Abstract
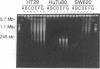
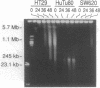
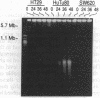
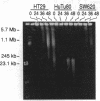
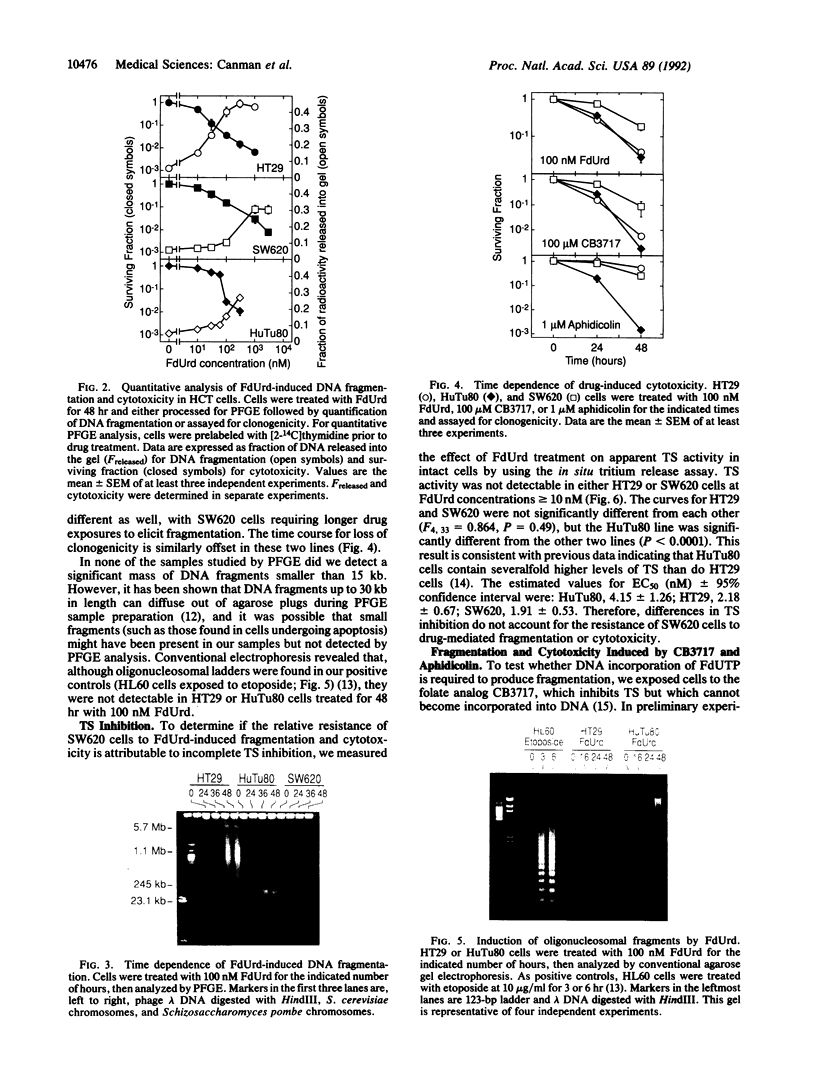
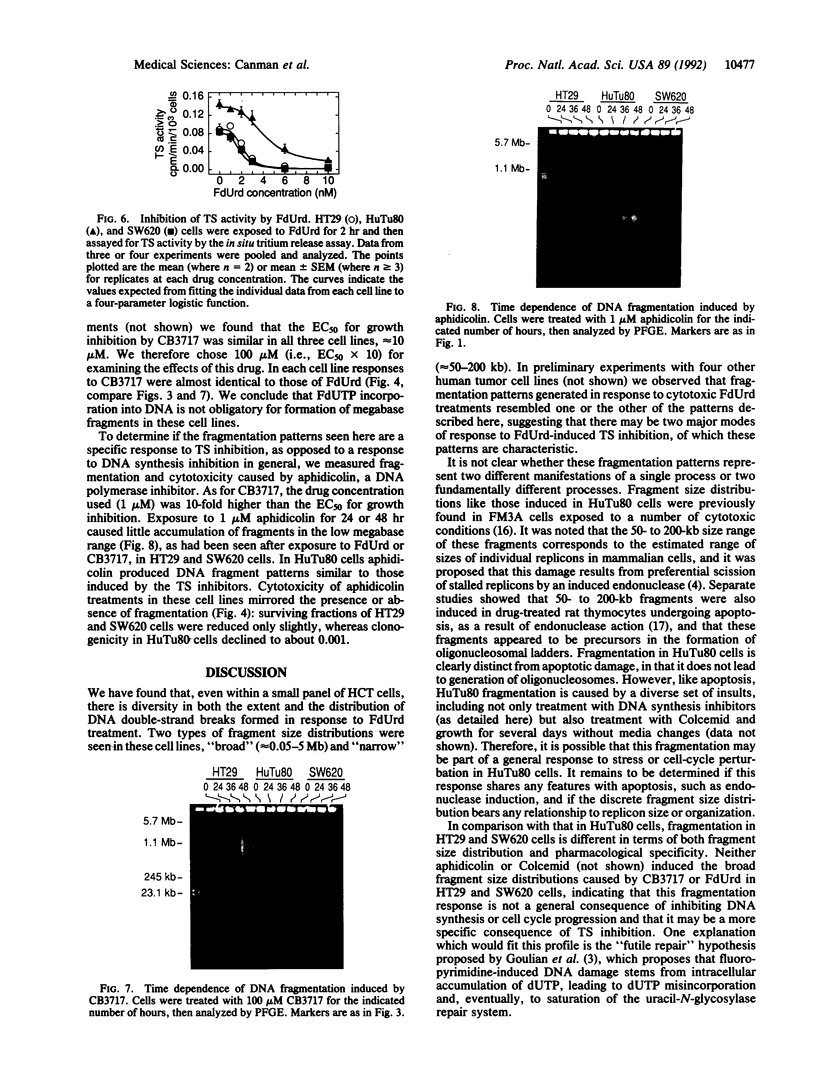
We have previously shown that treatment of the HT29 human colorectal tumor (HCT) cell line with 100 nM 5-fluorodeoxyuridine (FdUrd) induces DNA fragments ranging from 50 kilobases to 5 megabases. The studies reported here were conducted to characterize the kinetics, concentration dependence, and pharmacologic specificity of this process and to determine if such fragmentation varies among HCT cell lines. HT29 and SW620 cells yielded similar fragment size distributions upon treatment with either FdUrd or CB3717 [a folate analog inhibitor of thymidylate synthase (TS)]. With either of these agents the SW620 line required higher drug concentrations or longer incubation times than HT29 cells to achieve a given level of fragmentation or cytotoxicity, even though the two cell lines are equally sensitive to FdUrd-induced TS inhibition. These data indicate that SW620 resistance is not due to a lesion in the events leading up to TS inhibition but it may be due to a difference in the steps following TS inhibition. Aphidicolin, a DNA polymerase inhibitor, did not cause substantial fragmentation or cytotoxicity in these two cell lines, demonstrating that the fragmentation response to the other two drugs is not a general consequence of DNA synthesis inhibition. A third HCT line, HuTu80, gave rise only to a smaller and more discrete population of DNA fragments, ranging from approximately 50 to 200 kilobases, following exposure to FdUrd. Similar patterns were seen in this line upon treatment with CB3717 or aphidicolin, indicating that this fragmentation pattern is not specific to TS inhibition and may be characteristic of a more general response than that seen in the other two cell lines. DNA fragments induced by FdUrd in HuTu80 cells did not degrade into smaller pieces, demonstrating that the process by which they are formed is distinct from apoptosis. We conclude that the responses of HCT cells to FdUrd-induced TS inhibition vary significantly, that these differences may reflect heterogeneity in the mechanism of DNA damage formation, and that, in some cases, FdUrd resistance may be due to alterations in the fragmentation process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Arai H., Wataya Y., Seno T. A specialized form of chromosomal DNA degradation induced by thymidylate stress in mouse FM3A cells. Mutat Res. 1988 Jul-Aug;200(1-2):221–230. doi: 10.1016/0027-5107(88)90086-3. [DOI] [PubMed] [Google Scholar]
- Blöcher D. In CHEF electrophoresis a linear induction of dsb corresponds to a nonlinear fraction of extracted DNA with dose. Int J Radiat Biol. 1990 Jan;57(1):7–12. doi: 10.1080/09553009014550291. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dusenbury C. E., Davis M. A., Lawrence T. S., Maybaum J. Induction of megabase DNA fragments by 5-fluorodeoxyuridine in human colorectal tumor (HT29) cells. Mol Pharmacol. 1991 Mar;39(3):285–289. [PubMed] [Google Scholar]
- Fritz R. B., Musich P. R. Unexpected loss of genomic DNA from agarose gel plugs. Biotechniques. 1990 Nov;9(5):542, 544, 546-50. [PubMed] [Google Scholar]
- Goulian M., Bleile B. M., Dickey L. M., Grafstrom R. H., Ingraham H. A., Neynaber S. A., Peterson M. S., Tseng B. Y. Mechanism of thymineless death. Adv Exp Med Biol. 1986;195(Pt B):89–95. doi: 10.1007/978-1-4684-1248-2_15. [DOI] [PubMed] [Google Scholar]
- Grem J. L., Hoth D. F., Hamilton J. M., King S. A., Leyland-Jones B. Overview of current status and future direction of clinical trials with 5-fluorouracil in combination with folinic acid. Cancer Treat Rep. 1987 Dec;71(12):1249–1264. [PubMed] [Google Scholar]
- Jackson R. C., Jackman A. L., Calvert A. H. Biochemical effects of a quinazoline inhibitor of thymidylate synthetase, N-(4-(N-(( 2-amino-4-hydroxy-6-quinazolinyl)methyl)prop-2-ynylamino) benzoyl)-L-glutamic acid (CB3717), on human lymphoblastoid cells. Biochem Pharmacol. 1983 Dec 15;32(24):3783–3790. doi: 10.1016/0006-2952(83)90150-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. "Thymineless" death in androgen-independent prostatic cancer cells. Biochem Biophys Res Commun. 1989 Nov 30;165(1):73–81. doi: 10.1016/0006-291x(89)91035-8. [DOI] [PubMed] [Google Scholar]
- Lawrence T. S., Davis M. A., Maybaum J., Stetson P. L., Ensminger W. D. The effect of single versus double-strand substitution on halogenated pyrimidine-induced radiosensitization and DNA strand breakage in human tumor cells. Radiat Res. 1990 Aug;123(2):192–198. [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- VanDevanter D. R. Resolution of DNA fragments from 23 kilobases to 6 megabases by biphasic linear pulse ramping. Nucleic Acids Res. 1992 Mar 11;20(5):1148–1148. doi: 10.1093/nar/20.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. R., Smith C., Youdale T., Leblanc J., Whitfield J. F., Sikorska M. Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res. 1991 Feb 15;51(4):1078–1085. [PubMed] [Google Scholar]
- Washtien W. L. Increased levels of thymidylate synthetase in cells exposed to 5-fluorouracil. Mol Pharmacol. 1984 Jan;25(1):171–177. [PubMed] [Google Scholar]
- Wataya Y., Hazawa T., Watanabe K., Hirota Y., Yoshioka-Hiramoto A. Detection of deoxyribonucleoside-triphosphate imbalance death-induced DNA double strand breakage in FM3A cells by orthogonal-field-alternation gel electrophoresis (OFAGE). Nucleic Acids Symp Ser. 1988;(19):53–55. [PubMed] [Google Scholar]
- Yalowich J. C., Kalman T. I. Rapid determination of thymidylate synthase activity and its inhibition in intact L1210 leukemia cells in vitro. Biochem Pharmacol. 1985 Jul 1;34(13):2319–2324. doi: 10.1016/0006-2952(85)90788-9. [DOI] [PubMed] [Google Scholar]