Abstract
AIM
To describe the thromboelastography (TEG) “reference” values within a population of liver transplant (LT) candidates that underline the differences from healthy patients.
METHODS
Between 2000 and 2013, 261 liver transplant patients with a model for end-stage liver disease (MELD) score between 15 and 40 were studied. In particular the adult patients (aged 18-70 years) underwent to a first LT with a MELD score between 15 and 40 were included, while all patients with acute liver failure, congenital bleeding disorders, and anticoagulant and/or antiplatelet drug use were excluded. In this population of cirrhotic patients, preoperative haematological and coagulation laboratory tests were collected, and the pretransplant thromboelastographic parameters were studied and compared with the parameters measured in a previously studied population of 40 healthy subjects. The basal TEG parameters analysed in the cirrhotic population of liver candidates were as follows: Reaction time (r), coagulation time (k), Angle-Rate of polymerization of clot (α Angle), Maximum strenght of clot (MA), Amplitudes of the TEG tracing at 30 min and 60 min after MA is measured (A30 and A60), and Fibrinolysis at 30 and 60 min after MA (Ly30 and Ly60). The possible correlation between the distribution of the reference range and the gender, age, MELD score (higher or lower than 20) and indications for transplantation (liver pathology) were also investigated. In particular, a MELD cut-off value of 20 was chosen to verify the possible correlation between the thromboelastographic reference range and MELD score.
RESULTS
Most of the TEG reference values from patients with end-stage liver disease were significantly different from those measured in the healthy population and were outside the suggested normal ranges in up to 79.3% of subjects. Wide differences were found among all TEG variables, including r (41.5% of the values), k (48.6%), α (43.7%), MA (79.3%), A30 (74.4%) and A60 (80.9%), indicating a prevailing trend to hypocoagulability. The differences between the mean TEG values obtained from healthy subjects and the cirrhotic population were statistically significant for r (P = 0.039), k (P < 0.001), MA (P < 0.001), A30 (P < 0.001), A60 (P < 0.001) and Ly60 (P = 0.038), indicating slower and less stable clot formation in the cirrhotic patients. In the cirrhotic population, 9.5% of patients had an r value shorter than normal, indicating a tendency for faster clot formation. Within the cirrhotic patient population, gender, age and the presence of hepatocellular carcinoma or alcoholic cirrhosis were not significantly associated with greater clot firmness or enhanced whole blood clot formation, whereas greater clot strength was associated with a MELD score < 20, hepatitis C virus and cholestatic-related cirrhosis (P < 0.001; P = 0.013; P < 0.001).
CONCLUSION
The range and distribution of TEG values in cirrhotic patients differ from those of healthy subjects, suggesting that a specific thromboelastographic reference range is required for liver transplant candidates.
Keywords: Thromboelastography, Liver cirrhosis, Blood coagulation disorder, Liver transplantation, Reference values
Core tip: Thromboelastography provides a more comprehensive coagulation assessment than routine tests in cirrhotic patients. We evaluated the baseline thromboelastography (TEG) tracing and preoperative laboratory tests of cirrhotic patients undergoing liver transplant (LT) to generate a reliable picture of their coagulation profile. We also studied how TEG value distribution in cirrhotic patients could be modified by gender, age, model for end-stage liver disease score and liver disease characteristics. End-stage liver disease is associated with considerable changes in TEG variables, which should be allowed for when interpreting TEG traces in cirrhotic patients. TEG reference values derived from a healthy population could be misleading in the management of cirrhotic patients during LT.
INTRODUCTION
Laboratory evaluations of bleeding disorders have been conducted with standard clotting assays such as prothrombin time (PT) and activated partial thromboplastin time (PTT) for a long time. However, standard laboratory tests fail to give comprehensive information about the bleeding tendency of cirrhotic patients. Tripodi et al[1] showed that patients suffering from chronic liver disease as well as healthy subjects have the ability to generate the same amount of thrombin in stable liver disease conditions.
PT International Normalized Ratio (INR) tests performed in the absence of thrombomodulin are of little use in representing the real state of coagulation in cirrhotic patients. Furthermore, such tests are not standardized across centres when they are used for patients with liver disease[2,3].
Because of these limits, the interest in assays performed with thromboelastography (TEG), which offers a more targeted approach to assess the overall outcome of the interactions of clotting factors beyond the initiation of clot formation, has progressively increased. However, even though thromboelastography is a useful tool for measuring global haemostasis during hepatic surgery and liver transplant, allowing the optimization of blood product selection and usage, its methodology is not standardized. Normal TEG values, as reported by manufacturers and in the literature, are determined from the average clotting time of healthy volunteers[4]. Although investigators have tested the correlation between TEG values and the risk of bleeding in various surgical populations[5,6], it is possible that standard TEG cut-off values derived from a healthy population have a different and misleading meaning in the management of cirrhotic patients during liver transplantation (LT). Addressing the issue of the reference values, the TEG analyzer manufacturer suggests that each new user should tests 20 healthy volunteers to generate normal values to be used locally as reference values at each institution, prior to clinical use[7]. The consequence is that TEG suffers from a lack of proven reliability[8,9], also motivated by the large range of normal values. However, this wide normal range defined for healthy people, is unreliable when applied to patients with liver disease, making it necessary to define thromboelastographic “reference ranges” for cirrhotic patients.
Under physiological conditions, the haemostatic system of these patients reaches a new equilibrium determined by a parallel decline of the pro- and anticoagulant drivers, which is represented by specific thromboelastographic values[10]. The main aim of the present study was to describe the thromboelastographic preoperative coagulation condition of cirrhotic patients undergoing liver transplant to generate a more reliable picture of their common coagulation profile. A further aim of the study was to compare the TEG range distribution of cirrhotic patients with a population of healthy subjects, verifying that the range corrected for cirrhotic patients could be modified by gender, age and model for end-stage liver disease (MELD) score as well as liver disease characteristics.
MATERIALS AND METHODS
Between 2000 and 2013, 473 patients underwent LT in Liver Transplant Center of Policlinico di Modena (Italy). After the approval of the local Ethical Authority and the receipt of written informed consent, the thromboelastographic parameter distribution of a selected population of cirrhotic patients was studied according to the following inclusion and exclusion criteria: adult patients (aged 18-70 years), first LT, and MELD score between 15 and 40. The exclusion criteria were as follows: acute liver failure, congenital bleeding disorders (i.e., haemophilia A and B), and anticoagulant and/or antiplatelet drug use. Therefore, the analysis was performed in 261 (55%) patients who underwent LT. A MELD score between 15 and 40 was chosen because it is the most frequently used in the literature, and the AISF (Italian Association for Liver Study) also recommends it for listing a patient for LT[11]. In this population of cirrhotic patients, preoperative haematological and coagulation laboratory tests were collected, and thromboelastographic traces were studied and compared with those obtained from a previously studied population of 40 healthy subjects. The study protocol approved by the Institutional Review Board of Azienda Ospedaliera-Universitaria, Modena (N°:139/14 TRIGGER) was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Blood samples were collected with the double-syringe technique from a clean venipuncture. The first 6 mL of each sample was discarded. All the healthy subjects (20 males and 20 females), selected from among residents, students and nurses, had not taken drugs known to affect coagulation parameters or platelet aggregation for at least 1 wk before the collection of blood samples.
Distribution ranges of the basal TEG parameters (r, k, α, MA, A30, A60, Ly30 and Ly60) in the cirrhotic population of patients were analysed. The possible correlation of the distribution of reference ranges with gender, age, MELD score (higher and lower than 20) and indications for transplantation (liver pathology) were also investigated. In particular, a MELD cut-off value of 20 was chosen to verify the possible correlation between thromboelastographic reference range and MELD score. This cut-off is the most frequently used parameter in the literature for predicting mortality risk after LT[12,13]. Two TEG® 5000 Hemostasis Analyzers (Haemoscope Inc., Skokie, Illinois, United States) were used. The strength of clot formation is graphically represented over time as the tracing shown in Figure 1.
Figure 1.
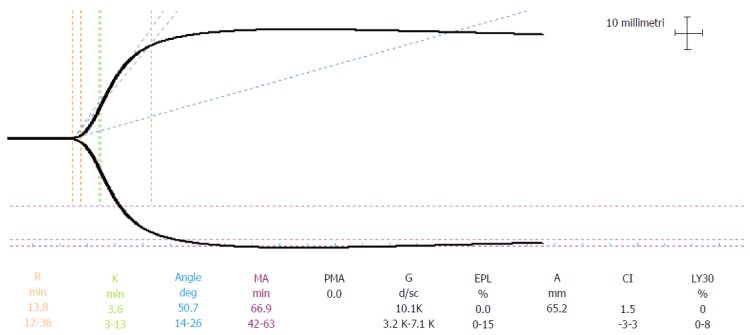
Normal trace. The reference ranges are those defined by manufacturer thromboelastography® 5000 Hemostasis Analyzers (Haemoscope Inc., United States). PMA: Projected MA.
Maintenance and quality controls were performed daily in accordance with manufacturer recommendations. Native arterial blood samples were collected from a radial artery cannulated before induction of anaesthesia and were analysed without adding anticoagulant or activator. We routinely use heparinase TEG, only after reperfusion in all cases and from the baseline only in patients with fulminant liver failure.
Blood samples were always handled by the same three anaesthesiologists. TEG tracings were started within 4 min after sampling. Clot formation was triggered by contact activation. TEG tracings were displayed before the surgical procedure in the operating room. Parameters normally used to assess the process of coagulation are as follows[8,14]: r (coagulation time) is the time from the start of the TEG tracing until the TEG trace amplitude reaches 2 mm. This represents the rate of initial fibrin formation and is functionally related to plasma clotting factors and circulating inhibitor activity. Prolongation of the r time may be a result of coagulation factor deficiencies or severe hypofibrinogenemia; k (Clot Formation time) is measured from r to the point where the amplitude of the tracing reaches 20 mm. This is the time taken to reach a standard clot firmness and is affected by the activity of the intrinsic clotting factors, fibrinogen and platelet; α Angle (Angle-Rate of polymerization of clot) is the angle formed by the slope of the TEG tracing from the r to the k value. This represents the rate of clot growth and describes the polymerization of the structural elements involved in clotting[15]; MA (Maximum Clot Firmness) is the maximum amplitude of the TEG tracing. This reflects the strength of the clot and is a direct result of the function of platelets and plasma factors and their interaction; the A30 and A60 parameters are the amplitudes of the TEG tracing at 30 min and 60 min after MA is measured; the Ly30 and Ly60 (Fibrinolysis at 30 and 60 min after MA) parameters measure percent lysis at 30 and 60 min after MA is reached. The Ly30 and Ly60 measurements are based on the reduction of the area under the TEG tracing from the time MA is measured until 30 (or 60) min after the MA.
Statistical analysis
Continuous data are reported as the mean ± SD (range) and/or median (reference ranges) and were compared using the two-sided Student’s t test for normally distributed parameters. Continuous non-normally distributed data were compared using the Wilcoxon-Mann-Whitney test. Comparisons between groups for categorical variables were performed using the χ2 test with Yates’ correction or Fisher’s exact test when appropriate. Descriptive methods were used to calculate the 2.5% and 97.5% percentiles according to the NCCLS guidelines to establish reference ranges[16]. Reference ranges were not calculable for groups of less than 40 cases. Statistical significance was set at P < 0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0., IBM Corp., Armonk, NY. The statistical review of the study was performed by a biomedical statistician.
RESULTS
The demographic profiles and laboratory data of the patient population and their indication for LT are shown in Table 1.
Table 1.
Demographic and laboratory data of the Patient Population and their indication for liver transplantation
| Study group (n = 261) | |
| Males/females (n/n), % | (193/68) 73.9%/26.1% |
| Age (yr) | 53.5 ± 9.4 |
| Body mass index (kg/m2) | 26.18 ± 6.40 |
| MELD score | 24 ± 6.5 |
| Indication for liver transplantation (n, %) | |
| Alcoholism | 40 (15.3 %) |
| Viral | 189 (72.4%) |
| Colestatic | 15 (5.7%) |
| Other | 17 (6.5 %) |
| HCC | 107 (41 %) |
| Laboratory data | |
| Hb (g/dL) | 11.3 ± 2.2 (nv:12-16) |
| Hct (%) | 3.4 ± 6.2 (nv: 36-46) |
| PLT (103/μL) | 83.2 ± 66.7 (nv: 150-450) |
| PT (%) | 53.6 ± 22.4 (nv: 70-100) |
| INR | 1.7 ± 0.7 (nv: 0.84-1.24) |
| aPTT ratio | 2.0 ± 9.3 (nv: 0.82-1.24) |
| Fibrinogen (mg/dL) | 190 ± 120 (nv: 200-400) |
| ATIII (%) | 50 ± 27(nv: 80-120) |
Data are expressed as the median ± SD. MELD: Model for end stage liver disease; HCC: Hepatocellular carcinoma; PLT: Platelets; PT: Prothrombin time; INR: International normalized ratio; nv: Normal values.
Reference value distribution in the whole population
Median, minimum and maximum value and reference ranges, for the whole population of cirrhotic patients undergoing LT and comparison with healthy subjects, are presented for r, k, α, MA, A30, A60, Ly30 and Ly60 in Table 2.
Table 2.
Medians, means, ranges and reference ranges (2.5%-97.5% percentiles) for thromboelastographic variables obtained from the study population (261 cirrhotic patients) and from the 40 healthy patients
| r (min) | k (min) | α (degree) | MA (mm) | A(30) mm | A(60) mm | Ly30 (%) | Ly60 (%) | |
| Cirrhotic patient population (n = 261) | ||||||||
| Reference values | 6.2-58.5 | 4.2-39.2 | 3.4-42.8 | 10.4-63.5 | 9.8-62 | 92-62 | 0-4 | 0-10 |
| Mean ± SD | 23.7 ± 12.5 | 14.9 ± 9.6 | 18.2 ± 10 | 35.3 ± 12.8 | 33.8 ± 12.8 | 32.3 ± 12.6 | 0.38 ± 1 | 2.28 ± 4.3 |
| Median (range) | 21.8 (2.2/75.4) | 12.3 (1.6/68.1) | 16.1 (1.7/67) | 33.6 (2.2/71.9) | 33 (2/86) | 31 (2.2/85.5) | 0.0 (0/11) | 0.40 (0/44) |
| Healthy population (n = 40) | ||||||||
| Reference values | 11-26 | 3-14 | 15-46 | 43-64 | 41-64 | 42-63 | 0-4 | 0-5 |
| Mean ± SD | 19.6 ± 1.3 | 9.8 ± 0.9 | 20.6 ± 1.2 | 43.7 ± 2.9 | 43.2 ± 3.1 | 42.9 ± 0.8 | 0.8 ± 2.5 | 0.9 ± 2.1 |
| Median (range) | 17.8 (8-27) | 7.2 (2-15) | 18.1 (13-48) | 41.5 (41-66) | 42 (39-67) | 41.7 (41-65) | 0.7 (0-5) | 0.76 (0-7) |
| 1P | 0.039 | < 0.001 | 0.131 | < 0.001 | < 0.001 | < 0 .001 | 0.06 | 0.038 |
| Number of tests below normal | 25 (9.5%) | 2 (0.76%) | 112 (42.9%) | 200 (77%) | 192 (74%) | 207 (79%) | 0 | 0 |
| Number of tests above normal | 84 (32%) | 125 (47.9%) | 2 (0.8%) | 6 (2.3%) | 5 (1.9%) | 5 (1.9%) | 2 (0.76%) | 28 (10.7%) |
| Total number of tests outside the healthy population range | 109 (41.5%) | 127 (48.6%) | 114 (43.7%) | 206 (79.3%) | 197 (74.4%) | 212 (80.9%) | 2 (0.76%) | 28 (10.7%) |
Number of test results outside the normal reference range proposed by the manufacturer. r: Time to initial fibrin formation; k: Time to clot formation; α: Alpha angle, rate of clot formation; MA: Maximum amplitude, absolute clot strength; A30: Maximum amplitude at 30 min after MA; Ly30: Fibrinolysis at 30 min after MA; Ly60: Fibrinolysis at 60 min after MA;
P value expresses the significant differences between the mean values obtained from the study population and from the healthy population.
Most TEG reference values from patients with end-stage liver disease (ESLD) were found to be outside the suggested normal ranges and were abnormal in up to 79.3% of subjects. Wide differences were found for all TEG variables, including r (41.5% of the values), k (48.6%), α (43.7%), MA (79.3%), A30 (74.4%) and A60 (80.9%), indicating a prevailing trend to hypocoagulability. The differences between mean TEG values obtained from healthy subjects and the cirrhotic population were statistically significant for r (P = 0.039), k (P < 0.001), MA (P < 0.001), A30 (P < 0.001), A60 (P < 0.001) and Ly60 (P = 0.038), indicating slower and less stable clot formation in cirrhotic patients (Table 2). In the cirrhotic population 25 (9.5%), patients had r values shorter than normal, indicating a tendency to faster clot formation.
Reference values distribution according to patient gender, age and liver disease characteristics
A comparison of the average values of TEG parameters in the cirrhotic patient population did not show any statistically significant difference for gender and age (Table 3). Gender and age were not significantly associated with greater clot firmness or with enhanced whole blood clot formation (Table 3).
Table 3.
Median and reference ranges for thromboelastography assay in the study population according to gender, age, model for end-stage liver disease, liver disease and presence of hepatocellular carcinoma
| r (min) | k (min) | α (degree) | MA (mm) | A (30) mm | A (60) mm | Ly30 (%) | Ly60 (%) | |
| Females (n = 68) | 22.7 (7.6-58.6) | 12.5 (3-38.5) | 16.5 (4.1-52.2) | 38.1 (10.3-70) | 37.7 (8.5-71.1) | 35.5 (6.7-71.1) | 0.0 (0-4) | 0.25 (0-26.5) |
| Males (n = 193) | 22.8 (5.8-61.5) | 13.5 (3.2-44.9) | 15.8 (3.9-49.8) | 34 (8.1-71.2) | 33.4 (8.1-75) | 3.3 (6.7-75) | 0.0 (0-4) | 0.4 (0-10) |
| P | 0.9 | 0.97 | 0.74 | 0.57 | 0.37 | 0.29 | 0.64 | 0.9 |
| < 60 yr (n = 181) | 21 (5.1-57.6) | 12.2 (4.1-40.9) | 16.7 (3.7-42.9) | 32.5 (10.4-62.6) | 32 (9.8-59.4) | 30.2 (9.2-57.7) | 0.0 (0.0-4.1) | 0.2 (0.0-9.8) |
| ≥ 60 yr (n = 80) | 22.7 (10.2-65.1) | 13 (5.3-40) | 15.6 (2.4-35.4) | 37.8 (6.7-70.7) | 37.2 (6.7-70.7) | 35.2 (6.7-70.7) | 0.0 (0-3.5) | 0.2 (0-9.8) |
| P | 0.08 | 0.8 | 0.1 | 0.2 | 0.2 | 0.12 | 0.76 | 0.54 |
| MELD < 20 (n = 90) | 19.4 (8-59.8) | 11.6 (2.6-40.5) | 18.3 (4.1-56.8) | 38.9 (19.9) | 38.4 (17-69.6) | 35.9 (8.2-71.6) | 0.0 (0-4.9) | 0.8 (0-25.3) |
| MELD ≥ 20 (n = 171) | 22.3 (5.7-58.6) | 13 (4.4-40.3) | 15.4 (3.2-42.2) | 31.3 (9-62.2) | 31 (9.1-61.5) | 30 (9.1-60) | 0.0 (0-4) | 0.10 (0-9.6) |
| P | 0.9 | 0.66 | 0.07 | < 0.001 | < 0.001 | < 0.001 | 0.19 | 0.76 |
| Not alcoholic (n = 216) | 21.2 (5.6-58.7) | 13.1 (4.1-41) | 15.4 (1.8-32.4) | 30.2 (2.8-70.4) | 30 (2.8-70.4) | 29 (2.8-70.4) | 0.0 (0-1.4) | 0.3 (0-6.9) |
| Alcoholic (n = 45) | 22.5 (10.4-63.3) | 12.8 (3.6-30.8) | 15 (2.2-45.3) | 33.9 (5.7-64.2) | 33.8 (5.7-64.2) | 33.1 (5.7-63.9) | 0 (0-10.4) | 0.4 (0-42.5) |
| P | 0.68 | 0.81 | 0.2 | 0.16 | 0.18 | 0.19 | 0.95 | 0.97 |
| HCV absence (n = 111) | 21.8 (7.4-68.5) | 11.5 (4.5-54.4) | 16.7 (3-40.6) | 37.6 (8.9-70.1) | 36.6 (8.9-70.1) | 33.9 (8.9-68.6) | 0.0 (0.0-3.6) | 0.4 (0-9.4) |
| HCV presence (n = 150) | 21.5 (5.2-53.7) | 13.1 (4.1-36) | 15.7 (4-43.3) | 31.4 (10.5-59.3) | 30.9 (9.9-59.1) | 30 (8.5-57.7) | 0 (0-4.2) | 0.15 (0-13.9) |
| P | 0.31 | 0.62 | 0.43 | 0.013 | 0.021 | 0.023 | 0.65 | 0.43 |
| HBV absence (n = 206) | 22.0 (6.4-57.9) | 13 (4.2-38.1) | 16 (3.8-42.8) | 33.9 (10.2-65.8) | 33.6 (9.1-67.3) | 31.2 (7.4-67) | 0 (0-4) | 0.3 (0-10.4) |
| HBV presence (n = 55) | 21 (3.8-65) | 11.2 (2-67.3) | 16.7 (3.2-56.1) | 33.3 (10.7-55.7) | 32.5 (10.7-55.6) | 30.1 (10.7-53.4) | 0 (0-4.1) | 0.4 (0-14.4) |
| P | 0.34 | 0.4 | 0.36 | 0.25 | 0.2 | 0.16 | 0.74 | 0.99 |
| Not cholestatic (n = 246) | 21.7 (6.2-57.9) | 12.3 (4.1-40) | 15.5 (3.4-42.4) | 33 (10.2-58.6) | 32.1 (9.6-58.5) | 30.2 (9-57.1) | 0.0 (0-3.9) | 0.4 (0-10.4) |
| Cholestatic (n = 15) | 22.2 (NA) | 11.5 (NA) | 18.1 (NA) | 53.4 (NA) | 53.4 (NA) | 53.3 (NA) | 0.0 (NA) | 0.3 (NA) |
| P | 0.15 | 0.53 | 0.38 | 0.001 | 0.001 | 0.001 | 0.26 | 0.5 |
| HCC absence (n = 154) | 2.7 (7.6-65.6) | 12.2 (4.2-42.2) | 16.5 (3.1-42.9) | 33.9 (10.7-65.1) | 33 (10.7-62.8) | 30.8 (9.7-62.8) | 0 (0-4) | 0.4 (0-9.1) |
| HCC presence (n = 107) | 23.2 (4.8-55.8) | 13 (4.1-39.6) | 15.8 (3.4-42.8) | 33.3 (9.3-64.9) | 33.3 (8-64.9) | 31.5 (8.3-64.6) | 0.0 (0-3.4) | 0.1 (0-12.5) |
| P | 0.56 | 0.6 | 0.3 | 0.76 | 0.84 | 0.82 | 0.6 | 0.87 |
MELD: Model for End-stage Liver Disease; HCV: Hepatitis C virus; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; r: Time to initial fibrin formation; k: Time to clot formation; α: Alpha angle, rate of clot formation; MA: Maximum amplitude, absolute clot strength; A30: Maximum amplitude at 30 min after MA; Ly30: Fibrinolysis at 30 min after MA; Ly60: Fibrinolysis at 60 min after MA.
Patients with a MELD score less than 20 showed greater clot firmness (higher MA, A30 and A60) compared with patients with a MELD score above 20, with MA (P < 0.001), A30 (P < 0.001) and A60 (Table 3, P < 0.001).
As shown in Table 3, the presence of hepatocellular carcinoma (HCC) or alcoholic cirrhosis did not result in faster coagulation activation (shorter r and k) or greater clot firmness (higher MA, A30, or A60). Patients with a MELD score under 20 showed no thromboelastographic difference based on the presence of HCC. Patients with HCV-related cirrhosis did not show faster activation of the coagulation process but showed significantly greater clot firmness compared with the other patients enrolled in the study because of end stage liver disease, according to MA (P = 0.013), A30 (P = 0.021) and A60 values (P = 0.023). Instead, hepatitis B virus-related cirrhosis did not appear to have any significant influence on clot activation or strength.
The clot strength of patients transplanted for cholestatic disease was enhanced (higher MA, A30, and A60; all with P < 0.001) compared with patients without cholestatic liver disease, and activation of the coagulation process did not result in faster activation (Table 3).
DISCUSSION
Several authors have found a relatively poor correlation between bleeding and laboratory indices of coagulation in patients with chronic liver disease[17,18]. INR and PTT explore only the first 5% of whole thrombin formation[19,20] and are performed without adding thrombomodulin, making these techniques less optimal for exploring the physiological mechanisms regulating thrombin formation. The inadequacy of laboratory methods and the production of technologies applied to blood coagulation analysis have increased interest in thromboelastography for the management of acute perioperative bleeding[21-24]. TEG offers a rapid and global view of the coagulation processes[15,25-27], but in spite of these advantages, users should keep in mind the poor reproducibility, the wide boundaries of normality, the lack of standardization[8,28] and the need to define local normal ranges[28].
Although TEG is a useful viscoelastic test for haemostatic monitoring, interpretation of its results requires care. In particular, the normal ranges of TEG variables may not apply under different operating and patient conditions such as in the cirrhotic patient population.
In the present study, we determined the range of distribution for TEG variables in a population of patients receiving a first liver graft for ESLD or HCC, with a MELD score between 15 and 40. We also underlined the differences in TEG values obtained from cirrhotic patients from those recorded in the normal, healthy population. In the cirrhotic population the r and k values were above the upper limit of normality in 32% and 47.9% of the population, respectively, indicating significant reduced activation of clot formation. In our population, the mean plasma fibrinogen concentration, PT, INR, aPTT and platelet number were outside the normal laboratory reference range, indicating a reduction in clotting factors and platelet number, which are typical features of ESLD and could be a possible explanation for prolonged r and k values. The heparin-like effect (HLE) may also be another possible explanation for the longer r time recorded in the baseline tracings. This effect is not often represented in the first basal tracing (before the beginning of the surgical operation) and is usually less pronounced than that observed after reperfusion or in patients with acute liver failure[29]. Because only 6% of patients undergoing LT have a severe HLE at baseline, which does not seem to correlate with an increase in blood requirements[30], we do not usually perform this test at baseline, and we can only argue that a basal prolongation of the r time may more often be related to coagulation factor deficiencies or hypofibrinogenaemia than to HLE, as shown in the laboratory data.
If a large percentage of r and k values were abnormally prolonged, then in 58.5% and 51.4% of cases, the same parameters were within the range of normality, expressing normal clot activation and firmness. This observation is in line with Stravitz’ study[31] that showed that the mean and median TEG parameters were within normal limits in a cohort of 273 patients with stable cirrhosis. Nevertheless, we studied a population of patients with decompensated cirrhosis, and we observed normal coagulation parameters in half of the cases and a shorter than normal r value in 9.5% of cases, indicating a tendency to faster clot activation. These observations are in line with the new concept of rebalanced haemostasis, which better describes the coagulation condition of cirrhotic patients and is usually not represented in conventional laboratory tests[10]. However, the haemostatic balance in a patient with liver disease is relatively unstable as evidenced by the occurrence of both bleeding and thrombotic complications[27]. The shorter r values observed in 9.5% of patients could indicate cirrhotic patients’ tendency to develop thromboembolic complications at appreciable rates (between 0.5% and 1.9%)[32,33]. Another observation derived from the comparison of the two studied groups was the reduced clot firmness observed in the cirrhotic patient group. MA, A30 and A60 values were below the lower limit of normality for healthy people in up to 77%, 74% and 79% of patients, respectively. Thrombocytopenia, a typical feature of chronic liver disease[34,35], may justify the high number of patients with lower values of MA, A30 and A60 compared with the normal population. Thrombocytopenia, i.e., platelet counts between 30 and 100 × 109/L[36], is usually a sign of advanced liver atrophy[37] and is frequently observed in cirrhotic patients arriving in the operating room for LT. Because of increased levels of von Willebrand factor and low levels of ADAMTS 13 metalloproteinase, cirrhotic patients can compensate for platelet abnormalities[38]. Another possible explanation for these deteriorating TEG parameters may be the hypo- and dysfibrinogenemia associated with liver disease[39,40]. In our patient population, the mean preoperative platelet number was 83.2 ± 66.7 × 109/L, which has been shown in experimental observations to be sufficient to secure in vitro thrombin generation[41], whereas the mean plasma fibrinogen concentration was 190 ± 122 mg/dL, a value that can require correction in cases of severe bleeding[24]. So, a possible explanation for the reduced MA amplitude observed in the study could be a reduction in plasma fibrinogen concentration or fibrinogen function. Specific thrombelastographic tests[42,43] may be helpful for determining the combined effects of thrombocytopenia and hypofibrinogenaemia. Unfortunately, we have only been using TEG functional fibrinogen assays to detect signs of functional fibrinogen deficit in our intraoperative management since 2013, and we did not have enough data to identify the role of platelets and fibrinogen in determining MA amplitude.
Ly30 and Ly60, unlike the other parameters studied, have been shown to differentiate between the values recorded in healthy patients in a smaller number of subjects. Ly30 and Ly60 reference ranges were different from the healthy population in 0.76% and 10.7% of samples that were above the upper limit of normality. Cirrhosis has been variably associated with an increased tendency to fibrinolysis; however, hypofibrinolysis can also be the result of reduced levels of plasminogen and increased levels of plasminogen activator inhibitor[34].
Therefore, although contrasting results have been reported, the balance of fibrinolytic processes is most likely restored in patients with liver disease by the parallel changes in the circulating levels of pro-fibrinolytic and anti-fibrinolytic agents[18]. This phenomenon could explain the low number of patients who showed abnormal Ly30 and Ly60 values. During liver transplant, primary hyperfibrinolysis may occur in up to 60% of cases but is usually confined to the phase of hepatectomy and reperfusion[44,45].
Because of the unique haemostatic behaviour of cirrhotic patients, specific thromboelastographic ranges have to be considered when managing liver transplant patients. Even if it was not the point of the study to demonstrate the clinical advantage of interpreting the TEG traces, taking into account the “reference ranges” for cirrhotic patients in term of blood products savage, we think that when managing bleeding during surgery, it would most likely be useful to correct TEG values while keeping in mind the reference ranges for this category of patients and not for healthy patients.
Realizing the wide variation in patient characteristics and in the causes of ESLD, we divided our cirrhotic population into subgroups of patients based on gender, age, MELD score and liver disease characteristics. For the potential effect of gender on TEG values, our analysis did not find any difference in coagulation activation and in clot firmness between females and males. Our results do not support the findings of Gorton et al[46] who showed enhanced coagulation activity in females with non-activated thromboelastography. Chronic liver disease induces a severe dysfunction of sex hormone metabolism, causing feminization in men and infertility and amenorrhoea in women[47]. This may explain the absence of difference in coagulation activation between males and females observed in our study. Lang et al[48] showed small differences in ROTEM variables between males and females that were not always statistically significant and argued that a sex-related definition of reference ranges in thromboelastometry is not necessary.
For age, we were not able to find any thromboelastographic signs of increased coagulability related to advanced age as otherwise described by Ng et al[49] who showed that hypercoagulability increases progressively beyond age sixty. In our study, r, k, α and MA were not dependent on age. The variables are functionally related to levels of plasma clotting factors, fibrinogen, platelets and activity of circulating inhibitors. It is possible that hypercoagulability, which is usually associated with advancing age due to increased plasma concentrations of fibrinogen, factor VII and factor IX, has not been observed in aged patients because of ESLD and coagulation factor synthesis impairment[49,50].
In accordance with another study[15], we found significantly higher clot firmness in cholestatic patients compared with cirrhotic patients undergoing liver transplant for other causes. Usually, patients with cholestatic cirrhosis show higher fibrinogen levels as well as stable or even increased platelet function[51], which can justify the significantly higher clot firmness observed in the group of patients transplanted for cholestatic disease.
Patients with HCV-related cirrhosis showed a significant tendency towards higher clot firmness (higher MA, A30 and A60), which was not observed in patients without HCV infection. In HCV liver diseases, Panasiuk et al[52] showed evidence of in vivo platelet activation, as suggested by the increased concentrations of b-thromboglobulin and platelet factor 4 in serum. Furthermore, plasma-soluble P-selectin levels have been shown to be markedly elevated in chronic hepatitis C[53], and this infection might be directly responsible for in vivo platelet activation and for the higher MA values observed in patients suffering from this disease.
The presence of HCC nodules has been associated by Samonakis et al[54] and by Krzanicki et al[55], even if with a very low prevalence of hypercoagulability, with a thrombophilic tendency and with thrombotic complications. For this reason, we would have expected to see faster coagulation activation (shorter r) and/or greater clot firmness (higher MA), but we did not observe any signs of hypercoagulation. HCC did not appear to be responsible for a higher thrombophilic tendency in the study population, even in subgroups of patients with a low MELD score (15-20) and a minor coagulation impairment.
Patients affected by alcoholic or hepatitis B cirrhosis did not show any significant difference in clot formation or strength.
Cirrhotic patients with a MELD score under 20 had significantly better MA, A30, and A60 values than patients with a score above 20 (P < 0.001), which could be an expression of greater stability of the clot related to less severe liver disease and better coagulation function[56,57]. In particular, r, k, and α were within normal limits, although the maximum amplitude was decreased. As previously showed by Stravitz et al[31] in patients with stable cirrhosis, global haemostasis is maintained, while the mean maximum amplitude of clot formation can be below normal limits. Our cohort of patients with a MELD score less than 20 represents a lower grade of liver disease severity and, for this reason, is more similar to the results described by Stravitz.
Our study showed how TEG value distribution in patients with ESLD is very different from that obtained from a healthy population. The coagulation system in healthy patients is characterized by a greater functional reserve of both procoagulants and anticoagulants, and it is unlikely that the thromboelastographic reference ranges of a healthy population are also representative of patients with ESLD. In healthy people, “normal” range also means normal coagulation balance. Patients with liver disease may show a satisfactory coagulation balance without spontaneous bleeding, even if their TEG values are outside the normal ranges observed in healthy people. However, this was a descriptive and not an outcome study, and we think that this study’s findings should always be kept in mind when TEG data are interpreted in patients with ESLD. It was not possible to directly demonstrate the clinical effect of interpreting the TEG in cirrhotic patients with or without taking these “normal” variations into account. Thromboelastographic ranges in liver transplant candidates are so different from normal subjects that specific ranges for cirrhotic patients have to be defined. Because of the unique coagulation condition of cirrhotic, TEG ranges representative of this category of patients, have probably to be considered in all bleeding conditions avoiding to correct these parameters to normal TEG ranges for healthy patients. In the last few years, several transfusion algorithms have been proposed, aiming at developing a better treatment for haemostasis in patients with coagulopathy and bleeding, but none of these algorithms have been built using values typically obtained from cirrhotic patient candidates. For this reason, our group has already shown how specific thromboelastographic cut off values, adapted for cirrhotic patients, can be used to guide blood product infusions before invasive procedures, ensuring patient safety and avoiding bleeding episodes[58]. Similarly, Wang et al[21] showed that TEG values higher than normal in transplant recipients may not have a reliable predictive value of increased blood loss during surgery. In their study, the authors adopted a TEG-guided transfusion protocol using higher threshold values to initiate transfusions, without observing any negative consequences. Therefore, standard TEG values obtained from healthy volunteers may be misleading for patients with liver disease.
This study presents the following possible limitations: TEG suffers from a lack of proven standardization[8,9], and pre-analytical factors such as sampling and sample handling could play a significant role in coagulation testing. Due to the manual steps, such as placement of pin and cup or pipetting a sample, operator-to-operator variability had to be considered. Another possible limitation is that the range of distribution described in this population could most likely only be applied to our reality and is not necessarily representative of other liver transplant centres.
In conclusion, the comparison between thromboelastographic parameters of cirrhotic patients and those of healthy subjects have shown many differences that are the ultimate expression of the different coagulation balance typical of cirrhotic subjects. The analysis of the cirrhotic population has also demonstrated how a MELD score greater than 20 and HCV infection-related cirrhosis may be related to the formation of a less stable clot, and patient candidates for LT due to cholestatic liver diseases are capable of forming more stable and durable clots. The TEG values described in this population of candidates for liver transplantation, although very different from those of a healthy population, are however an expression of a new haemostatic balance that cirrhotic patients reach and, in conditions of stability, does not result in spontaneous bleeding. The observation of a shorter than normal r value in 10% of cirrhotic patients should make the reader remember that such a population of patients can face thrombotic as well as haemorrhagic problems during surgery because of their unstable haemostatic balance. Determining a range of distribution for TEG values in a very specific population of cirrhotic patients could be important for the implementation of a transfusion protocol based on a point-of-care device that could help in properly guiding coagulation therapy. If the imperative is the correction of the thromboelastographic parameters only in the presence of active bleeding, aiming to restore TEG values to those suggested as “normal” could lead to an over-correction of the coagulation abnormalities typical of cirrhotic patients. This hypothesis needs to be confirmed by detailed clinical trials on the medical utility of new TEG reference ranges for the management of perioperative haemostasis in cirrhotic patient clinical settings.
COMMENTS
Background
Standard laboratory tests (international normalized ratio, activated partial thromboplastin time) fail to give comprehensive information about the bleeding tendency and coagulation status of cirrhotic patients because they are not standardized across centres when used for patients with liver disease and are performed in the absence of thrombomodulin. All of these limits have progressively increased the interest in thromboelastography (TEG), which assesses the overall coagulation process beyond the initiation of clot formation. However, this methodology is not standardized, and when defining reference values, the TEG analyzer manufacturer suggests that each new user should test 20 healthy volunteers to generate “his own” normal values to be used locally as reference values. The normal TEG values reported by manufacturers and the literature are determined from the average clotting time of healthy volunteers, making them unreliable and potentially misleading in the management of patients with liver disease. It is very important to try to generate a more reliable picture of a common cirrhotic patient coagulation profile to properly manage these patients during liver transplant (LT).
Research frontiers
Many publications have shown that TEG-based transfusion algorithms are useful in the management of blood products during LT, but the proposed cut-off value for transfusion is subject to great variability. The values proposed as indices of transfusion are often detected in patients with cirrhosis without being associated with bleeding. In this study, similar to reference values obtained from healthy people, the authors tried to study TEG value distribution in a group of patient candidates for LT. Stravitz, in a cohort of 273 patients with stable cirrhosis, found that the mean and median TEG parameters were within normal limits, although the maximum amplitude was decreased in proportion to the severity of thrombocytopenia due to hypersplenism. In contrast with this author, the authors studied patients with decompensated cirrhosis who arrived in the operating theatre with rebalanced haemostasis, which differs considerably from healthy people but can be “normal” for cirrhotic patients.
Innovations and breakthroughs
Stable cirrhotic patients do not have inherent bleeding diathesis but rather a reduced reserve that can be readily tipped towards a bleeding or thrombotic tendency. In the last few years, several transfusion algorithms have been proposed, aiming to develop a better treatment for haemostasis in patients with coagulopathy and bleeding, but none of these algorithms have been built using values typically obtained from cirrhotic patient candidates. In contrast with Stravitz, the authors studied a population of patients with decompensated cirrhosis, with candidates for liver transplant having normal coagulation parameters in almost half of cases and more rapid clot formation in a small percentage of patients. The authors could show which reference range distributions in a population of patient candidates for LT should be taken into account when administering blood products during LT. However, this is a descriptive and not an outcome study, and the authors think that these findings should always be kept in mind when TEG data are interpreted in patients with end-stage liver disease.
Application
Stable cirrhotic patients do not have an inherent bleeding diathesis but rather a reduced reserve that can be readily tipped towards a bleeding or thrombotic tendency. The liver disease patient has a new balanced haemostatic profile that corresponds with TEG values that are very different from those observed in healthy people but that are within the range of normality in almost half of the liver transplant candidates studied. Even if it was not possible to directly demonstrate the clinical effect of interpreting the TEG traces, taking into account the “reference ranges” for cirrhotic patients, the authors think that in cases of bleeding episodes or intraoperative haemorrhage, it would most likely be useful to correct TEG values while keeping in mind the reference ranges for this category of patients to avoid unnecessary blood product transfusions.
Terminology
TEG offers a more targeted approach for assessing the overall outcome of the interactions of clotting factors beyond the initiation of clot formation. Although TEG is a useful viscoelastic test for haemostatic monitoring, interpretation of its results requires care, especially in cirrhotic patients in whom they have already shown that specific cut off values are necessary to guide blood products infusion. Liver transplantation is the only therapeutic approach for end-stage liver disease. It is a surgical procedure characterized by deep haemodynamic, coagulation and biochemical repercussions that are different depending on the surgical stage (laparotomy, pre-anhepatic, anhepatic, or reperfusion phase) observed.
Peer-review
This is a very interesting observational study and the manuscript has been well written.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Azienda Ospedaliera-Universitaria, Modena (139/14 Trigger) and was conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Informed consent statement: All patients or their legal guardian, provided a written informed consent prior to study enrolment.
Conflict-of-interest statement: All the Authors have no conflict of interest related to the manuscript.
Data sharing statement: The original anonymous dataset is available on request from the corresponding author at lesley.depietri@yahoo.it.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 12, 2016
First decision: June 12, 2016
Article in press: August 16, 2016
P- Reviewer: Abdel-Razik A, Sharma M S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, Mannuccio Mannucci P. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553–558. doi: 10.1002/hep.20569. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs MJ, Wong A, MacKinnon K, Weir K, Keeney M, Boyle E, Cruickshank M. Assessment of the validity of the INR system for patients with liver impairment. Thromb Haemost. 1994;71:727–730. [PubMed] [Google Scholar]
- 3.Kenison JAR, Smith A, Brimhall B, Phillips C, Olson J. Interlaboratory variation in INR leads to clinically relevant changes in MELD score: survey of US clinical laboratories. Am J Transpl. 2006;6:333. [Google Scholar]
- 4.Ji HW, Ma L, Gao XR, Liu N, Zhang Y, Wang Y, Ma ZX, Wang Y, Wang J, Fu X, et al. Establishment of normal reference values for thromboelastography on Chinese population in Beijing. Zhonghua Yixue Zazhi. 2011;91:980–983. [PubMed] [Google Scholar]
- 5.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–1217. doi: 10.1590/s0100-879x2009001200015. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. TEG hemostasis analyzer-User manual. Niles: Haemoscope Corporation; 2004. [Google Scholar]
- 8.Vig S, Chitolie A, Bevan DH, Halliday A, Dormandy J. Thromboelastography: a reliable test? Blood Coagul Fibrinolysis. 2001;12:555–561. doi: 10.1097/00001721-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hobson AR, Agarwala RA, Swallow RA, Dawkins KD, Curzen NP. Thrombelastography: current clinical applications and its potential role in interventional cardiology. Platelets. 2006;17:509–518. doi: 10.1080/09537100600935259. [DOI] [PubMed] [Google Scholar]
- 10.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 11.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Bruns H, Lozanovski VJ, Schultze D, Hillebrand N, Hinz U, Büchler MW, Schemmer P. Prediction of postoperative mortality in liver transplantation in the era of MELD-based liver allocation: a multivariate analysis. PLoS One. 2014;9:e98782. doi: 10.1371/journal.pone.0098782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaib E, Figueira ER, Brunheroto A, Gatti AP, Fernandes DV, D’Albuquerque LA. Does the patient selection with MELD score improve short-term survival in liver transplantation? Arq Bras Cir Dig. 2013;26:324–327. doi: 10.1590/s0102-67202013000400014. [DOI] [PubMed] [Google Scholar]
- 14.Chitlur M, Sorensen B, Rivard GE, Young G, Ingerslev J, Othman M, Nugent D, Kenet G, Escobar M, Lusher J. Standardization of thromboelastography: a report from the TEG-ROTEM working group. Haemophilia. 2011;17:532–537. doi: 10.1111/j.1365-2516.2010.02451.x. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Ari Z, Panagou M, Patch D, Bates S, Osman E, Pasi J, Burroughs A. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol. 1997;26:554–559. doi: 10.1016/s0168-8278(97)80420-5. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS. How to Define and Determine Reference Intervals in the Clinical Laboratory; Approved Guideline. 2nd ed. Wayne, PA: NCCLS; 2000. [Google Scholar]
- 17.Tripodi A, Mannucci PM. Abnormalities of hemostasis in chronic liver disease: reappraisal of their clinical significance and need for clinical and laboratory research. J Hepatol. 2007;46:727–733. doi: 10.1016/j.jhep.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, Tripodi A, Sanyal AJ. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–1046. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 19.Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost. 2003;1:1504–1514. doi: 10.1046/j.1538-7836.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 20.McCully SP, Fabricant LJ, Kunio NR, Groat TL, Watson KM, Differding JA, Deloughery TG, Schreiber MA. The International Normalized Ratio overestimates coagulopathy in stable trauma and surgical patients. J Trauma Acute Care Surg. 2013;75:947–953. doi: 10.1097/TA.0b013e3182a9676c. [DOI] [PubMed] [Google Scholar]
- 21.Wang SC, Lin HT, Chang KY, Mandell MS, Ting CK, Chu YC, Loong CC, Chan KH, Tsou MY. Use of higher thromboelastogram transfusion values is not associated with greater blood loss in liver transplant surgery. Liver Transpl. 2012;18:1254–1258. doi: 10.1002/lt.23494. [DOI] [PubMed] [Google Scholar]
- 22.Saner FH, Gieseler RK, Akız H, Canbay A, Görlinger K. Delicate balance of bleeding and thrombosis in end-stage liver disease and liver transplantation. Digestion. 2013;88:135–144. doi: 10.1159/000354400. [DOI] [PubMed] [Google Scholar]
- 23.Lisman T, Porte RJ. Pitfalls in assessing platelet activation status in patients with liver disease. Liver Int. 2012;32:1027; author reply 1028. doi: 10.1111/j.1478-3231.2011.02737.x. [DOI] [PubMed] [Google Scholar]
- 24.Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA, De Robertis E, Filipescu DC, Fries D, Görlinger K, Haas T, Imberger G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 25.Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW, Starzl TE, Winter PM. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 26.Schaden E, Saner FH, Goerlinger K. Coagulation pattern in critical liver dysfunction. Curr Opin Crit Care. 2013;19:142–148. doi: 10.1097/MCC.0b013e32835ebb52. [DOI] [PubMed] [Google Scholar]
- 27.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878–885. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald SG, Luddington RJ. Critical factors contributing to the thromboelastography trace. Semin Thromb Hemost. 2010;36:712–722. doi: 10.1055/s-0030-1265288. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal B, Wright G, Gatt A, Riddell A, Vemala V, Mallett S, Chowdary P, Davenport A, Jalan R, Burroughs A. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol. 2012;57:780–786. doi: 10.1016/j.jhep.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal S, Senzolo M, Melikian C, Burroughs A, Mallett SV. The prevalence of a heparin-like effect shown on the thromboelastograph in patients undergoing liver transplantation. Liver Transpl. 2008;14:855–860. doi: 10.1002/lt.21437. [DOI] [PubMed] [Google Scholar]
- 31.Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol (NY) 2012;8:513–520. [PMC free article] [PubMed] [Google Scholar]
- 32.Northup PG, McMahon MM, Ruhl AP, Altschuler SE, Volk-Bednarz A, Caldwell SH, Berg CL. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101:1524–1528; quiz 1680. doi: 10.1111/j.1572-0241.2006.00588.x. [DOI] [PubMed] [Google Scholar]
- 33.Gulley D, Teal E, Suvannasankha A, Chalasani N, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53:3012–3017. doi: 10.1007/s10620-008-0265-3. [DOI] [PubMed] [Google Scholar]
- 34.Tripodi A. The coagulopathy of chronic liver disease: is there a causal relationship with bleeding? No. Eur J Intern Med. 2010;21:65–69. doi: 10.1016/j.ejim.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Violi F, Basili S, Raparelli V, Chowdary P, Gatt A, Burroughs AK. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55:1415–1427. doi: 10.1016/j.jhep.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Mannucci PM, Tripodi A. Liver disease, coagulopathies and transfusion therapy. Blood Transfus. 2013;11:32–36. doi: 10.2450/2012.0151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bleibel W, Caldwell SH, Curry MP, Northup PG. Peripheral platelet count correlates with liver atrophy and predicts long-term mortality on the liver transplant waiting list. Transpl Int. 2013;26:435–442. doi: 10.1111/tri.12064. [DOI] [PubMed] [Google Scholar]
- 38.Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53–61. doi: 10.1002/hep.21231. [DOI] [PubMed] [Google Scholar]
- 39.Green G, Thomson JM, Dymock IW, Poller L. Abnormal fibrin polymerization in liver disease. Br J Haematol. 1976;34:427–439. doi: 10.1111/j.1365-2141.1976.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 40.Martinez J, MacDonald KA, Palascak JE. The role of sialic acid in the dysfibrinogenemia associated with liver disease: distribution of sialic acid on the constituent chains. Blood. 1983;61:1196–1202. [PubMed] [Google Scholar]
- 41.Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell’Era A, Fabris F, Salerno F, Mannucci PM. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44:440–445. doi: 10.1002/hep.21266. [DOI] [PubMed] [Google Scholar]
- 42.Kalina U, Stöhr HA, Bickhard H, Knaub S, Siboni SM, Mannucci PM, Peyvandi F. Rotational thromboelastography for monitoring of fibrinogen concentrate therapy in fibrinogen deficiency. Blood Coagul Fibrinolysis. 2008;19:777–783. doi: 10.1097/MBC.0b013e32830ef90c. [DOI] [PubMed] [Google Scholar]
- 43.Herbstreit F, Winter EM, Peters J, Hartmann M. Monitoring of haemostasis in liver transplantation: comparison of laboratory based and point of care tests. Anaesthesia. 2010;65:44–49. doi: 10.1111/j.1365-2044.2009.06159.x. [DOI] [PubMed] [Google Scholar]
- 44.Görlinger K. Coagulation management during liver transplantation. Hamostaseologie. 2006;26:S64–S76. [PubMed] [Google Scholar]
- 45.Sabate A, Dalmau A, Koo M, Aparicio I, Costa M, Contreras L. Coagulopathy management in liver transplantation. Transplant Proc. 2012;44:1523–1525. doi: 10.1016/j.transproceed.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Gorton HJ, Warren ER, Simpson NA, Lyons GR, Columb MO. Thromboelastography identifies sex-related differences in coagulation. Anesth Analg. 2000;91:1279–1281. doi: 10.1097/00000539-200011000-00042. [DOI] [PubMed] [Google Scholar]
- 47.Burra P. Liver abnormalities and endocrine diseases. Best Pract Res Clin Gastroenterol. 2013;27:553–563. doi: 10.1016/j.bpg.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301–310. doi: 10.1097/01.mbc.0000169225.31173.19. [DOI] [PubMed] [Google Scholar]
- 49.Ng KF. Changes in thrombelastograph variables associated with aging. Anesth Analg. 2004;99:449–554, table of contents. doi: 10.1213/01.ANE.0000133140.75831.1E. [DOI] [PubMed] [Google Scholar]
- 50.Javorschi S, Richard-Harston S, Labrouche S, Manciet G, Freyburger G. Relative influence of age and thrombotic history on hemostatic parameters. Thromb Res. 1998;91:241–248. doi: 10.1016/s0049-3848(98)00104-2. [DOI] [PubMed] [Google Scholar]
- 51.Pihusch R, Rank A, Göhring P, Pihusch M, Hiller E, Beuers U. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002;37:548–555. doi: 10.1016/s0168-8278(02)00239-8. [DOI] [PubMed] [Google Scholar]
- 52.Panasiuk A, Prokopowicz D, Zak J, Matowicka-Karna J, Osada J, Wysocka J. Activation of blood platelets in chronic hepatitis and liver cirrhosis P-selectin expression on blood platelets and secretory activity of beta-thromboglobulin and platelet factor-4. Hepatogastroenterology. 2001;48:818–822. [PubMed] [Google Scholar]
- 53.Ferroni P, Mammarella A, Martini F, Paoletti V, Cardarello CM, Labbadia G, Donnarumma L, De Matteis A, Gazzaniga PP, Musca A, et al. Increased soluble P-selectin levels in hepatitis C virus-related chronic hepatitis: correlation with viral load. J Investig Med. 2001;49:407–412. doi: 10.2310/6650.2001.33785. [DOI] [PubMed] [Google Scholar]
- 54.Samonakis DN, Koutroubakis IE, Sfiridaki A, Malliaraki N, Antoniou P, Romanos J, Kouroumalis EA. Hypercoagulable states in patients with hepatocellular carcinoma. Dig Dis Sci. 2004;49:854–858. doi: 10.1023/b:ddas.0000030099.13397.28. [DOI] [PubMed] [Google Scholar]
- 55.Krzanicki D, Sugavanam A, Mallett S. Intraoperative hypercoagulability during liver transplantation as demonstrated by thromboelastography. Liver Transpl. 2013;19:852–861. doi: 10.1002/lt.23668. [DOI] [PubMed] [Google Scholar]
- 56.Esmat Gamil M, Pirenne J, Van Malenstein H, Verhaegen M, Desschans B, Monbaliu D, Aerts R, Laleman W, Cassiman D, Verslype C, et al. Risk factors for bleeding and clinical implications in patients undergoing liver transplantation. Transplant Proc. 2012;44:2857–2860. doi: 10.1016/j.transproceed.2012.09.085. [DOI] [PubMed] [Google Scholar]
- 57.Asrani SK, Kim WR. Model for end-stage liver disease: end of the first decade. Clin Liver Dis. 2011;15:685–698. doi: 10.1016/j.cld.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, Gerunda GE, di Benedetto F, Garcia-Tsao G, Villa E. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63:566–573. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]


