39 |
| 51–75 | 291; 27.30 | 6377; 31.80 | 279; 29.18 | 5777; 34.53 | 0.50 |
| 76–100 | 46; 4.32 | 1470; 7.33 | 31; 3.24 | 1675; 10.01 | 0.40 |
| Low socioeconomic status | 70; 6.57 | 2053; 10.24 | 50; 5.23 | 1364; 8.15 | 0.83 |
| Suffers from chronic disease | 168; 15.76 | 3666; 18.28 | 127; 13.28 | 3372; 20.15 | 0.84 |
| Farmer insurance system | 51; 4.4 | 1477; 5.8 | 49; 3.2 | 1672; 5.8 | 0.591 |
| Residential area | |||||
| Rural | 342; 32.08 | 5036; 25.11 | 374; 39.12 | 4674; 27.93 | 0.39 |
| Urban | 618; 57.97 | 12,597; 62.81 | 474; 49.58 | 9987; 59.69 | 0.64 |
| 106; 9.94 | 2423; 12.08 | 108; 11.30 | 2071; 12.38 | – |
Mean, SD.
Adjusted on GP as a random factor.
Discussion
Statement of principal findings
Our study found that populations at elevated risk of advanced melanoma (men, patients older than 50 years, patients with a low socioeconomic status, and patients suffering from a chronic disease) were less likely to be included in a targeted melanoma screening even though their GPs were actively recruiting patients. Our study also tested whether displaying an alarmist poster in the waiting room positively influenced the inclusion of these specific populations in the screening project. Despite appropriate sample sizes, no significant impact from the posters was observed.
Study strengths and weaknesses
The study strengths were its analysis of an on-going melanoma targeted screening intervention that has previously demonstrated benefits [13,14] and the basis of the study on a reproducible screening method using a validated tool.[15] Additional strengths were a large patient cohort and a population recruited from a primary care setting. The design based on data extracted from the national insurance system database allowed the inclusion of patients in the study analysis who might exhibit a low degree of concern regarding melanoma prevention. In total, the generalizability of the findings should be important.
A limitation of this study is that we were unable to provide a quantitative assessment of the abilities of the GPs to systematically identify the eligible patients. However, to collect data on the ability of GPs to select the right individuals, observation would have been necessary, which would have led to a major bias known as the Hawthorne effect.[23] Another limitation is that the abilities and practices of different GPs might differ, which is why we presented GP effect-adjusted data. A third limitation is the data collection process, which utilised the national health insurance system database: (1) 20% of the French population has another insurance provider (e.g., railway workers, lawyers, soldiers), resulting in the population included in the study not being entirely representative of the general population and (2) only administrative data could be collected.
Findings with respect to other studies
The overall rate of patients at high risk of melanoma included in the screening (4.73%) is consistent with the very high rate of melanoma diagnosed in the selected population.[14,15] The crude incidence of melanoma in the high risk population was reported to be 25-fold higher than incidence in the general population.[14] The corresponding 5% rate of high-risk patients in the population consulting the GP during the study period appears consistent with the SAMScore properties to concentrate the number of melanomas in a small population.[14,15]
Our study revealed associations between likelihood of inclusion in screening and sociodemographic characteristics, which might be a neglected causal factor explaining the associations between diagnoses of melanoma at advanced stages and patient sociodemographic characteristics.[5] Patients are diagnosed with melanoma at advanced stages for complex reasons. Researchers have previously suggested that inequalities in cancer survival might be due to inequalities in awareness and appraisal of cancer symptoms, which could increase the time interval between symptom onset and presentation to a doctor.[24] Andrulonis reported that only 25% of the patients who sought a consultation for a skin cancer screening were male.[11] Other authors have reported that men and low-income patients are less concerned with preventive procedures and follow-up strategies.[11,12,25,26] Other contributing factors to the diagnoses of advanced melanoma in these patients might be that men have types of melanoma that grow faster and are more often located on their backs compared with women. This study suggests that diagnosis at more advanced stages might also be due to fewer GP opportunistic screenings in certain populations (even though GPs were asked to concentrate on cancer screening). In our study, the probability of including men in the screening was 0.67-fold lower than the probability of including women (OR = 0.67 [0.61–0.73]), even though the SAMScore does not consider gender as a risk factor; a question is whether there might be a link with the lower frequency of advanced-stage melanoma in women.[5] In total, our results are consistent with previous findings that emphasized the major role of GPs in counselling on melanoma prevention.[27]
Our findings regarding the impact of co-morbidities are relevant. Gonzales reported that patients who were diagnosed at more advanced stages were more likely to have co-morbid diseases.[22] Some authors have argued that comorbidities may mask early cancer symptoms.[28] Our study demonstrates that the presence of other comorbid illnesses may favour non-inclusion in targeted screening for melanoma. GPs may not focus their attention and energy on skin examinations due to the presence of other health issues.[29] A hypothesis is that patients who are not concerned with skin cancer screening may also not be concerned with health and prevention in general. Thus, a higher involvement of GPs in these populations may first impact other health issues, in accordance with risk assessment and disease prevalence. Even though GPs were asked to concentrate their efforts on melanoma screening, their clinical priority was likely different at the time of the consultation. Paradoxically, Fleming reported that breast and colorectal cancers could be diagnosed earlier in patients with other pathologies.[30] However, these cancers can be identified fortuitously because of radiological exams that are required for other reasons whereas there would likely be no such secondary benefit for patients with melanoma (radiological exams do not favour melanoma diagnosis).
Most studies have reported that patients who experience difficulty with respect to healthcare access present with more advanced lesions because of less effective screening.[31,32] However, our study focused on the GP consultation, and access to primary care is less critical in France than access to the dermatologist. Stitzenberg reported that delay in diagnosis was associated with the distance to the dermatologist [32] but our study did not focus on this second part of the screening.
Although our findings concerning the poster did not reveal any statistical significance, we believe that our negative finding is relevant. Clinicians and policymakers charged with the task of designing melanoma screening messages should know that framing a message in either an alarmist or conventional manner is not imperative. This finding is consistent with the results of O’Keefe [33] and Gallagher,[34] who reported no effect of framing among studies that encouraged cancer detection. Based on our study findings, policy makers should avoid alarmist communications because a large proportion (44.4%) of the GPs who were invited to display alarmist posters in this study neglected to follow the protocol.
The impact of GPs on patient inclusion in screening programs has been demonstrated previously.[35] GPs are typically cited as the most trusted professionals in public surveys [36] and it is necessary to determine whether they could help minimize healthcare inequalities in screening. In a recent publication, Raine focused on the socioeconomic gradient in the uptake of bowel cancer screening, which potentially leads to inequalities in morbi-mortality.[37] These researchers evaluated whether the endorsement of cancer screening by an individual’s GP could reduce this gradient but did not demonstrate any significant impact. Although our study was based on an active inclusion procedure based on GP involvement, health inequalities toward screening remained significant.
Conclusion
This study demonstrated inequalities in inclusion in a targeted melanoma screening: patients at risk of advanced cancer were screened less often. The involvement of a GP, even one supported by a validated tool for melanoma screening, was insufficient to avoid screening inequalities. Further studies should focus on the ability of GPs to identify and screen these patients. Given the strong inequalities for this type of cancer with respect to socioeconomic status, old age and gender, these factors should be further integrated in future tools and scores to increase clinician awareness regarding the screening of these populations.
Acknowledgements
The authors wish to thank the GPs for their participation in the COPARIME targeted melanoma screening project.
Disclosure statement
No financial disclosures were reported by the authors.
Funding
COPARIME is a cohort study that is funded and supported by the French National Institute of Cancer (199,000€).
Registration number: NCT01610531.
References
- 1.Curiel-Lewandrowski C, Kim CC, Swetter SM, et al. Survival is not the only valuable end point in melanoma screening. J Invest Dermatol. 2012;132:1332–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piris A, Lobo AC, Duncan LM.. Melanoma staging: where are we now? Dermatol Clin. 2012;30:581–592. [DOI] [PubMed] [Google Scholar]
- 3.Grange F, Barbe C, Aubin F, et al. Clinical and sociodemographic characteristics associated with thick melanomas: a population-based, case-case study in France. Arch Dermatol. 2012;17:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Weyers W. The ‘epidemic’ of melanoma between under- and overdiagnosis. J Cutan Pathol. 2012;39:9–16. [DOI] [PubMed] [Google Scholar]
- 5.Lyratzopoulos G, Abel GA, Brown CH, et al. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol. 2013;24:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birch-Johansen F, Hvilsom G, Kjaer T, et al. Social inequality and incidence of and survival from malignant melanoma in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44:2043–2049. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford MJ, Ironmonger L, Ormiston-Smith N, et al. Estimating the potential survival gains by eliminating socioeconomic and sex inequalities in stage at diagnosis of melanoma. Br J Cancer. 2015;31:S116–S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Durme DJ, Ferrante JM, Pal N, et al. Demographic predictors of melanoma stage at diagnosis. Arch Fam Med. 2000;9:606–611. [DOI] [PubMed] [Google Scholar]
- 9.Mandalà M, Imberti GL, Piazzalunga D, et al. Association of socioeconomic status with Breslow thickness and disease-free and overall survival in stage I–II primary cutaneous melanoma. Mayo Clin Proc. 2011;86:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geller AC, Swetter SM, Oliveria S, et al. Reducing mortality in individuals at high risk for advanced melanoma through education and screening. J Am Acad Dermatol. 2011;65:S87–S94. [DOI] [PubMed] [Google Scholar]
- 11.Andrulonis R, Secrest AM, McGuire ST, et al. The influence of age and sex on reasons for seeking and expected benefits of skin cancer screening. Arch Dermatol. 2010;146:1097–1102. [DOI] [PubMed] [Google Scholar]
- 12.Merseyside and Cheshire Cancer Network Targeting men aged 50+ to increase early detection of melanoma local campaign planning guide. [Internet]; [cited 2014 Nov 1] Available from: http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@hea/documents/generalcontent/cr_045568.pdf (accessed 1 Nov 2014). [Google Scholar]
- 13.Rat C, Quereux G, Riviere C, et al. Targeted melanoma prevention intervention: a cluster-randomized controlled trial. Ann Fam Med. 2014;12:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rat C, Quereux G, Grimault C, et al. Melanoma incidence and patient compliance in a targeted melanoma screening intervention. One-year follow-up in a large French cohort of high-risk patients. Eur J Gen Pract. 2015;21:124–130. [DOI] [PubMed] [Google Scholar]
- 15.Quéreux G, N’guyen JM, Cary M, et al. Validation of the Self-Assessment of Melanoma Risk Score for a melanoma-targeted screening. Eur J Cancer Prev. 2012;21:588–595. [DOI] [PubMed] [Google Scholar]
- 16.Cokkinides VE, Geller AC, Jemal A.. Trends in melanoma mortality among non-Hispanic whites by educational attainment, 1993–2007. Arch Dermatol. 2012;148:587–591. [DOI] [PubMed] [Google Scholar]
- 17.Boxer P, Wilson S, Mathers N.. Short report: how often do UK primary care trials face recruitment delays? Fam Pract. 2007;24:601–603. [DOI] [PubMed] [Google Scholar]
- 18.Van der Wouden JC, Blankenstein AH, Huibers MJ, et al. Survey among 78 studies showed that Lasagna's law holds in Dutch primary care research. J Clin Epidemiol. 2007;60:819–824. [DOI] [PubMed] [Google Scholar]
- 19.Montella M, Crispo A, Grimaldi M, et al. An assessment of factors related to tumor thickness and delay in diagnosis of melanoma in southern Italy. Prev Med. 2002;35:271–277. [DOI] [PubMed] [Google Scholar]
- 20.Richard MA, Grob JJ, Avril MF, et al. Delays in diagnosis and melanoma prognosis (I): the role of patients. Int J Cancer. 2000;89:271–279. [PubMed] [Google Scholar]
- 21.Baumert J, Plewig G, Volkenandt M, et al. Factors associated with a high tumour thickness in patients with melanoma. Br J Dermatol. 2007;156:938–944. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez EC, Ferrante JM, Van Durme DJ, et al. Comorbid illness and the early detection of cancer. South Med J. 2001;94:913–920. [PubMed] [Google Scholar]
- 23.McCarney R, Warner J, Iliffe S, et al. The Hawthorne effect: a randomised controlled trial. BMC Med Res Methodol. 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon AE, Waller J, Robb K, et al. Patient delay in presentation of possible cancer symptoms: the contribution of knowledge and attitudes in a population sample from the United Kingdom. Cancer Epidemiol Biomarkers Prev. 2010;19:2272–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bränström R, Kristjansson S, Ullén H.. Risk perception, optimistic bias, and readiness to change sun related behaviour. Eur J Public Health. 2006;16:492–497. [DOI] [PubMed] [Google Scholar]
- 26.Hvidberg L, Pedersen AF, Wulff CN, et al. Cancer awareness and socio-economic position: results from a population-based study in Denmark. BMC Cancer. 2014;14:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falk M, Magnusson H.. Sun protection advice mediated by the general practitioner: an effective way to achieve long-term change of behaviour and attitudes related to sun exposure? Scand J Prim Health Care. 2011;29:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Søgaard M, Thomsen RW, Bossen KS, et al. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5:3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundu RV. Practice gaps. Barriers in melanoma detection in ethnic minorities: comment on anatomic distribution of malignant melanoma on the non-Hispanic black patient, 1998–2007. Arch Dermatol. 2012;148:801–802. [DOI] [PubMed] [Google Scholar]
- 30.Fleming ST, Pursley HG, Newman B, et al. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005;43:132–140. [DOI] [PubMed] [Google Scholar]
- 31.Roetzheim RG, Lee JH, Ferrante JM, et al. The influence of dermatologist and primary care physician visits on melanoma outcomes among medicare beneficiaries. J Am Board Fam Med. 2013;26:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stitzenberg KB, Thomas NE, Dalton K, et al. Distance to diagnosing provider as a measure of access for patients with melanoma. Arch Dermatol. 2007;143:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Keefe DJ, Jensen JD.. The relative persuasiveness of gain-framed and loss-framed messages for encouraging disease detection behaviors: a meta-analytic review. J Health Commun. 2007;12:623–644. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher KM, Updegraff JA.. Health message framing effects on attitudes, intentions, and behavior: a meta-analytic review. Ann Behav Med. 2012;43:101–116. [DOI] [PubMed] [Google Scholar]
- 35.Camilloni L, Ferroni E, Cendales BJ, et al. Methods to increase participation Working Group, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health. 2013;13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royal College of Physicians: trust in professions 2008: public awareness of trust in professions. London; 2008. [Google Scholar]
- 37.Raine R, Duffy SW, Wardle J, et al. Impact of general practice endorsement on the social gradient in uptake in bowel cancer screening. Br J Cancer. 2016;114:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]