Abstract
Nephropathy secondary to BK virus, a member of the Papoviridae family of viruses, has been recognized for some time as an important cause of allograft dysfunction in renal transplant recipients. In recent times, BK nephropathy (BKN) of the native kidneys has being increasingly recognized as a cause of chronic kidney disease in patients with solid organ transplants, bone marrow transplants and in patients with other clinical entities associated with immunosuppression. In such patients renal dysfunction is often attributed to other factors including nephrotoxicity of medications used to prevent rejection of the transplanted organs. Renal biopsy is required for the diagnosis of BKN. Quantitation of the BK viral load in blood and urine are surrogate diagnostic methods. The treatment of BKN is based on reduction of the immunosuppressive medications. Several compounds have shown antiviral activity, but have not consistently shown to have beneficial effects in BKN. In addition to BKN, BK viral infection can cause severe urinary bladder cystitis, ureteritis and urinary tract obstruction as well as manifestations in other organ systems including the central nervous system, the respiratory system, the gastrointestinal system and the hematopoietic system. BK viral infection has also been implicated in tumorigenesis. The spectrum of clinical manifestations from BK infection and infection from other members of the Papoviridae family is widening. Prevention and treatment of BK infection and infections from other Papovaviruses are subjects of intense research.
Keywords: BK viral infection, BK nephropathy, Cardiac transplant, Bone marrow transplant, Liver transplant, Pancreatic transplant, Lung transplant
Core tip: BK virus (BKV) is a member of a family of viruses that cause various diseases in animals and humans. Severe disease in transplanted kidneys was the first recognized human disease caused by BKV. In more recent times, BKV was also recognized as a cause of disease in the native kidneys of patients who had received bone marrow, heart, lung, liver and pancreas transplants, as well as in the kidneys of patients with loss of resistance to infection, such as patients with acquired immune deficiency syndrome or patients treated for malignant tumors. In addition to disease of the kidneys, BKV has also caused severe disease of the urinary bladder, the brain, the lungs, the gut and the blood. The diagnosis and particularly the management of infection by BKV present difficulties. Research for new medications specific for treating this infection is imperative.
INTRODUCTION
BK virus (BKV) is a human polyomavirus identified in 1971 when it was isolated from the urine of a Sudanese kidney transplant recipient with renal failure secondary to distal ureteral stenosis[1]. It belongs to the Papovaviridae family of viruses[2]. BKV along with other papovaviruses, e.g., JC virus (JCV), can cause disease in humans[3,4]. BKV is ubiquitous in the general population and serologic studies suggest that primary infection occurs in early childhood at a median age of 4-5 years[5,6]. BKV primary infection usually results in upper respiratory symptoms with rare reports of acute cystitis[5,6]. The route of transmission is not conclusively known. It is believed that transmission occurs via the respiratory pathway[5,6].
Latent infection with BKV typically causes clinical disease in the genitourinary tract since the virus has a tropism for renal tubular and transitional epithelial cells. In these cells BKV establishes a life-long latency[3,4,7]. Viral reactivation usually occurs in patients with immunosuppressed states resulting in viruria. A small percentage of patients with viruria develop an invasive infection of the kidney[3]. BKV infections involving the urinary tract were the first to be reported in kidney transplant recipients and are the most frequent manifestations of BKV. BKV infection in other organs is less frequent[2,3,8].
BK nephropathy (BKN) was recognized as an emerging problem in renal transplant recipients with the introduction of improved immunosuppressive treatments such as tacrolimus, mycophenolate, and antilymphocyte globulins[6,7,9]. Renal transplant failure rates, due to BKN, especially if diagnosed late, can reach as high as 50%-80% within 24 mo[7]. Therefore screening for BKV in renal transplant recipients has become routine[2,9]. Costa et al[10] reviewed the clinical and histologic features, diagnosis, monitoring of the virology and immunological picture and treatment of BKN. Their review was based primarily on reports of BKN involving renal allografts[10].
In recent years, reports of BKN in native kidneys and of BKV infection in other organ systems have emerged with increasing frequency in non-renal solid organ and bone marrow transplant patients[2,5,7,8,11] as well as in other immunosuppressed patients. The main purpose of this review is to summarize the clinical characteristics, diagnosis, pathophysiology and treatment of BKV infection in patients with solid organ and bone marrow transplantation. The spectra of manifestations of BKV infection and of patient groups developing BKV infection are enlarging. In addition to BKN in native kidneys of transplant recipients, this report will also address manifestations of BKV infection outside the urologic system and in patients without organ transplants. Several aspects of BKV infection, particularly the diagnosis, pathogenesis, and treatment of BKN have been studied extensively in kidney transplant recipients. This review will therefore include relevant studies of renal transplant recipients in these three areas.
The review has three major parts: (1) clinical manifestations of BKV infection; (2) diagnosis of BKN and pathogenesis of BKV infection; and (3) treatment of BKV infection and human diseases secondary to other members of the Papovavirus family. Key points each major part will be presented at its end.
PART A CLINICAL MANIFESTATIONS OF BKV INFECTION
Two cases will illustrate the clinical features and histology of BKN in native kidneys of transplant recipients.
Patient 1
A 30-year-old Hispanic man received a matched allogeneic bone marrow transplant from an unrelated donor approximately two years after the diagnosis of aplastic anemia. Six months after the transplant he developed post-transplant lymphoproliferative disorder (Ebstein Barr Virus associated diffuse large B cell lymphoma of the right tonsil). He underwent tonsillectomy, localized radiation, and one cycle of CHOP (cyclophosphamide, adriamycin, vincristine, prednisone) followed by two treatments with rituximab.
Two years after transplantation he developed graft vs host disease of his esophagus and small intestine which required initiation of immunosuppressive therapy. He was placed on tacrolimus. After ten months, tacrolimus was tapered and sirolimus was started because of concern for calcineurin inhibitor toxicity. After three months sirolimus was replaced by mycophenolate mofetil because his graft vs host disease was not improving.
The patient’s serum creatinine was 0.7-0.9 mg/dL pre-transplant and 1.2 mg/dL prior to the initiation of tacrolimus. Renal function worsened while he was on tacrolimus, which was discontinued when the serum creatinine reached 2.0 mg/dL. All blood tacrolimus trough levels were between 2 and 3 ng/mL. Despite discontinuation of tacrolimus, the patient’s renal function continued to decline. Approximately four years following the bone marrow transplant, his serum creatinine was 3.15 mg/dL (estimated glomerular filtration rate by CKD-EPI equation of 25 mL/min per 1.73 m2). Urine microscopy was bland and urine protein to creatinine ratio was 0.6 g/g. Renal ultrasound was unremarkable. Serum antinuclear antibodies, antineutrophil cytoplasmic antibodies (ANCA), hepatitis panel, and human immunodeficiency virus (HIV) test were negative. Serum complement levels (C3, C4) were normal. Serum BK viral load was 700000 copies/mL.
Percutaneous renal biopsy demonstrated morphologic features consistent with BKN. Light microscopy was notable for lymphocytic tubulitis and viral nuclear inclusions (Figure 1C and D). Immunohistochemical staining for SV 40 large T antigen showed positivity in tubular nuclei. There were no specific findings on immunofluorescence or electron microscopy.
Figure 1.
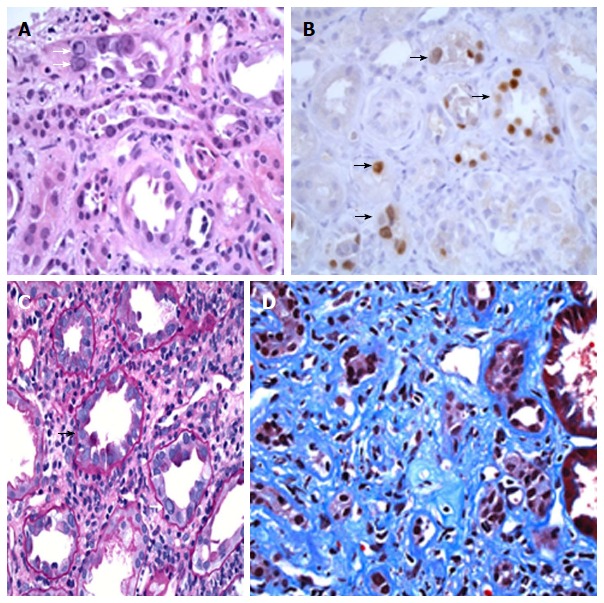
BK nephropathy in the native kidneys of a lung transplant recipient (patient 1 in this report, A and B) and in the native kidneys of a bone marrow transplant recipient (patient 2 in this report, C and D). Kidney biopsy showing BK nephropathy (BKN), taken from a 70-year-old male with a history of lung transplantation for pulmonary fibrosis. A renal biopsy was performed because of significant worsening in renal function over a one-month period. A: Kidney biopsy showing active BK virus infection of renal tubules, with multiple homogeneous-appearing viral nuclear inclusions (white arrows), and features of associated acute tubular injury, including sloughing of tubular cells (H and E stain, 400 × magnification); B: Multiple renal tubules show positive nuclear staining for the SV40 large T antigen by immunoperoxidase staining (black arrows), confirming infection of tubular cells by polyomavirus (400 × magnification); Kidney biopsy from a 30-year-old male with a history of an allogeneic bone-marrow transplantation for aplastic anemia, who developed sequentially post-transplant Epstein-Barr virus associated large B-cell lymphoma, graft vs host disease and progressive elevation of his serum creatinine. This patient died from pneumococcal pneumonia and invasive aspergillosis two months after the diagnosis of BKN; C: Biopsy of renal cortex showing mononuclear tubulitis (black arrow), intranuclear BK virus inclusions (white arrow), and a prominent interstitial chronic inflammatory infiltrate (PAS stain, 400 × magnification); D: Another area of the biopsy shows extensive interstitial fibrosis and tubular atrophy, consistent with late changes secondary to infection (Trichrome stain, 400 × magnification).
The patient’s BKN was treated with ciprofloxacin only because immunosuppression could not be lowered given his graft vs host disease and leflunomide could not be used due to preexisting leukopenia. During treatment with ciprofloxacin renal function and BKV titer continued to worsen. One month after the start of ciprofloxacin treatment, the patient was hospitalized with pneumococcal pneumonia and invasive aspergillosis. He became progressively septic and died one month later.
Patient 2
A 70-year-old Caucasian male with history of pulmonary fibrosis due to usual interstitial pneumonitis underwent a left sided lung transplant. His immunosuppressive regimen included tacrolimus, mycophenolic acid and prednisone. One year following the lung transplant, he suffered unprovoked pulmonary embolism and has remained on anticoagulation with warfarin since then. Serum creatinine levels were stable at 1.0-1.1 mg/dL until two years following the lung transplant when they began to rise. BK viremia was detected and mycophenolic acid was discontinued. However, renal function continued to decline and serum creatinine reached 3.0 mg/dL. His blood tacrolimus levels were between 5 and 8 ng/mL.
Urine microscopy was bland. Renal ultrasonogram demonstrated normal sized kidneys with multiple bilateral simple cysts. Serum BKV level was 10 million copies/mL. Renal biopsy showed active BKN, with visible viral inclusions, positive tubular nuclear staining for SV-40 large T antigen, and associated tubular cell injury/necrosis and mainly mononuclear tubulitis (Figure 1A and B). There was moderately severe interstitial fibrosis and tubular atrophy (about 40%-45%) and global glomerulosclerosis (13%). There were no specific findings on immunofluorescence microscopy.
Following the renal biopsy, administration of tacrolimus and prednisone was continued and Leflonomide was started at a dose of 10 mg daily and was slowly titrated up to 20 mg daily two months later. He also received three montly doses of intravenous immunoglobulin (IVIG) at a dose of 1 g/kg. However, despite improved BK viral titers (from 10 million to 3.5 million copies/mL), his serum creatinine continues to range between 2.8 and 3.0 mg/dL.
GENERAL CONCEPTS OF BKV INFECTION IN PATIENTS WITH ORGAN TRANSPLANTS
Evolution of BKN in kidney transplant recipients[12-22]
An early study by Hogan et al[12] detected polyomavirus excretion in the urine in 20% of renal transplant recipients. Approximately equal numbers of patients with viruria excreted JCV and BKV. The same study reported a tendency to more frequent complications related to the renal graft in patients with documented viral replication[12]. Subsequently, Rosen et al[13] described the development of tubulointerstitial nephritis secondary to BKV in a 6-year-old boy with a renal transplant. A few years later Randhawa et al[14] calculated that the incidence of BKN in renal transplant recipients was as high as 5%. Shinohara et al[15], using a BKV-specific antibody, found that the virus was localized in renal calyces, renal pelvis, ureter and the urinary bladder. These findings are consistent with the clinical manifestations of BKV infection in the urinary tract.
Hirsch et al[16] reported associations of BKN with high BK viral loads in the plasma of renal transplant recipients and with treatment for rejection, particularly with corticosteroids. Addionally, Hariharan[17] computed a high incidence (between 30% and 60%) of irreversible renal transplant failure in patients with BKN. Bohl et al[18] stressed the association between potent immunosuppression and BKN in renal transplant recipients and the need for screening for early detection and prevention of BKN.
In an analysis of a large cohort of renal transplant recipients reported to the Organ Procurement Transplant Network national registry of the United Sates, Dharnidharka et al[19] found an increasing Kaplan-Maier incidence of BKN with time and a higher risk of BKN with immunosuppressive regimens that included rabbit antithymocyte globulin and tacrolimus/mycophenolate combinations. Subsequently, the same group[20] stressed the risks of over-immunosuppression in respect to BKV infection and the lack of optimal methods for treating BKN. Nickeleit et al[21] reviewed recent developments in the diagnosis of BKN, including noninvasive diagnostic procedures, and the expanding role of polyomaviruses in oncogenesis in patients with organ transplants. Sawinski et al[22] identified male gender, advanced age of the recipient, previous rejection episodes, severity of leukocyte antigen mismatching, long cold ischemia time, serology for BKV and ureteral stent placement as additional risk factors for BKN.
Evolution of the concepts of BKN in native kidneys and of other manifestations of BKV infection[2,23-37]
The manifestations of BKV infection from the urinary tract may differ between transplanted organ recipients. BKN and ureteral stenosis were identified as the cardinal manifestations of BKV infection in kidney transplant recipients and hemorrhagic cystitis was recognized as a cardinal manifestation of BKV infection in recipients of bone marrow transplants[23-25]. The documented sites of BKV-associated disease include the urinary system, the lungs, the eyes, the brain, the retinae and the blood vessels[24].
Rates of BK viruria and viremia in recipients of organ transplants were reported from several geographical sites. In a study from Madrid[26], viruria was detected in 26.5% of kidney transplant recipients, 25.5% of heart recipients and 7.8% of liver recipients, while viremia was found in 12.2% of kidney recipients and 7.0% of heart transplant recipients. In Pittsburgh, BK viruria was detected in 8.7% of non-immunosuppressed controls, 34.9% of renal transplant recipients and 15.9% of liver transplant recipients, while BK viremia was detected only in renal transplant recipients (7.7%)[27]. In the same study, the BK viral load in urine was higher in the kidney transplant patients than in the liver transplant recipients or control patients; interestingly, JC viruria was observed in 34.7% of controls, 22.3% of renal transplant patients and 22.7% of liver transplant recipients. JC viremia was not detected in any group.
In a study from Mayo Clinic, Rochester, Minnesota and University of Toronto, Ontario[28], combined BK and JC viremia was found in 26% of kidney transplant patients, 7% of heart transplant patients and 4% of liver transplant recipients. In the same study, BK viremia was associated in certain instances with renal transplant rejection. A study combining findings from Philadelphia, Pennsylvania, and Seattle, Washington[29], found a 15% incidence of BK viruria in 34 recipients of lung, liver, heart, and heart-lung transplants with chronic renal dysfunction. In contrast, a study from Alberta, Edmonton[32], which also found an incidence of BK viruria in recipients of heart, liver or lung transplants, reported no association between renal dysfunction and BK viruria.
Sharma et al[34] presented the histological features of BKN, combined in one case with focal medullary JC viral co-infection, in patients with hematologic malignancies, with and without bone marrow transplants, or lung transplants. Several reviews[2,7,30,31,33,35,36] addressed the manifestations and pathogenesis of BKV infection. Finally, a recent systematic review[37] analyzed a large number of studies of BKV infection in non-renal solid organ transplant recipients. This study concluded that BK viremia was lower in non-renal than in renal transplant recipients and that BKN is rare in non-renal transplant recipients. In non-renal organ transplant recipients, overall incidence of BK viruria was 20% and incidence of BK viremia was 3%, with the highest incidence of BK viremia and BKN found in heart transplant recipients[37].
URINARY MANIFESTATIONS OF BKV INFECTION IN PATIENTS WITH BONE MARROW OR STEM CELL TRANSPLANTS AND SOLID TRANSPLANTS OTHER THAN KIDNEY
Table 1 shows the reported organ transplants, other than solitary kidney transplants, in which clinical manifestations of BKV infection have been described. An extensive list of references is attached to each transplanted organ with BKV infection indicating the rising interest in this topic.
Table 1.
BK infection studies in organ transplants other than solitary kidney transplants
| Transplanted organ | Ref. |
| Bone marrow stem cells | [2,5,8,39-81] |
| Heart | [11,82-96] |
| Lung | [97-102] |
| Liver | [103-113] |
| Pancreas, combined pancreas-kidney | [114-135] |
BK viral infections in the urinary system of recipients with bone marrow or stem cell transplants[2,5,8,38-81]
Hemorrhagic cystitis has been a frequent and serious complication of bone marrow transplantation. This condition had been attributed to the use of cyclophosphamide. Arthur et al[38] reported a substantially higher frequency of BK viruria in patients who developed hemorrhagic cystitis compared to those who did not develop hemorrhagic cystitis after bone marrow transplantation. They also identified a temporal association between the development of BK viruria and the onset of hemorrhagic cystitis. Bedi et al[41] concluded that prophylactic treatment with MESNA and forced diuresis directed at cyclophosphamide toxicity failed to prevent hemorrhagic cystitis in patients with BK viruria.
Subsequently, a large number of publications[5,39,41,43-45,47-50,52,53,55-57,59,61,62,66-68,71,75] provided firm evidence linking BKV infection and hemorrhagic cystitis in bone marrow or stem cell recipients and addressed various aspects of this syndrome.
Peinemann et al[45] reported that hemorrhagic cystitis in pediatric bone marrow transplant recipients is characterized by delayed onset, prolonged duration, viral reactivation and use of high doses of the alkylating agent busulfan. Bielorai et al[46] described patients with BKV-induced hemorrhagic cystitis triggered by cytomegalovirus (CMV) reactivation. Giraud et al[57] reported that the frequency of BK viruria and hemorrhagic cystitis was reduced in bone marrow recipients with related donors and in those receiving reduced intensity conditioning for the bone marrow transplant. The data analyzed by Koskevuo et al[71] suggest that BKV hemorrhagic cystitis may result from nosocomial transmission in pediatric bone marrow transplant recipients with very low or undetectable BKV antibodies. These authors raised the issues of infection control and prophylactic use of cidofovir.
Various malignancies and aplastic anemia were frequent underlying diseases leading to bone marrow transplantation in the reports of BKV hemorrhagic cystitis. Hereditary hematological diseases, including thalassemia and sickle cell anemia were reported in a few instances[66]. The severity of BKV hemorrhagic cystitis varies. Patients with life-threatening blood loss from hemorrhagic cystitis require drastic surgical interventions. Sébe et al[48] reported successful treatment of life-threatening blood loss by subtotal cystectomy in patients with BKV hemorrhagic cystitis.
The level of BK viruria[40,54,65,72,74,80] and plasma loads of BKV DNA[76] predict clinical manifestations of BKV infections, including hemorrhagic cystitis. However, BKV infection is not the only, or even the more common, cause of hemorrhagic cystitis[43]. Use of busulfan[44] and adenovirus infection[46] were also identified as other important causes of this entity.
Other manifestations from the urinary system of BKV infection in bone marrow or stem cell recipients include ureteritis with ureteral stenosis[78,80] and BKN[8,11,49,50,51,58,60,63,64,70,75,77,79]. One report[69] reviewed the features of BKN in bone marrow transplant recipients. Table 2 summarizes reported cases of BKN in recipients of bone marrow or stem cell transplant recipients. The majority of subjects developed end-stage renal disease (ESRD) and were placed on dialysis. Mortality was high in this patient sample. De laCruz et al[73] reviewed the clinical manifestations of BKV infection in hematopoietic stem cell transplantation.
Table 2.
BK nephropathy in recipients of bone marrow or stem cell transplants
| Ref. | Gender age | Renal function | Clinical associations |
| [8] | Female 36 yr | ESRD Dialysis | Relapsed Hodgkin’s lymphoma |
| [8] | Female 43 yr | ESRD Dialysis | Acute myelogenous leukemia |
| [11] | Male 47 yr | ESRD Dialysis | Non-Hodgkin’s lymphoma |
| [49] | Male 17 yr | ESRD Dialysis | Myelodysplastic syndrome Severe hemorrhagic cystitis No renal biopsy Death from multi-organ failure |
| [50] | Female 28 yr | ESRD Dialysis | Acute myelogenous leukemia Recurrent CMV reactivation |
| [51] | NR NR | ARF | Underlying disease NR Adenovirus pneumonia Adenovirus nephritis Death |
| [58] | Male 14 yr | Rising SCr | Acute myelogenous leukemia Death from multi-organ failure |
| [60] | Male 10 yr | GFR normalized | Acute myelogenous leukemia No renal biopsy |
| [63] | Male 51 yr | ESRD Dialysis | Myelodysplastic syndrome Hepatorenal syndrome GVHD Death from Pseudomonas sepsis |
| [64] | Male 10 yr | Peak SCr 3.5 mg/dL Scr at 1.7 mg/dL post-treatment | Chronic myelogenous leukemia Adenovirus and bacterial infections Severe GVHD |
| [64] | Male 13 yr | ESRD Declined dialysis | Fanconi’s anemia Gram-positive bacteremias Pulmonary aspergillosis Hyperacute GVHD Death |
| [70] | Female 10 yr | ESRD Dialysis | Recurrent metastatic neuroendocrine tumor Thrombocytopenia, leukopenia, lymphopenia Antineutrophil-antiplatelet antibodies Death from sepsis |
| [75] | Female 10 yr | Peak SCr 1.58 mg/dL SCr at 1.1-1.4 mg/dL post-treatment | Myelodysplastic syndrome Acute GVHD |
| [77] | Male 59 yr | CKD stage 5 not requiring dialysis | Myelodysplastic syndrome |
| [79] | Male 58 yr | Death due to sepsis eGFR stable at 20 at the time of death | Large B cell lymphoma Acute GVHD |
BK nephropathy was manifested at various times post-heart transplantation. Ages reported in this Table are the calculated ages at the time of diagnosis of BK nephropathy. ESRD: End-stage renal disease; ARF: Acute renal failure; SCr: Serum creatinine; GFR: Glomerular filtration rate; GVHD: Graft vs host disease; NR: Not report.
BK viral infections in the urinary system of recipients of heart transplants[11,82-96]
Table 3 summarizes reported cases of BKN in cardiac transplant recipients[11,84,85,86,89,90,91,93,94,96]. Rejection episodes of varying severity and frequency requiring increased immunosuppressive medications were reported in nine patients and ESRD in eight. Six patients underwent dialysis and three patients died. Lorica et al[94] describe two additional pediatric heart transplant recipients with BKN. A three-month-old girl was on peritoneal dialysis at the time of the report while a 3-year-old girl on peritoneal dialysis died from BK encephalomyelitis[94]. Thus, BKN has severe adverse effects on both renal function and overall state of health in cardiac transplant recipients. Persistent detection of the characteristic decoy cells in the urine indicating persistent BKV infection without any evidence of clinical manifestations was reported in one heart transplant recipient[83].
Table 3.
BK Nephropathy in heart transplant recipients
| Ref. | Gender age | Renal function | Clinical associations |
| [11] | Male, 65 yr | ESRD Refused dialysis | No rejection episodes Urothelial transitional cell carcinoma causing bilateral hydronephrosis Death following perforated gastric ulcer |
| [84] | Female 59 yr | SCr 5.0 mg/dL Awaiting dialysis | Three severe rejection episodes early |
| [85] | Male 57 yr | ESRD On dialysis | Repeated rejection episodes |
| [86] | Male 26 yr | ESRD On dialysis | Multiple rejection episodes |
| [89] | Male 54 yr | ESRD Dialysis | Persistent rejection Death from arrhythmia |
| [90] | Male 12 yr | Last SCr 2.0 mg/dL | Cardiomyopathy from chemotherapy for Ewing’s sarcoma One rejection episode |
| [91] | Male 8 yr | ESRD On dialysis | 8 rejection episodes in 1st heart transplant BK nephropathy after 2nd heart transplant |
| [93] | Female 9 yr | Peak SCr 1.9 mg/dL Last SCr 1.2 mg/dL | Rejection episodes not reported Reduction in BK viral load and improvement in renal function after leflunomide was started |
| [94] | Male 14 yr | ESRD Dialysis | Multiple rejection episodes Lymphoproliferative disorder in the 12th year BK nephropathy in the 16th year Death from multiple organ failure |
| [96] | Male 60 yr | ESRD On peritoneal dialysis | One rejection episode |
| [96] | Male 43 yr | eGFR 40 mL/min | Recurrent giant cell myocarditis in the transplanted heart One rejection episode |
BK nephropathy was usually manifested several years post-heart transplantation. Ages reported in this Table are the calculated ages at the time of diagnosis of BK nephropathy. ESRD: End-stage renal disease; SCr: Serum creatinine; eFGR: Estimated glomerular filtration rate.
Puliyanda et al[88] compared the incidence of BK viremia and the risk of BKN in patients with isolated kidney, heart, liver, and combined kidney-heart, kidney-liver, kidney-pancreas and kidney-heart-liver transplant recipients. These authors concluded that the risk of BKN is lower in patients with isolated heart or liver transplants than in those with kidney transplants. High levels of BK viremia were associated with BKN in this study. However, none of the patients with heart transplants exhibited BK viruria and the plasma levels of BKV were low in liver transplant recipients.
Ducharme-Smith et al[95] found BK viruria in approximately one third and BK viremia in only 7% of pediatric heart transplant recipients. One of the viremic patients developed BKN. Multivariate analysis identified history of Epstein-Barr infection as the only predictor of BK viruria in the same study[95]. In another study, Pendse et al[87] found definitive evidence of BK viruria in 13% of the heart transplant recipients but no signs of BKN. These authors proposed that a second organ-specific insult to the kidneys is needed for patients with BK viruria to develop BKN.
BK viral infections in the urinary system of recipients of lung transplants[97-102]
A small number of cases of BKN in lung transplant recipients has been reported[98,101,102]. Pertinent features of these patients are summarized in Table 4. Two of the three patients developed ESRD and were started on dialysis. One of these two patients died. One case of nephropathy secondary to a different polyomavirus (simian virus 40 or SV40) in a 32-year-old man with cystic fibrosis who had received a lung transplant was also reported[97]. This case progressed to ESRD. Another publication reported a case of BK hemorrhagic cystitis in a lung transplant recipient[99].
Table 4.
BK nephropathy in recipients of lung, liver and pancreas transplantation
| Ref. | Gender age | Renal function | Clinical associations |
| Lung | |||
| [98] | Male 40 yr | ESRD On dialysis | Metastatic seminoma treated successfully Three rejection episodes |
| [101] | Female 72 yr | Peak SCr 2.6 mg/dL Last SCr 2.2 mg/dL | Prolonged neutropenia post-transplant No rejection episodes |
| [102] | Male 9 yr | ESRD Dialysis | Collecting duct carcinoma Death from respiratory and cardiac failure |
| Liver | |||
| [112] | Male 59 yr | SCr 1.9 mg/dL at diagnosis | Multiple rejection episodes Follow-up after diagnosis not reported |
| Pancreas | |||
| [114] | Male 54 yr | SCr 2.2 mg/dL At diagnosis | Follow-up after diagnosis not reported |
ESRD: End-stage renal disease; SCr: Serum creatinine.
Thomas et al[100] studied longitudinally the frequency of viruria from three different polyomavirus species (BKV, JCV, SV40) in lung transplant recipients. Viruria, at least in one instance, was found for BKV in 42% of the patients, for JCV in 28% and for SV40 in 7%. Although no definitive evidence of clinical polyomavirus infection was detected in this study, patients with viruria had shorter survival.
BK viral infections in the urinary system of recipients of liver transplants[32,88,103-113]
We found only one reported case of BKN in a liver transplant recipient[112]. This case is summarized in Table 4. One man with combined liver-kidney transplants developed IgA nephropathy in his native kidneys and BKN in the transplanted kidney[110].
Several reports analyzed the frequency of BK viruria and viremia and its relationship with renal disease in liver transplant recipients[32,88,103-109,111,113]. Low frequencies of BK viruria and viruria and low risk of BKN were commonly reported[32,88,103,108]. Salama et al[104] concluded that BKV infection is not associated with a decline in renal function in liver transplant recipients. Rauschenfels et al[105] concluded that hepatotropic viruses, including BKV, do not have a major role in the pathogenesis of biliary atresia, which is the major condition leading to liver transplantation in pediatric populations.
Higher frequencies of BK viruria and viremia and a risk of kidney disease from BKV infection were reported in a few studies of liver transplant patients. Loeches et al[106] reported BK viruria in 21% and BK viremia in 18% of the liver recipients, the last one early after transplant, and concluded that persistent BK viremia may be associated with renal dysfunction. Demir-Onder et al[111] reported similar results. Higher frequency of BK viruria in pediatric than adult liver transplant recipients was described by Brinker et al[107]. Finally, Mitterhoffer et al[109] reported a higher frequency of BK viremia (56%) in prospective liver transplant recipients with preexisting chronic kidney disease than in those with normal kidney function (14%).
BK viral infections in the urinary system of recipients of pancreas and kidney-pancreas transplants[114-135]
We found only one confirmed case of BKN in a recipient of an isolated pancreatic transplant recipient[114]. This case is summarized in Table 4. BKN has been reported in renal transplants of several recipients of combined kidney-pancreas recipients[115,117-120,123-125,128,129,132-135]. The prevalence of BKV replication and BK viruria in those with combined kidney-pancreas transplants was high in several studies[116,126-128]. However, one study[120] reported a low incidence of BKN (2.9%) in recipients of combined kidney-pancreas transplants. CMV viremia may prevent reactivation of BKV in these patients and in recipients of solitary kidney transplants[130].
Preservation of pancreatic function was reported uniformly in recipients of combined kidney-pancreas transplants with BKV infection[115,117,119,120,124,128,129,133]. Preservation of normal kidney transplant function was reported in some studies[129,132-134], while other studies[117-119,123] concluded that BKN was an important cause of significant deterioration of the transplanted kidney function. A multivariate analysis performed by Heilman et al[121] identified BKN and a serum creatinine level at or above 1.6 mg/dL as independent correlates of renal graft fibrosis in kidney-pancreas transplant recipients. A recent study by Schachtner et al[135] concluded that in comparison to recipients of solitary kidney transplants, recipients of combined pancreas-kidney transplants exhibit a higher incidence and severity of BKN.
The diagnosis of BKN in recipients of combined kidney-pancreas transplants is complicated by the potential absence of decoy cells in the urine. Decoy cells are an important diagnostic clue for BKV infection in the urinary tract. High concentrations of pancreatic enzymes in the urine of transplanted patients may degrade these cells[122]. Quantitative nucleic acid testing for BKV may assist in the diagnosis of BKN in these subjects[131]. Kubal et al[125] reported renal transplant nephrectomies and subsequent successful combined kidney-pancreas transplants in two patients who had developed BKN and ESRD in the initial kidney allograft.
In general, BKN in native kidneys of patients with various transplanted organs is substantially less frequent than in transplanted kidneys, but like BKN in transplanted kidneys tends to lead to ESRD and is associated with significant mortality.
BKV INFECTIONS IN OTHER POPULATIONS
BKV infection with various clinical manifestations has been reported more frequently with diagnostic entities either causing immunosuppression or requiring therapeutic immunosuppression than in individuals without an apparent immunosuppressed state. The manifestations of BKV infection in immunosuppressed and non-immunossepressed states are briefly discussed below.
BKV infections in patients with HIV infection have been studied extensively[136-168]. Both BKN[139,147,151,154,155,159,164,166] and hemorrhagic cystitis[157,165] have been reported in HIV patients. A series of studies addressed rates of BK viremia and viruria[136,137,140,141,143,144,162-163,168], the pathogenesis of BKV infection[157,165] and various clinical aspects of this infection[138,140,145,146,149,150,152-154,156,158,160,167] in HIV-positive populations.
A patient under treatment for granulomatosis with polyangiitis developed BK hemorrhagic cystitis[169]. However, in a series of patients with vasculitis associated with ANCA, only those who had received a kidney transplant exhibited BK viremia[170]. Manifestations of BKV infection in patients with systemic lupus erythematosus (SLE) include viruria and viremia, and the presence of decoy cells in the urine of a patient with BK viruria, hemorrhagic cystitis and hemophagocytic syndrome[171,172]. The tendency of experimental animals with BKV infection to form anti-double stranded antibodies (anti-dsDNAs) has led to the hypothesis that BKV infection triggers SLE[171]. Life threatening hemorrhagic cystitis secondary to polyomavirus JC was reported in an adolescent with ataxia-telangiectasia[173].
BKV infection in patients with various malignancies has been a major focus of the literature[174-186]. An early study reported BK viruria in patients receiving chemotherapy for malignancy[174]. BKN has been diagnosed in patients with chronic lymphocytic leukemia[176,177,180,183], acute lymphocytic leukemia[178,180,185] and Hodgkin’s lymphoma[175]. BK cystitis was reported in patients with Hodgkin’s lymphoma[182,184,186]. One other patient with lymphoma[179] developed neurological manifestations of BKV infection.
The potential role of BKV infection in tumorigenesis has received great attention[187-248]. Urothelial malignancies in association with BKV infection were described in several recipients of kidney transplants[200,205,213,214,216,220-223,227,229,233,234,236,238,239,245,247] and one heart transplant recipient[243]. Malignancies in non-transplanted subjects in which BK infection may play a pathogenetic role include bladder carcinoma [201,211], renal cell carcinoma[192,230], prostatic carcinoma[212,217,235,245], Kaposi’s sarcoma[197], neuroblastoma[199], leukemia, non-Hodgkin’s lymphoma[205], colorectal carcinoma[215], gastrointestinal B-cell lymphoma[240], oral squamous cell carcinoma[244], cervical carcinoma[224], breast carcinoma[226] and melanoma[206].
The role of BKV in tumorigenesis has been disputed. Several studies[187,203,208,216,223] failed to find an association of BKV infection with various malignancies and published reviews of the role of BKV in malignancies[202,209,219,231,232,241,247] reflect the current uncertainty about this topic. However, in animal experiments BKV has been shown to play a role in tumorigenesis[190,191,193,195,196,198] and several reports[192,194,199,201,204,209,210,217,218,237,242] have addressed pathogenetic pathways potentially linking BKV infection and tumorigenesis. A commonly discussed mechanism is inactivation of the tumor suppressor proteins p53 and pRB family by the large T antigen T (T-Ag), which is a major antigen of BKV[199,204]. Other proposed pathways of tumorigenesis include the role of BKV as a cofactor in various malignancies[217,237] and BKV integration in the human genome[242].
Finally it is worth noticing that BKV infections with manifestations from the urinary system have been rarely reported in subjects without other systemic diseases. Examples of these infections include a case of BKN, urothelial ulceration and renal pelvic fibrosis with an imaging picture of a renal mass[249] and the association of BK, and to a greater extent JC, viruria with asymptomatic hematuria in a small sample of Koreans[250].
CLINICAL MANIFESTATIONS OF BKV INFECTION OTHER THAN NEPHROPATHY OR HEMORHAGIC CYSTITIS
Table 5 shows clinical manifestations of BKV infection that have been reported so-far. Manifestations other than BKN and hemorrhagic cystitis[15,78,81,145,146,251-287] will be reviewed in this section. In addition to the kidneys and urinary bladder, BKV was detected at autopsy in the epithelial cells of the ureters of a patient with non-Hodgkin’s lymphoma[15]. Ureteral involvement by BKV with various degrees of urinary obstruction was reported in patients with bone marrow or hematopoietic stem cell transplants[78,81,252,255] and renal transplants[251,253,254]. Fatal BK pneumonia was reported in three patients with hematopoietic stem cell transplants[257,259,260] and two patients under treatment for chronic lymphocytic leukemia[258,261]. BKV infection also accounted for 8% of the acute respiratory infections in non-immunocompromized children[256]. BKV infections in non-immunocompromized patients are probably under represented.
Table 5.
Clinical manifestations of BK virus infection
| Uropoietic system |
| Nephropathy |
| Hemorrhagic cystitis |
| Ureteritis - ureteral obstruction |
| Respiratory system |
| Upper respiratory infection |
| Pneumonia |
| Central nervous system |
| Meningoencephalitis |
| Progressive multifocal leukoencephalopathy (probable) |
| Retinae |
| Retinitis |
| Progressive outer retinal necrosis (questionable) |
| Blood vessels |
| Vasculitis |
| Gastrointestinal system |
| Intestinal ulcers |
| Lower gastrointestinal bleeding |
| Hematopoietic system |
| Pancytopenia |
| Neutropenia |
| Hemophagocytic syndrome |
| Polyclonal gammopathy |
| Malignancies |
| Urothelial tumors |
| Various other tumors |
Various neurological syndromes associated with BKV infection have been reported in patients with acquired immune deficiency syndrome (AIDS)[138,142,145,150,152,158,262,269]. In addition to AIDS patients, BK meningoencephalitis has been reported in non-immunocompromized subjects[263,264], in patients with malignancies including chronic lymphocytic leukemia[261], Hodgkin’s lymphoma[266], and in a kidney transplant recipient[273]. Progressive multifocal leukoencephalopathy, also often diagnosed in AIDS patients, has been associated primarily with the JCV[261,265], but has aso been reported in association with BKV infection in one patient[270]. However, this last association needs confirmation[271]. A case of progressive multifocal leukoencephalopathy associated with both JC and BKV infections in a non-immunocompromized patient has also been reported[272]. BK retinitis associated with other manifestations of BKV infection has been reported in AIDS patients[145,146]. Progressive outer retinal necrosis developed in a kidney transplant recipient with BKV and varicella zoster virus in the vitreous fluid[275]. This retinal disease was probably caused by varicella zoster virus. Neurological syndromes associated with BKV infection were analyzed in two reviews[268,274].
Deltoid muscle biopsy in a renal transplant recipient who developed progressive weakness and dyspnea, and died after several episodes of life-threatening arrhythmias revealed BK vasculitis[276]. A detailed description of the glomerular histologic changes in a large study of renal biopsy samples with BKN[277] failed to identify vascular changes. However, in other reports BKV was found to be localized in endothelial cells of both renal arteries and venules[278] and venous thrombosis associated with BKN was diagnosed in a renal allograft by[111] leukocyte imaging[279].
BKV is replicating in salivary glands[280]. High frequency of BKV shedding from the gastrointestinal tract in recipients of hematopoietic stem cell transplants has been reported[65]. Gastrointestinal bleeding associated with bowel lesions putatively caused by BKV infection was reported in a renal transplant recipient[281] and a hematopoietic stem cell transplant recipient[282]. Interestigly, high rates of BK viruria in patients with inflammatory bowel disease have been documented[283]. However, the clinical significance of this finding will require further study.
Pancytopenia or severe neutropenia associated with BKV infection have been found in kidney transplant recipients[284-286]. Hemophagocytic syndrome was diagnosed in one of these patients[286]. Polyclonal gammopathy triggered by BKV infection was reported in a hematopoietic stem cell transplant recipient suffering from B-cell lymphoblastic leukemia[287]. BKV DNA has been isolated in normal hepatic tissue and elevated hepatic enzymes were reported in bone marrow transplant recipients who had BK viruria[24].
Key points of part A
Clinical manifestations of BKV infection have been reported in patients with various immunosuppressed states and small numbers of subjects with apparent absence of immunosuppression. Although BKN is much less frequent in the native kidneys of organ transplant recipients than in transplanted kidneys, it is uniformly associated with poor outcomes. BKV infection causes a variety of clinical manifestations in addition to nephropathy and hemorrhagic cystitis.
PART B DIAGNOSIS OF BKN AND PATHOGENESIS OF BKV INFECTION DIAGNOSIS OF BKN[10,13,14,16,22,277,288-336]
BKN accounts only for a small fraction of the renal dysfunction encountered in transplant recipients. Its diagnostic features have been extensively studied in renal transplant recipients. Risk factors for the development of BKN including certain immunosuppressive agents, such as mycophenolate, and manifestations of BKV infection in the urinary tract, including BKN, ureteral obstruction, lymphocele, bacterial urinary tract infection, hematuria, and elevated serum creatinine levels have been studied in renal transplant populations[22,288]. A study from South Korea[336] identified an accompanying acute rejection episode, in addition to advanced histologic stage of BKN and elevated serum creatinine levels, as factors increasing the risk of renal transplant failure in renal transplant recipients. Reports involving renal transplant recipients constitute the main source of information reviewed in this section.
Renal biopsy constitutes the gold standard for the diagnosis of BKN. Various aspects of the renal biopsy in BKN have been studied[10,13,14,16,291,294,296,298,299,301-303,307,311,312,314,316,322,324,328]. An early report by Rosen et al[13] identified tubulo-interstitial nephritis as the main histologic picture of BKN. Viral replication in the tubular epithelial cells, starting in the renal medulla and extended later to the renal cortex, initiates cytopathic changes in the renal tubules that can be confirmed by immunohistochemistry, in situ hybridization, electron microscopy or polymerase chain reaction (PCR)[291,316].
The Maryland classification of BKN[291,296,298], which is based on the degree of interstitial inflammation and fibrosis, schematically recognizes three histological patterns which are considered to represent successive stages of BKN. The first pattern is characterized by absent or minimal interstitial inflammation and the presence of viral cytopathic changes, including karyomegaly, hyperchromasia, and basophilic nuclear inclusions, in a few tubular cells located primarily in the renal medulla. Cytolytic changes, including cell necrosis, apoptosis, smudged chromatin and hobnail nuclei with desquamation into the tubular lumen and formation of necrotic casts accompany often the cytopathic changes[291].
The second pattern of the Maryland classification is characterized by focal or diffuse clusters of tubules with cytopathic and cytosolic changes plus interstitial collections of inflammatory cells, primarily lymphocytes, with tubulitis and tubulo-interstitial atrophy in some cases. The third pattern is characterized by extensive interstitial fibrosis and tubular atrophy, lymphocytic infiltration and paucity of viral cytopathic changes. The course of renal dysfunction roughly correlates with the histological staging[291].
A key diagnostic feature of BKN is the finding of viral cytopathic changes, which are apparently identical for papovaviruses BKV, JCV and SV40[296]. The nuclei of the infected cells are large and contain a basophilic inclusion that either replaces the chromatin or displaces it to the periphery of the nucleus (Figure 1A and C). The presence of a halo around the BKV inclusion is used to differentiate between BKV infection and CMV infection. The BKV infected cells have larger nuclei in comparison to cytoplasm and no viral inclusions in their cytoplasm. Immunohistochemical staining for SV40 large T antigen (Figure 1B), which cross-reacts with BKV and JCV, identifies the presence of a papovavirus and allows its differentiation from adenovirus, which can also cause nephritis with intranuclear viral inclusions morphologically identical to those of papovaviruses. Transmission electron microscopy of cells infected with papovavirus shows characteristic intranuclear deposits of polyhedral virions with an average diameter of 40 nm and in some cases fibrillary or microtubular inclusions. Electron microscopy may assist in the differentiation of papovavirus virions from those of CMV, adenovirus and herpesvirus[296].
The proposed sequence of events leading to the histological changes of BKN is as follows[296]: Viral infection leads to cell death and disintegration with discharge of virions in the extracellular space. Entrance of virions into adjacent cells leads to spread of the infection. Infected renal tubular cells and virions exfoliate in the urine. If the tubular injury is severe, tubular basement mebranes rupture causing spillage of virions and viral proteins into the blood stream. Severe tubular injury also causes an inflammatory response with influx of tubulo-interstitial B cells, T cells, plasma cells and macrophages (Figure 1C). This histological picture can be confused with acute cellular rejection (ACR) in renal transplant recipients. When it is severe or persistent, the tubular injury leads to tubular atrophy and interstitial fibrosis.
The utility of the Maryland staging of BKN, modified by the American Society of Transplantation, has been successfully tested in clinical practice. In one study, the third pattern was associated with higher serum creatinine levels at presentation and greater renal function deterioration in follow-up measurements than the first or second pattern[299].
The histology of BKN has been reviewed in successive Banff group meetings[307,312,314,328]. The original Banff classification also recognizes three histologic patterns, characteristic of the stages of BKN: (1) an early stage without tubular cell necrosis; (2) a stage of active BKN with tubular cell necrosis (Figure 1A); and (3) a late stage characterized by fibrosis (Figure 1D)[307]. In one study, reasonable agreement between various nephropathologists was reported using this Banff classification[312]. However, another study failed to demonstrate superiority of the Banff staging of BKN over the Maryland classification[314]. The latest Banff group meeting stressed the need for improving the reproducibility of large SV40 T antigen immunostaining, which is proposed as an index of the BKV viral load and a potential predictor of the renal graft outcome in patients with BKN[328]. In situ hybridization may offer an alternative to immunohistochemistry in the diagnosis of BKN[316]. The diagnostic challenges associated with BKN were recently reviewed by Masutani[324].
In renal transplant biopsies with BKN, the presence of peritubular capillary staining for C4d raises the possibility of coexisting antibody-mediated (humoral) rejection. Some biopsies with BKN show staining of tubular basement membranes for C4d, and this finding is correlated with marked viral cytopathic effect[303]. Granular immune complex deposits in the tubular basement membranes[301] and in the subepithelial space of glomerular basement membranes[302] have been described in patients with BKN. In the latter, BKV was identified ultrastructurally in the immune deposits[302]. Glomerulonephritis attributed to BKV infection was found in a few renal transplant recipients[277,321].
The focal lesions of the early stages of BKN may be missed in a renal biopsy[298,324]. Several diagnostic pathways complementing the renal biopsy have been explored. The value of surveillance renal biopsies in early diagnosis of BKN has been discussed in several reports[22,310,313]. BK viruria[22,297,306,308,317,322,332,334] and viremia[22,289,308,309,317,322,323,332,335] provide another tool for the detection of BKN. High levels of viruria or viremia correlate reasonably with the presence of BKN. Cut-off levels for the diagnosis of BKN have been proposed and tested.
Detection in urinary samples of desquamated tubular or urothelial cells with BKV inclusions provide another tool for the diagnosis of BKV infection in the urinary system[291]. The cardinal features of these cells, known as “decoy cells”, because of their similarity to malignant cells, in a Papanicolaou stain include a greatly enlarged nucleus with a basophilic inclusion next to the chromatin producing a ground-glass or gelatinous look. A halo may surround the basophilic inclusion. Decoy cells may also be detected by phase-contrast microscopy[292]. Decoy cells led to the diagnosis of BKV infection in an immunocompetent child with otitis media followed by dysuria[315]. However, decoy cells may be absent from the urine of patients with documented BKN[333]. In one study, the positive predictive value of decoy cells was low, but improved by immunohistochemical staining of the urine for SV40 large T antigen[331]. Negative-stain electron microscopy and semi-quantitative identification of free BKV particles in the urine assists in the identification of patients at high risk of BKN[300]. Genotyping of BKV by an improved PCR method[327] and serologic tests[329] may help in the diagnosis of BKV infection. Ultrasonographic pictures suggesting BKN were recently reported[330].
In renal transplant recipients, the differentiation between ACR and BKN presents difficulties[294]. The histologic picture of tubulo-interstitial nephritis is indistinguishable between the two conditions[319]. Immunophenotyping of the mononuclear cells in the interstitial infiltrates was found to be promising in some studies[304,318], but could not differentiate between ACR and BKN in others[305]. Serial monitoring of donor-specific cell-free DNA in the urine may be a sensitive biomarker of acute kidney injury, but does not allow the distinction between ACR and BKN[320]. Urine analysis methodologies potentially allowing the differentiation of these two conditions are proteomics[325] and characterization of the percentages and absolute numbers of CD4(+) and CD8(+) effector memory T cells[326].
Several other questions related to the diagnosis of BK infection in the urinary tract have been investigated. Immunohistochemical analysis of renal biopsies revealed differences in the inflammatory infiltrate between different BKV strains[290]. Additionally, latent BKV and JCV were found in the urinary tract of immunocompetent subjects in an autopsy study[295]. One review[311] analyzed the diagnosis and pathogenesis of BK cystitis in hematopoietic cell transplant recipients. Another study found a high rate of mutations in the coding region VP-1 of BKV in HIV-infected patients with low CD4(+) counts[330]. The authors of this study postulated that these mutations could affect the clinical manifestations of BKV infection in HIV patients. Whether the diagnosis of BKV infection will require in the future an analysis of the mutations of the virus in various patient groups or individual patients is not clear.
PATHOGENESIS OF BKV INFECTION[10,20,35,126,336-436]
BKV is a small, unenveloped icosahedral DNA virus. Its genome sequence contains three functional regions. The early region encodes the large T antigen (T-Ag) and the small T antigen. These antigens are involved in BKV DNA replication and could be treatment targets. As noted earlier, interaction of T-Ag with p53 is believed to be the main pathway of tumorigenesis by BKV. The late region is responsible for the production of the proteins VP1, VP2 and VP3, the role of which in BKV infection will be examined later. Finally, the non-coding control region controls the expression of the viral genes[423].
The pathogenesis of BKV infection, and specifically of BKN, is a complex process that has not been elucidated completely. Costa et al[10] listed factors related to the patients, the transplanted organs, and the BKV genotypes as determinants of the development of BKN. The first contact with BKV occurs early in childhood. Antibodies against BKV are found in 50% of the subjects by age 3 and in 80%-90% by age 20 years, with decrease in the antibody titers in older age[20]. The incidence of primary infection is similar in immunosuppressed and non-immunosuppressed children[340].
Age older than 50 years is one of the patient-related risk factors for BKN[10]. In non-immunocompromised subjects, the rate of BK viruria is low below the age of 30 years and increases progressively after that age[35]. Older subjects excrete preferentially the BKV viral subtypes I and IV[35]. In a fraction of the subjects the virus persists without clinical manifestations, but in a state of active asymptomatic replication[35]. Organs harboring replicating BKV include the kidneys, the bone marrow and the brain[35]. Persistence of the virus in other tissues, including spleen, normal thyroid glands[429], pancreas[342], and lymphocytes in HIV-positive patients[344], has also been reported. Active BKV disease in various organs is more frequent if another insult to these organs has also occurred. Examples of this sequence include the relatively high frequencies of BKN in kidney transplant recipients and hemorrhagic cystitis in bone marrow or stem cell transplant recipients.
The mode of BKV transmission is not completely understood. Transplacental transmission was described in an early study[337]. Transmission through the transplanted kidneys has also been documented[351,430]. Replication of BKV in salivary glands was found in vitro suggesting oral transmission[367]. After the primary infection the virus remains latent in host tissues and is reactivated when an immunosuppressed state supervenes. Following renal transplantation, reactivation of BKV demonstrated by BK viruria is usually noticed after 3-6 mo while reactivation of JCV occurs as early as five days post transplantation[379]. Early BKV reactivation is associated with viremia[377] and worse transplant function[372].
Circulating BKV is taken up by cells. In experimental animals, endothelial cells in hepatic sinusoids and in the kidney were shown to remove rapidly blood-borne BK and JCV-like particles[409]. Upon contact with the cell membrane BKV is bound to membrane receptors[381]. The identified specific BKV receptors include polysialated ganglioside GT1b and (2,3)N-linked sialic acid[351]. Cellular entry of BKV is through caveolar endocytosis[357,369]. The GT1b receptor, which is involved in caveolar endocytosis[351], could provide a treatment target in the future.
Differences in cellular entry and trafficking exist between various cell types and viral genotypes[392]. The capsid proteins VP2 and VP3 have important roles in the nuclear entry of BKV[414]. BKV genotypes have different potential for pathogenicity[147,351,356,368,380,389]. The family of transforming-growth factors (TNF) plays a role in BKV gene expression[359]. BKV infection activates the TNF receptor system in BKN[432,433]. Monocyte and Th-2 cytokines, including IL-1 RA, IL-3, IL-6 and sIL-6R are elevated in the urine of renal transplant recipients with BK viruria and may be involved in the pathogenesis of BKN[370]. In general, BKV infection of renal tubular epithelial cells leads to activation of cellular genes involved in cell cycle regulation and apoptosis and downregulation of a small number of genes[373].
After entry into the cytoplasm, BKV is transported into the endoplasmic reticulum along the microtubules by a complex mechanism favored by acidic environment[368]. The ER associated degradation (ERAD) pathway, which is responsible for the transfer to the cytosol of ER secretory proteins that have not attained their proper conformation, where they are degraded by proteasomes, is responsible for transfering BKV into the cytosol, followed by entry of these proteins into the nucleus[372]. After entry of BKV into the nucleus, BKV genome release takes place[383]. The Derlin family of the ERAD translocation complex proteins is important for the trafficking of BKV and other polyomaviruses[370]. Proteasome action is also important in BKV trafficking[392].
Several reviews have stressed the role of innate immunity in the pathogenesis of BKV infection and the need to monitor both the BK viral load and the state of immunity in populations prone to BKV infection as the first step in the timely management of this infection[351,395,406]. A recent report reviewed potential preventive and therapeutic approaches for BKV infection related to the mechanisms of innate immunity[433]. Innate immunity compounds that inhibit BKV infection include lactoferrin[349], the antimicrobial defensins alpha-defensin human neutrophil protein 1 (HNP1) and human alpha-defensin 5 (HD5) which were shown in vitro to aggregate BKV virions thus blocking cellular entry[363], and the cellular DNA damage response (DDR) which modulates BKV replication[388,431]. Human leukocyte antigens (HLAs) that are associated with lower risk of BKV infection include HLA-A2, HLA-B44, HLA-DR5[397] and HLA cw7[421]. Expression in BKV-infected cells of p53, binding of which to the BKV large T-Ag is proposed as a mechanism of tumorigenesis, may provide a therapeutic target in the future[353]. In renal tissues, large T-Ag is expressed in decreasing frequency in medullary collecting ducts, distal and proximal convoluted tubules and Bowman’s capsule[350]. Viral replication pathways which could form the basis of therapeutic approaches in the future include agnoprotein, a viral phospho-protein[364], viral microRNA (miRNA)[394,410], and autophagy in host cells[401].
Disruption of adaptive immunity plays a major role in the pathogenesis of BKV infection. Both cellular and humoral aspects of adaptive immunity in BKV infection have been extensively studied. Age affects both the cellular and humoral immune responses to BKV infection[407]. BKV-specific cellular immunity is vital for control of viral replication and prevention of chronic viral disease[383]. Low levels of cytototoxic T cell (CTL) response correlate with high BKV loads and high anti-BKV antibody titers, while a high CTL response correlates with low viral loads and low anti-BKV antibody titers[347]. The finding that viral capsid epitopes of BKV share homology with other polyomaviruses, including JCV and SV40 suggests that infection with one of the viruses could establish cross-immunty against the other viruses via a cellular-immune response[348].
Loss of BKV-specific T cell immunity in the post-transplant period identifies kidney transplant recipients at high risk for BKV infection[427]. In patients with BKV infection, recovery of cellular immune responses to large T-Ag correlates with improvement of BKN[365,384]. However, in one study the percent of activated T cells correlated with the degree of BK viruria[396]. In the same study, patients with decreased renal function exhibited high levels of activated T cells and BK viruria.
Monitoring of both non-virus specific and virus-specific T cell responses in transplant patients has been advocated[405,417]. Monitoring these reponses post-transplantation may have a role in the detection of BKV reactivation[423]. T cells respond to different BKV antigens[419]. The nuclear factor of activated T cells (NFAT) binds to the viral promoter and regulates viral transcription. This factor is involved in a complex regulatory pathway that can affect the course of BKV infection[375]. The genetic variation of BKV strains is limited[425]. In HLA-A*0201 individuals, cytotoxic T-cell lymphocyte (CTL) responses are elicited towards two of the VP1 epitopes, VP1(p44) and VP1(p108)[347]. CTLs directed against VP1(p44) are more abundant than VP1(p108) in healthy individuals, while the opposite is true in kidney transplant patients who present with BKN. This suggests a shift in the epitope immunodominance in the setting of active BKV infection[347]. Flow-cytometry analysis of BKV-specific T cells also showed that VP3 is an important target of cellular immunity[386].
CD4 T cells have a role in BKV clearance[387,412]. Though the pattern of cellular response to BKV antigens has not been fully clarified, it has been discovered that in kidney allograft recipients, VP1-specific interferon-gamma producing T cells were more likely to be CD4+, while CD8+ lymphocytes are more frequently directed against the large T antigen[361]. Stimulation of CD28 in T cells is one of the rejection mechanisms blocked by belatarcept. Subpopulations of human T cells exposed to antigens may be activated by mechanisms different than CD28 and cause rejection resistant to belatarcept. In mice models polyomavirus exposure leads to reduced expression of CD28 in T cells and was proposed as a mechanism of resistant rejection[422]. Activated CD4 T cells upregulate CD30, another cell marker of B and T cells, causing an increase in serum soluble CD30 (sCD30), which plays a role in the pathogenesis of rejection[366]. Levels of sCD30 are associated with BK viremia and may be of use in the management of the immunosuppressive regimen for renal transplant patients as well as a prognostic factor for graft rejection[436].
The role of dendritic cells in antigen presentation to T cells is well known. Dendritic cell deficiency was shown to be a risk factor for reactivation of BKV infection after renal transplantation[382]. A genotypic analysis in renal transplant recipients found that low frequencies of the activating receptor KIR3DS1 are associated with the development of BKV infection and that there appears to be a genetic predisposition for BKN linked to natural killer cells[402].
The interplay between genetics and immunology is reflected in the finding that the NFAT can transcriptionally regulate BKV. During T-cell activation, NFAT translocates to the nucleus where it regulates the expression of various genes[341]. NFAT regulates BKV transcription, while NFAT inhibition with an NFAT inhibitor peptide, 11R-VIVIT, reduces BKV replication[375]. In addition there is growing evidence that epigenetic factors may contribute to the regulation of BKV and its tissue propagation. Viral microRNAs (miRNAs) are playing a crucial role in viral replication. BKV-encoded miRNAs (miR-B1) have been studied in patients with BKN. After BKV infection, miR-B1 levels are significantly increased and these miRNAs suppress T-ag-mediated autoregulation of BKV replication. Thus, miR-B1 offers a potential treatment strategy for controlling BKV infection[410].
In addition to cellular immune response, humoral immunity also plays an important role in BKV infection. Antibodies to various BKV antigens were detected in normal controls and patients suffering from various diseases; patients with urinary bladder carcinoma exhibited the highest frequency and titers of anti-BKV antibodies[338]. HIV patients with BK viruria and JC viruria have a low frequency of antibodies against these two viruses[343]. In renal transplant recipients, BKV-specific IgG levels were in the pre-transplant period lower in those who developed active BKV infection than in those who did not develop BKV infection; the rise in the antibody titers pos-transplant, however, was higher in patients who developed BKV infection[360]. In this last group, antibody titers correlated with the intensity of BKV infection. This suggested that specific antiBKV IgG response is not associated with viral clearance[360]. A prospective study concluded that determination of the serostatus of prospective kidney transplants and recipients allows stratification of the risk for BKV infection post-transplant[398].
Pre-kidney transplant levels of anti-BKV antibodies did not clearly predict post-transplant BK viremia in pediatric renal transplant recipients[374]. However, there is considerable evidence pointing to a link between antibody titers and BKV disease progression in the post-transplant period. Pediatric patients with hemagglutination-inhibition titers < 40 were found to be at greater risk of disease progression, and seronegative recipients were found to be at greater risk of developing BKN if seronegativity was demonstrated by the VP1 enzyme immunoassay[351]. In patients at different stages of BKN, BKV-specific IgG levels were higher in those who had recovered from BKN than in patients with acute infection. Interestingly, the density of plasma cells in the interstitial infiltrates of BKN was found to correlate with the levels of circulating anti-BKV IgM in one study[378]. BKV infection was fatal in a patient with hyper-IgM deficiency. This patient, whose class switching from IgM to IgG was impaired, was not able to produce the protective IgG antibodies against the virus[351]. This case suggests that immunoglobulin response has an important role in controlling BKV infection.
Measurement of the anti-BKV titers is an important tool to detect the onset of viral replication[352]. Further research is needed to determine the extent to which these antibodies can neutralize the virus or its active viral components, though some suggest that there are BKV neutralizing antibodies that target VP1[351,361,378]. In vitro coincubation of BKV with human intravenous IgG preparations caused 90% inhibition of viral DNA after 7 d in culture, a finding consistent with a direct neutralizing mechanism. This suggests a mechanism of protection against viral reactivation in an immunocompetent person by virus-specific antibodies[378].
Other aspects of antibody formation in BKV infection are of importance. In experimental animals, BKV infection induces the formation of anti-double stranded DNA antibodies[362]. This finding has led to the suggestion that BKV is implicated in the pathogenesis of SLE, as noted earlier in this report[171,172]. In a recent report, preemptive reduction of immunosuppression for BK viremia was found to be associated with high incidence of formation of HLA-specific antibodies (dnDSA)[420]. The authors of this report proposed that in order to prevent the consequences of rejection dnDSA levels should be monitored in renal transplant recipients subjected to reduction of immunosuppression for BK viremia.
The effects of interferon on BKV infection have also attracted attention. Exposure of interferon-sensitive cells infected with BKV to high concentrations of interferon resulted in significant reduction of the BKV load in an early study[339]. However administration of interferon to a renal transplant recipient with BK viremia and viruria had no appreciable effect in the same study. In vitro, interferon-γ inhibits the expression of the BKV T-ag and VP1[353]. Polymorphisms in the interferon-γ gene appear to affect the development of BKV infection in Hispanics[408].
A review of subversion mechanisms of several viruses causing kidney disease[354] stressed the role of immunosuppressed state in the pathogenesis of the viral kidney diseases, included BKN. The state of immunosuppression is the major mechanism of BKV reactivation and has been stressed in numerous reports[10,351,372,393,422]. Immunosuppressive medications that may increase the risk of BKV infection include tacrolimus[372,393,403], and mycophenolate[393]. ABO incompatible kidney transplantation is a risk factor for BKV infection[413,415]. Although immunoglobulin preparations inhibited BKV replication in vitro[378] and administration o fintravenous immunoglobulin was found to be effective and safe in treating BK viremia in one study[385], desensitization of ABO and HLA incompatible kidney transplant recipients with IVIG and rituximab was associated with higher incidence of BKV infection[126,391].
Other factors associated with increased risks for BKV infection and BKN include recipient age exceeding 50 years[10], male gender, comorbidities (diabetes mellitus)[10], negative recipient BKV serology prior to transplantation[10], prior rejection episodes[10,424], renal dysfunction[10], large BKV loads[10], deceased donor[403], positive CMV serology in donor and recipient[424], more than one transplant[424] and hypoxia[428]. In allogeneic stem cell transplant recipients, severe graft vs host reaction and oral mucositis are risk factors for BKV reactivation[434]. Mathematical modeling of the immune responses to BKV infection[432] could provide in the future new developments in the prevention and management of this disease.
Key points of part B
Renal biopsy is required for confirmation of the diagnosis and staging of BKN; BK viral loads in blood and urine and the presence of decoy cells in the use assist in the diagnosis of BKV replication; elucidation of the mechanism of BKV entry into cells and nuclei, factors affecting BKV replication and of the roles of cellular ald humoral immunity in BKV infection has the potential of leading to novel prevention and treatment strategies.
PART C TREATMENT OF BKV INFECTION: DISEASES CAUSED BY OTHER PAPOVAVIRUSES TREATMENT AND PROPHYLAXIS OF BKV INFECTION[10,19,20,79,92,385,437-493]
Current practices in the prevention and management of BKV infection are based on information obtained primarily from renal transplant recipients. In this patient group, reduction in immune responses to infection as a result of immunosuppression has been recognized as the universal risk factor for symptomatic BKV infection[10]. A large retrospective study of treatment of BKN in United States renal transplant recipients concluded that the incidence of BKN has been on the rise and is associated with increased risk of graft loss[19]. The same study reported that certain antirejection medications, including rabbit antithymocytic globulin and tacrolimus/mycophenolate combination, are risk factors for BKN.
Reducing the total immunosuppressive dose and converting to medications less prone to be associated with BKV infection has been reported to have beneficial effects on BK viremia and viruria in various renal transplant cohorts[447,451,455,471,480,481,484]. In a study from China, monitoring renal transplant recipients for BK viremia and preemptive reduction of immunosuppression was associated with resolution of the viremia and good graft function over five years of follow-up[481]. Reduction of immunosuppression, with careful monitoring for signs of rejection of the transplanted organ, and discontinuation of immunosuppressives that are associated with higher risk of BKV infection, e.g., mycophenolate, is currently the mainstay of management of BKV infection in transplant recipients.
Prevention and management of BKV infection in vulnerable populations is hampered by the absence of medications specific for papovaviruses. Certain drug classes have demonstrated antiviral properties in vitro and have been tried for preventing or treating BKV infection. The antiviral activity of cidofovir, an acyclic nucleoside phosphonate nucleotide analog, is linked to inhibition of viral DNA polymerases. The drug, which is approved for the treatment of CMV retinitis, was found to inhibit in vitro BKV replication in human cell series[439,483], although one study found modest antiviral activity and low selectivity of this compound[445]. Beneficial effects of cidofovir in transplant recipients with BKV infections, including BKN and hemorrhagic cystitis, have been reported in case reports and case series[438,443,446,473].
Cidofovir is administered parenterally. A review concluded that intravesicular administration of cidofovir is effective in cases of severe hemorrhagic BK cystitis[461]. The use of cidofovir in the management or prevention of BKN is limited by nephrotoxicity, which is the main adverse effect of the drug. Mitochondral changes in renal tubular epithelial cells[458] and renal dysfunction may develop in patients receiving the drug. Hydration prior to the injection and concomitant administration of probenecid reduce the risk of nephrotoxicity. Reduction of the dose of cidofovir without probenecid administration was reported to have beneficial effects on the renal function of a patient with BKN[443]. However, renal dysfunction has led to the discontinuation of the medication in several reports.
The issues raised by cidofovir have led to the search for compounds related to it, but with less toxicity and higher selectivity. A systematic in vitro study found several acyclic nucleoside phosphonates, including cidofovir, with inhibitory activity on BKV replication[459]. Brincidofovir, a compound derived by conjugating cidofovir with a lipid and designed to lead to intracellular release of cidofovir, has antiviral activities against several DNA viruses and was shown in vitro to inhibit BKV replication in human urothelial cells[489]. This compound was recently reported to reduce the viremia and stabilize the renal function without reduction of immunosuppression, which included mycophenolate, in a recipient of allogeneic hematopoietic stem cell transplant with BKN[79]. Despite the stabilization of the renal function, this patient, who had graft vs host disease, died from sepsis six months after the initiation of brincidofovir treatment. Treatment of BKV infection by bricidofovir will need further evaluation.
Leflunomide is a pyrimidine synthesis inhibitor used in the treatment of rheumatoid arthritis and has been shown to inhibit BKV replication in vitro in human tubular epithelial cells[452] and human salivary gland cells[483]. However, only modest antiviral activity and low selectivity of the drug were found in one in vitro study[439], while no antiviral activity of the compound was found in another in vitro study[459]. In case reports and case series, beneficial effects of leflunomide were reported for BK viremia[93,478], BKN[442,444,448] and hemorrhagic cystitis[465] in organ transplant recipients. In resistant cases, administration of cidofovir concomitantly with leflunomide[442] or ciprofloxacin followed by leflunomide[478] had apparent beneficial effects. The side effects of leflunomide include hepatotoxicity and neutropenia. Leflunonide treatment requires monitoring of its active metabolite in the blood to ensure therapeutic levels as well as monitoring of hepatic function tests and hematological parameters. A systematic review did not find any kidney transplant survival benefit by the use of leflunomide or cidofovir[455]. The need for prospective randomized studies was stressed even in studies reporting beneficial effects of leflunomide[465].
Fluoroquinolones inhibit in vitro the DNA topoisomerase of BKV. Levofloxacin and ofloxacin were reported to inhibit BKV replication in human renal tubular epithelial cells in vitro[457]. This effect of this class of antibiotics was criticized because of its low selectivity index[441]. Ciprofloxacin failed to inhibit BKV replication in another in vitro study[483]. Two retrospective studies in renal transplant recipients reported beneficial effects of fluoroquinolones on BKV infection. Reduction of BK viremia followed ciprofloxacin or levofloxacin administration in one study[440] and sequential treatment with ciprofloxacin and leflunomide in another study[478]. However, one retrospective study failed to show any benefit of ciprofloxacin or levofloxacin in the prevention of BKV infection in recipients of allogeneic hematopoietic stem cell transplants[469] and two randomized studies failed to show any effectiveness of levofloxacin in the prevention of BKV infection[472] or the treatment of BK viremia[474] in kidney transplant recipients.
HMG-CoA reductase inhibitors are another class of drugs that has been tried unsuccessfully for the treatment of BKV infection. After the original in vitro observation that pravastatin blocks BKV cellular entry[449], a retrospective multicenter study failed to show any effect of statin doses that maximize their cholesterol-lowering effect on BK viruria or the development of BKN in renal transplant recipients[479]. Intravenous (i.v.) immunoglobulin administration without reduction of the immunosuppression had beneficial effect in a pediatric case of BKN[450] and, in association with reduction of the immunosuppression, was associated with clearing of the BK viremia and good graft survival in a retrospective study of renal transplant recipients[385]. Issues associated with IVIG treatment were discussed in the section on pathogenesis. Following immunoglobulin infusion one kidney transplant recipient developed increase in BK viremia and BKN[464] and a second kidney transplant recipient with BKN developed severe antibody-mediated rejection[468]. A retrospective study found no difference in 1-year renal transplant outcomes between patients with BKN treated with reduction of the immunosuppression alone or with active treatment including administration of IVIG, leflunomide and ciprofloxacin[471]. Plasma exchange, along with intravenous immunoglobulin and cidofovir, has also been used for the treatment of BKV infection in renal transplant recipients[455]. A recent review concluded that reduction of the immunosuppression is the only proven effective treatment of BKN in renal transplant recipients, while cidofovir, leflunomide, fluoroquinolons and i.v. immunoglobulin have not been shown to offer any benefits[480].
The search for immunosuppressive agents lowering the risk of BKV infection has been the topic of several studies. Induction by alemtuzimab was associated with a higher risk of severe rejection and BKN than induction by antithymocytic globulin[487], even though antithymocytic globulin has been recognized as a risk factor for BKN[19]. Beneficial effects on BKV infection were reported with the use of the mTOR inhibitors everolimus[486,493], or sirolimus[488] instead of mycophenolate and tacrolimus in transplant recipients.
One report reviewed the conservative and surgical approaches to BK hemorrhagic cystitis in bone marrow transplant recipients[437]. Hyperhydration is sufficient for mild cases. Severe cases may require blood transfusions, suprapubic catheters, permanent bladder irrigation, or various surgical procedures[437]. Limited experience exists with certain other treatments. Successful combined kidney-liver transplant was reported in a patient with high grade BK viremia, fulminant hepatic failure and loss of his first kidney transplant to BKN[482]. The first kidney transplant was not removed in this case. Administration of the protease inhibitor bortezomib, which is used as a chemotherapeutic agent in multiple myeloma and mantle cell lymphoma, to a patient with severe BKN and plasma cell-rich infiltrates in the renal interstitium was associated with substantial improvement of the renal function and renal histology[491]. Treatment by hyperbaric oxygen was associated with resolution of the hematuria in 94% of a series of patients with BK hemorrhagic cystitis[462].
In a survey of European transplant centers, 66% of the responders stressed the need for new antiviral agents for BKV infection[485]. Agents that have been tested with some promise in experimental animals or in vitro include cyclosporine A[456], gamma interferon[460], two inhibitors of the ATPAse of the large T BKV antigen, bithionol and hexachlorophene[463], the small molecule Retro-2(cycl) which inhibits host retrograde viral trafficking[470], an expression plasmid for the Large BKV T antigen shRNA delivered by virus-like particles[475], gallic acid-based compounds[476] and the anti-malarial artesumate[477]. In a retrospective study in renal transplant recipients with BK viremia, switching the immunosppressive regime to a combination of low-dose cyclosporine plus an mTOR inhibitor was well tolerated and was associated with better short-term graft function than reduction of the immunosuppression alone[466].
The management of BKV infection in transplant recipients is currently based on reduction of the immunosuppression and, in some cases, substitution of mTOR inhibitors for mycophenolate and calcineurin inhibitors. The induction scheme that is best for prevention of BKV infection is not known. Systematic surveillance for BK viremia and viruria[335,400,451,492] will assist in the early detection and could benefit the outcome of BKV infections.
HUMAN DISEASE ASSOCIATED WITH OTHER PAPOVAVIRUSES[2-4,12,27,28,97,107,177,250,262,266,342,344,494-502]
BKV belongs to the Polyomaviridae family of viruses. Similar structure and animal species as natural hosts are the common features of the members of this family. Other human viruses in the same family that have been associated with human disease include the JCV, the Merkel virus and, probably, the SV40. The natural hosts of SV40 are monkeys and its role in human disease is disputed. The role of Polyomaviridae in human disease has been reviewed in several reports[2-4].
The structure of JCV has the closest association with BKV among all the known human Polyomaviridae. A 75% sequence homology between BKV and JCV has been found[500]. JCV infection has been studied extensively. Substantial rates of JC viremia, viruria and persistence in tissues of transplant recipients and other populations, including non-immunosuppressed subjects, have been reported[12,27,28,107,343,345,494,495,499,501]. Renal manifestations associated with JCV infection include a case of nephropathy in a patient with malignancy[177] and decreased renal function in kidney and liver transplant recipients with JC viruria[497]. The pathogenetic role of JCV in HIV-positive patients with progressive multifocal leukoencephalopathy has been established[261,265]. JCV is oncogenic in animal species, including primates. In humans JCV infection has been associated with brain tumors and carcinomas of the gastrointestinal tract, breast and cervix, but this association has not been found universally[496].
Merkel virus is oncogenic in humans. It is linked to Merkel carcinoma, a rare aggressive skin tumor affecting primarily older individuals[498,499]. Nephropathy associated with SV40 infection was reported in a lung transplant recipient[97].
The number of Polyomaviridae diseases attributed to this viral family is expanding. A recent revision of the taxonomy of the family recognized 76 viral species, 13 of which have humans as their natural hosts[502]. In this taxonomy, BKV is listed as human polyomavirus 1, abbreviated as BKVyV, JCV is listed as human polyomavirus 2, abbreviated as JCPyV, and Merkel virus is listed as human polyomavirus 8, abbreviated as MCPyV. No doubt this virus family will have a center stage in organ transplantation and probably in the immunocompromised states in the years to come.
Key points of part C
Reduction of immunosuppression is the first step in the treatment of symptomatic BKV infection; certain classes of anti-rejection medications are less prone to facilitate BKV replication; the clinical usefulness of drugs putatively inhibiting BKV replication is disputed. The toxicities of these drugs are important; the lists of papovaviruses and of human diseases attributed to them are expanding. Papovavirus-related diseases will be a major study topic in the future.
ACKNOWLEDGMENTS
We thank the Research Assistant by Research Service of the Raymond G. Murphy VA Medical Center.
Footnotes
Conflict-of-interest statement: Authors declare no conflicts of interest for this article.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: July 1, 2016
First decision: August 5, 2016
Article in press: September 8, 2016
P- Reviewer: Friedman EA, Marino IR, Puri K, Sureshkumar KK S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:179–188. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 3.Boothpur R, Brennan DC. Human polyoma viruses and disease with emphasis on clinical BK and JC. J Clin Virol. 2010;47:306–312. doi: 10.1016/j.jcv.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang M, Abend JR, Johnson SF, Imperiale MJ. The role of polyomaviruses in human disease. Virology. 2009;384:266–273. doi: 10.1016/j.virol.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41:11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong W, Chandraker A. BK virus nephropathy: a challenging complication in kidney transplant recipients. Nephrology Rounds. 2009;7:2. [Google Scholar]
- 7.Nickeleit V, Mihatsch MJ. Polyomavirus nephropathy in native kidneys and renal allografts: an update on an escalating threat. Transpl Int. 2006;19:960–973. doi: 10.1111/j.1432-2277.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell PH, Swanson K, Josephson MA, Artz AS, Parsad SD, Ramaprasad C, Pursell K, Rich E, Stock W, van Besien K. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant. 2009;15:1038–1048.e1. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiseman AC. Polyomavirus nephropathy: a current perspective and clinical considerations. Am J Kidney Dis. 2009;54:131–142. doi: 10.1053/j.ajkd.2009.01.271. [DOI] [PubMed] [Google Scholar]
- 10.Costa C, Cavallo R. Polyomavirus-associated nephropathy. World J Transplant. 2012;2:84–94. doi: 10.5500/wjt.v2.i6.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limaye AP, Smith KD, Cook L, Groom DA, Hunt NC, Jerome KR, Boeckh M. Polyomavirus nephropathy in native kidneys of non-renal transplant recipients. Am J Transplant. 2005;5:614–620. doi: 10.1046/j.1600-6143.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Hogan TF, Borden EC, McBain JA, Padgett BL, Walker DL. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980;92:373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- 13.Rosen S, Harmon W, Krensky AM, Edelson PJ, Padgett BL, Grinnell BW, Rubino MJ, Walker DL. Tubulo-interstitial nephritis associated with polyomavirus (BK type) infection. N Engl J Med. 1983;308:1192–1196. doi: 10.1056/NEJM198305193082004. [DOI] [PubMed] [Google Scholar]
- 14.Randhawa PS, Demetris AJ. Nephropathy due to polyomavirus type BK. N Engl J Med. 2000;342:1361–1363. doi: 10.1056/NEJM200005043421809. [DOI] [PubMed] [Google Scholar]
- 15.Shinohara T, Matsuda M, Cheng SH, Marshall J, Fujita M, Nagashima K. BK virus infection of the human urinary tract. J Med Virol. 1993;41:301–305. doi: 10.1002/jmv.1890410408. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 17.Hariharan S. BK virus nephritis after renal transplantation. Kidney Int. 2006;69:655–662. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 18.Bohl DL, Brennan DC. BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol. 2007;2 Suppl 1:S36–S46. doi: 10.2215/CJN.00920207. [DOI] [PubMed] [Google Scholar]
- 19.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019–1026. doi: 10.1097/TP.0b013e31819cc383. [DOI] [PubMed] [Google Scholar]
- 20.Dharnidharka VR, Abdulnour HA, Araya CE. The BK virus in renal transplant recipients-review of pathogenesis, diagnosis, and treatment. Pediatr Nephrol. 2011;26:1763–1774. doi: 10.1007/s00467-010-1716-6. [DOI] [PubMed] [Google Scholar]
- 21.Nickeleit V, Singh HK. Polyomaviruses and disease: is there more to know than viremia and viruria? Curr Opin Organ Transplant. 2015;20:348–358. doi: 10.1097/MOT.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30:209–217. doi: 10.1093/ndt/gfu023. [DOI] [PubMed] [Google Scholar]
- 23.Mylonakis E, Goes N, Rubin RH, Cosimi AB, Colvin RB, Fishman JA. BK virus in solid organ transplant recipients: an emerging syndrome. Transplantation. 2001;72:1587–1592. doi: 10.1097/00007890-200111270-00001. [DOI] [PubMed] [Google Scholar]
- 24.Reploeg MD, Storch GA, Clifford DB. Bk virus: a clinical review. Clin Infect Dis. 2001;33:191–202. doi: 10.1086/321813. [DOI] [PubMed] [Google Scholar]
- 25.Pahari A, Rees L. BK virus-associated renal problems--clinical implications. Pediatr Nephrol. 2003;18:743–748. doi: 10.1007/s00467-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz P, Fogeda M, Bouza E, Verde E, Palomo J, Bañares R. Prevalence of BK virus replication among recipients of solid organ transplants. Clin Infect Dis. 2005;41:1720–1725. doi: 10.1086/498118. [DOI] [PubMed] [Google Scholar]
- 27.Randhawa P, Uhrmacher J, Pasculle W, Vats A, Shapiro R, Eghtsead B, Weck K. A comparative study of BK and JC virus infections in organ transplant recipients. J Med Virol. 2005;77:238–243. doi: 10.1002/jmv.20442. [DOI] [PubMed] [Google Scholar]
- 28.Razonable RR, Brown RA, Humar A, Covington E, Alecock E, Paya CV. A longitudinal molecular surveillance study of human polyomavirus viremia in heart, kidney, liver, and pancreas transplant patients. J Infect Dis. 2005;192:1349–1354. doi: 10.1086/466532. [DOI] [PubMed] [Google Scholar]
- 29.Barton TD, Blumberg EA, Doyle A, Ahya VN, Ferrenberg JM, Brozena SC, Limaye AP. A prospective cross-sectional study of BK virus infection in non-renal solid organ transplant recipients with chronic renal dysfunction. Transpl Infect Dis. 2006;8:102–107. doi: 10.1111/j.1399-3062.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 30.Pavlakis M, Haririan A, Klassen DK. BK virus infection after non-renal transplantation. Adv Exp Med Biol. 2006;577:185–189. doi: 10.1007/0-387-32957-9_13. [DOI] [PubMed] [Google Scholar]
- 31.Vilchez RA, Kusne S. Molecular and clinical perspectives of polyomaviruses: emerging evidence of importance in non-kidney transplant populations. Liver Transpl. 2006;12:1457–1463. doi: 10.1002/lt.20915. [DOI] [PubMed] [Google Scholar]
- 32.Doucette KE, Pang XL, Jackson K, Burton I, Carbonneau M, Cockfield S, Preiksaitis JK. Prospective monitoring of BK polyomavirus infection early posttransplantation in nonrenal solid organ transplant recipients. Transplantation. 2008;85:1733–1736. doi: 10.1097/TP.0b013e3181722ead. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldo CH, Tylden GD, Sharma BN. The human polyomavirus BK (BKPyV): virological background and clinical implications. APMIS. 2013;121:728–745. doi: 10.1111/apm.12134. [DOI] [PubMed] [Google Scholar]
- 34.Sharma SG, Nickeleit V, Herlitz LC, de Gonzalez AK, Stokes MB, Singh HK, Markowitz GS, D’Agati VD. BK polyoma virus nephropathy in the native kidney. Nephrol Dial Transplant. 2013;28:620–631. doi: 10.1093/ndt/gfs537. [DOI] [PubMed] [Google Scholar]
- 35.White MK, Gordon J, Khalili K. The rapidly expanding family of human polyomaviruses: recent developments in understanding their life cycle and role in human pathology. PLoS Pathog. 2013;9:e1003206. doi: 10.1371/journal.ppat.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The Impact of Infection on Chronic Allograft Dysfunction and Allograft Survival After Solid Organ Transplantation. Am J Transplant. 2015;15:3024–3040. doi: 10.1111/ajt.13486. [DOI] [PubMed] [Google Scholar]
- 37.Viswesh V, Yost SE, Kaplan B. The prevalence and implications of BK virus replication in non-renal solid organ transplant recipients: A systematic review. Transplant Rev (Orlando) 2015;29:175–180. doi: 10.1016/j.trre.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 39.Apperley JF, Rice SJ, Bishop JA, Chia YC, Krausz T, Gardner SD, Goldman JM. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation. 1987;43:108–112. doi: 10.1097/00007890-198701000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Arthur RR, Shah KV, Charache P, Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988;158:563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- 41.Bedi A, Miller CB, Hanson JL, Goodman S, Ambinder RF, Charache P, Arthur RR, Jones RJ. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol. 1995;13:1103–1109. doi: 10.1200/JCO.1995.13.5.1103. [DOI] [PubMed] [Google Scholar]
- 42.Bogdanovic G, Priftakis P, Taemmeraes B, Gustafsson A, Flaegstad T, Winiarski J, Dalianis T. Primary BK virus (BKV) infection due to possible BKV transmission during bone marrow transplantation is not the major cause of hemorrhagic cystitis in transplanted children. Pediatr Transplant. 1998;2:288–293. [PubMed] [Google Scholar]
- 43.Azzi A, Cesaro S, Laszlo D, Zakrzewska K, Ciappi S, De Santis R, Fanci R, Pesavento G, Calore E, Bosi A. Human polyomavirus BK (BKV) load and haemorrhagic cystitis in bone marrow transplantation patients. J Clin Virol. 1999;14:79–86. doi: 10.1016/s1386-6532(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 44.Seber A, Shu XO, Defor T, Sencer S, Ramsay N. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant. 1999;23:35–40. doi: 10.1038/sj.bmt.1701523. [DOI] [PubMed] [Google Scholar]
- 45.Peinemann F, de Villiers EM, Dörries K, Adams O, Vögeli TA, Burdach S. Clinical course and treatment of haemorrhagic cystitis associated with BK type of human polyomavirus in nine paediatric recipients of allogeneic bone marrow transplants. Eur J Pediatr. 2000;159:182–188. doi: 10.1007/s004310050047. [DOI] [PubMed] [Google Scholar]
- 46.Bielorai B, Shulman LM, Rechavi G, Toren A. CMV reactivation induced BK virus-associated late onset hemorrhagic cystitis after peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;28:613–614. doi: 10.1038/sj.bmt.1703187. [DOI] [PubMed] [Google Scholar]
- 47.Sèbe P, Garderet L, Traxer O, Nouri M, Gluckman E, Gattegno B. Subtotal cystectomy with ileocystoplasty for severe hemorrhagic cystitis after bone marrow transplantation. Urology. 2001;57:168. doi: 10.1016/s0090-4295(00)00882-7. [DOI] [PubMed] [Google Scholar]
- 48.Akiyama H, Kurosu T, Sakashita C, Inoue T, Mori Si K, Tanikawa S, Sakamaki H, Onozawa Y, Chen Q, Zheng H, et al. Adenovirus is a key pathogen in hemorrhagic cystitis associated with bone marrow transplantation. Clin Infect Dis. 2001;32:1325–1330. doi: 10.1086/319992. [DOI] [PubMed] [Google Scholar]
- 49.Iwamoto S, Azuma E, Hori H, Hirayama M, Kobayashi M, Komada Y, Nishimori H, Miyahara M. BK virus-associated fatal renal failure following late-onset hemorrhagic cystitis in an unrelated bone marrow transplantation. Pediatr Hematol Oncol. 2002;19:255–261. doi: 10.1080/08880010252899424. [DOI] [PubMed] [Google Scholar]
- 50.Stracke S, Helmchen U, von Müller L, Bunjes D, Keller F. Polyoma virus-associated interstitial nephritis in a patient with acute myeloic leukaemia and peripheral blood stem cell transplantation. Nephrol Dial Transplant. 2003;18:2431–2433. doi: 10.1093/ndt/gfg361. [DOI] [PubMed] [Google Scholar]
- 51.Bruno B, Zager RA, Boeckh MJ, Gooley TA, Myerson DH, Huang ML, Hackman RC. Adenovirus nephritis in hematopoietic stem-cell transplantation. Transplantation. 2004;77:1049–1057. doi: 10.1097/01.tp.0000122421.71556.71. [DOI] [PubMed] [Google Scholar]
- 52.Erard V, Storer B, Corey L, Nollkamper J, Huang ML, Limaye A, Boeckh M. BK virus infection in hematopoietic stem cell transplant recipients: frequency, risk factors, and association with postengraftment hemorrhagic cystitis. Clin Infect Dis. 2004;39:1861–1865. doi: 10.1086/426140. [DOI] [PubMed] [Google Scholar]
- 53.Gorczynska E, Turkiewicz D, Rybka K, Toporski J, Kalwak K, Dyla A, Szczyra Z, Chybicka A. Incidence, clinical outcome, and management of virus-induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:797–804. doi: 10.1016/j.bbmt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Leung AY, Chan M, Cheng VC, Lie AK, Yuen KY, Kwong YL. Polyoma BK viruria in patients undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:1029–1030. doi: 10.1038/sj.bmt.1704944. [DOI] [PubMed] [Google Scholar]
- 55.Leung AY, Yuen KY, Kwong YL. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant. 2005;36:929–937. doi: 10.1038/sj.bmt.1705139. [DOI] [PubMed] [Google Scholar]
- 56.Fioriti D, Degener AM, Mischitelli M, Videtta M, Arancio A, Sica S, Sora F, Pietropaolo V. BKV infection and hemorrhagic cystitis after allogeneic bone marrow transplant. Int J Immunopathol Pharmacol. 2005;18:309–316. doi: 10.1177/039463200501800213. [DOI] [PubMed] [Google Scholar]
- 57.Giraud G, Bogdanovic G, Priftakis P, Remberger M, Svahn BM, Barkholt L, Ringden O, Winiarski J, Ljungman P, Dalianis T. The incidence of hemorrhagic cystitis and BK-viruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica. 2006;91:401–404. [PubMed] [Google Scholar]
- 58.Shapiro S, Robin M, Espérou H, Devergie A, Rocha V, Garnier F, Gluckman E, Socié G, Ribaud P, Oudot C, et al. Polyomavirus nephropathy in the native kidneys of an unrelated cord blood transplant recipient followed by a disseminated polyomavirus infection. Transplantation. 2006;82:292–293. doi: 10.1097/01.tp.0000226172.68372.f9. [DOI] [PubMed] [Google Scholar]
- 59.Wong AS, Chan KH, Cheng VC, Yuen KY, Kwong YL, Leung AY. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:830–837. doi: 10.1086/511863. [DOI] [PubMed] [Google Scholar]
- 60.Hoefele J, Rüssmann D, Klein B, Weber LT, Führer M. BK virus induced nephritis in a boy with acute myeloid leukaemia undergoing bone marrow transplantation. NDT Plus. 2008;1:336–339. doi: 10.1093/ndtplus/sfn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cesaro S, Facchin C, Tridello G, Messina C, Calore E, Biasolo MA, Pillon M, Varotto S, Brugiolo A, Mengoli C, et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:363–370. doi: 10.1038/sj.bmt.1705909. [DOI] [PubMed] [Google Scholar]
- 62.Federoff A. BK virus in hematopoietic stem cell transplantation recipients. Clin J Oncol Nurs. 2008;12:895–900. doi: 10.1188/08.CJON.895-900. [DOI] [PubMed] [Google Scholar]
- 63.Lekakis LJ, Macrinici V, Baraboutis IG, Mitchell B, Howard DS. BK virus nephropathy after allogeneic stem cell transplantation: a case report and literature review. Am J Hematol. 2009;84:243–246. doi: 10.1002/ajh.21358. [DOI] [PubMed] [Google Scholar]
- 64.Verghese PS, Finn LS, Englund JA, Sanders JE, Hingorani SR. BK nephropathy in pediatric hematopoietic stem cell transplant recipients. Pediatr Transplant. 2009;13:913–918. doi: 10.1111/j.1399-3046.2008.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong AS, Cheng VC, Yuen KY, Kwong YL, Leung AY. High frequency of polyoma BK virus shedding in the gastrointestinal tract after hematopoietic stem cell transplantation: a prospective and quantitative analysis. Bone Marrow Transplant. 2009;43:43–47. doi: 10.1038/bmt.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaziev J, Paba P, Miano R, Germani S, Sodani P, Bove P, Perno CF, Marziali M, Gallucci C, Isgrò A, et al. Late-onset hemorrhagic cystitis in children after hematopoietic stem cell transplantation for thalassemia and sickle cell anemia: a prospective evaluation of polyoma (BK) virus infection and treatment with cidofovir. Biol Blood Marrow Transplant. 2010;16:662–671. doi: 10.1016/j.bbmt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, Han XY, Tarrand J, Ribeiro R, Gulbis A, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–1190. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haines HL, Laskin BL, Goebel J, Davies SM, Yin HJ, Lawrence J, Mehta PA, Bleesing JJ, Filipovich AH, Marsh RA, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1512–1519. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 69.Raval M, Gulbis A, Bollard C, Leen A, Chemaly R, Shpall E, Lahoti A, Kebriaei P. Evaluation and management of BK virus-associated nephropathy following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1589–1593. doi: 10.1016/j.bbmt.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez-Pinto LN, Laskin BL, Jodele S, Hummel TR, Yin HJ, Goebel J. BK virus nephropathy in a pediatric autologous stem-cell transplant recipient. Pediatr Blood Cancer. 2011;56:495–497. doi: 10.1002/pbc.22860. [DOI] [PubMed] [Google Scholar]
- 71.Koskenvuo M, Dumoulin A, Lautenschlager I, Auvinen E, Mannonen L, Anttila VJ, Jahnukainen K, Saarinen-Pihkala UM, Hirsch HH. BK polyomavirus-associated hemorrhagic cystitis among pediatric allogeneic bone marrow transplant recipients: treatment response and evidence for nosocomial transmission. J Clin Virol. 2013;56:77–81. doi: 10.1016/j.jcv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Oshrine B, Bunin N, Li Y, Furth S, Laskin BL. Kidney and bladder outcomes in children with hemorrhagic cystitis and BK virus infection after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1702–1707. doi: 10.1016/j.bbmt.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.delaCruz J, Pursell K. BK Virus and Its Role in Hematopoietic Stem Cell Transplantation: Evolution of a Pathogen. Curr Infect Dis Rep. 2014;16:417. doi: 10.1007/s11908-014-0417-x. [DOI] [PubMed] [Google Scholar]
- 74.Abudayyeh A, Hamdi A, Lin H, Abdelrahim M, Rondon G, Andersson BS, Afrough A, Martinez CS, Tarrand JJ, Kontoyiannis DP, et al. Symptomatic BK Virus Infection Is Associated With Kidney Function Decline and Poor Overall Survival in Allogeneic Hematopoietic Stem Cell Recipients. Am J Transplant. 2016;16:1492–1502. doi: 10.1111/ajt.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aksenova M, Tsetlina V, Gutovskaya E, Mitrofanova A, Balashov D, Maschan A. BK virus nephropathy in a pediatric patient after hematopoietic stem cell transplantation. Pediatr Transplant. 2015;19:E29–E32. doi: 10.1111/petr.12411. [DOI] [PubMed] [Google Scholar]
- 76.Cesaro S, Tridello G, Pillon M, Calore E, Abate D, Tumino M, Carucci N, Varotto S, Cannata E, Pegoraro A, et al. A Prospective Study on the Predictive Value of Plasma BK Virus-DNA Load for Hemorrhagic Cystitis in Pediatric Patients After Stem Cell Transplantation. J Pediatric Infect Dis Soc. 2015;4:134–142. doi: 10.1093/jpids/piu043. [DOI] [PubMed] [Google Scholar]
- 77.Gagneux-Brunon A, Pillet S, Laurent B, Mariat C, Michalet M, Lucht F, Botelho-Nevers E. A case of BK virus nephropathy in a stem cell transplant recipient: a rare or under-recognized cause for Acute Kidney injury. Med Mal Infect. 2015;45:331–334. doi: 10.1016/j.medmal.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Haab AC, Keller IS, Padevit C, John H. BK virus associated pronounced hemorrhagic cystoureteritis after bone marrow transplantation. Can J Urol. 2015;22:8009–8011. [PubMed] [Google Scholar]
- 79.Papanicolaou GA, Lee YJ, Young JW, Seshan SV, Boruchov AM, Chittick G, Momméja-Marin H, Glezerman IG. Brincidofovir for polyomavirus-associated nephropathy after allogeneic hematopoietic stem cell transplantation. Am J Kidney Dis. 2015;65:780–784. doi: 10.1053/j.ajkd.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laskin BL, Singh HK, Beier UH, Moatz T, Furth SL, Bunin N, Witte D, Goebel J, Davies SM, Dandoy C, et al. The Noninvasive Urinary Polyomavirus Haufen Test Predicts BK Virus Nephropathy in Children After Hematopoietic Cell Transplantation: A Pilot Study. Transplantation. 2016 doi: 10.1097/TP.0000000000001085. Feb 18; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nigo M, Marin D, Mulanovich VE. The first case of acute unilateral pan-ureteritis caused by BK polyomavirus in an allogeneic stem cell transplant patient. Transpl Infect Dis. 2016;18:257–260. doi: 10.1111/tid.12504. [DOI] [PubMed] [Google Scholar]
- 82.Hirata Y, Katayama Y, Nakatani T. [A case of BK virus infection after heart transplantation] Kansenshogaku Zasshi. 1997;71:87–88. doi: 10.11150/kansenshogakuzasshi1970.71.87. [DOI] [PubMed] [Google Scholar]
- 83.Masuda K, Akutagawa K, Yutani C, Kishita H, Ishibashi-Ueda H, Imakita M. Persistent infection with human polyomavirus revealed by urinary cytology in a patient with heart transplantation. A case report. Acta Cytol. 1998;42:803–806. doi: 10.1159/000331852. [DOI] [PubMed] [Google Scholar]
- 84.Menahem SA, McDougall KM, Thomson NM, Dowling JP. Native kidney BK nephropathy post cardiac transplantation. Transplantation. 2005;79:259–260. doi: 10.1097/01.tp.0000145057.41418.22. [DOI] [PubMed] [Google Scholar]
- 85.Schmid H, Burg M, Kretzler M, Banas B, Gröne HJ, Kliem V. BK virus associated nephropathy in native kidneys of a heart allograft recipient. Am J Transplant. 2005;5:1562–1568. doi: 10.1111/j.1600-6143.2005.00883.x. [DOI] [PubMed] [Google Scholar]
- 86.Barber CE, Hewlett TJ, Geldenhuys L, Kiberd BA, Acott PD, Hatchette TF. BK virus nephropathy in a heart transplant recipient: case report and review of the literature. Transpl Infect Dis. 2006;8:113–121. doi: 10.1111/j.1399-3062.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- 87.Pendse SS, Vadivel N, Ramos E, Mudge GH, Von Visger T, Fang JC, Chandraker A. BK viral reactivation in cardiac transplant patients: evidence for a double-hit hypothesis. J Heart Lung Transplant. 2006;25:814–819. doi: 10.1016/j.healun.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Puliyanda DP, Amet N, Dhawan A, Hilo L, Radha RK, Bunnapradist S, Czer L, Martin P, Jordan S, Toyoda M. Isolated heart and liver transplant recipients are at low risk for polyomavirus BKV nephropathy. Clin Transplant. 2006;20:289–294. doi: 10.1111/j.1399-0012.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 89.Maddirala S, Pitha JV, Cowley BD, Haragsim L. End-stage renal disease due to polyomavirus in a cardiac transplant patient. Nat Clin Pract Nephrol. 2007;3:393–396. doi: 10.1038/ncpneph0512. [DOI] [PubMed] [Google Scholar]
- 90.Ali FN, Meehan SM, Pahl E, Cohn RA. Native BK viral nephropathy in a pediatric heart transplant recipient. Pediatr Transplant. 2010;14:E38–E41. doi: 10.1111/j.1399-3046.2008.01127.x. [DOI] [PubMed] [Google Scholar]
- 91.Sahney S, Yorgin P, Zuppan C, Cutler D, Kambham N, Chinnock R. BK virus nephropathy in the native kidneys of a pediatric heart transplant recipient. Pediatr Transplant. 2010;14:E11–E15. doi: 10.1111/j.1399-3046.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 92.Loeches B, Valerio M, Palomo J, Bouza E, Muñoz P. BK virus in heart transplant recipients: a prospective study. J Heart Lung Transplant. 2011;30:109–111. doi: 10.1016/j.healun.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 93.Butts RJ, Uber WE, Savage AJ. Treatment of BK viremia in a pediatric heart transplant recipient. J Heart Lung Transplant. 2012;31:552–553. doi: 10.1016/j.healun.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lorica C, Bueno TG, Garcia-Buitrago MT, Rusconi P, Gonzalez IA. BK virus nephropathy in a pediatric heart transplant recipient with post-transplant lymphoproliferative disorder: a case report and review of literature. Pediatr Transplant. 2013;17:E55–E61. doi: 10.1111/petr.12033. [DOI] [PubMed] [Google Scholar]
- 95.Ducharme-Smith A, Katz BZ, Bobrowski AE, Backer CL, Rychlik K, Pahl E. Prevalence of BK polyomavirus infection and association with renal dysfunction in pediatric heart transplant recipients. J Heart Lung Transplant. 2015;34:222–226. doi: 10.1016/j.healun.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 96.Joseph A, Pilichowska M, Boucher H, Kiernan M, DeNofrio D, Inker LA. BK Virus Nephropathy in Heart Transplant Recipients. Am J Kidney Dis. 2015;65:949–955. doi: 10.1053/j.ajkd.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 97.Milstone A, Vilchez RA, Geiger X, Fogo AB, Butel JS, Dummer S. Polyomavirus simian virus 40 infection associated with nephropathy in a lung-transplant recipient. Transplantation. 2004;77:1019–1024. doi: 10.1097/01.tp.0000119156.52197.ca. [DOI] [PubMed] [Google Scholar]
- 98.Schwarz A, Mengel M, Haller H, Niedermeyer J. Polyoma virus nephropathy in native kidneys after lung transplantation. Am J Transplant. 2005;5:2582–2585. doi: 10.1111/j.1600-6143.2005.01043.x. [DOI] [PubMed] [Google Scholar]
- 99.Elidemir O, Chang IF, Schecter MG, Mallory GB. BK virus-associated hemorrhagic cystitis in a pediatric lung transplant recipient. Pediatr Transplant. 2007;11:807–810. doi: 10.1111/j.1399-3046.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 100.Thomas LD, Milstone AP, Vilchez RA, Zanwar P, Butel JS, Dummer JS. Polyomavirus infection and its impact on renal function and long-term outcomes after lung transplantation. Transplantation. 2009;88:360–366. doi: 10.1097/TP.0b013e3181ae5ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Egli A, Helmersen DS, Taub K, Hirsch HH, Johnson A. Renal failure five years after lung transplantation due to polyomavirus BK-associated nephropathy. Am J Transplant. 2010;10:2324–2330. doi: 10.1111/j.1600-6143.2010.03265.x. [DOI] [PubMed] [Google Scholar]
- 102.Dufek S, Haitel A, Müller-Sacherer T, Aufricht C. Duct Bellini carcinoma in association with BK virus nephropathy after lung transplantation. J Heart Lung Transplant. 2013;32:378–379. doi: 10.1016/j.healun.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 103.Splendiani G, Cipriani S, Condò S, Paba P, Ciotti M, Favalli C, Vega A, Dominijanni S, Casciani CU. Polyoma virus BK and renal dysfunction in a transplanted population. Transplant Proc. 2004;36:713–715. doi: 10.1016/j.transproceed.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 104.Salama M, Boudville N, Speers D, Jeffrey GP, Ferrari P. Decline in native kidney function in liver transplant recipients is not associated with BK virus infection. Liver Transpl. 2008;14:1787–1792. doi: 10.1002/lt.21627. [DOI] [PubMed] [Google Scholar]
- 105.Rauschenfels S, Krassmann M, Al-Masri AN, Verhagen W, Leonhardt J, Kuebler JF, Petersen C. Incidence of hepatotropic viruses in biliary atresia. Eur J Pediatr. 2009;168:469–476. doi: 10.1007/s00431-008-0774-2. [DOI] [PubMed] [Google Scholar]
- 106.Loeches B, Valerio M, Pérez M, Bañares R, Ledesma J, Fogeda M, Salcedo M, Rincón D, Bouza E, Muñoz P. BK virus in liver transplant recipients: a prospective study. Transplant Proc. 2009;41:1033–1037. doi: 10.1016/j.transproceed.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 107.Brinkert F, Briem-Richter A, Ilchmann C, Kemper MJ, Ganschow R. Prevalence of polyomavirus viruria (JC virus/BK virus) in children following liver transplantation. Pediatr Transplant. 2010;14:105–108. doi: 10.1111/j.1399-3046.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- 108.Mitterhofer AP, Tinti F, Mordenti M, Pietropaolo V, Colosimo M, Ginanni Corradini S, Chiarini F, Rossi M, Ferretti G, Brunini F, et al. Polyomavirus BK replication in liver transplant candidates with normal renal function. Transplant Proc. 2011;43:1142–1144. doi: 10.1016/j.transproceed.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 109.Mitterhofer AP, Tinti F, Umbro I, Pietropaolo V, Fiacco F, Bellizzi A, Anzivino E, Ginanni Corradini S, Poli L, Rossi M, et al. Polyomavirus BK infection before liver transplantation in patients with chronic kidney disease. Transplant Proc. 2012;44:1934–1937. doi: 10.1016/j.transproceed.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 110.Ujire MP, Curry MP, Stillman IE, Hanto DW, Mandelbrot DA. A simultaneous liver-kidney transplant recipient with IgA nephropathy limited to native kidneys and BK virus nephropathy limited to the transplant kidney. Am J Kidney Dis. 2013;62:331–334. doi: 10.1053/j.ajkd.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 111.Demir-Onder K, Avkan-Oguz V, Unek T, Sarioglu S, Sagol O, Astarcioglu I. Monitoring the BK virus in liver transplant recipients: a prospective observational study. Exp Clin Transplant. 2014;12:429–436. doi: 10.6002/ect.2013.0224. [DOI] [PubMed] [Google Scholar]
- 112.Zeng Y, Magil A, Hussaini T, Yeung CK, Erb SR, Marquez-Alazagara V, Yoshida EM. First confirmed case of native polyomavirus BK nephropathy in a liver transplant recipient seven years post-transplant. Ann Hepatol. 2015;14:137–140. [PubMed] [Google Scholar]
- 113.Umbro I, Tinti F, Muiesan P, Mitterhofer AP. Different behaviour of BK-virus infection in liver transplant recipients. World J Gastroenterol. 2016;22:1532–1540. doi: 10.3748/wjg.v22.i4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haririan A, Ramos ER, Drachenberg CB, Weir MR, Klassen DK. Polyomavirus nephropathy in native kidneys of a solitary pancreas transplant recipient. Transplantation. 2002;73:1350–1353. doi: 10.1097/00007890-200204270-00030. [DOI] [PubMed] [Google Scholar]
- 115.Isaac J, Shihab FS. De novo C1q nephropathy in the renal allograft of a kidney pancreas transplant recipient: BK virus-induced nephropathy? Nephron. 2002;92:431–436. doi: 10.1159/000063313. [DOI] [PubMed] [Google Scholar]
- 116.Haririan A, Hamze O, Drachenberg CB, Ramos E, Weir MR, Klassen DK. Polyomavirus reactivation in native kidneys of pancreas alone allograft recipients. Transplantation. 2003;75:1186–1190. doi: 10.1097/01.TP.0000061597.09830.49. [DOI] [PubMed] [Google Scholar]
- 117.Lipshutz GS, Mahanty H, Feng S, Hirose R, Stock PG, Kang SM, Posselt AM, Freise CE. BKV in simultaneous pancreas-kidney transplant recipients: a leading cause of renal graft loss in first 2 years post-transplant. Am J Transplant. 2005;5:366–373. doi: 10.1111/j.1600-6143.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 118.Matłosz B, Durlik M, Wesołowska A, Mróz A, Zegarska J, Rowiński W, Szmidt J. Polyoma BK virus reactivation in kidney and pancreas-kidney recipients. Transplant Proc. 2005;37:947–948. doi: 10.1016/j.transproceed.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 119.Duclos AJ, Krishnamurthi V, Lard M, Poggio E, Kleeman M, Winans C, Fatica R, Nurko S. Prevalence and clinical course of BK virus nephropathy in pancreas after kidney transplant patients. Transplant Proc. 2006;38:3666–3672. doi: 10.1016/j.transproceed.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 120.Gupta G, Shapiro R, Thai N, Randhawa PS, Vats A. Low incidence of BK virus nephropathy after simultaneous kidney pancreas transplantation. Transplantation. 2006;82:382–388. doi: 10.1097/01.tp.0000228899.05501.a7. [DOI] [PubMed] [Google Scholar]
- 121.Heilman RL, Chakkera HA, Reddy KS, Colby TV, Moss AA, Williams JW, Mazur MJ, Petrides S, Mulligan DC. Clinical factors associated with graft fibrosis in kidney-transplant recipients on steroid-avoidance immunosuppression. Clin Transplant. 2008;22:309–315. doi: 10.1111/j.1399-0012.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- 122.Lard LR, van der Boog PJ, Veselic M, Vossen AC, de Fijter JW, Groeneveld JH. A pitfall in screening with decoy cells after simultaneous pancreas kidney transplantation. Clin Transplant. 2008;22:833–836. doi: 10.1111/j.1399-0012.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- 123.Ison MG, Parker M, Stosor V, Kaufman DB. Development of BK nephropathy in recipients of simultaneous pancreas-kidney transplantation. Transplantation. 2009;87:525–530. doi: 10.1097/TP.0b013e3181949629. [DOI] [PubMed] [Google Scholar]
- 124.Akpinar E, Ciancio G, Sageshima J, Chen L, Guerra G, Kupin W, Roth D, Ruiz P, Burke G. BK virus nephropathy after simultaneous pancreas-kidney transplantation. Clin Transplant. 2010;24:801–806. doi: 10.1111/j.1399-0012.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 125.Kubal S, Powelson JA, Taber TE, Goble ML, Fridell JA. Simultaneous pancreas and kidney transplantation with concurrent allograft nephrectomy for recipients with prior renal transplants lost to BK virus nephropathy: two case reports. Transplant Proc. 2010;42:2009–2010. doi: 10.1016/j.transproceed.2010.05.089. [DOI] [PubMed] [Google Scholar]
- 126.Kahwaji J, Sinha A, Toyoda M, Ge S, Reinsmoen N, Cao K, Lai CH, Villicana R, Peng A, Jordan S, et al. Infectious complications in kidney-transplant recipients desensitized with rituximab and intravenous immunoglobulin. Clin J Am Soc Nephrol. 2011;6:2894–2900. doi: 10.2215/CJN.03710411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kroth LV, Henkin CS, Peres LD, Paganella MC, Mazzali M, Duval VD, Traesel MA, Saitovitch D. Prevalence of urinary decoy cells and associated risk factors in a Brazilian kidney, pancreas, and kidney-pancreas transplant population. Transplant Proc. 2012;44:2394–2396. doi: 10.1016/j.transproceed.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Mindlova M, Boucek P, Saudek F, Skibova J, Jedinakova T, Lipar K, Adamec M, Hirsch HH. Prevalence and risk factors of polyomavirus BK replication in simultaneous pancreas/kidney transplant recipients from a single transplant center. Clin Transplant. 2012;26:267–274. doi: 10.1111/j.1399-0012.2011.01488.x. [DOI] [PubMed] [Google Scholar]
- 129.Mujtaba M, Fridell J, Sharfuddin A, Kandula P, Yaqub MS, Phillips CL, Mishler D, Taber T. BK virus nephropathy in simultaneous pancreas kidney transplant: a potentially preventable cause of kidney allograft loss. Clin Transplant. 2012;26:E87–E93. doi: 10.1111/j.1399-0012.2012.01599.x. [DOI] [PubMed] [Google Scholar]
- 130.Elfadawy N, Flechner SM, Liu X, Schold J, Srinivas TR, Poggio E, Fatica R, Avery R, Mossad SB. CMV Viremia is associated with a decreased incidence of BKV reactivation after kidney and kidney-pancreas transplantation. Transplantation. 2013;96:1097–1103. doi: 10.1097/TP.0b013e3182a6890d. [DOI] [PubMed] [Google Scholar]
- 131.Myint TM, Turner RM, Craig JC, Cross NB, Kable K, Nankivell BJ, Chapman JR, Webster AC, O’Connell P, Dwyer DE, et al. Test performance characteristics of quantitative nucleic acid testing for polyomaviruses in kidney and kidney-pancreas transplant recipients. Clin Transplant. 2013;27:E571–E579. doi: 10.1111/ctr.12195. [DOI] [PubMed] [Google Scholar]
- 132.Tai DS, Hong J, Busuttil RW, Lipshutz GS. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg. 2013;148:368–373. doi: 10.1001/2013.jamasurg.261. [DOI] [PubMed] [Google Scholar]
- 133.Elfadawy N, Flechner SM, Schold JD, Srinivas TR, Poggio E, Fatica R, Avery R, Mossad SB. Transient versus persistent BK viremia and long-term outcomes after kidney and kidney-pancreas transplantation. Clin J Am Soc Nephrol. 2014;9:553–561. doi: 10.2215/CJN.08420813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cadavid D, Mesa L, Schweineberg J, Posada JG. Polyomavirus-associated nephropathy in renal and simultaneous pancreas-kidney transplant recipients: report of three cases. Saudi J Kidney Dis Transpl. 2015;26:94–97. doi: 10.4103/1319-2442.148750. [DOI] [PubMed] [Google Scholar]
- 135.Schachtner T, Zaks M, Kahl A, Reinke P. Simultaneous pancreas/kidney transplant recipients present with late-onset BK polyomavirus-associated nephropathy. Nephrol Dial Transplant. 2016;31:1174–1182. doi: 10.1093/ndt/gfv441. [DOI] [PubMed] [Google Scholar]
- 136.Flaegstad T, Permin H, Husebekk A, Husby G, Traavik T. BK virus infection in patients with AIDS. Scand J Infect Dis. 1988;20:145–150. doi: 10.3109/00365548809032431. [DOI] [PubMed] [Google Scholar]
- 137.Markowitz RB, Thompson HC, Mueller JF, Cohen JA, Dynan WS. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J Infect Dis. 1993;167:13–20. doi: 10.1093/infdis/167.1.13. [DOI] [PubMed] [Google Scholar]
- 138.Vallbracht A, Löhler J, Gossmann J, Glück T, Petersen D, Gerth HJ, Gencic M, Dörries K. Disseminated BK type polyomavirus infection in an AIDS patient associated with central nervous system disease. Am J Pathol. 1993;143:29–39. [PMC free article] [PubMed] [Google Scholar]
- 139.Gluck TA, Knowles WA, Johnson MA, Brook MG, Pillay D. BK virus-associated haemorrhagic cystitis in an HIV-infected man. AIDS. 1994;8:391–392. doi: 10.1097/00002030-199403000-00019. [DOI] [PubMed] [Google Scholar]
- 140.Sundsfjord A, Flaegstad T, Flø R, Spein AR, Pedersen M, Permin H, Julsrud J, Traavik T. BK and JC viruses in human immunodeficiency virus type 1-infected persons: prevalence, excretion, viremia, and viral regulatory regions. J Infect Dis. 1994;169:485–490. doi: 10.1093/infdis/169.3.485. [DOI] [PubMed] [Google Scholar]
- 141.Agostini HT, Brubaker GR, Shao J, Levin A, Ryschkewitsch CF, Blattner WA, Stoner GL. BK virus and a new type of JC virus excreted by HIV-1 positive patients in rural Tanzania. Arch Virol. 1995;140:1919–1934. doi: 10.1007/BF01322682. [DOI] [PubMed] [Google Scholar]
- 142.Vago L, Cinque P, Sala E, Nebuloni M, Caldarelli R, Racca S, Ferrante P, Trabottoni G, Costanzi G. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:139–146. doi: 10.1097/00042560-199606010-00006. [DOI] [PubMed] [Google Scholar]
- 143.Degener AM, Pietropaolo V, Di Taranto C, Rizzuti V, Ameglio F, Cordiali Fei P, Caprilli F, Capitanio B, Sinibaldi L, Orsi N. Detection of JC and BK viral genome in specimens of HIV-1 infected subjects. New Microbiol. 1997;20:115–122. [PubMed] [Google Scholar]
- 144.Di Taranto C, Pietropaolo V, Orsi GB, Jin L, Sinibaldi L, Degener AM. Detection of BK polyomavirus genotypes in healthy and HIV-positive children. Eur J Epidemiol. 1997;13:653–657. doi: 10.1023/a:1007371320999. [DOI] [PubMed] [Google Scholar]
- 145.Bratt G, Hammarin AL, Grandien M, Hedquist BG, Nennesmo I, Sundelin B, Seregard S. BK virus as the cause of meningoencephalitis, retinitis and nephritis in a patient with AIDS. AIDS. 1999;13:1071–1075. doi: 10.1097/00002030-199906180-00010. [DOI] [PubMed] [Google Scholar]
- 146.Hedquist BG, Bratt G, Hammarin AL, Grandien M, Nennesmo I, Sundelin B, Seregard S. Identification of BK virus in a patient with acquired immune deficiency syndrome and bilateral atypical retinitis. Ophthalmology. 1999;106:129–132. doi: 10.1016/S0161-6420(99)90014-3. [DOI] [PubMed] [Google Scholar]
- 147.Smith RD, Galla JH, Skahan K, Anderson P, Linnemann CC, Ault GS, Ryschkewitsch CF, Stoner GL. Tubulointerstitial nephritis due to a mutant polyomavirus BK virus strain, BKV(Cin), causing end-stage renal disease. J Clin Microbiol. 1998;36:1660–1665. doi: 10.1128/jcm.36.6.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nebuloni M, Tosoni A, Boldorini R, Monga G, Carsana L, Bonetto S, Abeli C, Caldarelli R, Vago L, Costanzi G. BK virus renal infection in a patient with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1999;123:807–811. doi: 10.5858/1999-123-0807-BVRIIA. [DOI] [PubMed] [Google Scholar]
- 149.Cubukcu-Dimopulo O, Greco A, Kumar A, Karluk D, Mittal K, Jagirdar J. BK virus infection in AIDS. Am J Surg Pathol. 2000;24:145–149. doi: 10.1097/00000478-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 150.Lesprit P, Chaline-Lehmann D, Authier FJ, Ponnelle T, Gray F, Levy Y. BK virus encephalitis in a patient with AIDS and lymphoma. AIDS. 2001;15:1196–1199. doi: 10.1097/00002030-200106150-00026. [DOI] [PubMed] [Google Scholar]
- 151.Barouch DH, Faquin WC, Chen Y, Koralnik IJ, Robbins GK, Davis BT. BK virus-associated hemorrhagic cystitis in a Human Immunodeficiency Virus-infected patient. Clin Infect Dis. 2002;35:326–329. doi: 10.1086/341491. [DOI] [PubMed] [Google Scholar]
- 152.Garavelli PL, Boldorini R. BK virus encephalitis in an HIV-seropositive patient. Preliminary data. Recenti Prog Med. 2002;93:247. [PubMed] [Google Scholar]
- 153.Jørgensen GE, Hammarin AL, Bratt G, Grandien M, Flaegstad T, Johnsen JI. Identification of a unique BK virus variant in the CNS of a patient with AIDS. J Med Virol. 2003;70:14–19. doi: 10.1002/jmv.10370. [DOI] [PubMed] [Google Scholar]
- 154.Crum-Cianflone N, Quigley M, Utz G, Hale B. BK virus-associated renal failure among HIV patients. AIDS. 2007;21:1501–1502. doi: 10.1097/QAD.0b013e32823647d4. [DOI] [PubMed] [Google Scholar]
- 155.Sukov WR, Lewin M, Sethi S, Rakowski TA, Lager DJ. BK virus-associated nephropathy in a patient with AIDS. Am J Kidney Dis. 2008;51:e15–e18. doi: 10.1053/j.ajkd.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 156.Waldman M, Marshall V, Whitby D, Kopp JB. Viruses and kidney disease: beyond HIV. Semin Nephrol. 2008;28:595–607. doi: 10.1016/j.semnephrol.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Borissov K, Tsekov I, Gavazova R, Kalvatchev Z, Argirova R. Do human polyoma viruses and human immunodeficiency virus share common co-receptors? J Med Virol. 2010;82:8–13. doi: 10.1002/jmv.21674. [DOI] [PubMed] [Google Scholar]
- 158.Kinnaird AN, Anstead GM. Hemorrhagic cystitis and possible neurologic disease from BK virus infection in a patient with AIDS. Infection. 2010;38:124–127. doi: 10.1007/s15010-009-9201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Manabe M, Yoshii Y, Mukai S, Sakamoto E, Kanashima H, Shirano M, Goto T, Kubo Y, Fukushima H, Inoue T, et al. BK virus-associated nephropathy in an HIV-positive patient with gingival plasmablastic lymphoma. Int J Hematol. 2010;92:208–210. doi: 10.1007/s12185-010-0629-2. [DOI] [PubMed] [Google Scholar]
- 160.Jeffers L, Webster-Cyriaque JY. Viruses and salivary gland disease (SGD): lessons from HIV SGD. Adv Dent Res. 2011;23:79–83. doi: 10.1177/0022034510396882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Machado DM, Fink MC, Pannuti CS, Succi RC, Machado AA, Carmo FB, Gouvêa Ade F, Urbano PR, Beltrão SV, Santos IC, et al. Human polyomaviruses JC and BK in the urine of Brazilian children and adolescents vertically infected by HIV. Mem Inst Oswaldo Cruz. 2011;106:931–935. doi: 10.1590/s0074-02762011000800006. [DOI] [PubMed] [Google Scholar]
- 162.Nali LH, Centrone Cde C, Urbano PR, Penalva-de-Oliveira AC, Vidal JE, Miranda EP, Pannuti CS, Fink MC. High prevalence of the simultaneous excretion of polyomaviruses JC and BK in the urine of HIV-infected patients without neurological symptoms in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2012;54:201–205. doi: 10.1590/s0036-46652012000400004. [DOI] [PubMed] [Google Scholar]
- 163.Ledesma J, Muñoz P, Garcia de Viedma D, Cabrero I, Loeches B, Montilla P, Gijon P, Rodriguez-Sanchez B, Bouza E. BK virus infection in human immunodeficiency virus-infected patients. Eur J Clin Microbiol Infect Dis. 2012;31:1531–1535. doi: 10.1007/s10096-011-1474-9. [DOI] [PubMed] [Google Scholar]
- 164.Ogawa Y, Yoshida M, Kanashima H, Nakao T, Sirano M, Goto T, Fukushima H, Inoue T, Yamane T. [Human immunodeficiency virus-related non-hodgkin lymphoma: a clinical investigation at our hospital] Gan To Kagaku Ryoho. 2013;40:1027–1030. [PubMed] [Google Scholar]
- 165.Rossi F, Li X, Jacobson L, Levine AJ, Chen Y, Palella FJ, Margolick J, Viscidi R. BK virus capsid antibodies are associated with protection against subsequent development of PML in HIV-infected patients. Virology. 2015;485:467–472. doi: 10.1016/j.virol.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jung SW, Sung JY, Park SJ, Jeong KH. BK virus-associated nephropathy with hydronephrosis in a patient with AIDS: a case report and literature review. Clin Nephrol. 2016;85:173–178. doi: 10.5414/CN108482. [DOI] [PubMed] [Google Scholar]
- 167.Karalic D, Lazarevic I, Banko A, Cupic M, Jevtovic D, Jovanovic T. Molecular characterization of BK virus in patients infected with human immunodeficiency virus. Med Microbiol Immunol. 2016;205:185–193. doi: 10.1007/s00430-015-0439-5. [DOI] [PubMed] [Google Scholar]
- 168.Akhgari S, Mohraz M, Azadmanesh K, Vahabpour R, Kazemimanesh M, Aghakhani A, Jozpanahi M, Banifazl M, Bavand A, Ramezani A. Frequency and subtype of BK virus infection in Iranian patients infected with HIV. Med Microbiol Immunol. 2016;205:57–62. doi: 10.1007/s00430-015-0426-x. [DOI] [PubMed] [Google Scholar]
- 169.Amatruda J, Dieckhaus K, Hegde P, Taylor J. Bladder Cancer versus Hemorrhagic Cystitis: A Case of Mistaken Identity in a 34-Year-Old Male Undergoing Therapy for Granulomatosis with Polyangiitis. Case Rep Nephrol Urol. 2014;4:120–125. doi: 10.1159/000363692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Geetha D, Levine SM, Manno RL, Valsamakis A, Ghazarian S, Seo P. BK virus replication in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Am J Nephrol. 2014;39:20–26. doi: 10.1159/000357409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Colla L, Mesiano P, Morellini V, Besso L, Cavallo R, Bergallo M, Costa C, Merlino C, Marcuccio C, Fop F, et al. Human polyomavirus BK in patients with lupus nephritis: clinical and histological correlations. Lupus. 2007;16:881–886. doi: 10.1177/0961203307084169. [DOI] [PubMed] [Google Scholar]
- 172.Gupta N, Lawrence RM, Nguyen C, Modica RF. Review article: BK virus in systemic lupus erythematosus. Pediatr Rheumatol Online J. 2015;13:34. doi: 10.1186/s12969-015-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Christmann M, Heitkamp S, Lambrecht E, Doerries K, Schubert R, Zielen S. Haemorrhagic cystitis and polyomavirus JC infection in ataxia telangiectasia. J Pediatr Urol. 2009;5:324–326. doi: 10.1016/j.jpurol.2009.02.198. [DOI] [PubMed] [Google Scholar]
- 174.Reese JM, Reissing M, Daniel RW, Shah KV. Occurrence of BK virus and BK virus-specific antibodies in the urine of patients receiving chemotherapy for malignancy. Infect Immun. 1975;11:1375–1381. doi: 10.1128/iai.11.6.1375-1381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Silva LM, Bale P, de Courcy J, Brown D, Knowles W. Renal failure due to BK virus infection in an immunodeficient child. J Med Virol. 1995;45:192–196. doi: 10.1002/jmv.1890450214. [DOI] [PubMed] [Google Scholar]
- 176.Boudville N, Latham B, Cordingly F, Warr K. Renal failure in a patient with leukaemic infiltration of the kidney and polyomavirus infection. Nephrol Dial Transplant. 2001;16:1059–1061. doi: 10.1093/ndt/16.5.1059. [DOI] [PubMed] [Google Scholar]
- 177.Fogazzi GB, Furione M, Saglimbeni L, Gatti M, Cantù M, Tarantino A. BK and JC polyomavirus infection in a patient with chronic lymphocytic leukaemia and renal failure. Nephrol Dial Transplant. 2002;17:1534–1536. doi: 10.1093/ndt/17.8.1534. [DOI] [PubMed] [Google Scholar]
- 178.Inaba H, Jones DP, Gaber LW, Shenep JL, Call SK, Pui CH, Razzouk BI. BK virus-induced tubulointerstitial nephritis in a child with acute lymphoblastic leukemia. J Pediatr. 2007;151:215–217. doi: 10.1016/j.jpeds.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ferrari A, Luppi M, Marasca R, Potenza L, Morselli M, Volzone F, Santachiara R, Forghieri F, Barozzi P, Torelli G. BK virus infection and neurologic dysfunctions in a patient with lymphoma treated with chemotherapy and rituximab. Eur J Haematol. 2008;81:244–245. doi: 10.1111/j.1600-0609.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 180.van der Bij A, Betjes M, Weening J, Cornelissen J, Mes T, Osterhaus A, Beersma M. BK virus nephropathy in an immunodeficient patient with chronic lymphocytic leukemia. J Clin Virol. 2009;45:341–344. doi: 10.1016/j.jcv.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 181.Aoki K, Kotani S, Ichinohe T, Kondo T, Ishikawa T. Acute renal failure associated with systemic polyoma BK virus activation in a patient with peripheral T-cell lymphoma. Int J Hematol. 2010;92:638–641. doi: 10.1007/s12185-010-0694-6. [DOI] [PubMed] [Google Scholar]
- 182.Le Calloch R, Ianotto JC, Berthou C, Tempescul A. Hemorrhagic Cystitis due to BK Reactivation in a Young Female Treated for Hodgkin-Disease. Case Rep Hematol. 2011;2011:592470. doi: 10.1155/2011/592470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McCrory R, Gray M, Leonard N, Smyth J, Woodman A. Native kidney BK virus nephropathy associated with chronic lymphocytic leukaemia. Nephrol Dial Transplant. 2012;27:1269–1271. doi: 10.1093/ndt/gfs002. [DOI] [PubMed] [Google Scholar]
- 184.Alavi S, Yazdi MK, Parvin M, Zohrehbandian F, Azma R. Haemorrhagic cystitis due to BK virus in a child with ALL on standard chemotherapy without stem cell transplant. Ecancermedicalscience. 2013;7:350. doi: 10.3332/ecancer.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Filler G, Licht C, Haig A. Native kidney BK virus nephropathy associated with acute lymphocytic leukemia. Pediatr Nephrol. 2013;28:979–981. doi: 10.1007/s00467-013-2438-3. [DOI] [PubMed] [Google Scholar]
- 186.Perram J, Estell J. A Novel Case of Symptomatic BK Viraemia in a Patient Undergoing Treatment for Hodgkin Lymphoma. Case Rep Infect Dis. 2014;2014:909516. doi: 10.1155/2014/909516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fiori M, Di Mayorca G. Occurrence of BK virus DNA in DNA obtained from certain human tumors. Proc Natl Acad Sci USA. 1976;73:4662–4666. doi: 10.1073/pnas.73.12.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pyrhönen S, Mäntyjärvi R, Tykkä H, Sarna S, Tallberg T. BK and Herpes simplex virus antibodies in renal cell carcinoma. Med Biol. 1978;56:194–200. [PubMed] [Google Scholar]
- 189.Shah KV, Daniel RW, Stone KR, Elliott AY. Investigation of human urogenital tract tumors of papovavirus etiology: brief communication. J Natl Cancer Inst. 1978;60:579–582. doi: 10.1093/jnci/60.3.579. [DOI] [PubMed] [Google Scholar]
- 190.Corallini A, Altavilla G, Carra L, Grossi MP, Federspil G, Caputo A, Negrini M, Barbanti-Brodano G. Oncogenity of BK virus for immunosuppressed hamsters. Arch Virol. 1982;73:243–253. doi: 10.1007/BF01318078. [DOI] [PubMed] [Google Scholar]
- 191.Altavilla G, Carrà L, Alberti S, Corallini A, Cavazzini L, Fabris G, Aleotti A, Barbanti-Brodano G. BK virus-induced tumors in hamsters: a morphological, histochemical and ultrastructural study. Oncology. 1983;40:427–441. doi: 10.1159/000225777. [DOI] [PubMed] [Google Scholar]
- 192.Knepper JE, diMayorca G. Cloning and characterization of BK virus-related DNA sequences from normal and neoplastic human tissues. J Med Virol. 1987;21:289–299. doi: 10.1002/jmv.1890210313. [DOI] [PubMed] [Google Scholar]
- 193.Dalrymple SA, Beemon KL. BK virus T antigens induce kidney carcinomas and thymoproliferative disorders in transgenic mice. J Virol. 1990;64:1182–1191. doi: 10.1128/jvi.64.3.1182-1191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Theile M, Grabowski G. Mutagenic activity of BKV and JCV in human and other mammalian cells. Arch Virol. 1990;113:221–233. doi: 10.1007/BF01316675. [DOI] [PubMed] [Google Scholar]
- 195.Verhagen W, Hubert CM, Mohtaschem E. Tumour induction by transplacental infection with polyoma virus of the F1 generation of Wistar rats. Arch Virol. 1993;133:459–465. doi: 10.1007/BF01313783. [DOI] [PubMed] [Google Scholar]
- 196.Corallini A, Campioni D, Rossi C, Albini A, Possati L, Rusnati M, Gazzanelli G, Benelli R, Masiello L, Sparacciari V, et al. Promotion of tumour metastases and induction of angiogenesis by native HIV-1 Tat protein from BK virus/tat transgenic mice. AIDS. 1996;10:701–710. doi: 10.1097/00002030-199606001-00003. [DOI] [PubMed] [Google Scholar]
- 197.Monini P, Rotola A, de Lellis L, Corallini A, Secchiero P, Albini A, Benelli R, Parravicini C, Barbanti-Brodano G, Cassai E. Latent BK virus infection and Kaposi’s sarcoma pathogenesis. Int J Cancer. 1996;66:717–722. doi: 10.1002/(SICI)1097-0215(19960611)66:6<717::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 198.Barbanti-Brodano G, Martini F, De Mattei M, Lazzarin L, Corallini A, Tognon M. BK and JC human polyomaviruses and simian virus 40: natural history of infection in humans, experimental oncogenicity, and association with human tumors. Adv Virus Res. 1998;50:69–99. doi: 10.1016/s0065-3527(08)60806-4. [DOI] [PubMed] [Google Scholar]
- 199.Flaegstad T, Andresen PA, Johnsen JI, Asomani SK, Jørgensen GE, Vignarajan S, Kjuul A, Kogner P, Traavik T. A possible contributory role of BK virus infection in neuroblastoma development. Cancer Res. 1999;59:1160–1163. [PubMed] [Google Scholar]
- 200.Geetha D, Tong BC, Racusen L, Markowitz JS, Westra WH. Bladder carcinoma in a transplant recipient: evidence to implicate the BK human polyomavirus as a causal transforming agent. Transplantation. 2002;73:1933–1936. doi: 10.1097/00007890-200206270-00015. [DOI] [PubMed] [Google Scholar]
- 201.Robles C, Viscidi R, Malats N, Silverman DT, Tardon A, Garcia-Closas R, Serra C, Carrato A, Herranz J, Lloreta J, et al. Bladder cancer and seroreactivity to BK, JC and Merkel cell polyomaviruses: the Spanish bladder cancer study. Int J Cancer. 2013;133:597–603. doi: 10.1002/ijc.28053. [DOI] [PubMed] [Google Scholar]
- 202.Croul S, Otte J, Khalili K. Brain tumors and polyomaviruses. J Neurovirol. 2003;9:173–182. doi: 10.1080/13550280390194055. [DOI] [PubMed] [Google Scholar]
- 203.Knöll A, Stoehr R, Jilg W, Hartmann A. Low frequency of human polyomavirus BKV and JCV DNA in urothelial carcinomas of the renal pelvis and renal cell carcinomas. Oncol Rep. 2003;10:487–491. [PubMed] [Google Scholar]
- 204.Tognon M, Corallini A, Martini F, Negrini M, Barbanti-Brodano G. Oncogenic transformation by BK virus and association with human tumors. Oncogene. 2003;22:5192–5200. doi: 10.1038/sj.onc.1206550. [DOI] [PubMed] [Google Scholar]
- 205.Kausman JY, Somers GR, Francis DM, Jones CL. Association of renal adenocarcinoma and BK virus nephropathy post transplantation. Pediatr Nephrol. 2004;19:459–462. doi: 10.1007/s00467-003-1407-7. [DOI] [PubMed] [Google Scholar]
- 206.Boratyńska M, Rybka K. Malignant melanoma in a patient with polyomavirus-BK-associated nephropathy. Transpl Infect Dis. 2005;7:150–153. doi: 10.1111/j.1399-3062.2005.00109.x. [DOI] [PubMed] [Google Scholar]
- 207.Engels EA, Rollison DE, Hartge P, Baris D, Cerhan JR, Severson RK, Cozen W, Davis S, Biggar RJ, Goedert JJ, et al. Antibodies to JC and BK viruses among persons with non-Hodgkin lymphoma. Int J Cancer. 2005;117:1013–1019. doi: 10.1002/ijc.21277. [DOI] [PubMed] [Google Scholar]
- 208.Newton R, Ribeiro T, Casabonne D, Alvarez E, Touzé A, Key T, Coursaget P. Antibody levels against BK virus and prostate, kidney and bladder cancers in the EPIC-Oxford cohort. Br J Cancer. 2005;93:1305–1306. doi: 10.1038/sj.bjc.6602869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.White MK, Gordon J, Reiss K, Del Valle L, Croul S, Giordano A, Darbinyan A, Khalili K. Human polyomaviruses and brain tumors. Brain Res Brain Res Rev. 2005;50:69–85. doi: 10.1016/j.brainresrev.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 210.Weinreb DB. BK virus and carcinoma of the prostate, kidney and bladder. Br J Cancer. 2006;94:1948; author reply 1949–1950. doi: 10.1038/sj.bjc.6603124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Weinreb DB, Desman GT, Amolat-Apiado MJ, Burstein DE, Godbold JH, Johnson EM. Polyoma virus infection is a prominent risk factor for bladder carcinoma in immunocompetent individuals. Diagn Cytopathol. 2006;34:201–203. doi: 10.1002/dc.20429. [DOI] [PubMed] [Google Scholar]
- 212.Fioriti D, Russo G, Mischitelli M, Anzivino E, Bellizzi A, Di Monaco F, Di Silverio F, Giordano A, Chiarini F, Pietropaolo V. A case of human polyomavirus Bk infection in a patient affected by late stage prostate cancer: could viral infection be correlated with cancer progression? Int J Immunopathol Pharmacol. 2007;20:405–411. doi: 10.1177/039463200702000223. [DOI] [PubMed] [Google Scholar]
- 213.Narayanan M, Szymanski J, Slavcheva E, Rao A, Kelly A, Jones K, Jaffers G. BK virus associated renal cell carcinoma: case presentation with optimized PCR and other diagnostic tests. Am J Transplant. 2007;7:1666–1671. doi: 10.1111/j.1600-6143.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- 214.Emerson LL, Carney HM, Layfield LJ, Sherbotie JR. Collecting duct carcinoma arising in association with BK nephropathy post-transplantation in a pediatric patient. A case report with immunohistochemical and in situ hybridization study. Pediatr Transplant. 2008;12:600–605. doi: 10.1111/j.1399-3046.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 215.Giuliani L, Ronci C, Bonifacio D, Di Bonito L, Favalli C, Perno CF, Syrjänen K, Ciotti M. Detection of oncogenic DNA viruses in colorectal cancer. Anticancer Res. 2008;28:1405–1410. [PubMed] [Google Scholar]
- 216.Roberts IS, Besarani D, Mason P, Turner G, Friend PJ, Newton R. Polyoma virus infection and urothelial carcinoma of the bladder following renal transplantation. Br J Cancer. 2008;99:1383–1386. doi: 10.1038/sj.bjc.6604711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Russo G, Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Giordano A, Autran-Gomez A, Di Monaco F, Di Silverio F, Sale P, et al. p53 gene mutational rate, Gleason score, and BK virus infection in prostate adenocarcinoma: Is there a correlation? J Med Virol. 2008;80:2100–2107. doi: 10.1002/jmv.21312. [DOI] [PubMed] [Google Scholar]
- 218.Vats A. BK virus and neoplasia: an emerging role. Pediatr Transplant. 2008;12:499–502. doi: 10.1111/j.1399-3046.2008.00960.x. [DOI] [PubMed] [Google Scholar]
- 219.Abend JR, Jiang M, Imperiale MJ. BK virus and human cancer: innocent until proven guilty. Semin Cancer Biol. 2009;19:252–260. doi: 10.1016/j.semcancer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hill P, Slavin J, Goodman D. High-grade urothelial carcinoma in a kidney transplant recipient with BK virus infection. NDT Plus. 2009;2:246–249. doi: 10.1093/ndtplus/sfp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang HH, Liu KL, Chu SH, Tian YC, Lai PC, Chiang YJ. BK virus infection in association with posttransplant urothelial carcinoma. Transplant Proc. 2009;41:165–166. doi: 10.1016/j.transproceed.2008.08.138. [DOI] [PubMed] [Google Scholar]
- 222.Chen CH, Wen MC, Wang M, Lian JD, Cheng CH, Wu MJ, Yu TM, Chuang YW, Chang D, Shu KH. High incidence of malignancy in polyomavirus-associated nephropathy in renal transplant recipients. Transplant Proc. 2010;42:817–818. doi: 10.1016/j.transproceed.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 223.Galed-Placed I, Valbuena-Ruvira L. Decoy cells and malignant cells coexisting in the urine from a transplant recipient with BK virus nephropathy and bladder adenocarcinoma. Diagn Cytopathol. 2011;39:933–937. doi: 10.1002/dc.21579. [DOI] [PubMed] [Google Scholar]
- 224.Fraase K, Hart J, Wu H, Pang X, Ma L, Grant F, Li A, Lennon A, Hu PC, Dong J. BK virus as a potential co-factor for HPV in the development of cervical neoplasia. Ann Clin Lab Sci. 2012;42:130–134. [PubMed] [Google Scholar]
- 225.Groom HC, Warren AY, Neal DE, Bishop KN. No evidence for infection of UK prostate cancer patients with XMRV, BK virus, Trichomonas vaginalis or human papilloma viruses. PLoS One. 2012;7:e34221. doi: 10.1371/journal.pone.0034221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hachana M, Amara K, Ziadi S, Gacem RB, Korbi S, Trimeche M. Investigation of human JC and BK polyomaviruses in breast carcinomas. Breast Cancer Res Treat. 2012;133:969–977. doi: 10.1007/s10549-011-1876-5. [DOI] [PubMed] [Google Scholar]
- 227.Neirynck V, Claes K, Naesens M, De Wever L, Pirenne J, Kuypers D, Vanrenterghem Y, Poppel HV, Kabanda A, Lerut E. Renal cell carcinoma in the allograft: what is the role of polyomavirus? Case Rep Nephrol Urol. 2012;2:125–134. doi: 10.1159/000341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Salehipoor M, Khezri A, Behzad-Behbahani A, Geramizadeh B, Rahsaz M, Aghdaei M, Afrasiabi MA. Role of viruses in renal cell carcinoma. Saudi J Kidney Dis Transpl. 2012;23:53–57. [PubMed] [Google Scholar]
- 229.Bialasiewicz S, Cho Y, Rockett R, Preston J, Wood S, Fleming S, Shepherd B, Barraclough K, Sloots TP, Isbel N. Association of micropapillary urothelial carcinoma of the bladder and BK viruria in kidney transplant recipients. Transpl Infect Dis. 2013;15:283–289. doi: 10.1111/tid.12072. [DOI] [PubMed] [Google Scholar]
- 230.Bulut Y, Ozdemir E, Ozercan HI, Etem EO, Aker F, Toraman ZA, Seyrek A, Firdolas F. Potential relationship between BK virus and renal cell carcinoma. J Med Virol. 2013;85:1085–1089. doi: 10.1002/jmv.23559. [DOI] [PubMed] [Google Scholar]
- 231.Dalianis T, Hirsch HH. Human polyomaviruses in disease and cancer. Virology. 2013;437:63–72. doi: 10.1016/j.virol.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 232.Hrbacek J, Urban M, Hamsikova E, Tachezy R, Heracek J. Thirty years of research on infection and prostate cancer: no conclusive evidence for a link. A systematic review. Urol Oncol. 2013;31:951–965. doi: 10.1016/j.urolonc.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 233.McDaid J, Farkash EA, Steele DJ, Martins PN, Kotton CN, Elias N, Ko DS, Colvin RB, Hertl M. Transitional cell carcinoma arising within a pediatric donor renal transplant in association with BK nephropathy. Transplantation. 2013;95:e28–e30. doi: 10.1097/TP.0b013e31828235ec. [DOI] [PubMed] [Google Scholar]
- 234.Pino L, Rijo E, Nohales G, Frances A, Ubre A, Arango O. Bladder transitional cell carcinoma and BK virus in a young kidney transplant recipient. Transpl Infect Dis. 2013;15:E25–E27. doi: 10.1111/tid.12042. [DOI] [PubMed] [Google Scholar]
- 235.Delbue S, Ferrante P, Provenzano M. Polyomavirus BK and prostate cancer: an unworthy scientific effort? Oncoscience. 2014;1:296–303. doi: 10.18632/oncoscience.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Medani S, O’Kelly P, O’Brien KM, Mohan P, Magee C, Conlon P. Bladder cancer in renal allograft recipients: risk factors and outcomes. Transplant Proc. 2014;46:3466–3473. doi: 10.1016/j.transproceed.2014.06.075. [DOI] [PubMed] [Google Scholar]
- 237.Moens U, Van Ghelue M, Ehlers B. Are human polyomaviruses co-factors for cancers induced by other oncoviruses? Rev Med Virol. 2014;24:343–360. doi: 10.1002/rmv.1798. [DOI] [PubMed] [Google Scholar]
- 238.Oikawa M, Hatakeyama S, Fujita T, Murakami R, Hagiwara K, Narita T, Noro D, Tanaka T, Tanaka Y, Tobisawa Y, et al. BK virus-associated urothelial carcinoma of a ureter graft in a renal transplant recipient: a case report. Transplant Proc. 2014;46:616–619. doi: 10.1016/j.transproceed.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 239.Tsai HL, Chang JW, Wu TH, King KL, Yang LY, Chan YJ, Yang AH, Chang FP, Pan CC, Yang WC, et al. Outcomes of kidney transplant tourism and risk factors for de novo urothelial carcinoma. Transplantation. 2014;98:79–87. doi: 10.1097/TP.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 240.Tseng CE, Yeh CM, Fang CY, Shay J, Chen PL, Lin MC, Chang D, Wang M. Detection of human JCPyV and BKPyV in diffuse large B-cell lymphoma of the GI tract. Eur J Clin Microbiol Infect Dis. 2014;33:665–672. doi: 10.1007/s10096-013-2010-x. [DOI] [PubMed] [Google Scholar]
- 241.Keller EX, Delbue S, Tognon M, Provenzano M. Polyomavirus BK and prostate cancer: a complex interaction of potential clinical relevance. Rev Med Virol. 2015;25:366–378. doi: 10.1002/rmv.1851. [DOI] [PubMed] [Google Scholar]
- 242.Kenan DJ, Mieczkowski PA, Burger-Calderon R, Singh HK, Nickeleit V. The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome. J Pathol. 2015;237:379–389. doi: 10.1002/path.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lavien G, Alger J, Preece J, Alexiev BA, Alexander RB. BK Virus-Associated Invasive Urothelial Carcinoma With Prominent Micropapillary Carcinoma Component in a Cardiac Transplant Patient: Case Report and Review of Literature. Clin Genitourin Cancer. 2015;13:e397–e399. doi: 10.1016/j.clgc.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 244.Polz D, Morshed K, Stec A, Podsiadło Ł, Polz-Dacewicz M. Do polyomavirus hominis strains BK and JC play a role in oral squamous cell carcinoma? Ann Agric Environ Med. 2015;22:106–109. doi: 10.5604/12321966.1141378. [DOI] [PubMed] [Google Scholar]
- 245.Saleeb R, Faragalla H, Yousef GM, Stewart R, Streutker CJ. Malignancies arising in allograft kidneys, with a first reported translocation RCC post-transplantation: A case series. Pathol Res Pract. 2015;211:584–587. doi: 10.1016/j.prp.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 246.Taghavi A, Mohammadi-Torbati P, Kashi AH, Rezaee H, Vaezjalali M. Polyomavirus Hominis 1(BK virus) Infection in Prostatic Tissues: Cancer versus Hyperplasia. Urol J. 2015;12:2240–2244. [PubMed] [Google Scholar]
- 247.Papadimitriou JC, Randhawa P, Rinaldo CH, Drachenberg CB, Alexiev B, Hirsch HH. BK Polyomavirus Infection and Renourinary Tumorigenesis. Am J Transplant. 2016;16:398–406. doi: 10.1111/ajt.13550. [DOI] [PubMed] [Google Scholar]
- 248.Salvatore SP, Myers-Gurevitch PM, Chu S, Robinson BD, Dadhania D, Seshan SV. Polyoma (BK) virus associated urothelial carcinoma originating within a renal allograft five years following resolution of polyoma virus nephropathy. Clin Nephrol. 2016;85:179–183. doi: 10.5414/CN108410. [DOI] [PubMed] [Google Scholar]
- 249.Go S, Conlin M, Hooper JE, Troxell ML. Polyoma virus nephropathy-related mass lesion in an apparently immunocompetent patient. Int Urol Nephrol. 2012;44:1585–1588. doi: 10.1007/s11255-011-9985-y. [DOI] [PubMed] [Google Scholar]
- 250.Lee SH, Hong SH, Lee JY, Hwang TK, Kim KS, Lee H, Choi YJ. Asymptomatic hematuria associated with urinary polyomavirus infection in immunocompetent patients. J Med Virol. 2014;86:347–353. doi: 10.1002/jmv.23724. [DOI] [PubMed] [Google Scholar]
- 251.Coleman DV, Mackenzie EF, Gardner SD, Poulding JM, Amer B, Russell WJ. Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. J Clin Pathol. 1978;31:338–347. doi: 10.1136/jcp.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gaston KE, Gabriel DA, Lavelle JP. Rare cause of ureteral obstruction. Urology. 2005;66:1110. doi: 10.1016/j.urology.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 253.Cavallo R, Costa C, Bergallo M, Messina M, Mazzucco G, Segoloni GP. A case of ureteral lesions in a renal transplant recipient with a co-infection of BK virus and JC virus. Nephrol Dial Transplant. 2007;22:1275. doi: 10.1093/ndt/gfl725. [DOI] [PubMed] [Google Scholar]
- 254.Rajpoot DK, Gomez A, Tsang W, Shanberg A. Ureteric and urethral stenosis: a complication of BK virus infection in a pediatric renal transplant patient. Pediatr Transplant. 2007;11:433–435. doi: 10.1111/j.1399-3046.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 255.Hwang YY, Sim J, Leung AY, Lie AK, Kwong YL. BK virus-associated bilateral ureteric stenosis after haematopoietic SCT: viral kinetics and successful treatment. Bone Marrow Transplant. 2013;48:745–746. doi: 10.1038/bmt.2012.215. [DOI] [PubMed] [Google Scholar]
- 256.Goudsmit J, Wertheim-van Dillen P, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10:91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- 257.Sandler ES, Aquino VM, Goss-Shohet E, Hinrichs S, Krisher K. BK papova virus pneumonia following hematopoietic stem cell transplantation. Bone Marrow Transplant. 1997;20:163–165. doi: 10.1038/sj.bmt.1700849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Galan A, Rauch CA, Otis CN. Fatal BK polyoma viral pneumonia associated with immunosuppression. Hum Pathol. 2005;36:1031–1034. doi: 10.1016/j.humpath.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 259.Akazawa Y, Terada Y, Yamane T, Tanaka S, Aimoto M, Koh H, Nakane T, Koh KR, Nakamae H, Ohsawa M, et al. Fatal BK virus pneumonia following stem cell transplantation. Transpl Infect Dis. 2012;14:E142–E146. doi: 10.1111/tid.12011. [DOI] [PubMed] [Google Scholar]
- 260.Yapa HM, McLornan DP, Raj K, Streetly M, Kazmi M, Cuthill K, Laurie J, Menon PA, Macmahon E. Pneumonitis post-haematopoeitic stem cell transplant - cytopathology clinches diagnosis. J Clin Virol. 2012;55:278–281. doi: 10.1016/j.jcv.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 261.Ferrante P, Caldarelli-Stefano R, Omodeo-Zorini E, Vago L, Boldorini R, Costanzi G. PCR detection of JC virus DNA in brain tissue from patients with and without progressive multifocal leukoencephalopathy. J Med Virol. 1995;47:219–225. doi: 10.1002/jmv.1890470306. [DOI] [PubMed] [Google Scholar]
- 262.Voltz R, Jäger G, Seelos K, Fuhry L, Hohlfeld R. BK virus encephalitis in an immunocompetent patient. Arch Neurol. 1996;53:101–103. doi: 10.1001/archneur.1996.00550010121025. [DOI] [PubMed] [Google Scholar]
- 263.Stoner GL, Alappan R, Jobes DV, Ryschkewitsch CF, Landry ML. BK virus regulatory region rearrangements in brain and cerebrospinal fluid from a leukemia patient with tubulointerstitial nephritis and meningoencephalitis. Am J Kidney Dis. 2002;39:1102–1112. doi: 10.1053/ajkd.2002.32795. [DOI] [PubMed] [Google Scholar]
- 264.Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Bonington A. BKV-DNA and JCV-DNA in CSF of patients with suspected meningitis or encephalitis. Infection. 2003;31:374–378. doi: 10.1007/s15010-003-3078-5. [DOI] [PubMed] [Google Scholar]
- 265.Beck RC, Kohn DJ, Tuohy MJ, Prayson RA, Yen-Lieberman B, Procop GW. Detection of polyoma virus in brain tissue of patients with progressive multifocal leukoencephalopathy by real-time PCR and pyrosequencing. Diagn Mol Pathol. 2004;13:15–21. doi: 10.1097/00019606-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 266.Friedman DP, Flanders AE. MR Imaging of BK virus encephalitis. AJNR Am J Neuroradiol. 2006;27:1016–1018. [PMC free article] [PubMed] [Google Scholar]
- 267.Lintas C, Altieri L, Lombardi F, Sacco R, Persico AM. Association of autism with polyomavirus infection in postmortem brains. J Neurovirol. 2010;16:141–149. doi: 10.3109/13550281003685839. [DOI] [PubMed] [Google Scholar]
- 268.Lopes da Silva R. Polyoma BK virus: an emerging opportunistic infectious agent of the human central nervous system. Braz J Infect Dis. 2011;15:276–284. doi: 10.1016/s1413-8670(11)70189-1. [DOI] [PubMed] [Google Scholar]
- 269.Bárcena-Panero A, Echevarría JE, Van Ghelue M, Fedele G, Royuela E, Gerits N, Moens U. BK polyomavirus with archetypal and rearranged non-coding control regions is present in cerebrospinal fluids from patients with neurological complications. J Gen Virol. 2012;93:1780–1794. doi: 10.1099/vir.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 270.Daveson KL, Ong CW, Bowden S, Koina ME, Hallam LA. BK virus-associated progressive multifocal leukoencephalopathy. Med J Aust. 2013;198:216–218. doi: 10.5694/mja12.10072. [DOI] [PubMed] [Google Scholar]
- 271.Brew BJ, McLean CA, Major EO. Progressive multifocal leukoencephalopathy caused by BK virus? Med J Aust. 2013;198:179–180. doi: 10.5694/mja12.11798. [DOI] [PubMed] [Google Scholar]
- 272.Kastrup O, Göricke S, Kretzschmar H, Wauschkuhn B, Diener HC. Progressive multifocal leukoencephalopathy of the brainstem in an immunocompetent patient--JC and BK polyoma-virus coinfection? A case report and review of the literature. Clin Neurol Neurosurg. 2013;115:2390–2392. doi: 10.1016/j.clineuro.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 273.Rocha A, Faria S, Costa T, Marques L, Freitas C, Mota C. BK virus nephropathy complicated with meningoencephalitis after kidney transplantation. Pediatr Transplant. 2014;18:E48–E51. doi: 10.1111/petr.12209. [DOI] [PubMed] [Google Scholar]
- 274.Saylor D, Thakur K, Venkatesan A. Acute encephalitis in the immunocompromised individual. Curr Opin Infect Dis. 2015;28:330–336. doi: 10.1097/QCO.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 275.Turno-Kręcicka A, Boratyńska M, Tomczyk-Socha M, Mazanowska O. Progressive outer retinal necrosis in immunocompromised kidney allograft recipient. Transpl Infect Dis. 2015;17:400–405. doi: 10.1111/tid.12386. [DOI] [PubMed] [Google Scholar]
- 276.Petrogiannis-Haliotis T, Sakoulas G, Kirby J, Koralnik IJ, Dvorak AM, Monahan-Earley R, DE Girolami PC, DE Girolami U, Upton M, Major EO, et al. BK-related polyomavirus vasculopathy in a renal-transplant recipient. N Engl J Med. 2001;345:1250–1255. doi: 10.1056/NEJMoa010319. [DOI] [PubMed] [Google Scholar]
- 277.Celik B, Randhawa PS. Glomerular changes in BK virus nephropathy. Hum Pathol. 2004;35:367–370. doi: 10.1016/j.humpath.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 278.Nada R, Joshi K, Jha V. BK virus nephropathy and vascular endothelium. Hum Pathol. 2005;36:447–48; author reply 448. doi: 10.1016/j.humpath.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 279.Pucar D, Klein K, Corley J, Williams HT. BK Nephritis and Venous Thrombosis in Renal Transplant Recipient Detected by 111In Leukocyte Imaging. Clin Nucl Med. 2015;40:e382–e385. doi: 10.1097/RLU.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 280.Burger-Calderon R, Madden V, Hallett RA, Gingerich AD, Nickeleit V, Webster-Cyriaque J. Replication of oral BK virus in human salivary gland cells. J Virol. 2014;88:559–573. doi: 10.1128/JVI.02777-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kim GY, Peji J, Nuovo G, Thomas F. BK virus colonic ulcerations. Clin Gastroenterol Hepatol. 2004;2:175–177. doi: 10.1016/s1542-3565(03)00316-1. [DOI] [PubMed] [Google Scholar]
- 282.Koskenvuo M, Lautenschlager I, Kardas P, Auvinen E, Mannonen L, Huttunen P, Taskinen M, Vettenranta K, Hirsch HH. Diffuse gastrointestinal bleeding and BK polyomavirus replication in a pediatric allogeneic haematopoietic stem cell transplant patient. J Clin Virol. 2015;62:72–74. doi: 10.1016/j.jcv.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 283.Flores V, Rodríguez-Sánchez B, Marín-Jiménez I, Bouza E, Menchén L, Muñoz P. Prospective study of BK virus infection in patients with inflammatory bowel disease. ScientificWorldJournal. 2014;2014:970528. doi: 10.1155/2014/970528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gardeniers SH, Mekahli D, Levtchenko E, Lerut E, Renard M, Van Damme-Lombaerts R. Bone marrow aplasia and graft loss in a pediatric renal transplant patient with polyomavirus nephropathy. Pediatr Nephrol. 2010;25:2191–2192. doi: 10.1007/s00467-010-1519-9. [DOI] [PubMed] [Google Scholar]
- 285.Pambrun E, Mengelle C, Fillola G, Laharrague P, Esposito L, Cardeau-Desangles I, Del Bello A, Izopet J, Rostaing L, Kamar N. An Association between BK Virus Replication in Bone Marrow and Cytopenia in Kidney-Transplant Recipients. J Transplant. 2014;2014:252914. doi: 10.1155/2014/252914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yaich S, Charfeddine K, Hsairi D, Zaghdane S, Kammoun K, Makni S, Boudawara T, Hachicha J. BK virus-associated hemophagocytic syndrome in a renal transplant recipient. Saudi J Kidney Dis Transpl. 2014;25:610–614. doi: 10.4103/1319-2442.132205. [DOI] [PubMed] [Google Scholar]
- 287.Maximova N, Pizzol A, Sonzogni A, Gregori M, Granzotto M, Tamaro P. Polyclonal gammopathy after BKV infection in HSCT recipient: a novel trigger for plasma cells replication? Virol J. 2015;12:23. doi: 10.1186/s12985-015-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Howell DN, Smith SR, Butterly DW, Klassen PS, Krigman HR, Burchette JL, Miller SE. Diagnosis and management of BK polyomavirus interstitial nephritis in renal transplant recipients. Transplantation. 1999;68:1279–1288. doi: 10.1097/00007890-199911150-00011. [DOI] [PubMed] [Google Scholar]
- 289.Nickeleit V, Klimkait T, Binet IF, Dalquen P, Del Zenero V, Thiel G, Mihatsch MJ, Hirsch HH. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med. 2000;342:1309–1315. doi: 10.1056/NEJM200005043421802. [DOI] [PubMed] [Google Scholar]
- 290.Boldorini R, Omodeo-Zorini E, Suno A, Benigni E, Nebuloni M, Garino E, Fortunato M, Monga G, Mazzucco G. Molecular characterization and sequence analysis of polyomavirus strains isolated from needle biopsy specimens of kidney allograft recipients. Am J Clin Pathol. 2001;116:489–494. doi: 10.1309/GAUE-92W7-ACDV-X46M. [DOI] [PubMed] [Google Scholar]
- 291.Drachenberg RC, Drachenberg CB, Papadimitriou JC, Ramos E, Fink JC, Wali R, Weir MR, Cangro CB, Klassen DK, Khaled A, et al. Morphological spectrum of polyoma virus disease in renal allografts: diagnostic accuracy of urine cytology. Am J Transplant. 2001;1:373–381. [PubMed] [Google Scholar]
- 292.Fogazzi GB, Cantú M, Saglimbeni L. ‘Decoy cells’ in the urine due to polyomavirus BK infection: easily seen by phase-contrast microscopy. Nephrol Dial Transplant. 2001;16:1496–1498. doi: 10.1093/ndt/16.7.1496. [DOI] [PubMed] [Google Scholar]
- 293.Buehrig CK, Lager DJ, Stegall MD, Kreps MA, Kremers WK, Gloor JM, Schwab TR, Velosa JA, Fidler ME, Larson TS, et al. Influence of surveillance renal allograft biopsy on diagnosis and prognosis of polyomavirus-associated nephropathy. Kidney Int. 2003;64:665–673. doi: 10.1046/j.1523-1755.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 294.Nickeleit V, Singh HK, Mihatsch MJ. Polyomavirus nephropathy: morphology, pathophysiology, and clinical management. Curr Opin Nephrol Hypertens. 2003;12:599–605. doi: 10.1097/00041552-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 295.Boldorini R, Veggiani C, Barco D, Monga G. Kidney and urinary tract polyomavirus infection and distribution: molecular biology investigation of 10 consecutive autopsies. Arch Pathol Lab Med. 2005;129:69–73. doi: 10.5858/2005-129-69-KAUTPI. [DOI] [PubMed] [Google Scholar]
- 296.Drachenberg CB, Hirsch HH, Ramos E, Papadimitriou JC. Polyomavirus disease in renal transplantation: review of pathological findings and diagnostic methods. Hum Pathol. 2005;36:1245–1255. doi: 10.1016/j.humpath.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 297.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, Mihatsch MJ, Nickeleit V, Ramos E, Randhawa P, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 298.Drachenberg CB, Papadimitriou JC. Polyomavirus-associated nephropathy: update in diagnosis. Transpl Infect Dis. 2006;8:68–75. doi: 10.1111/j.1399-3062.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 299.Gaber LW, Egidi MF, Stratta RJ, Lo A, Moore LW, Gaber AO. Clinical utility of histological features of polyomavirus allograft nephropathy. Transplantation. 2006;82:196–204. doi: 10.1097/01.tp.0000226176.87700.a4. [DOI] [PubMed] [Google Scholar]
- 300.Singh HK, Madden V, Shen YJ, Thompson BD, Nickeleit V. Negative-staining electron microscopy of the urine for the detection of polyomavirus infections. Ultrastruct Pathol. 2006;30:329–338. doi: 10.1080/01913120600932347. [DOI] [PubMed] [Google Scholar]
- 301.Bracamonte E, Leca N, Smith KD, Nicosia RF, Nickeleit V, Kendrick E, Furmanczyk PS, Davis CL, Alpers CE, Kowalewska J. Tubular basement membrane immune deposits in association with BK polyomavirus nephropathy. Am J Transplant. 2007;7:1552–1560. doi: 10.1111/j.1600-6143.2007.01794.x. [DOI] [PubMed] [Google Scholar]
- 302.Brealey JK. Ultrastructural observations in a case of BK virus nephropathy with viruses in glomerular subepithelial humps. Ultrastruct Pathol. 2007;31:1–7. doi: 10.1080/01913120600854418. [DOI] [PubMed] [Google Scholar]
- 303.Batal I, Zainah H, Stockhausen S, Basu A, Tan H, Shapiro R, Zeevi A, Girnita A, Randhawa P. The significance of renal C4d staining in patients with BK viruria, viremia, and nephropathy. Mod Pathol. 2009;22:1468–1476. doi: 10.1038/modpathol.2009.118. [DOI] [PubMed] [Google Scholar]
- 304.Miller DC, Qazi Y, Smogorzewski M, Azen CG, Shah T, Koss MN. Foxp3 staining in BK virus allograft nephropathy and comparison with acute cellular rejection. Transplant Proc. 2009;41:4188–4192. doi: 10.1016/j.transproceed.2009.09.062. [DOI] [PubMed] [Google Scholar]
- 305.Rogers NM, Russ GR, Cooper J, Coates PT. Immunophenotyping of interstitial infiltrate does not distinguish between BK virus nephropathy and acute cellular rejection. Nephrology (Carlton) 2009;14:118–122. doi: 10.1111/j.1440-1797.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 306.Dadhania D, Snopkowski C, Ding R, Muthukumar T, Lee J, Bang H, Sharma VK, Seshan S, August P, Kapur S, et al. Validation of noninvasive diagnosis of BK virus nephropathy and identification of prognostic biomarkers. Transplantation. 2010;90:189–197. doi: 10.1097/TP.0b013e3181e2a932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, Bracamonte ER, Broecker V, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 308.Anzivino E, Bellizzi A, Mitterhofer AP, Tinti F, Barile M, Colosimo MT, Fioriti D, Mischitelli M, Chiarini F, Ferretti G, et al. Early monitoring of the human polyomavirus BK replication and sequencing analysis in a cohort of adult kidney transplant patients treated with basiliximab. Virol J. 2011;8:407. doi: 10.1186/1743-422X-8-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hu J, Zhao H, Huang Y, Zhang X, Gao H, Yang M, Fan J, Ma W. Prospective study of posttransplant polyomavirus infection in renal transplant recipients. Exp Clin Transplant. 2011;9:175–180. [PubMed] [Google Scholar]
- 310.Henderson LK, Nankivell BJ, Chapman JR. Surveillance protocol kidney transplant biopsies: their evolving role in clinical practice. Am J Transplant. 2011;11:1570–1575. doi: 10.1111/j.1600-6143.2011.03677.x. [DOI] [PubMed] [Google Scholar]
- 311.Yamanaka K, Oka K, Nakazawa S, Hirai T, Kishikawa H, Nishimura K, Kyo M, Ichikawa Y. Immunohistochemical features of BK virus nephropathy in renal transplant recipients. Clin Transplant. 2012;26 Suppl 24:20–24. doi: 10.1111/j.1399-0012.2012.01636.x. [DOI] [PubMed] [Google Scholar]
- 312.Sar A, Worawichawong S, Benediktsson H, Zhang J, Yilmaz S, Trpkov K. Interobserver agreement for Polyomavirus nephropathy grading in renal allografts using the working proposal from the 10th Banff Conference on Allograft Pathology. Hum Pathol. 2011;42:2018–2024. doi: 10.1016/j.humpath.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 313.Chapman JR. Do protocol transplant biopsies improve kidney transplant outcomes? Curr Opin Nephrol Hypertens. 2012;21:580–586. doi: 10.1097/MNH.0b013e32835903f4. [DOI] [PubMed] [Google Scholar]
- 314.Masutani K, Shapiro R, Basu A, Tan H, Wijkstrom M, Randhawa P. The Banff 2009 Working Proposal for polyomavirus nephropathy: a critical evaluation of its utility as a determinant of clinical outcome. Am J Transplant. 2012;12:907–918. doi: 10.1111/j.1600-6143.2012.03993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Palamas M, Rocher AE, Sardi-Segovia M, Harriet LA, Palaoro LA. Symptomatic BK virus infection in an immunocompetent child diagnosed on urine cytology. Cytopathology. 2012;23:274–275. doi: 10.1111/j.1365-2303.2011.00862.x. [DOI] [PubMed] [Google Scholar]
- 316.Wang Z, Portier BP, Hu B, Chiesa-Vottero A, Myles J, Procop GW, Tubbs RR. Diagnosis of BK viral nephropathy in the renal allograft biopsy: role of fluorescence in situ hybridization. J Mol Diagn. 2012;14:494–500. doi: 10.1016/j.jmoldx.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 317.Huang G, Chen WF, Wang CX, Fei JG, Deng SX, Qiu J, Chen LZ. Noninvasive tool for the diagnosis of polyomavirus BK-associated nephropathy in renal transplant recipients. Diagn Microbiol Infect Dis. 2013;75:292–297. doi: 10.1016/j.diagmicrobio.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 318.Li X, Sun Q, Chen J, Ji S, Wen J, Cheng D, Liu Z. Immunophenotyping in BK virus allograft nephropathy distinct from acute rejection. Clin Dev Immunol. 2013;2013:412902. doi: 10.1155/2013/412902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Menter T, Mayr M, Schaub S, Mihatsch MJ, Hirsch HH, Hopfer H. Pathology of resolving polyomavirus-associated nephropathy. Am J Transplant. 2013;13:1474–1483. doi: 10.1111/ajt.12218. [DOI] [PubMed] [Google Scholar]
- 320.Sigdel TK, Vitalone MJ, Tran TQ, Dai H, Hsieh SC, Salvatierra O, Sarwal MM. A rapid noninvasive assay for the detection of renal transplant injury. Transplantation. 2013;96:97–101. doi: 10.1097/TP.0b013e318295ee5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Alsaad KO, Aloudah N, Alhamdan HM, Alamir A, Fakeeh K. Acute diffuse proliferative post-infectious glomerulonephritis in renal allograft--a case report and literature review. Pediatr Transplant. 2014;18:E77–E82. doi: 10.1111/petr.12233. [DOI] [PubMed] [Google Scholar]
- 322.Funahashi Y, Kato M, Fujita T, Tsuruta K, Inoue S, Gotoh M. Correlation between urine and serum BK virus levels after renal transplantation. Transplant Proc. 2014;46:567–569. doi: 10.1016/j.transproceed.2013.11.154. [DOI] [PubMed] [Google Scholar]
- 323.Hassan S, Mittal C, Amer S, Khalid F, Patel A, Delbusto R, Samuel L, Alangaden G, Ramesh M. Currently recommended BK virus (BKV) plasma viral load cutoff of ≥4 log10/mL underestimates the diagnosis of BKV-associated nephropathy: a single transplant center experience. Transpl Infect Dis. 2014;16:55–60. doi: 10.1111/tid.12164. [DOI] [PubMed] [Google Scholar]
- 324.Masutani K. Current problems in screening, diagnosis and treatment of polyomavirus BK nephropathy. Nephrology (Carlton) 2014;19 Suppl 3:11–16. doi: 10.1111/nep.12254. [DOI] [PubMed] [Google Scholar]
- 325.Sigdel TK, Salomonis N, Nicora CD, Ryu S, He J, Dinh V, Orton DJ, Moore RJ, Hsieh SC, Dai H, et al. The identification of novel potential injury mechanisms and candidate biomarkers in renal allograft rejection by quantitative proteomics. Mol Cell Proteomics. 2014;13:621–631. doi: 10.1074/mcp.M113.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.van Doesum WB, Abdulahad WH, van Dijk MC, Dolff S, van Son WJ, Stegeman CA, Sanders JS. Characterization of urinary CD4+ and CD8+ T cells in kidney transplantation patients with polyomavirus BK infection and allograft rejection. Transpl Infect Dis. 2014;16:733–743. doi: 10.1111/tid.12273. [DOI] [PubMed] [Google Scholar]
- 327.Gard L, Niesters HG, Riezebos-Brilman A. A real time genotyping PCR assay for polyomavirus BK. J Virol Methods. 2015;221:51–56. doi: 10.1016/j.jviromet.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 328.Hara S. Banff 2013 update: Pearls and pitfalls in transplant renal pathology. Nephrology (Carlton) 2015;20 Suppl 2:2–8. doi: 10.1111/nep.12474. [DOI] [PubMed] [Google Scholar]
- 329.Kardas P, Leboeuf C, Hirsch HH. Optimizing JC and BK polyomavirus IgG testing for seroepidemiology and patient counseling. J Clin Virol. 2015;71:28–33. doi: 10.1016/j.jcv.2015.07.305. [DOI] [PubMed] [Google Scholar]
- 330.Dugo M, Mangino M, Meola M, Petrucci I, Valente ML, Laurino L, Stella M, Mastrosimone S, Brunello A, Virgilio B, et al. Ultrasound findings of BK polyomavirus-associated nephropathy in renal transplant patients. J Nephrol. 2016 doi: 10.1007/s40620-016-0327-0. Jun 24; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 331.Nankivell BJ, Renthawa J, Jeoffreys N, Kable K, O’Connell PJ, Chapman JR, Wong G, Sharma RN. Clinical Utility of Urinary Cytology to Detect BK Viral Nephropathy. Transplantation. 2015;99:1715–1722. doi: 10.1097/TP.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 332.Rennert H, Fernandes H, Gilani Z, Sipley J. Development of a BK virus real-time quantitative assay using the bioMérieux analyte-specific reagents in plasma specimens. Am J Clin Pathol. 2015;144:909–915. doi: 10.1309/AJCPXKUGLG3Q3MPX. [DOI] [PubMed] [Google Scholar]
- 333.Ruangkanchanasetr P, Pumchandh N, Satirapoj B, Termmathurapoj S, Pongthanapisith V. Biopsy-proven bk virus nephropathy without detectable bk viremia in a one-year post-kidney transplant recipient. Southeast Asian J Trop Med Public Health. 2015;46:657–661. [PubMed] [Google Scholar]
- 334.Singh HK, Reisner H, Derebail VK, Kozlowski T, Nickeleit V. Polyomavirus nephropathy: quantitative urinary polyomavirus-Haufen testing accurately predicts the degree of intrarenal viral disease. Transplantation. 2015;99:609–615. doi: 10.1097/TP.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yoon SH, Cho JH, Jung HY, Choi JY, Park SH, Kim YL, Kim HK, Huh S, Kim CD. Clinical impact of BK virus surveillance on outcomes in kidney transplant recipients. Transplant Proc. 2015;47:660–665. doi: 10.1016/j.transproceed.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 336.Lee HM, Jang IA, Lee D, Kang EJ, Choi BS, Park CW, Choi YJ, Yang CW, Kim YS, Chung BH. Risk factors in the progression of BK virus-associated nephropathy in renal transplant recipients. Korean J Intern Med. 2015;30:865–872. doi: 10.3904/kjim.2015.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Taguchi F, Nagaki D, Saito M, Haruyama C, Iwasaki K. Transplacental transmission of BK virus in human. Jpn J Microbiol. 1975;19:395–398. doi: 10.1111/j.1348-0421.1975.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 338.Corallini A, Barbanti-Brodano G, Portolani M, Balboni PG, Grossi MP, Possati L, Honorati C, La Placa M, Mazzoni A, Caputo A, et al. Antibodies to BK virus structural and tumor antigens in human sera from normal persons and from patients with various diseases, including neoplasia. Infect Immun. 1976;13:1684–1691. doi: 10.1128/iai.13.6.1684-1691.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Cheeseman SH, Black PH, Rubin RH, Cantell K, Hirsch MS. Interferon and BK Papovavirus--clinical and laboratory studies. J Infect Dis. 1980;141:157–161. doi: 10.1093/infdis/141.2.157. [DOI] [PubMed] [Google Scholar]
- 340.Baserga M, Borgatti M, Nicoli A, Portolani M, Rosito P, Paolucci G. [Infection by BK virus, a human papovavirus, in immunosuppressed children and in children with normal immunological defenses (author’s transl)] Pediatr Med Chir. 1981;3:177–184. [PubMed] [Google Scholar]
- 341.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 342.van der Noordaa J, van Strien A, Sol CJ. Persistence of BK virus in human foetal pancreas cells. J Gen Virol. 1986;67(Pt 7):1485–1490. doi: 10.1099/0022-1317-67-7-1485. [DOI] [PubMed] [Google Scholar]
- 343.Knowles WA, Pillay D, Johnson MA, Hand JF, Brown DW. Prevalence of long-term BK and JC excretion in HIV-infected adults and lack of correlation with serological markers. J Med Virol. 1999;59:474–479. [PubMed] [Google Scholar]
- 344.Kwak EJ, Vilchez RA, Randhawa P, Shapiro R, Butel JS, Kusne S. Pathogenesis and management of polyomavirus infection in transplant recipients. Clin Infect Dis. 2002;35:1081–1087. doi: 10.1086/344060. [DOI] [PubMed] [Google Scholar]
- 345.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, Miller E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 346.Kaneko T, Moriyama T, Tsubakihara Y, Horio M, Imai E. Prevalence of human polyoma virus (BK virus and JC virus) infection in patients with chronic renal disease. Clin Exp Nephrol. 2005;9:132–137. doi: 10.1007/s10157-005-0348-9. [DOI] [PubMed] [Google Scholar]
- 347.Chen Y, Trofe J, Gordon J, Du Pasquier RA, Roy-Chaudhury P, Kuroda MJ, Woodle ES, Khalili K, Koralnik IJ. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80:3495–3505. doi: 10.1128/JVI.80.7.3495-3505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Li J, Melenhorst J, Hensel N, Rezvani K, Sconocchia G, Kilical Y, Hou J, Curfman B, Major E, Barrett AJ. T-cell responses to peptide fragments of the BK virus T antigen: implications for cross-reactivity of immune response to JC virus. J Gen Virol. 2006;87:2951–2960. doi: 10.1099/vir.0.82094-0. [DOI] [PubMed] [Google Scholar]
- 349.Longhi G, Pietropaolo V, Mischitelli M, Longhi C, Conte MP, Marchetti M, Tinari A, Valenti P, Degener AM, Seganti L, et al. Lactoferrin inhibits early steps of human BK polyomavirus infection. Antiviral Res. 2006;72:145–152. doi: 10.1016/j.antiviral.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 350.Meehan SM, Kraus MD, Kadambi PV, Chang A. Nephron segment localization of polyoma virus large T antigen in renal allografts. Hum Pathol. 2006;37:1400–1406. doi: 10.1016/j.humpath.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 351.Prosser S, Hariharan S. Pathogenesis of BK virus infection after renal transplantation. Expert Rev Clin Immunol. 2006;2:833–837. doi: 10.1586/1744666X.2.6.833. [DOI] [PubMed] [Google Scholar]
- 352.Randhawa PS, Gupta G, Vats A, Shapiro R, Viscidi RP. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin Vaccine Immunol. 2006;13:1057–1063. doi: 10.1128/CVI.00114-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Weinreb DB, Desman GT, Burstein DE, Kim DU, Dikman SH, Johnson EM. Expression of p53 in virally infected tubular cells in renal transplant patients with polyomavirus nephropathy. Hum Pathol. 2006;37:684–688. doi: 10.1016/j.humpath.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 354.Abend JR, Low JA, Imperiale MJ. Inhibitory effect of gamma interferon on BK virus gene expression and replication. J Virol. 2007;81:272–279. doi: 10.1128/JVI.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Bruggeman LA. Viral subversion mechanisms in chronic kidney disease pathogenesis. Clin J Am Soc Nephrol. 2007;2 Suppl 1:S13–S19. doi: 10.2215/CJN.04311206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Dugan AS, Gasparovic ML, Tsomaia N, Mierke DF, O’Hara BA, Manley K, Atwood WJ. Identification of amino acid residues in BK virus VP1 that are critical for viability and growth. J Virol. 2007;81:11798–11808. doi: 10.1128/JVI.01316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Moriyama T, Marquez JP, Wakatsuki T, Sorokin A. Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J Virol. 2007;81:8552–8562. doi: 10.1128/JVI.00924-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhong S, Zheng HY, Suzuki M, Chen Q, Ikegaya H, Aoki N, Usuku S, Kobayashi N, Nukuzuma S, Yasuda Y, et al. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J Clin Microbiol. 2007;45:193–198. doi: 10.1128/JCM.01645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Abend JR, Imperiale MJ. Transforming growth factor-beta-mediated regulation of BK virus gene expression. Virology. 2008;378:6–12. doi: 10.1016/j.virol.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bohl DL, Brennan DC, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Storch GA. BK virus antibody titers and intensity of infections after renal transplantation. J Clin Virol. 2008;43:184–189. doi: 10.1016/j.jcv.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Comoli P, Hirsch HH, Ginevri F. Cellular immune responses to BK virus. Curr Opin Organ Transplant. 2008;13:569–574. doi: 10.1097/MOT.0b013e3283186b93. [DOI] [PubMed] [Google Scholar]
- 362.Costa C, Touscoz GA, Bergallo M, Sidoti F, Terlizzi ME, Astegiano S, Merlino C, Segoloni GP, Cavallo R. Non-organ-specific autoantibodies in renal transplant recipients: relation to BK virus infection. New Microbiol. 2008;31:175–180. [PubMed] [Google Scholar]
- 363.Dugan AS, Maginnis MS, Jordan JA, Gasparovic ML, Manley K, Page R, Williams G, Porter E, O’Hara BA, Atwood WJ. Human alpha-defensins inhibit BK virus infection by aggregating virions and blocking binding to host cells. J Biol Chem. 2008;283:31125–31132. doi: 10.1074/jbc.M805902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Johannessen M, Myhre MR, Dragset M, Tümmler C, Moens U. Phosphorylation of human polyomavirus BK agnoprotein at Ser-11 is mediated by PKC and has an important regulative function. Virology. 2008;379:97–109. doi: 10.1016/j.virol.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 365.Prosser SE, Orentas RJ, Jurgens L, Cohen EP, Hariharan S. Recovery of BK virus large T-antigen-specific cellular immune response correlates with resolution of bk virus nephritis. Transplantation. 2008;85:185–192. doi: 10.1097/TP.0b013e31815fef56. [DOI] [PubMed] [Google Scholar]
- 366.Saini D, Ramachandran S, Nataraju A, Benshoff N, Liu W, Desai N, Chapman W, Mohanakumar T. Activated effector and memory T cells contribute to circulating sCD30: potential marker for islet allograft rejection. Am J Transplant. 2008;8:1798–1808. doi: 10.1111/j.1600-6143.2008.02329.x. [DOI] [PubMed] [Google Scholar]
- 367.Jeffers LK, Madden V, Webster-Cyriaque J. BK virus has tropism for human salivary gland cells in vitro: implications for transmission. Virology. 2009;394:183–193. doi: 10.1016/j.virol.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 368.Jiang M, Abend JR, Tsai B, Imperiale MJ. Early events during BK virus entry and disassembly. J Virol. 2009;83:1350–1358. doi: 10.1128/JVI.02169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Moriyama T, Sorokin A. BK virus (BKV): infection, propagation, quantitation, purification, labeling, and analysis of cell entry. Curr Protoc Cell Biol. 2009;Chapter 26:Unit 26.2. doi: 10.1002/0471143030.cb2602s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sadeghi M, Daniel V, Schnitzler P, Lahdou I, Naujokat C, Zeier M, Opelz G. Urinary proinflammatory cytokine response in renal transplant recipients with polyomavirus BK viruria. Transplantation. 2009;88:1109–1116. doi: 10.1097/TP.0b013e3181ba0e17. [DOI] [PubMed] [Google Scholar]
- 371.Yogo Y, Sugimoto C, Zhong S, Homma Y. Evolution of the BK polyomavirus: epidemiological, anthropological and clinical implications. Rev Med Virol. 2009;19:185–199. doi: 10.1002/rmv.613. [DOI] [PubMed] [Google Scholar]
- 372.Ziedina I, Folkmane I, Chapenko S, Murovska M, Sultanova A, Jushinskis J, Rozental R. Reactivation of BK Virus in the Early Period After Kidney Transplantation. Transplant Proc. 2009;41:766–768. doi: 10.1016/j.transproceed.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 373.Abend JR, Low JA, Imperiale MJ. Global effects of BKV infection on gene expression in human primary kidney epithelial cells. Virology. 2010;397:73–79. doi: 10.1016/j.virol.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Bijol V, Cimic A, Viscidi RP, Hymes LC. Pretransplant IgG antibodies to polyoma BK virus in pediatric renal transplants. Pediatr Transplant. 2010;14:224–227. doi: 10.1111/j.1399-3046.2009.01201.x. [DOI] [PubMed] [Google Scholar]
- 375.Jordan JA, Manley K, Dugan AS, O’Hara BA, Atwood WJ. Transcriptional regulation of BK virus by nuclear factor of activated T cells. J Virol. 2010;84:1722–1730. doi: 10.1128/JVI.01918-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kemény E, Hirsch HH, Eller J, Dürmüller U, Hopfer H, Mihatsch MJ. Plasma cell infiltrates in polyomavirus nephropathy. Transpl Int. 2010;23:397–406. doi: 10.1111/j.1432-2277.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- 377.Mitterhofer AP, Pietropaolo V, Barile M, Tinti F, Fioriti D, Mischitelli M, Limonta A, Meçule A, Ferretti G, Poli L, et al. Meaning of early polyomavirus-BK replication post kidney transplant. Transplant Proc. 2010;42:1142–1145. doi: 10.1016/j.transproceed.2010.03.130. [DOI] [PubMed] [Google Scholar]
- 378.Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89:1462–1465. doi: 10.1097/tp.0b013e3181daaaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Saundh BK, Tibble S, Baker R, Sasnauskas K, Harris M, Hale A. Different patterns of BK and JC polyomavirus reactivation following renal transplantation. J Clin Pathol. 2010;63:714–718. doi: 10.1136/jcp.2009.074864. [DOI] [PubMed] [Google Scholar]
- 380.Tremolada S, Delbue S, Larocca S, Carloni C, Elia F, Khalili K, Gordon J, Ferrante P. Polymorphisms of the BK virus subtypes and their influence on viral in vitro growth efficiency. Virus Res. 2010;149:190–196. doi: 10.1016/j.virusres.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Tsai B, Qian M. Cellular entry of polyomaviruses. Curr Top Microbiol Immunol. 2010;343:177–194. doi: 10.1007/82_2010_38. [DOI] [PubMed] [Google Scholar]
- 382.Womer KL, Huang Y, Herren H, Dibadj K, Peng R, Murawski M, Shraybman R, Patton P, Clare-Salzler MJ, Kaplan B. Dendritic cell deficiency associated with development of BK viremia and nephropathy in renal transplant recipients. Transplantation. 2010;89:115–123. doi: 10.1097/TP.0b013e3181bc6096. [DOI] [PubMed] [Google Scholar]
- 383.Babel N, Volk HD, Reinke P. BK polyomavirus infection and nephropathy: the virus-immune system interplay. Nat Rev Nephrol. 2011;7:399–406. doi: 10.1038/nrneph.2011.59. [DOI] [PubMed] [Google Scholar]
- 384.Chakera A, Bennett S, Lawrence S, Morteau O, Mason PD, O’Callaghan CA, Cornall RJ. Antigen-specific T cell responses to BK polyomavirus antigens identify functional anti-viral immunity and may help to guide immunosuppression following renal transplantation. Clin Exp Immunol. 2011;165:401–409. doi: 10.1111/j.1365-2249.2011.04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Vu D, Shah T, Ansari J, Naraghi R, Min D. Efficacy of intravenous immunoglobulin in the treatment of persistent BK viremia and BK virus nephropathy in renal transplant recipients. Transplant Proc. 2015;47:394–398. doi: 10.1016/j.transproceed.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 386.Mueller K, Schachtner T, Sattler A, Meier S, Friedrich P, Trydzenskaya H, Hinrichs C, Trappe R, Thiel A, Reinke P, et al. BK-VP3 as a new target of cellular immunity in BK virus infection. Transplantation. 2011;91:100–107. doi: 10.1097/tp.0b013e3181fe1335. [DOI] [PubMed] [Google Scholar]
- 387.Trydzenskaya H, Sattler A, Müller K, Schachtner T, Dang-Heine C, Friedrich P, Nickel P, Hoerstrup J, Schindler R, Thiel A, et al. Novel approach for improved assessment of phenotypic and functional characteristics of BKV-specific T-cell immunity. Transplantation. 2011;92:1269–1277. doi: 10.1097/TP.0b013e318234e0e5. [DOI] [PubMed] [Google Scholar]
- 388.Jiang M, Zhao L, Gamez M, Imperiale MJ. Roles of ATM and ATR-mediated DNA damage responses during lytic BK polyomavirus infection. PLoS Pathog. 2012;8:e1002898. doi: 10.1371/journal.ppat.1002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Luo C, Hirsch HH, Kant J, Randhawa P. VP-1 quasispecies in human infection with polyomavirus BK. J Med Virol. 2012;84:152–161. doi: 10.1002/jmv.22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Acott PD. Natural killer cell response to BK virus infection in polyoma virus-associated nephropathy of renal transplant recipients. Kidney Int. 2013;84:233–235. doi: 10.1038/ki.2013.148. [DOI] [PubMed] [Google Scholar]
- 391.Barbosa D, Kahwaji J, Puliyanda D, Mirocha J, Reinsmoen N, Lai CH, Villicana R, Peng A, Jordan SC, Vo A, et al. Polyomavirus BK Viremia in Kidney Transplant Recipients After Desensitization With IVIG and Rituximab. Transplantation. 2013 doi: 10.1097/01.TP.0000437671.78716.f3. Nov 21; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 392.Bennett SM, Jiang M, Imperiale MJ. Role of cell-type-specific endoplasmic reticulum-associated degradation in polyomavirus trafficking. J Virol. 2013;87:8843–8852. doi: 10.1128/JVI.00664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Borni-Duval C, Caillard S, Olagne J, Perrin P, Braun-Parvez L, Heibel F, Moulin B. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation. 2013;95:1498–1505. doi: 10.1097/TP.0b013e3182921995. [DOI] [PubMed] [Google Scholar]
- 394.Broekema NM, Imperiale MJ. miRNA regulation of BK polyomavirus replication during early infection. Proc Natl Acad Sci USA. 2013;110:8200–8205. doi: 10.1073/pnas.1301907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Comoli P, Cioni M, Basso S, Gagliardone C, Potenza L, Verrina E, Luppi M, Zecca M, Ghiggeri GM, Ginevri F. Immunity to Polyomavirus BK Infection: Immune Monitoring to Regulate the Balance between Risk of BKV Nephropathy and Induction of Alloimmunity. Clin Dev Immunol. 2013;2013:256923. doi: 10.1155/2013/256923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Lee MC, Lu MC, Lai NS, Liu SC, Yu HC, Lin TY, Hung SP, Huang HB, Yin WY. Renal dysfunction by BK virus infection is correlated with activated T cell level in renal transplantation. J Surg Res. 2013;180:330–336. doi: 10.1016/j.jss.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 397.Masutani K, Ninomiya T, Randhawa P. HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia. Nephrol Dial Transplant. 2013;28:3119–3126. doi: 10.1093/ndt/gft298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Sood P, Senanayake S, Sujeet K, Medipalli R, Van-Why SK, Cronin DC, Johnson CP, Hariharan S. Donor and recipient BKV-specific IgG antibody and posttransplantation BKV infection: a prospective single-center study. Transplantation. 2013;95:896–902. doi: 10.1097/TP.0b013e318282ba83. [DOI] [PubMed] [Google Scholar]
- 399.Trydzenskaya H, Juerchott K, Lachmann N, Kotsch K, Kunert K, Weist B, Schönemann C, Schindler R, Nickel P, Melzig MF, et al. The genetic predisposition of natural killer cell to BK virus-associated nephropathy in renal transplant patients. Kidney Int. 2013;84:359–365. doi: 10.1038/ki.2013.59. [DOI] [PubMed] [Google Scholar]
- 400.Barbosa D, Kahwaji J, Puliyanda D, Mirocha J, Reinsmoen N, Lai CH, Villicana R, Peng A, Jordan SC, Vo A, et al. Polyomavirus BK viremia in kidney transplant recipients after desensitization with IVIG and rituximab. Transplantation. 2014;97:755–761. doi: 10.1097/01.TP.0000437671.78716.f3. [DOI] [PubMed] [Google Scholar]
- 401.Bouley SJ, Maginnis MS, Derdowski A, Gee GV, O’Hara BA, Nelson CD, Bara AM, Atwood WJ, Dugan AS. Host cell autophagy promotes BK virus infection. Virology. 2014;456-457:87–95. doi: 10.1016/j.virol.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Costa C, Mantovani S, Piceghello A, Di Nauta A, Sinesi F, Sidoti F, Messina M, Cavallo R. Evaluation of polyomavirus BK cellular immune response by an ELISpot assay and relation to viral replication in kidney transplant recipients. New Microbiol. 2014;37:219–223. [PubMed] [Google Scholar]
- 403.Huang G, Zhang L, Liang X, Qiu J, Deng R, Li J, Chen G, Dong Y, Chen L. Risk factors for BK virus infection and BK virus-associated nephropathy under the impact of intensive monitoring and pre-emptive immunosuppression reduction. Transplant Proc. 2014;46:3448–3454. doi: 10.1016/j.transproceed.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 404.Lubetzky M, Bao Y, O Broin P, Marfo K, Ajaimy M, Aljanabi A, de Boccardo G, Golden A, Akalin E. Genomics of BK viremia in kidney transplant recipients. Transplantation. 2014;97:451–456. doi: 10.1097/01.TP.0000437432.35227.3e. [DOI] [PubMed] [Google Scholar]
- 405.Nishikawa K, Mizuno S, Masui S, Kanda H, Yamada Y, Arima K, Isaji S, Sugimura Y. Usefulness of monitoring cell-mediated immunity for predicting post-kidney transplantation viral infection. Transplant Proc. 2014;46:552–555. doi: 10.1016/j.transproceed.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 406.Satyanarayana G, Marty FM, Tan CS. The polyomavirus puzzle: is host immune response beneficial in controlling BK virus after adult hematopoietic cell transplantion? Transpl Infect Dis. 2014;16:521–531. doi: 10.1111/tid.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Schmidt T, Adam C, Hirsch HH, Janssen MW, Wolf M, Dirks J, Kardas P, Ahlenstiel-Grunow T, Pape L, Rohrer T, et al. BK polyomavirus-specific cellular immune responses are age-dependent and strongly correlate with phases of virus replication. Am J Transplant. 2014;14:1334–1345. doi: 10.1111/ajt.12689. [DOI] [PubMed] [Google Scholar]
- 408.Schwarz A, Linnenweber-Held S, Heim A, Framke T, Haller H, Schmitt C. Viral Origin, Clinical Course, and Renal Outcomes in Patients With BK Virus Infection After Living-Donor Renal Transplantation. Transplantation. 2016;100:844–853. doi: 10.1097/TP.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 409.Simon-Santamaria J, Rinaldo CH, Kardas P, Li R, Malovic I, Elvevold K, McCourt P, Smedsrød B, Hirsch HH, Sørensen KK. Efficient uptake of blood-borne BK and JC polyomavirus-like particles in endothelial cells of liver sinusoids and renal vasa recta. PLoS One. 2014;9:e111762. doi: 10.1371/journal.pone.0111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Tian YC, Li YJ, Chen HC, Wu HH, Weng CH, Chen YC, Lee CC, Chang MY, Hsu HH, Yen TH, et al. Polyomavirus BK-encoded microRNA suppresses autoregulation of viral replication. Biochem Biophys Res Commun. 2014;447:543–549. doi: 10.1016/j.bbrc.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 411.Vu D, Sakharkar P, Shah T, Naraghi R, Yasir Q, Hutchinson I, Min D. Association of interferon gamma gene polymorphisms with BK virus infection among Hispanic renal allograft recipients. Transplantation. 2014;97:660–667. doi: 10.1097/01.TP.0000438115.20198.89. [DOI] [PubMed] [Google Scholar]
- 412.Weist BJ, Schmueck M, Fuehrer H, Sattler A, Reinke P, Babel N. The role of CD4(+) T cells in BKV-specific T cell immunity. Med Microbiol Immunol. 2014;203:395–408. doi: 10.1007/s00430-014-0348-z. [DOI] [PubMed] [Google Scholar]
- 413.Becker LE, Siebert D, Süsal C, Opelz G, Leo A, Waldherr R, Macher-Goeppinger S, Schemmer P, Schaefer SM, Klein K, et al. Outcomes Following ABO-Incompatible Kidney Transplantation Performed After Desensitization by Nonantigen-Specific Immunoadsorption. Transplantation. 2015;99:2364–2371. doi: 10.1097/TP.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 414.Bennett SM, Zhao L, Bosard C, Imperiale MJ. Role of a nuclear localization signal on the minor capsid proteins VP2 and VP3 in BKPyV nuclear entry. Virology. 2015;474:110–116. doi: 10.1016/j.virol.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Bentall A, Neil D, Sharif A, Ball S. ABO-incompatible kidney transplantation is a novel risk factor for BK nephropathy. Transplantation. 2015;99:e8–e9. doi: 10.1097/TP.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 416.Bethge T, Hachemi HA, Manzetti J, Gosert R, Schaffner W, Hirsch HH. Sp1 sites in the noncoding control region of BK polyomavirus are key regulators of bidirectional viral early and late gene expression. J Virol. 2015;89:3396–3411. doi: 10.1128/JVI.03625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Calarota SA, Aberle JH, Puchhammer-Stöckl E, Baldanti F. Approaches for monitoring of non virus-specific and virus-specific T-cell response in solid organ transplantation and their clinical applications. J Clin Virol. 2015;70:109–119. doi: 10.1016/j.jcv.2015.07.299. [DOI] [PubMed] [Google Scholar]
- 418.Cioni M, Leboeuf C, Comoli P, Ginevri F, Hirsch HH. Characterization of Immunodominant BK Polyomavirus 9mer Epitope T Cell Responses. Am J Transplant. 2016;16:1193–1206. doi: 10.1111/ajt.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Dekeyser M, François H, Beaudreuil S, Durrbach A. Polyomavirus-Specific Cellular Immunity: From BK-Virus-Specific Cellular Immunity to BK-Virus-Associated Nephropathy? Front Immunol. 2015;6:307. doi: 10.3389/fimmu.2015.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Dieplinger G, Everly MJ, Briley KP, Haisch CE, Bolin P, Maldonado AQ, Kendrick WT, Kendrick SA, Morgan C, Terasaki PI, et al. Onset and progression of de novo donor-specific anti-human leukocyte antigen antibodies after BK polyomavirus and preemptive immunosuppression reduction. Transpl Infect Dis. 2015;17:848–858. doi: 10.1111/tid.12467. [DOI] [PubMed] [Google Scholar]
- 421.Gheith O, Al-Otaibi T, Zakaria Z, Abdel Halim M, Nampoory N. Human leukocyte antigen Cw7-mediated protection against polyoma BK virus in renal transplant recipients who received grafts from antigen-positive donors. Exp Clin Transplant. 2015;13 Suppl 1:383–387. [PubMed] [Google Scholar]
- 422.Mou D, Espinosa JE, Stempora L, Iwakoshi NN, Kirk AD. Viral-induced CD28 loss evokes costimulation independent alloimmunity. J Surg Res. 2015;196:241–246. doi: 10.1016/j.jss.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Mutlu E, Köksoy S, Mutlu D, Yılmaz VT, Koçak H, Dinçkan A, Süleymanlar G, Gültekin M. Quantitative analysis of BKV-specific CD4+ T cells before and after kidney transplantation. Transpl Immunol. 2015;33:20–26. doi: 10.1016/j.trim.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 424.Pai D, Mann DM, Malik A, Hoover DR, Fyfe B, Mann RA. Risk Factors for the Development of BK Virus Nephropathy in Renal Transplant Recipients. Transplant Proc. 2015;47:2465–2469. doi: 10.1016/j.transproceed.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 425.Sahoo MK, Tan SK, Chen SF, Kapusinszky B, Concepcion KR, Kjelson L, Mallempati K, Farina HM, Fernández-Viña M, Tyan D, et al. Limited Variation in BK Virus T-Cell Epitopes Revealed by Next-Generation Sequencing. J Clin Microbiol. 2015;53:3226–3233. doi: 10.1128/JCM.01385-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Sawinski D, Forde KA, Trofe-Clark J, Patel P, Olivera B, Goral S, Bloom RD. Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol. 2015;26:966–975. doi: 10.1681/ASN.2014010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Schachtner T, Stein M, Babel N, Reinke P. The Loss of BKV-specific Immunity From Pretransplantation to Posttransplantation Identifies Kidney Transplant Recipients at Increased Risk of BKV Replication. Am J Transplant. 2015;15:2159–2169. doi: 10.1111/ajt.13252. [DOI] [PubMed] [Google Scholar]
- 428.Signorini L, Croci M, Boldorini R, Varella RB, Elia F, Carluccio S, Villani S, Bella R, Ferrante P, Delbue S. Interaction Between Human Polyomavirus BK and Hypoxia Inducible Factor-1 alpha. J Cell Physiol. 2016;231:1343–1349. doi: 10.1002/jcp.25238. [DOI] [PubMed] [Google Scholar]
- 429.Stamatiou D, Derdas SP, Symvoulakis EK, Sakorafas GH, Zoras O, Spandidos DA. Investigation of BK virus, Epstein-Barr virus and human papillomavirus sequences in postoperative thyroid gland specimens. Int J Biol Markers. 2015;30:e104–e110. doi: 10.5301/jbm.5000115. [DOI] [PubMed] [Google Scholar]
- 430.Verghese PS, Schmeling DO, Knight JA, Matas AJ, Balfour HH. The impact of donor viral replication at transplant on recipient infections posttransplant: a prospective study. Transplantation. 2015;99:602–608. doi: 10.1097/TP.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Verhalen B, Justice JL, Imperiale MJ, Jiang M. Viral DNA replication-dependent DNA damage response activation during BK polyomavirus infection. J Virol. 2015;89:5032–5039. doi: 10.1128/JVI.03650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Banks HT, Hu S, Link K, Rosenberg ES, Mitsuma S, Rosario L. Modeling Immune Response to BK Virus Infection and Donor Kidney in Renal Transplant Recipients. Inverse Probl Sci Eng. 2016;24:127–152. doi: 10.1080/17415977.2015.1017484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Kariminik A, Yaghobi R, Dabiri S. Innate Immunity and BK Virus: Prospective Strategies. Viral Immunol. 2016;29:74–82. doi: 10.1089/vim.2015.0099. [DOI] [PubMed] [Google Scholar]
- 434.Peterson L, Ostermann H, Fiegl M, Tischer J, Jaeger G, Rieger CT. Reactivation of polyomavirus in the genitourinary tract is significantly associated with severe GvHD and oral mucositis following allogeneic stem cell transplantation. Infection. 2016;44:483–490. doi: 10.1007/s15010-016-0872-4. [DOI] [PubMed] [Google Scholar]
- 435.Ribeiro A, Merkle M, Motamedi N, Nitschko H, Köppel S, Wörnle M. BK virus infection activates the TNFα/TNF receptor system in Polyomavirus-associated nephropathy. Mol Cell Biochem. 2016;411:191–199. doi: 10.1007/s11010-015-2581-1. [DOI] [PubMed] [Google Scholar]
- 436.Shamran HA, Malik SN, Al-Saffer JM, Jawad RS. BK Virus Load Associated with Serum Levels of sCD30 in Renal Transplant Recipients. Int J Microbiol. 2016;2016:9752097. doi: 10.1155/2016/9752097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Vögeli TA, Peinemann F, Burdach S, Ackermann R. Urological treatment and clinical course of BK polyomavirus-associated hemorrhagic cystitis in children after bone marrow transplantation. Eur Urol. 1999;36:252–257. doi: 10.1159/000068007. [DOI] [PubMed] [Google Scholar]
- 438.Held TK, Biel SS, Nitsche A, Kurth A, Chen S, Gelderblom HR, Siegert W. Treatment of BK virus-associated hemorrhagic cystitis and simultaneous CMV reactivation with cidofovir. Bone Marrow Transplant. 2000;26:347–350. doi: 10.1038/sj.bmt.1702487. [DOI] [PubMed] [Google Scholar]
- 439.Farasati NA, Shapiro R, Vats A, Randhawa P. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation. 2005;79:116–118. doi: 10.1097/01.tp.0000149338.97084.5f. [DOI] [PubMed] [Google Scholar]
- 440.Leung AY, Chan MT, Yuen KY, Cheng VC, Chan KH, Wong CL, Liang R, Lie AK, Kwong YL. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528–537. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 441.Randhawa PS. Anti-BK virus activity of ciprofloxacin and related antibiotics. Clin Infect Dis. 2005;41:1366–1367; author reply 1367. doi: 10.1086/497080. [DOI] [PubMed] [Google Scholar]
- 442.Williams JW, Javaid B, Kadambi PV, Gillen D, Harland R, Thistlewaite JR, Garfinkel M, Foster P, Atwood W, Millis JM, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352:1157–1158. doi: 10.1056/NEJM200503173521125. [DOI] [PubMed] [Google Scholar]
- 443.Araya CE, Lew JF, Fennell RS, Neiberger RE, Dharnidharka VR. Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy. Pediatr Transplant. 2006;10:32–37. doi: 10.1111/j.1399-3046.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 444.Josephson MA, Gillen D, Javaid B, Kadambi P, Meehan S, Foster P, Harland R, Thistlethwaite RJ, Garfinkel M, Atwood W, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81:704–710. doi: 10.1097/01.tp.0000181149.76113.50. [DOI] [PubMed] [Google Scholar]
- 445.Randhawa P, Farasati NA, Shapiro R, Hostetler KY. Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob Agents Chemother. 2006;50:1564–1566. doi: 10.1128/AAC.50.4.1564-1566.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Savona MR, Newton D, Frame D, Levine JE, Mineishi S, Kaul DR. Low-dose cidofovir treatment of BK virus-associated hemorrhagic cystitis in recipients of hematopoietic stem cell transplant. Bone Marrow Transplant. 2007;39:783–787. doi: 10.1038/sj.bmt.1705678. [DOI] [PubMed] [Google Scholar]
- 447.Kayler LK, Batal I, Mohanka R, Morgan C, Basu A, Shapiro R, Randhawa PS. Antirejection treatment in kidney transplant patients with BK viruria. Transplantation. 2008;86:797–803. doi: 10.1097/TP.0b013e3181837802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Mazzucco G, Costa C, Bergallo M, Segoloni GP, Monga G. Severe crescentic BK virus nephropathy with favourable outcome in a transplanted patient treated with Leflunomide. Clin Nephrol. 2008;70:163–167. doi: 10.5414/cnp70163. [DOI] [PubMed] [Google Scholar]
- 449.Moriyama T, Sorokin A. Repression of BK virus infection of human renal proximal tubular epithelial cells by pravastatin. Transplantation. 2008;85:1311–1317. doi: 10.1097/TP.0b013e31816c4ec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Sharma AP, Moussa M, Casier S, Rehman F, Filler G, Grimmer J. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr Transplant. 2009;13:123–129. doi: 10.1111/j.1399-3046.2008.00958.x. [DOI] [PubMed] [Google Scholar]
- 451.Bennett WM, Meyer L, Ridenour J, Batiuk TD. Surveillance and modification of immunosuppression minimizes BK virus nephropathy. Am J Nephrol. 2010;32:10–12. doi: 10.1159/000313888. [DOI] [PubMed] [Google Scholar]
- 452.Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol. 2010;84:2150–2156. doi: 10.1128/JVI.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Gabardi S, Waikar SS, Martin S, Roberts K, Chen J, Borgi L, Sheashaa H, Dyer C, Malek SK, Tullius SG, et al. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol. 2010;5:1298–1304. doi: 10.2215/CJN.08261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Ganguly N, Clough LA, Dubois LK, Mcguirk JP, Abhyankar S, Aljitawi OS, O’Neal N, Divine CL, Ganguly S. Low-dose cidofovir in the treatment of symptomatic BK virus infection in patients undergoing allogeneic hematopoietic stem cell transplantation: a retrospective analysis of an algorithmic approach. Transpl Infect Dis. 2010;12:406–411. doi: 10.1111/j.1399-3062.2010.00513.x. [DOI] [PubMed] [Google Scholar]
- 455.Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89:1057–1070. doi: 10.1097/TP.0b013e3181d0e15e. [DOI] [PubMed] [Google Scholar]
- 456.Li YJ, Weng CH, Lai WC, Wu HH, Chen YC, Hung CC, Yang CW, Tian YC. A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus. Transplantation. 2010;89:299–306. doi: 10.1097/TP.0b013e3181c9b51c. [DOI] [PubMed] [Google Scholar]
- 457.Talmon G, Cornell LD, Lager DJ. Mitochondrial changes in cidofovir therapy for BK virus nephropathy. Transplant Proc. 2010;42:1713–1715. doi: 10.1016/j.transproceed.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 458.Sharma BN, Li R, Bernhoff E, Gutteberg TJ, Rinaldo CH. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antiviral Res. 2011;92:115–123. doi: 10.1016/j.antiviral.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 459.Topalis D, Lebeau I, Krecmerová M, Andrei G, Snoeck R. Activities of different classes of acyclic nucleoside phosphonates against BK virus in primary human renal cells. Antimicrob Agents Chemother. 2011;55:1961–1967. doi: 10.1128/AAC.01809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wilson JJ, Lin E, Pack CD, Frost EL, Hadley A, Swimm AI, Wang J, Dong Y, Breeden CP, Kalman D, et al. Gamma interferon controls mouse polyomavirus infection in vivo. J Virol. 2011;85:10126–10134. doi: 10.1128/JVI.00761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Mackey MC. Intravesicular cidofovir for the treatment of polyomavirus-associated hemorrhagic cystitis. Ann Pharmacother. 2012;46:442–446. doi: 10.1345/aph.1Q430. [DOI] [PubMed] [Google Scholar]
- 462.Savva-Bordalo J, Pinho Vaz C, Sousa M, Branca R, Campilho F, Resende R, Baldaque I, Camacho O, Campos A. Clinical effectiveness of hyperbaric oxygen therapy for BK-virus-associated hemorrhagic cystitis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2012;47:1095–1098. doi: 10.1038/bmt.2011.228. [DOI] [PubMed] [Google Scholar]
- 463.Seguin SP, Ireland AW, Gupta T, Wright CM, Miyata Y, Wipf P, Pipas JM, Gestwicki JE, Brodsky JL. A screen for modulators of large T antigen’s ATPase activity uncovers novel inhibitors of Simian Virus 40 and BK virus replication. Antiviral Res. 2012;96:70–81. doi: 10.1016/j.antiviral.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Boonyapredee M, Knight K, Little D. Increased BK viremia and progression to BK-virus nephropathy following high-dose intravenous immunoglobulin for acute cellular rejection. Mil Med. 2014;179:e699–e702. doi: 10.7205/MILMED-D-13-00489. [DOI] [PubMed] [Google Scholar]
- 465.Chen XC, Liu T, Li JJ, He C, Meng WT, Huang R. Efficacy and safety of leflunomide for the treatment of BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Acta Haematol. 2013;130:52–56. doi: 10.1159/000345852. [DOI] [PubMed] [Google Scholar]
- 466.Jacobi J, Prignitz A, Büttner M, Korn K, Weidemann A, Hilgers KF, Heller K, Velden J, Knöll A, Wullich B, et al. BK viremia and polyomavirus nephropathy in 352 kidney transplants; risk factors and potential role of mTOR inhibition. BMC Nephrol. 2013;14:207. doi: 10.1186/1471-2369-14-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Kim YJ, Jeong JC, Koo TY, Kwon HY, Han M, Jeon HJ, Ahn C, Yang J. Impact of combined acute rejection on BK virus-associated nephropathy in kidney transplantation. J Korean Med Sci. 2013;28:1711–1715. doi: 10.3346/jkms.2013.28.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Mainra R, Xu Q, Chibbar R, Hassan A, Shoker A. Severe antibody-mediated rejection following IVIG infusion in a kidney transplant recipient with BK-virus nephropathy. Transpl Immunol. 2013;28:145–147. doi: 10.1016/j.trim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 469.Phipps C, Ng HY, Appan P, Loh Y, Koh M, Ho AY, Lee JJ, Linn YC, Tan BH, Goh YT, et al. BK-virus prophylaxis: still no answer. Bone Marrow Transplant. 2013;48:1362–1363. doi: 10.1038/bmt.2013.62. [DOI] [PubMed] [Google Scholar]
- 470.Carney DW, Nelson CD, Ferris BD, Stevens JP, Lipovsky A, Kazakov T, DiMaio D, Atwood WJ, Sello JK. Structural optimization of a retrograde trafficking inhibitor that protects cells from infections by human polyoma- and papillomaviruses. Bioorg Med Chem. 2014;22:4836–4847. doi: 10.1016/j.bmc.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Halim MA, Al-Otaibi T, Gheith O, Zkaria Z, Mosaad A, Said T, Nair P, Nampoory N. Active management versus minimization of immunosuppressives of BK virus-associated nephropathy after a kidney transplant. Exp Clin Transplant. 2014;12:528–533. [PubMed] [Google Scholar]
- 472.Knoll GA, Humar A, Fergusson D, Johnston O, House AA, Kim SJ, Ramsay T, Chassé M, Pang X, Zaltzman J, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA. 2014;312:2106–2114. doi: 10.1001/jama.2014.14721. [DOI] [PubMed] [Google Scholar]
- 473.Kuten SA, Patel SJ, Knight RJ, Gaber LW, DeVos JM, Gaber AO. Observations on the use of cidofovir for BK virus infection in renal transplantation. Transpl Infect Dis. 2014;16:975–983. doi: 10.1111/tid.12313. [DOI] [PubMed] [Google Scholar]
- 474.Lee BT, Gabardi S, Grafals M, Hofmann RM, Akalin E, Aljanabi A, Mandelbrot DA, Adey DB, Heher E, Fan PY, et al. Efficacy of levofloxacin in the treatment of BK viremia: a multicenter, double-blinded, randomized, placebo-controlled trial. Clin J Am Soc Nephrol. 2014;9:583–589. doi: 10.2215/CJN.04230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Lin MC, Wang M, Fang CY, Chen PL, Shen CH, Chang D. Inhibition of BK virus replication in human kidney cells by BK virus large tumor antigen-specific shRNA delivered by JC virus-like particles. Antiviral Res. 2014;103:25–31. doi: 10.1016/j.antiviral.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 476.O’Hara BA, Rupasinghe C, Yatawara A, Gaidos G, Mierke DF, Atwood WJ. Gallic acid-based small-molecule inhibitors of JC and BK polyomaviral infection. Virus Res. 2014;189:280–285. doi: 10.1016/j.virusres.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Sharma BN, Marschall M, Henriksen S, Rinaldo CH. Antiviral effects of artesunate on polyomavirus BK replication in primary human kidney cells. Antimicrob Agents Chemother. 2014;58:279–289. doi: 10.1128/AAC.01800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Zaman RA, Ettenger RB, Cheam H, Malekzadeh MH, Tsai EW. A novel treatment regimen for BK viremia. Transplantation. 2014;97:1166–1171. doi: 10.1097/01.TP.0000441825.72639.4f. [DOI] [PubMed] [Google Scholar]
- 479.Gabardi S, Ramasamy S, Kim M, Klasek R, Carter D, Mackenzie MR, Chandraker A, Tan CS. Impact of HMG-CoA reductase inhibitors on the incidence of polyomavirus-associated nephropathy in renal transplant recipients with human BK polyomavirus viremia. Transpl Infect Dis. 2015;17:536–543. doi: 10.1111/tid.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Gonzalez S, Escobar-Serna DP, Suarez O, Benavides X, Escobar-Serna JF, Lozano E. BK Virus Nephropathy in Kidney Transplantation: An Approach Proposal and Update on Risk Factors, Diagnosis, and Treatment. Transplant Proc. 2015;47:1777–1785. doi: 10.1016/j.transproceed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 481.Huang G, Wang CX, Zhang L, Fei JG, Deng SX, Qiu J, Li J, Chen GD, Fu Q, Chen LZ. Monitoring of polyomavirus BK replication and impact of preemptive immunosuppression reduction in renal-transplant recipients in China: a 5-year single-center analysis. Diagn Microbiol Infect Dis. 2015;81:21–26. doi: 10.1016/j.diagmicrobio.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 482.Huang J, Danovitch G, Pham PT, Bunnapradist S, Huang E. Kidney retransplantation for BK virus nephropathy with active viremia without allograft nephrectomy. J Nephrol. 2015;28:773–777. doi: 10.1007/s40620-015-0200-6. [DOI] [PubMed] [Google Scholar]
- 483.Jeffers-Francis LK, Burger-Clderon R, Webster-Cyriaque J. Effect of leflunomide, cidofovir and ciprofloxacin on replication of BKPyV in a salivary gland in vitro culture system. Antiviral Res. 2015;118:46–55. doi: 10.1016/j.antiviral.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Leventhal JR, Elliott MJ, Yolcu ES, Bozulic LD, Tollerud DJ, Mathew JM, Konieczna I, Ison MG, Galvin J, Mehta J, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99:288–298. doi: 10.1097/TP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 485.Pape L, Tönshoff B, Hirsch HH. Perception, diagnosis and management of BK polyomavirus replication and disease in paediatric kidney transplant recipients in Europe. Nephrol Dial Transplant. 2016;31:842–847. doi: 10.1093/ndt/gfv392. [DOI] [PubMed] [Google Scholar]
- 486.Polanco N, González Monte E, Folgueira MD, Morales E, Gutiérrez Martínez E, Bengoa I, Hernández A, Morales JM, Praga M, Andrés A. Everolimus-based immunosuppression therapy for BK virus nephropathy. Transplant Proc. 2015;47:57–61. doi: 10.1016/j.transproceed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 487.Saull HE, Enderby CY, Gonwa TA, Wadei HM. Comparison of alemtuzumab vs. antithymocyte globulin induction therapy in primary non-sensitized renal transplant patients treated with rapid steroid withdrawal. Clin Transplant. 2015;29:573–580. doi: 10.1111/ctr.12532. [DOI] [PubMed] [Google Scholar]
- 488.Tohme FA, Kalil RS, Thomas CP. Conversion to a sirolimus-based regimen is associated with lower incidence of BK viremia in low-risk kidney transplant recipients. Transpl Infect Dis. 2015;17:66–72. doi: 10.1111/tid.12347. [DOI] [PubMed] [Google Scholar]
- 489.Tylden GD, Hirsch HH, Rinaldo CH. Brincidofovir (CMX001) inhibits BK polyomavirus replication in primary human urothelial cells. Antimicrob Agents Chemother. 2015;59:3306–3316. doi: 10.1128/AAC.00238-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Mohamed M, Parajuli S, Muth B, Astor BC, Panzer SE, Mandelbrot D, Zhong W, Djamali A. In kidney transplant recipients with BK polyomavirus infection, early BK nephropathy, microvascular inflammation, and serum creatinine are risk factors for graft loss. Transpl Infect Dis. 2016;18:361–371. doi: 10.1111/tid.12530. [DOI] [PubMed] [Google Scholar]
- 491.Wu D, Zhang MC, Chen JS, Li X, Cheng DR, Xie KN, Ji SM, Liu ZH, Wen JQ. BK Virus-Associated Nephropathy with Plasma Cell-Rich Infiltrates Treated by Bortezomib-Based Regimen. Exp Clin Transplant. 2015;13:603–606. doi: 10.6002/ect.2014.0225. [DOI] [PubMed] [Google Scholar]
- 492.Yalci A, Celebi ZK, Ozbas B, Sengezer OL, Unal H, Memikoğlu KO, Sengul S, Tuzuner A, Keven K. Evaluation of Infectious Complications in the First Year After Kidney Transplantation. Transplant Proc. 2015;47:1429–1432. doi: 10.1016/j.transproceed.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 493.Belliere J, Kamar N, Mengelle C, Allal A, Sallusto F, Doumerc N, Game X, Congy-Jolivet N, Esposito L, Debiol B, et al. Pilot conversion trial from mycophenolic acid to everolimus in ABO-incompatible kidney-transplant recipients with BK viruria and/or viremia. Transpl Int. 2016;29:315–322. doi: 10.1111/tri.12718. [DOI] [PubMed] [Google Scholar]
- 494.Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 495.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 496.Maginnis MS, Atwood WJ. JC virus: an oncogenic virus in animals and humans? Semin Cancer Biol. 2009;19:261–269. doi: 10.1016/j.semcancer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Kusne S, Vilchez RA, Zanwar P, Quiroz J, Mazur MJ, Heilman RL, Mulligan D, Butel JS. Polyomavirus JC urinary shedding in kidney and liver transplant recipients associated with reduced creatinine clearance. J Infect Dis. 2012;206:875–880. doi: 10.1093/infdis/jis469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Carter JJ, Paulson KG, Wipf GC, Miranda D, Madeleine MM, Johnson LG, Lemos BD, Lee S, Warcola AH, Iyer JG, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:1510–1522. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Husseiny MI, Anastasi B, Singer J, Lacey SF. A comparative study of Merkel cell, BK and JC polyomavirus infections in renal transplant recipients and healthy subjects. J Clin Virol. 2010;49:137–140. doi: 10.1016/j.jcv.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Polz D, Stec A, Polz-Dacewicz M. BK-virus (BKV) – structure, epidemiology and pathogenesis. J Pre-Clin Clin Res. 2013;7:90–92. [Google Scholar]
- 501.Bassil N, Rostaing L, Mengelle C, Kallab S, Esposito L, Guitard J, Cardeau-Desangles I, Weclawiak H, Izopet J, Kamar N. Prospective monitoring of cytomegalovirus, Epstein-Barr virus, BK virus, and JC virus infections on belatacept therapy after a kidney transplant. Exp Clin Transplant. 2014;12:212–219. [PubMed] [Google Scholar]
- 502.Calvignac-Spencer S, Feltkamp MC, Daugherty MD, Moens U, Ramqvist T, Johne R, Ehlers B. A taxonomy update for the family Polyomaviridae. Arch Virol. 2016;161:1739–1750. doi: 10.1007/s00705-016-2794-y. [DOI] [PubMed] [Google Scholar]


