Abstract
Background:
Several lines of evidence suggest that the cholinergic system may be disrupted in schizophrenia and so this may contribute to the cognitive impairments of schizophrenic patients. Because such deficits do not respond to neuroleptic treatment, different approaches have been done by acetylcholinesterase inhibitors (AChEIs). The objective of the present assessment was to evaluate the safety and clinical effects of rivastigmine, as an adjunctive drug, on the clinical symptoms of schizophrenia.
Methods:
A total of 46 patients with a diagnosis of schizophrenia entered into a 12-week, double-blind, clinical trial for random assignment to rivastigmine or placebo, as adjuvant to their current antipsychotic medication. Positive and Negative Symptom Scale (PANSS) and Mini Mental State Examination (MMSE) had been used as the primary outcome measures. Clinical Global Impressions- Improvement (CGI-I) Scale and Extrapyramidal Symptom Rating Scale (ESRS) had been used as the secondary measures. Treatment efficacy was evaluated by a Student’s t test and repeated-measures analysis of variance (ANOVA). Statistical significance was defined as a two-sided p value ⩽ 0.05. Cohen’s standard (d) and correlation measures of effect size (r) had been calculated for comparing baseline to endpoint changes.
Results:
According to the findings, except for significant enhancement of MMSE by rivastigmine (p < 0.001), no significant improvement in PANSS (negative symptoms), PANSS (positive symptoms), and PANSS (general psychopathology) was evident in the target group. Also, except for significant improvement of CGI-I by rivastigmine in intragroup analysis, no significant effectiveness was evident in between-group analysis or repeated-measures ANOVA. ESRS, also, did not show any significant alteration in either group. Effect size (ES) analysis showed a large improvement in MMSE by rivastigmine.
Conclusions:
According to the findings, while rivastigmine could not induce significant improvement of positive and negative symptoms of schizophrenia, it caused significant enhancement of cognitive function in this group of patients.
Keywords: cholinesterase inhibitors, rivastigmine, schizophrenia
Introduction
Schizophrenia is a mental disorder, which is usually characterized by strange social behavior and lack of insight. Its diverse clinical features have caused a doubt that whether such a diagnosis characterizes a solitary disorder or a collection of separate syndromes [Buckley et al. 2009]. In addition, the mean life expectancy of persons suffering from this illness is 10–25 years lower than the mean life expectancy of people without that problem [Laursen et al. 2012]. Perhaps, this is due to a higher risk of suicide and missed somatic complications among this group of patients [Hor and Taylor, 2010]. In patients who progress to chronic schizophrenia, the acute symptoms have often largely resolved, with or without treatment [Kapur, 2009] and the characteristic picture becomes one of a ‘burnt out’ disease. These features are called negative symptoms [Shoja Shafti, 2015]. But the ‘positive–acute’ and ‘negative–chronic’ distinction is not absolute. Positive symptoms regularly persist or re-emerge in chronic cases, and some patients have negative symptoms in their first episode. Prominent, enduring negative symptoms are sometimes called deficit syndrome [Shoja Shafti, 2015; Shoja Shafti et al. 2015]. Residual schizophrenia is a transitional state between acute and chronic schizophrenia. It describes patients with positive symptoms within the past year who have also developed negative symptoms [World Health Organization, 1992]. Recently, lots of effort has been done to recognize and manage the prodromal phase of schizophrenia, which may be identified about 30 months in advance of overt psychosis [Hirsch and Weinberger, 2003]. While specific care has been paid to the role of dopamine in the mesolimbic circuit of the brain, the dopamine theory is now supposed to be imperfect [Marshall and Rathbone, 2011]. Specific attention, also, has been paid to the role of glutamate and its receptor in schizophrenia, mostly due to abnormal levels of glutamate receptors that have been found in the postmortem brains of schizophrenic patients [Jones and Pilowsky, 2002], and the finding that glutamate-blocking drugs such as ketamine and phencyclidine may simulate the signs and symptoms of schizophrenia [Konradi and Heckers, 2003]. Antipsychotics are effective in both the acute and maintenance treatment of schizophrenia and other psychotic disorders [Tandon et al. 2008; Shoja Shafti and Kaviani, 2015]. While antipsychotic medication remains the mainstay of treatment for schizophrenia and has been in use for a long time, as evidenced by ongoing research and partial effectiveness of the antipsychotics on cognitive and negative symptoms, the search is on for drugs that may improve these domains of functioning for someone suffering from schizophrenia. Acetylcholinesterase inhibitors (AChEIs) have long been in use for treating cognitive symptoms of dementia [Singh et al. 2012]. Cognitive deficits have been described in patients with schizophrenia from the first descriptions of dementia praecox to current concepts of cognitive disorders. Nevertheless, little is known about how to deal with them. In Alzheimer’s disease, cholinergic deficit is found and cholinesterase inhibitors have been used to delay the progression of memory and cognitive dysfunction. Several lines of evidence suggest that the cholinergic system may be disrupted in schizophrenia [Ribeiz et al. 2010]. It means that, alterations such as reduced muscarinic and nicotinic receptors in the central cholinergic system in patients with schizophrenia may contribute to these cognitive impairments. Because such deficits do not respond to neuroleptic treatment, different approaches have been developed regarding pharmacological treatments that enhance central cholinergic transmission, such as AChEIs [Voss et al. 2008]. Donepezil (Aricept), rivastigmine (Exelon), and galantamine (Reminyl) are cholinesterase inhibitors used to treat mild-to-moderate cognitive impairment in dementia of the Alzheimer’s type. They reduce the inactivation of the neurotransmitter acetylcholine and, thus, potentiate cholinergic neurotransmission, especially in the hippocampus and cerebral cortex, which in turn produces a modest improvement in memory and goal-directed thought [Qassem et al. 2008]. In long-term use, they slow the progression of memory loss and diminish apathy, depression, hallucinations, anxiety, euphoria, and purposeless motor behaviors [Qassem et al. 2008]. In this regard, cortical acetylcholine (ACh) depletion has also been supposed to be associated with visual hallucinations and the level of depletion is said to be related directly to the severity of the symptoms. Conditions with underlying cholinergic deficits and high rates of visual hallucinations include Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease. Current understanding of neurobiological visual processing and research in diseases with reduced cholinergic function, suggests that AChEIs may prove beneficial in treating visual hallucinations [Patel et al. 2010]. As augmentative agents, while according to some studies, cholinesterase inhibitors have shown useful influences respecting the improvement of cognitive function [Friedman, 2004], or enhancement of learning, memory, and attention [Lenzi et al. 2003] and finally, improvement of psychotic symptoms of schizophrenic patients [Qassem et al. 2008], contradictory or unsuccessful trials are existent in this regard [Voss et al. 2008; Stip et al. 2007; Chouinard et al. 2007; Ferreri et al. 2006; Kumari et al. 2006; Sharma et al. 2006]. Among available cholinesterase inhibitors, rivastigmine is generally well tolerated, but recommended dosages may need to be scaled back in the initial period of treatment to limit gastrointestinal and central nervous system adverse effects. Also, rivastigmine does not appear to cause hepatic, renal, hematologic, or electrolyte abnormalities [Qassem et al. 2008]. The aim of the present appraisal was to evaluate safety and clinical effects of rivastigmine as an adjunctive medication in conjunction with antipsychotic drugs, in patients with schizophrenia.
Methods
A total of 46 male inpatients with a diagnosis of schizophrenia, according to the Diagnostic and Statistical Manual of Mental Disorders [APA, 2013] after full description of the method and obtaining signed informed consent, entered into one of the designated groups, for random assignment to rivastigmine or placebo, as adjuvant to their current antipsychotic medication (which included one of the conventional antipsychotics like chlorpromazine, haloperidol, trifluperazine and perphenazine). Due to possible advantages of atypical antipsychotics on cognitive, depressive and negative symptoms of schizophrenia, on one hand, and definitively fewer extra-pyramidal adverse effects, on the other hand, their consumption was included in the exclusion criteria in the present assessment [Harrison et al. 2010]. Because the field of study was restricted to the chronic male district of the hospital, all samples were selected from the accessible male patients. This evaluation was approved by Academia’s Medical Ethics Commission . The assessment was performed based on a double-blind design. Separation of patients into groups was based on the number of the bed: odd numbers were placed into the target group and even numbers into the control group. Patients, staff and an assessor (a skilled clinical psychiatrist) were uninformed regarding the recommended medications, which were filled into similar capsules. Also, for the duration of study, no further psychotropic medication or psychosocial intervention was recommended or permitted. All the patients had been diagnosed with schizophrenia from at least 2 years previously, and the existence of any other identified psychiatric disorder, other than schizophrenia, in axis I, or any comorbid medical or neurological disorder, resulted in exclusion of the patient from the trial. Rivastigmine was started at a dosage of 3 mg per day in the first week, with biweekly increments of 3 mg per day up to 12 mg in the week 6. This dose was then held constant up to the end of the appraisal. Positive and Negative Symptom Scale (PANSS) [Kay et al. 1988] and Mini Mental State Examination (MMSE) [Folstein, 1975] were used as the primary outcome measures. Clinical Global Impressions-Improvement (CGI-I) Scale [Guy, 1976] and the Extrapyramidal Symptom Rating Scale (ESRS) [Chouinard et al. 1980] were used as the secondary measures. The length of the study was 12 weeks, and the cases were assessed by PANSS, MMSE, CGI-I and ESRS at baseline (week 0), and at weeks 6 and 12. Adverse effects of drugs were examined by another associate psychiatrist at weekly visits or were based on the staff reports.
Statistical analysis
Samples were compared regarding baseline characteristics with a Student’s t test. Treatment efficacy was evaluated by a Student’s t test and a repeated-measures analysis of variance (ANOVA) comparing both groups over 12 weeks. Statistical significance was defined as a two-sided p value ⩽ 0.05. With respect to the significant variations, Cohen’s standard (d) and correlation measures of effect size (r) were calculated for comparing baseline and endpoint changes. MedCalc Statistical Software version 15.2 was used as a statistical software tool for analysis.
Results
Groups were primarily analogous and demographic and diagnostic variables were comparable (Table1). Also, analysis for efficacy was based on information from an equal amount of samples in both groups. A total of 5 patients in the target group left the trial due to GI problems (n = 3) or reluctance (n = 2), and 5 patients in the control group left the trial due to reluctance (n = 3) or digestive complications (n = 2). According to the findings and intragroup analysis of data, and in comparison with the baseline, no significant improvement in mean total scores of PANSS was evident regarding positive, negative and general psychopathology in either the rivastigmine group or the placebo group (p < 0.18, p < 0.44, p < 0.53 and p < 0.24, p < 0.49, p < 0.71, respectively) (Table 2). Repeated-measures ANOVA did not show any significant change concerning the aforesaid assessments in both groups (F [2, 51] = 0.815, p < 0.44, SS = 6.04 MSe = 3.70; F [2, 51] = 0.663, p < 0.51, SS = 13.48, MSe = 10.17; F [2, 51] = 1.34, p < 0.27, SS = 104.15, MSe = 39.01; and F [2, 51] = 0.668, p < 0.51, SS = 4.59, MSe = 3.44; F [2, 51] = 0.833, p < 0.44, SS = 19.70, MSe = 11.83; F [2, 51] = 1.30, p < 0.28, SS = 125.04, MSe = 48.12, respectively). In addition, between-group analysis did not demonstrate any significant difference between the target group and control group with respect to the aforesaid assessments (Table 3). But with respect to MMSE, while a significant enhancement was evident in the target group at the end of the assessment (p < 0.001), no significant improvement was obvious in the control group (p < 0.92). Repeated-measures ANOVA, also, showed a significant improvement in the target group (F [2, 51] = 4.22, p < 0.02, SS = 20.26, MSe = 2.40) and insignificant alteration in the control group (F [2, 51] = 1.67, p < 0.19, SS = 32.15, MSe = 9.62). Between-group analysis also exhibited a significant difference between rivastigmine and placebo, with respect to the improvement of MMSE at the end of the trial (p < 0.0001), which was evident from the week 6 (p < 0.009) (Table 3). Moreover, a split-plot (mixed) design ANOVA revealed, once more, the significant influence of rivastigmine in comparison with placebo on MMSE (F (4, 153) = 3.89, p < 0.004, SS = 613.80, MSe = 39.48). With regard to CGI-I, while, according to intragroup analysis, a significant improvement was evident in the rivastigmine group (p < 0.05) (Table 2), it was not similarly significant in between-group analysis at the end of the study and in comparison with the control group, ( 0.09) (Table 3). In this regard, a split-plot (mixed) design ANOVA, also, did not show any significant difference between rivastigmine and placebo (F [4, 153] = 11.7, p < 0.08, SS = 420.73, MSe = 8.98). With regard to ESRS, intragroup analysis and between-group analysis did not show any significant alteration in either group. Since the sample size was small, the effect size (ES) was analyzed for changes in MMSE and CGI-I, at the end of assessment, which showed a large (d = 0.8 or r = 0.73) and around medium (d = 0.5 or r = 0.24) improvement with rivastigmine (1.18 and 0.50, and 0.66 and 0.31, as Cohen’s d, and ES correlation r, respectively). Post hoc power analysis showed an intermediary power = 0.43 on behalf of this trial, which turned to power = 0.77 in the frame of compromise power analysis. The most common adverse effects of rivastigmine in the present study included nausea (n = 2), vomiting (n = 1), dizziness (n = 1), and diarrhea (n = 1). The adverse effects in the control group also included nausea (n = 1), vomiting (n = 1), and dizziness (n = 1). A comparison of proportions did not show any significant difference between the two groups regarding the occurrence of adverse effects (z = 0.80, p = 0.42, CI 95% = −0.38, 0.16).
Table 1.
Demographic and clinical characteristics of participants.
| Variables | Rivastigmine n = 18 | Placebon = 18 | t | p | CI |
|---|---|---|---|---|---|
| Age | 44.56 ± 5.8 | 6.44 ± 4.11 | 0.22 | 0.82 | −3.69, 4.59 |
| Duration of illness | 23.56 ± 6.15 | 23.89 ± 5.46 | −0.17 | 0.86 | −4.27, 3.61 |
| PANSS-positive symptoms | 16.2 ± 4.4 | 15.4 ± 4 | 0.57 | 0.57 | −2.05, 3.65 |
| PANSS-negative symptoms | 19.6 ± 9.2 | 21.1 ± 9.1 | −0.49 | 0.62 | −7.70, 4.70 |
| PANSS-general psychopathology | 36.1 ± 7.6 | 38.5 ± 8.2 | −0.91 | 0.36 | −7.76, 2.96 |
| MMSE-baseline | 24.1 ± 2.9 | 23 ± 3.2 | 1.08 | 0.28 | −0.97, 3.17 |
| CGI-I-baseline | 3.03 ± 0.14 | 3.06 ± 0.26 | −0.43 | 0.66 | −0.17, 0.11 |
| ESRS | 8.7 ± 2.6 | 9 ± 2.6 | −0.34 | 0.73 | −2.06, 1.46 |
CGI-I, Clinical Global Impressions-Improvement; ESRS, Extrapyramidal Symptom Rating Scale; MMSE, Mini Mental State Examination; PANSS, Positive and Negative Symptom Scale.
Table 2.
Intragroup analysis of different outcome measures between baseline and week 12.
| Variables | Rivastigmine baseline | Rivastigmine week 12 | t | p | CI | Placebo baseline | Placebo week 12 | t | p | CI |
|---|---|---|---|---|---|---|---|---|---|---|
| PANSS-positive symptoms | 16.2 ± 4.4 | 14.2 ± 4.4 | 1.36 | 0.18 | −0.98, 4.98 | 15.4 ± 4 | 13.8 ± 4.1 | 1.18 | 0.24 | −1.14, 4.34 |
| PANSS-negative symptoms | 19.6 ± 9.2 | 17.2 ± 9.6 | 0.76 | 0.44 | −3.97, 8.77 | 21.1 ± 9.1 | 19 ± 9 | 0.69 | 0.49 | −4.03, 8.23 |
| PANSS-general psychopathology | 36.1 ± 7.6 | 34.6 ± 6.9 | 0.62 | 0.53 | −3.42, 6.42 | 38.5 ± 8.2 | 37.5 ± 7.8 | 0.37 | 0.71 | −4.42, 6.42 |
| MMSE | 24.1 ± 2.9 | 27.1 ± 2.1 | −3.55 | 0.001 | −4.72, –1.28 | 23 ± 3.2 | 23.1 ± 3.2 | −0.09 | 0.92 | −2.27, 2.07 |
| CGI-I | 3.03 ± 0.14 | 2.66 ± 0.78 | 1.98 | 0.05 | −0.01, 0.75 | 3.06 ± 0.26 | 3.01 ± 0.38 | 0.46 | 0.64 | −0.17, 0.27 |
| ESRS | 8.7 ± 2.6 | 10 ± 3.4 | −1.28 | 0.20 | −3.35, 0.75 | 9 ± 2.6 | 10 ± 2.5 | −1.17 | 0.24 | −2.73, 0.73 |
CGI-I, Clinical Global Impressions-Improvement; ESRS, Extrapyramidal Symptom Rating Scale; MMSE, Mini Mental State Examination; PANSS, Positive and Negative Symptom Scale.
Table 3.
Between-group analysis of different outcome measures in weeks 0, 6 and 12.
| Variables | Rivastigmine | Placebo | t | p | CI |
|---|---|---|---|---|---|
| PANSS-positive symptoms week 0 | 16.2 ± 4.4 | 15.4 ± 4 | 0.57 | 0.57 | −2.05, 3.65 |
| PANSS-positive symptoms week 6 | 15.2 ± 4.9 | 15.3 ± 3.8 | −0.06 | 0.94 | −3.07, 2.87 |
| PANSS-positive symptoms week 12 | 14.2 ± 4.4 | 13.8 ± 4.1 | 0.28 | 0.77 | −2.48, 3.28 |
| PANSS-negative symptoms week 0 | 19.6 ± 9.2 | 21.1 ± 9.1 | −0.49 | 0.62 | −7.70, 4.70 |
| PANSS-negative symptoms week 6 | 18.1 ± 9.1 | 20.4 ± 9.1 | −0.75 | 0.45 | −8.46, 3.86 |
| PANSS-negative symptoms week 12 | 17.2 ± 9.6 | 19 ± 9 | −0.58 | −0.58 | −8.10, 4.50 |
| PANSS-general psychopathology week 0 | 36.1 ± 7.6 | 38.5 ± 8.2 | −0.91 | 0.36 | −7.76, 2.96 |
| PANSS-general psychopathology week 6 | 35.3 ± 7.1 | 37.7 ± 7.9 | −0.95 | −0.95 | −7.49, 2.69 |
| PANSS-general psychopathology week 12 | 34.6 ± 6.9 | 37.5 ± 7.8 | −1.18 | 0.24 | −7.89, 2.09 |
| MMSE week 0 | 24.1 ± 2.9 | 23 ± 3.2 | 1.08 | 0.28 | −0.97, 3.17 |
| MMSE week 6 | 26.1 ± 2.9 | 23.3 ± 3.2 | 2.75 | 0.009 | 0.73, 4.87 |
| MMSE week 12 | 27.1 ± 2.1 | 23.1 ± 3.2 | 4.43 | 0.0001 | 2.17, 5.83 |
| CGI-I week 0 | 3.03 ± 0.14 | 3.06 ± 0.26 | −0.43 | 0.66 | −0.17, 0.11 |
| CGI-I week 6 | 3.00 ± 0.29 | 3.05 ± 0.13 | −0.66 | 0.50 | −0.20, 0.10 |
| CGI-I week 12 | 2.66 ± 0.78 | 3.01 ± 0.38 | −1.71 | 0.09 | −0.77, 0.07 |
| ESRS week 0 | 8.7 ± 2.6 | 9 ± 2.6 | −0.34 | 0.73 | −2.06, 1.46 |
| ESRS week 6 | 9.1 ± 3.4 | 10.1 ± 2.5 | −1.00 | 0.32 | −3.02, 1.02 |
| ESRS week12 | 10 ± 3.4 | 10 ± 2.5 | 0.00 | 1.00 | −2.02, 2.02 |
CGI-I, Clinical Global Impressions-Improvement; ESRS, Extrapyramidal Symptom Rating Scale; MMSE, Mini Mental State Examination; PANSS, Positive and Negative Symptom Scale.
Figure 1.
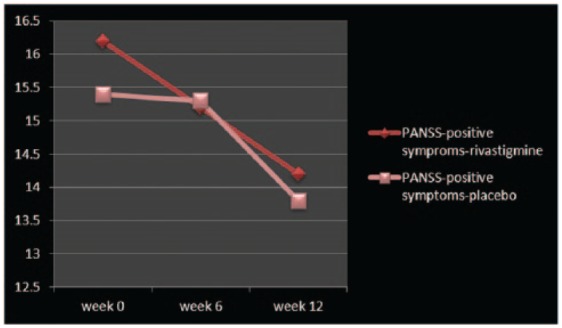
Changes of PANSS-positive symptoms between baseline and week 12.
PANSS, Positive and Negative Symptom Scale.
Figure 2.
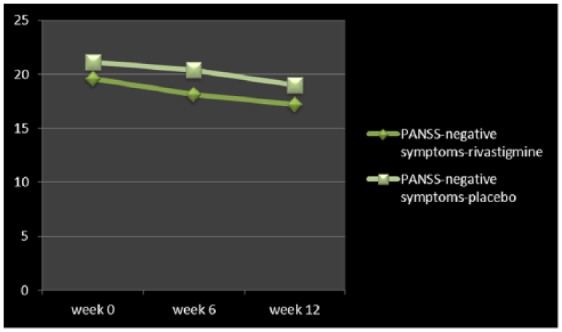
Changes of PANSS-negative symptoms between baseline and week 12.
PANSS, Positive and Negative Symptom Scale.
Figure 3.
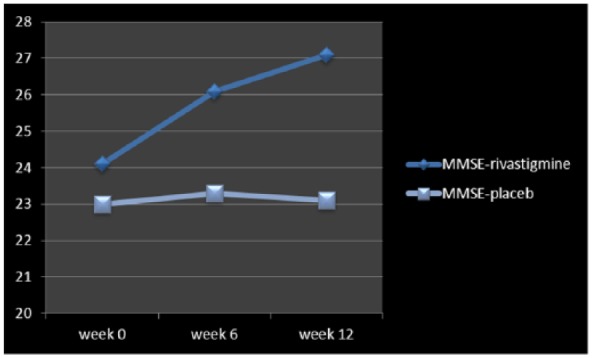
Changes of MMSE between baseline and week 12.
MMSE, Mini Mental State Examination.
Discussion
Schizophrenia is commonly considered a neurodevelopmental disorder that is associated with significant morbidity; however, unlike other neurodevelopmental disorders, the symptoms of schizophrenia often do not manifest for decades. In most patients, the formal onset of schizophrenia is preceded by prodromal symptoms, including positive symptoms, mood symptoms, cognitive symptoms, and social withdrawal. The proximal events that trigger the formal onset of schizophrenia are not clear but may include developmental biological events and environmental interactions or stressors [Jeffrey et al. 2001]. Because the clinical deterioration that occurs in schizophrenia may actually begin in the prepsychotic phase, early identification and intervention may favorably alter the course and outcome of schizophrenia [Jeffrey et al. 2001]. Research has shown that the symptoms of chronic schizophrenia fall more clearly into three clusters, rather than the two implied by the positive–negative distinction. The three are reality distortion (delusions and hallucinations), disorganization (thought disorder) and psychomotor poverty (similar to negative symptoms) [World Health Organization, 1992]. Problems with memory and attention are a neglected aspect of schizophrenia. The most affected domains are attention, working memory and semantic memory. These deficits are superimposed on a generalized intellectual deficit averaging about one standard deviation, though there is a wide range, and many patients score above normal [World Health Organization, 1992]. The impairments are, like the negative symptoms, basically stable and independent of the positive symptoms. However, the details are controversial. Some decline probably occurs well before the onset of illness, with a further decline around the first episode [World Health Organization, 1992].
Studies of individuals at risk for schizophrenia (such as first-degree relatives and individuals with schizotypal personality disorders) and first-episode patients have found that information-processing deficits are one of the earliest clinical and cognitive markers of vulnerability for schizophrenia [Jeffrey et al. 2001]. Attention deficits are present before the onset of the illness, and executive function impairments, including working memory, are also consistently demonstrated in patients with schizophrenia, as well as those with schizophrenia-spectrum disorders [Jeffrey et al. 2001].
Although attention and executive-function deficits have been associated with prefrontal cortical pathology in schizophrenia, other information-processing deficits suggest a significant breakdown in hippocampal and temporal cortical functions [Jeffrey et al. 2001]. Schizophrenia is a main reason of incapacity and is classified as the third most disabling illness subsequent to dementia and quadriplegia, and ahead of blindness and paraplegia [Miyamoto et al. 2005]. While antipsychotics are known as the principal treatment for schizophrenia, they cannot usually improve the cognitive dysfunction and negative symptoms. Nevertheless, continuous treatment with antipsychotics can decrease the risk of relapse [Zhang et al. 2013]. The main objective of this study was evaluating the effectiveness and safety of rivastigmine as an adjuvant to current antipsychotic treatment in schizophrenic patients. According to the findings, while rivastigmine did not show any significant influence regarding improvement of positive symptoms, negative symptoms and general psychopathology of schizophrenia in the present assessment, it could significantly enhance the cognitive ability of patients in the target group, which was evident in the second half of the trial. Such a conclusion may be favorable for enhancement of the outcome of psychosocial intervention or rehabilitation of this group of patients, which demands another methodical research for measurement of the pertained functional parameters. So, with respect to cognitive function, our finding was in harmony with the findings of Singh and colleagues [Singh et al. 2012], Friedman [Friedman, 2004] and Lenzi and colleagues [Lenzi et al. 2003], and in contrast with Voss and colleagues [Voss et al. 2008] and Sharma and colleagues [Sharma et al. 2006]. In this regard, Lenzi and colleagues, in an open study found that rivastigmine treatment could result in significant improvements in quality of life, cognitive function, learning, memory, attention, and finally ‘anergia’ in the Brief Psychiatric Rating Scale (BPRS) [Lenzi et al. 2003]. Friedman, too, had suggested that, based on the existent studies, specific cognitive deficits (memory, and the motor speed and attention part of executive function) of patients with schizophrenia and schizoaffective disorder are responsive to rivastigmine, donepezil and galantamine as adjunctive therapy. So he had concluded that while a cholinergic approach to ameliorating the cognitive dysfunction of schizophrenia appears viable, there is some preliminary data to support the efficacy of combined AChEIs and allosteric potentiators of the nicotinic receptor [Friedman, 2004]. But in contrast, Sharma and colleagues, in a 24-week randomized, placebo-controlled, double-blind study could not find any significant enhancement in any cognitive measurement by rivastigmine. According to this study, while some cognitive variables showed significant practice effects in both the placebo and rivastigmine groups, no significant effects were noted in symptoms or side effects ratings [Sharma et al. 2006]. Likewise, Voss and colleagues had concluded that thus far randomized, placebo-controlled studies exist only for donepezil and rivastigmine, and none could replicate the positive results of previous trials with open designs [Voss et al. 2008]; conclusions which are not in accordance with our inference; an attitude similar to Stip and colleagues [Stip et al. 2007], Chouinard and colleagues [Chouinard et al. 2007], Ferreri and colleagues [Ferreri et al. 2006], and Kumari and colleagues [Kumari et al. 2006], and in conflict with our inference. Regarding the positive symptoms, while the present trial could not find any beneficial influence, Sachin and colleagues, in their review of the literature, had found beneficial effects from AChEIs for the treatment of visual hallucinations in schizophrenia [Patel et al. 2010], an important finding that demands further methodical studies. Similarly, Singh and colleagues [Singh et al. 2012] had concluded that AChEIs plus antipsychotics had shown a benefit over antipsychotic and placebo in PANSS-negative symptoms, PANSS general psychopathology, in addition to cognitive function, and the results seemed to favor the use of AChEIs in combination with antipsychotics on a few domains of mental state and cognition, while due to various limitations in the studies the evidence was not strong enough [Singh et al. 2012]; a finding that was not comparably reproducible in the present evaluation regarding negative symptoms and general psychopathology of schizophrenia. Instead, while in our assessment the CGI-I of the target group had shown some significant improvement in the intragroup analysis, comparing the starting point with the endpoint, it was not significant in between-group analysis and a repeated-measures ANOVA. So, a reasonable or practical conclusion is not plausible for the moment. Regarding the cognitive measurement in the present assessment, it deserves to be mentioned that while the MMSE is one of the best known cognitive assessment scales in the mental health field, it has limited utility in those with schizophrenia. The MMSE was developed for those with organic disorders (such as dementia) who tend to have difficulties with orientation and language. Indeed, people with schizophrenia rated with the MMSE frequently obtain scores within the normal range. So, maybe other newer measures like ‘Brief Assessment of Cognition in Schizophrenia (BACS)’, with greater validity and reliability in people with schizophrenia would be a more reasonable choice (Keefe et al. 2003). While the most common adverse effects of rivastigmine are nausea, vomiting, diarrhea, dizziness, weight loss, headache, abdominal pain, anorexia, fatigue, and somnolence [Qassem et al. 2008], only the first four were evident in our trial. It should be noticed that the adverse effects of AChEIs are most likely to occur at the start of therapy or when the dose is increased. They are dose-related and tend to be transient. Urinary incontinence has also been reported [Dunn et al. 2000]. In view of their pharmacological action, AChEIs may have vagotonic effects on heart rate (i.e. bradycardia). The potential for this action may be of particular importance in patients with ‘sick sinus syndrome’ or other supraventricular cardiac conduction disturbances, such as sinoatrial or atrioventricular block [Rosenbloom et al. 2010]. But in contrast, rivastigmine appears to be least likely to cause problematic drug interactions, a factor that may be important in an elderly population subject to polypharmacy [Grossberg et al. 2000]. Short duration of study, small sample size, and gender-based sampling were among the weak points of the present assessment. No doubt, the fact of including only men as clinical samples and the limited power of the present design and recruitment are important limitations that disallow the careless generalization of results. Confirmatory studies, especially large, independent, well designed, randomized studies with higher numbers of patients, are needed to determine the clinical utility of this treatment strategy.
Conclusion
According to the findings, while rivastigmine could not induce significant improvement of positive and negative symptoms of schizophrenia, it caused significant enhancement of cognitive function in this group of patients.
Acknowledgments
The authors gratefully acknowledge dear colleagues, S. Mousavi, MD, P. Ghiasie, PhD, and the department of research for their practical and financial support of this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Saeed Shoja Shafti, University of Social Welfare and Rehabilitation Sciences (USWR), Razi Psychiatric Hospital, Tehran, Iran.
Abbas Azizi Khoei, Razi Psychiatric Hospital, Tehran, Iran.
References
- APA (2000) Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC: American Psychiatric Association, pp. 663–666. [Google Scholar]
- Buckley P., Miller B., Lehrer D., Castle D. (2009) Psychiatric comorbidities and schizophrenia. Schizophr Bull 35: 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard G., Ross-Chouinard A., Annable L., Jones B. (1980) Extrapyramidal rating scale. Can J Neurolog Sci 22: 259–263. [Google Scholar]
- Chouinard S., Stip E., Poulin J., Melun J., Godbout R., Guillem F., et al. (2007) Rivastigmine treatment as an add-on to antipsychotics in patients with schizophrenia and cognitive deficits. Curr Med Res Opin 23: 575–583. [DOI] [PubMed] [Google Scholar]
- Dunn N., Pearce G., Shakir S. (2000) Adverse effects associated with the use of donepezil in general practice in England. J Psychopharmacol 14: 406–408. [DOI] [PubMed] [Google Scholar]
- Ferreri F., Agbokou C., Gauthier S. (2006) Cognitive dysfunctions in schizophrenia: potential benefits of cholinesterase inhibitor adjunctive therapy. J Psychiatry Neurosci 31 :369–376. [PMC free article] [PubMed] [Google Scholar]
- Folstein M., Folstien S., McHugh P. (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189. [DOI] [PubMed] [Google Scholar]
- Friedman J. (2004) Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology (Berl) 174: 45–53. [DOI] [PubMed] [Google Scholar]
- Grossberg G., Stahelin H., Messina J., Anand R., Veach J. (2000) Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 15: 242–247. [DOI] [PubMed] [Google Scholar]
- Guy R. (1976) ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. (ADM). Rockville, MD: US Department of Health, Education, and Welfare, pp. 76–338. [Google Scholar]
- Harrison P., Geddes J., Sharpe M. (2010) Lecture Notes: Psychiatry (10th ed.). Oxford: Wiley-Blackwell. [Google Scholar]
- Hirsch S., Weinberger D. (2003) Schizophrenia. Oxford: Wiley-Blackwell, p. 481. [Google Scholar]
- Hor K., Taylor M. (2010) Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol 24: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H., Pilowsky L. (2002) Dopamine and antipsychotic drug action revisited. Br J Psychiatry 181: 271–275. [DOI] [PubMed] [Google Scholar]
- Kapur S. (2009) Schizophrenia. Lancet 374: 635–645. [DOI] [PubMed] [Google Scholar]
- Kay S., Opler L., Lindenmayer J. (1988) Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenia. Psych Res 23: 99–110. [DOI] [PubMed] [Google Scholar]
- Keefe R., Goldberg T., Harvey P. (2003) The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 68: 283–297. [DOI] [PubMed] [Google Scholar]
- Konradi C., Hecker S. (2003) Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Therapeut 2: 153–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V., Aasen I., Ffytche D., Williams S., Sharma T. (2006) Neural correlates of adjunctive rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind fMRI study. Neuroimage 29: 545–556. [DOI] [PubMed] [Google Scholar]
- Laursen T., Munk-Olsen T., Vestergaard M. (2012) Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry 25: 83–88. [DOI] [PubMed] [Google Scholar]
- Lenzi A., Maltinti E., Poggi E., Fabrizio L., Coli E. (2003) Effects of rivastigmine on cognitive function and quality of life in patients with schizophrenia. Clin Neuropharmacol 26: 317–321. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Perkins D., Belger A., Chakos M., Jarskog F., Boteva K., et al. (2001) The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry 50: 884–897. [DOI] [PubMed] [Google Scholar]
- Marshall M., Rathbone J. (2011) Early intervention for psychosis. Cochrane Database Syst Rev 6: CD004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Duncan G., Marx C., Lieberman J. (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10: 79–104. [DOI] [PubMed] [Google Scholar]
- Patel S., Attard A., Jacobsen P., Shergill S. (2010) Acetylcholinesterase inhibitors (AChEIs) for the treatment of visual hallucinations in schizophrenia: A review of the literature. BMC Psychiatry 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qassem A., Cross S., Hopkins F., Adelman A., Mehr D., Schellhase K., et al. (2008) Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 148: 370. [DOI] [PubMed] [Google Scholar]
- Ribeiz S., Bassitt D., Arrais J., Avila R., Steffens D., Bottino C. (2010) Cholinesterase inhibitors as adjunctive therapy in patients with schizophrenia and schizoaffective disorder: a review and meta-analysis of the literature. CNS Drugs 24: 303–317. [DOI] [PubMed] [Google Scholar]
- Rosenbloom M., Finley R., Scheinman M., Feldman M., Miller B. (2010) Donepezil-associated bradyarrhythmia in a patient with dementia with Lewy bodies (DLB). Alzheimer Dis Assoc Disord 24: 209–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T., Reed C., Aasen I., Kumari V. (2006) Cognitive effects of adjunctive 24-weeks rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind investigation. Schizophr Res 85: 73–83. [DOI] [PubMed] [Google Scholar]
- Shoja Shafti S. (2015) Odyssey of negative symptoms of schizophrenia: rehabilitation vs. stigmatization. Curr Psychopharmacol 4: 1–12. [Google Scholar]
- Shoja Shafti S., Jafarabad M., Azizi R. (2015) Amelioration of deficit syndrome of schizophrenia by norepinephrine reuptake inhibitor. Ther Adv Psychopharmacol 5: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoja Shafti S., Kaviani H. (2015) Quetiapine versus aripiprazole in the management of schizophrenia. Ther Adv Psychopharmacol 5: 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Kour K., Jayaram M. (2012) Acetylcholinesterase inhibitors for schizophrenia. Cochrane Database Syst Rev 18(1): CD007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stip E., Sepehry A., Chouinard S. (2007) Add-on therapy with acetylcholinesterase inhibitors for memory dysfunction in schizophrenia: a systematic quantitative review, part 2. Clin Neuropharmacol 30: 218–229. [DOI] [PubMed] [Google Scholar]
- Tandon R., Belmaker R., Gattaz W., Lopez-Ibor J., Okasha A., Singh B., et al. (2008) World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res 100: 20–38. [DOI] [PubMed] [Google Scholar]
- Voss B., Thienel R., Leucht S., Kircher T. (2008) Therapy of cognitive deficits in schizophrenia with acetylcholinesterase inhibitors. A systematic overview. [Article in German] Nervenarzt 79: 47–48, 50,–52, 54–59. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992) International Statistical Classification of Diseases and Related Health Problems (ICD-10). Geneva: World Health Organization, pp. 85–109. [Google Scholar]
- Zhang J., Gallego G., Robinson D., Malhotra A., Kane J., Correll C. (2013) Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol 16: 1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]


