Abstract
AIM
To investigate the therapeutic potential of vasculotide (VT) - a Tie2 activating therapeutic - in kidney transplantation.
METHODS
We performed a murine MHC-mismatched renal transplant model (C57Bl/6 male into Balb/c female) with 60 min cold and 30 min warm ischemia time. 500 ng VT was administered i.p. to donor mice 1 h before organ removal. In addition, recipients received 500 ng VT i.p. directly and 3 d after surgery. Survival was monitored and remaining animals were sacrificed 28 d after transplantation. In this model, we analyzed: (1) organ function; (2) Kaplan-Meier survival; (3) organ damage (periodic acid Schiff staining) via semi-quantitative scoring [0-4 (0 = no injury/inflammation to 4 = very severe injury/inflammation)]; (4) expression of renal endothelial adhesion molecules (ICAM-1) via immunofluorescence (IF) staining, immunoblotting and qPCR; (5) infiltration of inflammatory cells (IF Gr-1, F4/80); and (6) fibrosis via staining of α-smooth muscle actin (αSMA), Sirius red staining and immunoblotting of SMAD3 activation.
RESULTS
Exogenous activation of Tie2 with VT resulted in diminished expression of peritubular and glomerular endothelial adhesion molecules. Consequently, infiltration of inflammatory cells (analyzed as ICAM-1, Gr-1 and F4/80 positive cells) was reduced in VT-treated mice compared to controls. Additionally, VT was protective against fibrogenesis after kidney transplantation. Trends towards lower serum creatinine (vehicle: 142 ± 17 μmol/L vs VT: 94 ± 23 μmol/L), urea (vehicle: 76 ± 5 mmol/L vs VT: 60 ± 8 mmol/L) and lactate dehydrogenase (vehicle: 1288 ± 383 iU vs VT: 870 ± 275 iU) were observed on day 6 after transplantation. Kaplan-Meier survival analysis showed improved survival rates in the VT-treated mice that did not reach statistical significance (27% vs 54%, P = 0.24, n = 11 per group). Exogenous activation of Tie2 via VT might reduce infiltration of inflammatory cells into renal tissue thereby protecting the transplant from early graft dysfunction potentially affecting long-term function.
CONCLUSION
Protection of the endothelial microvasculature via the Tie2 axis in the early transplant setting might hold promise as a therapeutic target.
Keywords: Vasculotide, Tie2, Kidney transplantation, Endothelium, Angiopoietin
Core tip: Activation of the Tie2 receptor has been shown to be beneficial in different models of disease. Here, we demonstrate that agonistic stimulation of Tie2 via the drug-like putative therapeutic termed “vasculotide” (VT) ameliorates outcome in a murine MHC-mismatched kidney transplant model. VT treatment (i.e., activation of endothelial Tie2) prevented inflammation and fibrosis thereby preserving graft function. Moreover, single administration at the time of transplantation was also sufficient to prolong survival compared to control group.
INTRODUCTION
Graft failure and ultimately graft loss are still major problems in solid organ transplantation. The endothelium hereby plays a pivotal role in mediating inflammation and subsequent organ dysfunction. In general, a healthy endothelium is essential for vascular homeostasis, and preservation of endothelial cell (EC) function is critical for maintaining transplant allograft function. Damage to the microvascular ECs is a characteristic feature of acute vascular rejection, an important predictor of later graft function and loss[1]. Innovative therapeutic strategies preventing IRI and maintaining stable renal function are highly desirable.
The angiopoietin (Angpt)/Tie2 system consists of the transmembrane endothelial tyrosine kinase Tie2 and its four circulating ligands (Angpt1-4)[2-5]. This system regulates baseline endothelial barrier function and its response to injury[6,7]. Previous work has shown that the balance between the Tie2 agonist (Angpt-1) and the antagonist (Angpt-2) controls Tie2 phosphorylation[6]. Angpt-1 which is mainly secreted by pericytes binds Tie2 as a natural agonist thereby promoting vascular quiescence[8]. Canonical downstream effects of Tie2 signaling are activation of PI3K/Akt[9,10], inhibition of the inflammatory transcription factor NFκB[11] and consecutive control of adhesion molecule expression[12] as well as cytoskeletal regulation via the scaffolding protein IQGAP1[13]. All together Tie2 activation promotes an anti-inflammatory, pro-survival, and anti-permeability phenotype of the vasculature. In contrast, Angpt-2 which is released from ECs upon pro-inflammatory stimuli inhibits Tie2 phosphorylation and consequently disrupts protective Tie2 signaling[14].
Few data indicate a beneficial role of Tie2 activation in solid organ transplantation. In kidney transplant recipients, it has been shown that increased Angpt-2 levels (the natural Tie2 antagonist) correlate with mortality indicating that a dysbalanced Angpt/Tie2 system might be unfavorable in renal transplantation[15]. Interestingly, it has very recently been demonstrated that a chimeric Angpt-1 mimetic, termed COMP-Ang1, is able to reduce endothelial permeability and inflammation in a murine heart transplantation model[16].
Vasculotide (VT) - a PEGylated synthetic Tie2 agonistic peptide (CHHHRHSF) - has proven its potency to activate Tie2 in vivo even stronger and longer than its natural ligand Angpt-1. The therapeutic use of VT was first described in a murine diabetes model where it improved wound healing[17]. Additionally, we and others have shown that VT can reduce vascular leakage and endothelial inflammation in different murine models of acute systemic inflammation[18-21].
Given the beneficial properties of Tie2 activation on multiple levels of intracellular signaling with clinically relevant functional effects, we hypothesized that exogenous manipulation of the Angpt/Tie2 system might be protective in transplantation. To test this, we exogenously activated the Tie2 receptor with VT. The aim of our study was to investigate the potential beneficial effects of VT treatment in a murine kidney transplant model on graft function. We analyzed inflammation, fibrous tissue deposition, renal function and overall survival to better understand if Tie2 activation might improve outcome after transplantation.
MATERIALS AND METHODS
Mouse studies and experimental design
All experiments were approved by the local authorities and conducted in accordance with institutional and governmental guidelines. Mice were housed in a room with 12 h day/night cycle, constant temperature and humidity as well as water and food ad libitum. All appropriate measures were taken to minimize pain or discomfort. Eight-week-old male C57Bl/6 or Balb/c mice were purchased from Charles River Laboratories (Sulzfeld, Germany). Briefly, kidneys from C57Bl/6 male (donor) were transplanted into Balb/c female (recipient) (n = 23). Donor mice received 500 ng VT (n = 11) or vehicle (PBS) (n = 11) intraperitoneally (i.p.) 1h prior to surgery. Recipients were injected with 500 ng VT or vehicle directly and on day 3 after kidney transplantation i.p.. Dosage of VT was carefully adjusted before[18]. Mice were anesthetized with isofluorane and the donor kidney, ureter, and bladder were harvested en block, including the renal artery with a small aortic cuff and the renal vein. Cold ischemia time is 60, and warm ischemia time 30 min. After explantation, kidneys are stored in vehicle solution at 4 °C for 60 min. These ischemia times induce a moderate degree of ischemia-reperfusion injury (IRI) in this model. After left nephrectomy of the recipient, vascular cuff and vein are anastomosed to the recipient abdominal aorta and vena cava, respectively, below the level of the native renal vessels. The ureter is directly anastomosed into the bladder. A second dose of VT or vehicle was administered systemically (i.v.) 30 min post-transplantation. The right native kidney was removed on post-transplantation day 4 so that survival becomes graft dependent. Within a given experiments/analysis, we only used samples from single mice. We did not pool samples to increase protein amounts. Blood was taken on days 0, 6, 14, 21 and 28. Survivors were sacrificed 28 d after transplantation for further analysis. Renal function was estimated by serum lactate dehydrogenase (LDH), creatinine and urea measurements (Olympus).
Antibodies and reagents
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. GR-1 (AbD Serotec, MCA7716), F4/80 (Biolegend, 122602), Alexa Fluor 555 (Life Technologies), intercellular adhesion molecule (ICAM-1) (M-19) (Santa Cruz, sc-1511), α-smooth muscle actin (αSMA) (Abcam, ab7817) and pSMAD3 (Cell Signaling, C25A9) were utilized for immunoblot or immunohistochemistry. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (FL-335) (Santa Cruz, 25778) served as loading control for immunoblots.
Immunoblotting
Protein was extracted by using RIPA buffer [including 1 mmol/L Na3VO4, 50 mmol/L NaF, protease inhibitors (Roche Diagnostics, Mannheim, Germany)] and resolved with a 10% polyacrylamide gel, followed by blotting on a polyvinylidene fluoride membrane (Merck Millipore, Darmstadt, Germany). Membranes were blocked with 3% bovine serum albumin and incubated with a primary antibody overnight (4 °C). Incubation with the second antibody was performed for 1 h at room temperature. All washing steps were carried out in TBST [20 mmol/L Tris, 150 mmol/L NaCl, 0.1% Tween20 (Merck)]. Bands were visualized with SuperSignal™ West Pico Chemiluminescent Substrate (Life Technologies) and Versa Doc Imaging System Modell 3000 (BioRad).
Immunohistochemistry
Ice-cold acetone-fixed cryosections (6 μm) were blocked with 10% donkey serum (Dianova) and stained with primary antibodies against ICAM-1. Paraformaldehyde-fixed (Merck, Darmstadt, Germany) and paraffin-embedded tissue sections (1.5 μm) were dehydrated and rehydrated with ascending and descending ethanol series including deparaffinising with Histoclear (Biozym, Hessisch Oldendorf, Germany). After blocking with 10% donkey serum, paraffin sections were stained with primary and a secondary antibody. Mounting was accomplished with VectaShield DAPI (Vector Laboratories Inc., Burlingame, CA).
Periodic acid Schiff and sirius red staining
Paraffin-embedded sections were prepared as described above. Periodic acid Schiff staining was performed with periodic acid (0.5%) (Merck), Schiff’s reagent (Merck) and hematoxylin (Fluka). For Sirius red staining, sections were treated with 0.2% phosphomolybdic acid, 0.1% Sirius red in 3% picric acid, 0.01 mol/L HCl, 70% and 100% ethanol in the order specified.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from murine kidneys using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Equal amounts of total RNA were reverse transcribed with the Transcriptor First Strand cDNA Synthesis kit from Roche Diagnostics. Real-time-quantitative polymerase chain reaction (RT-qPCR) was performed by a LightCycler 480 II (Roche). Triplicate RT-qPCR analyses were executed for each sample, and the obtained threshold cycle values (CT) were averaged. Gene expression was normalized to the expression of the housekeeping gene, yielding the ∆CT value.
Statistical analysis
Statistical significance was assessed by independent samples and unpaired t test as well as Mann-Whitney test as indicated. Survival data were analyzed by Log-Rank test. All experimental results are presented as mean ± SEM or median and a two-tailed P value of less than 0.05 was considered to be statistical significant. Analysis and graph generation were performed in GraphPad Prism 6.0 (La Jolla, CA).
RESULTS
VT improves renal transplant function and survival
Given the beneficial properties of Tie2 activation on the endothelial function, we hypothesized that early exogenous activation of Tie2 might also be beneficial in long-term transplant function. Therefore, we established an MHC-incompatible murine kidney transplant model[22] and treated the mice with 2 doses of VT or vehicle control. Serial blood measurements after transplantation (day 3, 6, 14, 21, 28) showed that renal function was indeed slightly improved upon VT treatment at early time points (serum creatinine vehicle-treated (n = 8): 142 ± 17 μmol/L vs VT-treated (n = 9): 94 ± 23 µmol/L, P = 0.12; urea level vehicle-treated (n = 8): 76 ± 5 mmol/L vs VT-treated (n = 10): 60 ± 8 mmol/L, P = 0.13 using unpaired t test) was observed on day 6 (Figure 1A and B). LDH as a broad surrogate marker for cell death showed a similar trend in vehicle-treated animals compared to the VT group potentially indicating that VT might reduce apoptosis/necrosis [vehicle-treated (n = 7): 1288 ± 383 iU vs VT-treated (n = 8): 870 ± 275 iU, P = 0.38 using unpaired t test] (Figure 1C). Additionally, we analyzed survival after transplantation and observed a trend towards improved survival in VT- compared to vehicle-treated mice (27% vs 54%, P = 0.24, n = 11) (Figure 1D). Together, these data indicate that early VT treatment might improve kidney function after renal transplantation.
Figure 1.
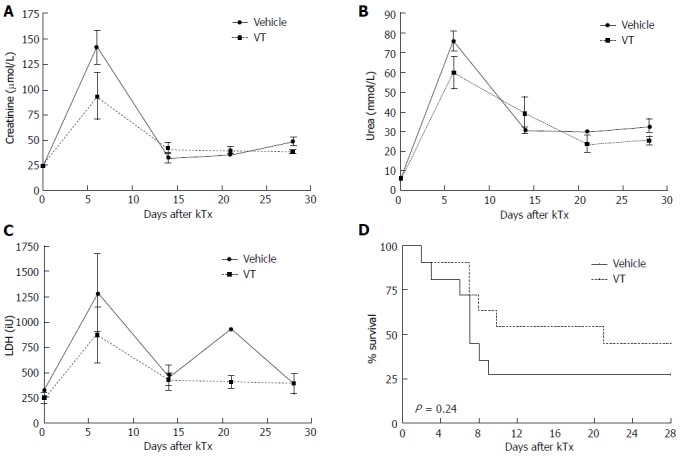
Vasculotide shows trends to improved kidney function and survival in an MHC-mismatched renal transplant model. C57Bl/6 donor mice received 500 ng VT or vehicle prior to surgery (-1 h). Balb/c recipients were injected with 500 ng VT i.p. or vehicle directly and on day 3 after kidney transplantation. A-C: Kidney function [serum creatinine, urea and lactate dehydrogenase (LDH) levels] was monitored on day 6, 14, 21 and 28 after transplantation in murine serum and analyzed with unpaired t test (day 6, n = 7-10); D: Kaplan-Meier survival after MHC-mismatch kidney transplantation (n = 11 per group). VT: Vasculotide.
Infiltration of inflammatory cells is diminished upon VT treatment
We next studied histological changes to investigate graft rejection and inflammation in transplanted kidneys. One can easily appreciate the glomerular as well as the interstitial inflammatory infiltrates in the vehicle-treated mice on day 28 after transplantation (Figure 2A and C, left side). In the lower left panel (Figure 2C, left side) almost no intact tubular structures are detectable anymore. VT treated mice exhibited a much weaker inflammatory burden both in the glomerulus as well as the interstitium (Figure 2, right side). These results were confirmed by a histological semi-quantification (Table 1) regarding interstitial inflammation [vehicle (n = 3): Median = 4.0 (25% quartile: 4.0%-75% quartile: 4.0) vs VT-treated (n = 5): 2.0 (2.0-2.0), P = 0.02 using Mann-Whitney test] and glomerular injury [vehicle (n = 3): 4.0 (3.0-4.0) vs VT-treated (n = 5): 2.0 (1.0-2.0), P = 0.04 using Mann-Whitney test] (Figure 2B and D). These results suggest that two early doses of VT are sufficient to reduce infiltration of inflammatory cells into the graft thereby potentially preventing graft dysfunction and rejection.
Figure 2.
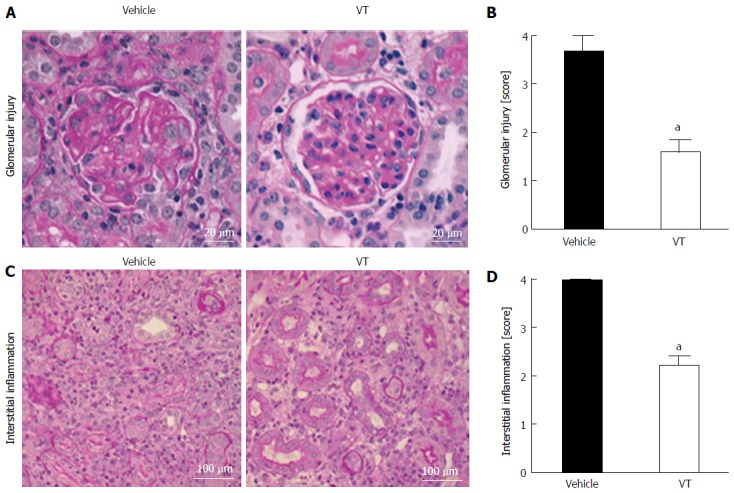
Vasculotide-treated mice show less infiltration of inflammatory cells in the kidney. A: Exemplary PAS staining of kidneys from mice treated with vehicle or VT. Glomerular injury was evaluated on day 28 after transplantation; B: Semi-quantification of glomerular injury by surveying kidney cross-sections. Scoring was done as described above (see Table 1) (vehicle n = 3; VT n = 5) (aP < 0.05); C: PAS staining for peritubular injury in transplanted kidneys from mice treated with vehicle or VT; D: Semi-quantification of interstitial inflammation by surveying kidney cross-sections from transplanted mice (aP < 0.05). Scoring was done as described above (Table 1) (vehicle n = 3; VT n = 5). Semi-quantification was assessed using Mann-Whitney test. VT: Vasculotide; PAS: Periodic acid Schiff.
Table 1.
Scoring system for semiquantification of periodic acid Schiff staining
| Interstitial inflammation | 0 = no interstitial inflammation, < 5% of interstitium affected 1 = mild interstitial inflammation, 5%-25% of interstitium affected 2 = moderate interstitial inflammation, 25%-50% of interstitium affected 3 = severe interstitial inflammation, 50%-75% of interstitium affected 4 = very severe interstitial inflammation, > 75% of interstitium affected |
| Glomerular injury | 0 = no glomerular injury 1 = mild glomerular injury, < 10% of glomeruli damaged 2 = moderate glomerular injury, 10%-50% of glomeruli damaged 3 = severe glomerular injury, 50%-75% of glomeruli damaged 4 = very severe glomerular injury, > 75% of glomeruli damaged |
VT reduces vascular inflammation and tissue infiltration
Keeping in mind the profound histological changes indicating that VT prevents infiltration of immune cells, we wanted to further analyze vascular inflammation and the infiltrative cell population. Therefore, we performed fluorescent immunohistochemistry for ICAM-1, for Gr-1 (a marker of granulocytes), as well as F4/80 (macrophages). Kidney cross-sections from VT-treated mice exhibit much less ICAM-1 expression than vehicle-treated mice (Figure 3A). Additionally, whole kidney homogenates also depicted less ICAM-1, as shown by immunoblotting (Figure 3D). Presumably as a consequence of less adhesion molecule expression we also noted a reduction of Gr-1 and F4/80 in the peritubular interstitium of VT-treated mice (Figure 3B and C). Together these data indicate that early VT regulates vascular adhesion molecule expression thereby reducing overwhelming tissue infiltration of inflammatory cells in the later post-transplant course.
Figure 3.
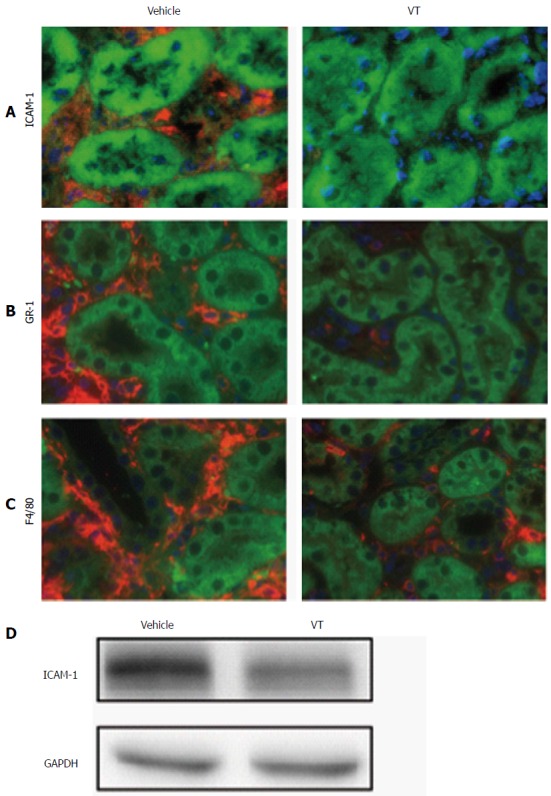
Vasculotide treatment reduces vascular inflammation and tissue infiltration in kidney transplantation. Fluorescent immunohistochemistry for (A) ICAM-1 (red), (B) Gr-1 (red) and (C) F4/80 (red) in kidney cross-sections of transplanted mice (vehicle or VT-treated) on day 28 after transplantation. Autofluorescence is shown in green. Images are exemplary for n = 5/condition; D: Immunoblot of murine kidney homogenate for ICAM-1 and GAPDH for the same conditions. VT: Vasculotide; ICAM-1: Intercellular adhesion molecule; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
VT ameliorates fibrosis progression
Fibrosis as a consequence of acute or chronic inflammation is a key contributor to organ dysfunction. After kidney transplantation we observed an increased expression of αSMA, a broad marker of fibrosis (Figure 4A and B, left side). However, upon VT treatment tubular as well as glomerular damage were reduced with regard to αSMA expression (Figure 4A and B, right side). To further substantiate our finding, we visualized collagen fibers by Sirius red (Figure 4C) and observed profound differences between vehicle and VT-treated mice. VT appears to prevent inflammation-driven collagen formation. Pathological phosphorylation of SMAD3 a canonical downstream target of TGFβ signaling after transplantation was also reduced in mice treated with VT (Figure 4D). Taken together, VT might prevent the induction of TGFβ signaling and collagen formation leading to reduced fibrosis and organ dysfunction.
Figure 4.
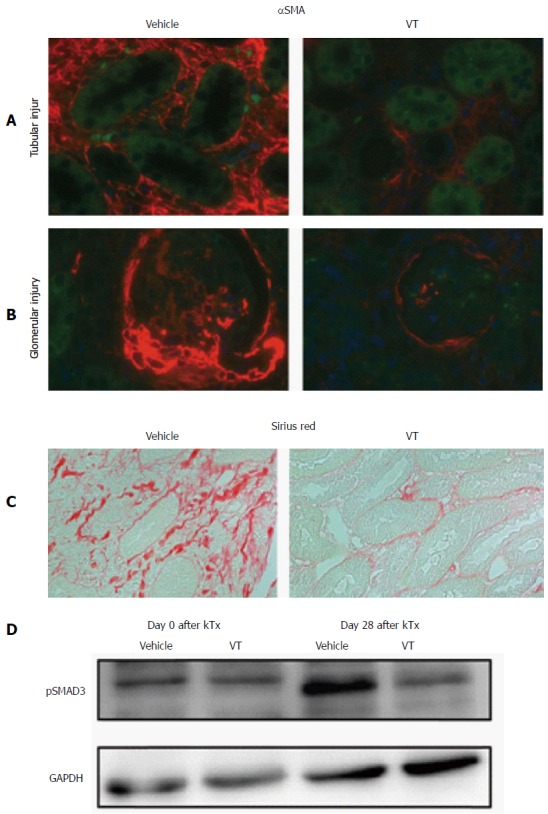
Vasculotide ameliorates fibrogenesis. Fluorescent immunohistochemistry of α-smooth-muscle-actin (red) regarding (A) tubular and (B) glomerular injury in kidney cross-sections of transplanted mice (vehicle or VT-treated) on day 28 after transplantation. Autofluorescence is shown in green. Images are exemplary for n = 5/condition; C: Sirius red staining for the same conditions (D) Immunoblot of murine kidney homogenates for pSMAD3 in healthy (day 0) and kidney transplanted mice (day 28) upon vehicle or VT treatment. VT: Vasculotide; αSMA: α-smooth-muscle-actin; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
VT does not prevent induction of inflammation on the transcriptional level
To further investigate the anti-inflammatory properties of VT in murine kidney transplantation, we analyzed different markers of inflammation (ICAM-1, VCAM-1, TGFβ, collagen-1, collagen-3 and fibronectin) on the transcriptional level. Notably, we detected a dramatic upregulation of these markers in vehicle- and VT-treated animals on day 28 after transplantation compared to explanted donor kidneys on day 0. Despite the differences on protein level demonstrating that VT-treatment indeed reduces inflammation in a murine renal transplant model, differences on the transcriptional are not present on day 28 after transplantation (Figure 5 analyzed using Mann-Whitney test, n = 3-5).
Figure 5.
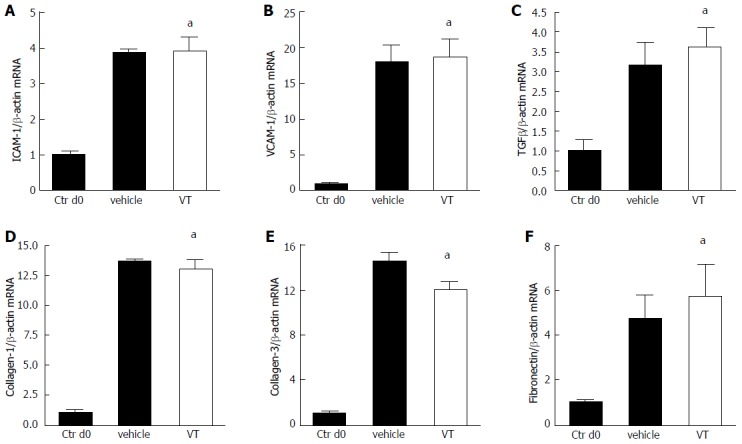
Anti-inflammatory properties of vasculotide are not regulated on the transcriptional level. A-F: Mice were treated with vehicle (n = 3) or VT (n = 5) and underwent kidney transplantation. Transplanted kidneys were harvested on day 28 after transplantation. Explanted donor kidneys from day 0 served as control (n = 3). Expression levels of Intercellular adhesion molecule (ICAM-1), Vascular cell adhesion protein 1 (VCAM-1), Transforming growth factor β (TGFβ), collagen-1, collagen-3 and fibronectin in kidney homogenates were determined via RT-qPCR and analyzed using Mann-Whitney test (aP < 0.05). VT: Vasculotide.
DISCUSSION
The endothelium plays an important role in maintaining organ function and homeostasis in health and disease. As part of the rejection process of solid organ transplants, the endothelium is characterized by a highly activated proinflammatory phenotype. In routine kidney transplant pathology this has nowadays been implicated in the grading of rejection by using a so-called C4d staining that does reflect complement activation in the endothelium[23]. We therefore hypothesized that pharmacological stabilization of the vasculature might be beneficial.
Our approach demonstrated that exogenous activation of the endothelium-stabilizing Tie2 receptor with the drug-like compound, termed VT, might prevent graft dysfunction and inflammation. Administration of VT showed trends toward improved organ function and survival in a renal transplant model. One beneficial effect of VT in kidney transplantation could be attributed to phosphorylation of the Tie2 receptor thereby activating the PI3K/Akt pathway and suppressing NFκB signaling. This assumed canonical mechanisms of action of VT resulted in reduced tissue infiltration of immune cells and expression of endothelial adhesion molecules. Furthermore, early VT administration was sufficient to ameliorate classical fibrogenic signaling (e.g., SMAD3/TGFβ) thereby reducing collagen formation and the development of fibrosis. Interestingly, we could not detect any transcriptional regulation of neither adhesion molecules nor inflammatory mediators. How VT regulates endothelial inflammation has to be thoroughly investigated in future projects.
Additionally, organ function in VT treated mice was slightly better at early time points exclusively. Due to the fact, that animals were treated at day 0 and 3 after transplantation, the beneficial effect of VT would be expected to decrease over time. Re-dosing could further ameliorate outcome after kidney transplantation. To investigate and improve the beneficial effects of VT in kidney transplantation, pharmacokinetics of this Tie2 agonist need to be further investigated. Most experimental data on VT that showed improved outcome are derived from acute short-term injury models, such as sepsis and influenza[19,21]. These data confirm however that the endothelium indeed plays an important role in the pathogenesis of various medical conditions and that maintaining endothelial homeostasis early in the pathogenesis might provide protection. Some work on slow-progressing disease models, such as diabetes and tumor growth used extensive re-dosing of VT to maintain beneficial effects at the highest possible level[17,24].
Due to the small number of animals that survived until day 28, we were not able to include more animals into our studies. Nevertheless, our VT-treated mice show a clear trend towards improvement after kidney transplantation indicating a potential type II error in our statistical analysis.
Another aspect that we did not investigate but that is - at least theoretically - of high relevance is the putative long-term effect of an early short-term VT treatment. It might very well be that an improved early graft function has relevant implications for long term graft performance, as our histological data at day 28 suggest and as it has been demonstrated for delayed graft function[25].
In summary, our study demonstrated that early VT treatment slightly improves graft function in an MHC-mismatched kidney transplant model potentially via regulation of endothelial activation and transmigration of harmful inflammatory cells into the transplant’s interstitium. The Tie2 agonistic strategy might hold promise as a potential therapeutic in transplant medicine and future examination of long-term results are highly desirable.
ACKNOWLEDGMENTS
We thank Yvonne Nicolai for technical assistance.
COMMENTS
Background
Early graft dysfunction as well as acute rejection after solid organ transplantation are characterized by a proinflammatory microvascular endothelium. The Angiopoietin/Tie2 system plays an important role in maintenance of baseline endothelial barrier function and its response to injury. Activation (i.e., phosphorylation) of the Tie2 receptor promotes endothelial homeostasis as well as anti-inflammatory properties. The authors therefore analyzed the potential of the Tie2 activating drug-like compound “vasculotide” (VT), as a novel therapeutic strategy in an MHC-mismatched renal transplant model.
Research frontiers
As long as the mystery of tolerance remains unsolved pharmacotherapy for transplanted patients is obligatory based on immunosuppressive regimens that come with a high burden of adverse events. Novel approaches aim to find therapeutic strategies that do not weaken the host or graft function. Here, the authors present a putative approach that promotes vascular stability/quiescence thereby preventing graft dysfunction and loss.
Innovations and breakthroughs
Targeting the endothelium as a direct interface between self and non-self offers the opportunity to interfere with graft specifically at the site of rejection.
Applications
The synthetic Tie2 agonist VT promotes vascular quiescence and improves graft function after allogenic solid organ transplantation. The potency of VT has recently been demonstrated in different models of vascular diseases underlining its potential therapeutic relevance. In the future, toxicity studies and first clinical trials are planned, specifically for the treatment of acute kidney injury.
Terminology
Angpt: Angiopoietin; αSMA: α-smooth muscle actin; EC: Endothelial cell; ICAM-1: Intercellular adhesion molecule; LDH: Lactate dehydrogenase; VT: Vasculotide.
Peer-review
This is an interesting study in which authors show that VT - a synthetic Tie2 agonist- may improved renal transplant outcome.
Footnotes
Supported by The Else-Kröner Fresenius Stiftung, No. 2013_A154; and the German Research Foundation, No. DA 1209/4-1 (to David S).
Institutional review board statement: The study was reviewed and approved by the MHH Institutional Review Board.
Institutional animal care and use committee statement: All experiments were approved by the local authorities and conducted in accordance with institutional and governmental guidelines (AZ12/0843).
Conflict-of-interest statement: Vasculotide has been patented and is being commercialized by Vasomune Therapeutics. Van Slyke P and Dumont DJ are co-inventors of Vasculotide and Van Slyke P is the Chief Scientific Officer, while Dumont DJ sits on the Scientific Advisory Board. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: March 31, 2016
First decision: May 17, 2016
Article in press: July 18, 2016
P- Reviewer: Gonzalez-Reimers E, Sun CK S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Piotti G, Palmisano A, Maggiore U, Buzio C. Vascular endothelium as a target of immune response in renal transplant rejection. Front Immunol. 2014;5:505. doi: 10.3389/fimmu.2014.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 3.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura M, Miki T, Yashima R, Yokoi N, Yano H, Sato Y, Seino S. Angiopoietin-3, a novel member of the angiopoietin family. FEBS Lett. 1999;448:254–256. doi: 10.1016/s0014-5793(99)00381-6. [DOI] [PubMed] [Google Scholar]
- 5.Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1999;96:1904–1909. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 7.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 9.Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–959. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 10.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O‘Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 11.Hughes DP, Marron MB, Brindle NP. The antiinflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-kappaB inhibitor ABIN-2. Circ Res. 2003;92:630–636. doi: 10.1161/01.RES.0000063422.38690.DC. [DOI] [PubMed] [Google Scholar]
- 12.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 13.David S, Ghosh CC, Mukherjee A, Parikh SM. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol. 2011;31:2643–2652. doi: 10.1161/ATVBAHA.111.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molnar MZ, Kümpers P, Kielstein JT, Schiffer M, Czira ME, Ujszaszi A, Kovesdy CP, Mucsi I. Circulating Angiopoietin-2 levels predict mortality in kidney transplant recipients: a 4-year prospective case-cohort study. Transpl Int. 2014;27:541–552. doi: 10.1111/tri.12293. [DOI] [PubMed] [Google Scholar]
- 16.Syrjälä SO, Nykänen AI, Tuuminen R, Raissadati A, Keränen MA, Arnaudova R, Krebs R, Koh GY, Alitalo K, Lemström KB. Donor Heart Treatment With COMP-Ang1 Limits Ischemia-Reperfusion Injury and Rejection of Cardiac Allografts. Am J Transplant. 2015;15:2075–2084. doi: 10.1111/ajt.13296. [DOI] [PubMed] [Google Scholar]
- 17.Van Slyke P, Alami J, Martin D, Kuliszewski M, Leong-Poi H, Sefton MV, Dumont D. Acceleration of diabetic wound healing by an angiopoietin peptide mimetic. Tissue Eng Part A. 2009;15:1269–1280. doi: 10.1089/ten.tea.2007.0400. [DOI] [PubMed] [Google Scholar]
- 18.David S, Ghosh CC, Kümpers P, Shushakova N, Van Slyke P, Khankin EV, Karumanchi SA, Dumont D, Parikh SM. Effects of a synthetic PEG-ylated Tie-2 agonist peptide on endotoxemic lung injury and mortality. Am J Physiol Lung Cell Mol Physiol. 2011;300:L851–L862. doi: 10.1152/ajplung.00459.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumpers P, Gueler F, David S, Slyke PV, Dumont DJ, Park JK, Bockmeyer CL, Parikh SM, Pavenstadt H, Haller H, et al. The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit Care. 2011;15:R261. doi: 10.1186/cc10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rübig E, Stypmann J, Van Slyke P, Dumont DJ, Spieker T, Buscher K, Reuter S, Goerge T, Pavenstädt H, Kümpers P. The Synthetic Tie2 Agonist Peptide Vasculotide Protects Renal Vascular Barrier Function In Experimental Acute Kidney Injury. Sci Rep. 2016;6:22111. doi: 10.1038/srep22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiyama MG, Armstrong SM, Wang C, Hwang D, Leong-Poi H, Advani A, Advani S, Zhang H, Szaszi K, Tabuchi A, et al. The Tie2-agonist Vasculotide rescues mice from influenza virus infection. Sci Rep. 2015;5:11030. doi: 10.1038/srep11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong S, Lewis AG, Kunter U, Haller H, Gueler F. A knotless technique for kidney transplantation in the mouse. J Transplant. 2012;2012:127215. doi: 10.1155/2012/127215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu FT, Lee CR, Bogdanovic E, Prodeus A, Gariépy J, Kerbel RS. Vasculotide reduces endothelial permeability and tumor cell extravasation in the absence of binding to or agonistic activation of Tie2. EMBO Mol Med. 2015;7:770–787. doi: 10.15252/emmm.201404193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geddes CC, Woo YM, Jardine AG. The impact of delayed graft function on the long-term outcome of renal transplantation. J Nephrol. 2002;15:17–21. [PubMed] [Google Scholar]


